JGP study suggests that Kv1 channels share a common mechanism of slow inactivation, but that some family members are less prone to inactivate than others.
Abstract
JGP study suggests that Kv1 channels share a common mechanism of slow inactivation, but that some family members are less prone to inactivate than others.
Voltage-activated potassium (Kv) channels repolarize neurons and other excitable cells following membrane depolarization. Sustained depolarization, however, inactivates Kv channels in two kinetically and mechanistically distinct ways: a rapid form of inactivation involving blockade of the internal pore, and a slower inactivation process linked to structural changes in the channel’s selectivity filter. These inactivation processes are crucial for shaping action potentials and regulating neuronal firing frequency. In this issue of JGP, Wu et al. (1) reveal that members of the Kv1 subfamily have different propensities to undergo slow inactivation, but they likely all do so via an identical structural rearrangement.
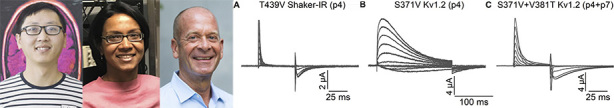
Xiaosa Wu, Kanchan Gupta, and Kenton Swartz (left to right) reveal that members of the Kv1 channel family have different propensities to slow inactivate, but use the same mechanism of selectivity filter dilation. The T439V mutation in the Shaker channel greatly accelerates slow inactivation (A) whereas the equivalent mutation in Kv1.2, S371V, has less of an effect (B). This difference is erased, however, when S371V is combined with a second mutation in a critical residue near the selectivity filter, V381T (C).
Initially described in the Drosophila Kv channel Shaker (2), slow inactivation has been shown to depend on a number of amino acids located in the selectivity filter in the external region of the pore. Based on observations of the bacterial potassium channel KcsA, the selectivity filter was initially proposed to collapse during slow inactivation. Earlier this year, however, Kenton Swartz and colleagues at the NIH obtained structural data indicating that the selectivity filter of Shaker actually dilates and inactivates the channel by blocking two potassium-binding sites in the outer pore (3). A recent crystal structure suggests that the selectivity filter of the mammalian Kv1.2 channel undergoes a similar dilation during slow inactivation (4).
A series of conserved amino acids around the selectivity filter form hydrogen bond networks that stabilize the conducting state of the channel (5), and their rearrangement underlies filter dilation (2, 3). Mutating these residues can either speed up or slow down slow inactivation, but some of these mutations have been reported to have different effects on Shaker and Kv1.2 channels (6). “We wanted to do a systematic comparison of how mutations influence slow inactivation in Kv1.2 and Shaker to get a sense of whether the two channels use similar mechanisms,” says Swartz.
Swartz and colleagues Xiaosa Wu and Kanchan Gupta therefore generated equivalent mutations in Shaker and Kv1.2 and measured their effects on channel inactivation (1). Several individual point mutations in Shaker accelerate slow inactivation, in some cases making the process so fast that the channels are effectively nonconducting (7). Wu et al. (1) consistently observed that the equivalent mutations in Kv1.2 have less dramatic phenotypes, only slightly accelerating slow inactivation or having no effect at all.
The researchers found that these differences depend on one key residue near the selectivity filter, which is a threonine (T449) in Shaker and a valine (V381) in Kv1.2. Mutations in Kv1.2 accelerated slow inactivation to the same extent as the equivalent mutations in Shaker when they were combined with a V381T mutation to make the Kv1.2 channel identical to Shaker at this position, extending a similar observation by others (6).
This suggests that due, in part, to the presence of valine at this critical position, Kv1.2 channels have a lower propensity than Shaker channels to slow inactivate, but the inactivation mechanism involves a similar dilation of the selectivity filter in both channels. “It’s a good example of how subtle differences between two channels can make the impact of mutations look very different and lead people to think they’re looking at distinct mechanisms when, in fact, they may be similar,” Swartz says.
Further work will be required to identify some of the other differences that make Kv1.2 channels less prone to slow inactivation. Swartz and colleagues are also interested in studying other Kv channels, such as Kv2.1, that may, indeed, slow inactivate through a different mechanism.
References
- 1.Wu, X., et al. 2022. J. Gen. Physiol. 10.1085/jgp.202213222 [DOI] [Google Scholar]
- 2.Hoshi, T., et al. 1991. Neuron. 10.1016/0896-6273(91)90367-9 [DOI] [Google Scholar]
- 3.Tan, X.F., et al. 2022. Sci. Adv. 10.1126/sciadv.abm7814 [DOI] [Google Scholar]
- 4.Reddi, R., et al. 2022. Sci. Adv. 10.1126/sciadv.abm8804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pless, S.A., et al. 2013. Elife. 10.7554/eLife.01289 [DOI] [Google Scholar]
- 6.Suarez-Delgado, E., et al. 2020. J. Gen. Physiol. 10.1085/jgp.201912499 [DOI] [Google Scholar]
- 7.Yang, Y., et al. 1997. J. Gen. Physiol. 10.1085/jgp.109.6.779 [DOI] [Google Scholar]


