Abstract
This non-interventional, observational, longitudinal study describes treatment patterns of atopic dermatitis (AD) in Sweden. Data from 3 Swedish registries were merged, and included patients who received an AD diagnosis (during the period 1997 to 2019) and had AD treatment prescribed (during the period 2006 to 2020). Treatment persistence, treatment sequencing, time-to-event analysis, and 12-month prevalence were analysed. Overall, data for 99,885 patients with AD were included, of whom 4,086 (4.1%) received systemic treatments. Median persistence rates were 12.6 (95% CI 11.9, 13.4) months for methotrexate, 10.8 (9.1, 13.0) months for azathioprine, 5.6 (3.8, 6.2) months for mycophenolate, 5.1 (4.4, 5.7) months for alitretinoin and 3.4 (3.2, 3.7) months for cyclosporine. Median (Q1, Q3) time from first secondary care visit for AD to first systemic treatment was 5.8 (2.2, 11.0) years overall and 4.4 (1.3, 9.1) years in the Stockholm region. Methotrexate was a prominent first- and second-line treatment used during the period 2006 to 2020. Dupilumab was introduced during the study period and was increasingly used as first- or second-line therapy over time. The 12-month prevalence of AD generally remained steady, with a gradual increase observed over time for the overall population. A steep increase was observed in Stockholm from 2011. This study shows that a small proportion of patients with AD are offered systemic treatments in Sweden, with long periods in secondary care prior to systemic treatments and low persistence on systemic treatments. Regional differences highlight a need for national treat ment guidelines.
Key words: atopic dermatitis, atopic eczema, registry, epidemiology, dispensed prescription, treatment patterns
Atopic dermatitis (AD) is a chronic relapsing inflammatory skin disease, which is commonly associated with other atopic manifestations, such as asthma, allergic rhinitis, or food allergy (1). AD is the most common skin disease in children, and its incidence has increased 2- to 3-fold in industrialized nations in the 21st century, affecting up to 25% of children and up to 18% of adults worldwide (2–5).
SIGNIFICANCE
Swedish registry data showed that the use and persistence of systemic treatments among patients with atopic dermatitis has generally been low, and that there is, even now, no clear treatment pattern for patients on systemic treatment. Methotrexate dispensations increased from 2006 to 2020, and the novel treatment dupilumab, which was used predominantly as a third- or fourth-line treatment early in the study, was increasingly used as a first- or second-line treatment over time. Overall, the findings imply sub-optimal treatment strategies for atopic dermatitis in Sweden, despite the current trend towards introduction of new and effective treatments. This study highlights the need for good management practices, including encouragement of treatment adherence, as well as the need for further treatment options.
European guidelines recommend using emollients and topical agents to treat mild and moderate AD, and suggest adding systemic treatments for severe AD, including the introduction of a biological therapy for severe disease in adults and children (6). In addition, narrowband ultraviolet (UV)B or medium-dose UVA may be used as a concomitant treatment across all severities of AD (6). Dupilumab was the first biological approved as first-line treatment for moderate-to-severe AD in the USA (2017) (7) and in Europe (2017) (8). In Sweden, dupilumab was approved in 2018 for the treatment of patients with severe AD who lack other treatment options due to lack of effect with conventional treatments (9). A survey of dermatologists from Nordic countries indicated that dupilumab tends to be introduced in sequence after a mean of 2 other therapies have been tried (10). At the time of this study several novel treatments for AD were being investigated in clinical trials, or were awaiting approval and/or inclusion in the European guidelines, including Janus kinase inhibitors and several treatments targeting different cytokines involved in the pathogenesis of AD (11, 12).
There is a degree of uncertainty among primary care clinicians as to how to manage AD, possibly due to lack of specialized dermatological knowledge, national guidelines, or familiarity with European guidelines. The lack of Swedish national guidelines for AD leads to the possibility that healthcare decisions are influenced at a secondary healthcare level by local or regional factors rather than national recommendations. Similarly, it is known that, in psoriasis, treatment patterns vary by region (13), and this has also been seen in studies in other countries. Evaluation of the treatment patterns of patients with AD in Sweden, especially in those with more severe disease, will provide insights into how AD is currently being managed and how much variation there is between regions.
Treatment patterns for AD in other countries have been assessed and indicate a lack of consistency in the treatment of AD compared with European guidelines. In Denmark, for example, the most frequently prescribed systemic therapies in the months immediately prior to the first hospital dermatologist visit (during admission or as an outpatient) for AD were systemic corticosteroids and dicloxacillin, although this was only assessed in the period between 2005 and 2012, which is a limitation of these findings (14). Approximately one-third of patients were prescribed a potent or moderately potent topical corticosteroid when referred for hospital-based treatment, and very few patients were treated with systemic therapies. In a UK study using data from The Health Improvement Network, patients with an index diagnosis (first systemic immunosuppressant prescription) between 2007 and 2009 were studied. Most were prescribed topical corticosteroids and/or emollients (15). Comparison of immunosuppressant exposure (> 75% vs ≤ 75%) revealed a significant association between greater exposure (with mycophenolate mofetil and methotrexate in particular) and ≥1 oral corticosteroid prescription (15). In a US study, real-world immunosuppressant utilization patterns were explored using data for patients diagnosed with AD with commercial or Medicare Supplemental insurance between 2010 and 2015, with (immunosuppressant group) or without (controls) a claim for systemic immunosuppressant (16). During the 12-month follow-up period, 69% of patients in the immunosuppressant group were non-persistent. Only 12% resuming immunosuppressant therapy after the treatment gap, and 85% received concomitant treatment, predominantly oral or systemic corticosteroids. The incidence of immunosuppressant-related hospitalizations was significantly higher for that group than for their control counterparts, demonstrating substantial treatment burden (16).
