Abstract
Objective:
To systematically review and meta-analyse studies of the efficacy of probiotics to reduce antenatal Group B Streptococcus (GBS) colonisation.
Participants:
Antenatal participants with known positive GBS colonisation or unknown GBS status.
Intervention:
Probiotic interventions containing species of Lactobacillus or Streptococcus.
Design:
Systematic review and meta-analysis.
Measurements and findings:
The systematic review included 10 studies. Five articles contained in vitro studies of probiotic interventions to determine antagonistic activity against GBS. Six clinical trials of probiotics to reduce antenatal GBS were systematically reviewed and meta-analysed. The meta-analysis revealed that the use of an antenatal probiotic decreased the probability of a positive GBS result by 44% (OR = 0.56, 95% CI = 8.7%, 194.1%, p = 0.02) (n = 709). However, only one clinical trial of 10 had a low risk of bias.
Key conclusions:
The probiotic interventions subjected to in vitro testing showed antagonistic activity against GBS through the mechanisms of acidification, immune modulation, and adhesion. The findings of the meta-analysis of the clinical trials revealed that probiotics are a moderately effective intervention to reduce antenatal GBS colonisation. More well-controlled trials with diverse participants and with better elucidation of variables influencing GBS colonisation rates are needed.
Implications for practice:
Probiotic interventions appear to be a safe and effective primary prevention strategy for antenatal GBS colonisation. Application of this low-risk intervention needs more study but may reduce the need for intrapartum antibiotic prophylaxis in countries or regions where antenatal GBS screening is used. Midwives can be instrumental in conducting and supporting larger well-controlled clinical trials.
Keywords: Probiotics, Antenatal, GBS, in vitro, Systematic Review, Meta-analysis
Introduction
Streptococcus agalactiae, more commonly known as Group B Streptococcus (GBS) is an encapsulated, beta-haemolytic, grampositive coccus, and a facultative anaerobe that is part of the commensal microbiome of humans. The gastrointestinal (GI) tract is the source for vaginal GBS colonisation in women (American College of Obstetrics and Gynaecology, ACOG, 2019; Picard and Bergeron, 2004). Prenatal GBS colonisation is generally asymptomatic (Armistead et al., 2019; Marziali et al., 2019), and may be transient or persistent (ACOG, 2019; Armistead et al., 2019; Marziali et al., 2019; Meyn et al., 2009; Picard and Bergeron, 2004). (Brzychczy-Włoch et al., 2014) conducted a descriptive study with a sample of 42 healthy adult pregnant women without signs of clinical genitourinary infection. Separate vaginal and rectal GBS swabs were collected in each trimester. Fifteen participants were GBS positive at some point in pregnancy and 27 were GBS negative. The researchers found that vaginal GBS colonisation was relatively stable throughout pregnancy, averaging 7.42 × 104 CFU/ml in the first trimester and 1.74 × 104 CFU/ml in the third trimester, while rectal colonisation changed substantially during pregnancy with an average 2.8 × 104 CFU/ml in the first trimester and 4.37 × 105 CFU/ml in the third trimester. Several risk factors have been associated with GBS colonisation, including but not limited to: people who are employed in healthcare, of African descent, overweight or obese, have low vitamin D levels, have poor vaginal hygiene, engaging in oral sex, or have frequent sexual intercourse (Akoh et al., 2017; Capan-Melser et al., 2015; Foxman et al., 2007; Le Doare and Heath, 2013; Stapleton et al., 2005).
During normal vaginal birth, GBS can be vertically transmitted to the fetus. Approximately 50% of neonates born to GBS culture positive women will become colonized with GBS but of these only 1–2% will develop Early Onset Group B Streptococcus Disease (EOG-BSD) (Chan et al., 2006; Illuzzi & Bracken, 2006; Virranniemi et al., 2019). EOGBSD can result in significant neonatal morbidity and mortality (Verani et al., 2010).
Worldwide, GBS colonizes up to 65% of healthy nonpregnant people and between 15-40% of pregnant people (Russell et al., 2017; Seale et al., 2017). The global estimate of antenatal GBS is 18% with a large range of regional prevalence; the lowest rates of colonisation (11–13%) are in Southern and Eastern Asia while the Caribbean has the highest rate (35%) (Russell et al., 2017). Seale et al. (2017) performed an extensive analysis of the worldwide burden of perinatal GBS. Of the 10 GBS serotypes, III is the most virulent and accounts for 48% of the EOGBSD cases worldwide, while serotypes 1a and 1b are responsible for 30%, and seven serotypes (II, IV, V, VI, VII, VIII, and IX) account for the remaining cases.
The United States (US) Centers for Disease Control and Prevention (CDC) 2010 guidelines recommended universal vaginal to rectal screening at 35-37 weeks gestation and intrapartum antibiotic prophylaxis (IAP) for those who test positive. Implementation of these guidelines resulted in an 80% reduction in EOGBSD in the US, from 1.8 newborns per 1,000 live births in the 1990s to 0.23 per 1,000 live births in 2015 (Nanduri et al., 2019; Verani et al., 2010). Residual GBS, including missed or transient GBS and late onset neonatal GBS disease, remain persistent challenges despite the introduction of the CDC guidelines (Berardi et al., 2013; Parente et al., 2017; Van Dyke et al., 2009). In 2019, ACOG American College of Obstetricians and Gynecologists (ACOG) 2019 American Academy of Pediatrics (AAP) 2019 assumed stewardship of GBS recommendations for all maternity care providers and newborns respectively, replacing and updating the CDC 2010 guidelines. Subsequently, the American Society for Microbiology (ASM) issued new recommendations for standard laboratory practices related to GBS (Filkins et al., 2020). An important change in the recent ACOG guidelines is that of later vaginal to rectal antenatal GBS screening between 36 0/7 and 37 6/7 weeks gestation to assure that the culture result is valid for a five-week period before anticipated birth (ACOG, 2019).
Strategies used to prevent EOGBSD vary based on the recommendations of guideline-setting organizations and by regional variations in GBS prevalence. Three main EOGBSD prevention strategies are used worldwide: (a) universal antenatal screening for GBS according to the ACOG guidelines, (b) the risk-based approach, or (c) a combination of both (Kolkman et al., 2013). Although considered a standard of care in the US, the CDC and ACOG/AAP universal screening approaches are both considered cost-effective in regions where the prevalence of newborn GBS infection is high (> 1.2/1,000 births) (Santhanam et al., 2017). In regions where the rate of newborn GBS infection is considered low, a risk-based approach is often used to determine candidates for IAP administration (Santhanam et al., 2017). Risk factors for GBS transmission and a comparison of the antenatal screening and risk-based approaches appear in Table 1.
Table 1.
Comparison of GBS prevention strategies on indications for intrapartum antibiotic prophylaxis.
| Screening Based Approach | Risk Based Approach | ||
|---|---|---|---|
|
| |||
| Care | Factors Considered | Intrapartum Antibiotic Prophylaxis Indicated | |
| Antepartum | Previously GBS affected infant | Yes | Yes |
| GBS Bacteriuria | Yes | Yes | |
| GBS positive in prior pregnancy | No | Possiblya | |
| GBS Culture obtained at 36 weeks gestation | Yes | Not applicable | |
|
| |||
| Intrapartum | GBS culture result is positive | Yes | Not applicable |
| Premature labor <37 weeks | Yesb | Yes | |
| Prolonged Rupture of Membranes | No | Yes | |
| Intrapartum fever 3100.4 | No | Yes | |
Notes:
If GBS status is unknown in current pregnancy, history of GBS colonization in prior pregnancy may serve as a rationale for IAP.
Unless a negative 36-week GBS test result is available.
Efforts aimed at primary prevention of antepartum GBS colonisation are of interest to healthcare consumers and providers, including midwives. Intrapartum vaginal chlorhexidine gel/cream or washes were examined in a systematic review that included four clinical trials (all graded as very low quality) with outcomes for 1,125 infants. Vaginal chlorhexidine did not reduce EOGBSD (Ohlsson et al., 2014). Although research, development, and testing continue, efforts to develop a GBS vaccine have not yet been successful (Hillier et al., 2019). Midwives have suggested probiotics as an approach to reduce GBS colonisation (Bishara, 2006; Singleton, 2007), but until very recently this strategy had not been scientifically studied.
Probiotics are live microorganisms, which when administered in sufficient amounts confer health benefits on the host (Food and Agriculture Organization of the United Nations, FAO, and World Health Organization, WHO, 2001). Probiotic interventions produce health benefits on mucosal surfaces like the GI tract and vagina. Mechanisms of action include secretion of organic acids, vitamins, and bacteriocins to prevent the adhesion of pathogens and work synergistically with the host immune system (Reid et al., 2013).
Probiotic names include genus and species (e.g., Lactobacillus acidophilus). Strains are designated by letter and/or numbers (e.g., NCFM, or North Carolina Food Microbiology). Probiotic intervention dosages are described in Colony Forming Units (CFUs). CFUs are the number of colonies multiplied by the dilutions on the plate, divided by the volume of culture on the plate, and are reported in scientific notation (Brugger et al., 2012).
