Abstract
Mycoplasma and Salmonella are serious pathogens threaten the poultry industry. This study aimed to prepare and evaluate an inactivated pentavalent vaccine targeting bacteria, including Salmonella enterica serovar Typhimurium (ST), Salmonella enterica serovar Enteritidis (SE), Salmonella enterica serovar Kentucky (SK), Mycoplasma gallisepticum (MG), and Mycoplasma synoviae (MS), from locally isolated strains. The prepared vaccine was adjuvanted with Montanide ISA70 oil and then tested for safety, sterility, and potency. The vaccine efficacy was evaluated in 110 specific pathogen-free, 1-day-old chicks, which were divided into three groups as follows: 1) vaccinated group (50 birds), which was subdivided into five subgroups of ten birds each; 2) control positive (challenged) group (50 birds), which was subdivided into five subgroups of ten birds each; and 3) control negative (blank) group, which included ten birds. Chicks in group 1 were administered the first dose of vaccine at 7 d of age followed by a booster dose after 3 wk. At 3 wk after booster vaccination, the chicks who were administered the booster dose were challenged and kept under observation until the end of the experiment when the chicks were approximately 10 wk. Details of clinical symptoms, daily mortality, weights, and postmortem lesions; serum samples; cloacal swabs; and nasal swabs were collected during the experiment. The humoral immune response to the prepared pentavalent vaccine was assessed using enzyme-linked immunosorbent assay. Our findings revealed that the prepared vaccine showed high protective antibody titers against Salmonella and Mycoplasma with 100% efficacy and no mortalities (100% survival rate) were recorded in vaccinated and challenged birds. The vaccine reduced both clinical signs and bacterial shedding post challenge in vaccinated birds in comparison with control positive group. The prepared vaccine did not affect the body weight gain of the vaccinated birds in comparison with control negative birds. The current study concluded that locally manufactured inactivated pentavalent vaccine offers protection to birds and could be employed as an effective tool along with biosecurity measures to overcome mycoplasmosis and salmonellosis in layer and breeder chicken farms in Egypt.
Key words: inactivated vaccine, Mycoplasma, montanide oil, pentavalent, Salmonella
INTRODUCTION
Diseases caused by Salmonella enterica serovar Typhimurium (ST), Salmonella enterica serovar Enteritidis (SE), Salmonella enterica serovar Kentucky (SK), Mycoplasma gallisepticum (MG), and Mycoplasma synoviae (MS), cause significant economic losses to the poultry industry worldwide, particularly in chicken and turkey populations and these diseases are linked to digestive, reproductive, locomotive, and respiratory illnesses; poor performance; decreased egg production; reduced fertility; and increased embryonic mortality (Eissa et al., 2014; Ibrahim et al., 2018; Marouf et al., 2020, 2022).
Salmonella species is a gram-negative, facultative bacterium, which infects birds, reptiles, and mammals, including humans (El-Saadony et al., 2022). Salmonella species and their contamination of chicken eggs, especially by SE, has become a significant public health hazard (Guard-Petter, 2001; Cogan and Humphrey, 2003). ST is a common contaminant isolated from broiler chicken flesh and eggs. Therefore, it is necessary to address both SE and ST infections in poultry (Ibrahim et al., 2018; El-Saadony et al., 2022). As long-term use of antibiotics in poultry production has become more prevalent, antibiotic resistance in Salmonellae has increased dramatically (Van Boeckel et al., 2019; Alvarez et al., 2020; Rabello et al., 2020), with increased risks to both animals and humans (Michael and Schwarz 2016; Pan et al., 2018). Therefore, application of biosecurity measures with proper vaccination and the usage of antibiotic natural alternatives is a global direction to minimize the usage of antibiotics (Abd El-Hack et al., 2022a).
Avian mycoplasma is an economically important disease, primarily due to MG and MS infections (El-Naggar et al., 2022). These infections cause significant economic losses due to the impact of several factors on poultry, such as weight loss, low feed conversion efficiency, lowered egg production, lowered hatchability, increased embryo mortality, increased carcass condemnation rates, increased costs for broiler prophylaxis, layer therapy costs, and increased costs associated with breeder flocks (El-Naggar et al., 2022). Infections caused by MG and MS are respiratory infections recognized by the World Organization for Animal Health (OIE, 2012). MG causes chronic breathing illness and inflammation sinusitis in chicken, and MS causes synovitis and avian airsacculitis. Horizontal and vertical transmission of both the infections has been observed (El-Naggar et al., 2022).
Biosecurity, therapy, and immunization are commonly used prevention and control strategies for addressing avian mycoplasmosis. Various avian immunization vaccines exist against both MG and MS and include inactivated bacteria, live attenuated, and recombinant live poxvirus vaccines (Yadav et al., 2021).
Mycoplasmosis and salmonellosis in hens are most frequently prevented through vaccination along with appropriate management and biosecurity techniques (Ibrahim et al., 2018; El-Naggar et al., 2022). Previous studies have suggested that when dealing with multiage commercial layer operations suffering from endemic infections, vaccination is the most practical option (Ibrahim et al., 2018). Currently, there are commercially available trivalent vaccines against Salmonella infants, ST, and SE in several countries (Crouch et al., 2020). Moreover, a trivalent Salmonella vaccine (ST, SE, and SK) is commercially available in Egypt (Ibrahim et al., 2018).
Vaccination against Salmonella cannot completely prevent infection in chickens (OIE, 2012). Nevertheless, vaccinations can reduce the risk of further infection, cause reduction in the excretion of the organism, and prevent vertical transmission, which can contribute to contamination of hatching or table eggs along with other negative outcomes for poultry (OIE, 2012). Due to its efficacy, the vaccination is effective in conjunction with other hygiene measures and eradication methods, such as all-out production and culling, and its benefits also extend to biosecurity and farm sanitation (OIE, 2012).
Previous studies have primarily focused on live attenuated, bacteria-based, and recombinant vaccines (Hussein et al., 2007; Rabie and Amin Girh, 2020), and previous trials were often conducted to prepare vaccines for use against mycoplasmosis (Fabricant, 1975). Subsequently, different types of MG vaccines are available as live attenuated vaccines, including 6/85 strain, ts-11, F strain, K strain, and K5054 (Leigh et al., 2019). These comprised attenuated, recombinant, and genetically modified MG vaccines (Leigh et al., 2019).
These vaccines are typically administered using spray or eye drop techniques, and some of them induce protective immunity against MG (Leigh et al., 2019). Unfortunately, they may produce lesions in immunosuppressed flocks, particularly in the presence of co-infections and stressors, and they may additionally increase the risk of harmful bacterial shedding. Enzyme-linked immunosorbent assay (ELISA) is an effective quantitative technique to evaluate the immune response of different vaccines (Elyazeed et al., 2020). In the prophylaxis of respiratory illnesses, inactivated vaccines are often more effective compared with live vaccines. However, an exception exists in the case of more expensive and complicated vaccines, which are difficult to administer and sometimes require two doses to be effective (Ley, 2003; Ishfaq et al., 2020).
