Abstract
Objectives
The aim of this study was to investigate the relationship between COVID-19 diagnosis and the risk of developing a first-ever vascular event (VE) compared with the same risk in those with respiratory tract infection (RTI).
Study design
This was a retrospective cohort study.
Methods
This study using data from Disease Analyzer Database (IQVIA) included patients aged ≥18 years with at least one visit to a German practice during the index period. VEs were defined as cardiovascular or cerebrovascular events. Two cohorts were created: patients with a diagnosis of COVID-19 and those diagnosed with RTI. These were matched using propensity scores. Kaplan–Meier curves were created for the purposes of time to event analysis. A Poisson model was used to calculate incidence rates and derive incidence rate ratios (IRRs).
Results
A total of 58,904 patients were matched. There was no significant association between COVID-19 diagnosis and increased incidence of VE events among females (IRR [95% confidence interval (CI)]: 0.96 [0.82–1.11] and 1.30 [0.88–1.81]) or males (IRR, 95% CI: 0.91 [0.78–1.05] and 1.13 [0.80–1.62]). Overall, no significant association between COVID-19 diagnosis and incidence of VE was observed across age categories except for cardiovascular vascular events in the age category ≥70 years (IRR [95% CI]: 0.78 [0.67–0.94]).
Conclusions
Overall, our study suggests that COVID-19 diagnosis was not associated with an increased risk of developing VE compared with RTI diagnosis. However, further research in a variety of healthcare settings and regions is needed to confirm these preliminary findings from our cohort, which is a good reflection of routine clinical practice in Germany.
Keywords: COVID-19, Cardiovascular disease, Cerebrovascular disease, Real-world evidence, German practice
Introduction
Vascular disease is a group of non-communicable disorders of the heart and blood vessels including cardiovascular events (CDVE) such as coronary heart disease, peripheral arterial disease, and cerebrovascular events (CVE)1 consisting of stroke and transient ischemic attack.2 In 2019, an estimated 17.9 million people died from this group of diseases, which translates to 32% of global deaths.1 More than 1.68 million of these deaths occurred in Europe alone. In many countries such as Germany, VEs are the leading cause of death and are also a significant cause of disability. This group of diseases accounted for 36% of all deaths in Germany in 2018, with a higher proportion of deaths observed in females vs males (38.8% vs 33.5%),3 and consequently, places a considerable burden on healthcare systems, adding to the escalating costs of care.4
Previous research suggests that there may be a relationship between the recent COVID-19 pandemic and an increased risk of CDVEs and CVEs in different subpopulations.5, 6, 7 The most common complications of COVID-19 include pulmonary and extrapulmonary symptoms, with frequent reports of fever, cough, and shortness of breath among symptomatic patients.8 It is estimated that among those who develop symptoms, approximately 80% recover without the need for hospital treatment or specialized care.9 However, COVID-19 can also cause cardiac and cerebral injuries as a result of mechanisms currently under investigation, including a combination of direct viral injury and the immunological response of the patient acting as a host.10 For example, cardiac and cerebral injury caused by COVID-19 may lead to the development of cardiovascular comorbidities such as myocardial infarction (MI) and other forms of CDVE including CVE.
A significant body of literature indicates that chronic systemic inflammation favors the development of atherosclerosis and predisposes individuals to clot formation by interfering with physiological hemostasis and by inducing a state of hypercoagulability.11 , 12 It is therefore evident that acute inflammation facilitates the development of vascular events.13 , 14 In particular, respiratory tract infections (RTIs), which evoke a broad systemic inflammatory reaction may be involved in the pathogenesis of cardiovascular complications.15, 16, 17 Patients with a pre-existing VE might even be at a higher risk of vascular events than those without.18 In the current debate, COVID-19 is suspected to have a different pathophysiological impact on the development of many disorders.19 In this context, it could be surmised that the systemic inflammatory response caused by SARS-CoV-2 exposure may have a different effect on the immune reaction than other unspecified RTIs. If this is confirmed, this difference could render a different pathophysiological impact in causing VE.
Therefore, the aim of this retrospective cohort study was to investigate the relationship between COVID-19 diagnosis and the risk of developing CDVE or CVE among patients without pre-existing VE in general practices in Germany compared with a contemporary cohort diagnosed with RTI.
