Prurigo nodularis (PN) is a chronic skin disease, characterized by single or multiple hyperkeratotic skin lesions, which are symmetrically distributed and often highly pruritic. The pathogenesis of PN is largely unknown, but it is linked to inflammation in the skin and neuronal plasticity (1). The patient group is usually quite heterogeneous, and several conditions have been described as associated with development of the disease, such as different types of dermatoses, e.g. atopic dermatitis, or itch due to systemic or neurogenic causes (1).
Treatment for PN usually aims to target itch and inflammation in the skin. Topical and intralesional corticosteroids are commonly used, as are immunosuppressants, such as methotrexate and cyclosporine. Neuromodulators, such as gabapentin and pregabalin, are also common treatments, as are antidepressants. Neurogenic dysregulation has been suggested to play a part in the pathogenesis, and there have been attempts to treat PN with NK-1 receptor antagonists, to block substance Pdependent signalling. However, randomized studies of serlopitant have not shown any significant effect (2). Other studies have suggested the involvement of an activated Th2 cytokine pathway (3).
In recent years, the Th2 cytokine pathway has been the focus of new biological treatments directed at interleukin (IL)-4, IL-13, and IL-31. An increasing number of patients with PN have been given dupilumab, a monoclonal antibody that inhibits IL-4 and IL-13 signalling, to target inflammation and itch in the skin, even in patients without atopic manifestations. There have been several case reports and a few case series that have shown promising results in patients with PN (4). For example, a recent case series in 16 patients with either non-atopic or atopic background showed good effect on itch and skin lesions, and a good safety profile (5).
The aim of the current study was to collect data on patients with PN treated with dupilumab from August 7, 2019 to November 30, 2021, and analyse quality of life scores, depression scores, and mutations in the filaggrin gene (FLG).
MATERIALS AND METHODS
All patients with PN who had received dupilumab and were registered in the local research register at Karolinska University Hospital, Stockholm, Sweden, between August 2019 and November 2021 were included.
The following outcome measurements were scheduled to be registered before treatment: numerical rating scale (NRS) for pruritus scores, Dermatology Life Quality Index (DLQI) scores, and Montgomery Åsberg Depression Rating Scale – Self-report (MADRS-S) scores. Peak pruritus NRS was defined as self-reported worst itch over the previous 24 h.
Additional data, such as the extent of disease at baseline (number of lesions based on visual assessment of clinical pictures), date of onset, various treatments, and comorbidities were collected from medical records (see Table I). Sixteen of 19 patients were analysed for FLG mutations. They were genotyped for the most common FLG mutations found in the European population, 2282del4, R501X, and R2447X (30), using the Taqman Allelic Discrimination method (6).
Table I.
Characteristics of the study population
| All patients | Patients included in statistical analysis | |
|---|---|---|
| Number | n = 19 | n = 10* |
| Sex: M:F | 8:11 | 6:4 |
| Age, years, median (IQR) | 58 (45–74) | 66.5 (56–77) |
| Number of previous treatments, median (IQR) | 5 (3–7) | 5 (4–7) |
| Previous treatments, n (%) | ||
| Topical corticosteroids | 19 (100) | 10 (100) |
| Phototherapy (PUVA, UVB, and/or UVB TL01) | 13 (68) | 6 (60) |
| Methotrexate | 14 (74) | 7 (70) |
| Cyclosporine | 6 (32) | 3 (30) |
| Other | 15 (79)** | 8 (80) |
| Atopic dermatitis, n (%) | 7 (37) | 5 (50) |
| Asthma/rhinitis, n (%) | 6 (32) | 2 (20) |
| Number of lesions at baseline (n)*** | 14 | 9 |
| > 50, n (%) | 10 (71) | 6 (67) |
| 10–50, n (%) | 3 (21) | 3 (33) |
| < 10, n (%) | 1 (7) | 0 |
Patients with values at baseline AND at follow-up after 1–3 months on treatment with dupilumab.
Antihistamines (n = 12), gabapentin (n = 5), pregabalin (n = 4), antidepressives (n = 4), omalizumab (n = 2), systemic corticosteroids (n = 2), apremilast (n = 1), lymecycline (n = 1), naltrexone (n = 1), aprepitant (n = 1).
Clinical pictures missing for 5 of the patients at the start of treatment.
IQR: interquartile range; PUVA: psoralen plus ultraviolet A; UVB: ultraviolet B.
