Abstract
Purpose
This study aimed to identify the clinical outcomes, microbiological features and risk factors for difficult-to-treat resistance (DTR) Klebsiella pneumoniae (Kp) infection.
Materials and Methods
A retrospective study was conducted at Peking University Third Hospital from January 2020 to March 2021. DTR was defined as resistance to ≥1 carbapenem, ≥1 extended-spectrum cephalosporin, and ≥1 fluoroquinolone. Hypervirulent Kp (HvKp) was defined as peg-344-, iroB-, iucA-, rmpA-, or rmpA2-positive. Clinical data were collected. Antimicrobial susceptibility testing and string tests were performed to determine resistance and hypermucoviscosity phenotype. Whole genome sequencing was performed to analyze the sequence type (ST), capsular serotypes, resistance and virulence genes. Risk factors for 30-day mortality were analyzed.
Results
Fifty DTR-Kp (50.0%) strains were identified among 100 patients. Compared to non-DTR-Kp group, a significant number of patients with DTR-Kp infection experienced ICU admission (44.0% versus 10.0%, P<0.001) and mechanical ventilation after Kp detection (26.0% versus 10.0%, P=0.037). Notably, the percentage of hvKp among the DTR-Kp isolates increased consistently over the 15 months evaluated. Most DTR-Kp strains belonged to ST11 (82.0%), followed by ST15 (12.0%), ST86 (2.0%), ST996 (2.0%), and ST3157 (2.0%). DTR-Kp isolates possessed various resistance genes, such as blaKPC-2, blaTEM-1D and fosA3 (90.0%, 80.0% and 72.0%, respectively). Importantly, the yersiniabactin genes were significantly clustered in DTR group (48/50, 96.0%). The 30-day mortality was significantly higher in patients with DTR-Kp infection than non-DTR-Kp group (38.0% versus 8.2%, P=0.001). DTR-Kp infection (odds ratio [OR] = 4.196) was an independent risk factor for the 30-day mortality of Kp-infected patients. Additionally, cerebrovascular disease (OR = 2.780) and Charlson comorbidity index (OR= 1.584) were independent risk factors for DTR-Kp infections.
Conclusion
DTR-hvKp is rapidly emerging. The DTR-Kp strains harbored various resistance genes and high rates of yersiniabactin siderophore genes. DTR-Kp infection was an independent risk factor for mortality, suggesting that enhanced awareness essential.
Keywords: Klebsiella pneumoniae, difficult-to-treat resistance, mortality, risk factor
Introduction
Klebsiella pneumoniae (Kp) is an important gram-negative bacterium that can cause various fatal community- and hospital-acquired infections, including pneumonia, bacteremia, pyogenic liver abscess and urinary tract infections.1 Increasing rates of antibiotic resistance among Kp strains have made current antibiotic therapy more challenging.
Previously, the classifiers of coresistance included multidrug resistance (MDR) as nonsusceptibility to ≥1 agent in ≥3 antimicrobial categories; extensive drug resistance (XDR) as susceptibility limited to ≤2 categories; and pandrug resistance (PDR) as nonsusceptibility to all agents in all antimicrobial categories.2 Notably, these classifiers weight all of the antibiotics equally regardless of their efficacy and toxicity and require testing for very broad classes of antibiotics.2,3 Additionally, studies reported that MDR and XDR infections were inconsistently associated with poorer patient outcomes.4–6 In 2018, Kadri et al proposed a new classification for antimicrobial resistance among gram-negative bacteria (GNB): difficult-to-treat resistance (DTR) is defined as in vitro resistance to all high-efficacy, low-toxicity (or first-line) agents, specifically β-lactams and fluoroquinolones.3 DTR focuses on resistance through the lens of a bedside provider who is interested in whether there are any relatively safe and effective antibiotics available to treat the patient rather than how many antibiotic categories a given pathogen is resistant to.7 A previous study reflected that the proportion of ICU stays associated with DTR GNB-infected inpatients reached 73%.8 Indeed, DTR represented an even higher level of resistance and was associated with worse outcomes, which could be useful for surveillance and prognostication.3,9,10
A previous study reported that the most common DTR Enterobacteriaceae was Klebsiella spp.11 Another study detected Klebsiella spp. The DTR rate was 2.8% among the gram-negative bloodstream isolates.12 Specifically, Karlowsky et al observed high DTR rates of 3.0% for Kp.13 Furthermore, Jean et al found that the DTR rate was 10.4% among the 316 Kp isolates collected in 2020 that caused bloodstream infections.14 Importantly, a previous study revealed that 226 (78%) of the isolates out of the 288 meropenem-resistant Kp strains were DTRs.15 A previous retrospective study showed that the most frequent target organism was Kp (17, 56.7%) in hospitalized DTR GNB-infected patients who received IV fosfomycin for ≥48 h.16 Of note, DTR-Kp is rapidly emerging, indicating that close attention should be given to it.
To the best of our knowledge, most previous studies have mainly focused on the DTR GNB, not on Kp exclusively. Data regarding the clinical and microbiological characteristics of DTR-Kp are scarce. Thus, we conducted a retrospective study to investigate the clinical and microbiological characteristics, all-cause death risk and risk factors for DTR-Kp infection.
Materials and Methods
Enrolled Patients
A retrospective study was focused on Kp culture-positive strains collected from January 2020 to March 2021 at Peking University Third Hospital. The average number of open beds in Peking University Third Hospital is about 1800. The clinical data and patient information were obtained from medical records and included basic demographics, medical history, Charlson comorbidity index (CCI), usage of invasive devices, infection site, infection type, blood examination, ICU admission, mechanical ventilation after Kp infections and sequential organ failure assessment (SOFA) score.