Various adjuvants have been used in previous studies, but the Montanide ISA70 water-in-oil (w/o) emulsion is considered a good option for use in bird vaccines (Cahyani et al., 2020; El-Jakee et al., 2020). In inactivated vaccines that have proven successful against ST, SE, SK, MG, and MS, one or more purified antigens, killed pathogens, or bacterins with an oil adjuvant can successfully induce an immune response (Ibrahim et al., 2018; El-Naggar et al., 2022).
Based on this foundation, the current study aimed to prepare and evaluate the efficacy of a pentavalent Mycoplasma and Salmonella vaccine comprising locally isolated ST, SE, SK, MG, and MS strains to overcome the previously encountered problems.
MATERIALS AND METHODS
Bacterial Strains
Locally isolated and well-identified strains of MG and MS were obtained from a previous study by Marouf et al. (2020), conducted at the Microbiology Department, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt. Salmonella strains (ST, SK, and SE) were kindly supplied by the Veterinary Serum and Vaccine Research Institute in Abbasia, Cairo, Egypt (Ibrahim et al., 2018).
Vaccine Preparation
MG and MS were cultured separately using Pleuro-Pneumonia Like Organisms (PPLO) broth (Becton Dickinson, Franklin Lakes, NJ, USA) and were incubated at 37°C in a CO2 incubator for 48 h, and the cultured bacterial colonies were adjusted to contain 1 × 108 colony-forming units (CFU) mL-1 in different groups of embryonated chicken eggs and hens. Calculations were performed using Rodwell and Whitcomb's (1983) standard techniques (Bekele and Assefa, 2018). ST, SK, and SE were each cultured on Salmonella Shigella (SS) agar (Lab M Limited, Lancashire, UK) at 37°C for 24 h. After being transferred into tryptone soya broth (Lab M Limited), the colonies were cultivated at 37°C for 24 h. After obtaining the total colony count, the bacterial solution was adjusted to contain 1 × 108 CFU mL-1.
All bacteria were pelleted after centrifugation at 12,000 x g for 30 min at 4°C. Using the total colony count approach, the bacterial counts of ST, SK, and SE were adjusted to 1 × 108 CFU 0.5 mL-1 of the final product by preparing separate final suspensions from ST, SK, and SE, respectively. Finally, all bacteria were inactivated using 0.3% formalin via agitation, and the bacterial suspension was combined with Montanide ISA70 (SEPPIC, Courbevoie, France) in a ratio of 70% volume of adjuvant to 30% volume of antigens according to Charles et al. (1994).
Quality Control of the Prepared Vaccine
Sterility Test for Oil Adjuvant and Trial Vaccine
According to Bekele and Assefa (2018), sterilization of the oil adjuvant (Montanide ISA 70) used in the immunization was achieved using a dry autoclave at 160°C for 1 h. These experiments were conducted at 37°C over a period of 7 d using thioglycolate (Lab M Limited), tryptic soy broth (Lab M Limited), tryptose agar (Lab M Limited), Sabouraud agar (Lab M Limited) and PPLO agar (Becton Dickinson). After the validation of the growth inactivation procedure, Montanide oil adjuvant was used to sufficiently emulsify the bacterial biomass. As a final step, the inactivated culture was sterilized and rendered safe.
Purity Test
The prepared vaccine was tested for bacterial and fungal contamination according to the OIE (2012).
Safety Test
In accordance with the OIE (2012), 20-day-old specific pathogen-free (SPF) chicks were administered a double field dose (1 mL) of the manufactured vaccine for two consecutive weeks. The chicks were observed daily for the appearance of any local responses, clinical symptoms, or mortality.
Experimental Design (Potency Test)
Experimental Birds
All experimental conditions and birds handling procedures were approved by the Ethics Committee, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt with number Vet CU12/10/2021/350.
All birds used in the study were initially examined to ensure that they were free of antibodies against Salmonella and Mycoplasma as well as their antigens. A total of 110 SPF 1-day-old chicks were obtained from Koom Osheem, Fayuom, Egypt. These chicks were housed in an area with a concrete floor with fresh wood shavings as bedding in separate pens on a deep litter system. The chicks were reared under optimal temperature, humidity, and ventilation, and were kept on a 24-h constant light schedule for a 10-wk observation period. Overall, they were kept under the same conditions as described by Evans et al. (2012).
Also, birds were provided with fresh, clean water and a balanced diet (containing starter and growth rations) without any interventions, that met or surpassed NRC (1994) recommendations.
Experimental Design
From 110 SPF 1-day-old chicks, three groups were formed, which were divided into subgroups, as summarized in Table 1. The first group comprised 50 birds, which were subdivided into five subgroups ( ten birds for each strain). Each chick in all subgroups was subcutaneously (S/C) injected at mid-neck region with 0.5 mL of the locally prepared inactivated pentavalent vaccine at 7 d of age. A booster dose was administered 3 wk after the administration of the first dose (28 days old); then, each subgroup was challenged 3 wK after the booster dose (49 days old) by administering 0.5 mL of each strain, MG and MS, which contained 1 × 108 CFU mL-1 of cells, via eye dropping in both the eyes as per the study by Bekele and Assefa (2018); moreover, 0.5 mL of each strain, ST, SE, and SK, which contained 1 × 108 CFU mL-1 of cells, was administered via crop gavage.
Table 1.
Experimental design.
| Groups | Subgroup (10 birds each) | First vaccine dose (0.5 mL via subcutaneous injection) | Booster dose (0.5 mL via subcutaneous injection) | Challenged bacteria (7 weeks = 49 days old) | |
|---|---|---|---|---|---|
| A total 50 bird (Vaccinated with pentavalent vaccine & challenged group) | MG | 7 days old | After 21 days (4 weeks = 28 days old) | 0.5 mL via eye dropping in both the eyes | MG |
| MS | MS | ||||
| ST | 0.5 mL via crop gavage | ST | |||
| SE | SE | ||||
| SK | SK | ||||
| A total 50 bird control positive (Challenged group) | MG | 0.5 mL via eye dropping in both the eyes | MG | ||
| MS | MS | ||||
| ST | - | - | 0.5 mL via crop gavage | ST | |
| SE | - | - | SE | ||
| SK | SK | ||||
| Control negative (Unvaccinated & unchallenged group) | - | - | - | - | - |
Salmonella enterica serovar Typhimurium (ST), Salmonella enterica serovar Enteritidis (SE), Salmonella enterica serovar Kentucky (SK), Mycoplasma gallisepticum (MG), and Mycoplasma synoviae (MS).
The second control positive challenged with ST, SE, SK, MG, and MS groups included 50 birds, which were subdivided into five subgroups (ten birds each). Each subgroup was individually challenged by administering 0.5 mL of MG and MS strains containing 1 × 108 CFU mL-1 of each strain into both the eyes via eye dropping, whereas 0.5 mL of ST, SE, and SK strains containing 1 × 108CFU mL-1 of each strain was administered via crop gavage. The third group included ten unvaccinated and unchallenged birds, which were kept as a blank control negative.
After the challenge, the inoculated chicks were observed for three consecutive weeks (10 wk = 70 days old). The degree of protection conferred by the vaccine was determined by the severity of the clinical signs; mortality; shedding of the challenge organisms from the tracheal swabs of MG and MS and cloacal samples of ST, SE, and SK.
