Highlights
-
•
Allergenicity of kiwifruit strongly associated with its physiological attributes.
-
•
10-day storage of kiwifruit at 20 °C increased the Act d 2 content by 50 %.
-
•
Act d 2 content in the seeds was 4-fold higher than that of peel.
-
•
Act d 2 content showed a negative relationship with antioxidant activity.
Keywords: Kiwifruit, Allergic potential, Maturity, Storage conditions, Antioxidant activity
Abstract
This work aimed to study the influences of physiological characteristics (variety, maturity, and fruit components) and storage conditions on the allergenic potential of kiwifruit. The results found kiwifruit allergen (Act d 2) is only measured in the green kiwifruit rather than golden kiwifruit. The Act d 2 content of seed is 2-fold and 4-fold higher than that of pulp and peel, respectively. The highest Act d 2 allergen content was determined in ripe kiwifruit, followed by overripe fruit and unripe fruit. A 50 % of enhancement in Act d 2 content was observed after 10-day storage at 20 °C. Further, the Act d 2 content showed a negative relationship with the ascorbic acid content and total antioxidant activity at various conditions. Thus, antioxidants as important factors may involve the regulation of Act d 2 in kiwifruit. These findings could provide a deep understanding in the distribution of Act d 2.
Introduction
Kiwifruit (Actinidia deliciosa) is considered as a nutrient-dense fruit due to its high levels of dietary fiber, sugars, vitamin C and vitamin E, minerals, omega-3 fatty acids, and antioxidants (Richardson et al., 2018, Wang et al., 2019). Over the last decade, extensive studies have reported that these nutrients present in kiwifruit can contribute to the prevention of functional gastrointestinal disorders, cancer, and cardiovascular related diseases). Studies found that consuming two kiwifruit (containing approximately 6 g of fibre) each day significantly contribute to the total daily fibre intake and increase the water retention capacity, which could benefit to the digestive function of human and animals(Mishra and Monro, 2012, Montoya et al., 2016). Furthermore, extensive research on the relationship between consumpting kiwifruit and repairing DNA damage in human cells showed that kiwifruit or kiwifruits extracts significantly reduced the DNA damage in fibroblasts, the viability of gastric cancer cells, lung cancer cells, Hep3B and HeLa cells (Lim et al., 2016, Platt et al., 2010). These reductions resulted from consuming kiwifruit or relevant products may be due to its high levels of antioxidants, which could lead to reducing the risk of surfing from cancers potentially (Skinner, Loh, Hunter, & Zhang, 2011). For the improvement of hyperlipidemia, Chang et al. (2009) treated 43 patients with a history of hyperlipidemia by consuming two kiwifruits each day during an eight-week evaluation. After the treatment, the volunteers showed a lower density in lipoprotein cholesterol, which may be the higher levels of Vitamin C and vitamin E present in the blood compared with previous stage (Chang & Liu, 2009). Thus, kiwifruit has various heath benefits for human.
However, kiwifruit is becoming one of the most common elicitor causing allergic symptoms except for the big eight around the world (Wang, Vanga, McCusker, et al., 2019). In a study conducted in Sweden and Denmark, self-reported surveys found that 50 % of people with a history of food allergy are also allergic to kiwifruit or relevant products (Eriksson, Moller, Werner, Magnusson, Bengtsson, & Zolubas, 2004). In Finland, kiwifruit was recognized as the top ten most common allergic foods in the school. The study found that most volunteers (65–75 %) with kiwifruit allergy have oral allergy syndrome (Ukleja-Sokołowska, Zacniewski, Lis, Żbikowska-Gotz, Kuźmiński, & Bartuzi, 2021). In Spain and France, 1.5–2.0 % of the total population are surfing from kiwifruit allergy (Mills et al., 2007, Rance et al., 2005). More interestingly, many studies have reported that kiwifruit and pollen could cause an allergic cross-reactivity resulting from the similar epitopes in their allergens (Wang, Vanga, McCusker, et al., 2019; Wang, Vanga, & Raghavan, 2019a). To date, 20–40 % of the global population are allergic to pollen including flower pollens, tree pollens, and weed pollens (Asam, Hofer, Wolf, Aglas, & Wallner, 2015). In a survey study, 55 volunteers with a history of kiwifruit allergy were diagnosed with the oral food challenge. The results found that 50 % of patients showed cross-reactivity to peaches, 31 % in peanuts, and 42 % in tree nuts (Sirvent et al., 2014). Thus, kiwifruit allergy has been considered as the most common allergy among fruit and vegetables causing health issues globally.
