Abstract
Background
Human immunodeficiency virus (HIV) infection during pregnancy is associated with reduced transplacental transfer of maternal antibodies and increased risk of severe infections in children who are exposed and uninfected with HIV. The basis of this reduced transfer of maternal immunity has not yet been defined but could involve modifications in the biophysical features of antibodies. The objective of this study was to assess the impact of maternal HIV infection on the biophysical features of serum IgG and transplacental antibody transfer.
Methods
Maternal serum IgG subclass levels, Fc glycosylation, Fc receptor (FcR) binding, and transplacental transfer of pathogen-specific maternal IgG were measured in pregnant women with HIV (WWH) and pregnant women testing negative for HIV (WNH) in Cape Town, South Africa.
Results
Maternal antibody profiles were strikingly different between pregnant WWH and WNH. Antibody binding to FcγR2a and FcγR2b, IgG1 and IgG3 antibodies, and agalactosylated antibodies were all elevated in WWH, whereas digalactosylated and sialylated antibodies were reduced compared to pregnant WNH. Antibody features that were elevated in WWH were also correlated with reduced transplacental transfer of vaccine antigen-specific antibodies.
Conclusions
HIV infection is associated with marked alterations of biophysical features of maternal IgG and reduced placental transfer, potentially impairing antimicrobial immunity.
Keywords: IgG, transplacental transfer, HIV infection, Fc receptors, pregnancy
This study demonstrates that maternal HIV infection is associated with major changes in the biophysical features of maternal antibodies that are potentially key to their transfer across the placenta.
While the prevention of mother to child transmission of human immunodeficiency virus (HIV) has greatly improved with the widespread use of antiretroviral therapy, UNAIDS estimates that the number of women of childbearing age with HIV (WWH) has increased from 12.4 million in 2002 to 20.2 million in 2021. Children who are exposed to HIV during gestation and are uninfected (CHEU) face higher levels of morbidity and mortality than HIV-unexposed infants [1, 2]. As of 2018, there are 14.8 million CHEU worldwide, the majority of whom live in sub-Saharan Africa [3], who face increased risk of morbidity and mortality due to such causes as enteric pathogens [4], respiratory infections [5], and neurodevelopmental [6] and growth deficits [7].
Although several factors could contribute to the increased infectious diseases-related morbidity and mortality in CHEU, immune alterations induced by maternal HIV infection are likely to play an important role. In particular, HIV infection during pregnancy is associated with a reduced transplacental transfer of maternal antibodies [8–10]. As young infants are critically dependent on maternal antibodies for the control of infectious pathogens [11], a reduction in the transfer of maternal antibodies across multiple microbial antigen specificities likely contributes to their vulnerability to diverse infectious pathogens [9, 10]. Furthermore, a reduction of maternal antibody transfer limits the benefit of immunization during pregnancy that is currently recommended in many countries to protect young infants against tetanus, influenza, and pertussis. Understanding the mechanism by which maternal HIV infection reduces the transplacental transfer of maternal antibodies is therefore essential to the prevention of infectious disease morbidity and mortality in CHEU worldwide.
Transplacental transfer of maternal immunoglobulin G (IgG) depends on the binding of the crystallizable fragment (Fc) domain to the neonatal Fc receptor (FcRn) expressed by syncytiotrophoblasts [12]. The mechanisms involved in the transport of maternal IgG across additional cellular barriers between maternal and fetal circulation, such as the fetal capillary endothelium [13, 14], is less well established. It has been proposed that other receptors, such as FcγRI, FcγRIIa and IIb, and FcγRIIIa, participate in maternal IgG transport beyond syncytiotrophoblasts [15–18]. Importantly, each of these receptors exhibits binding preferences across human IgG subclasses and Fc glycoforms [19, 20]. Thus, in addition to the well-established ability to modify the effector functions of antibodies, IgG Fc features may also impact the efficiency of transfer of different subpopulations of maternal IgG.
Both pregnancy and HIV infection are associated with important modifications in serum antibody features. Pregnancy increases the proportion of sialylated and galactosylated IgG Fc, isoforms that are associated with a reduced binding to class I Fc receptors and anti-inflammatory responses [21, 22]. Galactosylated maternal IgG are preferentially transferred across the placenta and may thereby contribute to the regulation of inflammatory responses in young infants [23, 24]. On the other hand, HIV infection is associated with polyclonal B-cell activation, resulting in IgG1-biased hypergammaglobulinemia and reduced IgG Fc galactosylation and sialylation, even in the context of effective antiretroviral therapy [25, 26]. HIV infection during pregnancy therefore has the potential to alter the features of maternal IgG Fc and thereby influence transfer to the newborn [27, 28]. In turn, alterations in the features of maternal IgG transferred to the newborn may modify interactions with the infant innate immune system and thereby alter effector functions against pathogens.
In this study, we performed a detailed analysis of maternal IgG features in pregnant WWH as compared to women testing negative for HIV (WNH) and assessed relationships between these features and with the efficiency of transfer of antigen-specific antibodies.
METHODS
Study Population
Serum samples were obtained from a previously described mother-infant study [29] in Khayelitsha, Western Cape Province, South Africa between March 2009 and April 2010. At the time of the study, no vaccines were routinely recommended during pregnancy. Mothers who tested negative for HIV during pregnancy had a rapid HIV test (Abbott Determine HIV-1/2) at enrollment with pretest and posttest counseling to confirm their HIV status. The HIV-exposed infants tested negative via HIV polymerase chain reaction (Amplicor HIV-a DNA kit, version 1.5; Roche Molecular Systems) performed at ages 4 and 16 weeks. Maternal and matching infant venous blood samples were collected at birth. Serum samples remaining after primary analysis to assess levels of Bordetella pertussis toxin (pertussis)-, Haemophilus influenzae B (Hib)-, Streptococcus pneumoniae capsular polysaccharides (pneumococcal)-, and tetanus toxoid (tetanus)-specific antibodies in infant and maternal blood and used to define the placental transfer efficiencies for each specificity. A cohort of maternal serum samples that were collected from 24 WWH and 24 WNH (Table 1), selected randomly from 109 original study participants based on serum sample availability and ensuring balance between groups, was analyzed throughout the present work. Ethical approval for the mother-infant study was granted by the University of Cape Town (382/2008) and the University of Stellenbosch (N08/10/278), South Africa, and the National Health Service Research Ethics Committee, England (07/H0720/178), including for storage of residual serum. Analysis of the stored samples was reviewed and approved by the Dartmouth College Institutional Review Board. All women provided written informed consent.