For the estimation of bacterial shedding, cloacal swabs were collected from all birds in Salmonella groups which were inoculated into tetrathionate broth and examined bacteriologically for the shedding of different groups of Salmonella. In addition, nasal swabs and synovial fluids were collected from all birds in Mycoplasma groups which were inoculated into PPLO broth according to Hofstad et al. (1997).
Humoral immune responses were measured and evaluated using microagglutination ELISA using coated plates for MG and MS (BioChek ELISA Kit, Ascot, UK) as recommended by Ali et al. (2015). The humoral immune response against ST and SE antigens in the pentavalent vaccine was assessed by ELISA using coated plates (Jordan Bio-Industries Center -JOVAC, Amman, Jordan) while traditional ELISA was applied to detect antibodies against SK antigens as described by Barrow (1992). Therefore, blood samples (2 mL bird-1) were collected from the wing vein to separate sera samples, before immunization then, regularly after administering each vaccine dose, and after the challenge for 3 wk (once per week) to measure and evaluate the developed humoral immune response.
Body Weight Gain of Experimental Birds
The body weight gain of the birds was evaluated every week by analyzing feed intake, average body weight gain, and cumulative feed data. These were estimated to detect the efficacy of the trial vaccine in the birds individually and to compare the results among the pentavalent vaccinated, control positive challenged, and control negative groups.
Statistical Analysis
Data are represented as mean ± standard deviation. We analyzed the normal distribution using the Shapiro–Wilk test and analyzed homoscedasticity using the Levene's test. Additionally, significance was tested using an independent sample t test to compare the ELISA titers between the vaccinated and unvaccinated groups challenged with the same pathogen.
One-way ANOVA was performed to compare body weight gain among the three groups. Multiple comparisons between groups were conducted using the Fisher's Least Significant Difference post-hoc test. All statistical analyses were performed and graphs were obtained using RStudio v1.3.1093 (RStudio Team, 2020) and R programming language v4.0.3 (R Core Team, 2020). Differences between the groups were considered significant when P-values were < 0.05.
RESULTS
Clinical Findings
Birds vaccinated with the locally prepared pentavalent vaccine and the blank control negative group exhibits no clinical signs during the observation period. While the control positive MG-challenged chicks displayed respiratory signs including coughing, sneezing, nasal discharge, and conjunctivitis, as well as nasal discharge. The hens in the MS-challenged control group showed retarded development, swelling of the joints, with respiratory manifestations. The ST, SE, and SK vaccinated group showed slight brownish diarrhea with low degree of enteritis. While the chickens in the ST, SE, and SK challenged control positive group showed severe brownish diarrhea (Figure 1), depression, decreased feed intake, lowered weight, ruffled feathers, sleepy appearance, recumbent, and unable to walk.
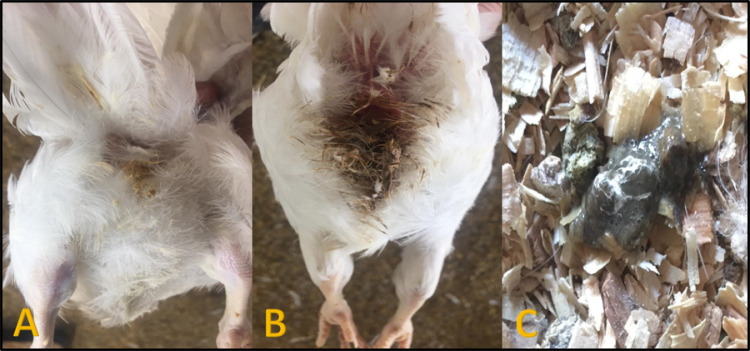
Figure 1.
(A) Chickens in blank control negative group showing apparently normal appearance. (B) Bird from Salmonella enterica serovar Enteritidis (SE) challenged non-vaccinated bird showing brown color dropping soiling the vent region. (C) Dropping in Salmonella enterica serovar Kentucky (SK) challenged non-vaccinated bird showing dark brown colored diarrhea.
Morbidity and Mortality Rates
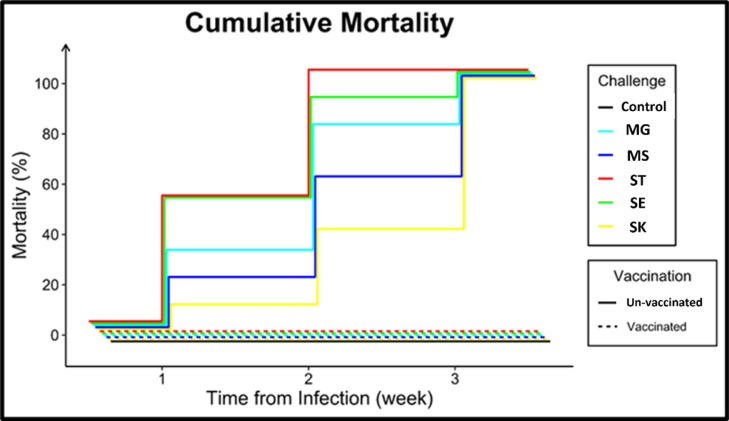
Low morbidity and mortality rates were noted in both the vaccinated and negative control groups. Significant morbidity and mortality rates were observed in the positive control challenged groups, as illustrated in Figure 2.
Figure 2.
The mortality rates in different experimental groups; influences of different pathogenic challenges on cumulative mortality of vaccinated and not vaccinated groups. Mycoplasma gallicepticum (MG), Mycoplasma synoviae (MS), Salmonella enterica serovar Typhimurium (ST), Salmonella enterica serovar Enteritidis (SE), and Salmonella enterica serovar Kentucky (SK).
Postmortem Lesions
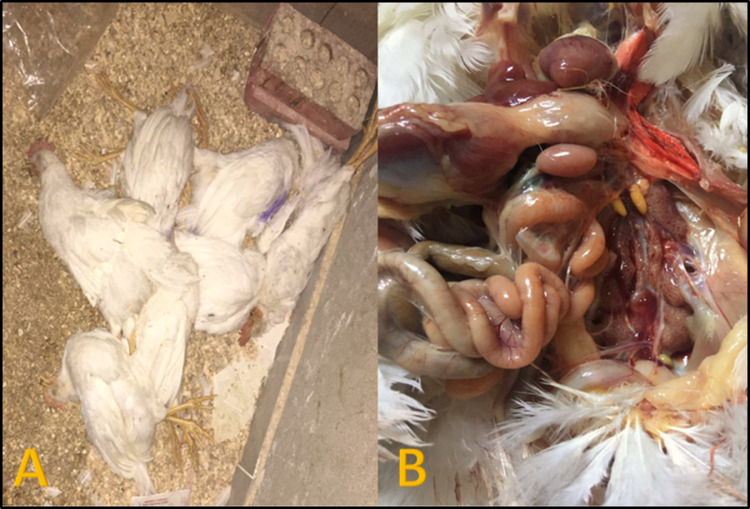
Postmortem examination for ST, SE, and SK challenged positive control group revealed severe degree of enteritis, enlarged spleen, liver, nephritis, impacted gallbladder with tiny hepatic necrotic foci, pericarditis, and septicemic picture was observed in some cases as shown in Figure 3, Figure 4, Figure 5. The postmortem examination of freshly dead chicken in positive control challenged group with MG showed slight foamy turbidity of air sacs as shown in Figure 6.