In recent years, extensive research has reported many factors including maturity, variety, storage conditions, and various components, affecting the allergic potential of the fruit. Pru p 3 is a major peach allergen that is strongly related to its ripening attributes (Brenna, Pastorello, Farioli, Pravettoni, & Pompei, 2004). During the ripening period, the SDS-PAGE protein bands intensities of Pru p 3 in fully ripened peaches were higher compared to unripe ones (Brenna et al., 2004). A similar result was observed in apples, the level of major apple allergen (Mal d 3) in the matured samples is two times higher than the immature ones (Sancho, Foxall, Rigby, et al., 2006). In 2011, López-Matas et al. evaluated the differences in the allergenicity among six varieties of tomatoes. The results found that ‘Rambo’ contained the highest level of allergen, whereas the lowest level of tomato allergen was observed in ‘Rama’. The levels of tomato allergen present in ‘Pera’, ‘Canario’, ‘Raf’, and ‘Kumato’ are similar (López-Matas et al., 2011). In respect to the influences of storage conditions on the allergenicity of fruit, studies have reported that the synthesis of apple allergen (Mal d 1) significantly increased after an 8-week storage (Sancho, Foxall, Browne, et al., 2006). Further, scientists found that allergic compounds might distribute in the peel, pulp, or/and seeds of fruits (Pravettoni et al., 2009). In mango, the majority of allergens were determined in the peel, and the content of mango allergen present in the peel is 16 times higher than that in the pulp (Knödler, Reisenhauer, Schieber, & Carle, 2009). In Rosaceae family fruits, such as peach and apple, studies have reported that fruit allergens were mainly determined in the peel compared with the pulp (Borges et al., 2006, Fernández-Rivas et al., 2006).
However, few reports are published regarding the relationship between kiwifruit allergenicity and its physiological characteristics such as variety, maturity, storage conditions, and fruit components. This work aims to study the effect of physiological characteristics (e.g. variety, maturity, and fruit components) and storage conditions on the kiwifruit allergenicity and nutritional properties. The relationship between kiwifruit allergen (Act d 2, a major kiwifruit allergen) and antioxidant compounds of fruit are also assessed. Hopefully, it can help to reveal the physiological synthesis mechanism of kiwifruit allergens.
Materials and methods
Plant material and treatment
As designed in Fig. S1, two varieties of kiwifruit i.e. green and golden kiwifruits were purchased from the local store (Costco, Montreal, Quebec, Canada). Then, the size and weight-related parameters of all the samples were measured immediately. The fresh firm kiwifruits are selected as unripe samples (soluble solids: 6.2 %-8%; firmness: 15–20 N) and the soft fruits selected as ripe stage (soluble solids:12–15 %; firmness: 6–8 N). 30 fresh kiwifruits will be stored at 4 °C, 20 °C, and −20 °C for 10 days, respectively, to explore the changes of nutritional properties and allergenicity of kiwifruit. For the different fruit components, the peel, pulp and seeds of 20 kiwifruits are separated, and stored at −20℃until further analysis. After finishing the collection all the samples, they were dried using a freeze drier (7420020, Labconco Corporation, Kansas City, USA), and then were stored at −20 °C. All treatments and analyses are performed in triplicates.
Total soluble solids, pH, and moisture content determination
In the study, a handheld refractometer (Cole-Parmer, QC, Canada) was used to determine the total soluble solids (TSS) of kiwifruit samples and the results were expressed as °Brix at room temperature. The pH values were determined using a handheld pH meter (Fisher Scientific, USA) at 25 °C. The moisture content of kiwifruit samples was measured using weight differences before and after drying using an oven at 75 °C.
Sodium dodecyl sulphate polyacrylamide gel Electrophoresis (SDS-GAGE) analysis
According to the previous study, kiwifruit samples are extracted with 0.01 mol. L-1 phosphate buffer (pH 7.0) for two hours at room temperature and centrifuged at 5000 × g for 10 min (Kinaciyan et al., 2018). The supernatants are analyzed by SDS-PAGE and the relevant analysis is performed through a Fisher brand™ FB-VE10-1 Vertical Electrophoresis System.
Enzyme-linked immunosorbent assay (ELISA) test
The rabbit polyclonal antibodies and recombinant protein (anti-Act d 2) were purchased from Elabscience (USA). The Immunodetection of protein (Act d 2) present in the kiwifruit samples are evaluated through ELISA test. All the experimental procedures followed according to the protocol provided by the company. After the reaction, the optical density (OD) was recorded at 450 nm. Recombinant protein will be used as a standard to analyze the Act d 2 content.
Total protein measurement and total antioxidant activity
In the study, the Pierce BCA protein assay kit purchased from Thermo Fisher Scientific was used to determine the total protein content of kiwifruit. According to the previous studies, a Ferric-reducing/antioxidant power (FRAP) assay was used to analyze the total antioxidant capacity of samples (Wang, Vanga, & Raghavan, 2019b). The FRAP working solution was obtained through dissolving 2,4,6-Tripyridyl-S-triazine into 20 mmol.L-1 ferric chloride solution and 40 mmol.L-1 acetic acid buffer by the ratio 10:1:1 (v/v/v). And then, 10 μL of extract was mixed with 250 μL of FRAP working solution and the mixture was incubated at 37 °C for 5 min in the dark. The absorbance changes were observed at 593 nm through a plate reader (Fisher Scientific, USA). An external standard, 1000 μmol.L-1 of ferrous sulphate solution was applied to make a calibration curve, and the results were expressed as μmol Fe(II)/100 mL kiwifruit samples.