Table 1.
Cohort Characteristics
| Characteristic | Women With HIV (n = 24) | Women Testing HIV Negative (n = 24) |
|---|---|---|
| Maternal age, y, median (IQR) | 27 (26–31) | 23 (20–27) |
| Primigravidity, n (%) | 5 (20) | 12 (50) |
| Infant delivery by vaginal delivery, n (%) | 24 (100) | 24 (100) |
| Female sex of infant, n (%) | 12 (50) | 13 (54) |
| Birth weight, kg, mean (SD) | 3.22 (0.35) | 3.30 (0.41) |
| CD4 count at delivery, cells/µL, mean (SD) | 493 (278.17) | … |
| Viral load at delivery, copies/mL, median (IQR) | 660 (357–3150) | … |
| CD4 < 200 cells/µL, n (%) | 4 (17) | … |
| Highly active antiretroviral therapy, n (%) | 4 (17) | … |
| Zidovudine in pregnancy, n (%) | 18 (75) | … |
| Nevirapine in pregnancy, n (%) | 23 (95) | … |
| HIV infection diagnosed in this pregnancy, n (%) | 13 (54) | … |
During the study period, the Prevention of Mother to Child Transmission program consisted of dual therapy for pregnant women, with the administration of zidovudine at 28 or more weeks’ gestation, and a single dose of nevirapine. Pregnant women were eligible for highly active antiretroviral treatment if their CD4 T-cell count was lower than 200 cells/µL.
Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range.
IgG Subclass and FcR Binding Characterization
Assessment of the subclass composition and Fc receptor binding profiles of total serum IgG was conducted in an adaptation of a previously described multiplex assay [30]. Briefly, FcR (FcγRI, FcγIIa, FcγRIIb, FcγRIIa, FcRn, FcRL2, and FcRL5) and anti-IgG subclass (IgG1, IgG2, IgG3, and IgG4) antibodies (Supplementary Table 1) were coupled to magnetic carboxylated fluorescent beads (Luminex Corporation). A bead mixture comprised of 500 beads per type (Fc receptor or anti-IgG subclass antibody) per well suspended in assay wash buffer consisting of 1× phosphate buffered saline (PBS) with 0.1% bovine serum albumin, 0.05% Tween 20, was added to serum samples diluted in 1× PBS to a concentration that was in a linear range of detection for each bead set, and incubated overnight at 4°C with orbital shaking (1000 rpm). After primary incubation, beads were washed 5 times with assay wash buffer using an automated plate-washer (biotek 405), then resuspended in assay wash buffer with 0.65 μg/mL of either subclass-specific or total IgG-specific phycoerythrin-conjugated detection reagents and incubated at room temperature for 1 hour on an orbital shaker (1000 rpm). Following a final wash, median fluorescent intensity data were acquired for each bead set on a Luminex array reader (FlexMap 3D, Luminex Xponent 4.2). Intravenous immunoglobulin, serum, or myeloma-derived IgG subclasses, and recombinant VRC01 monoclonal antibody [31] in IgG1, IgG2, IgG3, and IgG4 subclass forms [32] were used as controls.
IgG Fc Glycan Characterization
The relative abundance of major IgG glycan structures was quantified by capillary electrophoresis as previously described [33]. Total IgG was captured from serum with Lambda and Kappa magnetic beads (Merck) for an hour at room temperature with continuous mixing or end-over-end rotation, followed by washing in PBS. From the captured antibody, Fc fragments were released by digestion with 1 µL of IdeZ Protease (New England Biolabs) in a total volume of 20 µL of PBS at 37°C for an hour. Cleaved Fc fragments were deglycosylated and glycans were fluorescently labeled using a GlycanAssure APTS Kit (Thermo Scientific) according to the manufacturer’s instructions. Labeled glycans were loaded onto a 3130 XL genetic analyzer (Applied Biosystems) using a POP7 polymer in a 36-cm capillary. Peaks of 19 substructures were identified, and the relative abundance of a given structure was determined by calculating the area under the curve of each peak divided by the total area of all the peaks for each sample. N-glycan fucosyl, afucosyl, and mannose N-glycan libraries were evaluated in parallel to enable confident identification of the discrete glycan species. Individual glycan peaks on the capillary electrophoresis spectrum and glycan structures are shown in Supplementary Figure 1. For clarity in reporting results, the individual glycoforms were categorized into 6 major groups to define the overall levels of agalactosylated, monogalactosylated, digalactosylated, fucosylated, bisected, and mono- and disialylated glycans.
Data Analysis
Four missing measurements for disialic acid were imputed using K-nearest neighbors as implemented by the scikit-learn [34] default, which imputes missing values by taking the mean of the 5 nearest neighbors without missing values for the measurement in question. Antibody profiles were visualized using the python implementation of the uniform manifold projection (UMAP) dimensionality reduction algorithm [35]. Labels for clusters were generated with the spectral clustering algorithm implementation from scikit-learn. The features driving the clusters in the projection were explored using Welch t test feature-by-feature between clusters. For the volcano plot, fold change was calculated by ratio between cluster means by feature, and P value by Welch t tests. Imputed disialic acid measurements were only included in plots involving UMAP clusters, and not for evaluating glycosylation profiles according to HIV infection status.
