Abstract
Spindle cell hemangioma (SCH) is a benign vascular tumour, first identified by Weiss and Enzinger in 1986. Habitually, the SCH affects almost exclusively the dermis and subcutaneous tissues of distal extremities. So far, only 2 cases have been described in the lung. We describe herein the third case of SCH occurring in the lung in a 47 year‐old woman. The patient was successfully treated by right lower lobectomy. The histopathological and immunohistochemistry examination of the excised tumour leads to the definitive diagnosis. Our case is instructive by its different clinical and radiological presentation compared to the previous two cases.
Keywords: case report, hemangioma, lung tumour, spindle cell
SCHs are benign vascular tumor that occurs in young adults and typically arises in the subcutis of the distal extremities, particularly the hand. So far only two cases of pulmonary SCH were reported in the literature. Our case is instructive by its different clinical (hemoptysis) and radiological presentation (cavitary lesion) compared to the previous two cases.
INTRODUCTION
Spindle cell hemangioma, formerly known as spindle cell hemangioendothelioma, is a rare benign vascular tumour. It typically occurs in the subcutis of the distal extremities and appears as red‐brown lesions that can grow in both size and number over time.
SCH is predominantly found in young adults. SCH strikes both genders with a similar frequency. 1
SCHs which occur in lungs are exceeding rare. We describe herein the third case reported so far.
CASE REPORT
A 47 ‐year‐old, non‐smoking woman, with no history of major illness was admitted to our department with recurrent low‐abundance hemoptysis without fever or weight loss. She had no other respiratory symptoms (chest pain or shortness of breath). Physical examination and regular laboratory data showed no abnormalities. The chest computer tomography (CT) revealed a 12 mm long axis cavitary lesion of the right antero‐basal segment. It was surrounded by a small area of ground glass opacities. With prone position CT scans, no clear mobility of the image described above was detected (Figure 1).
FIGURE 1.
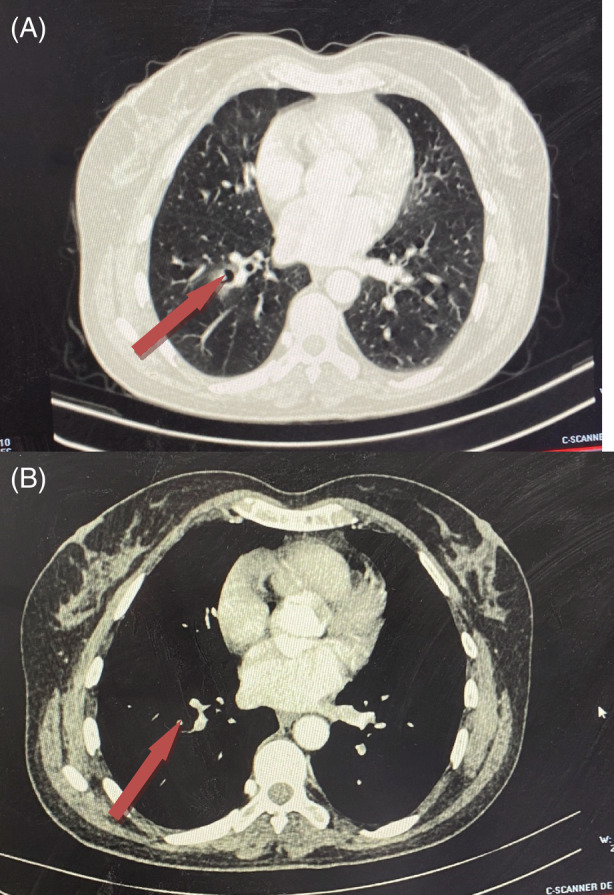
Chest CT scan: Lung parenchyma window (A) and mediastinal window (B) showing a cavitary lesion of the right antero‐basal segment (red arrows) surrounded by a small area of ground glass opacities
Sputum smear microscopy for detection of acid fast bacilli (AFB) was negative.
Aspergillus serology was negative. Flexible bronchoscopy showed a free bronchial tree. Pulmonary function tests were normal. To make a definitive diagnosis, a right lower lobectomy was done.
