Abstract
Background
Respiratory oscillometry is a promising complement to the traditional pulmonary function tests for its simplicity. The usefulness of oscillometry in adult clinical practice has not been clarified. This study aimed to analyse the characteristics and diagnostic performance of oscillometry in respiratory diseases, and explore the cut-offs of oscillometric parameters for severity grading.
Methods
In this multicentre registry of impulse oscillometry (IOS), IOS and spirometric data of healthy individuals and patients with respiratory diseases were collected and analysed. Linear mixed model analysis was performed to explore the effects of disease and forced expiratory volume in 1 s (FEV1) on oscillometric parameters.
Results
The study included 567 healthy subjects, 781 asthmatic patients, 688 patients with chronic obstructive pulmonary disease (COPD), 109 patients with bronchiectasis, 40 patients with upper airway obstruction (UAO) and 274 patients with interstitial lung disease (ILD) in the analysis. Compared at the same FEV1 level, asthma, COPD, bronchiectasis, UAO and ILD displayed different oscillometric characteristics. The z-score of resistance at 5 Hz (R5) was the best variable to identify respiratory diseases with a sensitivity of 62.4–66.7% and a specificity of 81.5–90.3%. With reference to the severity grading cut-offs of FEV1, R5 z-scores of 2.5 and 4 were defined as the cut-off values of moderately and severely increased R5.
Conclusion
Respiratory oscillometry is more appropriate to be a tool of evaluating, rather than of diagnosing, respiratory diseases. A severity grading system of oscillometric parameters was developed to help the interpretation of oscillometry in clinical practice.
Short abstract
This multicentre registry of impulse oscillometry revealed that respiratory oscillometry is more suitable to evaluate than to diagnose respiratory diseases. A severity grading system of oscillometry was developed. https://bit.ly/3KZdEi3
Introduction
Pulmonary function tests (PFTs) play an essential role in the diagnosis and management of respiratory diseases. For instance, spirometry, the prominent test of PFTs, is acknowledged as the gold standard for assessing airflow limitation in guidelines [1–3]. Despite its wide range of use, spirometry is sometimes hard to fulfil in practice, due to its requirements of rigorous and forceful breathing manoeuvres during the measurements, which is challenging for the very young, old or weak. For those who cannot co-operate with PFTs or those for whom they are contraindicated, alternative tools to provide lung function information are necessary for diagnosing and monitoring diseases.
Respiratory oscillometry is a noninvasive technique for measuring respiratory resistance and compliance of the respiratory system during quiet tidal breathing [4]. The impulse oscillometry system (IOS), a common device for respiratory oscillometry, can measure respiratory mechanics of different airway sites by imposing multiple-frequency sound waves over normal breaths [5, 6]. Resistance of the respiratory system (Rrs) measured at 5 Hz (R5) and 20 Hz (R20) mainly reflect the total respiratory resistance and the central airway resistance, respectively, while reactance at 5 Hz (X5) represents the elastance (i.e. the compliance) of the respiratory system. The frequency dependence of Rrs, determined by the difference between R5 and R20 (R5−R20), is considered primarily to be sensitive to heterogeneous narrowing in the peripheral airways, although it may also be affected by the heterogeneity of more central airways and time constants, and the upper airway shunt flow [6, 7]. These technical features allow oscillometry to provide additional information to the traditional PFTs. Coupled with its minimal demand for the co-operation of the patient, respiratory oscillometry is expected to be a potential complementary tool to traditional PFTs, especially for those who cannot perform spirometry [8, 9].
Despite its promising potential, oscillometry is currently more of a research tool than a routine test in practice, especially in adult clinical practice. The main hindrances to oscillometry being routinely applied lie in the uncertainty of its usefulness and the difficulty in its interpretation in routine clinical practice. Although prior studies have reported oscillometric data on respiratory diseases [10–14], most of these observations focused on one specific disease and its comparison of the raw oscillometric data with healthy individuals, without taking the effects of anthropometrics and the severity of the disease into consideration. In addition, data concerning comparisons among multiple respiratory diseases are lacking. Thus, the characteristics and usefulness of oscillometry for respiratory diseases in adult clinical practice have not been clearly clarified. More importantly, there is a lack of standardised cut-offs to determine the abnormality or severity of the oscillometric results, leading to difficulty of the interpretation of oscillometry.
Based on the multicentre registry of impulse oscillometry, the present study aimed to: 1) clarify oscillometric characteristics of the common respiratory diseases; 2) analyse the diagnostic performance of oscillometry in respiratory diseases; and 3) explore the cut-offs of oscillometric parameters for severity grading.
Methods
Study design and participants
The multicentre study of impulse oscillometry in China is a registry that collected data on impulse oscillometry from healthy individuals and patients with respiratory diseases, conducted in 20 hospitals across China from 2016 to 2018. In brief, ≥18-year-old healthy subjects and patients with a physician-assigned diagnosis or suspected diagnosis of asthma, stable chronic obstructive pulmonary disease (COPD), bronchiectasis, upper airway obstruction (UAO) or interstitial lung disease (ILD) were enrolled through lung function laboratories. The inclusion criteria of the healthy subjects and the details of study sites were published previously [15].
The study was conducted in accordance with the Helsinki Declaration and approved by the relevant institutional review boards and ethics committees. All participants provided written informed consent.
More information regarding the methods is provided in the supplementary material.
Measurements
Oscillometry measurements were performed using the MasterScreen IOS (CareFusion, Hochberg, Germany) according to the European Respiratory Society (ERS) 2003 guideline [16]. Impedance verification was performed daily and a criterion of error ≤10% or 0.01 kPa·s·L–1 was adopted. Briefly, participants were required to breathe normally for 30–60 s with their nose clipped and their cheeks supported by their hands. The average values of three technically acceptable measurements under the requirements of the ERS 2020 guideline [4] were used in the analysis. Oscillometric parameters analysed in the study included R5, R20, R5−R20, X5, resonant frequency (fres) and the area of reactance (AX).
