Abstract
Clinical studies on the relationship between pesticide exposure at home and infertility in the general population are scarce. Whether the antioxidant nutrients or other health-related factors affect the pesticide–infertility relationship remains unknown. This nationwide study screened 29,400 participants of the National Health and Nutrition Examination Surveys conducted between 2013 and 2018. The participants were subdivided according to dietary zinc intake based on the recommended dietary allowances as the low-zinc and high-zinc groups (< 8 and ≥ 8 mg/day, respectively), and according to body mass index (BMI; cut-off 28 kg/m2) as the low-BMI and high-BMI groups. Participants who were exposed to pesticides at home had an increased risk of infertility (odds ratio [OR] = 1.56, 95% confidence intervals [CI]: 1.06–2.29). The incidence of infertility differed in low-zinc and high-zinc groups (OR, 95% CI: 2.38, 1.40–4.06 vs. 0.98, 0.53–1.79, respectively), indicating an interaction between pesticide exposure and zinc intake in households (P = 0.047), which suggests that a zinc-rich diet may reduce the risk of pesticide-induced infertility. Similarly, the relationship between pesticide exposure and infertility risk differed in the low-BMI and high-BMI groups (OR, 95% CI: 0.90, 0.42–1.93 vs. 2.23, 1.39–3.58, respectively; P = 0.045), suggesting that high BMI may intensify the infertility risk caused by pesticide exposure. These new findings reveal the antagonistic and synergistic effect of zinc and obesity, respectively, in pesticide-induced infertility risk and suggest that individuals who are obese and on a low-zinc diet may be more susceptible to infertility induced by household pesticide exposure.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11356-022-23629-x.
Keywords: Infertility, Household pesticide exposure, Dietary zinc intake, Obesity, General population
Introduction
Worldwide, an estimated 2.3 billion kg pesticides are used in agriculture annually, and pesticides enter our daily lives in a variety of ways (Juntarawijit and Juntarawijit 2018; Nougadère et al. 2020; de Gavelle et al. 2016; Sieke et al. 2018; Ferré et al. 2018; Benbrook and Davis 2020). Research has increased the extant concerns that the permissible pesticide levels in food may be too high for humans, especially vulnerable groups, such as pregnant women (Vandenberg et al. 2012; Hayes et al. 2002). Fucic et al. showed that many pesticides are endocrine disruptors, and that exposure to even very low doses of pesticides may produce observable biological effects, such as diabetes, neurodegenerative diseases, cancer, and birth defects (Fucic et al. 2021). Recently, Chiu et al. reported that eating fruits or vegetables with pesticide that were within the normal human exposure range was associated with adverse reproductive outcomes in humans (Chiu et al. 2018).
Given the ubiquity of pesticide exposure, eating fewer fruits and vegetables seems like a ‘better’ option. However, evidence of a relationship between diet and female fertility and of a lower fruit intake with infertility has increasingly accummulated (Skoracka et al. 2021). To improve their chances of conception, many women request treatment with dietary supplements with variable ingredients (Vitagliano et al. 2021). A systematic review by Salas-Huetos et al. indicated that daily diets rich in some antioxidant nutrients, such as vitamin C, vitamin E, selenium, and zinc, were inversely associated with low semen quality parameters (Salas-Huetos et al. 2017). Owing to these conflicting results, the relationship between pesticide exposure and infertility needs to be further investigated. The risks of using pesticides in home have also been overlooked. Clinical studies of the relationship between household pesticide exposure and infertility are limited, and Morris emphasised the need to replicate the association between pesticide exposure and infertility (Morris 2018). In addition, obesity is strongly associated with infertility and is a major contributor to various causes of infertility (Sharma et al. 2013; Talmor and Dunphy 2015). Dietary factors and physical fitness might affect the relationship between pesticide exposure and infertility in infertile individuals who are exposed to pesticides.
Therefore, we aimed to explore whether effect modifiers, such as dietary zinc intake and body mass index (BMI), could modulate the relationship between pesticide exposure and infertility and to analyse how the dietary zinc intake and BMI affect this association.
Materials and methods
Study population and data sources
The nationally representative population and data included in this study were sourced from the National Health and Nutrition Examination Survey (NHANES), conducted by the National Center for Health Statistics (NCHS) and Centers for Disease Control and Prevention (CDC), that used a complex, multi-stage, stratified sample survey to obtain data representative of the entire non-institutionalised US population. Continuous public data from the NHANES database (three cycles: 2013–2014, 2015–2016, and 2017–2018) were included in the analysis because only three cycles containing the infertility questionnaire were completed before the coronavirus disease pandemic. We collected demographic, examination, dietary, and questionnaire data. During the data processing, we excluded individuals for whom data on pesticide exposure, infertility, dietary intakes, BMI, and other covariates were missing. The final analysis dataset included 2680 American women.
Ethical approval
The study was approved by the NCHS Ethics Review Board, and the original protocol is available online (https://www.cdc.gov/nchs/nhanes/irba98.htm). All participants aged 18 to 46 years were included and provided written informed consent.