Numerous authors have published claims about the variety of bacterial species that are considered effective as probiotics for human health benefits (Fijan, 2014). Commercially available probiotic supplements commonly include species of Lactobacillus and Bifidobacterium. Lactobacilli are frequently used for women’s health applications, since numerous species secrete acids, such as hydrogen peroxide that acidify the vaginal mucosa. The presence of Lactobacillus is a marker of vaginal health, as this species plays an integral role in the microbiologic homeostasis of the genitourinary tract referred to as eubiosis (Reid et al., 2016). Bifidobacterium species are less commonly used for women’s health applications, since their actions takes place in the GI tract. The presence and diversity of Bifidobacteria are markers of GI wellness (Mitsuoka, 1990).
The scientific premise of probiotic interventions to reduce antenatal vaginal and rectal GBS colonisation is based on the observation that women with higher counts of vaginal Lactobacilli have fewer GBS CFUs (Altoparlak et al., 2004; Moghaddam, 2010; Ronnqvist et al., 2006; Rosen et al., 2017; Whitney et al., 2004). Therefore, the administration of probiotic interventions aims to increase vaginal Lactobacilli. Several scientific investigations have demonstrated that an oral probiotic intervention may colonize the vagina in 2-10 days (Ehrström et al., 2010; Hemmerling et al., 2010).
The purpose of this article is to systematically review and meta-analyse studies of the efficacy of probiotics to reduce antenatal GBS. Both in vitro laboratory studies of probiotic supplements used to inhibit GBS and clinical trials of probiotic interventions used to reduce antepartum GBS colonisation are systematically reviewed. The GBS outcomes of the clinical trials are meta-analysed. The implications for clinical practice and future research are discussed.
Methods
In consultation with a health sciences librarian, the literature search was developed. Searches were completed using the following electronic databases: PubMed, CINAHL (Cumulative Index to Nursing and Allied Health Literature), Cochrane Library, and Web of Science. Structures of the search included terms related to probiotic interventions and reduction of antepartum GBS colonisation and its related vocabulary. Sample search terms included probiotics, prebiotics, pregnancy, antenatal care, Group B Streptococcus. Particular attention was used for transmission terminology, such as vertical infection transmission and maternal-child transmission. The initial search was conducted in November 2019. For a complete list of the literature search strategies see Appendix A.
The results were limited to English language only, with no limit on publication date range nor type of publication. The search strategy was first established in PubMed using a combination of MeSH (medical subject headings; database-controlled vocabulary) and key words. The MeSH headings were searched along with the keywords. Specific MeSH terminology included probiotics, “gastrointestinal agents,” “Bsp protein, group B Streptococcus,” and “Infectious Disease Transmission, Vertical.” From there, the other database search strategies were developed, and searches were conducted. With each database search, database-controlled vocabulary was searched in combination with keywords. The initial search ran in November 2019 and yielded 574 results with the removal of 34 duplicates. Additional searches were run using the ClinicalTrials.gov website, as well as hand searching and reviewing reference lists of articles. There were an additional eight articles found for a total of 540 titles screened with a final 10 articles chosen for the review, one of which (Martiń et al., 2019) included both types, in vitro and a RCT. Figure 1 contains the PRISMA diagram for reporting systematic reviews, according to the most recent update (Page et al., 2021).
Fig. 1.
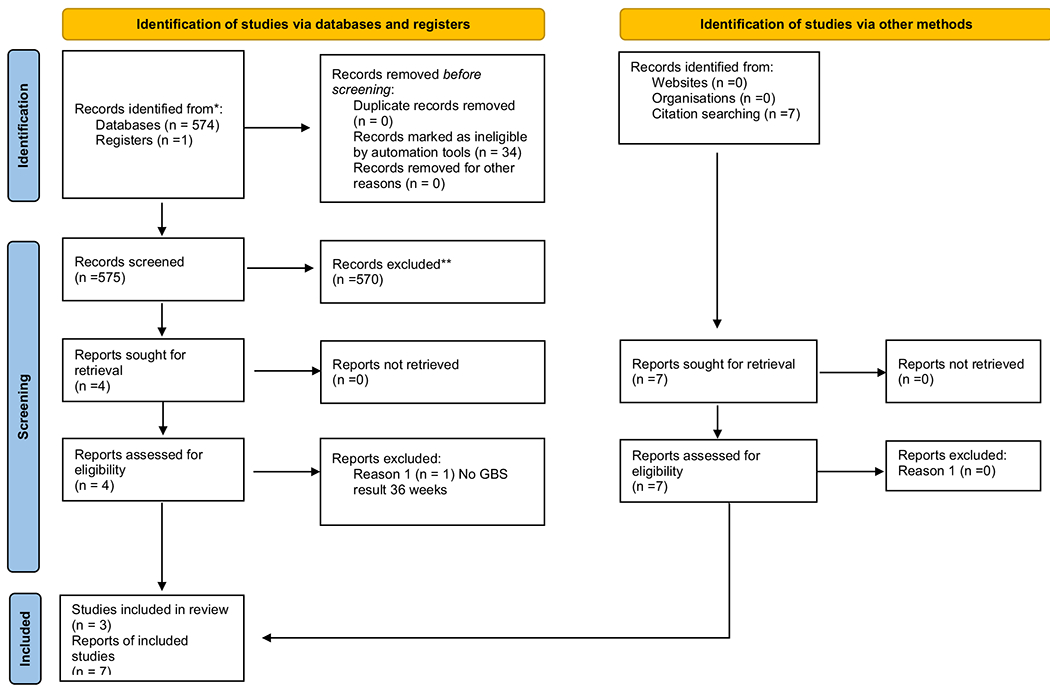
PRISMA diagram 04262021.
Clinical trials of probiotic interventions were included if they reported 36-week GBS results. In vitro studies were included in this systematic review if probiotics were tested for antagonist activity against GBS. Data were obtained from the five in vitro studies and six clinical trials of probiotics to reduce GBS. The first two authors extracted data using the Cochrane data form for RCTs and non-RCTs Cochrane Collaboration (2020). Only clinical trials were rated for quality using the Cochrane risk of bias criteria (Sterne et al., 2019). The first two authors performed these appraisals independently and reached consensus on the ratings where there was a difference. The final ratings were shared with the third and final authors who verified them. Table 2 contains the risk of bias ratings.
Table 2.
Risk of Bias assessment of clinical trials.
| Author/Year | Design | Random sequence generation (selection bias) | Allocation concealment (selection bias) | Blinding of personnel (performance bias) | Blinding of outcome assessment (detection bias) | Incomplete outcome data (attribution bias) | Selective reporting (reporting bias) | Other bias |
|---|---|---|---|---|---|---|---|---|
| Sharpe et al., 2019 | RCT | unclear | low | low | low | low | low | n/a |
| Olsen et al., 2018 | RCT | unclear | high | low | high | unclear | low | n/a |
| Ho et al., 2016 | RCT | low | low | low | low | low | low | SA |
| Martiń et al., 2019 | PC | high | high | high | unclear | unclear | unclear | SA |
| Di Pierro et al., 2016 | OL-PC | high | high | high | high | unclear | high | SA |
| Hanson et al., 2014 | OL-QE | high | high | high | high | low | low | n/a |
RCT = randomized controlled trial; PC = prospective cohort; OL = open-label; QE = Quasi- experiment; SA = Statistical analysis limited; n/a=not applicable.
Meta-analysis
Descriptive statistics and meta-analysis were completed in the software platform R (R Core Team, 2021), with the R package metafor (Viechtbauer, 2010). The Odds Ratio (OR) was used to calculate the effect size, or the difference between the control and intervention groups (probiotics), in relation to the prevalence of a negative GBS result. The OR, Confidence Interval (CI), and the percent weight contribution for each study were used to summarize the results estimated from the random effect model, implying that there is a population distribution of effect sizes, and the selected studies represent samples of this distribution Card (2012). The results present the random effect OR with a 95% CI across the six studies meta-analysed shown in a forest plot. Statistical heterogeneity was assessed in the meta-analysis using Cochrane’s Q t 2, and I 2 statistics (Viechtbauer, 2010).
