Highlights
-
•
Minimal dose of nitrite /nitrate protects against lipid oxidation.
-
•
Minimal dose of nitrite /nitrate limits NOCs formation.
-
•
Even in absence of nitrite/nitrate, non volatile N nitrosamines are formed in the stomach.
Keywords: Digestion, Nitrite, Nitrate, Processed meat, Dry-cured fermented sausages, Nitroso-compounds, Nitrosation, Nitrosylation, Oxidation
Abstract
In vitro digestions of dry-cured sausages formulated with four different rates of added sodium nitrite and sodium nitrate (NaNO2 / NaNO3, in ppm: 0/0; 80/80; 120/120; 0/200) were performed with a dynamic gastrointestinal digester (DIDGI®). The chemical reactivity of the potentially toxic nitroso-compounds (NOCs), oxidation reactions products and different iron types were evaluated over time. No nitrite nor nitrate dose effect was observed on NOCs’ chemical reactivity. Nitrosothiols were scarce, and nitrosylheme was destabilized for every conditions, possibly leading to free iron release in the digestive tract. Total noN-volatile N-nitrosamines concentrations increased in the gastric compartment while residual nitrites and nitrates remained stable. The minimal rate of 80/80 ppm nitrite/nitrate was enough to protect against lipid oxidation in the digestive tract. The present results provide new insights into the digestive chemistry of dry sausages, and into new reasonable arguments to reduce the load of additives in formulations.
Introduction
Processed meats are core parts of the French gastronomy, and dry sausages represented 8 % of the total food consumed during one week according to a study over more than 3000 individuals in 2015–2016 (Hébel, 2019). Indeed, dry-cured sausages are nutritionally relevant (highly digestible proteins, essential amino acids and fatty acids, B group vitamins, and highly bioavailable iron and zinc (Papuc, Goran, Predescu, & Nicorescu, 2017) and display unique organoleptic features, developed through ripening (De Mey et al., 2014, Marco et al., 2006, Ordonez et al., 1999). Nevertheless, they can be questioned when it comes to salt and additives intake. Those food additives relate to nitrite and nitrate, which are used in the formulation of dry-cured fermented sausages for multiple reasons. They contribute not only to preserve sausages during the entire process of aging by inhibiting the growth of spoilage and pathogenic bacteria (Marco et al., 2006, Ordonez et al., 1999), but they also contribute to the sensory qualities of the products in terms of red colour, typical cured flavour, and texture (Marco, Navarro, & Flores, 2006). Moreover, nitrite and nitrate display strong antioxidant features, and thus protect lipids from oxidation (Berardo, De Maere, Stavropoulou, Rysman, Leroy, & De Smet, 2016) and therefore from the development of rancid aromas in the product (Ordonez, Hierro, Bruna, & de la Hoz, 1999). In products with sufficiently long process time, nitrate can be used with nitrite or not. The nitrate reductase activity of the starters used in the formulations provide a progressive and continue delivery of nitrite throughout the drying step (Marco, Navarro, & Flores, 2006).
Nevertheless, awareness has been raised about processed meats preserved with nitrite and nitrate, as they may lead to health issues during digestion (Bouvard et al., 2015, Demeyer et al., 2016, IARC, 2018). Indeed, contrasted nutritional aspects highlight the benefit to risk equation of processed meats consumption and notably dry-cured sausages consumption, which justifies the work on nutritional guidelines proposed by safety authorities worldwide in the last few years (Johnston et al., 2019). These warnings concern potential impacts on health, because of their degradation in the digestive tract. Nitrite and nitrate are able to react with endogenous compounds present in meat, leading to the synthesis of nitroso-compounds (NOCs) (de La Pomélie et al., 2017, de La Pomélie et al., 2018). Yet, those molecules appear to be at stake in the potential effects assigned to processed meats’ toxicological impact, particularly because of their fate in the digestive tract.
NOCs gather nitrosamines, nitrosothiols and nitrosylheme (also called nitrosylated heme iron), and seem to be enhanced by oxidative substances and environment (de La Pomélie et al., 2017, de La Pomélie et al., 2018). N-nitrosation, where secondary amines react with nitrosonium ions to form nitrosamines, is promoted by an acidic environment. The evolution of nitrosamines during digestion is paramount to explore, considering the potential toxicity of these molecules, and the enhancement of the reactions in the digestive tract. Nitrosothiols stem from a reaction of S-nitrosation. Thiol groups of cysteine residues in meat tend to react either with nitrosonium ions or with nitric oxide to form nitrosothiols (Kuhnle et al., 2007). Nitrosylheme is formed by the attachment of nitric oxide on heme iron of myoglobin according to a reaction of nitrosylation (de La Pomélie, Santé-Lhoutellier, & Gatellier, 2018). Free iron, either endogenous, exogenous, or resulting of the release by heme, is known to catalyse lipid oxidation in the gastrointestinal tract, whereby the chain reaction leads to the synthesis of cytotoxic aldehydes (Gueraud et al., 2015, Min and Ahn, 2005).
Nitrosamines, nitrosylheme, nitrosothiols and lipid oxidation products may be the first chain trigger leading to the formation of DNA adducts through their degradation in the gastrointestinal tract, which emphasizes the importance to understand the chemical reactivity of NOCs during digestion (Kuhnle et al., 2007, Santarelli et al., 2010), in order to objectify the roles of nitrate and nitrite in the manufacture of dry-cured sausages.
The aim of this study was to provide consistent data on the chemical reactivity of nitrite and nitrate preserved dry-cured fermented sausages during in vitro modelized digestion, in order to provide new insights into the potential effects of these additives in processed meats and consistent data on the potential reduction of those food additives as well as the sources of nitrite oxide (leading to NOCs’ synthesis).
Materials and methods
Reagents
Every reagent, i.e. acetone, amano lipase A from Aspergillus niger (CAS: 9001-62-1; >120,000 U/g), ascorbic acid, butanol, citric acid, ethanol, ferrozin, ferrous sulphate, the Griess reagent kit for nitrite and nitrate assays (ref: 23479-1KT-F), hydrochloric acid, mercury chloride, pancreatin from porcine pancreas (CAS: 8049-47-6; 8xUSP Specifications), pepsin from porcine gastric mucosa (CAS: 9001-75-6; 3,200-4,500 U/mg of proteins), sodium chloride, thiobarbituric acid (TBA) (CAS: 504-17-6) and trichloroacetic acid (TCA) (CAS: 76-03-9) were from Sigma-Aldrich (Saint Louis, MO, USA).
Dry cured fermented sausages samples
The elaboration process of dry-cured fermented sausages used in the present paper and their composition have been previously described (Bonifacie, Gatellier et al., 2021). Briefly, the sausages studied differ in their content in nitrites (NaNO2) and nitrates (NaNO3), in mg/kg (ppm), and in sodium chloride (NaCl) (g/kg) (Table 1).
Table 1.