Infertility
Infertility was defined based on the response to the following item in the questionnaire: ‘Have you ever tried and failed to get pregnant for at least a year?’ The response ‘yes’ was defined as ‘ever infertile’, the response ‘no’ was defined as ‘fertile’, and participants with missing responses were excluded from the study (Gleason et al. 2019).
Household pesticide exposure
Household pesticide exposure was defined based on the response to the following item in the questionnaire: ‘In the past 7 days, were there any chemical products used in your/his/her home to control fleas, roaches, ants, termites, or other insects?’ The response ‘yes’ was defined as ‘household pesticide exposure’, the response ‘no’ was defined as ‘no household pesticide exposure’, and participants with missing responses were excluded from the study (Chen et al. 2020a, b). Moreover, to further understand the effects of pesticide exposure, we explored the relationship between pesticide metabolites in urine and infertility risk. With other covariates complete, a total of 616 participants with chlorophenol metabolites in urine (NHANES 2013–2014, and 2015–2016), and 738 participants with organophosphate metabolites in urine (NHANES 2015–2016, and 2017–2018) were available for the analysis.
Dietary antioxidant nutrient intake
The 24-h dietary antioxidant nutrient intake data were obtained by dietary recall interview, and all interview data were collected by trained professionals in mobile examination centres. The 24-h recall interview collection method is the most commonly used method to determine dietary intake in large surveys and has been used by the NHANES for many years based on consensus among expert panels (Ahluwalia et al. 2016). The recommended dietary allowances (RDA) for zinc, selenium, vitamin E, and vitamin C for women are 8 mg/day, 55 µg/day, 15 mg/day, and 75 mg/day, respectively. Based on the RDA values, the zinc, selenium, vitamin E, and vitamin C intake data were categorised into two groups (low and high groups).
Potential covariates
Our study considered the following variables as covariates: age, race/ethnicity, family income, smoking status, educational level, marital status, work activity, cotinine, waist circumference, BMI, alcohol consumption, and dietary intakes, such as zinc, selenium, vitamin E, vitamin C, caffeine, folate, sodium, potassium, phosphorus, calcium, magnesium, copper, iron, lycopene, beta-cryptoxanthin, beta-carotene, vitamin A, vitamin D, vitamin K, lutein-zeaxanthin, niacin, use of multivitamins, energy, protein, and fibre. In this study, we categorised race/ethnicity as non-Hispanic white, non-Hispanic black, other Hispanic, Mexican–American, and other races. Family income was defined using the poverty income ratio. Education level (as defined by the NHANES) was classified as ‘did not graduate from high school’, ‘high school graduate’, and ‘college graduate or above’. Marital status was classified as ‘married or lives with partner’ and ‘lives alone’. BMI was obtained from examination data, and the participants were categorised into two groups based on the value of 28 kg/m2. Participants were asked if they had smoked more than 100 cigarettes previously, and those who answered that they had been smoking over several days or daily were classified as ‘current smokers’; those who were not smoking currently were classified as ‘former smokers’; and those who had not smoked at least 100 cigarettes previously were regarded as ‘never smokers’. Work activity was categorised as vigorous, moderate, and light work activities.
Statistical analysis
The statistical software packages of R (http://www.R-project.org, The R Foundation) and Free Statistics software version 1.3 were used for all analyses (Yang et al. 2021). Categorical and continuous variables are represented by the number (n) and percentage (%), and mean and standard deviations, respectively. The statistical methods used in the analyses include the chi-square test, two-tailed Student’s t test, and logistic regression. Model 1 did not consider any variables in the univariate analysis. In model 2, age and race/ethnicity were considered in the multivariate analysis. In combination with previous evidence, and in order to obtain more robust results, the following covariates were further adjusted in model 3 based on model 2: family income, smoking status, educational level, marital status, work activity, cotinine, waist circumference, BMI, alcohol consumption, and dietary intakes, such as zinc, selenium, vitamin E, vitamin C, caffeine, folate, sodium, potassium, phosphorus, calcium, magnesium, copper, iron, lycopene, beta-cryptoxanthin, beta-carotene, vitamin A, vitamin D, vitamin K, lutein-zeaxanthin, niacin, use of multivitamins, energy, protein, and fibre. Odds ratios (ORs) and 95% confidence intervals (CI) were calculated. The subgroup analyses according to the dietary antioxidant nutrient intake and BMI were performed using the stratified multivariate logistic regression model. We tested for potential interaction among the subgroups by using a likelihood ratio test comparing the model with only common non-interaction term against adding a term to the model in which the two predictor variables [pesticide exposure at home × subgroup variable (dietary antioxidant nutrient intake or BMI)] are multiplied. A two-sided P < 0.05 was considered statistically significant.
