Abstract
Introduction
Nodules detected in the lung parenchyma should be considered as malignant until proven otherwise, and the necessary tests should be performed for diagnosis.
Aim
To calculate the preoperative platelet-to-lymphocyte ratio (PLR) in patients with malignant lung nodules and to investigate the diagnostic value of this ratio in determining the histopathology of the nodule.
Material and methods
Ninety-one patients who were operated on for a malignant nodule in the lung between September 2010 and September 2020 were included in the study. The PLR was calculated by dividing the absolute platelet count by the absolute lymphocyte count. These values were compared with the histopathological diagnoses of the resected tumor tissue. Patients with primary lung malignancy were classified as group 1 (n = 54), and lung metastases of other organs were classified as group 2 (n = 37).
Results
The mean PLR was 127.27 ±46.82 in the first group and 183.56 ±93.49 in the second group. There was a statistically significant difference in PLR values between the two groups, and PLR was higher in group 2. There was no statistically significant difference between the two groups in terms of lymph node positivity, nodule size and SuvMax values. A moderately strong, significant and same-sided correlation was observed between nodule size and SuvMax values in the first group of patients (r = 0.48, p = 0.001)
Conclusions
PLR values less than 89.41 indicate that the histopathological result may be a lung-derived malignancy. However, in cases where the PLR is detected above 165.6, it would be appropriate to interpret another previously detected malignancy as metastasis to the lung.
Keywords: platelet, lymphocyte, nodule, lung cancer, metastases
Introduction
Lung cancer is the most common type of cancer in the world, and in approximately 85% of cases it is non-small cell cancer [1]. Although surgery is the recommended treatment, especially in early-stage lung cancer, one of the most important factors in determining the stage is tumor size. Solitary pulmonary nodules (SPN) are defined as well-circumscribed and single lesions smaller than 3 cm in the lung parenchyma in radiological imaging [2]. The differential diagnosis of pulmonary nodules includes malignant lung cancers, metastases and carcinoid tumors, while benign lesions include infectious diseases, granulomas, hamartomas, sequelae of previous lung diseases, etc. A nodule detected in the lung should be considered malignant until proven otherwise, and the examinations should be arranged accordingly. With the increase in high-resolution imaging methods, the prevalence of incidentally detected solitary pulmonary nodules is approximately 31%, although this rate can reach 50% in high-risk patients [3].
Determining the possibility of malignancy of the patient is possible with both clinical and radiological evaluation. Although advanced age, smoking, history of another malignancy, and family history increase the possibility of malignancy of the nodule, it is not possible to exclude malignancy in their absence. Increasing the size of the nodule also increases the possibility of malignancy. While the probability of malignancy is 1% in nodules less than 5 mm in size, 50% of nodules larger than 20 mm are malignant [4]. Again, nodules with a doubling time of 20–400 days are also more likely to be malignant than others [5]. Even though the characteristic features of the nodule in thorax CT inform us about the possibility of malignancy, this information is often insufficient. Solid lesions are more common, but subsolid lesions are more likely to be malignant [6]. PET-CT is applied in 8 mm and larger nodules according to the ACCP guidelines [7]. However, it should be kept in mind that false negatives and false positives may occur.
Although the gold standard in diagnosis is tissue biopsy sampling, it is not always possible to diagnose due to the location of the nodule. If lung nodules are thought to be malignant, resection is recommended, and the width of the resection is determined according to the histopathological type of the nodule. Nodules in the lung may be primary lung carcinoma or may present as metastases from other organs to the lungs. Metastasectomy is recommended in cases where the primary tumor, such as colorectal and osteosarcoma, is under control and the lung metastases are few [8]. Many studies have been published suggesting that systemic inflammation seen in malignant patients may be associated with tumor burden, metastasis, invasion and progression. It is also known that patients with malignancy may have thrombocytosis and/or lymphopenia. In recent studies, the platelet/lymphocyte ratio (PLR) has been used as a diagnostic and prognostic marker for many diseases [9].