Methods
Database description
This retrospective cohort study used data from the Disease Analyzer Database (IQVIA). The Disease Analyzer contains de-identified electronic medical records from general and specialized practices in Germany including demographic, diagnosis (according to International Classification of Diseases, 10th revision [ICD-10]), and prescription patient data.20 As data are collected in a non-interventional manner, the database offers an accurate reflection of routine clinical practice and real-world settings. Approximately 3% of all German practices are included in the Disease Analyzer Database. The validity and representativeness of the data have been described extensively, demonstrating the suitability of the Disease Analyzer Database for the conduction of pharmacoepidemiological and pharmacoeconomic studies.21 In addition, the Disease Analyzer Database has previously been used in studies focusing on COVID-19 and cardiovascular outcomes.22
Study population
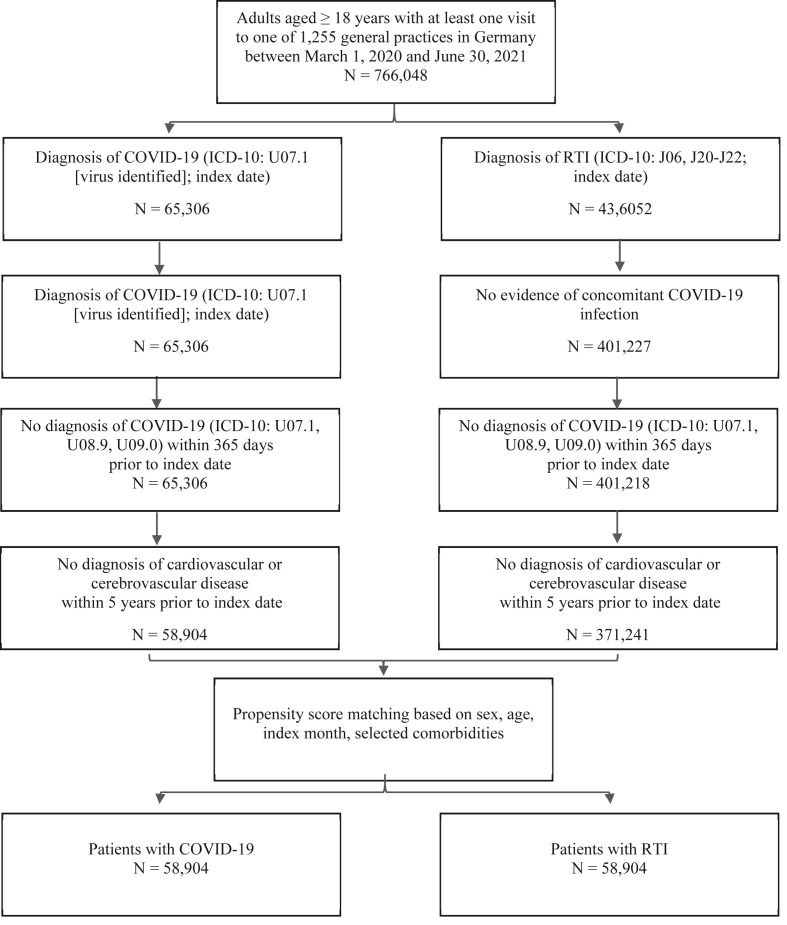
Patients aged ≥18 years with at least one visit to a German practice during the index period were included in the study. The index period was defined as March 1, 2020 (the start of the pandemic), to June 30, 2021. The study end was defined as December 31, 2021, allowing for a minimum follow-up time of 6 months. Patients for whom sex or age information was missing were excluded from the study. Two contemporary cohorts were defined: a cohort of patients with a diagnosis of COVID-19 (ICD-10: U07.1) and a cohort of patients with a diagnosis of acute lower or upper RTI (ICD-10: J06, J20, J21, J22). Any patient with a diagnosis of RTI who had also been diagnosed with COVID-19 during the index period was considered for inclusion in the COVID-19 cohort only. Care was taken to exclude patients with a diagnosis of COVID-19 before March 1, 2020, to avoid including patient records with a diagnosis code used to identify a disease other than COVID-19. Because this study does not include patients with pre-existing CDVE or CVE, all patients with pre-existing CDVE including CVE (see Appendix 1 for ICD-10 codes) up to 5 years before the index date were excluded from the study. Patient comorbidities, including diabetes, hypertension, obesity, and any type of cancer (see Appendix 2 for ICD-10 codes), were also retrieved for up to 5 years before the index date. After applying the inclusion and exclusion criteria, both cohorts were matched using a propensity score approach based on sex, age, index month of the infection, and identified comorbidities. The selection diagram for study patients is displayed in Fig. 1 .
Fig. 1.
Selection of study patients.