Results
Baseline characteristics of the study population
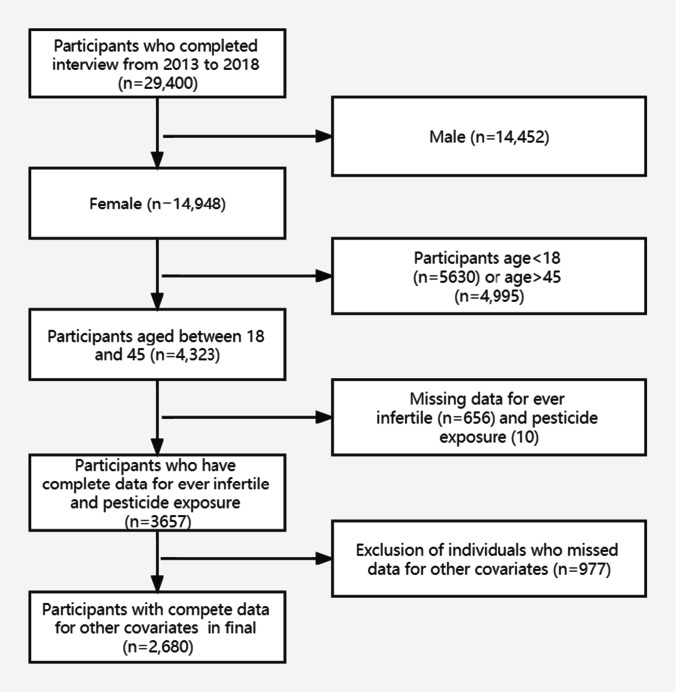
A flowchart of the exclusion and inclusion criteria is shown in Fig. 1. As shown in Table 1, among the 2860 participants who met the criteria for inclusion in the final analysis, 242 (9.0%) reported exposure to pesticides in their households. The mean age of participants in our study population was 32.7 ± 7.5 years, and 11.9% of the participants self-reported ever infertility. There were no significant differences between the pesticide exposure and non-exposure groups except for race/ethnicity, poverty income ratio, marital status, smoking status, cotinine, and infertility. The mean value of zinc, selenium, vitamin E, and vitamin C daily intake in the study population were 8.7 mg, 94.8 ug, 6.9 mg, and 45.1 mg, respectively, and the daily intake of vitamin E, and vitamin C was below the RDA value. Pesticide use in the study population was more likely to occur in lower-income households. Infertility rates in the pesticide non-exposure and exposure groups were 11.5% and 16.1%, respectively.
Fig. 1.
Flowchart of the study
Table 1.
Baseline characteristics of the study sample
| Variables | Total (n = 2680) | Pesticide unexposed (n = 2438) | Pesticide exposed (n = 242) | P value |
|---|---|---|---|---|
| Demographic | ||||
| Age, mean (SD), (years) | 32.7 ± 7.5 | 32.7 ± 7.5 | 32.8 ± 8.1 | 0.885 |
| Race, n (%) | 0.009 | |||
| Mexican American | 442 (16.5) | 396 (16.2) | 46 (19) | |
| Non-Hispanic White | 938 (35.0) | 866 (35.5) | 72 (29.8) | |
| Non-Hispanic Black | 568 (21.2) | 498 (20.4) | 70 (28.9) | |
| Other Hispanic | 266 ( 9.9) | 244 (10) | 22 (9.1) | |
| Other race | 466 (17.4) | 434 (17.8) | 32 (13.2) | |
| Poverty income ratio, median (IQR) | 1.9 (1.0, 3.7) | 2.0 (1.0, 3.8) | 1.6 (0.8, 2.8) | < 0.001 |
| Education_level, n (%) | 0.197 | |||
| College education or above | 1768 (66.0) | 1621 (66.5) | 147 (60.7) | |
| Graduated from high school | 514 (19.2) | 461 (18.9) | 53 (21.9) | |
| Did not graduate from high school | 398 (14.9) | 356 (14.6) | 42 (17.4) | |
| Marital_status, n (%) | 0.024 | |||
| Married or live with partner | 1573 (58.7) | 1448 (59.4) | 125 (51.7) | |
| Live alone | 1107 (41.3) | 990 (40.6) | 117 (48.3) | |
| BMI, (kg/m2) median (IQR) | 28.2 (23.3, 34.2) | 28.2 (23.4, 34.4) | 28.1 (23.2, 33.8) | 0.746 |
| Waist_circumference, (cm) Median (IQR) | 93.6 (82.1, 107.2) | 93.7 (82.1, 107.6) | 92.7 (81.8, 104.8) | 0.432 |
| Smoke, n (%) | 0.019 | |||
| Never | 1878 (70.1) | 1719 (70.5) | 159 (65.7) | |
| Former | 302 (11.3) | 280 (11.5) | 22 (9.1) | |
| Current | 500 (18.7) | 439 (18) | 61 (25.2) | |
| Work activity, n (%) | 0.291 | |||
| Light | 1482 (55.3) | 1356 (55.6) | 126 (52.1) | |
| Moderate | 709 (26.5) | 646 (26.5) | 63 (26) | |
| Vigorous | 489 (18.2) | 436 (17.9) | 53 (21.9) | |
| Cotinine, (ng/ml) median (IQR) | 0.0 (0.0, 2.3) | 0.0 (0.0, 1.6) | 0.1 (0.0, 36.3) | < 0.001 |
| Dietary | ||||
| Alcohol, (gm) median (IQR) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.807 |
| Caffeine, (mg) median (IQR) | 72.0 (5.0, 150.0) | 72.0 (5.0, 150.0) | 61.5 (8.0, 159.8) | 0.68 |