Ascorbic acid
According to the previous study, 2 g of frize dried kiwifruit samples were extracted with 10 mL of acetic acid buffer containing 3 % meta-phosphoric acid (Wang, Vanga, et al., 2019b). The ascorbic acid extact was obtained after centrifuging at 8000 × g, 4 °C for 15 min. In order to quantify the ascorbic acid content in the samples, an 1100 series HPLC system (Agilent Technologies, USA) equipped with a diode array detector (DAD, G131A) and a C18 column (250 × 4.60 mm, 5 μm; Sigma, USA) was used in the study. The flow rate was set at 0.5 mL/min. A gradient of mobile phase composed of two solvents, namely 0.17 % acetic acid (A), and acetonitrile (B) was used as the following program: from 0 to 9 % B in 3 min, from 9 to 81 % B in 4.5 min, from 81 to 76 % B in 5.5 min, from 76 to 70 % in 2 min, from 70 to 91 % B in 10 min (Wang, Vanga, et al., 2019b). The absorbance of ascorbic acid was recorded at 245 nm.
Results and discussion
Physiological parameters of ripe green and golden kiwifruit
The physiological parameters such as moisture content, firmness, pH, and total soluble solids of ripe green and golden kiwifruit were outlined in Table S1. The dimeter of green kiwifruit (43.98 mm) is smaller than golden kiwifruit (51.12 mm), while a heavier fresh weight was observed in green kiwifruit (73.8 g per fruit) compared to the golden one (61.2 g per fruit). No significant differences in the firmness and pH value were measured between green and golden kiwifruit. The moisture content of green kiwifruit was 83.24 %, followed by the golden kiwifruit with 79.31 %. A higher total soluble solids content was determined in green kiwifruit (16.17 Brix°) when compared with golden kiwifruit (14.13 Brix°). Thus, these advantages in physiological parameters may contribute to more consumption of green kiwifruit than the golden one.
Changes of kiwifruit allergenic potential under different varieties and fruit components
In the present study, SDS-PAGE and ELISA analyses were performed to evaluate the differences in allergen Act d 2 content between green and golden kiwifruit, and various fruit components (peel, pulp, and seed). As shown in Fig. 1a, a recombined Act d 2 was used as a standard during the SDS-PAGE analysis. SDS-PAGE measurement found that some clear intense protein bands, especially in seed, were observed in the samples obtained from green kiwifruit. The highest concentration of protein band at 24 KD was determined in seed, followed by pulp, and very limited intensity of band in peel samples. However, there no protein bands at 24 KD were observed in golden kiwifruit. ELISA analysis obtained similar results reported that Act d 2 allergen was mainly concentrated in seed of green kiwifruit (17.1 µg/g. DW), which is twofold and fourfold higher than that in pulp (8.5 µg/g. DW) and peel (4.2 µg/g. DW) samples, respectively (Fig. 1b). In comparison, the concentration of Act d 2 allergen was hardly determined in golden kiwifruit.
Fig. 1.
The Act d 2 content of green and golden kiwifruit in different fruit components (peel, pulp, and seed) was analyzed by SDS-PAGE (a) and ELISA test (b). Note: values with different letters in various columns are significantly different (p < 0.05) from each other. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Many studies have reported that the allergenicity of fruit varies among different varieties. In tomatoes, López-Matas et al. (2011) compared the allergenic potential in six tomato varieties including Rama, Rambo, Canario, Kumato, Pera, and Raf (López-Matas et al., 2011). The ranks of major tomato allergen, Lyc e 3, content was “Rambo > Pera > Canario > Raf > Kumato > Rama”. In strawberries, Tulipani et al. (2011) determined the differences in the allergenicity of four varieties of strawberries (Tulipani, Marzban, Herndl, Laimer, Mezzetti, & Battino, 2011). The results found that variety of ‘AN94.414.52’ showed the highest allergen content, followed by ‘Adria’ and ‘AN00.239.55’, whereas the variety of ‘Sveva’ contributed the lowest allergen content. In apples, Bolhaar et al. (2005) determined the allergenicity of twenty-one cultivars, the highest allergenic potential was ‘Golden Delicious’ apples, followed by Gala apples. Whereas the lowest level of allergenicity was observed in the ‘Santana’ apples (Bolhaar et al., 2005). In the present study, the concentration of Act d 2 was hardly measured using the SDS-PAGE and ELISA test (Fig. 1), which might be due to the lack of gene expression related to Act d 2 in golden kiwifruit. According to the data published by World Health Organization and International Union of Immunological Societies (WHO/IUIS), there four allergens including Act c1, Act c5, Act c8, and Act c10, have been recognized in golden kiwifruit (Wang, Vanga, et al., 2019a). Thus, patients with kiwifruit allergy history still may trigger allergenic reactions when consuming golden kiwifruit.