Findings
In vitro
Investigators in five laboratory studies were successful at inhibiting GBS in vitro (Ephraim et al., 2012; (Martiń et al., 2019; Marziali et al., 2019; Patras et al., 2015; Zárate and Nader-Macias, 2006). These studies are summarized in Table 3, including probiotic species tested, study duration, source of GBS, and findings specific to mechanisms of action. Lactobacillus was chosen as the probiotic bacterial genus for four of the five studies, while Streptococcus salivarius was chosen for use in one. Since adhesion to host epithelial cells is vital for pathogen colonisation and clinical outcomes, most investigators measured the ability of probiotic species and GBS to compete for adhesion to human vaginal epithelial cells (VEC) (Martiń et al., 2019; Patras et al., 2015; Zárate and Nader-Macias, 2006) or animal-derived epithelial cells (Ephraim et al., 2012). In addition, some researchers explored acidification and/or immune modulation interactions by coculturing bacterial cells in various culture media (Ephraim et al., 2012; Marziali et al., 2019; Patras et al., 2015), and/or within a murine model (Patras et al., 2015). All but one article (Zárate and Nader-Macias, 2006) contained a list of the GBS isolates used in the laboratory experiments. Most used GBS from an internationally available standard cell line manufacturer (American Type Culture Collection, ATCC; Spanish Type Culture Collection, STCC); others used locally derived GBS samples. In one of the articles, the researchers linked the GBS isolates studied to serotypes relevant to clinical practice (Marziali et al., 2019). Marziali and colleagues (2019) tested 14 strains of Lactobacilli for efficacy of eradicating GBS. They found no difference in probiotic inhibitory activity against three strains of GBS. Therefore, they only reported the findings of activity against one GBS strain (SA 24/serotype III). The researchers concluded that acidification by lactic acid was the driving mechanism of action for probiotic inhibition of GBS. Martiń et al. (2019) conducted extensive genotyping to choose 10 strains of Lactobacillus salivarius isolated from vaginal exudates for their in vitro testing. L. salivarius CECT 9145 (STCC) was selected as most effective against GBS for their pilot clinical trial, discussed later in this article. L. salivarius CECT 9145 showed high anti-GBS activity and produced lactic acid and hydrogen peroxide, corroborating Marziali’s conclusion that acidification is a key mechanism of action against GBS. In addition to acidification, strong L. salivarius CECT adhesion to VEC and ability to co-aggregate with GBS was found. Patras et al. (2015) tested nine strains of Streptococcus salivarius, an oral commensal, against 13 human GBS isolates to find the likely most effective strain (K12) to use in future clinical vaginal trials. K12 exhibited a higher adhesion affinity to in vitro human and in vivo murine VEC than GBS. In addition, interleukin-8 levels were lower in human VEC incubated with K12 and GBS compared to VEC incubated with GBS alone. Ephraim et al. (2012) studied the antagonistic effects of Lactobacillus rhamnosus HN001 and the combination product Florajen3® against GBS. Inhibition and complete exclusion of some GBS strains cocultured with Florajen3® was correlated with lower pH values and higher probiotic adhesion affinity to epithelial cells, however exact pH values were not reported. Finally, Zárate et al. (2006) focused exclusively on VEC adhesion to determine that Lactobacillus acidophilus (CRL 1259) and Lactobacillus paracasei (CRL 1289) were the most effective Lactobacilli species to inhibit GBS. The authors noted that L. acidophilus (CRL 1259) is a known lactic acid producer and L. paracasei (CRL 1289) is a known hydrogen peroxide producer, but they did not report pH measurements throughout the adhesion experiments.
Table 3.
Summary of in vitro laboratory testing of probiotics to reduce GBS.
| Author, year | Probiotic bacteriatested | GBS isolatestested | Antagonist activityagainst GBS | Mechanism of action |
|---|---|---|---|---|
| Marziali et al., 2019 | 14 Lactobacillus strains: • 7 strains of L. crispatus (BC1, BC3-BC8) • 5 strains of L. gasseri (BC9, BC10, BC12- BC14) • 2 strains of L. vaginalis (BC16, BC17). |
3 GBS strains: • derived from vaginal & peri-rectal swabs |
• L. crispatus (BC1, BC4, BC7, and BC8) cells or supernatants totally eradicated GBS in 60 minutes • L. crispatus BC6, L. gasseri (BC9, BC12-BC14) cells or supernatants inhibited GBS growth in 60 minutes • L. crispatus (BC3, BC5), L. gasseri (BC10), L. vaginalis (BC16, BC17) cells or supernatants had no activity against GBS or ↓ GBS viability in 60 minutes |
Acidification • pH<4.0 • pH <4.0-4.5 • pH≥4.5 |
| (Martiń et al., 2019) | 10 Lactobacillus salivarius strains (V3III-1, CECT 9145, V711-1, V711-62, V7IV-1; V7IV-60, V8III-62, V11I-60, V11IV-60, CELA2) | 4 GBS strains: • from vaginal exudates |
• L salivarius CECT 9145 cells showed best adherence to VEC, and was selected as most effective with 100% GBS eradication in 24 hours | Acidification: pH=4.01 (High lactic acid and hydrogen peroxide) Adherence |
| Patras et al., 2015 | 9 Streptococcus salivarius strains (K12, M18, Tove R, NR, 20P3, #5, MPS, P, CCHSS3) | 13 GBS strains: • ATCC • vaginally-derived |
• S. salivarius K12 and Tove R inhibited all 13 GBS strains (K12 was most effective) • S. salivarius K12 had good adherence to VEC. M18 showed little capacity to bind to VEC. • S. salivarius K12 and GBS decreased IL-8 levels in VEC compared to GBS alone |
Adherence Immune modulation Lower IL-8 levels |
| Ephraim et al., 2012 |
L. rhamnosus HN001Florajen3® • L. acidophilus NCFM • B. longum Bi05 • B. animalis subsp. lactis |
4 GBS strains: • ATCC • blood stream samples |
• L. rhamnosus HN001 significantly inhibited GBS strains 0191, 0192, 0193 (p<0.05) at 24 hours and 48 hours • Florajen3® significantly decreased 0192 by 5 logs (p<0.05); completely eliminated GBS 0191, the most hemolytic GBS isolate at 24 hours and 48 hours. |
Acidification pH not reported Adherence |
| (Zárate and Nader-Macias, 2006) | 4 Lactobacillus species: • L. acidophilis (CRL 1259) • L. crispatus (CRL 1266) • L. paracasei (CRL 1289) • L. salivarius (CRL 1328) |
Detail not provided • Urogenital origin |
• L. acidophilus (CRL 1259) significantly inhibited the adhesion of GBS to VEC (p<0.05) after 120 minutes. • L. acidophilus (CRL 1259) and L. paracasei (CRL 1289) significantly inhibited GBS after 120 minutes. • L. paracasei (CRL 1289) significantly displaced GBS from VEC (p<0.05) • L. crispatus (CRL 1266) & L. salivarius (CR 1328) not significant against GBS after 120 minutes. |
Adherence |
| (Marziali et al., 2019) | 14 Lactobacillus strains: • 7 strains of L. crispatus (BC1, BC3-BC8) • 5 strains of L. gasseri (BC9, BC10, BC12- BC14) • 2 strains of L. vaginalis (BC16, BC17). |
3 • derived from vaginal and peri-rectal swabs |
• L. crispatus (BC1, BC4, BC7, and BC8) cells or supernatants totally eradicated GBS in 60 minutes • L. crispatus BC6, L. gasseri (BC9, BC12-BC14) cells or supernatants inhibited GBS growth in 60 minutes • L. crispatus (BC3, BC5), L. gasseri (BC10), L. vaginalis (BC16, BC17) cells or supernatants had no activity against GBS or ↓ GBS viability in 60 minutes |
Acidification pH<4.0 pH<4.0-4.5 pH≥4.5 |
| (Martiń et al., 2019) | 10 Lactobacillus salivarius strains: (V3III-1, CECT 9145, V711-1, V711-62, V7IV-1; V7IV-60, V8III-62, V11I-60, V11IV-60, CELA2) | 4 • from vaginal exudates |
L salivarius CECT 9145 cells showed best adherence to VEC and was selected as most effective with 100% GBS eradication in 24 hours | AdherenceAcidification • pH=4.01 (High lactic acid and hydrogen peroxide) |
| Patras et al., 2015 | 9 Streptococcus salivarius strains: (K12, M18, Tove R, NR, 20P3, #5, MPS, P, CCHSS3) | 13 • ATCC • vaginally-dervied |
• S. salivarius K12 and Tove R inhibited all 13 GBS strains (K12 was most effective) • S. salivarius K12 had good adherence to VEC. M18 showed little capacity to bind to VEC. • S. salivarius K12 and GBS decreased IL-8 levels in VEC compared to GBS alone. |
InhibitionAdherenceImmune modulation • Lower IL-8 levels |
| Ephraim et al., 2012 | L. rhamnosus HN001 | 4 • ATCC • blood stream samples |
• L. rhamnosus HN001 significantly inhibited GBS strains 0191, 0192, 0193 (p<0.05) at 24 hours and 48 hours | CompetitionAcidification • pH not reported |
| Florajen3® • L. acidophilus NCFM • B. longum Bi05 • B. animalis subsp. lactis |
• Florajen3® significantly decreased 0192 by 5 logs (p<0.05); completely eliminated GBS 0191, the most hemolytic GBS isolate at 24 hours and 48 hours. | |||
| (Zárate and Nader-Macias, 2006) |
4 Lactobacillus species: • L. acidophilis (CRL 1259) • L. crispatus (CRL 1266) • L. paracasei (CRL 1289) • L. salivarius (CRL 1328) |
Detail not provided • urogenital origin |
• L. acidophilus (CRL 1259) significantly inhibited the adhesion of GBS to VEC (p<0.05) after 120 minutes. • L. acidophilus (CRL 1259) and L paracasei (CRL 1289) significantly inhibited GBS after 120 minutes. • L. paracasei (CRL 1289) significantly displaced GBS from VEC (p<0.05) • L. crispatus (CRL 1266) & L. salivarius (CR 1328) not significant against GBS after 120 minutes. |
• Exclusion • Competition • Displacement |
Notes: VEC = Vaginal epithelium cells
Together these five in vitro studies identified mechanisms of action of probiotics to reduce GBS, including acidification, immune modulation, and adhesion. Two of these laboratory studies (Ephraim et al., 2012; Martiń et al., 2019) were performed to determine the choice of probiotic intervention for subsequent clinical trials (Hanson et al., 2014; Martiń et al., 2019).