RESULTS
Two Distinct Antibody Profiles Among Study Participants
A cohort of 24 pregnant WWH and 24 control WNH were selected from a larger prior study [29]. Characteristics of this cohort are shown in Table 1. Maternal age and pregnancy characteristics were comparable in WWH and WNH. Most WWH received zidovudine and nevirapine during pregnancy, whereas the 4 pregnant women who had a CD4 T-cell count below 200 cells/µL received highly active antiretroviral therapy. HIV replication was only partially suppressed in this study population, as median viral load at delivery was 660 copies/mL.
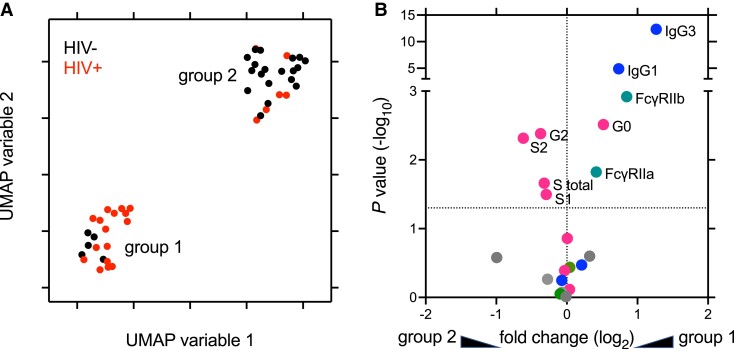
Maternal IgG subclasses, Fc receptor binding, and IgG Fc glycan composition data were analyzed using a UMAP method to determine how maternal antibody profiles of participants related to each other. Samples discriminated into 2 major clusters (Figure 1A), with the majority of samples from WWH in cluster 1 and samples from WNH in cluster 2. This result suggests that the most striking and consistent differences in antibody features across the cohort were highly, but imperfectly, linked to HIV infection status. To determine univariate differences in IgG features between the 2 clusters, statistical significance and fold-changes in means were determined for each feature (Figure 1B), identifying differences in IgG1, IgG3, FcγRIIa and FcγRIIb binding, and agalactosylated, digalactosylated, mono-, and disialylated glycan compositions as associated with UMAP-defined clusters.
Figure 1.
Overall distinctions among maternal serum IgG profiles strongly relate to HIV infection status. A, Unsupervised analysis of subjects based on maternal antibody Fc glycans, IgG subclass prevalence, and FcR-binding profiles. Position in UMAP variable space indicates similarity or distinctions in serum antibody phenotypes. Color indicates subject HIV infection status. Number indicates UMAP cluster group. B, Volcano plot showing the magnitude and statistical confidence (unpaired t test) of differences between subjects according to UMAP-defined subject groups. The dotted horizontal line represents a P value of .05 and dotted vertical line indicates no fold-change. Measurements with nominally significant P values are labeled and colored according to feature type. Glycans, including levels of agalactosylated (G0), monogalactosylated (G1), digalactosylated (G2), fucosylated (F), bisected (B), and mono- (S1) and di- (S2) sialylated glycans are indicated in pink, IgG subclasses in blue, FcγR in green, and other FcR in gray. Abbreviations: FcR, Fc receptor; HIV, human immunodeficiency virus; IgG, immunoglobulin G; UMAP, uniform manifold projection.
Features Associated With UMAP Clustering Significantly Differ Based on HIV Serostatus
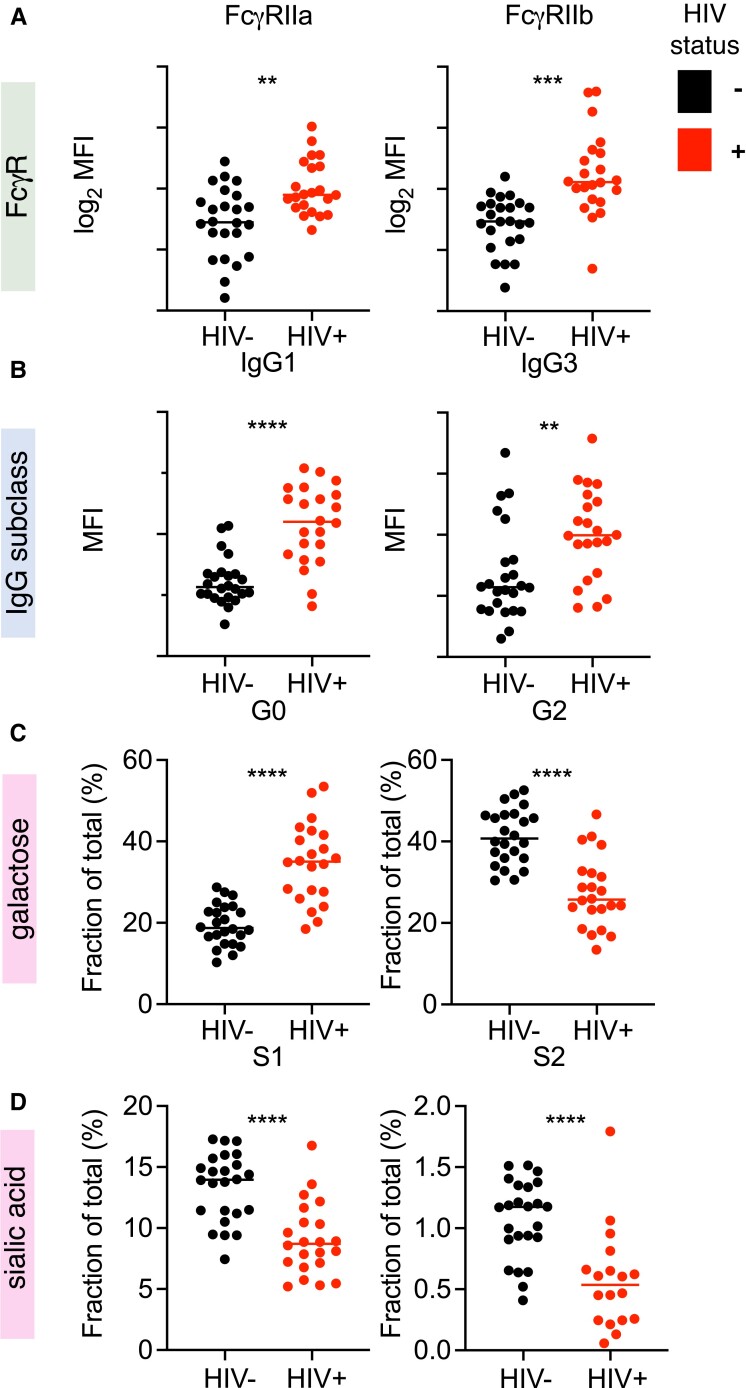
Each IgG feature defined as distinct among UMAP clusters was also distinct between mothers based on HIV status (Figure 2). FcγRIIa and FcγRIIb binding, IgG1, IgG3, and agalactosylated IgG Fcs were elevated in WWH compared to WNH. In contrast, digalactosylated, and mono- and disialylated IgG Fcs were reduced in serum from WWH. The magnitude of these changes was considerable. For example, while 40% of total serum IgG Fc glycans bore no galactose in WWH, 40% of glycans were fully (di) galactosylated in WNH. Similarly, the median levels of sialylated IgG Fc glycans were halved in WWH as compared to WNH. IgG3 levels, which were a major driver of UMAP cluster group, showed a bimodal distribution in both WWH and WNH.