The obvious differences in the allergen content present in various fruit components (peel, pulp, and seed) have been observed in apples (Fernández-Rivas et al., 2006, Marzban et al., 2005), peaches (Boyano‐Martínez, Pedrosa, Belver, Quirce, & García‐Ara, 2013), tomatoes (Larramendi et al., 2008), and mangoes (Knödler et al., 2009). For example, studies fund that major mano allergen, alk(en)ylresorcinol, is mainly concentrated in the peel (41.9 mg/100 g) which is 16-fold higher compared to that in pulp (2.6 mg/100 g) (Knödler et al., 2009). In one study, fifty-seven children (7.4 years old) were hired to evaluate their allergic reactions to peach extracts from peel and pulp using a skin prick test (Boyano‐Martínez et al., 2013). The results found that 58 % of the total volunteers showed positive result with peach peel extract, 35 % with peach pulp extract. However, in the present study, our results reported that kiwifruit allergen, Act d 2 was mainly distributed in the seed, less amount of Act d 2 in the pulp, and very limited level in the peel (Fig. 1b). This distribution of Act d 2 may be associate with total protein content. As shown in Fig. 2a, the total protein content in seed extract of green kiwifruit (195.1 mg/g. DW) was significantly higher than that in peel (146.8 mg/g. DW) and pulp (159.9 mg/g. DW) due to the presence of storage proteins in seed. Thus, a higher total protein concentration in seed of kiwifruit possibly contributes to a higher allergenic potential to the patient with kiwifruit allergy (Wang, Vanga, et al., 2019a).
Fig. 2.
Total soluble protein content of kiwifruit in various components (a) and different maturity stages (b). Note: values with different letters in various columns are significantly different (p < 0.05) from each other.
Changes of kiwifruit allergenic potential under different maturity stages
Maturity is one of the most important factors which affects the fruit quality parameters including sweetness, flavor, color attributes, and nutrients concentration (Fawole and Opara, 2013, Teka, 2013). Recently, many studies have reported that maturity plays an important role in affecting the allergenic potential of fruit. In apples, Sancho et al. (2006) observed that the expression of a major apple allergen, Mal d 3, significantly increased in ripe apples compared with the unripe fruit (Sancho, Foxall, Rigby, et al., 2006). Another major apple allergen, Mal d 1, in ripe apples showed a similar trend of 4-fold higher than the apples picked at an early date (not reaching it ripe stage). Similar results were obtained by Schmitz-Eiberger et al. (2011) in three different varieties of apple including ‘Topaz’, ‘Golden Delicious’ and ‘Braeburn’ (Schmitz-Eiberger & Matthes, 2011). In tomatoes, Kitagawa et al. (2006) found that increasing the expression of the ripening inhibitor Mutant gene could significantly reduce the syntheses of allergenic proteins in tomatoes (Kitagawa et al., 2006).
In the present study, our results observed that major kiwifruit allergen, Act d 2 presented differently at various maturity stages. The highest intensity of protein band at 24 KD was measured in extracts obtained from ripe kiwifruit using SDS-PAGE analysis, followed by overripe kiwifruit, and unripe kiwifruit (Fig. 3a). The quantifying results obtained from the ELISA test proved the trend described above. The allergen content significantly enhanced by 50 % from the unripe stage to the ripe stage (9.5 μg/g.DW) of kiwifruit, while a slight decrease of Act d 2 in the overripe kiwifruit (7.5 μg/g.DW) was observed (Fig. 3b). The enhancement or decrease in the allergen content at various maturity stages is strongly associated with the presence of ethylene (Yang, Song, Campbell-Palmer, Walker, & Zhang, 2012). Yang et al. (2012) found that the expression of Mal d 1 (an apple major allergen) related gens was significantly up-regulated in apples during ripening and further obvious enhancement was measured after certain concentration of ethylene treatment (Yang et al., 2012). Whereas, the application of 1-Methylcyclopropene, an ethylene action inhibitor, significantly reduced the expression those Mal d 1 related gens. It provides an evidence that ethylene plays a role in regulating the allergen related gene expression in fruit.
Fig. 3.
SDS-PAGE analysis (a) and ELISA test (b) of green kiwifruit at different maturity stages and storage conditions (c). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
As mentioned above, higher total protein content may contribute to a higher allergenic potential. In the present study, ripe kiwifruit (154.0 mg/g. DW) showed the highest total protein content, followed by overripe kiwifruit (148.6 mg/g. DW) and unripe kiwifruit (124.7 mg/g. DW), which agrees with the trend of allergen, Act d 2, content in various maturity stages (Fig. 2b). Further, many studies reported that the allergenic potential of fruit is strongly linked to bioactive components such as phenolics. In sweet cherries, Schmitz-Eiberger et al. (2012) reported that cherries with higher phenolic compounds and anthocyanin demonstrated a lower allergen content (Schmitz-Eiberger & Blanke, 2012). In strawberries, previous study found that large amount of accumulation in total antioxidant capacity, total phenolics, and total flavonoids contributed to an obvious reduction in synthesis of strawberry allergen Fra a 1 (Tulipani et al., 2011). As shown in Fig. 4, ripe kiwifruit presented the highest percentage of total antioxidant activity (42.9 %), compared to unripe kiwifruit (31.35 %) and overripe kiwifruit (25.75 %), which is consistent with the trend of allergenic potential of kiwifruit under difference maturity stages.