Outcomes
The primary outcome was the incidence of vascular events including CDVEs or CVEs. The secondary outcome was the time to first VE. A CDVE was considered to have occurred if the following diagnosis was recorded: angina pectoris, acute MI, subsequent MI, certain current complications following acute MI, other acute ischemic heart diseases, chronic ischemic heart disease, atrial fibrillation and flutter, and heart failure. A CVE was defined as stroke, cerebral infarction, or transient ischemic attack. A complete list of the ICD-10 codes used for the identification of these events can be found in Appendix 3.
Statistical methods
No statistical power calculation was conducted in this real-world study as the primary outcome was descriptive in nature. All study patients in the Disease Analyzer Database who met the inclusion and passed the exclusion criteria were included. Descriptive summary statistics (n [%], mean, standard deviation [SD], and interquartile range) were used to describe continuous variables. Counts and proportions were used to describe categorical variables. No imputation method was used for handling missing data as patients for whom age or sex information was missing were excluded from the cohort. Kaplan–Meier curves were used for the analysis of time to VE event, from index date until the first year of the follow-up period. Given the small proportion of events observed, a Poisson model approach was the preferred method for calculating the incidence rates of VEs per 1000 person-years and deriving incidence rate ratios (IRRs). P values <0.05 were considered statistically significant. Analyses were carried out using manufacturer is RStudio (Public Benefit Corporation) version 1.2.1235.
Results
Cohort description
In total, some 766,048 patients aged ≥18 years with at least one visit to one of 1255 general practices in Germany between March 1, 2020, and June 30, 2021, were available for inclusion, of which 1085 were excluded due to missing sex information. After applying further inclusion and exclusion criteria (including diagnosis of COVID-19 or RTI, no previous CDVE or CVE), 58,904 patients and 371,241 patients remained in the COVID-19 and RTI cohorts, respectively. These patients were matched using the propensity score approach, leading to a total of 58,904 patients diagnosed with COVID-19 and 58,904 patients with RTI for final inclusion in this study (Fig. 1). The mean (SD) age was 45.6 (17.4) and 45.4 (17.0) years, respectively, in the COVID-19 and RTI cohorts, with a higher proportion of females vs males in both groups (53.8% vs 46.4% in the COVID-19 cohort and 54.0% vs 46.0% in the RTI cohort). The mean (SD) follow-up time was 363.7 (17.3) days and 363.6 (16.8) days, respectively, in the COVID-19 and RTI cohorts, with a minimum follow-up time of 184 days for both. The baseline characteristics of both cohorts after 1:1 propensity score matching are summarized in Table 1 .
Table 1.
Baseline characteristics of study patients after 1:1 propensity score matching.
| Variable | Patients with COVID-19 (n = 58,832) | Patients with RTI (n = 58,832) |
|---|---|---|
| Age in database | ||
| Mean (SD) | 45.6 (17.4) | 45.4 (17.0) |
| Follow-up (days) | ||
| Mean (SD) | 363.7 (17.3) | 363.6 (16.8) |
| Age in database, n (%) | N (%) | N (%) |
| 18–30 | 13,390 (22.8) | 13,397 (22.8) |
| 31–40 | 11,474 (19.5) | 11,518 (19.6) |
| 41–50 | 11,094 (18.9) | 11,132 (18.9) |
| 51–60 | 12,211 (20.8) | 12,388 (21.0) |
| 61–70 | 5697 (9.7) | 5873 (10.0) |
| >70 | 4966 (8.4) | 4524 (7.7) |
| Sex | ||
| Male | 27,165 (46.4) | 27,066 (46.0) |
| Female | 31,667 (53.8) | 31,766 (54.0) |
| Index month | ||
| March 20 | 412 (0.7) | 412 (0.7) |
| April 20 | 1566 (2.7) | 1551 (2.6) |
| May 20 | 838 (1.4) | 834 (1.4) |
| June 20 | 640 (1.1) | 645 (1.1) |
| July 20 | 809 (1.4) | 797 (1.4) |
| August 20 | 1236 (2.1) | 1249 (2.1) |
| September 20 | 1300 (2.2) | 1308 (2.2) |
| October 20 | 4173 (7.1) | 4187 (7.1) |
| November 20 | 8688 (14.8) | 8604 (14.6) |
| December 20 | 10,187 (17.3) | 9949 (16.9) |
| January 21 | 7172 (12.2) | 7119 (12.1) |
| February 21 | 3617 (6.2) | 3535 (6.0) |
| March 21 | 5810 (9.9) | 5878 (9.9) |
| April 21 | 7223 (12.3) | 7463 (12.7) |
| May 21 | 3856 (6.6) | 4022 (6.8) |
| June 21 | 1305 (2.2) | 1279 (2.2) |
| Comorbidities in the last 5 years (not mutually exclusive) | ||
| Cancer | 1839 (3.1) | 1487 (2.5) |
| Diabetes | 3999 (6.8) | 3658 (6.2) |
| Hypertension | 10,629 (18.1) | 10,384 (17.7) |
| Lipid disorders | 6589 (11.2) | 7439 (12.6) |
| Obesity | 4359 (7.4) | 4278 (7.3) |
RTI, respiratory tract infection.