Routine hemogram evaluation is a test performed in almost all patients because it is inexpensive, minimally invasive, and provides predictions about many diseases after hospital admission. In recent years, the platelet/lymphocyte ratio obtained as a result of hemogram examinations has started to be used to determine the prognosis in patients followed up for lung cancer [10]. Although there are many studies to determine the relationship between cancer and platelet/lymphocyte ratio (PLR), no study was found in the literature to evaluate the differentiation of nodules seen in the lung into a primary lung tumor or lung metastasis of other organs.
Aim
In this study, we aimed to compare the PLR in the preoperative hemogram examinations of patients with malignant lung nodules and the histopathological diagnoses of postoperatively resected lung nodules, thus evaluating the usability of PLR as a biomarker in predicting the histopathology of lung nodules.
Material and methods
Ninety-one patients who were operated on for a malignant nodule in the lung between September 2010 and September 2020 were included in our study. Patient information was obtained from patient files and epicrisis information. Patients who did not have atelectasis, lymphadenopathy or pleurisy on thorax computed tomography (CT) and had lesions smaller than 3 cm in the lung parenchyma were included. The nodules were evaluated by a radiologist. All nodules in our study were solid. The patients were evaluated retrospectively in terms of age, gender, size of the lung nodule, postoperative histopathological diagnosis, preoperative platelet, lymphocyte counts and ratios to each other, and the operation performed. Preoperative complete blood count and routine biochemistry evaluation were performed in all patients. All hematological evaluations were performed in the same laboratory.
The patients were intubated in the supine position under general anesthesia with a single or double-lumen tube. They were operated on with thoracotomy and/or VATS with a lateral decubitus position. Lobectomy, segmentectomy, wedge resection or incision-circumcision were performed in the operated patients. After the operation, all patients were taken to the intensive care unit as extubated. Oxygen, intravenous analgesics, and bronchodilator treatment with nebula were given. Chest radiography was evaluated in the early postoperative period.
The patients were divided into 2 groups according to the postoperative histopathological diagnosis of the malignant pulmonary nodule. In the first group of patients, nodules were considered as the primary malignancy of the lung (n = 54), and in the second group of patients, the lung metastases (n = 37) of the malignancies of other organs in the body were under control. No extrapulmonary tumor was detected in group 1 patients. None of the patients had a history of oncologic treatment in the last year. In the preoperative hemogram evaluation of all patients, lymphocyte and platelet counts and the ratios of these values to each other were calculated. PLR was calculated by dividing the absolute platelet count in the peripheral blood by the lymphocyte count. Patients with concomitant infection in the preoperative evaluation were not included in the study. The results were analyzed with statistical analysis programs and correlated with postoperative histopathological diagnoses. Patients with lung nodules larger than 3 cm, benign nodules, atelectasis, and pleurisy were not included in the study. Inflammatory diseases, autoimmune diseases, stroke, metabolic syndrome, etc., were not detected in any patient.
An ethics committee decision was made for the study protocol. Informed consent for the operation was obtained from each patient in the study. This study was conducted in accordance with the Principles of the Declaration of Helsinki.
Statistical analysis
Statistical analysis was performed using SPSS version 24.0 for Windows (IBM Corp., Armonk NY, USA). Descriptive data were expressed as mean ± standard deviation (SD), median (min.–max.), number and frequency. Variable distributions and conformity to normal distribution analyses were checked with the Kolmogorov-Smirnov test. Student’s t test was used for comparison between groups. The relationship between variables was evaluated with Pearson correlation analysis. ROC (receiver operating characteristic) analysis was performed for the cut-off values. P < 0.05 was accepted as the cut-off value of significance. Sensitivity and specificity analyses were performed for PLR at 3 different cut-off values. The purpose of this is to determine the maximum specificity and maximum sensitivity, as well as to identify the optimum values.
Results
The mean age of 91 patients (21 female, 70 male) included in our study was 59.35 ±11.85 (between 20–81). The patients in the first group (n = 54) were 47 males and 7 females, with a mean age of 62.7 ±9.6 (between 23 and 81 years), while the second group (n = 37) consisted of 23 males and 14 females, with a mean age of 54.4 ±13.2 (between 20 and 73) years (Table I). Although high platelet and lymphocyte counts were observed in the first group, it was not statistically significant.
Table I.