| Energy, (kcal) median (IQR) | 1850.0 (1381.0, 2366.0) | 1843.0 (1380.0, 2359.0) | 1998.0 (1412.0, 2492.0) | 0.137 |
| Protein, (gm) median (IQR) | 67.5 (49.1, 91.6) | 67.6 (49.4, 91.5) | 65.7 (45.8, 91.7) | 0.575 |
| Fibre, (gm) median (IQR) | 13.4 (8.7, 19.7) | 13.5 (8.8, 19.9) | 12.7 (8.0, 18.5) | 0.106 |
| Folat, (mcg) median (IQR) | 306.0 (205.0, 445.0) | 304.0 (207.0, 445.0) | 311.5 (186.2, 440.8) | 0.672 |
| Sodium, (mg) median (IQR) | 3008.0 (2145.0, 3971.0) | 2994.0 (2146.0, 3963.0) | 3158.0 (2144.0, 4103.0) | 0.277 |
| Potassium, (mg) median (IQR) | 2136.0 (1525.0, 2841.0) | 2131.0 (1527.0, 2848.0) | 2174.0 (1471.0, 2824.0) | 0.612 |
| Phosphorus, (mg) median (IQR) | 1158.0 (832.8, 1530.0) | 1158.0 (834.8, 1528.0) | 1157.0 (821.2, 1541.0) | 0.656 |
| Calcium, (mg) median (IQR) | 783.0 (506.0, 1110.0) | 783.0 (505.0, 1109.0) | 781.0 (509.5, 1114.0) | 0.846 |
| Copper, (mg) median (IQR) | 1.0 (0.7, 1.4) | 1.0 (0.7, 1.4) | 1.0 (0.7, 1.3) | 0.96 |
| Iron, (mg) median (IQR) | 11.1 (7.8, 15.3) | 11.1 (7.8, 15.3) | 11.0 (7.4, 15.8) | 0.908 |
| Magnesium, (mg) median (IQR) | 247.0 (178.0, 331.2) | 247.5 (178.2, 332.0) | 238.5 (173.2, 315.0) | 0.186 |
| Lycopene, (mcg) median (IQR) | 1731.0 (13.8, 5177.0) | 1720.0 (12.0, 5177.0) | 1910.0 (111.0, 5004.0) | 0.566 |
| Beta-cryptoxanthin, (mcg) median (IQR) | 25.0 (7.0, 80.0) | 26.0 (7.0, 81.0) | 23.0 (6.0, 63.5) | 0.188 |
| Beta-carotene, (mcg) median (IQR) | 706.0 (262.0, 2240.0) | 718.5 (264.0, 2254.0) | 570.5 (248.5, 1923.0) | 0.153 |
| Vitamin A, (mcg) median (IQR) | 434.0 (231.8, 720.0) | 437.0 (234.2, 720.8) | 395.0 (209.2, 702.8) | 0.174 |
| Vitamin D, (mcg) median (IQR) | 2.6 (0.9, 5.2) | 2.6 (0.9, 5.2) | 2.7 (0.6, 4.9) | 0.331 |
| Vitamin K, (mcg) median (IQR) | 67.9 (37.1, 135.2) | 68.8 (37.4, 136.6) | 62.9 (36.2, 129.0) | 0.398 |
| Lutein-zeaxanthin, (mcg) median (IQR) | 689.5 (316.0, 1442.0) | 699.5 (317.2, 1452.0) | 555.0 (298.0, 1285.0) | 0.176 |
| Niacin, (mg) median (IQR) | 20.4 (14.1, 27.8) | 20.4 (14.2, 27.7) | 20.4 (13.5, 28.6) | 0.932 |
| Use of multivitamin, n (%) | 0.507 | |||
| No | 2653 (99.0) | 2412 (98.9) | 241 (99.6) | |
| Yes | 27 ( 1.0) | 26 (1.1) | 1 (0.4) | |
| Antioxidants | ||||
| Vitamin C, (mg) median (IQR) | 45.1 (18.8, 104.7) | 45.2 (18.8, 105.2) | 43.0 (19.4, 97.1) | 0.95 |
| Vitamin E, (mg) median (IQR) | 6.9 (4.5, 10.5) | 6.9 (4.5, 10.4) | 6.9 (4.3, 11.3) | 0.726 |
| Selenium, (mcg) median (IQR) | 94.8 (67.5, 129.4) | 94.8 (67.9, 129.1) | 95.5 (63.4, 131.5) | 0.998 |
| Zinc, (mg) median (IQR) | 8.7 (6.0, 12.0) | 8.7 (6.0, 11.9) | 8.6 (5.8, 12.2) | 0.564 |
| Infertile, n (%) | 0.044 | |||
| Fertile | 2361 (88.1) | 2158 (88.5) | 203 (83.9) | |
| Ever infertile | 319 (11.9) | 280 (11.5) | 39 (16.1) |
Association between covariates and infertility risk
The univariate analyses revealed that age, family income, BMI, waist circumference, marital status, and smoking status were associated with infertility. Dietary antioxidant intake, including zinc, selenium, vitamin E, and vitamin C, was un-associated with infertility (Supplementary Table 1).