The inclusion criteria were as follows: 1) age ≥ 18 years old; 2) Kp was positive on culture and was associated with the clinical infectious manifestations at the same time. The exclusion criteria included the following: 1) the bacterial strain was not viable after storage; and 2) the presence of duplicate isolates from the same patient within 3 months. The study outcomes were survival and all-cause death at 30 days after Kp infection.
The definitions of hospital-acquired infection and community-acquired infection were consistent with a previous study.17 Metastatic infection was defined as the presence of >1 infection site in the same patient.
The study protocol was approved by the Peking University Third Hospital Medical Science Research Ethics Committee (M2021545). Due to the retrospective nature of the study, the need for approval was waived and all the patient’s data enrolled in this study were anonymized. The need for written informed consent was waived by the Peking University Third Hospital Medical Science Research ethics committee due to retrospective nature of the study. We confirm that all methods were performed in accordance with the Declaration of Helsinki.
Clinical Kp Strains
Standardized isolation, culture and identification were performed in the Department of Clinical Microbiology. The identification of the strains relied on the Vitek 2 system (bioMérieux, Marcy-l’Étoile, France). All strains were stored at − 80 °C.
Antimicrobial Susceptibility Testing (AST) and String Test
Antimicrobial susceptibility testing was conducted by the Vitek 2 system (bioMérieux, Marcy-l’Étoile, France) using the N335 card, and the AST results were interpreted according to the 2020 Clinical and Laboratory Standards Institute (CLSI) guidelines.18 The tested antimicrobial agents included piperacillin/tazobactam, cefoperazone/sulbactam, ceftazidime, cefepime, imipenem, meropenem, levofloxacin, amikacin, minocycline, and trimethoprim/sulfamethoxazole. DTR was defined as an intermediate or resistant phenotype to all reported agents in the carbapenem, beta-lactam, and fluoroquinolone categories.3 The 2015 Centers for Disease Control and Prevention (CDC) surveillance definitions were used to classify the isolates into individual resistance phenotypes: carbapenem resistant (CR), extended-spectrum cephalosporin resistant (ESCR) or fluoroquinolone resistant (FQR).19 Therefore, DTR was defined as resistance to piperacillin/tazobactam, cefoperazone/sulbactam, ceftazidime, cefepime, imipenem, meropenem and levofloxacin in this study. The definition of an MDR strain is the resistance to three or more different antimicrobial categories as previously described.2
The hypermucoviscous phenotype was determined by the string test.20 Briefly, the Kp strains were inoculated onto Columbia agar with sheep blood (PB0123A, OXOID, Beijing, China) and were incubated at 37°C overnight. By touching and pulling a single colony upward, generating a viscous string > 5 mm in length was considered a positive string test.
DNA Extraction and Whole Genome Sequencing (WGS)
The DNA of all the Kp isolates was extracted by using the GenePure Pro Automatic Nucleic Acid Purification System (NPA-32P, Bioer Technology, Hangzhou, Zhejiang, China) and MagaBio Bacterium DNA Fast Purification Kit (BSC45S1E, Bioer Technology, Hangzhou, Zhejiang, China). DNA concentration and purity were evaluated by Nanodrop (ThermoFisher, Waltham, America).
All strains were sequenced using the Illumina HiSeq 2500 platform by constructing paired-end libraries to obtain 150 bp reads. The clean data were obtained using fast QC and were assembled using SPAdes (v.3.13) with the default parameters.
Bioinformation Analysis
Whole-genome sequencing was used to analyze the multilocus sequence typing (MLST), capsular serotypes, antimicrobial resistance (AMR) genes and virulence-associated genes. Raw data were filtered to remove the low-quality reads and then were assembled using SPAdes (v.3.13). MLST was distinguished by MLST (v.2.0) (Center for Genomic Epidemiology). The capsular serotypes were analyzed using Kleborate software (v.0.3.0). The AMR and virulence genes were annotated by comparison with relevant databases (ResFinder, Virulence Factor Database) using BLAST software (v.2.2.18). We performed a subgroup analysis between classic Kp (cKp) and hypervirulent Kp (hvKp). HvKp was defined as the combination of peg-344, iroB, iucA, rmpA, or rmpA2 positivity as described previously.21
Statistical Analysis
Data analysis was performed by SPSS software (v.25.0). Measurement data were evaluated as the mean ± standard deviation, and count data were reported as percentages. t-tests and Wilcoxon tests were performed for the analysis of continuous variables. We used the χ2 or Fisher’s exact test for categorical variables. A P value < 0.05 was considered statistically significant, and all tests were 2-tailed. The Kaplan–Meier curve and Log rank tests were performed to evaluate the all-cause mortality within 30 days between the two groups. Univariate logistic regression analysis was performed to identify the risk factors associated with DTR-Kp infection and death. A multivariable logistic regression analysis was conducted for independent risk factors for DTR-Kp infection and death (the variables with P < 0.05 were included).
Results
Clinical Characteristics of the DTR-Kp-Infected Patients
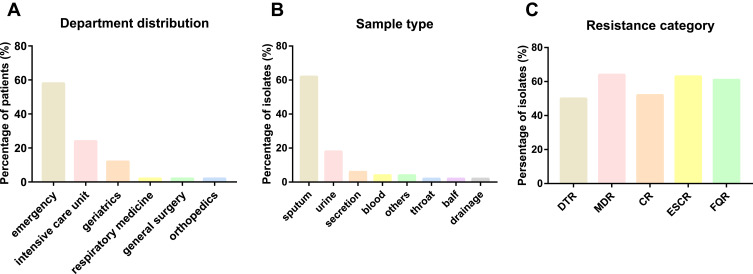
In total, 100 Kp infection patients were collected from January 2020 to March 2021. Among the strains, 50 strains (50.0%, 50/100) were classified as DTR-Kp. The median age of the DTR-Kp-infected patients was 79.54±12.22 years, and 29 patients (58.0%) were male (Table 1). Fifty DTR-infected patients were admitted to six departments, including the emergency department (29/50), ICU (12/50), geriatrics (6/50), respiratory medicine (1/50), general surgery (1/50) and orthopedics department (1/50) (Figure 1A). In this study, most of the DTR-Kp isolates were isolated from the sputum (62.0%), urine (18.0%), secretions (6.0%), blood (4.0%) and so on (Figure 1B). Among all the Kp strains, the MDR, CR, ESCR, and FQR Kp isolates accounted for 64.0%, 52.0%, 63.0%, and 61.0%, respectively (Figure 1C).