Figure 2.
Maternal HIV infection status is associated with changes in total serum IgG characteristics. Antibody features that were distinct according to UMAP group plotted according to HIV infection status (n = 43–48). Distinct FcγR-binding (A), IgG subclass (B), and IgG Fc galactose (C), and sialic acid (D) content were observed. HIV status is indicated by color. Statistical significance was determined by unpaired t test, unadjusted for multiple comparisons. ** P < .005; *** P < .001; **** P < .0001. MFI is reported for multiplex assay results. Lines indicate group mean. Levels of agalactosylated (G0), digalactosylated (G2), mono- (S1) and di- (S2) sialylated glycans are reported. Abbreviations: FcγR, Fcγ receptor; HIV, human immunodeficiency virus; IgG, immunoglobulin G; MFI, mean fluorescence intensity; UMAP, uniform manifold projection.
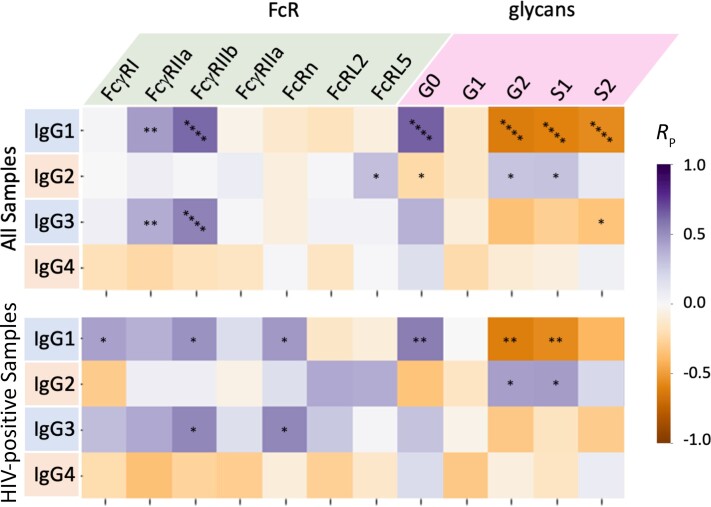
Correlations Between IgG Subclass, Fc Receptor Binding, and IgG Glycosylation Persist Despite HIV Infection
Given distinctions in multiple antibody features between clinical groups, we then investigated correlations between features of serum IgGs (Figure 3). A number of expected relationships were observed; for example, levels of IgG1 and IgG3, subclasses with enhanced ability to interact with FcγR, were positively correlated with FcγR binding, particularly FcγRIIa and FcγRIIb. These receptors are known to be relatively insensitive to the specific Fc glycoform, but highly impacted by subclass. In contrast, strong relationships between subclass and binding to FcγRIIIa, which is highly sensitive to glycoform, were not observed. In terms of relationships with IgG Fc glycoforms, IgG1 and IgG3 subclasses showed inverse correlations with the degree of Fc galactosylation and sialylation. Given that IgG1 is the dominant serum subclass in most individuals, it is likely that the strong correlative relationships between IgG1 levels and Fc glycans reflect glycosylation of the IgG1 pool. Levels of IgG4, a minor component of total IgG, were not strongly correlated with either FcγR binding or IgG glycosylation. The level of serum IgG2 tended to show no significant relationships with FcγR binding and glycan profiles, but correlated with increased FcRL5 binding across the whole cohort, and showed a trend toward a direct association with FcRL2 and FcRL5 binding within WWH. With respect to infection status, total serum IgG binding to FcRL2 and FcRL5 tended to be lower among WWH as compared to WNH, although this trend was not statistically significant (Supplementary Figure 2A). Because relatively little is known about these FcR-like molecules, we further investigated their subclass binding preferences and observed highest binding with serum-derived IgG2 and IgG1 subclasses (Supplementary Figure 2B). Intriguingly, no binding was observed with recombinant subclasses, suggesting a role for posttranslational modifications that differ between in vitro and in vivo expression. While binding to FcRn was moderately well correlated with IgG1 and IgG3 levels in WWH, strong relationships were not observed across the whole cohort.
Figure 3.