Dry-cured fermented sausages formulations in nitrites and nitrates summary.
| Sample identification | Justification of the study | NaNO2 / NaNO3 (ppm) | Nitrite salt / Nitrate salt (g/kg) | NaCl (g/kg) | Total salt (g/kg) |
|---|---|---|---|---|---|
| S-0 | Negative control | 0/0 | 0.00 / 0.00 | 26.00 | 26.00 |
| S-80 | Short-term goal of the manufacturers | 80/80 | 13.33 / 0.10 | 12.67 | 26.10 |
| S-120 | Maximum authorized amount in products with both nitrite and nitrate | 120/120 | 0.00 / 0.24 | 26.00 | 26.24 |
| S-200 | Maximum amount authorized in products with only nitrate | 0/200 | 20.00 / 0.14 | 6.00 | 26.14 |
The sausages were made at the Technical Institute ADIV (Clermont-Ferrand, France), with 87 % of porcine shoulder meat and 13 % of porcine back fat. Meat were grinded at 6 mm diameter then stuffed in porcine casings of 55 mm diameter. Products were aromatized with 1.50 g/kg of grounded grey pepper. Starters; (namely Lactobacillus sakei, Staphylococcus carnosus, Staphylococcus xylosus (Lallemand, France)), dextrose and lactose (0.15 g/kg; 5.50 g/kg and 6.00 g/kg, respectively) were added to acidify the sausages during the fermentation step. It should be noticed that no ascorbate was added in the formulation. The fermentation lasted 3 days and included 3 main steps:
-
•
A first step to increase temperature from 8 °C to 24 °C in 14 h with high hygrometry (85 %–95 %).
-
•
A fermentation step during 35 h at 24 °C to induce starters multiplication and acidification with a slight reduction of hygrometry.
-
•
A temperature drop during 24 h to reach the drying temperature with high hygrometry during 12 h to induce mould germination (Penicillium nalgovensis).
After the fermentation step, products were dried at 13 °C during 24 days. During fermentation, the decrease in pH was classical for sausages manufacturing, with an initial pH comprised between 5.75 and 5.85 and a decrease to pH 5.10 within 3 days (more information are provided in Supplementary Data 1). After the fermentation step, the pH increased slowly to a final value of 5.4. No significant pH difference was noticed between the different trials, as well as for weight losses (Supplementary Data 2).
In vitro digestion experiments
Dynamic gastro-intestinal digester (DIDGI) system
Digestions were performed on an in vitro dynamic gastro-intestinal digester, named DIDGI® (Inrae; Paris, France), allowing to model with good reliability the environment and the progress made by the chyme during an adult digestion (Menard, Picque, & Dupont, 2015). DIDGI® consists of three different bioreactors maintained at 37 °C, representing the stomach, the duodenum/jejunum, and the ileum. In order to represent the human pylorus, a Teflon stopper vented with 2 mm holes was set before the transfer pump between the gastric and the duodenum compartments, allowing to sieve the gastric bolus towards the modelized small intestine. The mechanical action of the digestive tract was recreated by stirring the contained substances with a blade in every reactor. The flows between the three reactors and the liberated enzymes was regulated by computer thanks to the software StoRM (Guillemin, Perret, Picque, Menard, & Cattenoz, 2010) to ensure a dynamical kinetic and a reliable evolution of the biochemical digestive environment. This regulation was achieved thanks to pumps (Verder; France), and probes (Mettler Toledo, ref: 59902194 K; Columbus, USA) monitoring the main physico-chemical parameters (temperature, pH and ionic strength). Two pH probes were used: one for the gastric compartment and the other for the duodenum/jejunum compartment. A setpoint pH curve was set to optimally rely to the kinetics of pH during digestion; in order to monitor the set-up pH curve, HCl (1 M) and Na2HCO3 (1 M) were delivered gradually in the stomach and in the duodenum/jejunum, respectively. The equation described by Elashoff et al. (Equation (1)) provides with the transit time in the different compartments, where f is the fraction in one of the three compartments, t is the delivery time and where β is the coefficient describing the curve for the chyme delivery among the different bioreactors (herein the gastric one and the duodenum/jejunum one) (Elashoff et al., 1982).
| (1) |
Application of DIDGI to dry cured sausage
Every dry cured sausage was minced with a cooking mixer to simulate the disruption of food in the mouth and was aliquoted to freeze at −80 °C. The simulated fluids used for the in vitro digestions are based on the international consensus work issued from Minekus et al. (Minekus, Alminger, Alvito, Ballance, Bohn, Bourlieu, et al., 2014).
Gastric digestion. The simulated gastric fluid (SGF) (KCl 6.9 mM; KH2PO4 0.9 mM; NaHCO3 25 mM; NaCl 47.2 mM; MgCl2 0.1 mM; (NH4)2CO3 0.5 mM) adjusted to a pH of 2 was introduced in the empty gastric compartment at 190 ml, with 3 ml of residual pepsin, prior the addition of 20 g of dry-cured sausages sample. The gastric enzymes, namely pepsin and lipase, were prepared in SGF. The half time of the delivery in the gastric compartment was set at 85 min, with β equal to 1.8.
Intestinal digestion. The intestinal enzymes, (pancreatin and bile salts) were prepared in simulated intestinal fluid (SIF) (KCl 6.9 mM; KH2PO4 0.9 mM; NaHCO3 85 mM; NaCl 38.4 mM; MgCl2 0.33 mM). Bile salts (50 % cholic acid sodium salt and 50 % deoxycholic acid sodium salt) were selected instead of bile to prevent potential interactions with the subsequent nitroso-compounds assays (de La Pomélie, Santé-Lhoutellier, Sayd, & Gatellier, 2018). All the enzymes were kept in an ice bath to prevent autolysis from happening while digestion was running.
The delivery of the enzymes was gradual although pepsin, lipase and pancreatin were dispensed at a constant flow rate of 0.25 ml/min and bile salts at a flow rate of 0.5 ml/min. For the ileal part, the half time of the delivery was 250 min, with β equal to 2.5.
Samples collection
Five chyme samples of 10 ml were collected over time to analyse the chemical reactivity kinetics during digestion. Three of them corresponded to samples from the gastric compartment, collected at 40, 80 and 120 min after the beginning of the digestion, and are presented as G40, G80 and G120 in the present paper. Those samples were used to analyse lipid oxidation; total heme iron; nitrosylated iron; free iron; residual nitrites and nitrates; non-volatile N-nitrosamines and nitrosothiols. Two samples were collected in the ileal reactor, at 90 and 200 min after the beginning of the digestion, were named I90 and I200, and were used to analyse lipid oxidation, total heme iron, nitrosylated iron and free total iron. After the collection from the reactors thanks a 10 ml syringe, the samples were filtrated through a cotton swab (Ref. 010811; 7.5x7.5 cm; 4 plies/17 threads; from Raffin-Medical, Saint Romain de Popey, France). Then, the pH was neutralized by added either sodium hydroxide or hydrochloric acid, before aliquoting for the subsequent biochemical assays. Digestions of dry-cured sausages have been performed independently in triplicates for each formulation.
Biochemical assays of the digestates
All the following biochemical assays have been performed in the products in a previous work (Bonifacie, Gatellier et al., 2021) and the results from this study are used to present the concentrations found at the initial time (noted as T0) in the different products.
Lipid oxidation
The TBARS assay developed by Folch et al. was employed to assess lipid oxidation products in the digestates (Folch, Lees, & Sloane-Stanley, 1957). The samples (300 µl of digestates) were incubated with 150 µl of TBA and 150 µl of TCA before boiling for 10 min in a water bath. Butanol (1.2 ml) was added prior to centrifugation (4000 rpm; 15 min; 4 °C). The absorbance of the organic phase was measured by spectrophotometry (Jasco V-770) at 535 nm and 760 nm. Six independent measurements were performed, and results are presented as mean ± standard deviation.