Table 1.
Clinical Characteristics of the Patients with DTR vs Non-DTR Klebsiella pneumoniae
| Clinical Characteristics | DTR (n=50) | Non-DTR (n=50) | P value |
|---|---|---|---|
| Basic demographics | |||
| Age | 79.54±12.22 | 75.26±10.75 | 0.066 |
| Male | 29(58.0%) | 31(62.0%) | 0.683 |
| Medical history | |||
| Diabetes | 18(36.0%) | 16(32.0%) | 0.673 |
| Pulmonary disease | 13(26.0%) | 14(28.0%) | 0.822 |
| Cardiovascular disease | 46(92.0%) | 36(72.0%) | 0.009 |
| Cerebrovascular disease | 36(72.0%) | 20(40.0%) | 0.001 |
| Digestive disease | 21(42.0%) | 19(38.0%) | 0.683 |
| Urinary disease | 27(54.0%) | 15(30.0%) | 0.015 |
| Cancer | 8(16.0%) | 8(16.0%) | 1.000 |
| CCIa | 3.84±1.56 | 2.48±1.45 | 0.000 |
| Surgery within 3 months | 2(4.0%) | 3(6.0%) | 1.000 |
| Antibiotics exposure within 90 days | 49(98.0%) | 28(56.0%) | 0.000 |
| Usage of invasive catheters | 50(100.0%) | 26(52.0%) | 0.000 |
| Urinary catheter | 45(90.0%) | 19(38.0%) | 0.000 |
| Gastrostomy tube | 45(90.0%) | 17(34.0%) | 0.000 |
| Endotracheal tube | 15(30.0%) | 3(6.0%) | 0.002 |
| Central intravenous catheter | 33(66.0%) | 6(12.0%) | 0.000 |
| Drainage tube | 12(24.0%) | 6(12.0%) | 0.118 |
| Metastatic infection | 18(36.0%) | 4(8.0%) | 0.001 |
| Infection type | |||
| Hospital acquired infection | 50(100.0%) | 36(72.0%) | 0.000 |
| Community acquired infection | 0(0.0%) | 14(28.0%) | 0.000 |
| Laboratory examination | |||
| Red blood cell | 2.87±0.82 | 3.79±0.69 | 0.000 |
| Hemoglobin | 90.14±24.62 | 114.90±20.49 | 0.000 |
| White blood cell | 9.6704±4.77709 | 10.9890±5.80403 | 0.218 |
| Platelet | 170.70±105.19 | 215.62±93.65 | 0.026 |
| NEU%b | 77.464±14.9145 | 78.956±18.0857 | 0.270 |
| Total protein | 62.46±7.737 | 64.76±9.502 | 0.201 |
| ALbumin | 30.992±4.5367 | 32.950±5.6690 | 0.062 |
| Hematocrit | 0.70±3.00 | 0.35±0.06 | 0.000 |
| Vasoactive drugs after Kp detection | 11(22.0%) | 6(12.0%) | 0.183 |
| Admitted to the ICUc | 22(44.0%) | 5(10.0%) | 0.000 |
| Mechanical ventilation after Kp detection | 13(26.0%) | 5(10.0%) | 0.037 |
| SOFAd | 6.12±5.85 | 1.98±2.53 | 0.000 |
| 30-day mortality | 19(38.0%) | 4(8.2%) | 0.000 |
Notes: Bold text: P value<0.05. cPatients infected kp were then transferred to the ICU.
Abbreviations: aCCI, Charlson comorbidity index; bNEU%, Neutrophil percentage; dSOFA, sequential organ failure assessment.
Figure 1.
(A) Department distribution of the DTR-Kp-infected patients. (B) Sample type distribution of the DTR-Kp strains. (C) Resistance category distribution of all the enrolled Kp isolates.
Abbreviations: DTR, difficult-to-treat resistance; MDR, multidrug resistance; CR, carbapenem resistance; ESCR, extended-spectrum cephalosporin resistance; FQR, fluoroquinolone resistance.
Compared with the non-DTR group, a greater number of patients with DTR-Kp infections had cardiovascular disease (92.0% versus 72.0%, P=0.009), cerebrovascular disease (72.0% versus 40.0%, P=0.001), and urinary disease (54.0% versus 30.0%, P=0.015) as their underlying diseases. Furthermore, the DTR group was associated with a higher CCI (3.84±1.56 versus 2.48±1.45, P<0.001). In addition, the DTR group had significantly more antibiotic exposure within 90 days (98.0% versus 56.0%, P<0.001). More patients who had invasive catheters were infected by DTR-Kp isolates (100.0% versus 52.0%, P<0.001), and the types of catheters included urinary catheters (90.0% versus 38.0%, P<0.001), gastrostomy tubes (90.0% versus 34.0%, P<0.001), endotracheal tubes (30.0% versus 6.0%, P=0.002) and central intravenous catheters (66.0% versus 12.0%, P<0.001). Notably, a significant number of the DTR-Kp-infected patients presented with metastatic infection (36.0% versus 8.0%, P=0.001). DTR-Kp was significantly associated with hospital-acquired infections (100.0% versus 72.0%, P<0.001). In contrast, more community-acquired infections occurred in the non-DTR group (0.0% versus 28.0%, P<0.001). Furthermore, the red blood cell, hemoglobin and platelet counts of the DTR-Kp-infected patients were significantly lower than those of the non-DTR group (P< 0.001, P< 0.001 and P=0.026, respectively). However, the DTR-Kp-infected patients had a higher hematocrit (P<0.001). In addition, a significant number of the DTR-Kp-infected patients experienced ICU admission (44.0% versus 10.0%, P<0.001) and mechanical ventilation (26.0% versus 10.0%, P=0.037). Furthermore, the SOFA score was significantly higher in the patients with DTR-Kp infection than in the patients in the non-DTR group (P<0.001) (Table 1).