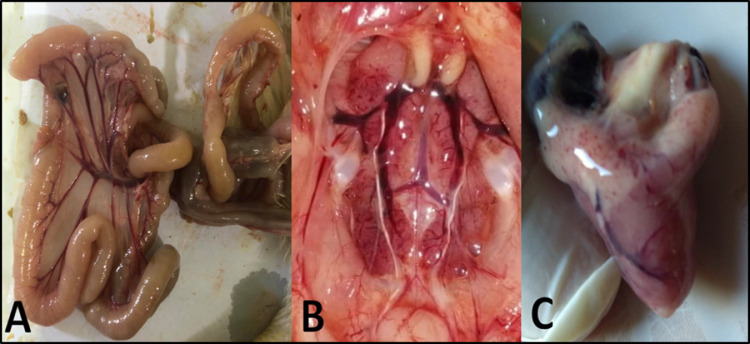
Figure 3.
(A) Chickens in Salmonella enterica serovar Typhimurium (ST), challenged non-vaccinated bird showing high mortalities after challenge. (B) Postmortem examination of freshly dead chickens in Salmonella enterica serovar Typhimurium (ST), challenged group showing nephrosis in kidney with distended ureters with presence of enteritis with congested mesenteric blood vessels.
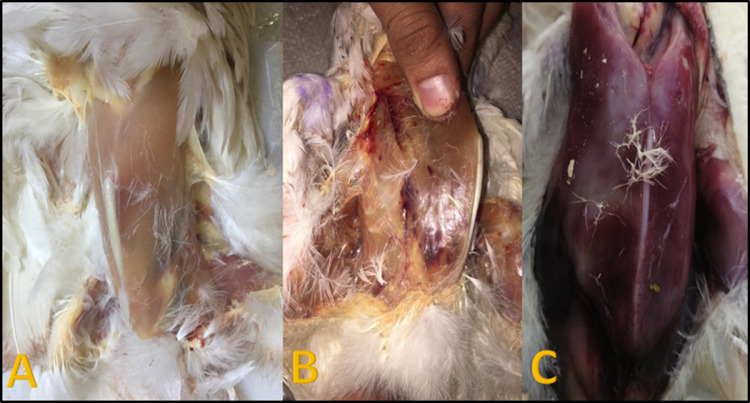
Figure 4.
(A) Apparently normal breast muscle appearance in control negative birds. (B) Postmortem in control positive Salmonella enterica serovar Typhimurium (ST), challenged birds showing congestion and hemorrhages in breast muscle. (C) Postmortem in control positive Salmonella enterica serovar Enteritidis (SE), challenged birds showing severe congestion of breast muscle with severe septicemic picture.
Figure 5.
(A) Intestine of Salmonella enterica serovar Enteritidis (SE), control positive challenged birds showing enteritis with engorgement of mesenteric blood vessels. (B) Kidney of Salmonella enterica serovar Enteritidis (SE), control positive challenged birds showing nephritis and nephrosis with distended ureters. (C) Heart of Salmonella enterica serovar Enteritidis (SE), control positive challenged birds showing hemorrhage on coronary fat.
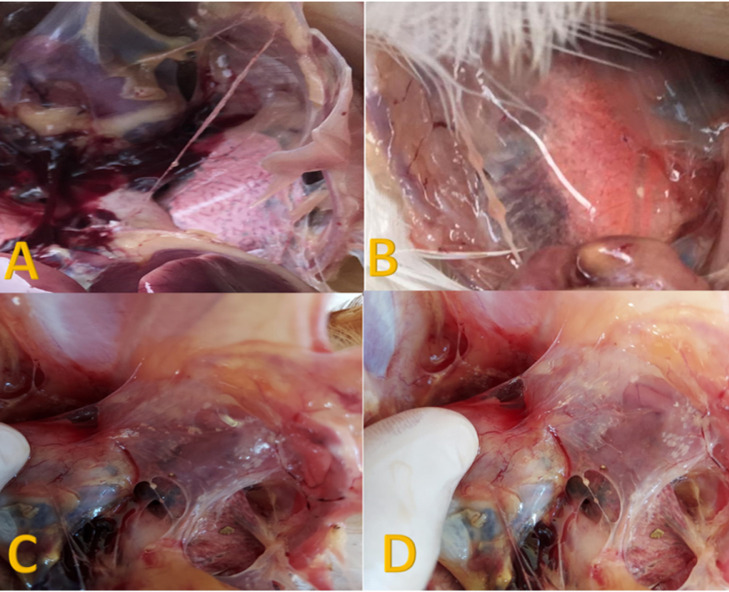
Figure 6.
(A) Postmortem examination of sacrificed birds in blank control negative group at the end of the experiment showing normal transparent and clear air sac. (B) Postmortem examination in vaccinated challenged birds with Mycoplasma gallicepticum (MG) showing normal air sac appearance. (C and D) Postmortem examination of freshly dead chicken in control positive challenged group with Mycoplasma gallicepticum (MG) showing foamy slight turbidity of air sacs.
Mycoplasma and Salmonella Shedding
Detection of Mycoplasma Shedding
Both MG and MS were re-isolated from tracheal swabs and synovial fluid samples in the primary study groups (control positive challenged group and vaccinated group). The control positive group was positive for fried egg colonies of MG and MS for 2 consecutive weeks after the challenge, whereas the pentavalent group revealed Mycoplasma shedding only at 1-wk postchallenge.
Detection of Salmonella Shedding
In the first, second, and third weeks after the challenge, the pentavalent vaccine shedding rates of ST and SE from chickens vaccinated with the inactivated vaccine were 30, 20, and 0%, respectively. In contrast, the fecal shedding rates of SK were 30, 10, and 0%, respectively. In each of the first, second, and third weeks after the challenge, the re-isolation rate in the control positive unvaccinated birds was 100% (Table 2).
Table 2.
Salmonella shedding rates.