Maternal serum Fc glycosylation and FcR binding relate to IgG subclass levels. Heatmaps depicting magnitude and statistical significance of Pearson correlation coefficients (RP) between IgG subclasses and Fc receptor binding and the prevalence of major Fc glycoforms for the whole cohort (upper) and among HIV-positive mothers only (lower). Color indicates correlation coefficient magnitude and direction. *P < .05; **P < .01; ****P < .0001. Relationships with agalactosylated (G0), monogalactosylated (G1), digalactosylated (G2), mono- (S1) and di- (S2) sialylated glycans are reported. Abbreviations: FcR, Fc receptor; HIV, human immunodeficiency virus; IgG, immunoglobulin G.
Overall, relationships among IgG subclass levels, Fc receptor binding propensity, and glycosylation of IgG molecules among WWH were similar to those observed across the cohort. Congruence of these correlative relationships suggest that their covariation may be independent of HIV status, and that correlative relationships between antibody features are not affected by maternal HIV infection.
Placental Transfer Efficiency of Pathogen-Specific IgG Is Associated With Maternal HIV Status and Characteristics of Total Serum IgG
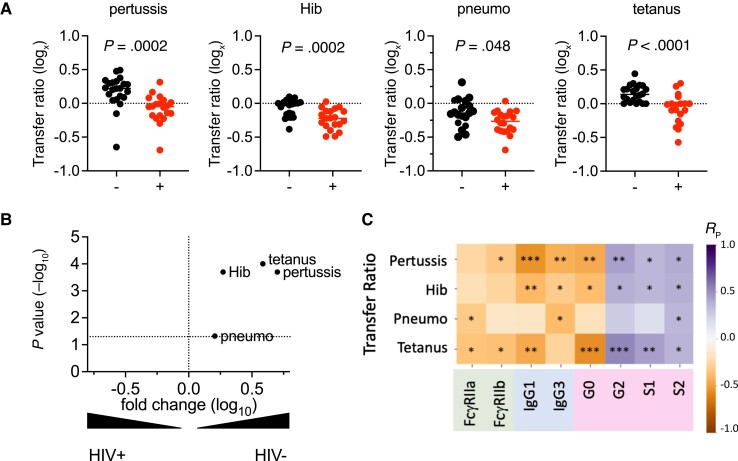
We next investigated how serum antibody profiles related to the efficiency of placental transfer of antigen-specific IgG. Concentrations of antibodies specific to a number of early life pathogens, including Bordetella pertussis toxin (pertussis), Haemophilus influenzae B (Hib), Streptococcus pneumoniae capsular polysaccharides (pneumococcus), and tetanus toxoid (tetanus), as previously measured in maternal and infant blood [29] were analyzed here. As reported for the larger study from which these samples were selected [29], IgG transfer ratios, defined as the relative level in cord versus maternal blood, were significantly lower among WWH for all specificities tested, compared to those among WNH (Figure 4A), with varying differences between the median transfer ratios across pathogens/antigens (Figure 4B). Decreased transfer was consistent for both specificities that were lower among WWH (Hib and pneumococcus) as well as for maternal specificities that did not differ between WLH and WNH (pertussis and tetanus). Similar differences in transfer ratios were observed when analyzing by UMAP group (Supplementary Figure 3A). Transfer efficiency was also observed to vary by antigen specificity, with pertussis- and tetanus-specific antibodies showing higher transfer ratios compared to antibodies recognizing Hib and pneumococcal polysaccharide antigens. These differences between antigen specificities were not linked to maternal vaccination status.
Figure 4.
Transfer of antigen-specific antibodies is associated with maternal HIV status and serum IgG profiles. A, Transfer ratios for pertussis toxoid (pertussis), Haemophilus influenza b (Hib), pneumococcus (pneumo), and tetanus toxin (tetanus) specific antibodies according to HIV infection status. Statistical significance determined by unpaired t test. Dotted line indicates a transfer ratio of 1. B, Volcano plot showing the magnitude and statistical confidence (unpaired t test) of differences in antibody transfer ratios between subjects according to HIV status. The dotted horizontal line represents a P value of .05 and dotted vertical line indicates no fold-change. C, Heatmap depicting magnitude and statistical significance of Pearson correlation coefficients (RP) between transfer ratios and IgG subclasses, Fc receptor binding, and the prevalence of major Fc glycoforms across the cohort. Color indicates correlation coefficient magnitude and direction. *P < .05; **P < .01; ***P < .001. Relationships with agalactosylated (G0), digalactosylated (G2), mono- (S1) and di- (S2) sialylated glycans are reported. Abbreviations: HIV, human immunodeficiency virus; IgG, immunoglobulin G.
To more directly relate transfer efficiency with antibody profiles, we next evaluated correlative relationships between the transfer efficiency of each antigen-specific IgG antibody subset and total serum IgG subclass, FcR binding, and Fc glycans (Figure 4C). While relationships between select individual features and transfer efficiency were strongest and most confident when considering the whole cohort, trends generally held within UMAP subsets (Supplementary Figure 3B). Galactosylated and sialylated antibodies were positively correlated with transfer, whereas agalactosylated antibodies were associated with reduced transfer. In general, features that were elevated in samples from WNH were positively correlated with antigen-specific antibody transfer ratios (digalactosylated, monosialylated, disialylated). Conversely, features that were elevated in serum samples from WWH (FcγR2a, FcγR2b, IgG1, IgG3, agalactosylated) were associated with reduced transfer efficiency.
Collectively, these data establish correlative relationships between features of total maternal serum IgG repertoires with the placental transfer efficiency of multiple antigen-specific antibody subsets. Because these correlative relationships hold across the whole cohort as well as within cohort subgroups, these data suggest that these features relate to antibody transfer independent of the placental disruption associated with HIV infection.