Association between pesticide exposure at home and infertility risk
As shown in Table 2, in model 1, individuals exposed to pesticides in their households had a higher risk of infertility than those unexposed (OR 1.48, 95% CI: 1.03–2.13, P = 0.035). Similar results were obtained using model 2, which was adjusted for sociodemographic data (OR 1.5, 95% CI: 1.03–2.17, P = 0.033), and a higher risk was observed in model 3, which was adjusted for all the covariates that are listed in Table 1 (OR 1.56, 95% CI 1.06–2.29, P = 0.024). Among associations between urinary concentrations of pesticide metabolites and infertility risk, Dimethylphosphate was associated with infertility risk (OR = 1.05, 95% CI: 1.00–1.09, P = 0.043) (Supplementary Table 2).
Table 2.
Association between pesticide exposure at home and infertility risk
| N (%) | OR | 95% CI | P value | |
|---|---|---|---|---|
| Model 1a | ||||
| No pesticide exposure | 2483 (11.5) | Reference | ||
| Pesticide exposure | 242 (16.1) | 1.48 | 1.03–2.13 | 0.035 |
| Model 2b | ||||
| No pesticide exposure | 2483 (11.5) | Reference | ||
| Pesticide exposure | 242 (16.1) | 1.50 | 1.03–2.17 | 0.033 |
| Model 3c | ||||
| No pesticide exposure | 2483 (11.5) | Reference | ||
| Pesticide exposure | 242 (16.1) | 1.56 | 1.06–2.29 | 0.024 |
OR, odds ratio; CI, confidence intervals
aModel 1: unadjusted
bModel 2: adjusted for sociodemographic covariates
cModel 3: adjusted for all covariates listed in Table 1
High dietary zinc intake weakens the association between pesticide exposure and infertility risk
A significant interaction was observed between zinc intake and household pesticide exposure for infertility (interaction likelihood ratio test: P = 0.047; Table 3). Stratified analysis based on zinc intake showed that, in the high dietary zinc group, infertility rates in the pesticide-exposed and -unexposed groups were 11.5% and 11.6%, respectively. The association between pesticide exposure and infertility risk was not statistically significant in the multivariate logistic analysis (OR 0.98, 95% CI: 0.53–1.79, P = 0.937). However, in the low dietary zinc group, infertility rates in the exposed and unexposed groups were 21.4% and 11.3%, respectively. Among participants with low zinc intake, exposure to pesticides was associated with a 138% higher risk of infertility (OR 2.38, 95% CI: 1.40–4.06, P = 0.001). Significant interactions were not observed between the intake of other antioxidant nutrients (selenium, vitamin E, and vitamin C) and pesticide exposure with regard to infertility (interaction likelihood ratio tests: P = 0.334, P = 0.356, and P = 0.560, respectively; Supplementary Tables 3–5).
Table 3.
Association between pesticide exposure at home and infertility risk by dietary zinc intake
| Subgroup | N (%) | OR | 95% CI | P value | P value for interaction |
|---|---|---|---|---|---|
| Zinc intake (< 8 mg/day) | 0.047 | ||||
| No pesticide exposure | 1069 (11.3) | Reference | |||
| Pesticide exposure | 112 (21.4) | 2.38 | 1.40–4.06 | 0.001 | |
| Zinc intake (≥ 8 mg/day) | |||||
| No pesticide exposure | 1369 (11.6) | Reference | |||
| Pesticide exposure | 130 (11.5) | 0.98 | 0.53–1.79 | 0.937 |
OR, odds ratio; CI, confidence intervals
Adjusted for all covariates listed in Table 1
High BMI strengthens the association between pesticide exposure and infertility risk
Table 4 shows an interaction between BMI and household pesticide exposure for infertility (interaction likelihood ratio test: P = 0.045). In the low BMI (< 28 kg/m2) group, infertility rates in the pesticide-exposed and -unexposed groups were 7.4% and 8.6%, respectively. The association between pesticide exposure and infertility risk was not statistically significant in the multivariate logistic analysis (OR 0.90, 95% CI: 0.42–1.93, P = 0.790). However, in the high-BMI (≥ 28 kg/m2) group, infertility rates in the exposed and unexposed groups were 24.8% and 14.2%, respectively. Among participants with a higher BMI, exposure to pesticides was associated with a 123% increased risk of infertility (OR 2.23, 95% CI: 1.39–3.58, P = 0.001).
Table 4.