Clinical trials
Table 4 contains a summary of the six clinical trials of probiotics to reduce antenatal GBS colonisation: three randomized controlled trials (RCTs) (Ho et al., 2016; Olsen et al., 2018; Sharpe et al., 2019), two prospective cohort studies (Di Pierro et al., 2016; Martiń et al., 2019), and one quasi experiment (Hanson et al., 2014). Each study is briefly described and critically analysed, then the findings are synthesized and meta-analysed.
Table 4.
Summary of Clinical Trials
| Primary author/year | Country | Probiotic intervention | Total CFU | Study design | N(I/C) | Startweek | BaselineGBS ++ | GBS Findings |
|---|---|---|---|---|---|---|---|---|
| Sharpe et al., 2019 | Canada | UREX™ • L. rhamnosus GR-1 • L. reuteri RC-14 |
5 × 109 | RCT | 114 (58/ 56) | 23-25 | Not done | NS (p=.48) at 35-37 weeks |
| Olsen et al., 2018 | Australia | L rhamnosus GR-1 | 1 × 108 | RCT | 34 (21/ 13) | 36 | 100% | NS (p = 0.7, FET) |
| Ho et al., 2016 | Taiwan | UREX™ • L rhamnosus GR-1, • L reuteri RC-14 |
2 × 109 | RCT | 99 (49/50) | 36 | 100%/ | Significant ↓ in GBS (p=0.007); 50% ↓ in probiotic vs 18% placebo |
| (Martiń et al., 2019) | Spain | L salivarius CECT 9145 | 9 × 109 | Pros. cohort | 57 (39/18) | 26 | 100%/ 0% | ~70% ↓ in GBS; CFU counts significantly ↓ |
| Di Pierro et al., 2016 | Italy | iNatal® • Enterococcus faecium L3 LMG P-27496 • Bifidobacterium animalis ssp. lactis BB12 DSM 15954 • Lactobacillus casei R025 CNCM I-3429 • Lactococcus lactis ssp. lactis SP 38 DSM 26868 |
14 × 109 | Cohort open-label | 406 (127/279) | 30 | NA | 6% ↓ in GBS; 30% ↓ in PROM (p<.001); ↓ GI symptoms |
| Hanson et al., 2014 | USA | Florajen3® • Lactobacillus acidophilus NCFM • Bifodobacterium longum Bl-05 • Bifidobacterium lactis Bi-07 |
15 × 109 | Quasi-exper. | 20 (10/10) | 28 ±2 | NA | ↓GI symptoms ↓GBS colony counts |
CFU = colony forming units; I = intervention group; C = control group; GBS = Group B streptococcus; RCT = randomized controlled trial; NS = non-significant; FET = Fischer’s exact test; PROM = premature rupture of membranes; Pros. = prospective study; NA = not available; GI = gastrointestinal; Quasi-exper. = quasi-experiment.
No significant differences were found between groups for demographic characteristics such as age and parity in any of the clinical trials. However, participant race and ethnicity were only reported in two (Di Pierro et al., 2016; Hanson et al., 2014) of the six studies.
All the studies included an oral probiotic intervention with at least one Lactobacillus species. The total dosages of the oral probiotic interventions varied from 1 × 109 to 15 × 109. The timing of the onset of the intervention also varied between studies. In two of the RCTs (Di Pierro et al., 2016; Sharpe et al., 2019) and the one quasi-experiment (Hanson et al., 2014), the intervention was initiated during the second trimester (26-28 weeks gestation) with the goal of reducing colonisation at 36 weeks. In the remaining three studies (Ho et al., 2016; Martiń et al., 2019; Olsen et al., 2018), the intervention was initiated between 26-36 weeks in GBS positive participants with the goal of eradicating it before the time of birth. None of the studies reported adverse events.
GBS outcomes were reported using standard of care vaginal to rectal swabs (qualitative positive or negative) and/or quantitative GBS colony counts in CFUs using separate swabs of the vagina and rectum. One study collected only vaginal swabs (Olsen et al., 2018).
Probiotics to prevent antenatal colonisation
In three of the clinical trials, researchers used probiotic interventions to reduce antenatal GBS colonisation at 36 weeks. Hanson and colleagues (2014) conducted an open-label quasi-experiment and feasibility study of a probiotic intervention to reduce GBS colonisation at 35–37 weeks in a midwifery practice. The 10 probiotic group participants were enrolled first, followed by the 10 controls. The standard of care vaginal to rectal GBS swab was collected at 35–37 weeks. Vaginal and rectal quantitative GBS swabs were collected at baseline (28 ± 2 weeks) and at 35–37 weeks in both groups. The probiotic intervention was initiated at 28 ± 2 weeks. Two participants in each group had positive GBS cultures at 36 weeks. Although the study was not powered to show efficacy of the intervention, participants in the probiotic group had lower quantitative GBS colony counts. The eight GBS negative participants in the probiotic group averaged 90% probiotic adherence compared to 68% adherence in the two who were GBS positive. The probiotic was well tolerated and five participants in the intervention group reporting improved GI symptoms. Outcomes related to yogurt ingestion, vaginal cleaning, and sexual practices were collected as potentially confounding variables. Yogurt ingestion was inversely related to GBS colonisation (p = 0.02). No other statistical differences between groups were found in analysis of these variables.
Di Pierro et al. (2016) performed a prospective cohort study of antenatal probiotic outcomes, including GBS colonisation. Of all 406 antenatal clients who received care in the study setting, 166 who reported third trimester vaginitis, bacterial vaginosis, constipation, colitis, diarrhea, or bladder infections, were offered treatment with the probiotic. The intervention group was formed by 127 women who had accepted the probiotic. The 39 who declined probiotics plus the 240 women (n = 279) who did not experience symptoms related to the inclusion criteria were considered controls. The intervention was administered from 30–40 weeks gestation. Participants who received therapeutic antibiotics in their pregnancy were instructed to stop the probiotics intervention and resume it once treatment was completed. Vaginal-rectal swabs for GBS were taken at 36–37 weeks in all participants. Among the participants in the probiotic group, 27 (21.3%) were GBS positive at 36–37 weeks, compared to 76 (27.3%) of controls (p < 0.05). Di Pierro et al. (2016) found that GI symptoms were lessened in the probiotic group participants. Intervention adherence was not monitored in this study. No baseline GBS measures were collected. The addition of 2 previous years of GBS outcomes as combined points of comparison inflated the control group with known higher GBS rates and confounded the statistical analysis. The researchers reported statistically significant positive intrapartum outcomes in the probiotic group compared to controls including premature rupture of membranes (0% versus 31.2%; p < 0.01), caesarean section (5.51% vs. 10.39%; p < 0.05), and umbilical cord <7.2 (0% versus 6.09%; p < 0.01). Although the researchers attributed the positive intrapartum outcomes of the probiotic intervention to the “antimicrobial effects…[and] improved…general condition of the mother and fetus” (p. 263), the probiotic group was formed by including women with infections and inflammatory states.
Sharpe et al. (2019) randomized 139 participants from 19 midwifery practices to probiotic or placebo capsules beginning at 23–25 weeks gestation in a double-blind manner. The recruitment rate was described as low (12%) owing to participants’ desire to take their own probiotic product and/or the exclusion criteria of antibiotic use. While no baseline GBS tests were used in this study, the population rate of GBS colonisation was approximated at 21%. The study was concluded before the desired sample size (n = 200) was achieved, due to expiration of the probiotics. Based on pill counts, the average adherence to the intervention was 87%. Among the 113 cases analysed, the GBS colonisation positive rate at 36 weeks was 9 of 57 (15.8%) in the probiotic group versus 12 of 56 (21.4%) in the placebo group (p = 0.48).
Probiotic interventions administered to GBS positive participants
Researchers in three investigations administered oral probiotic interventions targeted to women positive for GBS during pregnancy. In two RCTs (Ho et al., 2016; Olsen et al., 2018), the probiotics were administered to women following the 36-week positive GBS screen, with intrapartum GBS cultures obtained as the major study outcome. These final GBS results were not available until after the woman gave birth, therefore they did not alter intrapartum care to study participants. Study methods and outcomes are described below.