DISCUSSION
Decreased placental IgG transfer, resulting in lower levels of diverse pathogen-specific antibodies, that is associated with maternal HIV infection may contribute to the increased morbidity and mortality observed in CHEU. We sought to identify the molecular characteristics of maternal antibodies that might be associated with the reduced placental transfer efficiency and increased risk that CHEU face in early life. Numerous serum antibody features differed between pregnant WWH compared to WNH. These differences reflected a profile seen in other settings of persistent inflammation, including a dominance of IgG1 and IgG3 subclasses, and antibody Fc domains with reduced levels of galactose and sialic acid. This profile is consistent with previous reports of antibody profiles associated with HIV infection [25, 26]. In contrast, during a healthy pregnancy, the levels of galactosylated and sialylated antibodies typically increase [36–38]. Despite previous demonstration of marginally improved binding to FcγR and increased effector function [20], it is believed that these increases may promote an anti-inflammatory environment in the mother and infant [39, 40]. While pregnancy can alter an inflammatory phenotype in those with rheumatoid arthritis [21, 41], our data suggest that its impact on the inflammatory prolife associated with HIV infection is limited, and that the humoral immune system of WWH may not adapt to pregnancy in the same ways as in WNH. Importantly, beyond the reduction in transport across the placenta, changes in maternal serum IgG glycosylation state associated with maternal HIV infection are likely to modify their interactions infant innate immune cells and may thereby alter maternal antibody-dependent immunity to pathogens [20, 42].
Additionally, while the role of FcRn in transplacental antibody transfer is well established, its impact on speciation of transferred antibodies remains controversial. An effect of glycosylation profile on FcRn binding and/or placental transfer has been suggested from some [27, 40, 43–45] but not other [46, 47] studies. When glycoprofile-associated differences have been observed, they have suggested that galactose contributes positively to FcRn binding and transfer. Indeed, these observations, as well as the possible involvement of other IgG receptors in transfer, raise the possibility that rather than being driven by hypergammaglobulinemia, the reduced galactosylation of antibodies observed in WWH may play a more direct role in reduced transfer efficiency. Relevant to this possibility, saturation of FcRn has been proposed as a candidate mechanism whereby hypergammaglobulinemia reduces the transfer of antibodies across the placenta. However, correlative relationships between placental FcRn expression levels at delivery and antibody transfer have not been observed [48]. Furthermore, while saturation might account for lower efficiency of transfer (lower infant/cord levels relative to the mother’s elevated level), it is unclear how it could result in lower absolute levels of IgG.
On the other hand, the differing efficiency of transfer of IgG subclasses observed in maternal-infant studies, with highest transfer of IgG1 and lowest transfer of IgG4, [48] shows somewhat better agreement with relative binding affinity for FcγR than for FcRn. Thus, present knowledge of processes and receptors that affect maternal antibody transfer may well be incomplete. Indeed, there is considerable complexity to the cellular boundaries between the maternal and fetal circulation. Multiple Fc receptors are expressed in the syncytiotrophoblast, placental macrophages, stromal cells, and fetal endothelium [15–17], and each may play a role. Among the activating (FcγRI, FcγRIIa, FcγRIIIa, FcRL2, FcRL5) and inhibitory (FcγRIIb, FcRL2, FcRL5) Fc receptors evaluated here, relatively little is known about the role of FcRL2 and FcRL5. These 2 receptors were included in this study based on their observation in a proteomic study of placental villous tissue samples (Proteomics Identifications Archive PXD009825 [49]). Although FcRL2 and FcRL5 expression has been mainly described in memory B cells, B cells are not present in placental villous tissue, suggesting that they are expressed by other placental cells. FcRL2 and FcRL5 have both immunoreceptor tyrosine-based activation and inhibition motifs, which could allow them to preferentially up- or downregulate immune and inflammatory signaling, in addition to modulating transplacental transfer. Coupled to the variable transfer efficiency of antibodies with different antigen and pathogen specificities, it is clear that many unresolved questions remain as to mechanisms of transfer.
Antibody features elevated in serum from WWH were associated with reduced placental transfer of antigen-specific antibodies. Correlative relationships generally existed not only between groups but within them, suggesting that they were not driven by HIV infection status. Interestingly, while unsupervised IgG profile analysis clearly differentiated primarily according to HIV status, there were a few WNH whose profiles were most similar to those seen in the context of HIV infection, and a few WWH whose antibodies appeared indistinguishable from WNH. In greater numbers, these cases could represent an opportunity to better resolve the effects of HIV infection and its associated antibody profile changes on transplacental antibody transfer. This work supports the notion that correcting the alterations of antibody features associated with HIV infection could improve the transfer of protective antibodies to infants. Correcting these alterations also has the potential to normalize the features of antibodies transferred to the newborn and their interactions with the immune system in early life. Combined antiretroviral treatment was associated with improved maternal antibody transfer and reduced risk of severe infections in CHEU when initiated before pregnancy [50]. Future studies should determine whether the positive impact of combined antiretroviral treatment of WWH involves a correction of the alterations in maternal antibody features identified in this study.
In conclusion, this study demonstrates that maternal HIV infection is associated with major changes in the biophysical features of maternal antibodies that are potentially key to their transfer across the placenta. Beyond antibody levels, the quality of maternal antibodies appears to be an important determinant of the transfer of maternal immunity to the newborn.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Supplementary Material
Contributor Information
Sean A Taylor, Thayer School of Engineering, Dartmouth College, Hanover, New Hampshire, USA.
Shilpee Sharma, Institute for Medical Immunology, Université Libre de Bruxelles, Brussels, Belgium.
Christopher A L Remmel, Thayer School of Engineering, Dartmouth College, Hanover, New Hampshire, USA.
Beth Holder, Institute of Reproductive and Developmental Biology, Department of Metabolism, Digestion and Reproduction, Imperial College, London, United Kingdom.
Christine E Jones, Faculty of Medicine and Institute for Life Sciences, University of Southampton, Southampton, UK; NIHR Southampton Clinical Research Facility, Southampton, UK; NIHR Southampton Biomedical Research Centre, University Hospital Southampton NHS Foundation Trust, Southampton, UK.
Arnaud Marchant, Institute for Medical Immunology, Université Libre de Bruxelles, Brussels, Belgium.
Margaret E Ackerman, Thayer School of Engineering, Dartmouth College, Hanover, New Hampshire, USA.