Nitrosylation
The methodology used to dose total heme iron and nitrosylated heme iron has been previously described (Bonifacie et al., 2021, de La Pomélie et al., 2018) and is based on the methodology developed by Hornsey in 1956. To extract nitrosylheme, 500 µl of digestate was diluted with 2 ml of acetone (allowing to solubilize the pigments) and 150 µl of water. To extract total heme iron, 100 µl of water, 50 µl of hydrochloric acid and 2 ml of acetone were added to 500 µl of digestate from DIDGI®. After 15 min of stirring in the dark, the mixtures were filtrated on syringe filters with 0.45 µm regenerated cellulose membranes (Interchim®, France). The measurements were realized on a Jasco V-770 spectrophotometer at 760 nm and 540 nm for nitrosylated iron (ε tantamount to 11.3 mM), and 760 nm and 512 nm for total heme iron (ε tantamount to 9.52 mM). The analyses were performed in triplicates for both total heme iron and nitrosylated iron, and are presented as mean ± standard deviation.
Free iron
The methodology described by Stolze et al. was used to assess the part of free iron in the digestates (non-heme iron) (Stolze, Dadak, Liu, & Nohl, 1996). A sample of 500 µl of digestate was mixed with 1 ml of citric acid/chloride sodium buffer (adjusted to pH 7.4) before their dispense in Vivaspin® systems filled with membrane cut-off of 5 kDa. The samples were centrifugated for 105 min at 18 °C; allowing to isolate free iron from heme iron. The filtrated samples were then incubated with ascorbic acid (100 mM) and ferrozin solution (10 mM) for 15 min before spectrophotometric measurements at 562 nm and 760 nm (with a Jasco V-770). A concentration range from 0 to 0.03 mM of iron (II) sulfate was used to determine iron (II) sulfate concentrations. The measurements were performed in triplicates, and results are given as mean ± standard deviation.
Residual nitrites, residual nitrates, nitrosothiols and non-volatile nitrosamines
Griess reagent kits were used to dose residual nitrites, residual nitrates, nitrosothiols and total apparent non-volatile nitrosamines, according to the method described by Bonifacie et al. (Bonifacie, Aubry, Gatellier, Santé-Lhoutellier, & Théron, 2021). Those analysis were realized with 1 ml of digestate stemming from all the gastric kinetic points (G40, G80, G120). Digestates were diluted in 2 ml of water and extracted for 15 min in a dry water bath at 50 °C. Then, the samples were dispensed in Vivaspin® systems (equiped with cut-off membranes of 5 kDa; ref: VS0212; Sartorius, Göttingen, Germany) and were ultra-centrifugated at 16 °C, 4.000 rpm for 75 min (SL 40R centrifuge; Thermo-Scientific, Waltham, USA). Then, the ultra-filtrates were split into four samples for the assays (400 µl for residual nitrites, 400 µl for residual nitrates, 500 µl for nitrosothiols, 1.5 ml for nitrosamines), which were all evaluated by UV–visible spectrophotometry.
Nitrosothiols were evaluated after the breaking of the S-NO bound by mixing the filtrates with 5 mg of mercury chloride for 10 min. Measuring the nitrite concentrations in this sample and removing the concentration of residual nitrites allows to quantify the part of nitrite resulting from the disruption of nitrosothiols. The samples were then filtrated with syringe filters on regenerated cellulose membranes of 0.22 µm (ref: 17761; Sartorius; Göttingen, Germany). Total apparent non-volatile nitrosamines were assessed by measuring a kinetics of irradiation for 2 h under a UV lamp (LF 215.S, 254 nm, 2x15 W). Residual nitrates and irradiated samples for nitrosamines assay were reduced in nitrite thanks to a nitrate reductase, and its cofactor from the kit. Then, all those samples along with those set apart for residual nitrites and S-NO bound assays were mixed with two reagents (named A and B) from the Griess kit. The concentrations, expressed in µM, were evaluated thanks to nitrites and nitrates calibration curves, realized with standard solutions from the kit. The absorbance wavelengths for the measurements were 540 nm and 760 nm. A Multiskan Spectrum spectrophotometer (Thermo Scientific, Waltham, USA) was employed for the experiment. The analysis were done in triplicates and are expressed as mean ± standard deviation.
Statistical analysis
Data were analysed with the software XLStats (2020.3.1.2.; Microsoft; Albuquerque, USA)) via repeated measures variance analysis (ANOVA) and a Tukey post-hoc test. The significances were given by p-values < 0.05 (*), p-values < 0.01 (**), or p-values < 0.001 (***). The concentrations measured in the gastric compartment were analysed separately from those measured in the intestinal compartment, as the composition of the medium was strictly different. The concentrations measured after 40; 80 and 120 min in the gastric compartment, and after 90 and 200 min in the duodenum compartments were used as variables. Kinetic times were respectively noted as G40, G80, G120, I90 and I200. The selected factor was the different formulations of the dry-cured sausages. For each kinetic time, homogenous groups were identified by different letters for the two distinct compartments from a to c. Every biochemical analysis has been performed in triplicates, and the results are systematically given as mean ± standard deviation.
Results and discussion
The study aimed to elucidate the chemical reactivity of nitrite and nitrate during in vitro digestion of dry-cured fermented sausages, by quantitatively assessing key markers through digestive kinetics. The results are presented in the Table 2A, Table 2B, next to those obtained in the products (Bonifacie, Gatellier et al., 2021) to assess the evolution of this reactivity during digestion.
Table 2A.
Characterization of the dose–effect of nitrite and nitrate curing on the chemical reactivity of dry-cured fermented sausages during in vitro gastric and ileal digestion.