Treatment patterns may be influenced by patient and clinician perceptions of the current severity of the condition. This may be a consequence of the fact that patients, despite continued disease burden, become used to their condition throughout their life and do not necessarily consider their AD to be a severe disease at that time. In the US, a higher proportion of patients rated their AD as less severe than did their treating clinician (17). In a qualitative semi-structured interview study, young adults suggested that, despite their AD being a constant in their lives, most had become used to living with the condition (18). In addition, patients, despite having experience of AD since childhood, may nevertheless feel uncertainty about options for treatment of the disease.
The primary objective of this study was to present real-world evidence (RWE) regarding treatment patterns of AD in Sweden, with emphasis on treatment persistence and treatment sequencing. Secondary objectives included the generation of RWE on the prevalence of AD in secondary healthcare in Sweden.
MATERIALS AND METHODS
Study design
This was a retrospective, non-interventional, observational, and longitudinal analysis of Swedish patients with AD, using individual-level data from 3 Swedish national administrative registries (the National Patient Register (NPR), the Swedish Prescribed Drug Register (SPDR) and the Cause of Death (COD) register), which were merged into a single dataset.
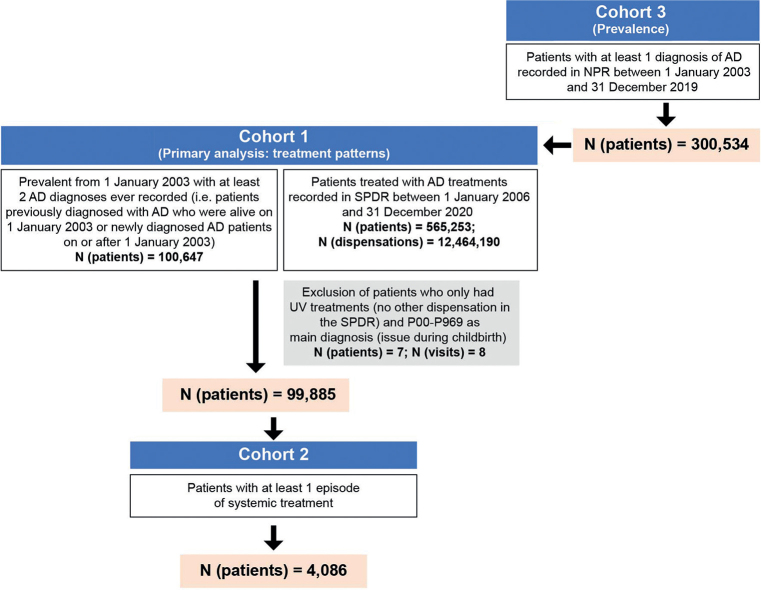
A total of 300,534 patients with AD as primary or secondary diagnosis for a secondary care visit in Sweden during 1 January 2003 to 31 December 2019 were identified and referred to as cohort 3 (Fig. 1 and Appendix S1; SFig. 1). This population was used to estimate the 12-month prevalence of AD. As the collection of data into the outpatient register (NPR) started in 2001, patients were followed from January 2003 to avoid a steep increase in the prevalence of AD in the earlier years of the study period.
Fig. 1.
Patient flow for cohort 1 for the treatment pattern analysis, cohort 2 for analysis of patients with an episode of systemic treatment, and cohort 3 for analysis of prevalence. AD: atopic dermatitis; NPR: National Patient Register; SDPR: Swedish Prescribed Drug Register.
To select a population with higher ascertainment of AD and active treatment, patients with ≥ 2 AD diagnoses as a main diagnosis recorded in the NPR that were prevalent on 1 January 2003 (i.e. previously diagnosed patients with AD who were alive on 1 January 2003 or newly diagnosed patients with AD on or after 1 January 2003) and AD treatment dispensed, as recorded in the SPDR between 1 January 2006 and 31 December 2020, were selected (Appendix S1; SFig. 1). In total, 99,885 patients with these criteria were identified and referred to as cohort 1 (Fig. 1). Furthermore, 4,086 patients with at least 1 dispensation of systemic treatment specifically were selected and referred to as cohort 2. In cohorts 1 and 2, patients were followed from January 2006, since this is when the first full year of data in the SPDR is available. However, the AD diagnosis could be much earlier, as the data capture for the NPR in this study started as early as January 1997.
This study was designed, implemented and reported in accordance with the Guidelines for Good Pharmacoepidemiology Practice (GPP) of the International Society for Pharmacoepidemiology (19), the S trengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (20), and the principles of the Declaration of Helsinki (21).
Primary treatment patterns analyses
Analyses of treatment patterns were conducted, and included an estimation of treatment persistence, descriptive analysis of treatment sequencing and time-to-event analysis. The duration of treatment persistence was defined as the time from the first dispensation of treatment to discontinuation. Patients were considered persistent with a treatment if the gap between dispensations (i.e. from the end of supply plus days supplied of the former dispensation to the dispensation date of the next) was less than the “grace period” (Appendix S1). Grace periods of 180 days (topical treatments, methotrexate, and azathioprine) and 60 days (all other treatments) were used to define drug persistence. An individual patient might have several treatments dispensed with varying durations of the supply period for a given drug, such as if they discontinued therapy for longer than the grace period then re-started treatment, or if they switched therapy. Treatment episodes were not determined for systemic corticosteroids and systemic antibiotics, since these were considered as on-demand treatments.