Incidence rate and IRR
Table 2 presents the incidence of VE calculated per 1000 person-years (overall and stratified by sex and age category) and IRRs with a confidence interval (CI) of 95% for each of the cohorts. Overall, no significant association was observed between COVID-19 diagnosis and increased incidence of VE (IRR, 95% CI: 0.92 [0.84; 1.03] and 1.20 [0.93; 1.55]). Similarly, no significant association was observed between COVID-19 diagnosis and increased incidence of VE among females (IRR, 95% CI: 0.96 [0.82; 1.11] and 1.30 [0.88; 1.81]) or males (IRR, 95% CI: 0.91 [0.78; 1.05] and 1.13 [0.80; 1.62]). In addition, there was no significant association between COVID-19 diagnosis and increased incidence of VE by age category except for CDVE events in the oldest age category, ≥70 years (IRR, 95% CI: 0.78 [0.67; 0.94]).
Table 2.
Incidence of CDVE and CVE per 1000 person-years in patients with COVID-19 and RTI.
| Patient cohort | Incidence per 1000 person-years in patients with COVID-19 (n = 58,832) | Incidence per 1000 person-years in patients with RTI (n = 58,832) | Incidence rate ratio (95% CI) | P value |
|---|---|---|---|---|
| Cardiovascular events (CDVE) | ||||
| Overall | 11.6 | 12.5 | 0.92 (0.84; 1.03) | 0.1727 |
| Female sex | 10.7 | 12.7 | 0.96 (0.82; 1.11) | 0.5425 |
| Male sex | 12.7 | 14.0 | 0.91 (0.78; 1.05) | 0.1887 |
| Age 18–30 | 1.3 | 0.9 | 1.42 (0.68; 2.99) | 0.3429 |
| Age 31–40 | 3.2 | 2.4 | 1.34 (0.82; 2.22) | 0.2425 |
| Age 41–50 | 5.9 | 6.8 | 0.86 (0.62; 1.20) | 0.3793 |
| Age 51–60 | 13.3 | 14.5 | 0.92 (0.74; 1.24) | 0.4376 |
| Age 61–70 | 25.9 | 26.4 | 0.98 (0.78; 1.24) | 0.8686 |
| Age >70 | 53.0 | 66.4 | 0.78 (0.67; 0.94) | 0.0083 |
| Cerebrovascular events (CVE) | ||||
| Overall | 2.3 | 1.9 | 1.20 (0.93; 1.55) | 0.1613 |
| Female sex | 2.1 | 1.7 | 1.30 (0.88; 1.81) | 0.2012 |
| Male sex | 2.5 | 2.2 | 1.13 (0.80; 1.62) | 0.4818 |
| Age 18–30 | 0.3 | 0.1 | 4.62 (0.52; 41.35) | 0.1318 |
| Age 31–40 | 0.6 | 0.6 | 1.01 (0.35; 2.88) | 0.9868 |
| Age 41–50 | 1.0 | 1.4 | 0.74 (0.34; 1.61) | 0.4465 |
| Age 51–60 | 2.9 | 2.5 | 1.15 (0.70; 1.88) | 0.5709 |
| Age 61–70 | 4.1 | 4.5 | 0.90 (0.51; 1.61) | 0.7358 |
| Age >70 | 11.3 | 7.6 | 1.49 (0.96; 2.31) | 0.0705 |
CI, confidence interval; RTI, respiratory tract infection.
Time to first VE
A total of 806 and 835 events were observed in the COVID-19 and RTI cohorts, respectively, accounting for less than 1% of events, with a higher proportion of CDVEs vs CVEs. The mean (SD) time to the first event was 412 (0.7) days for both cohorts (Table 3 ). A Kaplan–Meier analysis of the time to first VEs showed no significant differentiation of the overall survival probability between both cohorts as the curves crossed. Based on the log-rank test, there was no significant difference in the Kaplan–Meir curves for both cohorts for the time to first VE overall and by type of event in the first year of follow-up (Fig. 2 ).
Table 3.