Spirometry measurements were made using a spirometer (CareFusion) under the recommendations of ERS/American Thoracic Society (ATS) guideline [17]. Spirometric parameters analysed were forced expiratory volume in the first second (FEV1), forced vital capacity (FVC), FEV1/FVC, maximum mid-expiratory flow (MMEF) and peak expiratory flow (PEF).
Statistical analysis
Data are presented as mean±sd, median (interquartile range) or number (percentage), as appropriate. Between-group differences in oscillometric parameters were compared using the Kruskal–Wallis test. Significance for multiple testing was adjusted by the Bonferroni method.
To exclude the influences of anthropometrics, standardised z-scores were applied in the analysis. References values and z-scores of oscillometric parameters and spirometric parameters were derived from the previous reports [15, 18]. The effects of diseases and FEV1 on oscillometric parameters were analysed using a linear mixed model with disease, FEV1 z-score and their interaction as the fixed effects, and centre and its interaction with FEV1 z-score as the random effects. As the z-scores of oscillometric parameters exhibited skewed distribution and contained negative values, oscillometric data were analysed in linear mixed models after logarithmic transformations: ln(5+Rrs) or ln(42+X5). Estimates of the fixed effects in the linear mixed model were used to establish the equations of the oscillometric z-scores predicted by the FEV1 z-score. Diagnostic performances of the oscillometry were evaluated by receiver operating characteristic (ROC) curve analysis, using the MedCalc application (version 15.8). Other analysis was performed using R software (version 4.0.5).
Results
Anthropometrics and spirometry
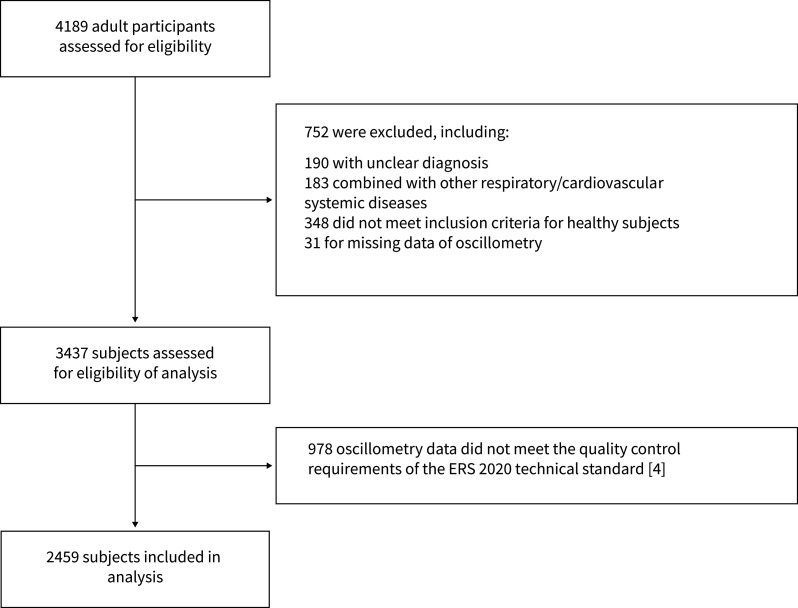
Between December 2016 and September 2018, a total number of 4189 adult participants was enrolled. After eligibility assessment, 567 healthy subjects, 781 patients with asthma, 688 patients with COPD, 109 patients with bronchiectasis, 40 patients with UAO and 274 patients with ILD were included in the analysis (figure 1).
FIGURE 1.
Study flowchart. ERS: European Respiratory Society.
The anthropometric and spirometric parameters of the analysed population are shown in table 1. The FEV1 z-score (mean±sd) of the healthy group, asthma group, COPD group, bronchiectasis group, UAO group and ILD group was 0.291±0.914, −1.784±1.902, −4.12±1.796, −2.506±1.942, −2.034±2.34, and −1.766±1.664, respectively.
TABLE 1.
Anthropometrics and spirometric parameters of the analysed population
| Healthy | ILD | Asthma | COPD | Bronchiectasis | UAO | |
| Subjects, N | 567 | 274 | 781 | 688 | 109 | 40 |
| Females, n (%) | 300 (52.9) | 124 (45.3) | 441 (56.5) | 58 (8.4) | 66 (60.6) | 19 (47.5) |
| Age, years | 38.3±14.3 | 57.4±11.6 | 45.9±12.9 | 63.2±8.6 | 52.9±11.1 | 54.5±14.9 |
| Height, cm | 164.3±8.3 | 161.6±8.0 | 162.2±8.2 | 165.2±7.0 | 160.0±8.4 | 160.7±7.5 |
| Weight, kg | 62.9±11.1 | 64.8±10.9 | 63.7±12.5 | 62.7±11.5 | 58.0±12.1 | 61.7±10.7 |
| BMI, kg·m−2 | 23.2±3.1 | 24.7±3.3 | 24.1±3.7 | 22.9±3.6 | 22.5±3.7 | 23.8±3.3 |
| FEV1, L# | 3.32±0.72 | 2.15±0.64 | 2.39±0.83 | 1.5±0.64 | 1.93±0.74 | 2.11±0.85 |
| FEV1 z-score# | 0.291±0.914 | −1.766±1.664 | −1.784±1.902 | −4.12±1.796 | −2.506±1.942 | −2.034±2.34 |
| FVC, L# | 3.96±0.87 | 2.71±0.84 | 3.52±0.95 | 3.02±0.80 | 2.84±0.86 | 3.22±0.84 |
| FVC z-score# | 0.310±0.978 | −1.800±1.753 | −0.225±1.442 | −1.517±1.690 | −1.363±1.482 | −0.407±1.526 |
| FEV1/FVC# | 83.8±5.5 | 80.1±8.7 | 67.2±13.0 | 48.5±12.8 | 67.2±14.1 | 64.2±16.0 |
| FEV1/FVC z-score# | −0.047±0.791 | 0.01±1.507 | −2.572±2.16 | −4.849±2.047 | −2.329±2.382 | −2.882±2.948 |
| MMEF, L·s−1¶ | 3.45±1.06 | 1.89±0.88 | 1.63±0.97 | 0.59±0.39 | 1.21±0.89 | 1.76±0.82 |
| MMEF z-score¶ | 0.153±0.993 | −0.904±1.347 | −1.917±1.172 | −2.901±0.552 | −2.094±1.304 | −1.399±1.067 |
| PEF, L·s−1# | 8.02±1.93 | 5.12±2.19 | 6.08±1.98 | 4.00±2.21 | 7.03±1.94 | 4.08±2.27 |
| PEF z-score# | 0.378±0.995 | −1.212±1.537 | −2.653±1.311 | −0.233±1.349 | −1.623±1.464 | −2.658±1.661 |
Data are presented as mean±sd unless otherwise stated. ILD: interstitial lung disease; UAO: upper airway obstruction; BMI: body mass index; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; MMEF: maximum mid-expiratory flow; PEF: peak expiratory flow. #: 11 data were missing from this analysis; ¶: 177 data were missing from this analysis.