Fig. 4.
Total antioxidant activity of kiwifruit in various components (a) and different maturity stages (b). Note: values with different letters in various columns are significantly different (p < 0.05) from each other.
Changes of kiwifruit allergenic potential under different storage conditions
In the present study, unripe green kiwifruits were stored at 4 °C, 20 °C, and −20 °C for 10 days. As shown in Fig. 3a, the SDS-PAGE analysis indicated that kiwifruit stored at 20 °C presented the highest intensity of Act d 2 protein band, followed by the kiwifruit stored at 4 °C and −20 °C. Similar results were observed by the ELISA test. After 10-day storage at different temperatures, the Act d 2 allergen content was significantly enhanced compared with the initial level (Fig. 3c). The highest concentration of Act d 2 allergen was obtained from the fruit stored at 20 °C for 10 days (9.8 μg/g. DW), followed by kiwifruit stored at 4 °C (7.5 μg/g. DW) and −20 °C (6.6 μg/g. DW). Whereas, no significant differences in Act d 2 allergen content were observed in fruit stored at −20 °C when compared with the initial level (5.0 μg/g. DW). Thus, the metabolisms of each compound are still preforming during the storages of kiwifruit even at a very low temperature (-20 °C). Similarly, previous study reported that a major apple allergen, Mal d 1, present in apples increased during cold storage at 4 °C (Hsieh, Moos, & Lin, 1995). The increase of related fruit allergens may be due to the improvement of ripeness of fruit, which results in more syntheses of bioactive compounds in the fruit (Wang, Vanga, et al., 2019a). Further, the enhancement in total protein content after 10-day storage potential contributed the increase of allergenic potential of kiwifruit (Table 1). However, Sancho et al. (2006) stored apples at room temperature and 2 °C for 0–5 months. The results found that the concentration of Mal d 3, a major apple allergen, significantly decreased compared with the initial level (Sancho, Foxall, Rigby, et al., 2006). Thus, the properties of fruit allergens are different between various fruit types.
Table 1.
Total soluble protein content (mg/g. DW) of green and golden kiwifruits after 10-day storage under different temperatures. Note: values with different letters in the same column are significantly different (p < 0.05) from each other.
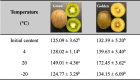 |
Ascorbic acid content of kiwifruit
Kiwifruit consistently ranks at the top of fruit due to its essential nutrition density, especially high ascorbic acid content (Wang, Vanga, McCusker, et al., 2019). In the present study, we compared the distribution differences of ascorbic acid in various fruit components including peel, pulp, and seed. As shown in Fig. 5a, the ascorbic acid content in green and golden kiwifruit showed a decreasing trend from the peel to the seed. In green kiwifruit, the highest concentration of ascorbic acid was found in the peel (165.42 mg/100 g.DW), followed by pulp (125.8 mg/100 g.DW) and seed samples (49.8 mg/100 g.DW). The ascorbic acid content in golden kiwifruit was up to 187.7 mg/100 g.DW, which is more than 2-fold higher than that in seed samples (81.8 mg/100 g.DW). Similar results were reported in pomegranate (Li, Guo, Yang, Wei, Xu, & Cheng, 2006), peach tomato and pear apple (Contreras-Calderón, Calderón-Jaimes, Guerra-Hernández, & García-Villanova, 2011). Peel component contains a higher ascorbic acid compared with other fruit components, which is due to the receptibility of peel resisting the external stresses such as high temperature and drought (Wang, Vanga, McCusker, et al., 2019). Further, more color-related compounds are synthesized in the peel resulting in a high ascorbic acid when compared with pulp and seed.
Fig. 5.
Ascorbic acid content of kiwifruit in various components (a) and correlation between Act d 2 content, total protein, ascorbic acid, and total antioxidant activity (TAA) of green kiwifruit (b). Note: *values in various columns are significantly different (p < 0.05) from each other. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Correlation between Act d 2 content, ascorbic acid, and antioxidant activity
In the present study, the results found that Act d 2 allergen content showed a positive correlation with total protein content, while a negative with the ascorbic acid content and total antioxidant activity were observed (Fig. 5b). The correlation coefficient of Act d 2 with the total antioxidant, ascorbic acid, and total protein is −0.97, −0.93, and 0.99, respectively. Thus, antioxidants (e.g., ascorbic acid) present in the kiwifruit may involve the metabolic regulation of Act d 2 allergen in various fruit components, which leads to a lower Act d 2 allergen synthesis in the peel as a result of the higher antioxidants compared with pulp and seed component (Fig. 4a). In contrast, the higher total protein concentration in the seed of green kiwifruit possibly contributed to a higher Act d 2 content present in seed compared with peel and pulp. Therefore, peeling and removing the seed from the green kiwifruit may reduce the allergenic potential of patients with a history of kiwifruit allergy.