| Weeks for shedding of Salmonella |
Salmonella enterica serovar Typhimurium (ST) |
Salmonella enterica serovar Enteritidis (SE) |
Salmonella enterica serovar Kentucky (SK) |
|||
|---|---|---|---|---|---|---|
| Pentavalent vaccinated group | Control positive challenged group | Pentavalent vaccinated group | Control positive challenged group | Pentavalent vaccinated group | Control positive challenged group | |
| 1st wk | 30 | 100 | 30 | 100 | 30 | 100 |
| 2nd wk | 20 | 100 | 20 | 100 | 10 | 100 |
| 3rd wk | 0 | 100 | 0 | 100 | 0 | 100 |
Humoral Immune Response in Different Groups
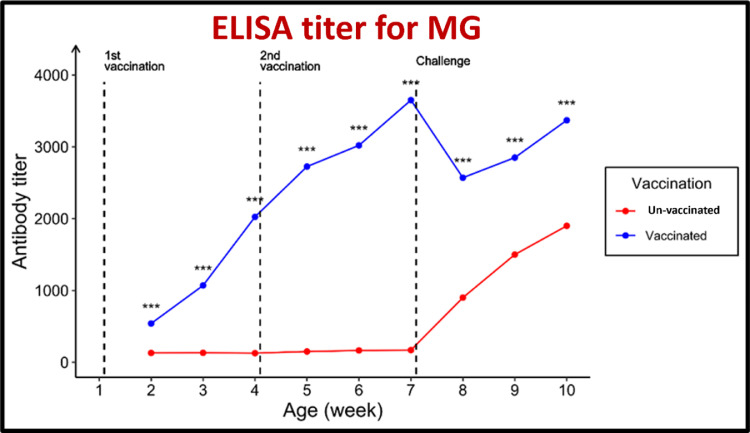
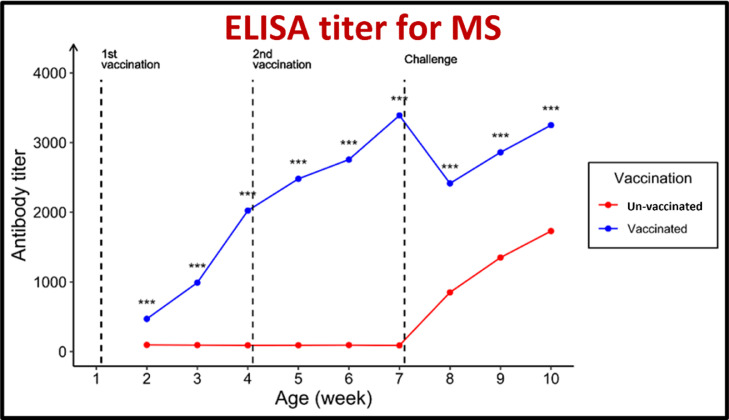
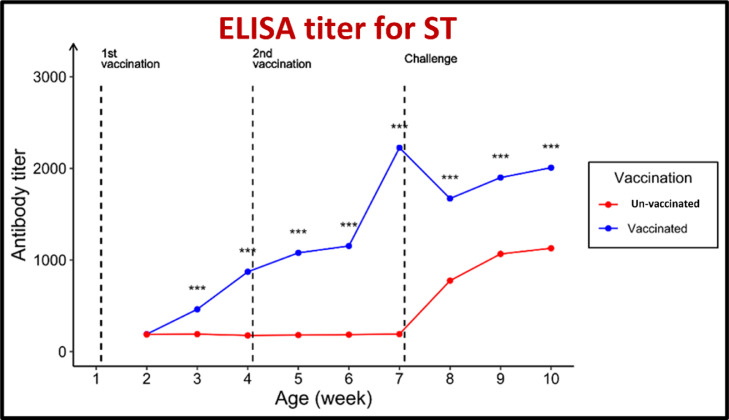
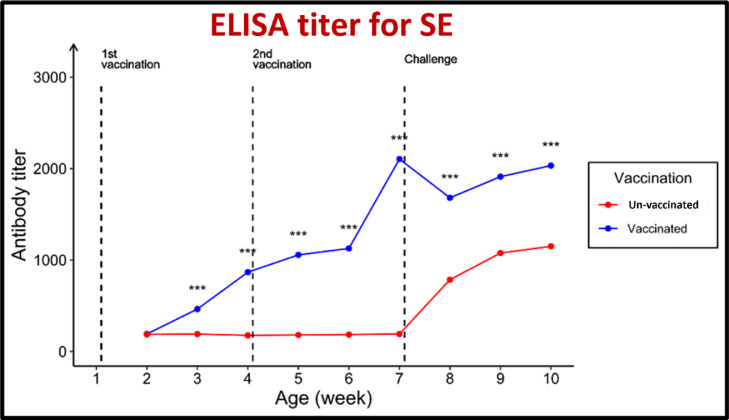
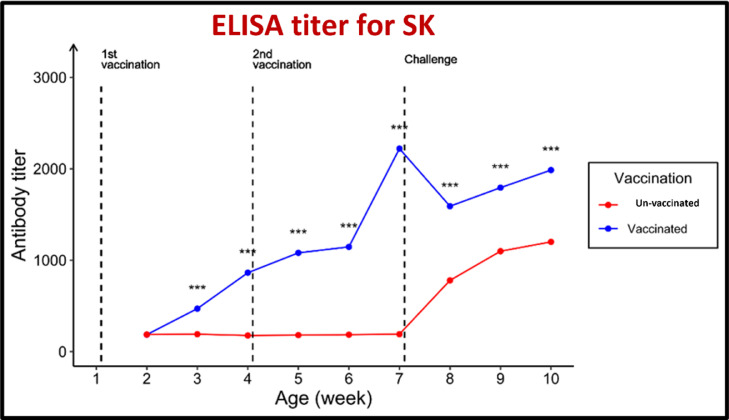
For antibodies against both MG and MS, the geometric mean antibody titers (GMT) were beyond the cutoff values of 843.0 and 737.0, respectively. The pentavalent vaccination showed an excellent protective response to the challenge posed by MG and MS. The serum ST antibody titers (GMT) of the vaccinated group increased from 190.0 before vaccination to 871.3 in the third week after the first immunization and 2225.1 in the third week after the booster administration.
The GMT increased to 2007 in the third week after the challenge. The GMT against SK increased from 186.0 before vaccination to 863.1 after the first immunization and 2,220.5 in the third week after the booster administration. The GMT increased to 1,985.7 in the third week after the challenge. In contrast, the GMT against SE increased from 191.0 before vaccination to 867.8 in the third week after the initial immunization and 2,104.3 in the third week after the booster administration. The GMT increased to 2,032.7 in the third week after the challenge. In contrast, the serum ELISA antibody titer in the unvaccinated chicks was 187.0.
Furthermore, for ST, a sudden increase in antibody titers was noted in the third week after exposure, with antibody titers of 176.8, 191.1, and 1,128.0. For SK, the value was 187.0. For SE, the increase in antibody titers was also noted in the third week after exposure, with antibody titers of 176.8, 191.1, and 1,200.7, and finally a figure of 187.0. Lastly, the increase in antibody titers was also noted in the third week after exposure, with antibody titers of 176.8, 191.1, and 1,150.4, as shown in Figure 7, Figure 8, Figure 9, Figure 10, Figure 11.
Figure 7.
Effect of vaccination and challenge with Mycoplasma gallicepticum (MG) on the ELISA titer MG antibodies. Significance between titers in the same age is expressed as *** (P-value < 0.001) (n = 5).
Figure 8.
Effect of vaccination and challenge with Mycoplasma synoviae (MS) on the ELISA titer MS antibodies. Significance between titers in the same age is expressed as *** (P-value < 0.001) (n = 5).
Figure 9.
Effect of vaccination and challenge with Salmonella enterica serovar Typhimurium (ST), on the ELISA titer ST antibodies. Significance between titers in the same age is expressed as *** (P-value < 0.001) (n = 5).
Figure 10.
Effect of vaccination and challenge with Salmonella enterica serovar Enteritidis (SE), on the ELISA titer SE antibodies. Significance between titers in the same age is expressed as *** (P-value < 0.001) (n = 5).
Figure 11.
Effect of vaccination and challenge with Salmonella enterica serovar Kentucky (SK) on the ELISA titer SK antibodies. Significance between titers in the same age is expressed as *** (P-value < 0.001) (n = 5).