Time to first event by type of event.
| Variable | Patients with COVID-19 |
Patients with RTI |
|---|---|---|
| (N = 58,832) | (N = 58,832) | |
| Time to first event (days) | ||
| Mean (SD) | 412 (0.7) | 412 (0.7) |
| Type of event a | N (%) | N (%) |
| CDVE | 674 (0.011) | 725 (0.012) |
| CVE | 132 (0.002) | 110 (0.002) |
CDVE, cardiovascular events; CVE, cerebrovascular event; RTI, respiratory tract infection.
CDVE and CVE are mutually exclusive.
Fig. 2.
Kaplan–Meier analysis of time to first event during first year of follow-up: (A) overall, (B) cardiovascular events (CDVE) only, (C) cerebrovascular events (CVE) only.
Discussion
Main findings
This retrospective study, conducted in a real-world setting in German primary care practices, showed that overall, there was no significant association between COVID-19 diagnosis and increased incidence of cardiovascular or cerebrovascular outcome in comparison to patients suffering from a different RTI. However, these results need to be interpreted with caution as the incidence of CVE was much higher in the COVID-19 cohort but non-significant due to the small total number of events. The latter was observed in all age groups except for CDVE events in the oldest age category ≥70 years, where a significant association was found.
Interpretation of results
The mean age in our study cohorts was approximately 45 years (equal to median age in our study), which is in line with that of the general German population (45.7 years in 2020).23 A study using data from the Swedish Public Health Agency database previously described the association between COVID-19 and cardiovascular outcomes in a COVID-19 cohort with a median age of 48 years.5 This study concluded that COVID-19 diagnosis was associated with a higher risk of developing an event. Although our overall results do not reflect those of the previous authors, it is important to highlight that our study cohorts excluded patients with a previous history of VE, which caused a lower number of events to be observed. When looking at the older age category ≥70 years, we observed an IRR of above one in the COVID-19 cohort (IRR, 95% CI: 1.49 [0.96; 2.31]), although this was still non-significant. This is comparable with the study of Modin et al., who used Danish registers to identify all patients diagnosed with COVID-19 in hospital settings.6 They found that in a population cohort with a mean age of 77 years, incidence rates for ischemic stroke after COVID-19 diagnosis were significant, ranging between 6.6 (3.6–11.9) and 12.9 (7.1–23.5) depending on varying COVID-19 risk intervals. Our study results can be interpreted as a confirmation of the latter; nevertheless, the relationship with COVID-19 diagnosis might be confounded by the fact that the elderly population is already at increased risk of suffering from cerebrovascular complications as previously described in the literature.24 , 25
In our study, we also observed that the IRR among females was higher than in males but still non-significant. Several studies that have investigated how the risk of developing a CDVE or CVE can vary based on sex.26, 27, 28 Although the evidence shows that females have a lower overall age-adjusted stroke incidence than men, they tend to experience more stroke events due to their longer life expectancy.29 This could potentially explain the higher IRR of females vs males both for CDVE and CVE (IRR, 95% CI: 0.96 [0.82; 1.11] vs. 0.91 [0.78; 1.05] in patients with CDVE and 1.30 [0.88; 1.81] vs 1.13 [0.80; 1.62] in patients with CVE).
It is important to note that our study compared patients diagnosed with COVID-19 with a contemporary cohort rather than a historical cohort of patients. This would contribute to the discrepancy between our results and those of previous research conducted in the field.5 , 30 However, as using a historical cohort might not account for changes in clinical practice and patient behavior since the start of the pandemic,31 , 32 we considered the use of a contemporary cohort as a suitable comparator. Furthermore, the comparator cohort in our study included patients with a diagnosis of RTI rather than the general population. Literature findings suggest that patients diagnosed with RTI have a higher incidence of developing a CDVE,15 , 16 which would explain the resulting IRR of below one observed in the overall results for CDVE (IRR, 95% CI: 0.92 [0.84; 1.03]), although this value is non-significant.
Finally, the minimum follow-up time in this study was 6 months, with some patients having a follow-up time of over 1 year. We identified a non-significant difference in the time to first VE event during the first year of follow-up. These results should be interpreted with caution, however, given that in the presence of crossing survival curves (non-proportional hazards), the performance of the log-rank test might be affected by the type of crossing observed.