| (A) | Kinetic time | S-0 | S-80 | S-200 | S-120 | p-value |
|---|---|---|---|---|---|---|
| TBARS (µM) | T0 | 21 ± 1.6a | 12 ± 1.6b | 13 ± 3.3b | 10 ± 3.3b | ** |
| G40 | 9.5 ± 2.4a | 1.5 ± 1.3c | 4.2 ± 0.8b | 1.1 ± 0.9c | *** | |
| G80 | 27.0 ± 14.1a | 4.0 ± 1.6b | 7.8 ± 0.7b | 3.6 ± 0.7b | *** | |
| G120 | 69.1 ± 33.9a | 11.3 ± 2.2b | 19.7 ± 1.9b | 8.6 ± 1.5b | *** | |
| I90 | 26.3 ± 2.7a | 19.9 ± 4.0b | 23.2 ± 1.8ab | 21.3 ± 1.3b | ** | |
| I200 | 52.5 ± 7.3a | 29.1 ± 3.5bc | 36.8 ± 5.2b | 26.5 ± 3.9c | *** | |
| Total heme iron (µM) | T0 | 190.0 ± 5.6a | 230.0 ± 12.0b | 230.0 ± 3.6b | 230.0 ± 4.8b | ** |
| G40 | 30.89 ± 8.9b | 56.9 ± 6.9a | 8.9 ± 5.9c | 11.9 ± 4.7c | *** | |
| G80 | 86.7 ± 19.3a | 97.9 ± 8.9a | 84.4 ± 4.7a | 14.9 ± 4.1b | *** | |
| G120 | 188.9 ± 7.3a | 170.2 ± 10. 9ab | 225.2 ± 1.4ab | 140.4 ± 42.3b | ** | |
| I90 | 132.2 ± 37.0a | 122.7 ± 3.4a | 218.2 ± 79.7a | 146.0 ± 7.4a | NS | |
| I200 | 181.0 ± 13.1b | 181.7 ± 6.7b | 248.6 ± 15.9a | 223.2 ± 27.9ab | ** | |
| Nitrosylated iron (µM) | T0 | 40.0 ± 1.9 a | 150.0 ± 5.3b | 120.0 ± 7.6c | 170.0 ± 35.1b | ** |
| G40 | 0b | 0b | 0b | 5.7 ± 1.3a | *** | |
| G80 | 0c | 0c | 4.9 ± 0.5b | 7.0 ± 1.4a | *** | |
| G120 | 16.7 ± 10.1c | 65.8 ± 2.7 a | 60.3 ± 13.1ab | 41.3 ± 2.3b | *** | |
| I90 | 0c | 0c | 19.3 ± 2.6b | 28.4 ± 3.8 a | *** | |
| I200 | 20.36 ± 20.3c | 45.6 ± 3.6b | 42.0 ± 3.8b | 62.1 ± 4.1 a | *** | |
| Total free iron (µM) | T0 | 48.0 ± 2.0 a | 11.0 ± 1.0b | 22.0 ± 21.0b | 16.0 ± 6b | ** |
| G40 | 15.5 ± 0.5 a | 7.9 ± 1.0 ab | 6.0 ± 0.5b | 10.0 ± 0.6 ab | * | |
| G80 | 16.7 ± 2.8 a | 16.1 ± 0.7 a | 10.4 ± 0.5b | 3.1 ± 1.2c | *** | |
| G120 | 32.8 ± 2.3 a | 30.7 ± 2.4 ab | 26.5 ± 1.3 bc | 23.5 ± 2.0c | ** | |
| I90 | 128.2 ± 1.0a | 123.5 ± 11.9a | 112.8 ± 2.9a | 119.2 ± 1.3a | NS | |
| I200 | 165.5 ± 6.8a | 141.4 ± 0.9b | 149.9 ± 4.0ab | 150.3 ± 11.0ab | * | |
| Kinetic time | S-0 | S-80 | S-200 | S-120 | p-value | |
| Residual nitrites (µM) | T0 | 3.6 ± 8.9a | 79.1 ± 4.2b | 96.0 ± 17.4b | 183.3 ± 26.1c | ** |
| G40 | 0c | 9.5 ± 1.3b | 7.9 ± 1.5b | 22.7 ± 0.9a | *** | |
| G80 | 0c | 15.8 ± 3.3b | 21.1 ± 1.1b | 40.5 ± 4.5a | *** | |
| G120 | 0d | 87.4 ± 2.7c | 104.7 ± 6.0b | 172.5 ± 5.9a | *** | |
| Residual nitrates (µM) | T0 | 67.6 ± 10.0a | 110.5 ± 82.1a | 211.9 ± 23.9b | 343.5 ± 51.3c | ** |
| G40 | 73.2 ± 14.1a | 56.0 ± 1.5ab | 55.3 ± 11.1ab | 44.5 ± 8.4b | * | |
| G80 | 80.0 ± 13.8a | 96.4 ± 11.7a | 90.6 ± 17.6a | 91.39 ± 35.2a | * | |
| G120 | 70.5 ± 9.5c | 178.9 ± 26.6ab | 159.6 ± 44.9b | 245.3 ± 18.1a | *** | |
| Total non volatile N-nitrosamines (µM) | T0 | ND | ND | 204.0 ± 80.5a | 123.0 ± 74.7a | NS |
| G40 | 0b | 62.5 ± 16.5a | 81.2 ± 36.4a | 79.7 ± 10.6a | *** | |
| G80 | 22.4 ± 8.3c | 58.6 ± 6.0b | 74.9 ± 4.1a | 89.1 ± 8.4a | *** | |
| G120 | 36.6 ± 9.3b | 100.9 ± 15.8 a | 141.5 ± 43.0a | 129.1 ± 5.7a | *** | |
| Nitrosothiols (µM) | T0 | 5.7 ± 3.2a | 0.4 ± 1.1a | 38.6 ± 9.6a | 34.2 ± 11.4a | NS |
| G40 | 0b | 3.3 ± 1.4a | 1.5 ± 1.9a | 3.7 ± 0.5a | ** | |
| G80 | 0c | 4.2 ± 1.0ab | 3.5 ± 1.3bc | 7.0 ± 2.6a | *** | |
| G120 | 0b | 5.8 ± 1.5ab | 5.4 ± 0.6ab | 8.9 ± 6.8a | * |
Legend: (A): S-0, S-80, S-120, and S-200 corresponded to 0/0, 80/80, 0/200, and 120/120 ppm of NaNO2/NaNO3 added, respectively. The concentrations measured in the products are given at time T0 (Bonifacie, Gatellier et al., 2021), when the concentrations measured after 40, 80 and 120 min in the gastric compartment are given as G40, G80 and G120. The amounts of compounds found after 90 and 200 min of digestion in the ileal compartment are designated as I90 and I200. Values are reported as means ± standard deviation of independent determinations. Homogeneous groups are to be read by kinetic time in order to identify the effect of nitrite/nitrate curing dose on the biochemical assay. The significant differences between the samples for each time are given such as: p-value > 0.05: non significative (NS); p-value < 0.05: *; p-value < 0.01: **; p-value < 0.001: ***. ND: non-detected value.
Table 2B.
Significant differences for both between subjects and within subjects. Interactions are provided.
| (B) | Digestive compartment | Between subject effect | Within subject effect | Interaction |
|---|---|---|---|---|
| Total heme iron (µM) | Gastric | *** | *** | *** |
| Intestinal | * | *** | NS | |
| Nitrosylated iron (µM) | Gastric | *** | *** | *** |
| Intestinal | *** | *** | *** | |
| TBARS (µM) | Gastric | *** | *** | *** |
| Intestinal | *** | *** | *** | |
| Total free iron (µM) | Gastric | *** | *** | *** |
| Intestinal | * | *** | NS | |
| Total non volatile N-nitrosamines (µM) | Gastric | *** | *** | NS |
| Nitrosothiols (µM) | Gastric | *** | * | * |
| Residual nitrites (µM) | Gastric | *** | *** | *** |
| Residual nitrates (µM) | Gastric | ** | *** | *** |
Legend: (B): Table synthetizing the p-values for the gastric and ileal compartments for effects between subjects (nitrite and nitrate dose–effect), and for effects within subjects (significant differences due to digestive time). Interaction between the nitrite/nitrate dose and time is also provided.
Lipid oxidation
Lipid oxidation was assessed by the TBARS assay, in order to quantify the quantity of secondary aldehydes generated through lipid oxidation chain reaction. The evolution of the TBARS concentration over digestion allows to distinguish the different formulations of the dry cured sausages according to their nitrite/nitrate formulations. Indeed, in the absence of nitrite and nitrate a significant larger amount of TBARS was measured both at the end of the gastric digestion and at the end of the intestinal phase, compared to the three other formulas. Lipid oxidation was up to seven times more important in absence of additives at G120, and up to twice more at I200. Moreover, none of the three amounts studied through the different formulations of nitrite and nitrate could be discriminated, as the TBARS concentrations were equivalent, and not statistically different from one another.