Associations between pesticide exposure at home and infertility risk by BMI
| Subgroup | N (%) | OR | 95% CI | P value | P value for interaction |
|---|---|---|---|---|---|
| BMI (< 28 kg/m2) | 0.045 | ||||
| No pesticide exposure | 1193 (8.6) | Reference | |||
| Pesticide exposure | 121 (7.4) | 0.90 | 0.42–1.93 | 0.79 | |
| BMI (≥ 28 kg/m2) | |||||
| No pesticide exposure | 1245 (14.2) | Reference | |||
| Pesticide exposure | 121 (24.8) | 2.23 | 1.39–3.58 | 0.001 |
OR, odds ratio; CI, confidence intervals
Adjusted for all covariates listed in Table 1
Discussion
In a nationally representative sample of adult women in the US general population, we explored the association between pesticide exposure at home and infertility risk, and then examined the modifiers of this relationship. In the low dietary zinc intake group, pesticide exposure was associated with a 1.38-fold increased risk of infertility compared with the pesticide-unexposed group. No association was found between pesticide use at home and infertility in the high dietary zinc intake group, which indicates that zinc intake may play a protective role against pesticide-exposure-induced infertility. Conversely, in the high-BMI group, pesticide exposure induced a 1.23-fold increase in the risk of infertility, whilst no association between pesticide exposure and infertility was found in the low-BMI group, thereby suggesting a synergistic effect of BMI on the infertility risk that is triggered by household pesticide exposure. Therefore, our results indicate that dietary zinc intake and BMI act as modifiers of the association between household pesticide exposure and infertility.
Numerous studies have shown that occupational exposure or residence near agricultural environments where pesticides are frequently used confers a higher risk of adverse reproductive health outcomes (Shirangi et al. 2008; Naidoo et al. 2011; Razi et al. 2016; Settimi et al. 2008; Rahimi et al. 2020). In the few studies that found no such association (Bell et al. 2001; Willis et al. 1993), dietary zinc intake might not have been considered. Approximately 20% of the world’s population and 10% of the US population have zinc deficiency (Chen et al. 2020a, b). Moreover, studies exploring the influence of pesticides on infertility in the general population are limited. Our results further explored that pesticide exposure at home is associated with infertility risk in the general population. Our results are in line with the results of a recent study by Chiu et al., which showed that dietary pesticide exposure, even through fruits and vegetables, is associated with infertility (Chiu et al. 2018), which raises widespread concerns about dietary pesticide exposure. Pesticides demonstrate their toxic influence on humans and animals via oxidative stress-mediated pathways (Weis et al. 2021; Sharma et al. 2020; Pašková et al. 2011). However, fruits and vegetables, as part of our healthy diet, contain many antioxidant nutrients, such as zinc, selenium, vitamin C, and vitamin E (Millen et al. 2016). However, it remains unknown whether these antioxidant nutrients or other health-related factors affect the pesticide–infertility relationship.
Our results showed a significant association between pesticide exposure and infertility in the low-zinc diet group but not in the high-zinc diet group, suggesting a beneficial effect of daily zinc intake as recommended based on the RDA. We found that other antioxidant nutrients did not have the same effect as zinc. Different nutrient elements may not have the same effect intensity, and further experimental studies are needed to elucidate their effects. Dietary supplements containing zinc are important for fertility (Garolla et al. 2020; Mumford et al. 2020; Vickram et al. 2021). Kerns et al. proposed that zinc is an essential ion for the ability of mammalian sperm to fertilise an ovum (Kerns et al. 2018). Similarly, Ebisch et al. concluded that zinc is important in the pathogenesis and prevention of infertility, considering its antioxidant properties (Ebisch et al. 2007). However, a new rigorous randomised controlled trial conducted by Schisterman et al. revealed that zinc supplementation did not significantly improve live birth rates among couples seeking infertility treatment (Schisterman et al. 2020), which disregarded the effect of some important potential factors, such as pesticide exposure. Despite these inconsistent results, given that zinc supplementation can alleviate infertility caused by pesticide exposure in daily life, we believe that zinc supplementation is necessary for the infertile population. In addition, zinc, as a cofactor, is essential for the functioning of more than 300 enzymes (Vallee and Falchuk 1993). Zinc is the only metallic element that is required in all six classes of enzymes, i.e., lyases, ligases, transferases, isomerases, oxidoreductases, and hydrolases (Kerns et al. 2018). Upon entering the body, pesticides are metabolised by a number of biological metabolic enzymes, including paraoxonase and glutathione S-transferase (Volk et al. 2011), and zinc supplementation may be beneficial in accelerating the metabolic elimination of pesticides. Furthermore, zinc interacts with cell membranes to counter the effects of various harmful substances that cause oxidative damage (Malhotra and Dhawan 2014). Goel et al. found that zinc supplementation played a potentially protective role in alleviating toxicity induced by chlorpyrifos in rats (Goel et al. 2005).