Public health implications
Previous research has confirmed the transient increase in the risk of cardiovascular and cerebrovascular complications following the diagnosis of several respiratory diseases, including influenza, pneumonia, acute bronchitis, and others.15 To the best of our knowledge, this is one of the first studies to have compared the effects of COVID-19 diagnosis on VE outcomes with the effects of RTI diagnosis. Our preliminary findings help increase the pool of evidence focusing on RTI, considered prevalent in many countries and different healthcare settings.33 The non-significant association between COVID-19 diagnosis and cardiovascular or cerebrovascular outcomes observed in our study can be interpreted in the context of the drop in hospital admissions due to acute coronary syndromes and stroke during the first wave of the pandemic.34 , 35 Further research in varying healthcare settings and regions will help to confirm or disprove our preliminary findings. Notwithstanding the above, there should be a focus on the general prevention of respiratory diseases, as the complications resulting from respiratory failure have represented a great public health burden since the start of the pandemic.
Strengths and limitations
The main strengths of the present study are the large sample size used and the fact that the study reflects routine clinical practice in Germany, accounting for the shift in clinical practice and patient behavior with the use of a contemporary cohort based on data from the Disease Analyzer. In addition, the relatively large sample allowed subgroup analyses by age and sex to be performed.
In addition to these strengths, however, this study is also subject to a number of limitations, which need to be discussed. Because the real-world database used in this study does not cover hospital data including information on mechanical ventilation and does not capture mortality associated with hospitalization, no patients were censored before the end of the study period (December 31, 2021). As a result, the IRR calculated for VE may be biased. However, the magnitude of this bias may have been reduced by the fact that the study included a relatively young population (mean 45 years), as literature findings indicate that the case fatality rate of COVID-19 among patients younger than 50 years is less than 1%.36 Similarly, the study did not account for database enrollment time or drop-outs. Therefore, patients were assumed to have contributed person-time until the end of the study, which might have introduced additional bias by increasing the person-time denominator and thus leading to the underestimation of incidence rates. Because it is not necessary for COVID-19 cases to be confirmed in a primary care practice in Germany, the number of confirmed cases might have been underreported, which may also introduce bias to the results. The latter could have caused the number of patients in the RTI cohort also diagnosed with COVID-19 to be underestimated, thus decreasing the incidence of RTI patients, and resulting in IRRs of below one. Furthermore, given the general setup of the COVID-19 reporting systems in European countries, those patients who approached a primary care practice in Germany to receive care (either for COVID-19 or RTI) might have introduced additional selection bias. In addition, vaccination status (vaccination was broadly implemented in Germany starting in 2021, approximately 1 year after the pandemic started in March 2020)37 was not considered for the propensity score matching in the present study because vaccination information is only captured by a subgroup of German practices included in the study.38 Therefore, given that this study considers a continued index period extending from 2020 to 2021, patients included in the COVID-19 cohort could have had different levels of immunity, which could have influenced the outcomes observed. Similarly, with the identification of new COVID-19 variants39 throughout the index period, patients in the COVID-19 cohort could have been exposed to variable infectiousness levels, which could have also affected the viral injury they suffered, influencing the primary outcomes observed.
Conclusions
Overall, our study suggests that COVID-19 diagnosis was not associated with an increased risk of developing a VE compared with RTI diagnosis. However, further research in a variety of healthcare settings and regions is needed to confirm these preliminary findings from our cohort, which is a good reflection of routine clinical practice in Germany.
Author statements
Acknowledgments
The authors would like to thank Salome Adam from IQVIA Real World Solutions (Basel, Switzerland) for her support with the critical appraisal of the article.
Ethical approval
The database used includes only anonymized data in compliance with the regulations set forth in the applicable data protection laws. German law allows the use of anonymous electronic medical records for research purposes under certain conditions. In accordance with this legislation, it is not necessary to obtain informed consent from patients or approval from a medical ethics committee for this type of observational study that contains no directly identifiable data. Because patients were only queried as aggregates and no protected health information was available for queries, no institutional review board approval was required for the use of this database for the completion of this study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interests
The authors declare that they have no conflicting interests.
Author contributions
S.Z. and K.K. developed the study concept and design. S.Z. conducted the analysis and drafted the article. A.C. ensured the quality control for the programs. All authors contributed to the interpretation of data and critically reviewed the article for intellectual content. All authors were involved in the final approval of the article for submission.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.puhe.2022.10.005.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.World Health Organization. Cardiovascular diseases, https://www.who.int/health-topics/cardiovascular-diseases [accessed 2 May 2022].
- 2.National Health System (NHS). Cardiovascular disease, https://www.nhs.uk/conditions/cardiovascular-disease/[accessed 6 May 2022].