The results from the digestive study may contrast with those obtained into the products, as the digestion modeling lead to a complete deconstruction of dry-cured sausages, thus separating the lipid-rich fraction from the muscle-rich fraction, leading to a more random sampling. Nevertheless, not only those results are consistent with the literature, according which nitrite and nitrate are major antioxidant additives (Berardo, De Maere, Stavropoulou, Rysman, Leroy, & De Smet, 2016), but they are also relevant with the quantities measured in the dry-cured fermented sausages prior to the digestive study (Bonifacie, Gatellier et al., 2021). The same protective effect of nitrites and nitrates was observed in the cured fermented sausages during digestion, which is in accordance with the results from Van Hecke et al. (Van Hecke et al., 2016).
This feature has to be related with the capacity of nitric oxide to bind to lipoperoxyde radicals, to form stable compounds called nitroso-lipoperoxydes (Kanner, 1994, Min and Ahn, 2005). It has been previously estimated that 1 to 15 % of the introduced nitrites in meat products bind to lipids (Honikel, 2008). The complex chemistry of nitrites in contact with lipids has been described by Bonifacie et al. in dry-cured fermented sausages (Bonifacie, Gatellier et al., 2021). The present results indicate that the lipidic nitroso-peroxydes remain stable over the digestive physico-chemical environment, and ensure the antioxidant trait of the additives even through digestion. Nitrite and nitrate therefore protect from the generation of free cytotoxic aldehydes (notably malondialdehyde (MDA), 4-hydroxy-2-nonenal (4-HNE) and 4-hydroxy-2-hexenal (4-HHE) during digestion. The results from Van Hecke et al. showed that MDA was by far the main aldehyde synthetized during digestions of dry-cured fermented sausages (Van Hecke, Vanden Bussche, Vanhaecke, Vossen, Van Camp, & De Smet, 2014). Yet, aldehydes and mainly MDA are toxic compounds able to react according to three principal paths. MDA can be absorbed through the small intestine as a free molecule, then leading to MDA-DNA-adducts. It can also bind to bacterial cellular components, either proteins, then leading to Schiff bases, or to bacterial DNA, leading to DNA-adducts; which is of great stake when studying fermented products (Montel, 1999). Those adducts generally promote mutagenesis, and through multiple reactions, may trigger carcinogenesis (Kuhnle et al., 2007, Van Hecke et al., 2014).
It is therefore paramount to take heed of the role of nitrites and nitrates in the protection against lipid oxidation, as in addition to prevent from the degradation of the organoleptic characteristics of the products (lipid oxidation conducing to rancid aromas), it predominantly protects them from the generation of toxic molecules that can react in the digestive tract, impeding the nutritional quality of nitrite and nitrate cured processed meats. Notwithstanding, the present results highlight an important point for processed meat manufacturers, which is the dose–response of the addition of nitrite and nitrate in sausages, as no significant difference was observed on the formation of TBARS between the three formulations. Therefore, even the minimal dose of nitrite and nitrate (80/80) may protect the products against lipid oxidation chain reactions, which is paramount considering the global reactivity of those additives in processed meats products. It is even more accurate to consider nitrite and nitrate for their antioxidant activity, as it was previously suggested that heme iron, contained in every muscle cell, catalysed the synthesis of such cytotoxic aldehydes (Corpet, 2011).
Total heme iron and nitrosylated iron
Nitrosylation is the reaction between nitric oxide and heme iron, to form nitrosylated heme iron (also called nitrosylheme). In the digestates, both nitrosylheme and total heme iron were assessed, in order to get a global perspective of the nitrosylation degree.
First, concerning total heme iron, it is worth noticing that there is no concrete distinction between the different nitrite and nitrate formulations for every digestion tests. This contrasts with the results from the products, where the absence of nitrite was correlated with less total heme iron. This effect seems to be erased throughout the digestion process. Yet, those results have to be taken with caution considering the variability of the data.
Then, regarding nitrosylated iron, the detection of the compound through digestion was very progressive according to the different formulas, and reached at the end of digestion 62.1 ± 4.1 µM for S-120, which is even less than half the amount found initially in the dry-cured products. S-80 and S-200 had comparable amounts of nitrosylated irons, 45.6 ± 3.6 and 42.0 ± 3.8 µM respectively, when S-0 had very few of it.
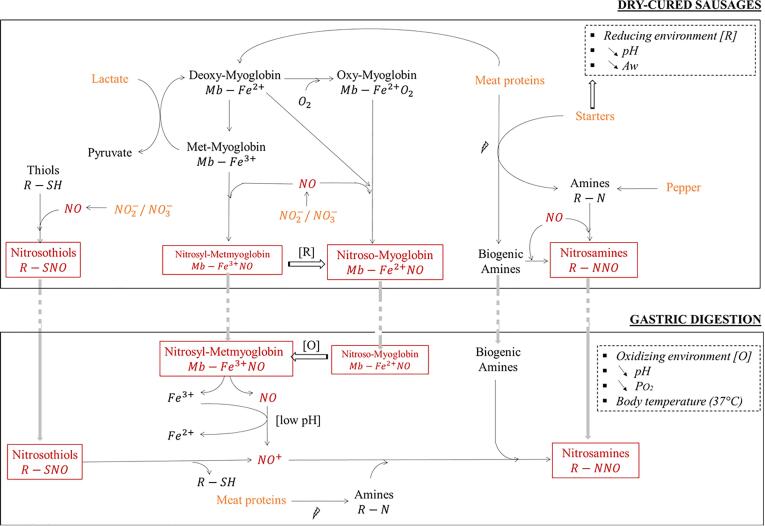
Nevertheless, the striking point with those results comes from the difference between the amounts of nitrosylheme recovered in the digestates compared to those in the sausages. This huge decrease suggests that this compound was either unstable through the digestive process or not accessible. When quantifying nitrosylheme, the methodology does not allow to discriminate the redox state of iron and its ligand. Indeed, several pathways may lead to nitrosylheme’s synthesis: nitric oxide is able to bind to the iron atom of deoxy-myoglobin (Mb-Fe2+) or metmyoglobin (Mb-Fe3+), or to exclude dioxygen from oxy-myoglobin (Mb-Fe2+–O2) (de La Pomélie et al., 2018, Montel, 1999). Nevertheless, the redox environment will prevail over the one or the other form of nitrosylheme (Montel, 1999), as more as no ascorbate was added in dry-cured fermented sausages formulations, contrary to some other processed meats formulas. Nitric oxide has indeed a major ability to bind to the iron atom compared to dioxygen (Skibsted, 2011). Therefore, two forms of nitrosylheme may exist simultaneously: nitrosyl-metmyoglobin (Mb-Fe3+–NO) and nitroso-myoglobin (Mb-Fe2+–NO). Hence, in dry-cured sausages products, the physico-chemical environment is highly prone to create a reducing environment (Montel, 1999). The microbial starters are key contributors in this (Hammes, 2012). In addition, the carbohydrates used in the formulation of the sausages are also part of the existing redox environment. Indeed, lactose is used as substrate for the microbial starters, of which lactic acid bacteria Lactobacillus sakei, to form lactic acid. This compound benefits the product by its anti-microbial features, by the organoleptic aspects procured to the sausages (colour, flavour, tenderness), and by the enhancement of a reductive environment (McClure, Sebranek, Kim, & Sullivan, 2011; Skibsted, 2011). Indeed, it was shown to promote the form of deoxymyoglobin within the products (McClure, Sebranek, Kim, & Sullivan, 2011). As a consequence, the nitroso-myoglobin form of nitrosylheme prevails in the products over nitrosyl-metmyoglobin, and thus ensure a longer desirable red color of the sausages (McClure, Sebranek, Kim, & Sullivan, 2011; Skibsted, 2011) nitrosyl-metmyoglobin is moreover very unstable (Fox and Thomson, 1963, Montel, 1999). Those conditions are also advantageous in a sensorial perspective, as nitroso-myoglobin is a red pigment, and in this way ensure a desirable colour for dry-cured sausages consumers (Skibsted, 2011).