Microscopically, the tumour was composed of thin‐walled vessels lined by flattened and cubic endothelial cells. Stromal cells between vascular spaces were spindled or round. They were associated with many siderophages (Figure 2).
FIGURE 2.
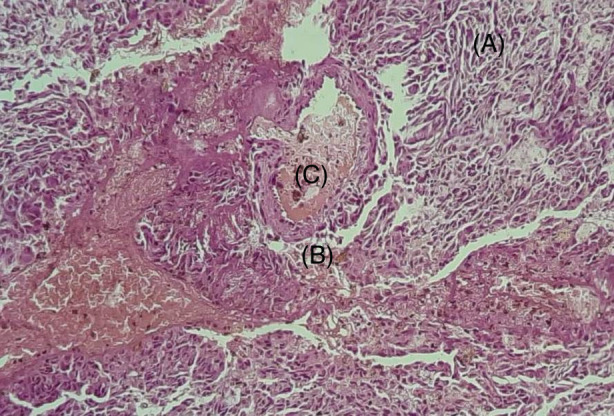
Histopathological features. The tumour is composed of bland spindle cells proliferation (A), dilated blood vessels (B), and hemosiderine‐laden macrophages (C)
Immunohistochemical stains for vascular markers CD31, CD34 and vimentin were positive. Thyroid transcription factor‐1 (TTF1) and pancytokeratin were negative. There were no histological signs of malignancy. These findings led to the diagnosis of SCH. The postoperative course was uneventful. On follow‐up 2 months after the surgery, the patient was asymptomatic, no signs of recurrence have been noted.
DISCUSSION
In 1986, Weiss and Enzinger described a new variant of the vascular tumour designated “spindle cell hemangioendothelioma,” which had limited malignant potential. 2 By the advent of immunochemistry, this tumour was no longer considered as malignant. 3 SCH generally occurs in young adults and typically arises in the subcutis of the distal extremities, particularly the hand. It has a slow rate of growth and usually reaches 1–2 cm. 4 It typically develops as a solitary nodule or multiple masses located in the dermal or subcutaneous layers of the distal extremities.
In a large case series of 78 patients, Perkins and Weiss evaluated its pathogenesis and long‐term behaviour. It affected all ages (range 8–78 years, median age 32 years, mean age 34 years). 5 There is an equal gender distribution or a very slight female predominance. 4 Clinically, this neoplasm is characterized by an indolent but progressive growth, local recurrence and multifocality. 6
Rare cases presenting in non‐cutaneous regions have also been described, including, mediastinum, pleura, spinal cord, muscles, thyroid, spleen, retroperitoneum and even infratemporal fossa. 1 , 6 , 7 , 8 , 9 , 10 , 11
So far only two cases of pulmonary SCH were reported in the literature. 2 , 12 The first case was reported in 2019, where SHC presented as multiple round nodules with smooth borders and uniform density in both lungs, and the diagnosis was made by video‐assisted thoracoscopic biopsy of the largest and most accessible lesion. 2 In the second case, SCH presented as a single enlarging pulmonary nodule on a background of multiple smaller stable nodules and ground‐glass opacities.
Between 2013 and 2018, its size increased to 13 mm and its morphology changed from smooth to irregular density and speculated margins. A right middle lung lobectomy was performed. 12
In our case, SCH presented under another different radiological aspect: a single cavitary lesion. The major differential diagnosis of pulmonary cavity lesion was pulmonary aspergillosis. Aspergillus was not isolated in bronchoalveolar lavage fluid, and the diagnosis of pulmonary aspergillosis was ruled out.
After discussing with the patient, a right lower lung lobectomy were performed.