Conclusion
In this study, the influences of variety, fruit components, maturity and storage conditions on the allergenic potential were evaluated. The results found that Act d 2 allergen is mainly observed in the green kiwifruit, while no detectable level of Act d 2 was determined in golden kiwifruit. The Act d 2 allergen present in seed is 2-fold and 4-fold higher when compared with pulp and peel, respectively. The highest Act d 2 allergen content was determined in ripe kiwifruit, followed by overripe fruit and unripe fruit. During the 10-day storages at different temperatures, the results found that a 50 % enhancement in Act d 2 content was observed when stored at 20 °C compared with the initial level, while no significant increases in the allergen concentration were observed after 10-day storage at −20 °C. Further, the Act d 2 content showed a negative with the ascorbic acid content and total antioxidant activity at various conditions. Therefore, antioxidants as important factors may involve the regulation of Act d 2 allergen of kiwifruit. Further studies regarding the mechanism of synthesis and regulation in Act d 2 allergen are still in need.
CRediT authorship contribution statement
Jin Wang: Conceptualization, Methodology, Software, Writing – original draft. Lili Zhang: Writing – review & editing. Xin Dong: Investigation, Writing – review & editing. Jun Wang: Supervision, Writing – review & editing. Vijaya Raghavan: Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
All the authors would like to thank the National Natural Science Foundation of China [32102093], Natural Science Foundation of Jiangsu Province [BK20210226], Fundamental Research Funds for the Central Universities, Natural Sciences and Engineering Research Council of Canada (NSERC) for supporting this work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100467.
Contributor Information
Jin Wang, Email: jinwang_2020@seu.edu.cn, jin.wang6@mail.mcgill.ca.
Jun Wang, Email: jun.wang@nwafu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Asam C., Hofer H., Wolf M., Aglas L., Wallner M. Tree pollen allergens—an update from a molecular perspective. Allergy. 2015;70(10):1201–1211. doi: 10.1111/all.12696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolhaar S.T., van de Weg W.E., van Ree R., Gonzalez-Mancebo E., Zuidmeer L., Bruijnzeel-Koomen C.A.…Knulst A.C. In vivo assessment with prick-to-prick testing and double-blind, placebo-controlled food challenge of allergenicity of apple cultivars. Journal of Allergy and Clinical Immunology. 2005;116(5):1080–1086. doi: 10.1016/j.jaci.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Borges J.-P., Jauneau A., Brule C., Culerrier R., Barre A., Didier A., Rougé P. The lipid transfer proteins (LTP) essentially concentrate in the skin of Rosaceae fruits as cell surface exposed allergens. Plant Physiology and Biochemistry. 2006;44(10):535–542. doi: 10.1016/j.plaphy.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Boyano-Martínez T., Pedrosa M., Belver T., Quirce S., García-Ara C. Peach allergy in Spanish children: Tolerance to the pulp and molecular sensitization profile. Pediatric Allergy and Immunology. 2013;24(2):168–172. doi: 10.1111/pai.12037. [DOI] [PubMed] [Google Scholar]
- Brenna O.V., Pastorello E.A., Farioli L., Pravettoni V., Pompei C. Presence of allergenic proteins in different peach (Prunus persica) cultivars and dependence of their content on fruit ripening. Journal of Agricultural and Food Chemistry. 2004;52(26):7997–8000. doi: 10.1021/jf0491052. [DOI] [PubMed] [Google Scholar]
- Chang W.-H., Liu J.-F. Effects of kiwifruit consumption on serum lipid profiles and antioxidative status in hyperlipidemic subjects. International Journal of Food Sciences and Nutrition. 2009;60(8):709–716. doi: 10.3109/09637480802063517. [DOI] [PubMed] [Google Scholar]
- Contreras-Calderón J., Calderón-Jaimes L., Guerra-Hernández E., García-Villanova B. Antioxidant capacity, phenolic content and vitamin C in pulp, peel and seed from 24 exotic fruits from Colombia. Food Research International. 2011;44(7):2047–2053. [Google Scholar]
- Eriksson N.E., Moller C., Werner S., Magnusson J., Bengtsson U., Zolubas M. Self-reported food hypersensitivity in Sweden, Denmark, Estonia, Lithuania and Russia. Journal of Investigational Allergology and Clinical Immunology. 2004;14(1):70–79. [PubMed] [Google Scholar]