Microbiological Characteristics of the DTR-Kp Strains
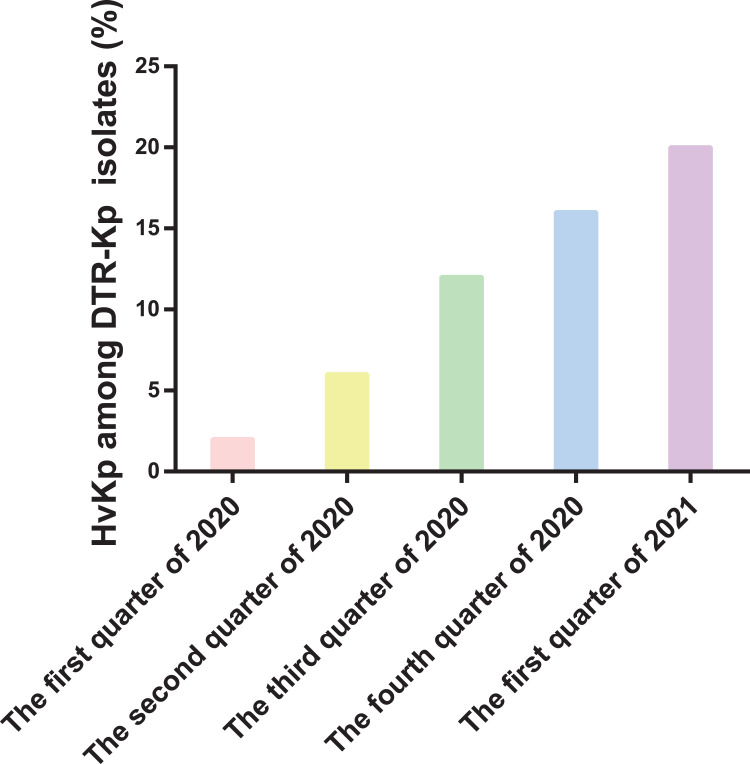
In this study, the percentage of hvKp among the DTR-Kp isolates that were investigated increased consistently from 2020 to 2021 (Figure 2). Twenty-eight isolates (56.0%) were recognized as hvKp in the DTR group, and 26 DTR-Kp strains (52.0%) presented with the hypermucoviscous phenotype. Remarkably, the resistance rate of the DTR-Kp isolates to all the tested antimicrobial agents was higher than that of the non-DTR group. Additionally, the overall resistance rate to these antibiotics was significantly lower than 30% in the non-DTR-Kp strains (Table S1).
Figure 2.
Percentage of the hypervirulent Klebsiella pneumoniae (hvKp) strains among the identified DTR-Kp strains from January 2020 to March 2021.
A significant number of the DTR-Kp strains belonged to ST11 (82.0%), followed by ST15 (12.0%), ST86 (2.0%), ST996 (2.0%), and ST3157 (2.0%) (Figure S1A). The most common capsule serotypes of the DTR-Kp strains were KL64 (42.0%) and KL47 (40.0%) (Figure S1B).
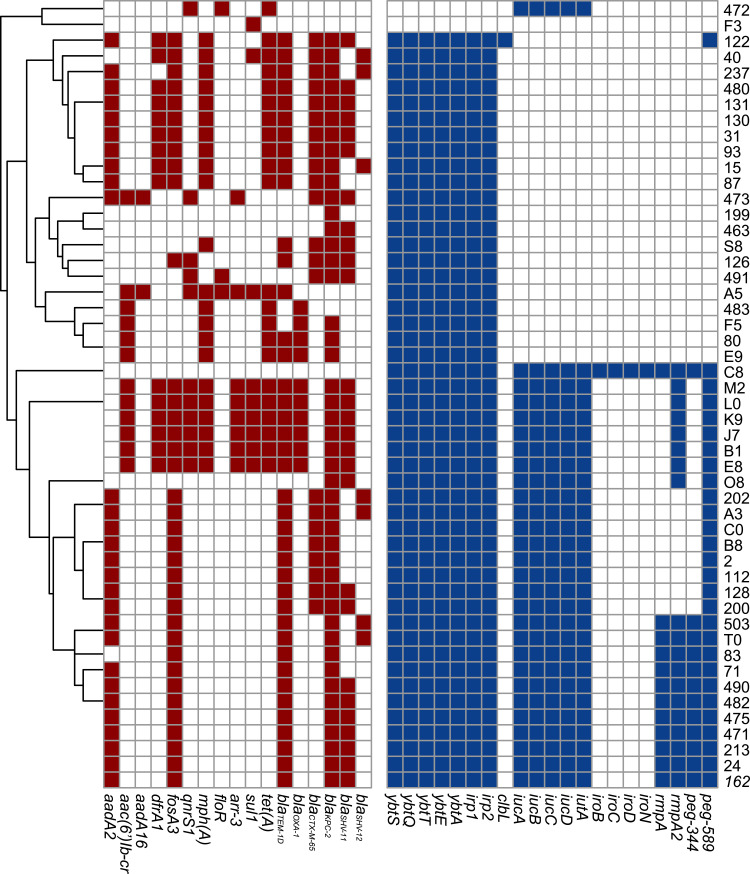
In terms of resistance genes, a greater number of DTR-Kp strains harbored the aminoglycoside resistance gene aadA2, the fosfomycin resistance gene fosA3, the macrolide resistance gene mphA, the tetracycline resistance gene tet(A), the diaminopyrimidine resistance gene dfrA1 than non-DTR group (P<0.01). The beta-lactamase genes blaKPC-2, blaTEM-1D, blaCTX-M-65 and blaSHV-12 were present in 90.0%, 80.0%, 44.0% and 14.0% of the isolates in the DTR group, which accounted for 4.0%, 12.0%, 0.0%, 0.0% in the non-DTR group, respectively (P<0.05). For virulence genes, the yersiniabactin genes (irp1-2 and ybtAEQST) were significantly clustered in the DTR group (96.0% versus 60.0%, P<0.001). Notably, 28 DTR-Kp isolates (56.0%) harbored the aerobactin gene (iucABCD-iutA). Similarly, over half of the DTR-Kp strains possessed peg589, which was associated with poor prognosis, as confirmed in animal experiments. (Figure 3, Table S2).