Oscillometric parameters in respiratory diseases
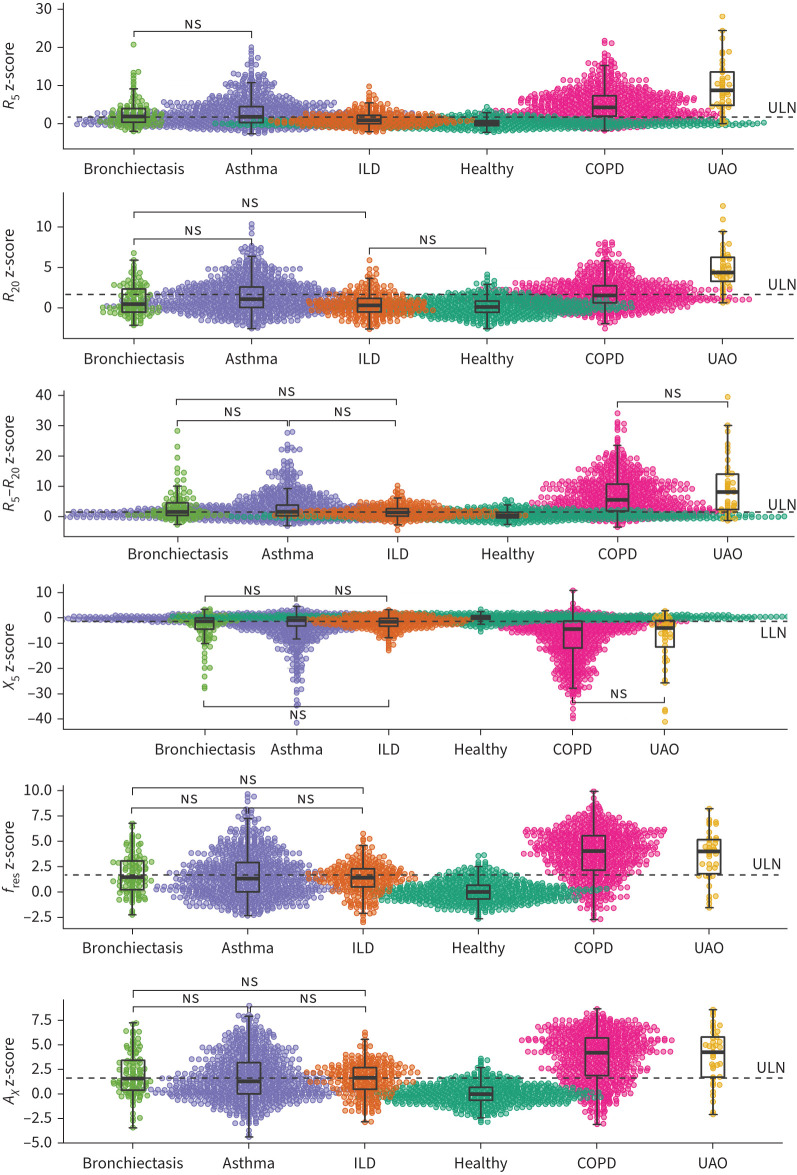
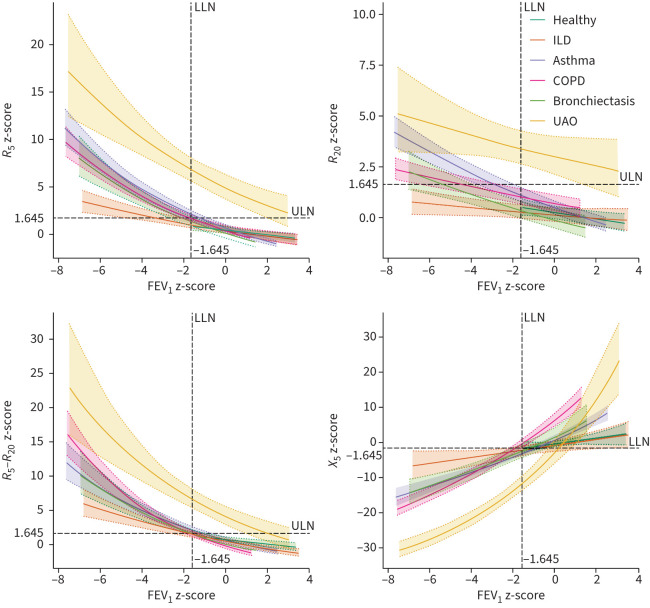
As shown in figure 2, R5 z-scores, R20 z-scores, R5−R20 z-scores, fres z-scores and AX z-scores of the five disease groups (except for the R20 z-score of ILD) were all significantly higher than those of the healthy group (p<0.05), while X5 z-scores of the disease groups were more negative than that of the healthy group (p<0.05). Moderate to high correlations were shown between oscillometric z-scores and FEV1 z-score (figures S1 and S2). Estimates of the functional relationships between oscillometric z-scores and FEV1 z-score analysed by the linear mixed model are displayed in table 2, where the intercepts represent the oscillometric z-score values when the FEV1 z-score is 0 and the slopes reflect the changes in oscillometric z-scores with FEV1 z-score. There was no significant difference between the healthy group and the ILD group in the intercepts and slopes, except for the slope of the R5−R20 z-score. For the R20 z-score, asthma was the only disease group that presented a significantly different intercept and slope from the healthy group. With the estimates of intercepts and slopes, equations of the z-scores of oscillometric parameters predicted by the FEV1 z-score were established by different diseases and are shown in figure 3.