Patients’ characteristics
| Parameter | Group 1 (n = 54) | Group 2 (n = 37) |
|---|---|---|
| Age [years] | 62.7 ±9.6 | 54.4 ±13.2 |
| Gender: | ||
| Male | 47 | 23 |
| Female | 7 | 14 |
| PLR | 127.27 ±46.82 | 183.56 ±93.49 |
| Size of the nodule [mm] | 20.05 ±7.4 | 15.02 ±5.79 |
| SUVmax values | 8.24 ±4.28 | 5.35 ±2.64 |
| WBC [× 103/mm] | 7.43 ±2.65 | 6.89 ±1.87 |
| Platelets [× 103/mm] | 255.1 ±62.3 | 252 ±77.4 |
| Lymphocytes [× 103/mm] | 2.17 ±0.65 | 1.65 ±0.73 |
PLR – platelet/lymphocyte ratio, WBC – white blood cells.
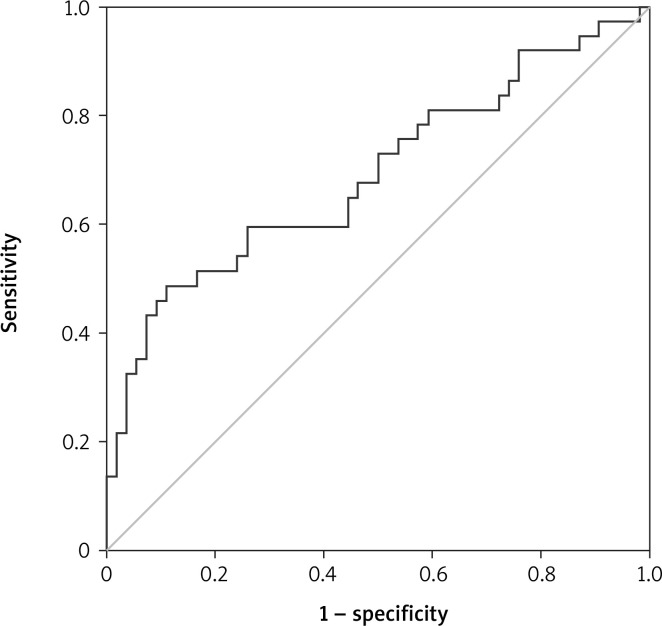
No correlation was found between age and gender and PLR. The mean PLR was 127.27 ±46.82 in the first group and 183.56 ±93.49 in the second group. There was a statistically significant difference in PLR values between the two groups, and PLR was higher in group 2. In ROC analysis, AUC for PLR was 0.692, p = 0.002, 95% CI: 0.577–0.807 (Figure 1). For PLR, sensitivity and selectivity analyses were performed at 3 different cut-off values. The cut-off value at maximum sensitivity (91.9%) was 89.41, and the cut-off value at maximum selectivity (80%) was 165.6. The cut-off value was calculated as 113.84 under optimal (sensitivity 73%, selectivity 49%) conditions (Table II).
Figure 1.
Receiver operating characteristic (ROC) analysis for PLR
Table II.
Cut-off values for PLR
| Varaiable | Cut-off | Sensitivity | Specificity |
|---|---|---|---|
| Optimal | 113.84 | 73% | 49% |
| Max sensitivity | 89.41 | 91.9% | |
| Max specificity | 165.6 | 80% |
PLR – platelet/lymphocyte ratio.
In the radiological evaluation, the size of the nodule was 20.05 ±7.40 mm in the first group of patients and 15.02 ±5.79 mm in the second group of patients. There was no statistically significant difference between the two groups in terms of lymph node positivity, nodule size and SuvMax values (p = 0.001). A moderately strong, significant and positive correlation was observed between nodule size and SuvMax values in the first group of patients (r = 0.48, p = 0.001)
In the histopathological evaluation of the nodules, group 1 consisted of 35 adenocarcinomas, 15 squamous cell carcinomas, 2 large cell carcinomas and 2 neuroendocrine tumors. Group 2 consisted of lung metastasis of the colon, breast, sarcomas, thyroid, hepatocellular carcinoma, malignant melanoma, renal cell carcinoma, gastric and pancreatobiliary cancers. No correlation was found between histopathological evaluation of the nodules and PLR (p = 0.07) (Table III).