Background characteristics and outcome measures at baseline were expressed as percentages of the total number of individuals observed, or median values, and interquartile ranges (IQRs). The Wilcoxon matched-pairs signed-rank test was used to compare outcome measures (pruritus-NRS, DLQI, MADRS-S) at baseline and at follow-up, after 1–3 months of treatment. All statistical calculations were performed with Stata statistical software (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX, USA: StataCorp LLC).
RESULTS
In total, 19 patients (8 males, 11 females) with PN were treated with dupilumab and followed in the registry. One patient was excluded from the analysis because of several missing values. Overall, the treatment was well-tolerated: 4 patients reported dry eyes, 1 presented with redness at the injection site, and 1 reported headache.
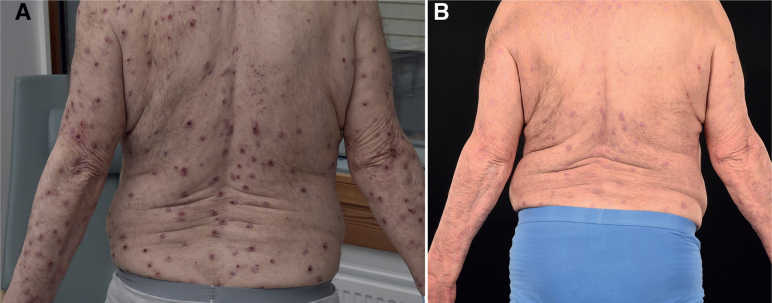
At the time of data collection, the patients had received treatment for a median of 16 months (range 4–30 months). All of the 18 included patients improved clinically (Fig. 1), and 16 patients were still on treatment in November 2021. During the study period, 1 patient discontinued dupilumab due to side-effects after 4 months and another 1 discontinued dupilumab due to lack of effect after 18 months. Among patients included in the statistical analysis (n = 10), median pruritus NRS scores were shown to be significantly decreased after 3 months of treatment, from 8.5 (IQR 7–9.5) to 3 (IQR 2–6) (n = 9, p = 0.016). There were also significant improvements in health-related quality of life (DLQI) scores, from 15 (IQR 10–20) to 6 (IQR 2–8) (n = 10, p = 0.002), and depression scores (MADRS-S), from 13 (IQR 8–19) to 6 (IQR 2–10) (n = 10, p = 0.002). Longitudinal data for a subgroup of patients (n = 14) who reported outcome measurements at baseline are shown in Table II. All patients analysed (n = 16) for FLG mutation were shown to have the wild type, thus no FLG mutations were found in this patient group.
Fig. 1.
(A) Patient before treatment. (B) After 5 months’ treatment with dupilumab.
Table II.
Outcome measurements among patients with prurigo nodularis receiving dupilumab (n = 14), from baseline to 1 year of treatment
| DLQI | Pruritus-NRS | MADRS-S | ||||
|---|---|---|---|---|---|---|
| Median (IQR) | n | Median (IQR) | n | Median (IQR) | n | |
| Baseline | 15 (10–20) | 14 | 8.5 (7–9.5) | 12 | 13 (8–19) | 14 |
| 1–3 months | 6.5 (3–8) | 10 | 3 (2–6) | 9 | 6 (4–10) | 10 |
| 4–6 months | 8 (2–16) | 8 | 4 (2–7) | 7 | 5 (3–7.5) | 8 |
| 7–9 months | 5 (4–11) | 5 | 2.5 (0–6) | 4 | 5 (5–8) | 5 |
| 10–12 months | 3 (1–4) | 5 | 1.5 (0.5–3) | 4 | 4 (2–7) | 5 |
| > 12 months | 6 (4–14) | 5 | 5 (3–5.5) | 4 | 3 (0–14) | 5 |
Only patients who reported DLQI (n = 14), Pruritus-NRS (n = 12), and MADRS-S (n = 14) at baseline were included in this analysis. When patients had multiple assessments in the interval the last value was chosen, with exception of >12 months when the first value (closest to 1 year of treatment) was included. Six patients had follow-up <12 months. Missing values range 3–9.
DLQI: Dermatology Life Quality Index; Pruritus-NRS: numerical rating scale; MADRS-S: Montgomery Åsberg Depression Rating Scale-Self-report; IQR: interquartile range.
All patients had previously received topical corticosteroids to treat PN. Also, all patients except 1 had received at least 1 systemic treatment prior to starting dupilumab. The most common treatments were methotrexate, cyclosporine, and ultraviolet (UV) (Table I). Most patients had received their PN diagnosis several years prior to treatment with dupilumab (median 10 years; IQR 6–14, 2 missing values). More than half (n = 11) of the patients had been hospitalized due to their skin disease.