FIGURE 2.
Comparisons of the z-scores of oscillometric parameters between different groups. R5: resistance at 5 Hz; R20: resistance at 20 Hz; X5: reactance at 5 Hz; fres: resonant frequency; AX: reactance area; ILD: interstitial lung disease; UAO: upper airway obstruction; ULN: upper limit of normal (1.645); LLN: lower limit of normal (−1.645). ns: nonsignificant between-group difference (p>0.05); other between-group differences without this notation were all significant (p<0.05).
TABLE 2.
Intercepts and slopes of the predictive equations of oscillometric parameters (ln z-scores) estimated by the linear mixed model analysis
| ln(5+R5 z-score) | ln(5+R20 z-score) | ln(5+R5−R20 z-score) | ln(42+X5 z-score) | |||||
| Estimate (95% CI) | p-value # | Estimate (95% CI) | p-value # | Estimate (95% CI) | p-value # | Estimate (95% CI) | p-value # | |
| Intercept | ||||||||
| Healthy | 1.67 (1.60–1.73) | <0.001 | 1.66 (1.59–1.72) | <0.001 | 1.76 (1.70–1.82) | <0.001 | 3.73 (3.71–3.75) | <0.001 |
| ILD | 1.68 (1.63–1.74) | 0.555 | 1.64 (1.59–1.69) | 0.463 | 1.71 (1.64–1.77) | 0.081 | 3.71 (3.67–3.75) | 0.095 |
| Asthma | 1.71 (1.68–1.75) | 0.014 | 1.70 (1.67–1.73) | 0.008 | 1.73 (1.69–1.78) | 0.215 | 3.77 (3.74–3.79) | <0.001 |
| COPD | 1.66 (1.61–1.72) | 0.933 | 1.74 (1.69–1.79) | 0.001 | 1.56 (1.49–1.62) | <0.001 | 3.89 (3.85–3.93) | 0.412 |
| Bronchiectasis | 1.61 (1.52–1.70) | 0.182 | 1.59 (1.51–1.67) | 0.091 | 1.65 (1.55–1.75) | 0.027 | 3.78 (3.72–3.85) | 0.095 |
| UAO | 2.29 (2.17–2.42) | <0.001 | 2.08 (1.97–2.19) | <0.001 | 2.21 (2.07–2.35) | <0.001 | 3.68 (3.60–3.77) | 0.007 |
| Slope (against FEV1 z-score) | ||||||||
| Healthy | −0.0514 (−0.0788– −0.0240) |
<0.001 | −0.0309 (−0.0532– −0.0086) |
0.006 | −0.0619 (−0.0956– −0.0282) |
<0.001 | 0.0175 (−0.0039–0.0390) |
0.1081 |
| ILD | −0.0639 (−0.0956– −0.0322) |
0.439 | −0.0159 (−0.0438–0.0121) |
0.292 | −0.1005 (−0.1364– −0.0648) |
0.036 | 0.0214 (−0.0019–0.0447) |
0.743 |
| Asthma | −0.1391 (−0.1661– −0.1121) |
<0.001 | −0.0672 (−0.0909– −0.0435) |
0.003 | −0.1427 (−0.1734– −0.1121) |
<0.001 | 0.0638 (0.0440–0.0837) |
<0.001 |
| COPD | −0.1333 (−0.1610– −0.1056) |
<0.001 | −0.0337 (−0.0579– −0.0095) |
0.821 | −0.1951 (−0.2266– −0.1636) |
<0.001 | 0.0972 (0.0769–0.1175) |
<0.001 |
| Bronchiectasis | −0.1370 (−0.1737– −0.1003) |
<0.001 | −0.0887 (−0.0242– −0.1207) |
0.121 | −0.1532 (−0.1948– −0.1116) |
<0.001 | 0.0657 (0.0389–0.0925) |
<0.001 |
| UAO | −0.1072 (−0.1526– −0.0618) |
0.016 | −0.0309 (−0.0708–0.0089) |
0.998 | −0.1492 (−0.2007– −0.0977) |
0.001 | 0.1646 (0.1316–0.1975) |
<0.001 |
R5: resistance at 5 Hz; R20: resistance at 20 Hz; X5: reactance at 5 Hz; ILD: interstitial lung disease; UAO: upper airway obstruction; FEV1: forced expiratory volume in 1 s. #: p-values of the healthy groups were derived from the comparisons with zero; p-values of disease groups were derived from the comparisons with the healthy group.
FIGURE 3.
Equations of the z-scores of oscillometric parameters predicted by the forced expiratory volume in 1 s (FEV1) z-score. The solid lines represent medians and the shaded areas 95% confidence intervals. R5: resistance at 5 Hz; R20: resistance at 20 Hz; X5: reactance at 5 Hz; ILD: interstitial lung disease; UAO: upper airway obstruction.