Ho and colleagues (2016) randomized 110 GBS positive pregnant participants who were midwifery clients to either the probiotic or placebo condition until the time of birth. Three women gave birth precipitously and therefore GBS cultures were not obtained. Among the 99 women who completed the study, 21 women (42.9%) of the GBS positive participants in the probiotic group tested negative at time of birth, while only 9 (18%) were negative in the control group (Chi-square, p = 0.0007). Researchers reported an average of 20 days of probiotic intervention and placebo duration but did not report individual participant adherence.
A similar study was conducted as a pilot in a setting where pregnant women routinely self-collected lower vaginal GBS swabs at 36 weeks gestation. Olsen and colleagues (2018) randomized 34 women, who were GBS positive at 36 weeks gestation, to receive usual care or to ingest a daily oral probiotic capsule for 3 weeks or until the time of birth. Probiotic adherence was not reported. Repeat GBS testing was done 3 weeks post intervention. However, the use of vaginal only swabs provided no GBS culture of the rectal reservoir. Of the 21 participants in the probiotic group, only 7 (30%) completed 14 or more days of the allocated treatment. Of those who had 2 weeks of probiotics, 4 (57%) of the seven were negative at 39 weeks or later, while two (15%) of the 13 control group participants were negative in that same time period. There was no significant in difference in GBS between the two groups (Fisher’s exact test, p = 0.7). However, use of the probiotic intervention significantly increased the probability of commensal vaginal organisms, such as Lactobacilli (Fisher’s exact test, p = 0.048).
Martin et al. (2019) used Lactobacillus salivarius (CECT 9145) as the probiotic intervention in a prospective cohort study. The probiotic group was comprised of 25 GBS positive women who were 12–26 weeks gestation, with the intervention initiated at 26 weeks. Adherence was not reported. One of the two control cohorts consisted of 14 GBS positive participants; the other included 18 GBS negative participants. Separate vaginal and rectal quantitative GBS swabs were collected at frequent intervals (26, 28, 30, 32, 35, and 38 weeks gestation), with the study ending at 38 weeks gestation. Of the 25 participants in the probiotic cohort, 18 (72%) had negative GBS rectal swabs and 17 (68%) had negative vaginal swabs. The average quantitative CFUs decreased from 5.14 (26 weeks) to 3.80 (38 weeks). Although the researchers described this finding as significant, a p value was not provided. The GBS status of participants in both control groups (GBS positive or GBS negative) remained unchanged. The findings were not statistically analysed. The number of group comparisons and measures detracted from the clarity of the findings and interpretation.
Meta-analysis
The six published clinical trials of probiotics against GBS were meta-analysed, including a total of 709 participants. Overall, there was significant statistical homogeneity between studies [Cochrane’s Q (df=5) = 9.39, p = 0.09. τ 2 = 0.08, SE = 0.22]. Based on the meta-analysis, probiotics significantly decreased the likelihood of a positive GBS test by 44% (OR = 0.56, 95% CI = [0.34, 0.92], p = 0.02). The random effects test for the meta-analysis indicates that the overall effect is different from 0 (estimate = −0.582, SE = 0.253, p = 0.022, 95% CI = [−1.08, −0.09]). A forest plot, with the OR and 95% CI, and the percent weight contribution for each study and Random Effects (RE) Model summarizing the results are presented (Figure 2).
Fig. 2.
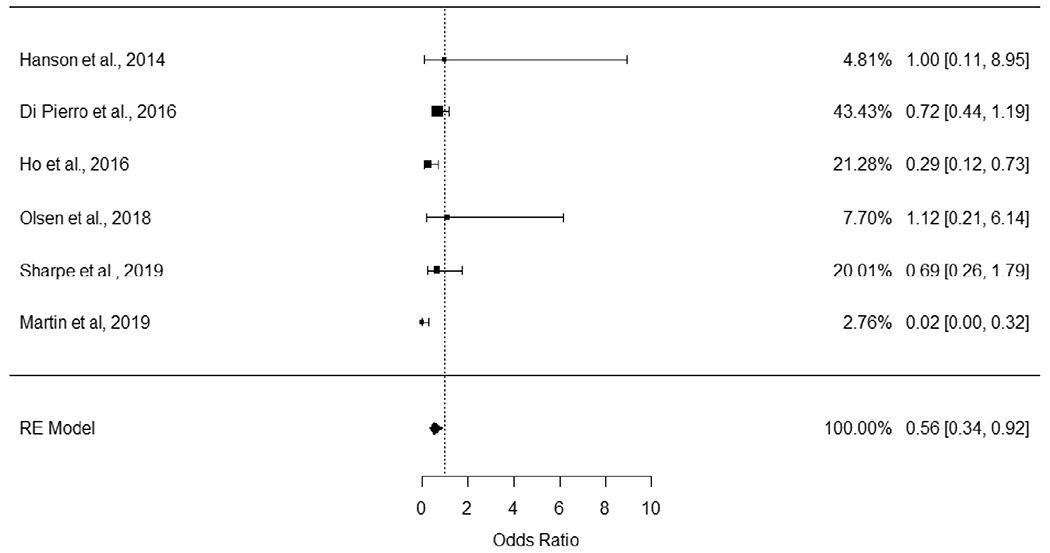
Forest plot of meta-analysis.
Discussion
This systematic review and meta-analysis included both RCTs and non-RCTs. The inclusion of non-RCTs has become more common when evaluating the efficacy of therapeutic interventions (Faber et al., 2016). Three of the studies were led by midwifery researchers (Hanson et al., 2014; Olsen et al., 2018; Sharpe et al., 2019). Sharpe and colleagues (2019) included participants who gave birth at home, birth centers, and hospitals in their multisite study, while the other five studies included only hospital birth participants. Only one of clinical trials had a statistically significant GBS outcome (Ho et al., 2016), while two others described reduced GBS without supporting statistics (Di Pierro et al., 2016; Martiń et al., 2019). When results were pooled, the meta-analysis demonstrated that probiotic interventions significantly decreased the probability of a positive GBS result. These findings suggest that probiotic interventions have a moderate effect on GBS colonisation. Probiotics are a primary prevention strategy that could reduce the need for IAP.
Researchers themselves offered possible explanations for nonsignificant findings. Examples included: length of probiotic intervention was too short, the dose of probiotic was too low, and/or ineffective strains were used (Olsen et al., 2018). Sharpe et al. (2019) described recruitment challenges and overly strict inclusion criteria as possible explanations. Properly collected GBS cultures are critical to valid and reliable GBS study outcomes. Self-collected GBS swabs are considered equivalent to provider collected swabs and may be preferable to participants (Arya et al., 2008); however, the sampling procedure described by Olsen and colleagues (2018) was a “routine lower vaginal swab.” This differs from those recommended by the CDC (n.d.) Centers for Disease Control and Prevention (CDC), n.d 2021, which includes the swab being inserted into the vaginal canal to 2cm, then into the rectum to a depth of 1cm.
The systematic review of the in vitro studies demonstrated that numerous probiotic strains or combination products showed antagonist activity against GBS. These in vitro studies established possible mechanisms of action as acidification, immune modulation, and adhesion to epithelial cells. Limitations of these studies include large variations in the probiotic species explored, laboratory methods used, and timepoints of data collection to measure adhesion and acidity. Advances in laboratory analysis procedures including DNA sequencing (Malloy et al., 2020) will facilitate future reproduceable in vitro studies. Selecting probiotics with in vitro antagonist activity against GBS should facilitate the selection of interventions with potential for clinical efficacy.
For clinicians, there are other relevant considerations for probiotic choice since the clinical outcomes of one probiotic strain cannot necessarily be to be attributed to another (Reid et al., 2009). Among the six clinical trials reviewed, there were five different single or combination probiotic products studied. Commercial over-the-counter probiotic labels may not include species, strain, and isolate identification. Availability of probiotic products varies by country.
Exclusion criteria varied between studies and included current probiotic use (Hanson et al., 2014; Sharpe et al., 2019) and/or antibiotic use (Ho et al., 2016; Sharpe et al., 2019). Olsen and colleagues (2018) removed participants from the study if they developed an infection after enrolment, while Di Pierro et al. (2016) instructed study participants to suspend the probiotic intervention during antibiotic administration. Antibiotics are a well-known factor in the disruption of vaginal flora (Sullivan et al., 2001). Simultaneous administration of antibiotics and probiotics could destroy the probiotic within the digestive tract (Boyanova & Mitov, 2012). Current recommendations include using probiotics during antibiotic therapy to decrease antibiotic associated diarrhea, but to separate the respective doses by 2-4 hours (Boyanova & Mitov, 2012). In one study, researchers collected information related to yogurt ingestion (Hanson et al., 2014). Nutrition appears to play a role in the health of the vaginal and rectal microbiome (Martiń et al., 2019). The association between diet and outcomes of probiotics interventions used during pregnancy needs more controlled clinical scientific investigation.