Table III.
Histopathological evaluation of the nodules
| Group 1 (n = 54) | Group 2 (n = 37) | ||
|---|---|---|---|
| Adenocarcinoma | 35 | Colon | 12 |
| Breast | 7 | ||
| Squamous cell carcinoma | 15 | Sarcomas | 9 |
| Thyroid | 1 | ||
| Large cell carcinoma | 2 | Hepatocellular | 1 |
| Malignant melanoma | 2 | ||
| Renal cell carcinoma | 1 | ||
| Neuroendocrine tumor | 2 | Gastric cancer | 2 |
| Pancreatobiliary cancers | |||
Discussion
Lung cancer is one of the leading causes of cancer-related deaths worldwide. Non-small cell lung cancers correspond to approximately 85% of all lung cancers. For this reason, many studies are carried out for the early detection of lung cancer. Nodules detected in peripheral or central areas of the lung can be diagnosed with interventional methods (fiberoptic bronchoscopy, transthoracic needle biopsy, etc.). However, histopathological evaluation is a serious problem for nodules that cannot be diagnosed by these methods. In our study, we used a hemogram, which is an easily accessible and inexpensive examination, in such nodules detected in thorax CT. In this way, we had the chance to easily count platelets and lymphocytes, which are blood elements.
Platelets are cells of hematopoietic stem cell origin and are involved in blood coagulation through aggregation and interaction with endothelial cells when tissue is damaged. In addition to hemostasis, these cells bind to cells involved in immunity and have a modulation role in the immune response [11]. They also play a role in inflammation and tumor development. Inflammation, on the other hand, is a common condition in cancer and has a relationship with inflammatory cells and mediators in the microenvironment of most tumors [12]. Platelets secrete cytokines such as platelet-derived growth factor, platelet factor 4, transforming growth factor-β, VEGFR, and thrombospondin-1, which allow tumor cells to increase and adhere to other cells [13, 14]. Platelets increase angiogenesis by secreting vascular endothelial growth factor (VEGF), which accelerates tumor growth. In their study, Wiesner et al. reported that VEGF-A levels were significantly higher in cancer patients compared to healthy adults [15]. As a result of these studies, we predicted high platelet counts in both groups of patients. IL-1 and IL-6 stimulate the differentiation of megakaryocytes into platelets and ensure the recruitment of neutrophils [16]. It is also known that IL-6 stimulates thrombopoietin production and causes thrombocytosis associated with poor prognosis in patients with cancer. In our hypothesis, we thought that platelet counts would be higher and lymphocyte counts lower in group 2 patients because metastasis was an indicator of advanced cancer. However, as a result of our study, mean platelet counts were higher in group 1. This value was not statistically significant. It is known that platelets interact directly with tumor cells and contribute to tumor growth, invasion and angiogenesis [17]. Platelets not only allow primary tumors to grow but also allow cancer cells to escape from the immune system and extravasate to secondary sites. Furthermore, platelets protect tumor cells from being destroyed by natural killer cells by releasing TGF-β and may facilitate metastasis. In the light of this information, studies have shown that antiplatelet therapies can give better oncological responses in head and neck cancers [18].
However, lymphocytes play a role in cancer surveillance to prevent tumor development [19]. Cancer-associated inflammation suppresses anti-tumor immunity by recruitment of regulatory T cells and activation of chemokines. Even in breast cancer and melanoma, the abundance of tumor-infiltrated lymphocytes is an indicator of a good prognosis [20]. Lymphocytopenia is known to be associated with poor prognosis in pancreatic and other gastrointestinal cancers [21]. In our study, mean lymphocyte counts were calculated to be lower in group 2 patients compared to group 1 patients (2.17 > 1.65). Although the platelet counts between the two groups were almost the same, the reason for the high PLR in group 2 patients was the low number of lymphocytes in group 2.