Among the disease groups, UAO exhibited the highest z-scores of R5 and R20. For asthma, COPD and bronchiectasis, COPD presented higher mean z-scores of R5, R20 and R5−R20 along with a more negative mean X5 z-score than asthma or bronchiectasis (figure 2). However, when compared at the same FEV1 z-score level with FEV1 z-scores below the lower limit of normal (LLN) (figure 3), R5 z-score and R20 z-score of asthma were higher than those of COPD or bronchiectasis, R5−R20 z-score of COPD was higher than that of asthma or bronchiectasis, and X5 z-score was similar in these three groups. For ILD, R5 z-score began to exceed the upper limit of normal (ULN) when FEV1 z-score was approximately < −3.5, while R5−R20 z-score and X5 z-score tended to exceed the ULN or LLN when FEV1 z-score was approximately <LLN. R20 z-score remained within the ULN at any FEV1 z-score level (figure 3).
Diagnostic values of oscillometric parameters
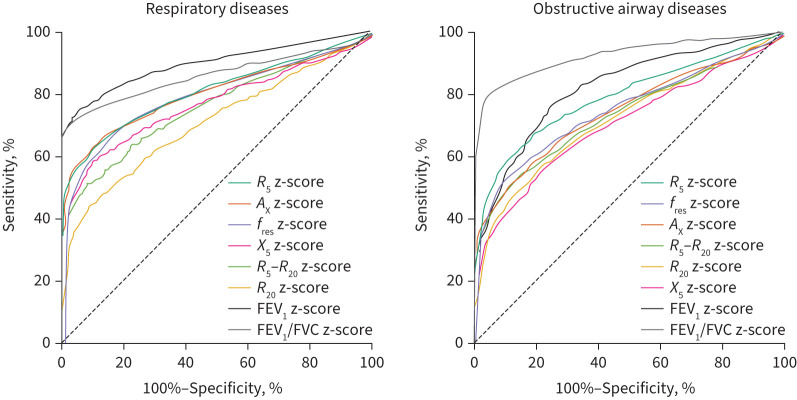
Among the analysed oscillometric parameters, R5 z-score was the best parameter to identify respiratory diseases (all disease groups) and obstructive airway diseases (disease groups except for ILD) with sensitivities of 62.4% and 66.7%, and specificities of 81.5 and 90.3%. However, compared with spirometry, oscillometric parameters showed lower diagnostic values than the FEV1 z-score or FEV1/FVC z-score (table 3 and figure 4).
TABLE 3.
Comparisons of the diagnostic values between R5 and spirometric parameters
| AUC (95% CI) | p-value | Cut-off | Sensitivity | Specificity | |
| Respiratory diseases | |||||
| R5 z-score | 0.807 (0.790–0.824) | <0.01 | >1.426 | 62.4% | 90.3% |
| FEV1 z-score | 0.900 (0.888–0.912) | <0.01 | ≤ −1.112 | 74.0% | 95.4% |
| FEV1/FVC z-score | 0.861 (0.847–0.875) | <0.01 | ≤ −1.410 | 70.5% | 97.4% |
| Obstructive airway diseases | |||||
| R5 z-score | 0.788 (0.770–0.806) | <0.01 | >1.426 | 66.7% | 81.5% |
| FEV1 z-score | 0.820 (0.803–0.837) | <0.01 | ≤ −1.069 | 76.2% | 75.2% |
| FEV1/FVC z-score | 0.922 (0.912–0.933) | <0.01 | ≤ −1.401 | 80.72% | 94.5% |
| UAO | |||||
| R20 z-score | 0.895 (0.852–0.938) | <0.01 | >2.300 | 87.5% | 79.0% |
| FEV1 z-score | 0.509 (0.420–0.598) | 0.84 | ≤ −0.230 | 82.1% | 26.6% |
| 0.557 (0.473–0.640) | 0.18 | ≤ −0.595 | 87.2% | 32.2% | |
| PEF z-score | 0.728 (0.657–0.799) | <0.01 | ≤−0.835 | 89.47% | 47.87% |
AUC: area under the curve; respiratory diseases: interstitial lung disease+asthma+COPD+bronchiectasis+upper airway obstruction (UAO) versus healthy; R5: resistance at 5 Hz; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; obstructive airway diseases: asthma+COPD+bronchiectasis+UAO versus healthy+ILD; R20: resistance at 20 Hz; PEF: peak expiratory flow.
FIGURE 4.
Diagnostic performances of oscillometric and spirometric parameters in diagnosing respiratory diseases (interstitial lung disease (ILD)+asthma+COPD+bronchiectasis+upper airway obstruction (UAO) versus healthy) and airway obstructive diseases (asthma+COPD+bronchiectasis+UAO versus healthy+ILD). R5: resistance at 5 Hz; AX: reactance area; fres: resonant frequency; X5: reactance at 5 Hz; R20: resistance at 20 Hz; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity.
For identifying specific respiratory disease, the highest discriminative capacity was in using R20 z-score to diagnose UAO, of which the sensitivity and specificity reached 87.5% and 78.9% (table S1 and figure S3), presenting a superior diagnostic value to the z-score of FEV1, FEV1/FVC or PEF (table 3 and figure S4).
Severity grading cut-offs of oscillometric parameters
According to the equations of oscillometric z-scores predicted by the FEV1 z-score, z-score values of oscillometric parameters corresponded to the spirometric severity grading cut-offs (FEV1 z-score −2.5 and −4) proposed by the ATS/ERS [19] were derived (table 4). As the corresponding z-score values of R5, R5−R20 and X5 of asthma, COPD and bronchiectasis were relatively similar, the average values of these three groups were applied as the grading cut-offs of the z-scores of R5, R5−R20 and X5, taking account of the generalisation of the cut-offs. No cut-off of R20 was derived as the corresponding R20 z-score values of different diseases varied and most were within the ULN.