DISCUSSION
There was significant improvement in the patients regarding all analysed parameters, including pruritus, health-related quality of life, and depressive symptoms. This is in line with previous reports, showing clinical improvement and suggesting this as a promising treatment option for PN (4). Overall, the treatment was well-tolerated and there was a rapid improvement in all parameters after starting dupilumab. It is well-known that PN has a negative impact on patients’ quality of life (7) and the disease has also been associated with an increased rate of depression (8). The fact that more than half of the patients had been hospitalized shows the severity of the skin disease and the difficulty of finding an effective treatment regimen. The current study shows that dupilumab not only has an effect on the disease and pruritus per se, but also effectively decreases patients’ depressive symptoms. However, no data on any objective measurement of PN-severity and no longitudinal data on the extent of PN were included, which is a limitation of this study.
Furthermore, no FLG mutations were found in any of the patients analysed in the current case series. No evidence was found for the importance of this specific mutation in patients with PN, although the study group of patients was quite small. In this context, one group recently reported a small patient material (n = 9) of patients with PN, in which 11% had FLG mutations (heterozygous). However, their study showed a deficiency in filaggrin expression in lesional skin even in the absence of FLG mutations, indicating that other factors may influence the expression of filaggrin and that filaggrin might play a role in PN (9).
In conclusion, the findings of this case series imply that dupilumab is a promising treatment for patients with PN, with significant reductions in both itch and depressive symptoms. Larger studies are needed to confirm the effectiveness and safety of dupilumab treatment among patients with PN.
Footnotes
The authors have no conflicts of interest to declare
REFERENCES
- 1.Zeidler C, Yosipovitch G, Ständer S. Prurigo nodularis and its management. Dermatol Clin 2018; 36: 189–197. [DOI] [PubMed] [Google Scholar]
- 2.Williams KA, Roh YS, Brown I, Sutaria N, Bakhshi P, Choi J, et al. Pathophysiology, diagnosis, and pharmacological treatment of prurigo nodularis. Expert Rev Clin Pharmacol 2021; 14: 67–77. [DOI] [PubMed] [Google Scholar]
- 3.Fukushi S, Yamasaki K, Aiba S. Nuclear localization of activated STAT6 and STAT3 in epidermis of prurigo nodularis. Br J Dermatol 2011; 165: 990–996. [DOI] [PubMed] [Google Scholar]
- 4.Toffoli L, Farinazzo E, Zelin E, Agozzino M, Dianzani C, Di Meo N, et al. Dupilumab as promising treatment for prurigo nodularis: current evidences. J Dermatolog Treat 2021; 15: 1–6. [DOI] [PubMed] [Google Scholar]
- 5.Calugareanu A, Jachiet M, Tauber M, Nosbaum A, Aubin F, Misery L, et al. Effectiveness and safety of dupilumab for the treatment of prurigo nodularis in a French multicenter adult cohort of 16 patients. J Eur Acad Dermatol Venereol 2020; 34: 74–76. [DOI] [PubMed] [Google Scholar]
- 6.Sandilands A, Terron-Kwiatkowski A, Hull PR, O’Regan GM, Clayton TH, Watson RM, et al. Comprehensive analysis of the gene encoding filaggrin uncovers prevalent and rare mutations in ichthyosis vulgaris and atopic eczema. Nat Genet 2007; 39: 650–654. [DOI] [PubMed] [Google Scholar]
- 7.Janmohamed SR, Gwillim EC, Yousaf M, Patel KR, Silver-berg JI. The impact of prurigo nodularis on quality of life: a systematic review and meta-analysis. Arch Dermatol Res 2021; 313: 669–677. [DOI] [PubMed] [Google Scholar]
- 8.Jørgensen KM, Egeberg A, Gislason GH, Skov L, Thyssen JP. Anxiety, depression and suicide in patients with prurigo nodularis. J Eur Acad Dermatol Venereol 2017; 31: 106–107. [DOI] [PubMed] [Google Scholar]
- 9.Fölster-Holst R, Reimer R, Neumann C, Proksch E, Rodriguez E, Weidinger S, et al. Comparison of epidermal barrier integrity in adults with classic atopic dermatitis, atopic prurigo and non-atopic prurigo nodularis. Biology (Basel) 2021; 10: 1008. [DOI] [PMC free article] [PubMed] [Google Scholar]