Treatment sequencing was determined following initiation of first systemic treatments and after the first AD visit in secondary care, with comparisons made at different time intervals (2006–2009, 2010–2014 and 2015–2020; starting on 1 January with the range end up to 31 December in each instance). The time interval cohorts were created based on when the first ever systemic treatment was dispensed. Time-to-event analysis assessed the time from the first secondary care visit for AD to the first systemic treatment, and time to switch to another systemic treatment. Time to switch was calculated as the time from starting 1 systemic treatment to starting another systemic treatment, regardless of treatment sequence.
Secondary analyses
The 12-month prevalence of AD in secondary care in Sweden was estimated (Appendix S1).
Statistical analyses
Descriptive statistics were used for analyses of treatment patterns and 12-month prevalence. In addition, several endpoints were analysed by subgroups, based on treatment, age group, and region: data for treatment persistence and time to switch to another systemic treatment were stratified by treatment and region; prevalence data were stratified by age group and region. The 4 age groups for age stratification (22) were infants (< 2 years of age), children (2–11 years), adolescents (12–17 years) and adults (≥ 18 years). The 4 regions for geographical stratification were “Stockholm”, “south”, “west” and “other”; see further detail in Appendix S1; STable I.
Drug persistence was estimated using Kaplan–Meier methodology (Appendix S1). Patients with missing defined daily dose of the package were excluded from analysis (except for alitretinoin, for which missing data could be imputed manually based on the available dosage information). Treatment episodes were not created for on-demand treatments, which included systemic corticosteroids, topical antibiotics, oral antibiotics, antihistamines, and ultraviolet (UV) light therapy. Treatment episodes (i.e. an event that relates to a change in treatment status) exceeding end of follow-up, which was set as 1 November 2020 (31 December 2020 to data cut, minus 60 days) for the 60-day grace period and 4 July 2020 (31 December 2020 to data cut, minus 180 days) for the 180-day grace period, were marked as censored, and the censor date was set as the end date of the treatment episode. Treatment episodes initiated after the end of follow-up were also included in the analysis as censored and with the censor date set as the end of treatment episode. Patients were also censored at the date of death.
RESULTS
Study population
Demographics and disease characteristics for the populations are provided as Appendix S1; STable II. In brief, the 3 cohorts were well balanced with respect to sex, with a higher proportion of females than males in all cohorts. However, while cohorts 1 and 3 had a similar age at diagnosis (median, 7 years for both), patients treated with systemics (cohort 2) were much older (median 32 years).
Distribution of treatments
There were 4,086 (4.1%) patients with ≥1 episode of systemic treatment (cohort 2) who had a total of 8,816 systemic treatment episodes. For systemic treatments, the most frequently used drug was methotrexate (received by 2,608 patients, with 3,621 episodes recorded), followed by cyclosporine (used by 961 patients, with 2,120 episodes) (Table I). Dupilumab was used by 607 patients for 696 treatment episodes. Dispensed emollients and corticosteroids were the most frequently used topical treatments of patients with ≥1 episode of systemic treatment, with each being received by approximately 4,000 patients.
Table I.
Distribution of patients and treatment episodes for patients in cohort 2
| Treatment | Treatment episodes, n |
|
|---|---|---|
| Patientsa | Treatment episodes | |
| Systemic treatment | ||
| Alitretinoin | 218 | 285 |
| Azathioprine | 783 | 1,187 |
| Cyclosporine | 961 | 2,120 |
| Dupilumab | 607 | 696 |
| Methotrexate | 2,608 | 3,621 |
| Mycophenolate mofetil | 184 | 907 |
| Topical agents for dermatitis | ||
| Dispensed emollients | 3,891 | 9,877 |
| Crisaborole | – | – |
| Pimecrolimus | 713 | 919 |
| Tacrolimus | 2,387 | 4,705 |
| Topical corticosteroids | 4,002 | 12,180 |
Patients may have had a systemic treatment episode with >1 systemic treatment with patient numbers reflecting each occurrence.
Treatment patterns in Sweden for patients with atopic dermatitis
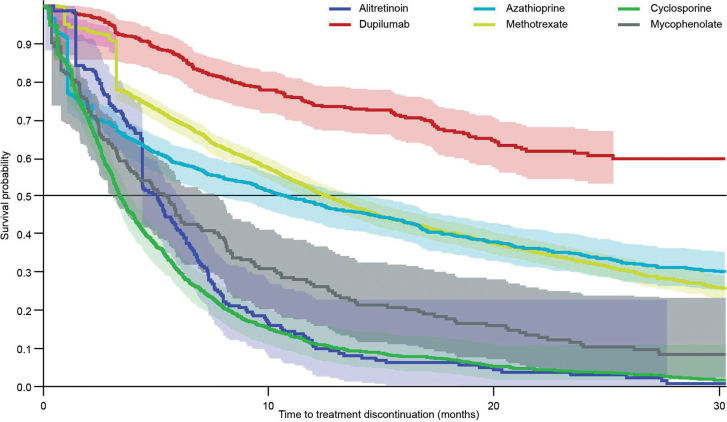
Methotrexate, a commonly used systemic treatment, had a median treatment persistence of 12.6 (95% CI 11.9, 13.4) months (Table II). In contrast, median treatment persistence with cyclosporine was 3.4 (3.2, 3.7) months. As less than 50% of patients discontinued dupilumab treatment before the end of the study period, the median treatment persistence could not be estimated. Drug persistence for all systemic treatments is shown in Fig. 2. Median treatment persistence for dispensed emollients and corticosteroids was ˜18 months for patients who received systemic treatment (Table II). Variation in persistence for systemic treatments was observed across the different regions, although there was very little difference observed in use of topical treatments across regions (Table III). When stratified by age, an obvious variation in persistence on azathioprine was observed, with little difference observed with methotrexate across age groups (Table IV). There was very little difference in persistence for each stratified age group for dispensed emollients and corticosteroids, except for the infant subgroup, which showed a trend towards longer persistence with dispensed emollients.