Figure 3.
Genomic distribution of the DTR Klebsiella pneumoniae strains. Red: antimicrobial resistance genes. Blue: Virulence genes.
For phylogenetic relationships, DTR-ST11 Kp strains clustered into two separate clades, including 21 and 20 isolates separately. Among DTR-Non-ST11 strains, four distinguished clades were identified (Figure S3).
All-Cause Death Risk of DTR-Kp Infection
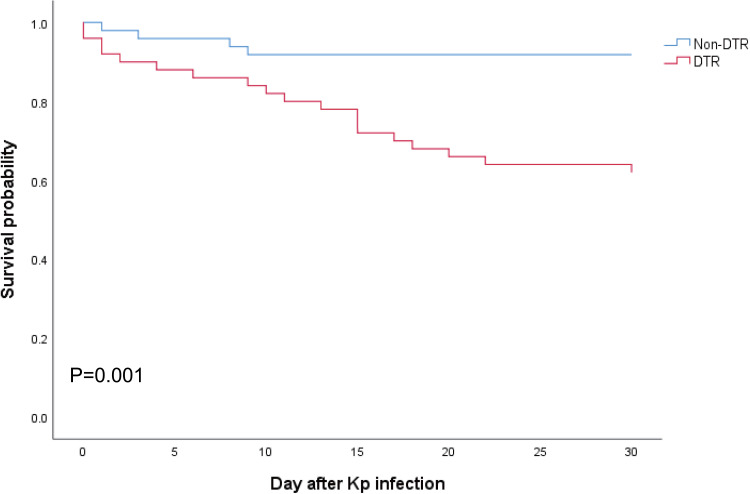
The survival curve revealed that 30-day mortality was significantly higher in the patients with DTR-Kp infection than in those without DTR-Kp infection (38.0% versus 8.2%, P=0.001) (Figure 4). Similar results were observed in the subgroups of hvKp and cKp (P=0.029 and 0.008, respectively) (Figure S2A and S2B). Of note, the mortality among the patients with cKp and hvKp infections was similar in the DTR group (P=0.253) (Figure S2C).
Figure 4.
Kaplan–Meier curves for all-cause 30-day mortality. Statistical significance was determined by the Log rank test.
Risk Factors for DTR-Kp Infection
In this study, the univariate regression analysis results showed that DTR-Kp infection (odds ratio [OR]=6.895), usage of invasive catheters (OR = 8.963) and mechanical ventilation (OR = 3.520) were statistically significant risk factors associated with 30-day mortality. Moreover, multivariate analysis revealed that DTR-Kp infection (OR = 4.196) was an independent risk factor for the 30-day mortality of Kp-infected patients (Table 2).
Table 2.
Risk Factors for Death
| Variable | Univariate | P value | Multivariate | P value |
|---|---|---|---|---|
| ORa (95% CIb) | OR (95% CI) | |||
| DTR | 6.895(2.137–22.244) | 0.001 | 4.196(1.089–16.173) | 0.037 |
| Usage of invasive catheters | 8.963(1.137–70.634) | 0.037 | 2.796(0.267–29.245) | 0.391 |
| Mechanical ventilation | 3.520(1.189–10.424) | 0.023 | 2.580(0.809–8.223) | 0.109 |
Note: Bold text: P value<0.05.
Abbreviations: aOR, odds ratio; bCI, confidence interval.
Furthermore, the univariate regression analysis results showed that cardiovascular disease (OR=4.472), cerebrovascular disease (OR = 3.857), urinary disease (OR = 2.739) and CCI (OR = 1.878) were risk factors associated with DTR-Kp infections. In addition, the multivariate analysis results showed that cerebrovascular disease (OR = 2.780) and CCI (OR= 1.584) appeared to be independent risk factors for DTR-Kp infections (Table 3).
Table 3.
Risk Factors for DTR-Kp Infection
| Variable | Univariate | P value | Multivariate | P value |
|---|---|---|---|---|
| ORa (95% CIb) | OR (95% CI) | |||
| Cardiovascular disease | 4.472(1.355–14.755) | 0.014 | 1.716(0.436–6.753) | 0.440 |
| Cerebrovascular disease | 3.857(1.670–8.911) | 0.002 | 2.780(1.109–6.969) | 0.029 |
| Urinary disease | 2.739(1.204–6.230) | 0.016 | 1.906(0.760–4.778) | 0.169 |
| CCI | 1.878(1.359–2.595) | 0.000 | 1.584(1.117–2.245) | 0.010 |
Note: Bold text: P value<0.05.
Abbreviations: aOR, odds ratio; bCI, confidence interval.
Discussion
In this study, the clinical and microbiological characteristics of the DTR-Kp isolates were demonstrated in detail. We found that the CCI and SOFA scores were significantly higher in the patients with DTR-Kp infections than in the non-DTR group. All of the DTR-Kp isolates caused hospital-acquired infections in this study. In addition, our data revealed that the DTR group was highly associated with the usage of catheters, ICU admission and mechanical ventilation after Kp detection. This result indicated that DTR-Kp might cause more serious and complex infections than non-DTR-Kp. Importantly, the nosocomial dissemination of DTR-Kp isolates raised the alarm that enhancing surveillance is essential in the hospital.