Despite the increased chance of survival in cancer patients in recent years, it has been attempted to develop diagnostic methods with increased sensitivity and specificity for early and accurate detection of cancer and determining the prognosis [22]. The available data indicate that there is no examination to distinguish lung nodules other than biopsy. For this, both clinicians and researchers are working on biomarkers that can detect cancer early and predict disease progression, response to treatment and survival. Blood tests and biopsy materials which were taken from the tumor are frequently used to determine the histopathology of cancer. Biopsy materials allow direct examination of the tumor. However, blood samples are materials that indirectly show cancer progression. At the same time, they can be repeated because they are cheap and easily accessible, and they are not affected by the heterogeneous structure within the tumor. Platelet count and platelet/lymphocyte ratio are used as prognostic factors in many types of cancer. Blood tests at diagnosis or before treatment can help show tumor-related inflammation. C-reactive protein (CRP), albumin, neutrophil/lymphocyte ratio and platelet/lymphocyte ratio can be used to evaluate inflammation in the body [23]. In these studies, lymph node positivity was found to be high in colon cancer patients with high PLR. Moreover, PLR was reported to be a marker of residual disease and stage in patients with ovarian cancer [24]. In general, high PLR is a marker of poor survival. Zhou et al. found in their meta-analysis that higher PLR values were associated with poorer survival. Cut-off values for PLR applied in the studies they evaluated ranged between 100 and 300. In some studies, even triple cut-off values were applied [25]. In our study, maximum specificity and sensitivity were evaluated separately. We conducted PLR investigations at different cut-off values. As a result of all these evaluations, hemogram examination performed in patients with pulmonary nodules detected in thorax CT gives us information about the histopathology of the nodule. With these results, we believe that the PLR calculated in the complete blood count in lung nodules that cannot be histopathologically diagnosed may be useful in preoperative diagnosis as a biomarker. PLR values less than 89.41 indicate with a sensitivity of 91.9% that the histopathological result may be a lung-derived malignancy. However, in cases where PLR is detected above 165.6, it would be appropriate to interpret another previously detected malignancy as lung metastasis with 80% specificity. It was thought that the values in the range 89.41–165.6 could be statistically misleading.
Our study has been a start to be able to use PLR as a biomarker. However, the study had some limitations. Studies with higher patient numbers are important in terms of determining cut-off values more clearly. Although PLR values are not sufficient to make definite inferences, we think that they can provide predictions.
Disclosure
The authors report no conflict of interest.
Biography

References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statics in China. CA Cancer J Clin 2016; 66: 115-132. [DOI] [PubMed] [Google Scholar]
- 2.Ost DE, Gould MK. Decision making in patients with pulmonary nodules. Am J Respir Crit Care Med 2012; 185: 363-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gould MK, Tang T, Liu IL, Lee JS, Zheng C, Danforth K, Kosco AE, Di Fiore JL, Suh DE. Recent trends in the identification of incidental pulmonary nodules. Am J Respir Crit Care Med 2015; 192: 1208-1214. [DOI] [PubMed] [Google Scholar]
- 4.Chan EY, Gaur P, Ge Y, Kopas L, Santacruz JF, Gupta N, Munden RF, Cagle PT, Kim MP. Management of the solitary pulmonary nodule. Arch Pathol Lab Med 2017; 141: 927-931. [DOI] [PubMed] [Google Scholar]