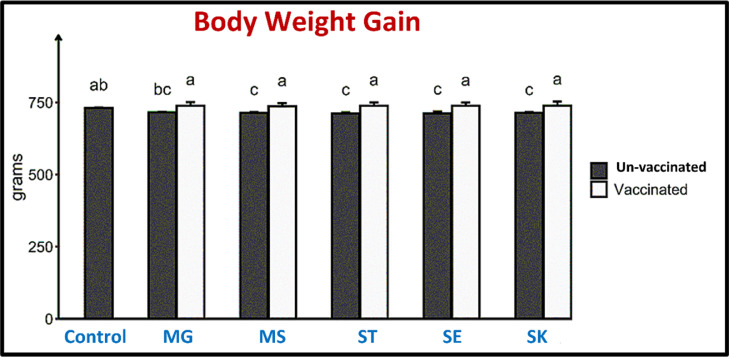
Body Weight Gain of the Experimental Birds
The statistical analysis showed no detectable difference between the pentavalent vaccinated group and the negative control group. Despite this result, there remained a difference between the positive and negative control groups, as shown in Figure 12.
Figure 12.
The performance of the birds in the different experimental groups; effect of vaccination on the body weight gain in different pathogenic challenges. Mycoplasma gallicepticum (MG), Mycoplasma synoviae (MS), Salmonella enterica serovar Typhimurium (ST), Salmonella enterica serovar Enteritidis (SE), and Salmonella enterica serovar Kentucky (SK). Data are expressed as mean ± SEM. Bars without shared letters indicate a significant difference between them (P-value < 0.05) (n = 5).
Protective Efficacy of the Prepared Vaccine
According to mortality after challenge, the manufactured vaccine had a 100% protection rate as the survival rate of the vaccinated birds were 100% postchallenge, the prepared vaccine diminished the severity of the clinical signs in vaccinated birds, reduced bacterial shedding, provided protective antibody titers, and did not affect body weight gain in the vaccinated birds in comparison with control group.
DISCUSSION
There has been a rapid increase in the world's principal poultry products (meat and eggs) output (Abd El-Hack et al., 2022a). In turn, this reflects customer need and acceptance for those high-quality items and the comparatively low price due to the efficiency of the manufacturing process (Abd El-Hack et al., 2022b). Between 1995 and 2005, worldwide consumption and production have grown (Scanes, 2007). In the chicken industry, respiratory and enteric infection due to bacterial, viral, parasitic, or managemental causes are common devastating problems that results in severe economic losses in the poultry industry (Setta et al., 2018; Abd El Hamid et al., 2019; Salem and Attia, 2021; Salem et al., 2021). When it comes to poultry, Mycoplasma and Salmonella are responsible for considerable economic losses. They are connected to respiratory and enteric diseases, poor performance in the field, decreased egg production, decreased fertility, and increased embryonic mortalities worldwide (Murugkar et al., 2005; Marouf et al., 2020, 2022).
In the current study, the chicks immunized by the prepared pentavalent vaccine showed a protective titer against MG, MS, ST, SE, and SK after challenge with 100% protection percentage. This protection percentage indicated the promising efficacy of using the prepared vaccine as mentioned by Heddleston (1975) and Knight-Jones et al. (2014). These studies confirmed that if the produced vaccine gave more than 80% protection value, it is considered acceptable for the usage.
Three weeks post challenge, the vaccinated group of hens had fecal shedding of 8.33%, whereas the unvaccinated control group had fecal shedding of 25%. In the vaccinated group, no shedding was identified in the fourth week after the challenge. While the unvaccinated control group shed 16.66%, the vaccinated group shed 16.66%. Sayed (2010) and Ibrahim et al. (2018) showed similar fecal shedding rates.
It is well known that Mycoplasma infections are widespread prevalent in commercial layer and broiler hens and backyard chickens in rural regions (Marouf et al., 2022). Birds with Mycoplasma infection generally exhibited nasal discharge, synovitis, and dyspnea with mouth breathing, as well as gross postmortem lesions of a congested lung, hyperemic mucoid trachea, and swollen air sacs after death (Talha, 2003). Increased mortality, carcass condemnation, lowered egg production, and hatchability are all expenses associated with MG-related diseases where depopulation of animals is not practicable on large commercial multiage layer farms (Ley, 2008). So, the most practical approach to disease management is vaccination (Bermudez and Kalbac, 1988; Branton et al., 2002).
From our findings, the pentavalent prepared inactivated Montanide ISA70-based Salmonella and Mycoplasma vaccine showed accepted antibodies levels when tested in chickens using the ELISA technique. The tested vaccine protected challenged birds and neither showed mortalities nor clinical signs. The results from the current study are supported by Ferguson-Noel et al. (2012), who found that Mycoplasma inactivated vaccines can reduce vertical transmission, lessen the severity of illness, and provide significant protection for the respiratory system and reproductive system. Also, El-Naggar et al. (2022) concluded that bivalent MG and MS inactivated vaccines adjuvanted with Montanide ISA70 revealed a protective antibodies titer and gave 80% protection against MG and 90% against MS in challenged birds. In the same way, Ibrahim et al. (2018) found that the inactivated trivalent Salmonella vaccine revealed protection and provoked protective antibodies titer in vaccinated chickens. Feberwee et al. (2000) also found that the inactivated SE vaccine resulted in the reduction of SE reinfection in broiler breeder flocks.
On the other hand, Evans and Hafez (1992) noticed that SPF layer hens whose were given an inactivated MG vaccination at 30 wk and subsequently challenged with a virulent R strain of MG revealed fair protection. However, the usage of inactivated Mycoplasma vaccinations is somewhat effective, and can reduce the spread of MG germs, but it cannot prevent horizontal transmission of the infection (El-Naggar et al., 2022). Also, Jacob et al. (2014) concluded that in commercial layer production facilities with diverse age groups, some feel bacterins have a limited impact in protecting against MG infection. Reviewing the available literature, no available data about pentavalent Salmonella and Mycoplasma in poultry were found; thus, from our finding, we recommend this vaccine to be applied to protect poultry flocks in Egypt or elsewhere.
CONCLUSIONS
The Egyptian locally prepared pentavalent vaccine against Mycoplasma and Salmonella infections is both safe and effective and provide 100% protection, reduce the clinical signs, produce protective antibody titers, lower the bacterial shedding, and did not affect the body weight gain with 100% survival rate in the vaccinated birds. Further studies are recommended to evaluate the efficacy of the prepared vaccine under field condition, especially those with layer or breeder flocks, to combat these major problems that threaten poultry industry in Egypt.
ACKNOWLEDGMENTS
The authors extend their appreciation to the Researchers Supporting Project number (RSP2022R439), King Saud University, Riyadh, Saudi Arabia for funding this research.
DISCLOSURES
The authors declare no conflict of interests.