Yet, when the digestion process begins, the redox environment is dramatically different, as the gastric compartment may be compared to an oxidative bioreactor (Kanner & Lapidot, 2001). Therefore, nitroso-myoglobin, which in the case of dry-cured fermented sausages is not denatured in nitrosylhemochrom (the pink pigment desired in cooked processed meats like cooked ham) and is therefore still able to change of conformation, may tend to be oxidized in nitrosyl-metmyoglobin (Skibsted, 2011). But as mentioned, this form of nitrosylheme is very unstable (Fox & Thomson, 1963). The loss in nitrosylated iron over digestion may therefore by explained by the destabilization of nitrosyl-metmyoglobin into free iron and nitrosonium ions (promoted by a low pH in the gastric compartment).
Total free iron
Results in Table 2A, Table 2B presents the evolution of total free iron (Fe2+ and Fe3+) concentrations in the gastric compartment and in the ileum one. In both of them, the concentrations were increasing over time. At the end of the gastric digestion, the values of total free iron were included between approximately 23.5 ± 2.0 µM and 32.8 ± 2.3 µM. The values obtained in the ileal compartment are way more important, reaching 150.3 ± 11.0 µM to 165.5 ± 6.8 µM; yet potential interferences between the composition of the ileal medium and the assay may be responsible for this increase (based on practical observations where turbidity was appearing). More investigation would be required regarding alternative furniture in free iron from the food matrix of the medium, notably in the case of a fermented product.
Though, a tendency emerges when looking at the extreme conditions of nitrite and nitrate at the end of the gastric digestion. Little free total iron was recovered in absence of nitrite (S-0) than in its presence at G120, at its maximal amount (S-120). This joins the observations in the products where nitrite stabilize heme iron through its nitrosylation (Bonifacie, Gatellier et al., 2021). This was explained by the iron atom at the center of the heme, which was more eager to destabilization and to turn into free iron when not bound to nitric oxide (Bechaux, de La Pomélie, Théron, Santé-Lhoutellier, & Gatellier, 2018).
Nevertheless, total free iron recovered in the digestates was two to three times more important for the three formulations of sausages with additives (S-80, S-120 and S-200). This rise indicates a liberation of the compound through the digestive process. Those results are consistent with the hypothesis on the destabilization of the nitrosylated heme iron of the cured sausages, whereby the poor stability of nitrosyl-metmyoglobin leads to the liberation of free iron on one side, and nitrosonium ions on the other side.
Residual nitrites and residual nitrates
Residual nitrites and residual nitrates were assessed by spectrophotometry in the digestates, as the free part of the additives which remained unreacted in the product.
Residual nitrites concentrations rose during the gastric digestion of the three conditions nitrites and nitrates cured sausages, until values tightly close to those obtained in the products. No residual nitrite was detected in absence of the additive in the formulation of the product. The residual amounts were progressive along with the quantities introduced: S-80, S-200 and S-120 had respective growing concentrations of residual nitrites at the end of the gastric digestion. This observation is consistent among the study from Hospital et al. (Hospital, Carballo, Fernández, Arnau, Gratacós, & Hierro, 2015) who observed a dose response linking the quantities of introduced nitrites with the concentrations of residual nitrites in the products. The present paper indicates that those concentrations found in the products remain stable over the digestive process, yet this unexpected point should be clarified in future experiments in order to understand the underlying mechanisms behind this apparent stability. Those results are contradictory with those of Kim et al. who observed a significant fall in residual nitrites concentrations during digestion (Kim & Hur, 2018).
Regarding residual nitrates, a basal quantity was recovered even in absence of additives in the formulations (67.6 ± 10.0 µM), and was stable all over the gastric digestion around, as no major significant difference could be noticed (between 70.5 ± 9.5 µM to 80.0 ± 13.8). In the same way as for residual nitrites, the amounts of residual nitrates are correlated with the amounts used for the formulations: significantly more compounds were recovered for S-120 then for S-80 and S-200. The basal concentration found in S-0 relates to the intrinsic chemistry of the meat used in the formulation. Indeed, in pork meat, auto-proteolysis can lead to the production of nitrogenous molecules, then leading to the production of nitrates (Karwowska & Kononiuk, 2020).
Nitrosothiols and nitrosamines
The assay developed to assess non-volatiles fractions of nitroso-compounds allows to detect on the one hand nitrosothiols, and nitrosamines on the other hand. In the product, very little nitrosothiols were recovered by Bonifacie et al.: almost none was detected in S-0 and S-80; but around 35.00 ± 10.00 µM for S-120 and S-200 (Bonifacie, Gatellier et al., 2021). During digestion, the amounts found of nitrosothiols were very low and tantamount to the low concentrations found in the products S-0 and S-80. This was the case for the gastric digestion. Those results suggest that nitrosothiols initially present in S-120 and S-200 have been disassociated under digestive conditions. In that case, nitrosonium ions would have been liberated in the medium, from the decoupling of the NO group from the thiol radical. This hypothesis seems relevant considering the poor stability of nitrosothiols: the bound linking nitric oxide to the thiol radical is very labile, and therefore these compounds appear to be unstable, as they are quickly reduced into low molecular weight molecules such as glutathiones (Jain, von Toerne, Lindermayr, & Bhatla, 2018).
Total non-volatile nitrosamines were not found in sausages S-0 and S-80, only in S-120 and S-200. The fact that dry-cured sausages do not go through heat treatments may be the reason for the minimal encounter with nitrosamines within the products (Honikel, 2008). Yet, during digestion, the amounts of nitrosamines went growing for all the nitrite and nitrate conditions, until the end of the gastric digestion. It is worth noticing that even for the negative control, some nitrosamines have been measured at G120, whereas none of them was initially in the product. Moreover, about 100.9 ± 15.8 µM of nitrosamines were estimated at the end of the gastric digestion of S-80. Yet, none was detected in the products. This indicated an endogenous synthesis of about 25 % non-volatile nitrosamines during digestion. Nitrosamines stem from the reaction of secondary amines with nitrosonium ions. The gastric compartment is propitious to these ions for its low pH (de La Pomélie, Santé-Lhoutellier, & Gatellier, 2017), and to these amines for protease’s activity in the gastric compartment. The endogenous synthesis of non-volatile nitrosamines is therefore enhanced, especially by the physico-chemical environment of the gastric compartment.