- 5.Thalanayar PM, Altintas N, Weissfeld JL, Fuhrman CR, Wilson DO. Indolent, potentially inconsequential lung cancers in the Pittsburgh lung screening study. Ann Am Thorac Soc 2015; 12: 1193-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McWilliams A, Tammemagi MC, Mayo JR, Roberts H, Liu G, Soghrati K, Yasufuku K, Martel S, Laberge F, Gingras M, Atkar-Khattra S, Berg CD, Evans K, Finley R, Yee J, English J, Nasute P, Goffin J, Puksa S, Stewart L, Tsai S, Johnston MR, Manos D, Nicholas G, Goss GD, Seely JM, Amjadi K, Tremblay A, Burrowes P, MacEachern P, Bhatia R, Tsao MS, Lam S. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med 2013; 369: 910-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel VK, Naik SK, Naidich DP, Travis WD, Weingarten JA, Lazzaro R, Gutterman DD, Wentowski C, Grosu HB, Raoof S. A practical algorithmic approach to the diagnosis and management of solitary pulmonary nodules: part 2: pretest probability and algorithm. Chest 2013; 143: 840-846. [DOI] [PubMed] [Google Scholar]
- 8.Pastorino U, Buyse M, Friedel G, Ginsberg RJ, Girard P, Goldstraw P, Johnston M, McCormack P, Pass H, Putnam JB Jr.; International Registry of Lung Metastases . Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997; 113: 37-49. [DOI] [PubMed] [Google Scholar]
- 9.Balta S, Ozturk C. The platelet-lymphocyte ratio: a simple, inexpensive and rapid prognostic marker for cardiovascular events. Platelets 2015; 26: 660-681. [DOI] [PubMed] [Google Scholar]
- 10.Huang Q, Diao P, Li CL, Peng Q, Xie T, Tan Y, Lang JY. Preoperative platelet-lymphocyte ratio is a superior prognostic biomarker to other systemic inflammatory response markers in non-small cell lung cancer. Medicine 2020; 99: e18607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Semple JW, Italiano JE, Freedman JE. Platelets and the immune continuum. Nat Rev Immunol 2011; 11: 264-274. [DOI] [PubMed] [Google Scholar]
- 12.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation and cancer. Cell 2010; 140: 883-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubernard V, Arbeille BB, Lemesle MB, Legrand C. Evidence for alpha-granular pool of the cytoskeletal protein alpha-actinin in human platelets that redistributes with the agresive glycoprotein thrombospondin-1 during the exocytotic process. Arterioscler Thromb Vasc Biol 1997; 17: 2293-305. [DOI] [PubMed] [Google Scholar]
- 14.Qian X, Tuszynski GP. Expression of the thrombospondin-1 in cancer: a role in tumor progression. Proc Soc Exp Biol Med 1996; 212: 199-207. [DOI] [PubMed] [Google Scholar]
- 15.Wiesner T, Bugl S, Mayer F, Hartman JT, Kopp HG. Differential changes in platelet VEGF,Tsp, CXCL12 and CXCL4 in patients with metastatic cancer. Clin Exp Metastasis 2010; 27: 141-149. [DOI] [PubMed] [Google Scholar]
- 16.Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol 2013; 14: 218-228. [DOI] [PubMed] [Google Scholar]
- 17.Jain S, Harris J, Ware J. Platelets: linking hemostasis and cancer. Arterioscler Thromb Vasc Biol 2010; 30: 2362-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furlan C, Steffan A, Polesel J, Trovo M, Gobitti C, Vaccher E, Serraino D, Barzan L, Franchin G. Lower platelet counts and antiplatelet therapy independently predict better outcomes in patients with head and neck squamous cell carcinoma: a retrospective analysis. Biomark Res 2015; 3: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004; 21: 137-148. [DOI] [PubMed] [Google Scholar]
- 20.Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, Ellis IO, Green AR. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 2011; 29: 1949-1955. [DOI] [PubMed] [Google Scholar]
- 21.Fogar P, Sperti C, Basso D, Sanzari MC, Greco E, Davoli C, Navaglia F, Zambon CF, Pasquali C, Venza E, Pedrazzoli S, Plebani M. Decreased total lymphocyte counts in pancreatic cancer: an index of adeverse outcome. Pancreas 2006; 32: 22-28. [DOI] [PubMed] [Google Scholar]
- 22.Paul D, Kumar A, Gajbhiye A, Santra MK, Srikanth R. Mass spectrometry-based proteomics in molecular diagnostics: discovery of cancer biomarkers using tissue culture. Biomed Res Int 2013; 4: 783131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care 2009; 12: 223-226. [DOI] [PubMed] [Google Scholar]
- 24.Raungkaewmanee S, Tangjitgamol S, Manusirivithaya S, Srijaipracharoen S, Thavaramara T. Platelet to lymphocyte ratio as a prognostic factor for epithelial ovarian cancer. J Gynecol Oncol 2012; 23: 265-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou X, Du Y, Huang Z, Xu J, Qiu T, Wang J. Prognostic value of PRL in various cancers: a meta-analysis. PLos One 2014; 9: e101119. [DOI] [PMC free article] [PubMed] [Google Scholar]