Table II.
Treatment persistence of systemic and topical treatments for atopic dermatitis in the overall population of cohort 2
| Treatment | Patients, n | Events, n (discontinuation) | Treatment persistence |
|
|---|---|---|---|---|
| Median (months) | 95% CI | |||
| Systemic treatments | ||||
| Alitretinoin | 218 | 197 | 5.1 | 4.4–5.7 |
| Azathioprine | 783 | 647 | 10.8 | 9.1–13.0 |
| Cyclosporine | 961 | 921 | 3.4 | 3.2–3.7 |
| Dupilumab | 607 | 162 | NRa | – |
| Methotrexate | 2,608 | 2,008 | 12.6 | 11.9–13.4 |
| Mycophenolate | 184 | 184 | 5.6 | 3.8–6.2 |
| Topical treatments | ||||
| Dispensed emollients | 3,891 | 3,298 | 18.1 | 17.6–19.4 |
| Pimecrolimus | 713 | 594 | 7.0 | 5.9–7.8 |
| Tacrolimus | 2,387 | 2,210 | 7.7 | 7.1–8.3 |
| Topical corticosteroids | 4,002 | 3,576 | 17.9 | 17.4–19.0 |
Duration of treatment persistence was defined as the time from the first dispensation of treatment to discontinuation (discontin.). Patients were considered persistent with a treatment if the gap between dispensations (i.e. from the end of supply plus days supplied of the former dispensation to the dispensation date of the next) was less than the “grace period” (Appendix S1). A discontinuation was recorded if the patient switched to another drug or had no treatment dispensed after the grace period
Follow-up time too short to estimate the median treatment persistence for dupilumab.
CI: confidence interval; NR: not reached.
Fig. 2.
Kaplan–Meier plot for treatment persistence of systemic treatments for all patients in cohort 2. Shading around each persistence curve represents the 95% confidence intervals (95% CI).
Table III.
Treatment persistence of systemic and topical treatments for atopic dermatitis stratified by region
| Treatment | Treatment persistence |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stockholm |
South |
West |
Other |
|||||||||
| Patients, n | Median (months) | 95% CI | Patients, n | Median (months) | 95% CI | Patients, n | Median (months) | 95% CI | Patients, n | Median (months) | 95% CI | |
| Systemic treatments | ||||||||||||
| Alitretinoin | 87 | 5.4 | 4.4–6.8 | 43 | 4.4 | 3.8–6.1 | 27 | 6.1 | 3.7–7.4 | 60 | 4.4 | 3.9–5.7 |
| Azathioprine | 201 | 9.9 | 7.2–15.4 | 162 | 12.0 | 6.9–16.2 | 105 | 15.7 | 7.3–22.7 | 314 | 10.4 | 6.1–13.8 |
| Cyclosporine | 217 | 3.2 | 2.6–3.5 | 141 | 4.0 | 3.4–5.4 | 187 | 3.2 | 2.6–3.6 | 413 | 3.7 | 3.2–4.3 |
| Dupilumab | 180 | NRa | – | 135 | NRa | – | 105 | NRa | – | 187 | NRa | – |
| Methotrexate | 760 | 11.2 | 10.2–12.9 | 523 | 13.2 | 12.0–15.9 | 375 | 14.0 | 11.5–17.0 | 944 | 12.9 | 11.4–14.5 |
| Mycophenolate | 32 | 6.4 | 3.7–10.4 | 32 | 5.8 | 2.5–9.2 | 57 | 4.9 | 2.6–7.8 | 61 | 5.1 | 2.5–8.1 |
| Topical treatments | ||||||||||||
| Dispensed emollients | 1,117 | 18.4 | 17.4–20.1 | 744 | 17.3 | 15.3–18.0 | 602 | 17.9 | 16.4–20.3 | 1,420 | 20.3 | 17.9–22.1 |
| Pimecrolimus | 242 | 6.1 | 5.9–8.1 | 153 | 7.6 | 5.9–10.5 | 112 | 7.5 | 5.9–10.2 | 203 | 6.6 | 5.9–8.3 |
| Tacrolimus | 720 | 8.4 | 7.2–9.6 | 444 | 7.2 | 5.9–8.5 | 405 | 6.4 | 5.9–7.6 | 811 | 8.3 | 7.4–9.9 |
| Topical corticosteroids | 1,132 | 16.7 | 15.5–17.8 | 771 | 18.3 | 16.6–21.0 | 631 | 17.8 | 15.7–20.9 | 1,460 | 19.4 | 17.7–21.8 |
Duration of treatment persistence was defined as the time from the first dispensation of treatment to discontinuation. Patients were considered persistent with a treatment if the gap between dispensations (i.e. from the end of supply plus days supplied of the former dispensation to the dispensation date of the next) was less than the “grace period” (Appendix S1). A discontinuation was recorded if the patient switched to another drug or had no treatment dispensed after the grace period. For some patients, information on region was not recorded in the register and so the total number of patients for stratification by region was lower for all treatments, except dupilumab, than the overall number of patients included in cohort 2 for each treatment. Further detail for the 4 regions of “Stockholm”, “south”, “west” and “other” is provided as Appendix SI; STable I.
Follow-up time too short to estimate the median treatment persistence for dupilumab.
CI: confidence interval; NR: not reached.
Table IV.