TABLE 4.
z-scores of oscillometric parameters corresponded to forced expiratory volume in 1 s (FEV1) z-score −2.5 and −4
| R5 z-score | R20 z-score | R5−R20 z-score | X5 z-score | |||||
| FEV1 z-score | −2.5 | −4 | −2.5 | −4 | −2.5 | −4 | −2.5 | −4 |
| Healthy | 1.024 | 1.506 | 0.656 | 0.925 | 1.787 | 2.448 | −2.129 | −3.162 |
| ILD | 2.859 | 4.682 | 1.475 | 2.162 | 3.089 | 5.02 | −5.2 | −8.558 |
| Asthma | 2.374 | 4.006 | 1.196 | 1.518 | 2.737 | 5.367 | −3.606 | −8.815 |
| COPD | 1.318 | 1.954 | 0.35 | 0.479 | 2.072 | 3.223 | −3.146 | −4.374 |
| Bronchiectasis | 2.022 | 3.624 | 0.632 | 1.129 | 2.608 | 4.573 | −4.683 | −8.185 |
| UAO | 7.932 | 10.188 | 3.645 | 4.055 | 8.209 | 11.52 | −15.639 | −21.406 |
| Average of asthma+COPD+bronchiectasis | 2.418 | 4.104 | 2.811 | 4.987 | −4.496 | −8.519 | ||
R5: resistance at 5 Hz; R20: resistance at 20 Hz; X5: reactance at 5 Hz; ILD: interstitial lung disease; UAO: upper airway obstruction.
Discussion
Based on the multicentre, large-sample oscillometric data of healthy subjects and patients with respiratory disease, this study presents an overview of oscillometric characteristics in asthma, COPD, bronchiectasis, UAO and ILD. ROC curve analysis revealed that oscillometry displayed a lower diagnostic value than spirometry in identifying respiratory diseases and obstructive airway diseases with high specificity and moderate sensitivity. According to the equations of oscillometric z-scores predicted by the FEV1 z-score, severity grading cut-offs of oscillometric parameters were developed: for R5 and R5−R20, z-scores ≤1.645 are normal, 1.645<R5 z-scores≤2.5 or 1.645<R5−R20 z-scores≤3 are mildly increased, 2.5<R5 z-scores≤4 or 3<R5−R20 z-scores≤5 are moderately increased and R5 z-scores >4 or R5−R20 z-scores >5 are severely increased; for X5, z-scores ≥ −1.645 are normal, −1.645<z-scores≤ −4.5 are mildly decreased, −1.645<z-scores≤ −8.5 are moderately decreased and z-scores > −8.5 are severely decreased.
Characteristics of oscillometry in asthma, COPD, bronchiectasis, UAO and ILD
Increased airway resistance is a hallmark of obstructive airway diseases. The present study showed that although the total respiratory resistance assessed by oscillometry (R5) of asthma, COPD and bronchiectasis were all increased (compared with the healthy subjects), when compared at the same FEV1 level, asthma presented a higher R5 than COPD or bronchiectasis, mainly owing to a higher central airway resistance (R20). In contrast, COPD displayed a higher peripheral airway resistance (assessed by R5−R20) than asthma or bronchiectasis at the same FEV1 level. These characteristics are consistent with the underlying pathophysiology of the diseases, as the airflow limitation manifested in asthma is a result of the pathological changes of both the large and small airways [20], while the airflow limitation of COPD largely refers to the pathological changes of the peripheral lung and airways [2]. These findings also indicate that oscillometry can offer more detailed information on the pathophysiology of obstructive airway diseases than spirometry, suggesting its potential usefulness in clinical evaluation.
Although ILD is recognised as a group of disorders that primarily affects the lung interstitium instead of the airways, R5 was found to be mildly increased when FEV1 z-score was approximately < −3.5 in the present study, and this increase of R5 was mostly due to the increase of the R5−R20. This finding is in keeping with the report of van Noord et al. [13] in which Rrs presented a negative frequency dependence in diffuse ILD patients with total lung capacity (TLC) <80% pred, and a small increase at low frequencies as well as a more marked frequency dependence of Rrs was found in those with TLC <50% pred. The manifestation of frequency dependence of Rrs in ILD patients may owe to the occurrence of small airway dysfunction in some ILD patients [21, 22]. Although X5 is defined as an indicator of the dynamic compliance of the respiratory system, which is basically determined by the lung elasticity and the airway resistance [4, 5], this study demonstrated that at the same FEV1 level with FEV1<LLN, the X5 of ILD (i.e. an X5 determined by the lung elasticity) was less negative than the X5 of the airway obstructive diseases. This indicates that X5 is more sensitive to the changes of airway obstruction than lung elasticity.
As the resistance of the central airway accounts for most of the total airway resistance [23], it is reasonable that UAO exhibited the highest R5 and R20 among the obstructive airway diseases. This distinct oscillometric characteristic enables oscillometry to discriminate UAO from other respiratory diseases with relatively high sensitivity and specificity, suggesting that oscillometry may be an easy screening tool for UAO in clinical practice.
Diagnostic performance of oscillometry
The present study showed that oscillometry had a lower diagnostic value than spirometry in identifying patients with respiratory diseases with high specificity but moderate sensitivity. The reason for the low sensitivity may be due to the overlap in oscillometric parameters between healthy subjects and disease patients (figure 2). Since COPD was diagnosed with the criteria of FEV1/FVC <0.70, it may increase the diagnostic value of spirometry to identify respiratory diseases in this study. Except for UAO, the capacity of oscillometry to diagnose other respiratory diseases was less than satisfactory. Sensitivities and specificities of oscillometric parameters in diagnosing COPD in the present study were lower than those of the previous studies [24, 25]. This may be attributed to the different analysed populations, as the present study included not only healthy subjects and patients with COPD, but also patients with other respiratory diseases in the ROC analysis, which we believe to be more in agreement with the practical situation in lung function laboratories. The present findings suggest that oscillometry may be not suitable to apply as a diagnostic tool in laboratories given its unsatisfied sensitivity. However, it does offer valuable information for those with airway obstruction as its high specificity. Thus, oscillometry may be served as an alternative when spirometric information is not available.