According to the previously mentioned hypothesis (Fig. 1), nitrosonium ions concentrations in the gastric compartment may be risen by the destabilization of nitrosyl-metmyoglobin and nitrosothiols. Simultaneously, the bioavailability in secondary amines also rises while proteolysis is undergoing during digestion. Therefore, endogenous N-nitrosation may be enhanced by the rise in the reagents of this reaction.
Fig. 1.
Proposed mechanism for the chemical reactivity of nitroso-compounds in the gastro-intestinal tract studied in the present paper. Legend: The dry-cured sausages formulation compounds are represented in orange, the nitroso-compounds in red-squared text (different sizes represent different proportions: major quantity with bigger text, minor quantity with smaller text) and the proteolysis with the thunderbolt symbol. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Conclusion
Additives in food products are of high technological relevance. Nonetheless, the role they play on human health must be considered in order to wisely weigh the benefits against the risks of those products’ consumption. This is of major importance when considering nitrites and nitrates in processed meats, as increasingly more data are provided on their chemistry. To address this matter, the study of the chemical reactivity happening in the gastro-intestinal tract allows to provide key data on how the chemistry linking technological and organoleptical tools (i.e. nitrites and nitrates) to nutritional risks (i.e. DNA adducts chain formation) could be assessed. To do so, in vitro dynamic digestions is a useful tool to model the chemical reactivity over time. To complete the data herein obtained through digestion time, similar studies should be conducted in vivo, thereby allowing to scheme a more complete overview of the digestive chemical reactivity of nitrite and nitrate cured processed meats.
Funding
This research was funded by the consortium ADDUITS and INRAE.
CRediT authorship contribution statement
Eléna Keuleyan: Investigation, Data curation, Formal analysis, Validation, Supervision, Conceptualization, Project administration. Aline Bonifacie: Data curation, Formal analysis, Validation. Thierry Sayd: Data curation, Formal analysis, Validation. Angéline Duval: Investigation. Laurent Aubry: Investigation. Sylvie Bourillon: Investigation. Philippe Gatellier: Data curation, Formal analysis, Validation, Supervision, Conceptualization, Project administration, Funding acquisition. Aurélie Promeyrat: Data curation, Formal analysis, Validation, Supervision, Conceptualization, Project administration, Funding acquisition. Gilles Nassy: Data curation, Formal analysis, Validation, Supervision, Conceptualization, Project administration, Funding acquisition. Valérie Scislowski: Data curation, Formal analysis, Validation, Supervision, Conceptualization, Project administration, Funding acquisition. Laurent Picgirard: Data curation, Formal analysis, Validation, Supervision, Conceptualization, Project administration, Funding acquisition. Laëtitia Théron: Data curation, Formal analysis, Validation, Supervision, Conceptualization, Project administration. Véronique Santé-Lhoutellier: Data curation, Formal analysis, Validation, Supervision, Conceptualization, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank Célia Nigon for her contribution in the experimental work, and the ADIV staff for taking care of the fermented sausages production. The members of the ADDUITS leading committee are thanked for their review and editing help on this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100474.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Bechaux J., de La Pomélie D., Théron L., Santé-Lhoutellier V., Gatellier P. IroN-catalysed chemistry in the gastrointestinal tract: Mechanisms, kinetics and consequences. A review. Food Chemistry. 2018;268:27–39. doi: 10.1016/j.foodchem.2018.06.018. [DOI] [PubMed] [Google Scholar]
- Berardo A., De Maere H., Stavropoulou D.A., Rysman T., Leroy F., De Smet S. Effect of sodium ascorbate and sodium nitrite on protein and lipid oxidation in dry fermented sausages. Meat Science. 2016;121:359–364. doi: 10.1016/j.meatsci.2016.07.003. [DOI] [PubMed] [Google Scholar]
- Bonifacie A., Aubry L., Gatellier P., Santé-Lhoutellier V., Théron L. Determination of nitroso-compounds in food products. MethodsX. 2021;8 doi: 10.1016/j.mex.2021.101289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacie A., Gatellier P., Promeyrat A., Nassy G., Picgirard L., Scislowski V.…Theron L. New Insights into the chemical reactivity of dry-cured fermented sausages: Focus on nitrosation, nitrosylation and oxidation. Foods. 2021;10(4) doi: 10.3390/foods10040852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvard V., Loomis D., Guyton K.Z., Grosse Y., Ghissassi F.E., Benbrahim-Tallaa L.…Straif K. Carcinogenicity of consumption of red and processed meat. The Lancet Oncology. 2015;16(16):1599–1600. doi: 10.1016/S1470-2045(15)00444-1. [DOI] [PubMed] [Google Scholar]
- Corpet D.E. Red meat and colon cancer: Should we become vegetarians, or can we make meat safer? Meat Science. 2011;89(3):310–316. doi: 10.1016/j.meatsci.2011.04.009. [DOI] [PubMed] [Google Scholar]
- de La Pomélie D., Santé-Lhoutellier V., Gatellier P. Mechanisms and kinetics of tryptophan N-nitrosation in a gastro-intestinal model. Food Chemistry. 2017;218:487–495. doi: 10.1016/j.foodchem.2016.08.131. [DOI] [PubMed] [Google Scholar]
- de La Pomélie D., Santé-Lhoutellier V., Gatellier P. Mechanisms and kinetics of heme iron nitrosylation in an in vitro gastro-intestinal model. Food Chemistry. 2018;239:86–93. doi: 10.1016/j.foodchem.2017.06.092. [DOI] [PubMed] [Google Scholar]
- de La Pomélie D., Santé-Lhoutellier V., Sayd T., Gatellier P. Oxidation and nitrosation of meat proteins under gastro-intestinal conditions: Consequences in terms of nutritional and health values of meat. Food Chemistry. 2018;243:295–304. doi: 10.1016/j.foodchem.2017.09.135. [DOI] [PubMed] [Google Scholar]
- De Mey E., De Klerck K., De Maere H., Dewulf L., Derdelinckx G., Peeters M.C.…Paelinck H. The occurrence of N-nitrosamines, residual nitrite and biogenic amines in commercial dry fermented sausages and evaluation of their occasional relation. Meat Science. 2014;96(2 Pt A):821–828. doi: 10.1016/j.meatsci.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Demeyer D., Mertens B., De Smet S., Ulens M. Mechanisms linking colorectal cancer to the consumption of (processed) red meat: A review. Critical Reviews in Food Science and Nutrition. 2016;56(16):2747–2766. doi: 10.1080/10408398.2013.873886. [DOI] [PubMed] [Google Scholar]
- Folch J., Lees M., Sloane-Stanley G.H. A simple method for the isolation and purification of total lipides from animal tissues. Journal of Biological Chemistry. 1957;226(1):497–509. [PubMed] [Google Scholar]
- Fox J.B., Jr., Thomson J.S. Formation of bovine nitrosylmyoglobin. Biochemistry. 1963;2(3):465–470. doi: 10.1021/bi00903a012. I. pH 4.5-6.5. [DOI] [PubMed] [Google Scholar]
- Gueraud F., Tache S., Steghens J.P., Milkovic L., Borovic-Sunjic S., Zarkovic N.…Priymenko N. Dietary polyunsaturated fatty acids and heme iron induce oxidative stress biomarkers and a cancer promoting environment in the colon of rats. Free Radical Biology and Medicine. 2015;83:192–200. doi: 10.1016/j.freeradbiomed.2015.02.023. [DOI] [PubMed] [Google Scholar]
- Guillemin, H., Perret, B., Picque, D., Menard, O., & Cattenoz, T. (2010). Logiciel StoRM – Stomach and duodenum Regulation and Monitoring. IDDN.FR.001.30009.000.R.P.2010.000, 31235, 290.