Probiotic dosage and administration are notable elements of comparison and contrast between studies. In the six clinical trials, the probiotic interventions were once daily oral capsules with dosages ranging from 18 CFU to 159 CFU. Ho et al. (2016) demonstrated clinically significant conversion from positive for GBS at 36 weeks gestation to negative GBS colonisation at the time of labour and birth after an average 20 days of probiotics. Yet Sharpe et al. (2019) were unable to demonstrate significant findings using more than twice the dose of the same probiotic combination given for 13–14 weeks. In both studies, the capsules that contained the probiotics were made of gelatin, excluding vegetarians and persons following Kosher or Halal diets, from study participation.
No adverse events related to probiotic interventions were reported in any of the studies. Di Pierro et al. (2016) compared minor symptoms and found no significant difference between probiotic versus control groups (p < 0.01). Both Di Pierro et al. (2016) and Hanson et al. (2014) reported reduced GI symptoms in the participants treated with probiotics.
In five of the studies (Di Pierro et al., 2016; Hanson et al., 2014; Ho et al., 2016, (Martiń et al., 2019; Olsen et al., 2018), the probiotic intervention was continued until the time of labour and birth, in one (Sharpe et al., 2019), it was stopped after the 36-week GBS culture. Cessation of the probiotic intervention before labour and birth has the potential to alter the GBS colonisation of study participants at this critical time. Adherence to the intervention was measured in two studies and found to be highly similar, 87% (Sharpe et al., 2019) and 86% (Hanson et al., 2014). In one small study, increased probiotic intervention adherence was associated with a trend towards reduced GBS colonisation (Hanson et al., 2014).
Two clinical trials were designed as feasibility studies to determine sample size for larger studies (Hanson et al., 2014; Sharpe et al., 2019). Variations in GBS carrier status by country impacted recruitment and sample size requirements in clinical trials. Studies in populations that include higher rates of GBS colonisation could require smaller sample sizes to demonstrate intervention efficacy. Further studies with pregnant participants require an ample dropout rate for anticipated pregnancy complications, such as premature birth, that can necessitate study withdrawal prior to the measurement of the primary GBS outcome. Similarly, investigators who sought to measure conversion of positive GBS to negative by the time of birth were challenged by precipitous labour and birth before GBS could be collected (Ho et al., 2016).
Limitations
This study was limited by the paucity of in vitro and clinical trials of probiotics to reduce GBS colonisation in pregnancy. Only one clinical trial had a low risk of bias (Sharpe et al., 2019). Several of the studies were complex to interpret (Martiń et al., 2019) and presented limited statistical analyses (Di Pierro et al., 2016; Ho et al., 2016; Martiń et al., 2019). All included relatively small samples and antenatal participants with unknown GBS status, and/or those with known GBS colonisation. Race, ethnicity, and factors, such as diet, infection, and antibiotic use, were inconsistently reported in the studies reviewed. In one study, participants with infections or inflammatory conditions who accepted probiotics formed the intervention group (Di Pierro et al., 2016). Future research using diverse populations with critical measures of characteristics and confounding variables relevant to GBS colonisation will strengthen the evidence for antenatal probiotic interventions.
One of the clinical trials was conducted in the US (Hanson et al., 2014) where clinical investigations of probiotics to reduce antenatal GBS require Food and Drug Administration (FDA, 2021) Investigational New Drug (IND) applications. This process is rigorous and requires corporate engagement in research to share proprietary information about the manufacture of probiotics. The reduction of GBS colonisation meets the FDA (2017) definition of a “drug use” of an over-the-counter product. The special population of pregnancy also has been determined to signal the need for an IND application. A review of the clinicaltrials.gov website indicated that several clinical trials are in process in the US and other countries.
Conclusion
Efforts to prevent EOGBSD vary by country and region. Where the universal screening approach is used, up to 30% of birth givers are exposed to IAP. Given the call for antibiotic stewardship and the potential perinatal microbiologic sequela of exposure, primary prevention of EOGBSD is needed. Several probiotic species have antagonist activity against GBS. Although a total of six clinical trials were included, this systematic review and meta-analysis demonstrated that antenatal probiotic interventions are low risk and significantly increase the probability of a negative GBS culture by 79%. Future double-blind, randomized controlled clinical trials are needed with larger diverse samples. Midwives can be instrumental in conducting and supporting these trials.
Supplementary Material
Funding sources
1. Ascension Sister Rosalie Klein Endowed Professor; 2. Dr Hanson is PI of the funded study, “The efficacy of probiotics to reduce antepartum GBS colonization” R21HD095320; 3. University of New Mexico Department of OBGYN Research Grant
Footnotes
Conflict of Interest
Drs Lisa Hanson, Leona VandeVusse and Nasia Safdar conducted the open-label quasi experiment published in 2014 with pilot funding and probiotic product provided from American Lifelines the makers of Florajen3. The American Lifelines company currently supplies Florajen3 and placebo capsules for Dr. Hanson’s NIH funded RCT (NCT04721912) and Florajen Digestion for her open-label quasi experiment. The American Lifelines company did not have any input into the design, conduct, analysis or dissemination of these studies.
Ethical approval
Not applicable.
CRediT authorship contribution statement
Lisa Hanson: Supervision, Conceptualization, Visualization, Investigation, Formal analysis, Writing – original draft. Leona VandeVusse: Conceptualization, Visualization, Investigation, Formal analysis, Writing – original draft. Emily Malloy: Writing – original draft, Writing – review & editing. Mauricio Garnier-Villarreal: Formal analysis, Writing – original draft. Lauren Watson: Writing – original draft. Alissa Fial: Conceptualization, Formal analysis, Writing – original draft. Marie Forgie: Writing – review & editing. Katrina Nardini: Writing – review & editing. Nasia Safdar: Writing – review & editing.
Midwifery Probiotic interventions to reduce antepartum Group B Streptococcus colonisation: A systematic review and meta-analysis –Manuscript Draft– Manuscript Number: YMIDW-D-21-00295 Article Type: Original Research
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.midw.2021.103208.
References
- American Academy of Pediatrics (AAP), 2019. Prevention of Group B Streptococcal Early-Onset Disease in Newborns. Pediatrics 144 (2), e20191882. doi: 10.1542/peds.2019-1882. [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists (ACOG), 2019. American College of Obstetricians and Gynecologists Committee Opinion. Prevention of Group B Streptococcal Early-Onset Disease in Newborns. Obstet. Gynecol 134 (1), e19–e40. [Google Scholar]
- Akoh CC, Pressman EK, Cooper E, Queenan RA, Pillittere J, O’Brien KO, 2017. Prevalence and risk factors for infections in a pregnant adolescent population. J. Pediatr. Adolesc. Gynecol 30 (1), 71–75. doi: 10.1016/j.jpag.2016.08.001. [DOI] [PubMed] [Google Scholar]
- Altoparlak U, Kadanali A, Kadanali S, 2004. Genital flora in pregnancy and its association with group B streptococcal colonization. Int. J. Gynaecol. Obstet 87 (3), 245–246. doi: 10.1016/j.ijgo.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Armistead B, Oler E, Waldorf KA, Rajagopal L, 2019. The double life of Group B Streptococcus: asymptomatic colonizer and potent pathogen. J. Mol. Biol 431 (16), 2914–2931. doi: 10.1016/j.jmb.2019.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya A, Cryan B, O’Sullivan K, Greene RA, Higgins JR, 2008. Self-collected versus health professional-collected genital swabs to identify the prevalence of group B Streptococcus: a comparison of patient preference and efficacy. Eur. J. Obstet. Gynecol. Reprod. Biol 139 (1), 43–45. doi: 10.1016/j.ejogrb.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Berardi A, Rossi C, Creti R, China M, Gherardi G, Venturelli C, Rumpianesi F, Ferrari F, 2013. Group B streptococcal colonization in 160 mother-baby pairs: a prospective cohort study. J. Pediatr 163 (4), 1099–1104. doi: 10.1016/j.jpeds.2013.05.064, e1. [DOI] [PubMed] [Google Scholar]
- Bishara RM, 2006. GBS in a homebirth setting. Midwifery Today Int. Midwife 79, 32–34 67 Available at: https://pubmed.ncbi.nlm.nih.gov/17024899 [Accessed Sep 20 2019]. [PubMed] [Google Scholar]
- Boyanova L, Mitov I, 2012. Coadministration of probiotics with antibiotics: why, when and for how long? Expert Rev. Anti Infect. Ther 10 (4), 407–409. doi: 10.1586/eri.12.24. [DOI] [PubMed] [Google Scholar]
- Brugger SD, Baumberger C, Jost M, Jenni W, Brugger U, Mühlemann K, 2012. Automated counting of bacterial colony forming units on agar plates. PLoS One 7 (3), e33695. doi: 10.1371/journal.pone.0033695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzychczy-Włoch M. Pabian W. Majewska E . Zuk MG , Kielbik J. Gosiewski T . Bulanda MG. 2014. Dynamics of colonization with group B streptococci in relation to normal flora in women during subsequent trimesters of pregnancy. New Microbiol 37 (3), 307–319. [PubMed] [Google Scholar]
- Capan-Melser M, Ngoma GM, Akerey-Diop D, Basra A, Würbel H, Groger M, Mackanga JR, Zoleko-Manego R, Schipulle U, Schwing J, Lötsch F, Rehman K, Matsiegui P-B, Selidji T, Agnandji AA, Adegnika SB, González R, Kremsner PG, Menendez C, Ramharter M, 2015. Evaluation of intermittent preventive treatment of malaria against group B Streptococcus colonization in pregnant women: a nested analysis of a randomized controlled clinical trial of sulfadoxine/pyrimethamine versus mefloquine. J. Antimicrob. Chemother 70 (6), 1898–1902. doi: 10.1093/jac/dkv041. [DOI] [PubMed] [Google Scholar]
- Card NA, 2012. Applied meta-analysis for social science research. Guilford Press, New York. [Google Scholar]
- Centers for Disease Control and Prevention (CDC), n.d. Instructions for the collection of a genital swab for the detection of a group B streptococcus (GBS) [Pictorial representation]. Available at: https://www.cdc.gov/groupbstrep/downloads/gbs_swab_sheet21.pdf [Accessed Mar 25 2021].