Previous studies reported that the worldwide spread of MDR Kp is mainly driven by ST11, which is endemic and most typically encountered in KPC-producing isolates, especially in China.22–24 As our previous study reported, all the ST11 Kp isolates (100.0%) were CR and MDR.25 Similarly, in this study, our data revealed that ST11 was the dominant clone (82.0%) of DTR-Kp strains, and KL64 and KL47 were common within the DTR group. Regarding resistance determinants, our data revealed that compared to the non-DTR-Kp isolates, a significantly higher number of the DTR-Kp strains harbored all kinds of AMR genes that were associated with resistance to aminoglycosides, fosfomycins, macrolides, tetracyclines, diaminopyrimidines, penams, cephalosporins, carbapenems and monobactam. Of note, we observed that the prevalence of the beta-lactamase genes blaKPC-2 and blaTEM-1D was very high in the DTR-Kp strains (90.0% and 80.0%, respectively). The detected AMR genes were consistent with the resistance phenotype in all the tested antimicrobial agents in our study. Furthermore, we need to be alert to the presence of some other AMR genes, such as fosA3, mphA, and tet(A), that were not validated by our study. Further investigations on the AMR genes of DTR-Kp are needed.
In terms of virulence, the yersiniabactin siderophore system is a key bacterial virulence factor responsible for scavenging iron from host transport proteins, thereby enhancing the ability of bacteria to survive and replicate within the host.26–28 A previous study demonstrated that yersiniabactin was prevalent among KPC-producing isolates, and it could cause the isolates from escape binding to the human immune protein lipocalin 2, thus promoting respiratory tract infections.29 Fostervold et al observed a higher prevalence of the yersiniabactin gene in extended-spectrum β-lactamase (ESBL) (37.8%) than in the non-ESBL (17.3%) Kp isolates.30 Another study reported that genes encoding yersiniabactin were identified in 83.7% (123/147) of the CRKP isolates.31 Sherif et al found that yersiniabactin was the most common virulence factor (69.2%) in 39 randomly selected, geographically diverse MDR Kp isolates.32 Furthermore, aerobactin was the dominant siderophore produced by the hvKp strains and the critical siderophore that enhances virulence ex vivo and in vivo.33 Aerobactin significantly enhances the survival of isolates in human ascites, in the serum, and in outbred mouse systemic and pulmonary infection models, which accounts for >80 to 90% of the total siderophore production.34 Yersiniabactin and aerobactin could enhance invasiveness, posing a threat to vulnerable populations.35,36 Importantly, we found that the yersiniabactin-associated genes were highly prevalent in the DTR-Kp strains. Additionally, over half of the DTR-Kp isolates harbored aerobactin-associated genes. The frequent detection of yersiniabactin and aerobactin genes among the DTR-Kp isolates indicated the potential convergence of antimicrobial resistance and virulence determinants. The emergence and spread of these DTR isolates carrying virulence determinants poses a great challenge for public health and threatens the current treatment and management strategies.
A previous retrospective cohort study at 173 US hospitals showed that Gram-negative bloodstream infections (GNBSIs) with DTR were associated with decreased survival, and its crude mortality was 43%, among which Klebsiella spp. accounted for 22% of the mortality cases.3 In addition, Huh et al found that the crude mortality for GNBSI caused by DTR was 50.3%. Their results showed that DTR was significantly associated with mortality (adjusted OR [aOR]= 3.58) and was also a significant predictor for mortality (aOR=3.48).9 Santoro et al confirmed that DTR was an independent risk factor for 7-, 30-, and 90-day mortality in patients with GNBSIs.37 Importantly, our results revealed that the 30-day mortality of DTR-Kp-infected patients was significantly higher than that of the non-DTR-Kp group. Furthermore, among the DTR-Kp-infected patients, our subgroup analysis showed that the mortality was similar between cKp and hvKp infections. Notably, it further confirmed that a DTR-Kp infection might be responsible for the poor prognosis instead of cKp or hvKp. Moreover, DTR-Kp infection was independently associated with 30-day mortality in patients with Kp infection. Previous studies focused on GNBSIs, and this study did not distinguish the specific Kp species and only focused on bloodstream infections. Remarkably, our attention should be given to the rapid emergence of DTR-Kp as previously described.14,15 Our study was the first to demonstrate the poor outcomes of DTR-Kp infection, suggesting an urgent need to enhance the clinical awareness.
A previous study showed that urban healthcare and higher baseline illness were predictors of DTR GNBSIs.3 Our results indicated that cerebrovascular disease and CCI were independent risk factors for DTR-Kp infections. It is noted that patients with complex underlying diseases and comorbidities were more susceptible to DTR-Kp infection. Medical staff needs to monitor these patients to avoid the development of DTR-Kp infections.
Our study has some limitations. First, it was a retrospective study conducted at a single center in Beijing, and this study included a small number of Kp strains, which might lead to statistical bias. Therefore, prospective multicenter studies that include more isolates are needed to better investigate the characteristics of DTR-Kp infection. Second, apart from virulence genes, in vitro and in vivo experiments are needed to further identify the Kp virulence phenotype, including the measurement of siderophore production, Galleria mellonella lethality assay, outbred mouse model, or human neutrophil assay.
Conclusion
In summary, our study demonstrated that DTR-Kp infection was an independent risk factor for mortality. The DTR-Kp-infected patients presented with a serious condition and poor prognosis. DTR-hvKp is rapidly emerging. The DTR-Kp strains harbored various resistance genes and possessed high rates of yersiniabactin siderophore genes, indicating the convergence of antimicrobial resistance and virulence determinants. It is of great significance to enhance clinical awareness and the control and management of DTR-Kp infections.