Cut-offs for defining abnormality and grading severity of oscillometric parameters
The lack of relevant cut-offs in the interpretation of respiratory oscillometry is an unsolved problem in clinical practice. For IOS, H.J. Smith proposed to use 150% of predicted R5 as the cut-off of abnormality, and 200% and 300% of predicted R5 as the moderate and severe grading cut-offs [26]. Our previous report has shown that the ULN of Rrs derived from the reference equations were lower than the 150% of predicted Rrs [15]. In addition, the latest ATS/ERS technical standard recommended using z-scores but not per cent of the predicted values for the severity grading of lung function impairment, as the latter is influenced by sex and age [19]. This indicates that the previous cut-offs may be inappropriate for adoption in clinical practice. The present study developed a grading system of oscillometry in the form of z-scores with reference to the grading cut-offs of spirometry. The drawback of this method lies in the limited consistency of oscillometry and spirometry. Ideal cut-offs ought to be based on the distribution of oscillometric parameters without reference to spirometry, and should be relevant to the prognosis of patients for guiding the clinical practice [19]. However, in this aspect, various cut-offs may be derived concerning different clinical outcomes of different diseases. The present study was designed to provide generalised grading criteria that could be applied to the majority of respiratory diseases. Considering spirometry is currently recognised as and may remain the gold standard of airflow limitation in the foreseeable future, oscillometric cut-offs developed by referring to spirometry could offer reference information for physicians in clinical decision making. This is of particular importance in those who cannot perform spirometry. Further studies are needed to prospectively validate the grading cut-offs developed in the present study, taking account of the possible device-dependent difference, or to develop oscillometric cut-offs that are more associated with the clinical outcomes.
Strengths and limitations
The strength of this study is the large-sample and broad-scale oscillometric data of multiple diseases, which we believe can cover the common spectrum of oscillometric data encountered in routine clinical practice. However, limitations exist for the lack of data on those who are in the pre-disease stage, such as symptomatic individuals, severe smokers or those with occupational exposure. This may bias the analysed sensitivity of oscillometry in the study when compared with the actual situation in laboratories. In addition, the number of patients with UAO was relatively small; thus, the data on UAO in this study may be less representative of the whole UAO population. Lastly, longitudinal oscillometric data and data regarding the bronchodilator and bronchoconstrictor responses were lacking; these data may offer additional insights into the disease characteristics or clinical application of oscillometry.
Conclusions
This multicentre registry of impulse oscillometry in adult patients with respiratory diseases revealed that at the same level of FEV1, asthma, COPD, bronchiectasis, UAO and ILD display different characteristics on oscillometry. Respiratory oscillometry can offer more detailed information on the pathophysiology of obstructive airway diseases than spirometry. Instead of being a diagnostic tool, oscillometry is more appropriate as a tool for clinical evaluation, especially when spirometric data are unavailable. Severity grading cut-offs of oscillometric parameters were developed, which may be helpful for the interpretation of oscillometry in clinical practice. Further research is needed for the validation of these cut-offs, and to explore more potential application value of oscillometry in clinical evaluation.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00080-2022.SUPPLEMENT (714.4KB, pdf)
Acknowledgements
The authors would like to thank to the following centres and investigators for offering data to this manuscript: Tieying Sun and Yi-Meng Yang (Beijing Hospital); Li Xiang (Beijing Children's Hospital, Capital Medical University); Cheng Zhang (Guizhou Provincial People's Hospital); Na Zhang (Henan Provincial People's Hospital); Mingjuan Zhou (Guangdong Provincial Hospital of Traditional Chinese Medicine); Chunyan Zheng and Dexiang Wang (Qilu Hospital of Shandong University); and Xiaoju Liu and Hairong Bao (The First Hospital of Lanzhou University).
This study is registered at www.clinicaltrials.gov with identifier number NCT03467880. The data are available from the corresponding author on reasonable request.
Provenance: Submitted article, peer-reviewed.
Author contributions: All authors contributed to the conception and design of the work. Y. Gao and J. Zheng contributed to the funding acquisition. X. Liang, W. Han, J. Du, Y. Lu, L. Chen, T. Wang, J. Liu, G. Huang, B. Zhao, G. Zhao, X. Zhang, Y. Peng, X. Chen and N. Zhou contributed to the data acquisition. X. Liang and Z. Zhang contributed to the data analysis. All authors contributed substantially to the interpretation of the data results and the writing of the manuscript. All authors read and approved this study before submission.
Conflict of interest: The authors declare that they have no conflicts of interest.
Support statement: This work was supported by the National Science and Technology Support Plan (grant number 2015BAI12B10) and the National Key Research and Develop Program (grant 2018YFC1311900) of China. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Graham BL, Steenbruggen I, Barjaktarevic IZ, et al. . Standardization of spirometry 2019 update. An official American Thoracic Society and European Respiratory Society technical statement. Am J Respir Crit Care Med 2019; 200: E70–E88. doi: 10.1164/rccm.201908-1590ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Initiative for Chronic Obstructive Lung Disease . Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: 2022 Reports. https://www.goldcopd.org/2022-gold-reports-2/. Date last updated: 22 November 2021. Date last accessed: 11 December 2021.
- 3.Global Initiative for Asthma . Global Strategy for Asthma Management and Prevention, 2021. Available from: https://www.ginasthma.org/reports/. Date last updated: 28 April 2021. Date last accessed: 11 December 2021.