A significant association between pesticide exposure and infertility was found in the high BMI-group but not in the low-BMI group, suggesting the potential impact of obesity on this association. Obesity is associated with various adverse reproductive outcomes, including anovulation, infertility, and an increased risk of miscarriage (Talmor and Dunphy 2015). The mechanism of obesity-related decline in female fertility occurs through influences on endometrial receptivity, oocyte quality, and the hypothalamic-pituitary-ovarian axis (Ramlau-Hansen et al. 2007; Van Der Steeg et al. 2008). A clinical study conducted by Zhang et al. found a positive association between pesticide exposure and obesity (Zhang et al. 2019). However, the exact mechanism whereby obesity interacts with pesticides to cause infertility is still unclear. Notably, Gutgesell et al. concluded that, given their lipophilic nature, many pesticides can accumulate in fatty tissue (Gutgesell et al. 2020). Therefore, it is reasonable to believe that the synergistic effect of obesity and pesticide exposure on infertility risk occurs because the excess fat in the body delays the excretion of pesticides, thereby exacerbating their toxic effects. Further experimental studies are necessary to verify our hypothesis.
Our study had some limitations. First, the inherent nature of cross-sectional studies precludes the inference of a temporal cause-effect relationship. Second, detailed information, such as the type, intensity, frequency, and duration of household pesticide exposure, which may have various effects on infertility, was unavailable in the NHANES database. Third, self-reported data may have introduced recall bias in mobile examination centre interviews. However, the incidence of infertility and pesticide exposure was generally consistent with that reported in the existing literature (Chen et al. 2020a, b; Glazer et al. 2019). Fourth, though the multi-stage stratified probability design method was used, the participants in the NHANES database are American citizens and are not fully representative of people living in other regions of the world. Given these limitations, well-designed multi-centre, controlled trials may be needed to confirm our findings.
Conclusion
In summary, we found an association between household pesticide exposure and infertility risk in the general adult female population. For the first time, we have reported the antagonistic effects of zinc and the synergistic effects of obesity in the mediation of pesticide-induced infertility. Though these new findings raise the concern that obese individuals who are on a low-zinc diet may be more susceptible to infertility triggered by the chronic toxicity of inevitable pesticide exposure in daily life, more evidence from randomised controlled studies is needed. Furthermore, experimental studies are essential to elucidate the exact interplay among zinc, obesity, pesticides, and infertility.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Dr. Zhang Jing (Shanghai Tongren Hospital, Shanghai, China) for helping collect the data for this study.
Abbreviations
- NHANES
National Health and Nutrition Examination Survey
- NCHS
National Center for Health Statistics
- CDC
Centers for Disease Control and Prevention
Author contribution
JGH drafted the manuscript, LQH collected the clinical data, and JY reviewed data analyses. All authors read and approved the final manuscript.
Funding
The present work was supported by the Scientific Research and Sharing Platform Construction Project of Shaanxi Province (2022PT-07).
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Declarations
Ethics approval and consent to participate
The study was approved by the NCHS Ethics Review Board, and the original protocol is available online (https://www.cdc.gov/nchs/nhanes/irba98.htm). All participants aged 18 to 46 years were included and provided written informed consent.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jungao Huang, Email: jungaohuang@sina.com.
Liqin Hu, Email: HuLiQin6611@163.com.
Juan Yang, Email: hhyhmy@sina.com.
References
- Ahluwalia N, Dwyer J, Terry A, Moshfegh A, Johnson C. Update on NHANES dietary data: focus on collection, release, analytical considerations, and uses to inform public policy. Adv Nutr. 2016;7:121–134. doi: 10.3945/an.115.009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell EM, Hertz-Picciotto I, Beaumont JJ. Case-cohort analysis of agricultural pesticide applications near maternal residence and selected causes of fetal death. Am J Epidemiol. 2001;154:702–710. doi: 10.1093/aje/154.8.702. [DOI] [PubMed] [Google Scholar]
- Benbrook CM, Davis DR. The dietary risk index system: a tool to track pesticide dietary risks. Environ Health. 2020;19:103. doi: 10.1186/s12940-020-00657-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Chen L, Hao G. Exercise attenuates the association between household pesticide exposure and depressive symptoms: evidence from NHANES, 2005–2014. Environ Res. 2020;188:109760. doi: 10.1016/j.envres.2020.109760. [DOI] [PubMed] [Google Scholar]