Acknowledgments
We would like to thank Dr. Pengcheng Du from the Qitan technique for providing instructions for the bioinformation analysis.
Funding Statement
This work was supported by the Clinical Cohort Construction Program of Peking University Third Hospital (BYSYDL2019007) and Beijing Key Clinical Specialty Program (010071) and the National Natural Science Foundation of China (82200012).
Data Sharing Statement
The genome sequences in this study were deposited into the NCBI database under BioProject accession no. PRJNA838135.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Paczosa MK, Mecsas J. Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol Mol Biol Rev. 2016;80(3):629–661. doi: 10.1128/mmbr.00078-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- 3.Kadri SS, Adjemian J, Lai YL, et al. Difficult-to-treat resistance in gram-negative bacteremia at 173 US hospitals: retrospective cohort analysis of prevalence, predictors, and outcome of resistance to all first-line agents. Clin Infect Dis. 2018;67(12):1803–1814. doi: 10.1093/cid/ciy378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vardakas KZ, Rafailidis PI, Konstantelias AA, Falagas ME. Predictors of mortality in patients with infections due to multi-drug resistant Gram negative bacteria: the study, the patient, the bug or the drug? J Infect. 2013;66(5):401–414. doi: 10.1016/j.jinf.2012.10.028 [DOI] [PubMed] [Google Scholar]
- 5.Tabah A, Koulenti D, Laupland K, et al. Characteristics and determinants of outcome of hospital-acquired bloodstream infections in intensive care units: the EUROBACT International Cohort Study. Intensive Care Med. 2012;38(12):1930–1945. doi: 10.1007/s00134-012-2695-9 [DOI] [PubMed] [Google Scholar]
- 6.Zilberberg MD, Nathanson BH, Sulham K, Fan W, Shorr AF. Multidrug resistance, inappropriate empiric therapy, and hospital mortality in Acinetobacter baumannii pneumonia and sepsis. Crit Care. 2016;20(1):221. doi: 10.1186/s13054-016-1392-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strich JR, Kadri SS. Difficult-to-treat antibiotic-resistant gram-negative pathogens in the intensive care unit: epidemiology, outcomes, and treatment. Article. Semin Respir Crit Care Med. 2019;40(4):419–434. doi: 10.1055/s-0039-1696662 [DOI] [PubMed] [Google Scholar]
- 8.Kadri SS. Recognizing the unique role of critical care providers in confronting antimicrobial resistance. Editorial. Am J Respir Crit Care Med. 2018;198(5):560–562. doi: 10.1164/rccm.201805-0962ED [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huh K, Chung DR, Ha YE, et al. Impact of difficult-to-treat resistance in gram-negative bacteremia on mortality: retrospective analysis of nationwide surveillance data. Article. Clin Infect Dis. 2020;71(9):E487–E496. doi: 10.1093/cid/ciaa084 [DOI] [PubMed] [Google Scholar]
- 10.Giannella M, Bussini L, Pascale R, et al. Prognostic utility of the new definition of difficult-to-treat resistance among patients with gram-negative bloodstream infections. Article. Open Forum Infect Dis. 2019;6(12). doi: 10.1093/ofid/ofz505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strich JR, Warner S, Lai YL, et al. Needs assessment for novel Gram-negative antibiotics in US hospitals: a retrospective cohort study. Article. Lancet Infect Dis. 2020;20(10):1172–1181. doi: 10.1016/S1473-3099(20)30153-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kadri SS, Lai YLE, Ricotta EE, et al. External validation of difficult-to-treat resistance prevalence and mortality risk in gram-negative bloodstream infection using electronic health record data from 140 US hospitals. Article. Open Forum Infect Dis. 2019;6(4). doi: 10.1093/ofid/ofz110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karlowsky JA, Lob SH, Raddatz J, et al. In vitro activity of imipenem/relebactam and ceftolozane/tazobactam against clinical isolates of gram-negative bacilli with difficult-to-treat resistance and multidrug-resistant phenotypes-study for monitoring antimicrobial resistance trends, United States 2015–2017. Article. Clin Infect Dis. 2021;72(12):2112–2120. doi: 10.1093/cid/ciaa381 [DOI] [PubMed] [Google Scholar]
- 14.Jean SS, Ko WC, Lu MC, Lee WS, Hsueh PR. Multicenter surveillance of in vitro activities of cefepime-zidebactam, cefepime-enmetazobactam, omadacycline, eravacycline, and comparator antibiotics against Enterobacterales, Pseudomonas aeruginosa, and Acinetobacter baumannii complex causing bloodstream infection in Taiwan, 2020. Article. Expert Rev Anti Infect Ther. 2022;20(6):941–953. doi: 10.1080/14787210.2022.2021876 [DOI] [PubMed] [Google Scholar]
- 15.Mouna H, Stylianos C, Linda H, et al. Inactivation of mgrB gene regulator and resistance to colistin is becoming endemic in carbapenem-resistant Klebsiella pneumoniae in Greece: a nationwide study from 2014 to 2017. Article. Int J Antimicrob Agents. 2020;55(4):105930. doi: 10.1016/j.ijantimicag.2020.105930 [DOI] [PubMed] [Google Scholar]
- 16.Abdallah TAK, Elajez R, Ibrahim TB, Alimam AB, Omrani AS. Efficacy and safety of intravenous fosfomycin for the treatment of difficult-to-treat Gram-negative bacterial infections. Article. J Infect Public Health. 2021;14(11):1620–1622. doi: 10.1016/j.jiph.2021.09.025 [DOI] [PubMed] [Google Scholar]
- 17.Li W, Sun G, Yu Y, et al. Increasing occurrence of antimicrobial-resistant hypervirulent (hypermucoviscous) Klebsiella pneumoniae isolates in China. Clin Infect Dis. 2014;58(2):225–232. doi: 10.1093/cid/cit675 [DOI] [PubMed] [Google Scholar]
- 18.Institute CaLS. Performance standards for antimicrobial susceptibility testing. 30th ed. In: CLSI Supplement M100 CLSI. Wayne, PA: Institute CaLS; 2020. [Google Scholar]
- 19.Centers for Disease Control and Prevention. Healthcare-Associated Infections (HAIs). Antibiotic resistance patient safety atlas: phenotype definitions. 2015. Available from: https://arpsp.cdc.gov/resources/AR-PhenotypeDefinitions.pdf. Accessed 14 October 2022.