- Fawole O.A., Opara U.L. Changes in physical properties, chemical and elemental composition and antioxidant capacity of pomegranate (cv. Ruby) fruit at five maturity stages. Scientia Horticulturae. 2013;150:37–46. [Google Scholar]
- Fernández-Rivas M., Bolhaar S., González-Mancebo E., Asero R., van Leeuwen A., Bohle B.…Sancho A.I. Apple allergy across Europe: How allergen sensitization profiles determine the clinical expression of allergies to plant foods. Journal of Allergy and Clinical Immunology. 2006;118(2):481–488. doi: 10.1016/j.jaci.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Hsieh L.-S., Moos M., Lin Y. Characterization of apple 18 and 31 kd allergens by microsequencing and evaluation of their content during storage and ripening. Journal of Allergy and Clinical Immunology. 1995;96(6):960–970. doi: 10.1016/s0091-6749(95)70234-2. [DOI] [PubMed] [Google Scholar]
- Kinaciyan T., Nagl B., Faustmann S., Frommlet F., Kopp S., Wolkersdorfer M.…Berger U. Efficacy and safety of 4 months of sublingual immunotherapy with recombinant Mal d 1 and Bet v 1 in patients with birch pollen–related apple allergy. Journal of Allergy and Clinical Immunology. 2018;141(3):1002–1008. doi: 10.1016/j.jaci.2017.07.036. [DOI] [PubMed] [Google Scholar]
- Kitagawa M., Moriyama T., Ito H., Ozasa S., Adachi A., Yasuda J.…Ishiguro Y. Reduction of allergenic proteins by the effect of the ripening inhibitor (rin) mutant gene in an F1 hybrid of the rin mutant tomato. Bioscience, Biotechnology, and Biochemistry. 2006;70(5):1227–1233. doi: 10.1271/bbb.70.1227. [DOI] [PubMed] [Google Scholar]
- Knödler, M., Reisenhauer, K., Schieber, A., & Carle, R. (2009). Quantitative determination of allergenic 5-alk (en) ylresorcinols in mango (Mangifera indica L.) peel, pulp, and fruit products by high-performance liquid chromatography. Journal of Agricultural and Food Chemistry, 57(9), 3639-3644. [DOI] [PubMed]
- Larramendi C., Ferrer A., Huertas A., García-Abujeta J., Andreu C., Tella R.…Pagán J. Sensitization to tomato peel and pulp extracts in the Mediterranean Coast of Spain: Prevalence and co-sensitization with aeroallergens. Clinical and Experimental Allergy. 2008;38(1):169–177. doi: 10.1111/j.1365-2222.2007.02865.x. [DOI] [PubMed] [Google Scholar]
- Li Y., Guo C., Yang J., Wei J., Xu J., Cheng S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chemistry. 2006;96(2):254–260. [Google Scholar]
- Lim S., Han S.H., Kim J., Lee H.J., Lee J.G., Lee E.J. Inhibition of hardy kiwifruit (Actinidia aruguta) ripening by 1-methylcyclopropene during cold storage and anticancer properties of the fruit extract. Food chemistry. 2016;190:150–157. doi: 10.1016/j.foodchem.2015.05.085. [DOI] [PubMed] [Google Scholar]
- López-Matas M.Á., Larramendi C.H., Ferrer Á., Huertas Á.J., Pagán J.A., García-Abujeta J.L.…Carnés J. Identification and quantification of tomato allergens: In vitro characterization of six different varieties. Annals of Allergy, Asthma & Immunology. 2011;106(3):230–238. doi: 10.1016/j.anai.2010.11.022. [DOI] [PubMed] [Google Scholar]
- Marzban G., Puehringer H., Dey R., Brynda S., Ma Y., Martinelli A.…Kolarich D. Localisation and distribution of the major allergens in apple fruits. Plant Science. 2005;169(2):387–394. [Google Scholar]
- Mills E.C., Mackie A., Burney P., Beyer K., Frewer L., Madsen C.…Van Ree R. The prevalence, cost and basis of food allergy across Europe. Allergy. 2007;62(7):717–722. doi: 10.1111/j.1398-9995.2007.01425.x. [DOI] [PubMed] [Google Scholar]
- Mishra S., Monro J. Kiwifruit remnants from digestion in vitro have functional attributes of potential importance to health. Food chemistry. 2012;135(4):2188–2194. doi: 10.1016/j.foodchem.2012.06.102. [DOI] [PubMed] [Google Scholar]
- Montoya C.A., Rutherfurd S.M., Moughan P.J. Kiwifruit fibre level influences the predicted production and absorption of SCFA in the hindgut of growing pigs using a combined in vivo–in vitro digestion methodology. British Journal of Nutrition. 2016;115(8):1317–1324. doi: 10.1017/S0007114515002883. [DOI] [PubMed] [Google Scholar]