- 3.Eurostat, Statistics of the European Union . 2018. Causes of death - diseases of the circulatory system.https://ec.europa.eu/eurostat/statistics-explained/index.php?title=File:Causes_of_death_%E2%80%94_diseases_of_the_circulatory_system _residents,_2018.png. [Google Scholar]
- 4.European Society of Cardiology: cardiovascular disease statistics. 2021. https://academic.oup.com/eurheartj/article/43/8/716/6472699?login=true [Google Scholar]
- 5.Katsoularis I., Fonseca-Rodríguez O., Farrington P., Lindmark K., Fors Connolly A.M. Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: a self-controlled case series and matched cohort study. Lancet Lond Engl. 2021;398(10300):599–607. doi: 10.1016/S0140-6736(21)00896-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Modin D., Claggett B., Sindet-Pedersen C., Lassen M.C.H., Skaarup K.G., Jensen J.U.S., et al. Acute COVID-19 and the incidence of ischemic stroke and acute myocardial infarction. Circulation. 2020;142(21):2080–2082. doi: 10.1161/CIRCULATIONAHA.120.050809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azevedo R.B., Botelho B.G., Hollanda JVG de, Ferreira L.V.L., Junqueira de Andrade L.Z., Oei S.S.M.L., et al. Covid-19 and the cardiovascular system: a comprehensive review. J Hum Hypertens. 2021;35(1):4–11. doi: 10.1038/s41371-020-0387-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta A., Madhavan M.V., Sehgal K., Nair N., Mahajan S., Sehrawat T.S., et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leung N.H.L., Xu C., Ip D.K.M., Cowling B.J. The fraction of influenza virus infections that are asymptomatic: a systematic review and meta-analysis. Epidemiol Camb Mass. 2015;26(6):862–872. doi: 10.1097/EDE.0000000000000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siripanthong B., Nazarian S., Muser D., Deo R., Santangeli P., Khanji M.Y., et al. Recognizing COVID-19-related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17(9):1463–1471. doi: 10.1016/j.hrthm.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansson G.K. Inflammation, atherosclerosis, and coronary Artery disease. N Engl J Med. 2005;352(16):1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 12.Piazza G., Goldhaber S.Z. Venous thromboembolism and atherothrombosis: an integrated approach. Circulation. 2010;121(19):2146–2150. doi: 10.1161/CIRCULATIONAHA.110.951236. [DOI] [PubMed] [Google Scholar]
- 13.Pei J., You X., Fu Q. Inflammation in the pathogenesis of ischemic stroke. Front Biosci-Landmark. 2015;20(4):772–783. doi: 10.2741/4336. [DOI] [PubMed] [Google Scholar]
- 14.Han K., Shi D., Yang L., Wang Z., Li Y., Gao F., et al. Prognostic value of systemic inflammatory response index in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Ann Med. 2022;54(1):1667–1677. doi: 10.1080/07853890.2022.2083671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davidson J.A., Warren-Gash C. Cardiovascular complications of acute respiratory infections: current research and future directions. Expert Rev Anti Infect Ther. 2019;17(12):939–942. doi: 10.1080/14787210.2019.1689817. [DOI] [PubMed] [Google Scholar]
- 16.Clayton T.C., Thompson M., Meade T.W. Recent respiratory infection and risk of cardiovascular disease: case-control study through a general practice database. Eur Heart J. 2008;29(1):96–103. doi: 10.1093/eurheartj/ehm516. [DOI] [PubMed] [Google Scholar]
- 17.Lee J.J., Koshiaris C., Hobbs F.R., Sheppard J.P. Beyond COVID-19: respiratory infection and cardiovascular events. Br J Gen Pract. 2021;71(709):342–343. doi: 10.3399/bjgp21X716477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hessami A., Shamshirian A., Heydari K., Pourali F., Alizadeh-Navaei R., Moosazadeh M., et al. Cardiovascular diseases burden in COVID-19: systematic review and meta-analysis. Am J Emerg Med. 2021;46:382–391. doi: 10.1016/j.ajem.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang T., Yan M.Z., Li X., Lau E.H.Y. Sequelae of COVID-19 among previously hospitalized patients up to 1 year after discharge: a systematic review and meta-analysis. Infection. 2022:1–43. doi: 10.1007/s15010-022-01862-3. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rathmann W., Bongaerts B., Carius H.J., Kruppert S., Kostev K. Basic characteristics and representativeness of the German Disease Analyzer database. Int J Clin Pharmacol Ther. 2018;56(10):459–466. doi: 10.5414/CP203320. [DOI] [PubMed] [Google Scholar]