Notes
Acknowledgments. The authors thank the mothers and their infants who participated in this study and who allowed us to store remaining samples for further analysis. We thank Beate Kampmann and Anneke Hesseling who were instrumental in the mother-infant study and Rohan Lewis for his contribution to the development of the experiments.
Author contributions. C. E. J. and B. H. contributed to conceptualization and securing funding. S. A. T. and S. S. performed experimental work. B. H., C. E. J., R. L., A. M., and M. E. A. advised on development of the experiments. S. A. T. and M. E. A. wrote the first draft of the manuscript. S. A. T., S. S., and C. A. L. R. performed data analysis. All authors contributed to manuscript revisions and approved the final version.
Financial support. This work was supported by National Institute of Allergy and Infectious Diseases (grant number U19AI145825); and Medical Research Council and Biotechnology and Biological Sciences Research Council (Global Challenges Research Fund Networks in Vaccines Research, Immunising Pregnant Women and Infants Network [IMPRINT]).
References
- 1. Arikawa S, Rollins N, Newell ML, Becquet R. Mortality risk and associated factors in HIV-exposed, uninfected children. Trop Med Int Health 2016; 21:720–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brennan AT, Bonawitz R, Gill CJ, et al. A meta-analysis assessing all-cause mortality in HIV-exposed uninfected compared with HIV-unexposed uninfected infants and children. AIDS 2016; 30:2351–60. [DOI] [PubMed] [Google Scholar]
- 3. Slogrove AL, Powis KM, Johnson LF, Stover J, Mahy M. Estimates of the global population of children who are HIV-exposed and uninfected, 2000–18: a modelling study. Lancet Glob Health 2020; 8:e67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Slogrove AL, Esser MM, Cotton MF, et al. A prospective cohort study of common childhood infections in South African HIV-exposed uninfected and HIV-unexposed infants. Pediatr Infect Dis J 2017; 36:e38– 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weinberg A, Mussi-Pinhata MM, Yu Q, et al. Excess respiratory viral infections and low antibody responses among HIV-exposed, uninfected infants. AIDS 2017; 31:669–79. [DOI] [PubMed] [Google Scholar]
- 6. Kerr SJ, Puthanakit T, Vibol U, et al. Neurodevelopmental outcomes in HIV-exposed-uninfected children versus those not exposed to HIV. AIDS Care 2014; 26:1327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Malaba TR, Phillips T, Le Roux S, et al. Antiretroviral therapy use during pregnancy and adverse birth outcomes in South African women. Int J Epidemiol 2017; 46:1678–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Moraes-Pinto MI, Almeida AC, Kenj G, et al. Placental transfer and maternally acquired neonatal IgG immunity in human immunodeficiency virus infection. J Infect Dis 1996; 173:1077–84. [DOI] [PubMed] [Google Scholar]
- 9. Evans C, Jones CE, Prendergast AJ. HIV-exposed, uninfected infants: new global challenges in the era of paediatric HIV elimination. Lancet Infect Dis 2016; 16:e92– 107. [DOI] [PubMed] [Google Scholar]
- 10. Abu-Raya B, Smolen KK, Willems F, Kollmann TR, Marchant A. Transfer of maternal antimicrobial immunity to HIV-exposed uninfected newborns. Front Immunol 2016; 7:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jennewein MF, Abu-Raya B, Jiang Y, Alter G, Marchant A. Transfer of maternal immunity and programming of the newborn immune system. Semin Immunopathol 2017; 39:605–13. [DOI] [PubMed] [Google Scholar]
- 12. Leach JL, Sedmak DD, Osborne JM, Rahill B, Lairmore MD, Anderson CL. Isolation from human placenta of the IgG transporter, FcRn, and localization to the syncytiotrophoblast: implications for maternal-fetal antibody transport. J Immunol 1996; 157:3317–22. [PubMed] [Google Scholar]
- 13. Antohe F, Radulescu L, Gafencu A, Ghetie V, Simionescu M. Expression of functionally active FcRn and the differentiated bidirectional transport of IgG in human placental endothelial cells. Hum Immunol 2001; 62:93–105. [DOI] [PubMed] [Google Scholar]
- 14. Kiskova T, Mytsko Y, Schepelmann M, et al. Expression of the neonatal Fc-receptor in placental-fetal endothelium and in cells of the placental immune system. Placenta 2019; 78:36–43. [DOI] [PubMed] [Google Scholar]
- 15. Kristoffersen EK. Human placental Fc gamma-binding proteins in the maternofetal transfer of IgG. APMIS Suppl 1996; 64:5–36. [DOI] [PubMed] [Google Scholar]
- 16. Bright NA, Ockleford CD, Anwar M. Ontogeny and distribution of Fc gamma receptors in the human placenta. Transport or immune surveillance? J Anat 1994; 184:297–308. [PMC free article] [PubMed] [Google Scholar]
- 17. Lyden TW, Robinson JM, Tridandapani S, et al. The Fc receptor for IgG expressed in the villus endothelium of human placenta is Fc gamma RIIb2. J Immunol 2001; 166:3882–9. [DOI] [PubMed] [Google Scholar]
- 18. Martinez DR, Fouda GG, Peng X, Ackerman ME, Permar SR. Noncanonical placental Fc receptors: what is their role in modulating transplacental transfer of maternal IgG? PLoS Pathog 2018; 14:e1007161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bruhns P, Iannascoli B, England P, et al. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood 2009; 113:3716–25. [DOI] [PubMed] [Google Scholar]
- 20. Dekkers G, Treffers L, Plomp R, et al. Decoding the human immunoglobulin G-glycan repertoire reveals a spectrum of Fc-receptor- and complement-mediated-effector activities. Front Immunol 2017; 8:877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van de Geijn FE, Wuhrer M, Selman MH, et al. Immunoglobulin G galactosylation and sialylation are associated with pregnancy-induced improvement of rheumatoid arthritis and the postpartum flare: results from a large prospective cohort study. Arthritis Res Ther 2009; 11:R193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pincetic A, Bournazos S, DiLillo DJ, et al. Type I and type II Fc receptors regulate innate and adaptive immunity. Nat Immunol 2014; 15:707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Anthony RM, Wermeling F, Ravetch JV. Novel roles for the IgG Fc glycan. Ann N Y Acad Sci 2012; 1253:170–80. [DOI] [PubMed] [Google Scholar]