- Platt K., Edenharder R., Aderhold S., Muckel E., Glatt H. Fruits and vegetables protect against the genotoxicity of heterocyclic aromatic amines activated by human xenobiotic-metabolizing enzymes expressed in immortal mammalian cells. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2010;703(2):90–98. doi: 10.1016/j.mrgentox.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Pravettoni V., Primavesi L., Farioli L., Brenna O.V., Pompei C., Conti A.…Pastorello E.A. Tomato allergy: Detection of IgE-binding lipid transfer proteins in tomato derivatives and in fresh tomato peel, pulp, and seeds. Journal of Agricultural and Food Chemistry. 2009;57(22):10749–10754. doi: 10.1021/jf9022367. [DOI] [PubMed] [Google Scholar]
- Rance F., Grandmottet X., Grandjean H. Prevalence and main characteristics of schoolchildren diagnosed withfood allergies in France. Clinical & Experimental Allergy. 2005;35(2):167–172. doi: 10.1111/j.1365-2222.2005.02162.x. [DOI] [PubMed] [Google Scholar]
- Richardson D.P., Ansell J., Drummond L.N. The nutritional and health attributes of kiwifruit: A review. European journal of nutrition. 2018;57(8):2659–2676. doi: 10.1007/s00394-018-1627-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho A.I., Foxall R., Browne T., Dey R., Zuidmeer L., Marzban G.…Laimer M. Effect of postharvest storage on the expression of the apple allergen Mal d 1. Journal of agricultural and food chemistry. 2006;54(16):5917–5923. doi: 10.1021/jf060880m. [DOI] [PubMed] [Google Scholar]
- Sancho A.I., Foxall R., Rigby N.M., Browne T., Zuidmeer L., van Ree R.…Mills E.C. Maturity and storage influence on the apple (Malus domestica) allergen Mal d 3, a nonspecific lipid transfer protein. Journal of agricultural and food chemistry. 2006;54(14):5098–5104. doi: 10.1021/jf0530446. [DOI] [PubMed] [Google Scholar]
- Schmitz-Eiberger M., Matthes A. Effect of harvest maturity, duration of storage and shelf life of apples on the allergen Mal d 1, polyphenoloxidase activity and polyphenol content. Food Chemistry. 2011;127(4):1459–1464. [Google Scholar]
- Schmitz-Eiberger M.A., Blanke M.M. Bioactive components in forced sweet cherry fruit (Prunus avium L.), antioxidative capacity and allergenic potential as dependent on cultivation under cover. LWT-Food. Science and Technology. 2012;46(2):388–392. [Google Scholar]
- Sirvent S., Cantó B., Cuesta-Herranz J., Gómez F., Blanca N., Canto G.…Palomares O. Act d 12 and Act d 13: Two novel, masked, relevant allergens in kiwifruit seeds. Journal of Allergy and Clinical Immunology. 2014;133(6):1765–1767. doi: 10.1016/j.jaci.2014.01.035. e1764. [DOI] [PubMed] [Google Scholar]
- Skinner M.A., Loh J.M., Hunter D.C., Zhang J. Gold kiwifruit (Actinidia chinensis ‘Hort16A’) for immune support. Proceedings of the Nutrition Society. 2011;70(2):276–280. doi: 10.1017/S0029665111000048. [DOI] [PubMed] [Google Scholar]
- Teka T.A. Analysis of the effect of maturity stage on the postharvest biochemical quality characteristics of tomato (Lycopersicon esculentum Mill.) fruit. International Research Journal of Pharmaceutical and Applied Sciences (IRJPAS) 2013;3(5):180–186. [Google Scholar]
- Tulipani S., Marzban G., Herndl A., Laimer M., Mezzetti B., Battino M. Influence of environmental and genetic factors on health-related compounds in strawberry. Food Chemistry. 2011;124(3):906–913. [Google Scholar]
- Ukleja-Sokołowska N., Zacniewski R., Lis K., Żbikowska-Gotz M., Kuźmiński A., Bartuzi Z. Exercise induced anaphylaxis in kiwi allergic patient: Case report. Allergy, Asthma & Clinical Immunology. 2021;17(1):1–7. doi: 10.1186/s13223-021-00595-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Vanga S.K., McCusker C., Raghavan V. A comprehensive review on kiwifruit allergy: Pathogenesis, diagnosis, management, and potential modification of allergens through processing. Comprehensive reviews in food science and food safety. 2019;18(2):500–513. doi: 10.1111/1541-4337.12426. [DOI] [PubMed] [Google Scholar]
- Wang J., Vanga S.K., Raghavan V. Effect of pre-harvest and post-harvest conditions on the fruit allergenicity: A review. Critical reviews in food science and nutrition. 2019;59(7):1027–1043. doi: 10.1080/10408398.2017.1389691. [DOI] [PubMed] [Google Scholar]
- Wang J., Vanga S.K., Raghavan V. High-intensity ultrasound processing of kiwifruit juice: Effects on the ascorbic acid, total phenolics, flavonoids and antioxidant capacity. LWT. 2019;107:299–307. [Google Scholar]
- Yang X., Song J., Campbell-Palmer L., Walker B., Zhang Z. Allergen related gene expression in apple fruit is differentially controlled by ethylene during ripening. Postharvest Biology and Technology. 2012;63(1):40–49. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