- 4.King GG, Bates J, Berger KI, et al. . Technical standards for respiratory oscillometry. Eur Respir J 2020; 55: 1900753. doi: 10.1183/13993003.00753-2019 [DOI] [PubMed] [Google Scholar]
- 5.Smith HJ, Reinhold P, Goldman MD. Forced oscillation technique and impulse oscillometry. In: Stam H, Gosselink R, eds. Lung Function Testing (ERS Monograph). Sheffield, European Respiratory Society, 2005; pp. 72–105. [Google Scholar]
- 6.Bickel S, Popler J, Lesnick B, et al. . Impulse oscillometry: interpretation and practical applications. Chest 2014; 146: 841–847. doi: 10.1378/chest.13-1875 [DOI] [PubMed] [Google Scholar]
- 7.Kaminsky DA, Simpson SJ, Berger KI, et al. . Clinical significance and applications of oscillometry. Eur Respir Rev 2022; 31: 210208. doi: 10.1183/16000617.0208-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zimmermann SC, Tonga KO, Thamrin C. Dismantling airway disease with the use of new pulmonary function indices. Eur Respir Rev 2019; 28: 180122. doi: 10.1183/16000617.0122-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jordon LH, Gore RB, Rusk RA, et al. . The role of impulse oscillometry in the management of asthma when forced expiratory maneuvers are contraindicated: case series and literature review. J Asthma 2021; 59: 1577–1583. [DOI] [PubMed] [Google Scholar]
- 10.Crim C, Celli B, Edwards LD, et al. . Respiratory system impedance with impulse oscillometry in healthy and COPD subjects: ECLIPSE baseline results. Respir Med 2011; 105: 1069–1078. doi: 10.1016/j.rmed.2011.01.010 [DOI] [PubMed] [Google Scholar]
- 11.Cavalcanti JV, Lopes AJ, Jansen JM, et al. . Detection of changes in respiratory mechanics due to increasing degrees of airway obstruction in asthma by the forced oscillation technique. Respir Med 2006; 100: 2207–2219. doi: 10.1016/j.rmed.2006.03.009 [DOI] [PubMed] [Google Scholar]
- 12.Guan W, Gao Y, Xu G, et al. . Impulse oscillometry in adults with bronchiectasis. Ann Am Thorac Soc 2015; 12: 657–665. doi: 10.1513/AnnalsATS.201406-280OC [DOI] [PubMed] [Google Scholar]
- 13.van Noord JA, Clément J, Cauberghs M, et al. . Total respiratory resistance and reactance in patients with diffuse interstitial lung disease. Eur Respir J 1989; 2: 846–852. [PubMed] [Google Scholar]
- 14.van Noord JA, Wellens W, Clarysse I, et al. . Total respiratory resistance and reactance in patients with upper airway obstruction. Chest 1987; 92: 475–480. doi: 10.1378/chest.92.3.475 [DOI] [PubMed] [Google Scholar]
- 15.Liang XL, Gao Y, Guan WJ, et al. . Reference values of respiratory impedance with impulse oscillometry in healthy Chinese adults. J Thorac Dis 2021; 13: 3680–3691. doi: 10.21037/jtd-20-3376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oostveen E, MacLeod D, Lorino H, et al. . The forced oscillation technique in clinical practice: methodology, recommendations and future developments. Eur Respir J 2003; 22: 1026–1041. doi: 10.1183/09031936.03.00089403 [DOI] [PubMed] [Google Scholar]
- 17.Miller MR, Hankinson J, Brusasco V, et al. . Standardisation of spirometry. Eur Respir J 2005; 26: 319–338. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 18.Jian W, Gao Y, Hao C, et al. . Reference values for spirometry in Chinese aged 4–80 years. J Thorac Dis 2017; 9: 4538–4549. doi: 10.21037/jtd.2017.10.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stanojevic S, Kaminsky DA, Miller M, et al. . ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J 2021; 60: 2101499. doi: 10.1183/13993003.01499-2021 [DOI] [PubMed] [Google Scholar]
- 20.Kaminsky DA, Chapman DG. Asthma and lung mechanics. Compr Physiol 2020; 10: 975–1007. doi: 10.1002/cphy.c190020 [DOI] [PubMed] [Google Scholar]
- 21.Mikamo M, Fujisawa T, Oyama Y, et al. . Clinical significance of forced oscillation technique for evaluation of small airway disease in interstitial lung diseases. Lung 2016; 194: 975–983. doi: 10.1007/s00408-016-9949-1 [DOI] [PubMed] [Google Scholar]
- 22.Bonifazi M, Sverzellati N, Negri E, et al. . Increased prevalence of small airways dysfunction in patients with systemic sclerosis as determined by impulse oscillometry. Rheumatol 2020; 59: 641–649. [DOI] [PubMed] [Google Scholar]
- 23.Green M. How big are the bronchioles? St Thomas Hosp Gaz 1965; 63: 136–139. [Google Scholar]
- 24.Chaiwong W, Namwongprom S, Liwsrisakun C, et al. . Diagnostic ability of impulse oscillometry in diagnosis of chronic obstructive pulmonary disease. COPD 2020; 17: 635–646. doi: 10.1080/15412555.2020.1839042 [DOI] [PubMed] [Google Scholar]
- 25.Liu Z, Lin L, Liu X. Clinical application value of impulse oscillometry in geriatric patients with COPD. Int J Chron Obstruct Pulmon Dis 2017; 12: 897–905. doi: 10.2147/COPD.S129974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bednarek M, Grabicki M, Piorunek T, et al. . Current place of impulse oscillometry in the assessment of pulmonary diseases. Respir Med 2020; 170: 105952. doi: 10.1016/j.rmed.2020.105952 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00080-2022.SUPPLEMENT (714.4KB, pdf)