- 21.Becher H., Kostev K., Schröder-Bernhardi D. Validity and representativeness of the “Disease Analyzer” patient database for use in pharmacoepidemiological and pharmacoeconomic studies. Int J Clin Pharmacol Ther. 2009;47(10):617–626. doi: 10.5414/cpp47617. [DOI] [PubMed] [Google Scholar]
- 22.Tanislav C., Rosenbauer J., Zingel R., Kostev K. No increased incidence of venous thrombosis or pulmonary embolism after SARS-CoV-2 vaccination in Germany. Publ Health. 2022;207:14–18. doi: 10.1016/j.puhe.2022.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.United Nations, Department of Economic and Social Affairs, Population Division . 2019. World Population Prospects 2019, custom data acquired via website. [Google Scholar]
- 24.Kelly-Hayes M. Influence of age and health behaviors on stroke risk: lessons from longitudinal studies. J Am Geriatr Soc. 2010;58(Suppl 2):S325–S328. doi: 10.1111/j.1532-5415.2010.02915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yousufuddin M., Young N. Aging and ischemic stroke. Aging. 2019;11(9):2542–2544. doi: 10.18632/aging.101931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mosca L., Barrett-Connor E., Wenger N.K. Sex/gender differences in cardiovascular disease prevention what a difference a decade makes. Circulation. 2011;124(19):2145–2154. doi: 10.1161/CIRCULATIONAHA.110.968792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao Z., Chen Z., Sun A., Deng X. Gender differences in cardiovascular disease. Med Nov Technol Devices. 2019;4 doi: 10.1016/j.medntd.2019.100025. [DOI] [Google Scholar]
- 28.Appelman Y., Rijn BB van, Haaf ME ten, Boersma E., Peters S.A.E. Sex differences in cardiovascular risk factors and disease prevention. Atherosclerosis. 2015;241(1):211–218. doi: 10.1016/j.atherosclerosis.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 29.Reeves M.J., Bushnell C.D., Howard G., Gargano J.W., Duncan P.W., Lynch G., et al. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7(10):915–926. doi: 10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie Y., Xu E., Bowe B., Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28(3):583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tu K., Sarkadi Kristiansson R., Gronsbell J., de Lusignan S., Flottorp S., Goh L.H., et al. Changes in primary care visits arising from the COVID-19 pandemic: an international comparative study by the International Consortium of Primary Care Big Data Researchers (INTRePID) BMJ Open. 2022;12(5) doi: 10.1136/bmjopen-2021-059130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ekman B., Arvidsson E., Thulesius H., Wilkens J., Cronberg O. Impact of the Covid-19 pandemic on primary care utilization: evidence from Sweden using national register data. BMC Res Notes. 2021;14(1):424. doi: 10.1186/s13104-021-05839-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.GBD Chronic Respiratory Disease Collaborators Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2020;8(6):585–596. doi: 10.1016/S2213-2600(20)30105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meza H.T., Gil Á Lambea, Saldaña A.S., Martínez-Zabaleta M., Juez P.D.L.R., Martínez E.L.C., et al. Impact of COVID-19 outbreak on ischemic stroke admissions and in-hospital mortality in North-West Spain. Int J Stroke. 2020;15(7):755–762. doi: 10.1177/1747493020938301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mafham M.M., Spata E., Goldacre R., Gair D., Curnow P., Bray M., et al. COVID-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet. 2020;396(10248):381–389. doi: 10.1016/S0140-6736(20)31356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bae S., Kim S.R., Kim M.N., Shim W.J., Park S.M. Impact of cardiovascular disease and risk factors on fatal outcomes in patients with COVID-19 according to age: a systematic review and meta-analysis. Heart. 2021;107:373–380. doi: 10.1136/heartjnl-2020-317901. [DOI] [PubMed] [Google Scholar]
- 37.Zheng C., Shao W., Chen X., Zhang B., Wang G., Zhang W. Real-world effectiveness of COVID-19 vaccines: a literature review and meta-analysis. Int J Infect Dis. 2022;114:252–260. doi: 10.1016/j.ijid.2021.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohl N., Zingel R., Kostev K. Investigation of the representativeness of the German IQVIA vaccine Analyzer database. Int J Clin Pharmacol Ther. 2022;60(2):79–86. doi: 10.5414/CP204098. [DOI] [PubMed] [Google Scholar]
- 39.Iacopetta D., Ceramella J., Catalano A., Saturnino C., Pellegrino M., Mariconda A., et al. COVID-19 at a glance: an up-to-date overview on variants, Drug design and therapies. Viruses. 2022;14(3):573. doi: 10.3390/v14030573. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.