Treatment persistence of systemic and topical treatments for atopic dermatitis stratified by age subgroups
| Treatment | Treatment persistence |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Infants (ages <2 y) |
Children (ages 2–11 y) |
Adolescents (ages 12–17 y) |
Adults (ages ≥18 y) |
|||||||||
| Patients, n | Median (months) | 95% CI | Patients, n | Median (months) | 95% CI | Patients, n | Median (months) | 95% CI | Patients, n | Median (months) | 95% CI | |
| Systemic treatments | 63 | 294 | 340 | 3,389 | ||||||||
| Alitretinoin | – | – | – | – | – | – | 5 | 7.7 | 1.5–11.7 | 213 | 5.1 | 4.4–5.7 |
| Azathioprine | 1 | NRa | – | 47 | 37.3 | 19.7–58.0 | 106 | 16.5 | 9.9–22.5 | 629 | 8.4 | 6.2–10.8 |
| Cyclosporine | 5 | 3.6 | 0.7–7.5 | 40 | 2.9 | 1.3–5.4 | 47 | 4.1 | 2.9–5.4 | 869 | 3.5 | 3.2–3.8 |
| Dupilumab | – | – | – | 6 | NRa | – | 17 | NRa | – | 584 | NRa | – |
| Methotrexate | 58 | 16.7 | 10.6–24.7 | 215 | 13.3 | 11.0–17.1 | 197 | 12.7 | 10.6–16.0 | 2,138 | 12.5 | 11.6–13.4 |
| Mycophenolate | 2 | 15.6 | 3.8–27.4 | 22 | 5.7 | 2.0–10.6 | 14 | 8.2 | 1.6–20.6 | 146 | 4.9 | 3.4–6.1 |
| Topical treatments | 36 | 348 | 329 | 3,325 | ||||||||
| Dispensed emollients | 30 | 32.8 | 15.2–73.7 | 353 | 14.0 | 10.5–15.6 | 331 | 12.9 | 11.5–15.3 | 3,177 | 20.3 | 18.8–21.7 |
| Pimecrolimus | 7 | 5.9 | 5.9–17.8 | 49 | 11.7 | 7.6–13.7 | 65 | 5.9 | 5.9–7.5 | 592 | 7.1 | 5.9–7.8 |
| Tacrolimus | 5 | 37.4 | 7.4–6.2 | 139 | 11.8 | 8.0–13.9 | 179 | 6.6 | 5.9–10.1 | 2,064 | 7.5 | 6.9–8.2 |
| Topical corticosteroids | 34 | 14.9 | 10.7–31.1 | 274 | 15.5 | 13.6–18.4 | 305 | 14.1 | 11.8–16.4 | 3,389 | 19.0 | 17.8–20.5 |
Duration of treatment persistence was defined as the time from the first dispensation of treatment to discontinuation. Patients were considered persistent with a treatment if the gap between dispensations (i.e. from the end of supply plus days supplied of the former dispensation to the dispensation date of the next) was less than the “grace period” (Appendix S1). A discontinuation was recorded if the patient switched to another drug or had no treatment dispensed after the grace period.
Follow-up time too short to estimate the median treatment persistence for dupilumab.
y: years; CI: confidence interval; NR: not reached.
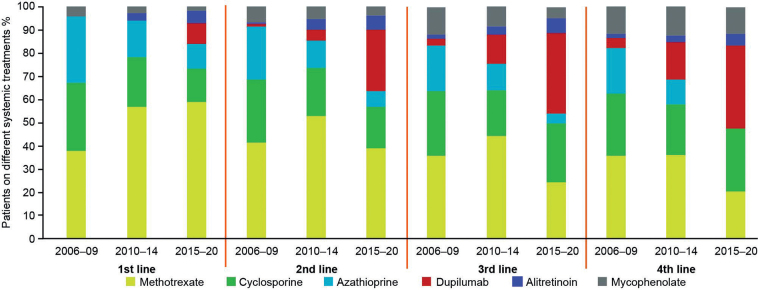
Methotrexate was a prominent first- and second-line treatment used in all time interval cohorts up to 2020; cyclosporine and azathioprine were also common first- and second-line treatments used in all time interval cohorts up to 2020 (Fig. 3 and Appendix SI; SFig. 4). In the time interval cohort defined from 1 January 2010 to 31 December 2014, dupilumab use was more noticeable as a third- and fourth-line choice of systemic treatment. A shift in use of systemic treatments across 4 lines of treatment was observed, particularly in the most recent time interval cohort (2015–2020; Fig. 3 and Appendix SI; SFig. 4). Dupilumab, in the most recent time interval cohort, was being used first-line by 9% of patients. The proportion of patients receiving dupilumab increased further in later lines of treatment in the most recent time interval cohort, which includes patients who may have been receiving systemic treatment since 2006; 27% had dupilumab dispensed second-line, and ˜35% had dupilumab dispensed as a third- and fourth-line treatment.
Fig. 3.
Percentage of patients on different systemic treatment lines over different time-periods (after initiation of first ever systemic treatment). Year ranges shown on the x-axis represent time interval ranges (starting on 1 January with the range end up to 31 December in each instance) of when first-line systemic treatment was initiated; subsequent lines were initiated at some time-point after this time interval up to end of 2020. As an example, for third line in 2010 to 2014, it is not describing third-line therapies introduced in 2010 to 2014, but is instead describing patients initiating any first-line therapy in 2010 to 2014 that has been switched to the third-line treatment, represented by the key at a later time-point (until the end of 2020).
Median (Q1, Q3) time from first secondary care visit for AD to (any) first systemic treatment was 5.8 (2.2, 11.0) years (Table V). When stratified by region, median (Q1, Q3) time to initiate systemic treatment ranged from 4.4 (1.3, 9.1) years in the Stockholm region to 6.8 (2.8, 12.4) years in the south region.
Table V.