- Hammes W.P. Metabolism of nitrate in fermented meats: The characteristic feature of a specific group of fermented foods. Food Microbiology. 2012;29(2):151–156. doi: 10.1016/j.fm.2011.06.016. [DOI] [PubMed] [Google Scholar]
- Hébel P. La consommation de charcuteries en France. Cahiers de Nutrition et de Diététique. 2019;54(5):5S16-15S22. [Google Scholar]
- Honikel K.O. The use and control of nitrate and nitrite for the processing of meat products. Meat Science. 2008;78(1–2):68–76. doi: 10.1016/j.meatsci.2007.05.030. [DOI] [PubMed] [Google Scholar]
- Hospital X.F., Carballo J., Fernández M., Arnau J., Gratacós M., Hierro E. Technological implications of reducing nitrate and nitrite levels in dry-fermented sausages: Typical microbiota, residual nitrate and nitrite and volatile profile. Food Control. 2015;57:275–281. [Google Scholar]
- IARC. (2018). Red meat and processed meat. IARC monographs on the evaluation of carcinogenic risks to humans, 114.
- Jain P., von Toerne C., Lindermayr C., Bhatla S.C. S-nitrosylation/denitrosylation as a regulatory mechanism of salt stress sensing in sunflower seedlings. Physiologia Plantarum. 2018;162(1):49–72. doi: 10.1111/ppl.12641. [DOI] [PubMed] [Google Scholar]
- Johnston B.C., Zeraatkar D., Han M.A., Vernooij R.W.M., Valli C., El Dib R.…Guyatt G.H. Unprocessed red meat and processed meat consumption: Dietary guideline recommendations from the nutritional recommendations (NutriRECS) consortium. Annals of Internal Medicine. 2019;171(10):756–764. doi: 10.7326/M19-1621. [DOI] [PubMed] [Google Scholar]
- Kanner J. Oxidative processes in meat and meat products: Quality implications. Meat Science. 1994;36:169–189. doi: 10.1016/0309-1740(94)90040-X. [DOI] [PubMed] [Google Scholar]
- Kanner J., Lapidot T. The stomach as a bioreactor: dietary lipid peroxidation in the gastric fluid and the effects of plant-derived antioxidants. Free Radic Biol Med. 2001;31(11):1388–1395. doi: 10.1016/s0891-5849(01)00718-3. [DOI] [PubMed] [Google Scholar]
- Karwowska M., Kononiuk A. Nitrates/nitrites in food-risk for nitrosative stress and benefits. Antioxidants. 2020;9(3) doi: 10.3390/antiox9030241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.S., Hur S.J. Effect of six different starter cultures on the concentration of residual nitrite in fermented sausages during in vitro human digestion. Food Chemistry. 2018;239:556–560. doi: 10.1016/j.foodchem.2017.06.160. [DOI] [PubMed] [Google Scholar]
- Kuhnle G.G., Story G.W., Reda T., Mani A.R., Moore K.P., Lunn J.C., Bingham S.A. Diet-induced endogenous formation of nitroso compounds in the GI tract. Free Radical Biology and Medicine. 2007;43(7):1040–1047. doi: 10.1016/j.freeradbiomed.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Marco A., Navarro J.L., Flores M. The influence of nitrite and nitrate on microbial, chemical and sensory parameters of slow dry fermented sausage. Meat Science. 2006;73(4):660–673. doi: 10.1016/j.meatsci.2006.03.011. [DOI] [PubMed] [Google Scholar]
- McClure B.N., Sebranek J.G., Kim Y.H., Sullivan G.A. The effects of lactate on nitrosylmyoglobin formation from nitrite and metmyoglobin in a cured meat system. Food Chemistry. 2011;129(3):1072–1079. doi: 10.1016/j.foodchem.2011.05.077. [DOI] [PubMed] [Google Scholar]
- Menard, O., Picque, D., & Dupont, D. (2015). The DIDGI((R)) System. In K. Verhoeckx, P. Cotter, I. Lopez-Exposito, C. Kleiveland, T. Lea, A. Mackie, T. Requena, D. Swiatecka & H. Wichers (Eds.), The impact of food bioactives on health: in vitro and ex vivo models, (pp. 73-81). Cham (CH). [PubMed]
- Min B., Ahn D.U. Mechanism of lipid peroxidation in meat and meat products – A review. Food Science Biotechnology. 2005;14(1):152–163. [Google Scholar]
- Montel M.C. Fermented Foods – Fermented meat products. Encyclopedia of Food Microbiology. 1999:744–753. [Google Scholar]
- Ordonez J.A., Hierro E.M., Bruna J.M., de la Hoz L. Changes in the components of dry-fermented sausages during ripening. Critical Reviews in food science and nutrition. 1999;39(4):329–367. doi: 10.1080/10408699991279204. [DOI] [PubMed] [Google Scholar]
- Papuc C., Goran G.V., Predescu C.N., Nicorescu V. Mechanisms of oxidative processes in meat and toxicity induced by postprandial degradation products: A review. Comprehensive Reviews in Food Science and Food Safety. 2017;16(1):96–123. doi: 10.1111/1541-4337.12241. [DOI] [PubMed] [Google Scholar]
- Santarelli R.L., Vendeuvre J.-L., Naud N., Taché S., Guéraud F., Viau M.…Pierre F.H. Meat processing and colon carcinogenesis: Cooked, nitrite-treated, and oxidized high-heme cured meat promotes muciN-depleted foci in rats. Cancer Prevention Research. 2010;3(7):852–864. doi: 10.1158/1940-6207.CAPR-09-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolze K., Dadak A., Liu Y., Nohl H. Hydroxylamine and phenol-induced formation of methemoglobin and free radical intermediates in erythrocytes. Biochemical Pharmacology. 1996;52(12):1821–1829. doi: 10.1016/s0006-2952(96)00460-1. [DOI] [PubMed] [Google Scholar]
- Van Hecke T., Vanden Bussche J., Vanhaecke L., Vossen E., Van Camp J., De Smet S. Nitrite curing of chicken, pork, and beef inhibits oxidation but does not affect N-nitroso compound (NOC)-specific DNA adduct formation during in vitro digestion. Journal of Agricultural and Food Chemistry. 2014;62(8):1980–1988. doi: 10.1021/jf4057583. [DOI] [PubMed] [Google Scholar]
- Van Hecke T., Wouters A., Rombouts C., Izzati T., Berardo A., Vossen E.…De Smet S. Reducing compounds equivocally influence oxidation during digestion of a high-fat beef product, which promotes cytotoxicity in colorectal carcinoma cell lines. Journal of Agricultural and Food Chemistry. 2016;64(7):1600–1609. doi: 10.1021/acs.jafc.5b05915. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.