Post‐operative pathological analysis showed characteristic features of spindle cell hemangioma: endothelial cells and spindled fibroblastic cells (lacking significant atypia and with low mitotic activity). Histologically, SCH shows a proliferation of spindle cells composed of endothelial cells, pericytes and fibroblasts between dilated vascular spaces. Lesional cells show immunoreactivity for endothelial markers such as CD34, CD31, vimentin and factor VIII‐related antigen. 6 SCH shows no or only a low level of mitotic activity. The distribution and percentage of the main histologic components may be highly variable. 6 Cavernous hemangioma and the nodular stage of Kaposi sarcoma should be considered in the differential diagnosis. Cavernous hemangioma is distinguished from SCH by the absence of solid spindle‐cell areas. Kaposi sarcoma lacks epithelioid endothelial cells with vacuolization and cavernous vascular structures. 1
SCH can be multifocal or can appear as part of Maffucci syndrome in 5% of cases. 13
Mafucci's syndrome is a rare congenital mesodermal dysplasia, which results in multiple enchondromas combined with SCHs of the dermis, subcutis, or visceral tissues. 14 , 15 It is not race or sex related, and usually presents before the onset of puberty, and patients are usually normal at birth. 14
Until recent years, it was thought that no genetic mutations or molecular characteristics were associated to SCH. But recent studies have shown that most sporadic SCH not associated with Maffucci syndrome also harbour mutations in isocitrate dehydrogenase (IDH). 6
Mutations in exon 4 of the IDH1(encoding an R132C substitution or R132H substitution) or IDH2 (encoding R172S) were identified in 71% of schemes. 13 , 16
This mutation seems to be unique to SCH, because a wide range of other vascular tumours that have been studied lack mutations in IDH. 6 Its presence in a vascular tumour with a spindle cell component should prompt consideration of a diagnosis of a scheme. 13
Management and prognosis of pulmonary SCH had no consensus yet. Thus, the management philosophy could be borrowed by extrapolating the management of SCH elsewhere. SCH is a benign lesion and the most widely acceptable treatment option presently is conservative excision without adjuvant chemotherapy or radiotherapy. 6
Following surgical excision, local recurrence rate of up to 58% has been reported. 6
Most reports in the literature have not specified the timeframe for local recurrence, it may develop many years after the initial excision. 17
Recurrences occur more commonly in patients with multiple lesions at presentation, occurring near surgical sites rather than within them. It is likely related to local intravascular propagation of the tumour. 6
Despite conservative excisions in most patients, the prognosis was excellent with no metastasis or death attributed to Scheme. 12
Our case is consistent with the literature; it highlights the difficulty in preoperative diagnosis of SCH, the diagnosis relying essentially on anatomo‐pathological examination and the immunohistochemistry analysis of the surgical specimen.
A further follow up of our patient is needed to evaluate the risk of recurrences.
Due to the lack of consensus regarding the time to recurrence and recommended radiological follow‐up; collaboration between the different centers is essential and a global registry can be proposed to collect data on patients from all over the world in order to answer unresolved questions and propose a management and follow‐up strategy for this rare tumour.
In conclusion, SCHs are benign vascular tumour that can occur at any age in males and females.
The lesions may develop as a solitary nodule or as multiple masses located in the dermal or subcutaneous layers of the distal extremities. Cases involving deep soft tissues are rare. The present case represents the third documented case of pulmonary SCH.
Although it is rare, it is important for clinicians, radiologists, surgeons and pathologists to consider the SCH as a possible etiological diagnosis of benign lung tumours. Our case is instructive by its different clinical and radiological presentation compared to the previous two cases. It is necessary to follow up these tumours in order to study the prognostic factors for recurrence.
AUTHOR CONTRIBUTIONS
Hamdi Mariem: Writing– original draft; writing – review & editing; visualization. Sabrine Louhaichi, Agnès Hamzaoui: Writing – review & editing. Emna Braham, Mahdi Abdennadhir, Besma Hamdi, Jamel Ammar, Faouzi El Mezni, Adel Mareghli: Validation; supervision. All authors have read and agreed to the published version of the manuscript.
CONFLICT OF INTEREST
None declared.
ETHICS STATEMENT
The authors declare that appropriate written informed consent was obtained from the patient for publication of this manuscript and accompanying images.