REFERENCES
- Abd El Hamid M.I., Abd El-Moaty D.A.M., El-Sergany E.F., Salem H.M., El-Sawy H., Abbas A.M. Utility of molecular biology tools for identification and characterization of Egyptian Riemerella anatipestifer duck isolates. Int. J. Vet. Sci. 2019;8:335–341. [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Saad A.M., Salem H.M., Ashry N.M., Abo Ghanima M.M., Shukry M., Swelum A.A., Taha A.E., El-Tahan A.M., AbuQamar S.F., El Tarabily K.A. Essential oils and their nanoemulsions as green alternatives to antibiotics in poultry nutrition: a comprehensive review. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Salem H.M., El-Tahan A.M., Soliman M.M., Youssef G.B.A., Taha A.E., Soliman S.M., Ahmed A.E., El-kott A.F., Al Syaad K.M., Swelum A.A. Alternatives to antibiotics for organic poultry production: types, modes of action and impacts on bird's health and production. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M.Z., Rahman M.M., Sultana S. Seroprevalence of Mycoplasma gallisepticum antibody by ELISA and serum plate agglutination test of laying chicken. Vet. World. 2015;8:9–14. doi: 10.14202/vetworld.2015.9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez J., Lopez G., Muellner P., de Frutos C., Ahlstrom C., Serrano T., Moreno M.A., Duran M., Saez J.L., Dominguez L., Ugarte-Ruiz M. Identifying emerging trends in antimicrobial resistance using Salmonella surveillance data in poultry in Spain. Transbound. Emerg. Dis. 2020;67:250–262. doi: 10.1111/tbed.13346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow P.A. Further observations on the serological response to experimental Salmonella typhimurium in chickens measured by ELISA. Epidemiol. Infect. 1992;108:231–242. doi: 10.1017/s0950268800049712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekele L., Assefa T. Inactivated vaccine trial of Mycoplasma gallisepticum in Ethiopia. Open J. Vet. Med. 2018;8:75–85. [Google Scholar]
- Bermudez A.J., Kalbac M. Control of Mycoplasma gallisepticum infection in commercial layers: a field study. J. Am. Vet. Med. Assoc. 1988;192:1783. [Google Scholar]
- Branton S.L., Bearson S.M., Bearson B., Lott B.D., Maslin W.R., Collier S.D., Pharr G.T., Boykin D.L. The effects of 6/85 live Mycoplasma gallisepticum vaccine in commercial layer hens over a 43-week laying cycle on egg production, selected egg quality parameters, and egg size distribution when challenged before beginning of lay. Avian. Dis. 2002;46:423–428. doi: 10.1637/0005-2086(2002)046[0423:TEOLMG]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Cahyani J.I., Widyarini S., Wibowo M.H. Comparative safety and efficacy of two bivalent vaccines containing Newcastle disease LaSota and avian influenza H9N2 Sidrap isolate formulated with different oil adjuvants. Vet. World. 2020;13:2493–2501. doi: 10.14202/vetworld.2020.2493-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles C.D., Rind D., Jouzel J., Koster R.D., Fairbanks R.G. Glacial-interglacial changes in moisture sources for Greenland: influence on the ice core record of climate. Science. 1994;263:508–511. doi: 10.1126/science.263.5146.508. [DOI] [PubMed] [Google Scholar]
- Cogan T.A., Humphrey T.J. The rise and fall of Salmonella enteritidis in the UK. J. Appl. Microbiol. 2003;94:114S–119S. doi: 10.1046/j.1365-2672.94.s1.13.x. [DOI] [PubMed] [Google Scholar]
- Crouch C.F., Nell T., Reijnders M., Donkers T., Pugh C., Patel A., Davis P., van Hulten M.C.W., de Vries S.P.W. Safety and efficacy of a novel inactivated trivalent Salmonella enterica vaccine in chickens. Vaccine. 2020;38:6741–6750. doi: 10.1016/j.vaccine.2020.08.033. [DOI] [PubMed] [Google Scholar]
- Eissa S.I., Metwally A.M., Hashem Y.M., Khalifa R.A., Refaie M.K. Molecular comparative analysis of Mycoplasma gallisepticum field and vaccine strains in Egypt. Eur. J. Vet. Med. 2014;2014:9. [Google Scholar]
- El-Jakee K.J., Moussa I.M., Omran M.S., Ahmed B.M., Elgamal M.A., Hemeg H.A., Mubarak A.S., Al-Maary K.S., A.Kabli S., Marouf S.A., Alhaaji J.H. A novel bivalent pasteurellosis-RHD vaccine candidate adjuvanted with Montanide ISA70 protects rabbits from lethal challenge. Saudi J. Biol. Sci. 2020;27:996–1001. doi: 10.1016/j.sjbs.2019.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Naggar M.S., Ibrahim H.M., Salem H.M., Marouf S. A novel locally prepared inactivated bivalent mycoplasma vaccine for chicken flocks in Egypt. Adv. Anim. Vet. Sci. 2022;10:55–61. [Google Scholar]
- El-Saadony M.T., Salem H.M., El-Tahan A.M., Abd El-Mageed T.A., Soliman S.M., Khafaga A.F., Swelum A.A., Ahmed A.E., Alshammari F.A., Abd El-Hack M.E. The control of poultry salmonellosis using organic agents: an updated overview. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elyazeed H.A., Al-Atfeehy N.M., Abotaleb R., Sayed R., Marouf S.H. Preparation of ELISA and lateral flow kits for rapid diagnosis of Mycoplasma gallisepticum in poultry. Sci. Rep. 2020;10:9056. doi: 10.1038/s41598-020-65848-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J.D., Leigh S.A., Purswell J.L., Jacob R., Peebles E.D., Collier S.D., Branton S.L. A comparative study of live attenuated F strain-derived Mycoplasma gallisepticum vaccines. Avian Dis. 2012;56:396–401. doi: 10.1637/9951-092711-Reg.1. [DOI] [PubMed] [Google Scholar]
- Evans R.D., Hafez Y.S. Evaluation of a Mycoplasma gallisepticum strain exhibiting reduced virulence for prevention and control of poultry mycoplasmosis. Avian Dis. 1992;36:197–201. [PubMed] [Google Scholar]
- Fabricant J. Immunization of chickens against Mycoplasma gallisepticum infection. Am. J. Vet. Res. 1975;36:566–567. [PubMed] [Google Scholar]
- Feberwee A., de Vries T.S., Elbers A.R.W., de Jong W.A. Results of a Salmonella enteritidis vaccination field trial in Broiler-Breeder flocks in the Netherlands. Avian Dis. 2000;44:249–255. [PubMed] [Google Scholar]
- Ferguson-Noel N., Cookson K., Laibinis V.A., Kleven S.H. The efficacy of three commercial Mycoplasma gallisepticum vaccines in laying hens. Avian Dis. 2012;56:272–275. doi: 10.1637/9952-092711-Reg.1. [DOI] [PubMed] [Google Scholar]
- Guard-Petter J. The chicken, the egg and Salmonella enteritidis. Environ. Microbiol. 2001;3:421–430. doi: 10.1046/j.1462-2920.2001.00213.x. [DOI] [PubMed] [Google Scholar]
- Heddleston KL. Pasteurellosis: isolation and identification of avian pathogens. Am. Assoc. Avian Pathol. 1975;1:38–51. [Google Scholar]
- Hofstad M.S., Calnek B.U., Heimboldts G.F., Ried M.W., Yoder H.W. 10th Ed. State University Press; Ames, IA: 1997. Diseases of Poultry. [Google Scholar]