Time from first secondary care visit for AD to the first systemic treatment for the overall population of cohort 2 and stratified by regiona
| Overall | Region |
||||
|---|---|---|---|---|---|
| Stockholm | South | West | Other | ||
| Observations, n | 3,579 | 968 | 697 | 569 | 1,345 |
| Time, months, mean (SD) | 82.6 (63.9) | 68.1 (60.7) | 93.2 (67.8) | 90.0 (64.1) | 84.2 (62.1) |
| Time, months, median (Q1, Q3) | 69.3 (26.0, 132.0) | 52.2 (15.3, 109.4) | 81.4 (34.0, 149.0) | 81.0 (34.7, 140.3) | 72.6 (30.0, 131.4) |
| Min-max time, months | 0–238.5 | 0–232.1 | 0–237.6 | 0–237.6 | 0–238.5 |
For some patients, information on region was not recorded in the register and so the total number of patients for stratification by region was lower than the overall number of patients included in cohort 2. Further detail for the 4 regions of “Stockholm”, “south”, “west” and “other” is provided as Appendix SI; STable I.
max: maximum; min: minimum; Q: quartile; SD: standard deviation.
12-month prevalence of atopic dermatitis in Sweden
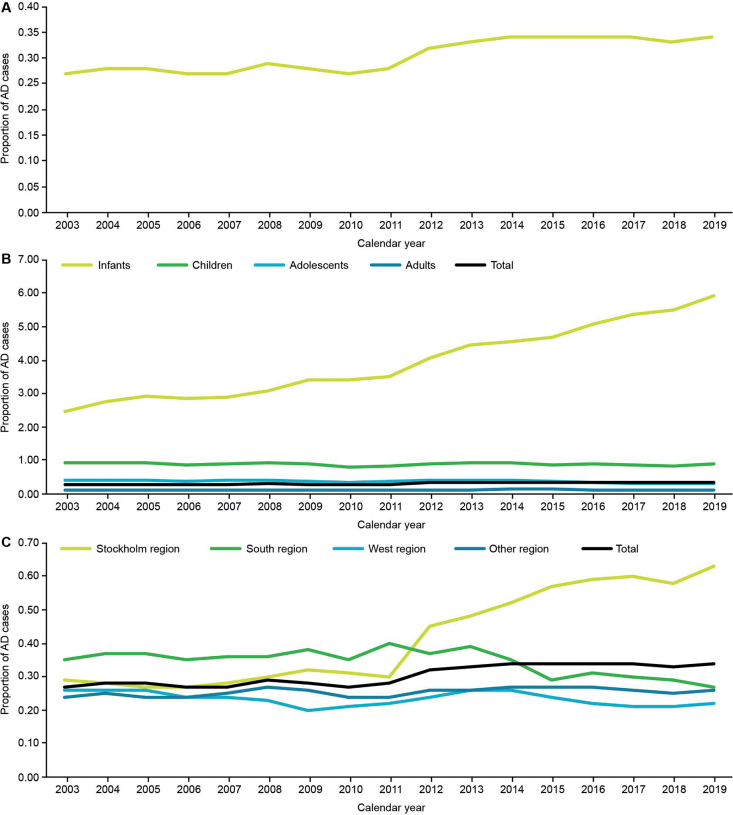
The 12-month prevalence of AD (newly and previously diagnosed cases) in the overall population showed a gradual increase observed over the study period (Fig. 4A). Analysis of 12-month prevalence by age subgroups showed that increase in prevalence was only observed in infants (Fig. 4B). Twelve-month prevalence of AD was shown to be stable in all Swedish regions, except in the Stockholm region, where a steep increase in 12-month prevalence has been observed since 2011 (Fig. 4C).
Fig. 4.
The 12-month prevalence of AD in Sweden for (A) the overall population of cohort 3, and stratified by (B) age subgroups and (C) region.
DISCUSSION
To our knowledge, this is the first study to describe the treatment patterns of systemic treatments for AD in Sweden. This study highlighted the fact that relatively few patients with AD are prescribed systemic treatment, as also observed in real-world studies from other countries (14, 23). Most systemic treatments had low persistence. Of the systemic treatments dispensed in Sweden, methotrexate had the longest treatment persistence, with patients remaining on this treatment for approximately 1 year. Including both systemic and topical agents, no AD treatment had a median persistence greater than 1.5 years, despite dermatologists usually suggesting that patients with AD remain indefinitely on emollients with judicious use of topical corticosteroids. The observed low persistence of all agents may highlight an unmet need for more effective and/or tolerable therapies and better information and support for patients with AD in Sweden.
In AD, treatments are often discontinued because of perceived lack of effectiveness, occurrence of side-effects, poor adherence, or a switch in therapy when newer treatment options become available (24). In this study, the reasons for treatment discontinuation were not recorded as part of the analysis, since this information was not available in the registries. Likewise, information relating to escalation or reduction in treatment intensity was not provided by the registries. There are no national guidelines for monitoring disease activity and no national guidelines for treatment decisions. More recently a register (Svenskt Kvalitetsregister för Atopisk Dermatit; SwedAD) has been established with the aim of collecting data about patients with AD on systemic therapy, i.e. presumably those with more severe AD.
The median treatment persistence with dupilumab could not be determined, since many subjects remained on dupilumab, the most recently approved treatment in this analysis, at the end of the study period. RWE from the US suggests that dupilumab persistence at 12 months is high in adult patients (25). In another US cohort, however, 22% of patients discontinued dupilumab treatment within 6 months (26). Further study will be required to confirm median treatment persistence with dupilumab in a Swedish population. With the introduction of newer treatment options, it is likely there will be changes in treatment patterns over time. This was already observed to some extent in the current analysis in the form of a shift in use of systemic treatments across treatment sequences, particularly for the most recent time interval cohort (2015 to 2020), which includes patients who initiated systemic treatment after dupilumab was first approved in Europe.