- Centers for Disease Control and Prevention (CDC), 2010. Prevention of Perinatal Group B Streptococcal Disease: Revised Guidelines from CDC, 2010. Mortality and Mortality Weekly Report (MMWR) 59 (RR-10), 1–32. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5910a1.htm [Accessed Apr 25, 2021]. [PubMed] [Google Scholar]
- Chan KL, Levi K, Towner KJ, Weston VC, Ramsay MM, Kean LH, 2006. Evaluation of the sensitivity of a rapid polymerase chain reaction for detection of group B streptococcus. J. Obstet. Gynaecol 26 (5), 402–406. doi: 10.1080/01443610600719925. [DOI] [PubMed] [Google Scholar]
- Cochrane Collaboration, 2020. Data extration form: data collection form for intervention reviews: RCTs and non-RCTs - template, Version 3, 2014 April. Available at: https://dplp.cochrane.org/dataextraction-forms [Accessed Aug 20 2020].
- Di Pierro F, Parolari A, Brundu B, Nigro R, 2016. Positive clinical outcomes derived from using a proprietary mixture of selected strains during pregnancy. Acta Biomed 87 (3), 259–265. [PMC free article] [PubMed] [Google Scholar]
- Ehrström S, Daroczy K, Rylander E, Samuelsson C, Johannesson U, Anzén B, Påhlson C, 2010. Lactic acid bacteria colonization and clinical outcome after probiotic supplementation in conventionally treated bacterial vaginosis and vulvovaginal candidiasis. Microbes Infect 12 (10), 691–699. doi: 10.1016/j.micinf.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Ephraim E. Schultz RD, Duster M, Warrack S, Spiegel CA, Safdar N. 2012. In-vitro evaluation of antagonistic effects of the probiotics Lactobacillus rhamnosus HN001 and Florajen3 against Group B streptococci. Int. J. Probiotics Prebiotics 7 (3/4), 113–120. [Google Scholar]
- Faber T, Ravaud P, Riveros C, Perrodeau E, Dechartres A, 2016. Meta-analyses including nonrandomized studies of therapeutic interventions: a methodological review. BMC (BioMed Central) Med. Res. Methodol 16 (35). doi: 10.1186/s12874-016-0136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fijan S, 2014. Microorganisms with claimed probiotic properties: an overview of recent literature. Int J Environ Res Public Health 11 (5), 4745–4767 doi: 10.3390/ijerph11050474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filkins L, Hauser J, Robinson-Dunn B, Tibbetts R, Boyanton B, Revell P, 2020. Guidelines for the Detection and Identification of Group B Streptococcus. American Society for Microbiology Available at https://asm.org/Guideline/Guidelines-for-the-Detection-and-Identification-of. [DOI] [PMC free article] [PubMed]
- Food and Agriculture Organization (FAO) of the United Nations & World Health Organization (WHO), 2001. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria: Report of a joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Available at: http://www.fao.org/3/a0512e/a0512e.pdf [Accessed Mar 25 2021].
- Food and Drug Administration (FDA), 2017. Drugs@FDA Glossary of Terms. Available at: https://www.fda.gov/drugs/drug-approvals-and-databases/drugsfda-glossary-terms [Accessed Mar 25 2021].
- Food and Drug Administration (FDA), 2021. Investigational New Drug (IND) application. Available at: https://www.fda.gov/drugs/types-applications/investigational-new-drug-ind-application [Accessed Mar 25 2021].
- Foxman B, Gillespie BW, Manning SD, Marrs CF, 2007. Risk factors for group B streptococcal colonization: potential for different transmission systems by capsular type. Ann. Epidemiol 17 (11), 854–862. doi: 10.1016/j.annepidem.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson L, VandeVusse L, Duster M, Warrack S, Safdar N, 2014. Feasibility of oral prenatal probiotics against maternal group B Streptococcus vaginal and rectal colonization. J. Obstet. Gynecol. Neonatal Nurs 43 (3), 294–304. doi: 10.1111/1552-6909.12308. [DOI] [PubMed] [Google Scholar]
- Hemmerling A, Harrison W, Schroeder A, Park J, Korn A, Shiboski S, Foster-Rosales A, Cohen C, 2010. Phase 2a study assessing colonization efficiency, safety, and acceptability of Lactobacillus crispatus CTV-05 in women with bacterial vaginosis. Sex. Transm. Dis 37 (12), 745–750. doi: 10.1097/OLQ.0b013e3181e50026. [DOI] [PubMed] [Google Scholar]
- Hillier SL, Ferrieri P, Edwards MS, Ewell M, Ferris D, Fine P, Carey V, Meyn L, Hoagland D, Kasper DL, Paoletti LC, Hill H, Baker CJ, 2019. A phase 2, randomized, control trial of Group B Streptococcus (GBS) Type III capsular polysaccharide-tetanus toxoid (GBS III-TT) vaccine to prevent vaginal colonization with GBS III. Clin. Infect. Dis 68 (12), 2079–2086. doi: 10.1093/cid/ciy838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M, Chang Y-Y, Chang W-C, Lin H-C, Wang M-H, Lin W-C, Chiu T-H, 2016. Oral Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 to reduce Group B Streptococcus colonization in pregnant women: a randomized controlled trial. Taiwan. J. Obstet. Gynecol 55 (4), 515–518. doi: 10.1016/j.tjog.2016.06.003. [DOI] [PubMed] [Google Scholar]
- Illuzzi JL, Bracken MB, 2006. Duration of intrapartum prophylaxis for neonatal group B Streptococcal disease: a systematic review. Obstet. Gynecol 108 (5), 1254–1265. doi: 10.1097/01.AOG.0000241539.86451.11. [DOI] [PubMed] [Google Scholar]
- Kolkman DGE, Rijnders MEB, Wouters MGAJ, van den Akker-van Marle ME, van der Ploeg CPBK, de Groot CJM, Fleuren MAH, 2013. Implementation of a cost-effective strategy to prevent neonatal early-onset group B haemolytic streptococcus disease in the Netherlands. BMC Preg. Childbirth 13, 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Doare K, Heath PT, 2013. An overview of global GBS epidemiology. Vaccine 31 (Suppl 4), D7–D12. doi: 10.1016/j.vaccine.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Malloy E, Kates A, Watson L, VandeVusse L, Safdar N, Hanson L, 2020. Laboratory analysis techniques for the perinatal microbiome: implications for studies of probiotic interventions. J. Perinat. Neonatal Nurs 34 (3), 239–250. doi: 10.1097/JPN.0000000000000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martiń V, Cárdenas N, Ocaña S, Mariń M, Arroyo R, Beltrán D, Badiola C, Fernández L, Rodriguez JM, 2019. Rectal and vaginal eradication of Streptococcus agalactiae (GBS) in pregnant women by using Lactobacillus salivarius CECT 9145, a target-specific probiotic strain. Nutrients 11, 810. doi: 10.3390/nu1104810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marziali G, Foschi C, Parolin C, Vitali B, Marangoni A, 2019. In-vitro effect of vaginal lactobacilli against group B streptococcus. Microb. Pathog 136, 103692. doi: 10.1016/j.micpath.2019.103692. [DOI] [PubMed] [Google Scholar]
- Meyn LA, Krohn MA, Hillier SL, 2009. Rectal colonization by group B Streptococcus as a predictor of vaginal colonization. Am. J. Obstet. Gynecol 201 (1). doi: 10.1016/j.ajog.2009.02.011, 76.e71–76.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuoka T, 1990. Bifidobacteria and their role in human health. J. Ind. Microbiol 6 (4), 263–267. doi: 10.1007/BF01575871. [DOI] [Google Scholar]
- Moghaddam MN, 2010. Recto-vaginal colonization of group B Streptococcus in pregnant women referred to a hospital in Iran and its effect on Lactobacillus normal flora. J. Biol. Sci 10 (2), 166–169. [Google Scholar]