- Hussein A., El-Shaib T.M., Saoud S.M., Shalaby N.A., Sultan H., Ragab A.M. Protective immune response of Mycoplasma gallisepticum vaccines in poultry. Egypt. J. Immunol. 2007;14:93–99. [PubMed] [Google Scholar]
- Ibrahim H.M., Sayed R.H., Shereen A.M. Efficacy of a locally prepared inactivated trivalent vaccine against salmonellosis in poultry. Int. J. Vet. Sci. 2018;7:82–87. [Google Scholar]
- Ishfaq M., Hu W., Khan M.Z., Ahmad I., Guo W., Li J. Current status of vaccine research, development and challenges of vaccines for Mycoplasma gallisepticum. Poult. Sci. 2020;99:4195–4202. doi: 10.1016/j.psj.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob R., Branton S.L., Evans J.D., Leigh S.A., Peebles E.D. Effects of live and killed vaccines against Mycoplasma gallisepticum on the performance characteristics of commercial layer chickens. Poult. Sci. 2014;93:1403–1409. doi: 10.3382/ps.2013-03748. [DOI] [PubMed] [Google Scholar]
- Knight-Jones T.J., Edmond D.K., Gubbins S., Paton D.J. Veterinary and human vaccine evaluation methods. Proc. Royal Soc. B. 2014;281 doi: 10.1098/rspb.2013.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh S.A., Evans J.D., Branton S.L. Complete genome sequences of two vaccine strains and one field isolate of Mycoplasma gallisepticum. Microbiol. Resour. Announc. 2019;8 doi: 10.1128/MRA.01237-19. :e01237-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley D.H. In: Diseases of Poultry. 11th ed. Saif Y.M., Barnes H.J., Glisson J.R., Fadly A.M., McDougal L.R., Swayne D.E., editors. Iowa State University Press; Ames, IA: 2003. Mycoplasma gallisepticum infection; pp. 722–743. [Google Scholar]
- Ley D.H. In: Pages 807–834 in Disease of Poultry. 12th ed. Saif Y.M., Fadly A.M., Glisson J.R., McDougald L.R., Nolan L.K., Swayne D.E., editors. Blackwell Publishing Press; Ames, IA: 2008. Mycoplasma gallisepticum infection. [Google Scholar]
- Marouf S.H., Moussa I.M., Salem H.S., Sedeik M.E., Elbestawy A.R., Hemeg H.A., Dawoud T.M., Mubarakb A.S., Mahmouda H., Alsubki R.A., Bahkali A.H. A picture of Mycoplasma gallisepticum and Mycoplasma synoviae in poultry in Egypt: phenotypic and genotypic characterization. J. King Saud Univ. Sci. 2020;32:2263–2268. [Google Scholar]
- Marouf S., Khalf M.A., Alorabi M., El-Shehawi A.M., El-Tahan A.M., AbdEl-Hack M.E., El-Saadony M.T., Salem H.M. Mycoplasma gallisepticum: a devastating organism for the poultry industry in Egypt. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael G.B., Schwarz S. Antimicrobial resistance in zoonotic nontyphoidal Salmonella: an alarming trend? Clin. Microbiol. Infect. 2016;22:968–974. doi: 10.1016/j.cmi.2016.07.033. [DOI] [PubMed] [Google Scholar]
- Murugkar H.V., Rahman H., Kumar A., Bhattacharyya D. Isolation, phage typing and antibiogram of Salmonella from man and animals in northeastern India. Indian. J. Med. Res. 2005;122:237–242. [PubMed] [Google Scholar]
- National Research Council (NRC) 9th rev. ed. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Office International des Epizooties (OIE). 2012. Salmonellosis in Manual of diagnostic tests and vaccines for Terrestrial animals.
- Pan H., Paudyal N., Li X., Fang W., Yue M. Multiple food-animal-borne route in transmission of antibiotic-resistant Salmonella Newport to humans. Front. Microbiol. 2018;9:23. doi: 10.3389/fmicb.2018.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. 2020. R: A Language and Environment for Statistical Computing (4.0.3). R Foundation for Statistical Computing. Accessed Mar. 2022. https://www.r-project.org/
- Rabello R.F., Bonelli R.R., Penna B.A., Albuquerque J.P., Souza R.M., Cerqueira A.M.F. Antimicrobial resistance in farm animals in Brazil: an update overview. Animals. 2020;10:552. doi: 10.3390/ani10040552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabie N.S., Amin Girh Z. Bacterial vaccines in poultry. Bull. Natl. Res. Cent. 2020;44:15. doi: 10.1186/s42269-019-0260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodwell A.W., Whitcomb R.F. In: Pages 185–196 in Methods in Mycoplasmology. 1st ed. Razin S., Tully J.G., editors. Academic Press; New York, NY: 1983. Methods for direct and indirect measurement of Mycoplasma growth. [Google Scholar]
- RStudio Team. 2020. RStudio: integrated development environment for R (1.3.1093). RStudio, PBC. Accessed Mar. 2022. http://www.rstudio.com/
- Salem H.M., Attia M.M. Accidental intestinal myiasis caused by Musca domestica L. (Diptera: Muscidae) larvae in broiler chickens: a field study. Int. J. Trop. Insect. Sci. 2021;41:2549–2554. [Google Scholar]
- Salem H.M., Ismael E., Shaalan M. Evaluation of the effects of silver nanoparticles against experimentally induced necrotic enteritis in broiler chickens. Int. J. Nanomed. 2021;16:6783–6796. doi: 10.2147/IJN.S319708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayed, R. H. 2010. Preparation and evaluation of combined inactivated vaccine against Salmonella enteritidis, Salmonella typhimurium and Clostridium perfringens type A and C toxins in chickens, M.VSc. Thesis, Faculty of Veterinary Medicine, Cairo University, Egypt.
- Scanes C.G. The global importance of poultry. Poult. Sci. 2007;86:1057–1058. doi: 10.1093/ps/86.6.1057. [DOI] [PubMed] [Google Scholar]
- Setta A., Salem H.M., Elhady M., El-Hussieny A., Arafa A.S. Molecular and genetic characterization of infectious bronchitis viruses isolated from commercial chicken flocks in Egypt between 2014 and 2016. J. World Poult. Res. 2018;8:01–08. [Google Scholar]
- Talha, A. F. S. M. 2003. Investigation on the prevalence and significance of M. gallisepticum in village chickens and possibility of establishing M. gallisepticum free flocks and significance of M. gallisepticum on different production parameters in layer chickens in Bangladesh. MSc Thesis, The Royal Veterinary and Agricultural University, Denmark and Bangladesh Agricultural University, Mymensingh, Bangladesh.
- Van Boeckel T.P., Pires J., Silvester R., Zhao C., Song J., Criscuolo N.G., Gilbert M., Bonhoeffer S., Laxminarayan R. Global trends in antimicrobial resistance in animals in low- and middle-income countries. Science. 2019;365:eaaw1944. doi: 10.1126/science.aaw1944. [DOI] [PubMed] [Google Scholar]
- Yadav J.P., Tomar P., Singh Y., Khurana S.K. Insights on Mycoplasma gallisepticum and Mycoplasma synoviae infection in poultry: a systematic review [e-pub ahead of print] Anim. Biotechnol. 2022 doi: 10.1080/10495398.2021.1908316. 10.1080/10495398.2021.1908316 doi: [DOI] [PubMed] [Google Scholar]