According to Wollenberg and colleagues, European treatment guidelines recommend a step-wise approach to treatment, with treatment escalated with increasing disease severity or lack of response (6). Presumably, most patients with AD in the wider community have mild disease (3), whereas patients referred to secondary care might be expected to have more severe disease and thus be candidates for systemic therapy. We observed in the current study, however, a median time of almost 6 years between first visit in secondary care and the first systemic treatment, which may be a consequence of there being few effective and registered systemic treatments for AD during the study period, or may suggest a systematic undertreatment of AD. Alternatively, it is possible that milder cases of AD that do not require systemic treatment are being referred to secondary care in Sweden. The hesitancy to prescribe systemic treatments could again reflect a lack of national treatment guidelines for the management of AD, with healthcare decisions being made at the regional level rather than reflecting recommendations at a national level. Interestingly, there is a shorter delay between first diagnosis and systemic treatment in the Stockholm region, which may be because of the introduction of “Vårdval” policy in 2011, resulting in better access to secondary care for patients in that region (27). The Vårdval policy means that all patients in the Stockholm region with inflammatory skin disorders are allowed access to secondary care following referral from primary care, regardless of disease severity. The large growth in prevalence that is observed in the Stockholm region may be difficult to explain, although the Vårdval policy may provide a partial explanation where we observed a steep increase in the number of new cases of AD between 2011 and 2012, which will include AD cases that otherwise would have remained unregistered in primary care. However, other factors influencing this steep increase cannot be ruled out. The occurrence of this steep rise in the Stockholm region may also be responsible for the increase in 12-month prevalence that is observed in the overall population. Equally, the increase in overall prevalence may have been driven by the increased 12-month prevalence that is observed in the infants’ age subgroup over time. Overall, the findings illustrate the potential influence of regional factors and age in healthcare utilization.
Study limitations
Although this analysis provides some interesting information on treatment patterns for AD in Sweden, the study has several limitations including the general limitations of RWE, which apply to this study. As this was a retrospective observational study, the data utilized were not specifically collected for the purpose of the study. Diagnosis codes were also not validated due to study design. The data collected were from secondary care with no primary-care data included, which is likely to have resulted in a more severe AD patient population spectrum being included. Furthermore, the estimated prevalence is “secondary care prevalence” and not “true prevalence” due to the study design. Within each of the registries, reasons for treatment discontinuation were not recorded, and so this information was not available for analysis. It cannot be excluded that some patients might have been dispensed treatment for a diagnosis other than AD; since treatments such as methotrexate and azathioprine have other indications (28). This would be particularly relevant in infants, who may have received systemic treatments for AD only on rare occasions; in which case, the use of the treatment could be misclassified as AD treatment. We do not, however, believe treatments for other conditions will have occurred in a large number of cases from our data, as this would have been captured in the current analysis of comorbidities (data not shown). Other limitations include the inability of the data to differentiate between oral and topical steroids, and the lack of data on switching products within the same class (e.g. between brands of emollient or corticosteroid). In addition, no information on whether patients purchased over-the-counter medications (e.g. emollients) is provided by the registry. Whereas topical emollients are used continuously, other treatments, such calcineurin inhibitors, may be used more to treat flares, so some patients may have long gaps between use.
This study used only descriptive statistics to describe treatment patterns. Differences between treatments were not statistically evaluated; however, the trends observed provide insights into the real-world treatment patterns for AD in Sweden. Finally, as the study was conducted in Swedish centres, the findings are only relevant to Sweden and may not be extrapolated to other countries.
Conclusion
This analysis of Swedish registry data shows that AD treatment persistence, on average, has always been low. Furthermore, the data showed no clear treatment pathway for patients on systemic treatment. This may be the consequence of a lack of national guidelines and/or consensus on treatment in Sweden. As the same holds true for the data stratified by region, the results indicate a need for the development of national treatment guidelines, which could be based on the current European guidelines. Overall, the findings suggest that suboptimal control of disease may still exist despite the introduction of new and effective treatments and, for those patients, attention is needed to promote good management, including encouraging compliance. These results highlight a need for further treatment options for AD.
ACKNOWLEDGEMENTS
Editorial assistance was provided by Simon Rhead, PhD, and Sue Neville, MSc, of Parexel International, with funding from LEO Pharma AB.
This paper has been presented as a poster POSA228 at Virtual ISPOR Europe November 2021 (“Treatment patterns of atopic dermatitis patients in Sweden”).
Footnotes
Conflicts of interest: EKJ has received speaker honoraria and/or been a consultant for AbbVie, ACO, Galenica, LEO-Pharma, Novartis, and Sanofi-Genzyme. NS and JF are employees of Parexel International Limited. AB and DJT are employees of LEO Pharma. CDA has received speaker honoraria and/or been a consultant for Vaxxas, AbbVie, ACO, LEO-Pharma, Novartis, and Sanofi-Genzyme.
REFERENCES
- 1.Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet 2020; 396: 345–360. [DOI] [PubMed] [Google Scholar]
- 2.Bylund S, Kobyletzki LB, Svalstedt M, Svensson A. Prevalence and incidence of atopic dermatitis: a systematic review. Acta Derm Venereol 2020; 100: adv00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan LN, Magyari A, Ye M, Al-Alusi NA, Langan SM, Margolis D, et al. The epidemiology of atopic dermatitis in older adults: a population-based study in the United Kingdom. PLoS One 2021; 16: e0258219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eichenfield LF, Tom WL, Chamlin SL, Feldman SR, Hanifin JM, Simpson EL, et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol 2014; 70: 338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab 2015; 66: 8–16. [DOI] [PubMed] [Google Scholar]
- 6.Wollenberg A, Christen-Zach S, Taieb A, Paul C, Thyssen JP, de Bruin-Weller M, et al. ETFAD/EADV eczema task force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J Eur Acad Dermatol Venereol 2020; 34: 2717–2744. [DOI] [PubMed] [Google Scholar]
- 7.Food and Drug Administration (FDA) . FDA approves new eczema drug dupixent. 2017. [cited 10 November 2021]. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-new-eczema-drug-dupixent.