- Nanduri SA, Petit S, Smelser C, Apostol M, Alden NB, Harrison LH, Lynfield R, Vagnone PS, Burzlaff K, Spina NL, Dufort EM, Schaffner W, Thomas AR, Farley MM, Jain JH, Pondo T, McGee L, Beall BW, Schrag SJ, 2019. Epidemiology of invasive early-onset and late-onset group B streptococcal disease in the United States, 2006 to 2015: multistate laboratory and population-based surveillance. JAMA Pediatr 173 (3), 224–233. doi: 10.1001/jamapediatrics.2018.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson A, Shah VS, Stade BC, 2014. Vaginal chlorhexidine during labour to prevent early-onset neonatal group B streptococcal infection. Cochrane Database Syst. Rev 12, 1–38. doi: 10.1002/14651858.CD003520.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen P, Williamson M, Traynor V, Georgiou C, 2018. The impact of oral probiotics on vaginal Group B Streptococcal colonisation rates in pregnant women: a pilot randomised control study. Women Birth 31 (1), 31–37. doi: 10.1016/j.wombi.2017.06.012. [DOI] [PubMed] [Google Scholar]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D, 2021. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Brit. Med. J 372, n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parente V, Clark RH, Ku L, Fennell C, Johnson M, Morris E, Romaine A, Utin U, Benjamin DK, Messina JA, Smith PB, Greenberg R, 2017. Risk factors for group B streptococcal disease in neonates of mothers with negative antenatal testing. J. Perinatol 37 (2), 157–161. doi: 10.1038/jp.2016.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patras KA, Wescombe PA, Rösler B, Hale JD, Tagg JR, Doran KS, 2015. Streptococcus salivarius K12 Limits Group B Streptococcus Vaginal Colonization. Infect Immun 83 (9), 3438–3444. doi: 10.1128/IAI.00409-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard FJ. Bergeron MG , 2004. Laboratory detection of group B Streptococcus for prevention of perinatal disease. Eur. J. Clin. Microbiol. Infect. Dis 23 (9), 665–671 doi: 10.1007/s10096-004-1183-8. [DOI] [PubMed] [Google Scholar]
- R Core Team, 2021. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: Available at https://www.R-project.org. [Google Scholar]
- Reid G, Dols J, Miller W, 2009. Targeting the vaginal microbiota with probiotics as a means to counteract infections. Curr. Opin. Clin. Nutr. Metab. Care 12 (6), 583–587. doi: 10.1097/MCO.0b013e328331b611. [DOI] [PubMed] [Google Scholar]
- Reid G, Kumar H, Khan AI, Rautava S, Tobin J, Salminen S, 2016. The case in favour of probiotics before, during and after pregnancy: insights from the first 1,500 days. Benef. Microbes 7 (3), 353–362. doi: 10.3920/BM2015.0140. [DOI] [PubMed] [Google Scholar]
- Reid JNS, Bisanz JE, Monachese M, Burton JP, Reid G, 2013. The rationale for probiotics improving reproductive health and pregnancy outcome. Am. J. Reprod. Immunol 69 (6), 558–566. doi: 10.1111/aji.12086. [DOI] [PubMed] [Google Scholar]
- Ronnqvist PDJ, Forsgren-Brusk UB, Grahn-Hakansson EE, 2006. Lactobacilli in the female genital tract in relation to other genital microbes and vaginal pH. Acta Obstet. Gynecol. Scand 85, 726–735. doi: 10.1080/00016340600578357. [DOI] [PubMed] [Google Scholar]
- Rosen GH, Randis TM, Desai PV, Sapra KJ, Ma B, Gajer P, Humphrys MS, Ravel J, Gelber SE, Ratner AJ, 2017. Group B Streptococcus and the vaginal microbiota. J Infect. Dis 216 (6), 744–751. doi: 10.1093/infdis/jix395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell NJ, Seale AC, O’Driscoll M, O’Sullivan C, Bianchi-Jassir F, Gonzalez-Guarin J, Lawn JE, Baker CJ, Bartlett L, Cutland C, Gravett MG, Heath PT, Le Doare K, Madhi SA, Rubens CE, Schrag S, Sobanjo-ter Meulen A, Vekemans J, Saha SK, Ip M, 2017. for the GBS Maternal Colonization Investigator Group, 2017. Maternal colonization with Group B Streptococcus and serotype distribution worldwide: systematic review and meta-analyses. Clin. Infect. Dis 65 (suppl2), S100–S111. doi: 10.1093/cid/cix658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhanam S, Jose R, Sahni RD, Thomas N, Beck MM, 2017. Prevalence of group B Streptococcal colonization among pregnant women and neonates in a tertiary hospital in India. J. Turk. Ger. Gynecol. Assoc 18 (4), 181–184. doi: 10.4274/jtgga.2017.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale AC, Blencowe H, Bianchi-Jassir F, Embleton N, Bassat Q, Ordi J, Menéndez C, Cutland C, Briner C, Berkley JA, Lawn JE, Baker CJ, Bartlett L, Gravett MG, Heath PT, Ip M, Le Doare K, Rubens CE, Saha SK, Schrag S, Sobanjo-ter Meulen A, Vekemans J, Madhi SA, 2017. Stillbirth with Group B Streptococcus disease worldwide: systematic review and metaanalyses. Clin. Infect. Dis 65 (suppl_2), S125–S132. doi: 10.1093/cid/cix585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe M, Shah V, Freire-Lizama T, Cates EC, McGrath K, David I, Cowan S, Letkeman J, Stewart-Wilson E, 2019. Effectiveness of oral intake of Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 on Group B Streptococcus colonization during pregnancy: a midwifery-led double-blind randomized controlled pilot trial. J. Matern. Fetal Neonatal Med doi: 10.1080/14767058.2019.1650907. [DOI] [PubMed] [Google Scholar]
- Singleton ML. 2007. Group B strep prophylaxis: what are we creating? Midwifery Today Int. Midwife 81, 18–20. [PubMed] [Google Scholar]
- Stapleton RD, Kahn JM, Evans LE, Critchlow CW, Gardella CM, 2005. Risk factors for group B streptococcal genitourinary tract colonization in pregnant women. Obstet. Gynecol 106 (6), 1246–1252. doi: 10.1097/01.AOG.0000187893.52488.4b. [DOI] [PubMed] [Google Scholar]
- Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng H-Y, Corbett MS, Eldridge SM, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT, 2019. RoB 2: a revised tool for assessing risk of bias in randomised trials. Brit. Med. J 366, 14898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- Sullivan A, Edlund C, Nord CE, 2001. Effect of antimicrobial agents on the ecological balance of human microflora. Lancet Infect. Dis 1 (2), 101–114. doi: 10.1016/S1473-3099(01)00066-4. [DOI] [PubMed] [Google Scholar]
- Van Dyke MK, Phares CR, Lynfield R, Thomas AR, Arnold KE, Craig AS, Mohle-Boetani J, Gershman K, Schaffner W, Petit S, Zansky SM, Morin CA, Spina NL, Wymore K, Harrison LH, Shutt KA, Bareta J, Bulens SN, Zell ER, Schuchat A, Schrag SJ, 2009. Evaluation of universal antenatal screening for group B streptococcus. N. Engl. J. Med 360 (25), 2626–2636. doi: 10.1056/NEJMoa0806820. [DOI] [PubMed] [Google Scholar]
- Verani JR, McGee L, Schrag SJ, 2010. Prevention of perinatal group B streptococcal disease: revised guidelines from CDC, 2010. MMWR. Morbid. Mortal. Weekly Rep. Recomm. Rep 59 (Rr-10), 1–36. Available at http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5910a1.htm?s. [Accessed Jul 20 2014]. [PubMed] [Google Scholar]
- Viechtbauer W, 2010. Conducting meta-analyses in R with the metafor package. J. Stat. Softw 36 (3), 1–48. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- Virranniemi M, Raudaskoski T, Haapsamo M, Kauppila J, Renko M, Peltola J, Risteli L, Laatio L, 2019. The effect of screening-to-labor interval on the sensitivity of late-pregnancy culture in the prediction of group B streptococcus colonization at labor: a prospective multicenter cohort study. Acta Obstet. Gynecol. Scand 98 (4), 494–499. doi: 10.1111/aogs.13522. [DOI] [PubMed] [Google Scholar]
- Whitney CG, Daly S, Limpongsanurak S, Festin MR, Thinn KK, Chipato T, Lumbiaganon P, Sauvarin J, Andrews W, Tolosa JEand for the Global Network for Perinatal and Reproductive Health., 2004. The International Infections in Pregnancy Study: group B streptococcal colonization in pregnant women. J. Matern. Fetal Neonatal Med 15 (4), 267–274. doi: 10.1080/14767050410001668617. [DOI] [PubMed] [Google Scholar]
- Zárate G, Nader-Macias M, 2006. Influence of probiotic vaginal lactobacilli on in vitro adhesion of urogenital pathogens to vaginal epithelial cells. Lett. Appl. Microbiol 43, 174–180. doi: 10.1111/j.1472-765X.2006.01934.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