Hamdi M, Braham E, Louhaichi S, Hamdi B, Abdennadher M, Ammar J, et al. Spindle cell hemangioma of the lung: An unusual presentation. Respirology Case Reports. 2022;10:e01057. 10.1002/rcr2.1057
Associate Editor: Tracy Leong
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERNCES
- 1. Gao BQ, Zhou DK, Qian XH, Zhang W, Ying LX, Wang WL. Spindle cell hemangioma of the spleen: a case report. Medicine (Baltimore). 2019;98(9):e14555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Duqing X, Zhaohong W, Gefei W. Multiple spindle cell hemangiomas in both lungs: a rare case report and review of the literature. J Cardiothorac Surg. 2019;14(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fletcher CDM, Beham A, Schmid C. Spindle cell haemangioendothelioma: a clinicopathological and immunohistochemical study indicative of a non‐neoplastic lesion. Histopathology. 1991;18(4):291–301. [DOI] [PubMed] [Google Scholar]
- 4. Marušić Z, Billings SD. Histopathology of spindle cell vascular tumors. Surg Pathol Clin. 2017;10(2):345–66. [DOI] [PubMed] [Google Scholar]
- 5. Perkins P, Weiss SW. Spindle cell hemangioendothelioma. An analysis of 78 cases with reassessment of its pathogenesis and biologic behavior. Am J Surg Pathol. 1996;20(10):1196–204. [DOI] [PubMed] [Google Scholar]
- 6. Oukessou Y, Lyoubi M, Hammouda Y, Rouadi S, Abada RL, Roubal M, et al. Spindle cell hemangioma in the infratemporal fossa: a unique case report. Int J Surg Case Rep. 2021;78:38–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Papez J, Starha J, Zerhau P, Pavlovska D, Jezova M, Jurencak T, et al. Spindle cell hemangioma and atypically localized juxtaglomerular cell tumor in a patient with hereditary BRIP1 mutation: a case report. Genes. 2021;12(2):220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hakozaki M, Tajino T, Watanabe K, Yamada H, Kikuchi S, Hojo H, et al. Intraosseous spindle cell hemangioma of the calcaneus: a case report and review of the literature. Ann Diagn Pathol. 2012;16(5):369–73. [DOI] [PubMed] [Google Scholar]
- 9. Nasser R, Ashayeri K, Legatt AD, Houten JK. Intramedullary spindle cell hemangioma: case report. J Neurosurg Spine. 2016;25(3):379–82. [DOI] [PubMed] [Google Scholar]
- 10. Sapariya BJ, Udhreja PR. Spindle cell hemangioma of thyroid. Indian J Cancer. 2015;52(3):349–50. [DOI] [PubMed] [Google Scholar]
- 11. Boyraz B, Hung YP. Spindle cell tumors of the pleura and the peritoneum: pathologic diagnosis and updates. APMIS Acta Pathol Microbiol Immunol Scand. 2022;130(3):140–54. [DOI] [PubMed] [Google Scholar]
- 12. Nimkar A, Mandel M, Buyuk A, Stavropoulos C, Naaraayan A. Spindle cell hemangioma of the lung: a case report. Cureus. 2022;14(1):e21191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kurek KC, Pansuriya TC, van Ruler MAJH, van den Akker B, Luks VL, Verbeke SLJ, et al. R132C IDH1 mutations are found in spindle cell hemangiomas and not in other vascular tumors or malformations. Am J Pathol. 2013;182(5):1494–500. [DOI] [PubMed] [Google Scholar]
- 14. Cai Y, Wang R, Chen XM, Zhao YF, Sun ZJ, Zhao JH. Maffucci syndrome with the spindle cell hemangiomas in the mucosa of the lower lip: a rare case report and literature review. J Cutan Pathol. 2013;40(7):661–6. [DOI] [PubMed] [Google Scholar]
- 15. Gupta V, Mridha AR, Khaitan BK. Unsatisfactory response to sirolimus in Maffucci syndrome‐associated spindle cell hemangiomas. Dermatol Ther [Internet]. 2019;32(3):e12851. 10.1111/dth.12851 [DOI] [PubMed] [Google Scholar]
- 16. Pansuriya TC, van Eijk R, d'Adamo P, van Ruler MAJH, Kuijjer ML, Oosting J, et al. Somatic mosaic IDH1 and IDH2 mutations are associated with enchondroma and spindle cell hemangioma in Ollier disease and Maffucci syndrome. Nat Genet. 2011;43(12):1256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tosios KI, Gouveris I, Sklavounou A, Koutlas IG. Spindle cell hemangioma (hemangioendothelioma) of the head and neck: case report of an unusual (or underdiagnosed) tumor. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2008;105(2):216–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