- 20.Shon A, Bajwa R, Russo T. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: a new and dangerous breed. Virulence. 2013;4(2):107–118. doi: 10.4161/viru.22718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russo TA, Olson R, Fang CT, et al. Identification of biomarkers for differentiation of hypervirulent Klebsiella pneumoniae from classical K. pneumoniae. J Clin Microbiol. 2018;56(9). doi: 10.1128/jcm.00776-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tóth A, Damjanova I, Puskás E, et al. Emergence of a colistin-resistant KPC-2-producing Klebsiella pneumoniae ST258 clone in Hungary. Eur J Clin Microbiol Infect Dis. 2010;29(7):765–769. doi: 10.1007/s10096-010-0921-3 [DOI] [PubMed] [Google Scholar]
- 23.Wang Q, Wang X, Wang J, et al. Phenotypic and genotypic characterization of carbapenem-resistant enterobacteriaceae: data from a longitudinal large-scale CRE study in China (2012–2016). Clin Infect Dis. 2018;67(suppl_2):S196–S205. doi: 10.1093/cid/ciy660 [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Zhang X, Cai L, Zong Z. Enhanced survival of ST-11 carbapenem-resistant Klebsiella pneumoniae in the intensive care unit. Infect Control Hosp Epidemiol. 2020;41(6):740–742. doi: 10.1017/ice.2020.68 [DOI] [PubMed] [Google Scholar]
- 25.Yang P, Wu Z, Liu C, et al. Clinical outcomes and microbiological characteristics of sequence type 11 Klebsiella pneumoniae infection. Front Med. 2022;9:889020. doi: 10.3389/fmed.2022.889020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lam MMC, Wick RR, Wyres KL, et al. Genetic diversity, mobilisation and spread of the yersiniabactin-encoding mobile element ICEKp in Klebsiella pneumoniae populations. Microb Genom. 2018;4(9):e000196. doi: 10.1099/mgen.0.000196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holden VI, Bachman MA. Diverging roles of bacterial siderophores during infection. Metallomics. 2015;7(6):986–995. doi: 10.1039/c4mt00333k [DOI] [PubMed] [Google Scholar]
- 28.Lawlor MS, O’Connor C, Miller VL. Yersiniabactin is a virulence factor for Klebsiella pneumoniae during pulmonary infection. Infect Immun. 2007;75(3):1463–1472. doi: 10.1128/IAI.00372-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bachman MA, Oyler JE, Burns SH, et al. Klebsiella pneumoniae yersiniabactin promotes respiratory tract infection through evasion of lipocalin 2. Infect Immun. 2011;79(8):3309–3316. doi: 10.1128/IAI.05114-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fostervold A, Hetland MAK, Bakksjø R, et al. A nationwide genomic study of clinical Klebsiella pneumoniae in Norway 2001–15: introduction and spread of ESBLs facilitated by clonal groups CG15 and CG307. J Antimicrob Chemother. 2022;77(3):665–674. doi: 10.1093/jac/dkab463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cienfuegos-Gallet AV, Zhou Y, Ai W, Kreiswirth BN, Yu F, Chen L. Multicenter genomic analysis of carbapenem-resistant Klebsiella pneumoniae from Bacteremia in China. Microbiol Spectr. 2022;10(2):e0229021. doi: 10.1128/spectrum.02290-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sherif M, Palmieri M, Mirande C, et al. Whole-genome sequencing of Egyptian multidrug-resistant Klebsiella pneumoniae isolates: a multi-center pilot study. Eur J Clin Microbiol Infect Dis. 2021;40(7):1451–1460. doi: 10.1007/s10096-021-04177-7 [DOI] [PubMed] [Google Scholar]
- 33.Russo TA, Olson R, MacDonald U, Beanan J, Davidson BA, Camilli A. Aerobactin, but not yersiniabactin, salmochelin, or enterobactin, enables the growth/survival of hypervirulent (Hypermucoviscous) Klebsiella pneumoniae ex vivo and in vivo. Infect Immun. 2015;83(8):3325–3333. doi: 10.1128/iai.00430-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Russo TA, Olson R, Macdonald U, et al. Aerobactin mediates virulence and accounts for increased siderophore production under iron-limiting conditions by hypervirulent (hypermucoviscous) Klebsiella pneumoniae. Infect Immun. 2014;82(6):2356–2367. doi: 10.1128/IAI.01667-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Russo TA, Marr CM. Hypervirulent Klebsiella pneumoniae. Clin Microbiol Rev. 2019;32(3). doi: 10.1128/cmr.00001-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El Fertas-Aissani R, Messai Y, Alouache S, Bakour R. Virulence profiles and antibiotic susceptibility patterns of Klebsiella pneumoniae strains isolated from different clinical specimens. Pathol Biol. 2013;61(5):209–216. doi: 10.1016/j.patbio.2012.10.004 [DOI] [PubMed] [Google Scholar]
- 37.Santoro A, Franceschini E, Meschiari M, et al. Epidemiology and risk factors associated with mortality in consecutive patients with bacterial bloodstream infection: impact of MDR and XDR bacteria. Article. Open Forum Infect Dis. 2020;7(11). doi: 10.1093/ofid/ofaa461 [DOI] [PMC free article] [PubMed] [Google Scholar]