- 8.Committee for Medicinal Products for Human Use (CHMP) . Assessment report: dupixent. 2017. [cited 10 November 2021]. Available from: https://www.ema.europa.eu/en/documents/assessment-report/dupixent-epar-public-assessment-report_en.pdf.
- 9.The Dental and Pharmaceutical Benefits Agency (TLV) . Dupixent is included in the drug benefits with limitation. 2018. [cited 10 November 2021]. Available from: https://www.tlv.se/download/18.2ec090df16367c5c52cd5e37/1527144790875/bes180517_dupixent.pdf.
- 10.Thyssen JP, Berents T, Bradley M, Deleuran M, Grimstad O, Korhonen L, et al. Clinical management of atopic dermatitis in adults: mapping of expert opinion in 4 Nordic countries using a modified Delphi process. Acta Derm Venereol 2020; 100: adv00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jappe U, Beckert H, Bergmann KC, Gulsen A, Klimek L, Philipp S, et al. Biologics for atopic diseases: indication, side effect management, and new developments. Allergol Select 2021; 5: 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai HR, Lu JW, Chen LY, Chen TL. Application of Janus kinase inhibitors in atopic dermatitis: an updated systematic review and meta-analysis of clinical trials. J Pers Med 2021; 11: 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The National Board of Health and Welfare . National guidelines for psoriasis care. Support for control and management. 2019. [cited 10 November 2021]. Available from: https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/nationella-riktlinjer/2019-3-11.pdf.
- 14.Egeberg A, Thyssen JP, Wu JJ, Pierce E, Terres JAR. Treatment patterns in Danish patients with atopic dermatitis before and after hospital referral. Dermatol Ther (Heidelb) 2021; 11: 499–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eckert L, Amand C, Gadkari A, Rout R, Hudson R, Ardern-Jones M. Treatment patterns in UK adult patients with atopic dermatitis treated with systemic immunosuppressants: data from The Health Improvement Network (THIN). J Dermatolog Treat 2020; 31: 815–820. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong AW, Huang A, Wang L, Miao R, Patel MY, Gadkari A, et al. Real-world utilization patterns of systemic immunosuppressants among US adult patients with atopic dermatitis. PLoS One 2019; 14: e0210517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei W, Anderson P, Gadkari A, Blackburn S, Moon R, Piercy J, et al. Discordance between physician- and patient-reported disease severity in adults with atopic dermatitis: a US cross-sectional survey. Am J Clin Dermatol 2017; 18: 825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lundin S, Jonsson M, Wahlgren CF, Johansson E, Bergstrom A, Kull I. Young adults’ perceptions of living with atopic dermatitis in relation to the concept of self-management: a qualitative study. BMJ Open 2021; 11: e044777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Epstein M, International Society of Pharmacoepidemiology. Guidelines for good pharmacoepidemiology practices (GPP). Pharmacoepidemiol Drug Saf 2005; 14: 589–595. [DOI] [PubMed] [Google Scholar]
- 20.Vandenbroucke JP, von Elm E, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Ann Intern Med 2007; 147: W163–194. [DOI] [PubMed] [Google Scholar]
- 21.World Medical Association . WMA Declaration of Helsinki – ethical principles for medical research involving human subjects. 2013. [cited 10 November 2021]. Available from: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/. [DOI] [PubMed]
- 22.Swedish MeSH . Age groups (Age groups). 2021. [cited 10 November 2021]. Available from: https://mesh.kib.ki.se/term/D009273/age-groups.
- 23.Gorritz M, Boytsov NN, Goldblum OM, Malatestinic WN, Wang X, Wade RL. Inadequate response and treatment patterns in adults diagnosed with atopic dermatitis and treated with topical therapy. J Dermatolog Treat 2022; 33: 2510–2517. [DOI] [PubMed] [Google Scholar]
- 24.Pino Lopez J, Kromer C, Herr R, Schmieder A, Bayerl C, Schaarschmidt ML. Drug survival rates and reasons for drug discontinuation in patients with atopic dermatitis: a retrospective study of adult outpatients. Eur J Dermatol 2021; 31: 233–238. [DOI] [PubMed] [Google Scholar]
- 25.Silverberg JI, Guttman-Yassky E, Gadkari A, Kuznik A, Mallya UG, Mastey V, et al. Real-world persistence with dupilumab among adults with atopic dermatitis. Ann Allergy Asthma Immunol 2021; 126: 40–45. [DOI] [PubMed] [Google Scholar]
- 26.Eichenfield LF, DiBonaventura M, Xenakis J, Lafeuille MH, Duh MS, Fakih I, et al. Costs and treatment patterns among patients with atopic dermatitis using advanced therapies in the United States: analysis of a retrospective claims database. Dermatol Ther (Heidelb) 2020; 10: 791–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Region Stockholm. The caregiver guide. Specialized skin care . 2020. [cited 10 November 2021]. Available from: https://vardgivarguiden.se/avtal/vardavtal/avtal-vardval-lov/lovvardval-stockholm/specialiserad-hudsjukvard-i-oppenvard/.
- 28.Smolen JS, Landewe RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020; 79: 685–699. [DOI] [PubMed] [Google Scholar]