- 24. Karsten CM, Pandey MK, Figge J, et al. Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcgammaRIIB and dectin-1. Nat Med 2012; 18:1401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moore JS, Wu X, Kulhavy R, et al. Increased levels of galactose-deficient IgG in sera of HIV-1-infected individuals. AIDS 2005; 19:381–9. [DOI] [PubMed] [Google Scholar]
- 26. Ackerman ME, Crispin M, Yu X, et al. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J Clin Invest 2013; 123:2183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jennewein MF, Goldfarb I, Dolatshahi S, et al. Fc glycan-mediated regulation of placental antibody transfer. Cell 2019; 178:202–15.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Martinez DR, Fong Y, Li SH, et al. Fc characteristics mediate selective placental transfer of IgG in HIV-infected women. Cell 2019; 178:190–201.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jones CE, Naidoo S, De Beer C, Esser M, Kampmann B, Hesseling AC. Maternal HIV infection and antibody responses against vaccine-preventable diseases in uninfected infants. JAMA 2011; 305:576–84. [DOI] [PubMed] [Google Scholar]
- 30. Boesch AW, Brown EP, Cheng HD, et al. Highly parallel characterization of IgG Fc binding interactions. MAbs 2014; 6:915–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wu X, Yang ZY, Li Y, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 2010; 329:856–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boesch AW, Osei-Owusu NY, Crowley AR, et al. Biophysical and functional characterization of rhesus macaque IgG subclasses. Front Immunol 2016; 7:589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mahan AE, Tedesco J, Dionne K, et al. A method for high-throughput, sensitive analysis of IgG Fc and Fab glycosylation by capillary electrophoresis. J Immunol Methods 2015; 417:34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pedrogosa F, Varoquaux G, Gramfort A, et al. Scikit-learn: machine learning in python. J Mach Learn Res 2011; 12:2825–30. [Google Scholar]
- 35. McInnes L, Healy J, Melville J. UMAP: uniform manifold approximation and projection for dimension reduction. arXiv:1802.03426v3; 2020.
- 36. Selman MH, Derks RJ, Bondt A, et al. Fc specific IgG glycosylation profiling by robust nano-reverse phase HPLC-MS using a sheath-flow ESI sprayer interface. J Proteomics 2012; 75:1318–29. [DOI] [PubMed] [Google Scholar]
- 37. Bondt A, Rombouts Y, Selman MH, et al. Immunoglobulin G (IgG) Fab glycosylation analysis using a new mass spectrometric high-throughput profiling method reveals pregnancy-associated changes. Mol Cell Proteomics 2014; 13:3029–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Twisselmann N, Bartsch YC, Pagel J, et al. IgG Fc glycosylation patterns of preterm infants differ with gestational age. Front Immunol 2018; 9:3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. de Haan N, Reiding KR, Driessen G, van der Burg M, Wuhrer M. Changes in healthy human IgG Fc-glycosylation after birth and during early childhood. J Proteome Res 2016; 15:1853–61. [DOI] [PubMed] [Google Scholar]
- 40. Jansen BC, Bondt A, Reiding KR, Scherjon SA, Vidarsson G, Wuhrer M. MALDI-TOF-MS reveals differential N-linked plasma- and IgG-glycosylation profiles between mothers and their newborns. Sci Rep 2016; 6:34001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bondt A, Selman MH, Deelder AM, et al. Association between galactosylation of immunoglobulin G and improvement of rheumatoid arthritis during pregnancy is independent of sialylation. J Proteome Res 2013; 12:4522–31. [DOI] [PubMed] [Google Scholar]
- 42. Subedi GP, Barb AW. The immunoglobulin G1 N-glycan composition affects binding to each low affinity Fc gamma receptor. MAbs 2016; 8:1512–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Williams PJ, Arkwright PD, Rudd P, et al. Short communication: selective placental transport of maternal IgG to the fetus. Placenta 1995; 16:749–56. [DOI] [PubMed] [Google Scholar]
- 44. Kibe T, Fujimoto S, Ishida C HT, et al. Glycosylation and placental transport of immunoglobulin G. J Clin Biochem and Nutr 1996; 21:57–63. [Google Scholar]
- 45. Dashivets T, Thomann M, Rueger P, Knaupp A, Buchner J, Schlothauer T. Multi-angle effector function analysis of human monoclonal IgG glycovariants. PLoS One 2015; 10:e0143520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Borghi S, Bournazos S, Thulin NK, et al. FcRn, but not FcgammaRs, drives maternal-fetal transplacental transport of human IgG antibodies. Proc Natl Acad Sci USA 2020; 117:12943–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Einarsdottir HK, Selman MH, Kapur R, et al. Comparison of the Fc glycosylation of fetal and maternal immunoglobulin G. Glycoconj J 2013; 30:147–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Clements T, Rice TF, Vamvakas G, et al. Update on transplacental transfer of IgG subclasses: impact of maternal and fetal factors. Front Immunol 2020; 11:1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lofthouse EM, Torrens C, Manousopoulou A, et al. Ursodeoxycholic acid inhibits uptake and vasoconstrictor effects of taurocholate in human placenta. FASEB J 2019; 33:8211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Goetghebuer T, Smolen KK, Adler C, et al. Initiation of antiretroviral therapy before pregnancy reduces the risk of infection-related hospitalization in human immunodeficiency virus-exposed uninfected infants born in a high-income country. Clin Infect Dis 2019; 68:1193–203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.