- Chen W, Eisenberg R, Mowrey WB, Wylie-Rosett J, Abramowitz MK, Bushinsky DA, Melamed ML. Association between dietary zinc intake and abdominal aortic calcification in US adults. Nephrol Dial Transplant. 2020;35:1171–1178. doi: 10.1093/ndt/gfz134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YH, Williams PL, Gillman MW, Gaskins AJ, Mínguez-Alarcón L, Souter I, Toth TL, Ford JB, Hauser R, Chavarro JE. Association between pesticide residue intake from consumption of fruits and vegetables and pregnancy outcomes among women undergoing infertility treatment with assisted reproductive technology. JAMA Intern Med. 2018;178:17–26. doi: 10.1001/jamainternmed.2017.5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gavelle E, de Lauzon-Guillain B, Charles MA, Chevrier C, Hulin M, Sirot V, Merlo M, Nougadère A. Chronic dietary exposure to pesticide residues and associated risk in the French ELFE cohort of pregnant women. Environ Int. 2016;92–93:533–542. doi: 10.1016/j.envint.2016.04.007. [DOI] [PubMed] [Google Scholar]
- Ebisch IM, Thomas CM, Peters WH, Braat DD, Steegers-Theunissen RP. The importance of folate, zinc and antioxidants in the pathogenesis and prevention of subfertility. Hum Reprod Update. 2007;13:163–174. doi: 10.1093/humupd/dml054. [DOI] [PubMed] [Google Scholar]
- Ferré DM, Quero AAM, Hernández AF, Hynes V, Tornello MJ, Lüders C, Gorla NBM. Potential risks of dietary exposure to chlorpyrifos and cypermethrin from their use in fruit/vegetable crops and beef cattle productions. Environ Monit Assess. 2018;190:292. doi: 10.1007/s10661-018-6647-x. [DOI] [PubMed] [Google Scholar]
- Fucic A, Duca RC, Galea KS, Maric T, Garcia K, Bloom MS, Andersen HR, Vena JE. Reproductive health risks associated with occupational and environmental exposure to pesticides. Int J Environ Res Public Health. 2021;18:6576. doi: 10.3390/ijerph18126576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garolla A, Petre GC, Francini-Pesenti F, De Toni L, Vitagliano A, Di Nisio A, Foresta C. Dietary supplements for male infertility: a critical evaluation of their composition. Nutrients. 2020;12:E1472. doi: 10.3390/nu12051472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer CH, Eisenberg ML, Tøttenborg SS, Giwercman A, Flachs EM, Bräuner EV, Vassard D, Pinborg A, Schmidt L, Bonde JP. Male factor infertility and risk of death: a nationwide record-linkage study. Hum Reprod. 2019;34:2266–2273. doi: 10.1093/humrep/dez189. [DOI] [PubMed] [Google Scholar]
- Gleason JL, Shenassa ED, Thoma ME. Self-reported infertility, metabolic dysfunction, and cardiovascular events: a cross-sectional analysis among U.S. women. Fertil Steril. 2019;111:138–146. doi: 10.1016/j.fertnstert.2018.10.009. [DOI] [PubMed] [Google Scholar]
- Goel A, Dani V, Dhawan DK. Protective effects of zinc on lipid peroxidation, antioxidant enzymes and hepatic histoarchitecture in chlorpyrifos-induced toxicity. Chem Biol Interact. 2005;156:131–140. doi: 10.1016/j.cbi.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Gutgesell RM, Tsakiridis EE, Jamshed S, Steinberg GR, Holloway AC. Impact of pesticide exposure on adipose tissue development and function. Biochem J. 2020;477:2639–2653. doi: 10.1042/BCJ20200324. [DOI] [PubMed] [Google Scholar]
- Hayes TB, Collins A, Lee M, Mendoza M, Noriega N, Stuart AA, Vonk A. Hermaphroditic, demasculinized frogs after exposure to the herbicide atrazine at low ecologically relevant doses. Proc Natl Acad Sci U S A. 2002;99:5476–5480. doi: 10.1073/pnas.082121499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juntarawijit C, Juntarawijit Y. Association between diabetes and pesticides: a case-control study among Thai farmers. Environ Health Prev Med. 2018;23:3. doi: 10.1186/s12199-018-0692-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns K, Zigo M, Sutovsky P. Zinc: a necessary ion for mammalian sperm fertilization competency. Int J Mol Sci. 2018;19:E4097. doi: 10.3390/ijms19124097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra A, Dhawan DK. Current view of zinc as a hepatoprotective agent in conditions of chlorpyrifos induced toxicity. Pestic Biochem Physiol. 2014;112:1–6. doi: 10.1016/j.pestbp.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Millen BE, Abrams S, Adams-Campbell L, Anderson CA, Brenna JT, Campbell WW, Clinton S, Hu F, Nelson M, Neuhouser ML, et al. The 2015 Dietary Guidelines Advisory Committee Scientific Report: development and major conclusions. Adv Nutr. 2016;7:438–444. doi: 10.3945/an.116.012120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A. Reproductive endocrinology: exposure to pesticide residues linked to adverse pregnancy outcomes. Nat Rev Endocrinol. 2018;14:4. doi: 10.1038/nrendo.2017.156. [DOI] [PubMed] [Google Scholar]
- Mumford SL, Johnstone E, Kim K, Ahmad M, Salmon S, Summers K, Chaney K, Ryan G, Hotaling JM, Purdue-Smithe AC, et al. A prospective cohort study to evaluate the impact of diet, exercise, and lifestyle on fertility: design and baseline characteristics. Am J Epidemiol. 2020;189:1254–1265. doi: 10.1093/aje/kwaa073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo S, London L, Burdorf A, Naidoo R, Kromhout H. Spontaneous miscarriages and infant deaths among female farmers in rural South Africa. Scand J Work Environ Health. 2011;37:227–236. doi: 10.5271/sjweh.3133. [DOI] [PubMed] [Google Scholar]
- Nougadère A, Sirot V, Cravedi JP, Vasseur P, Feidt C, Fussell RJ, Hu R, Leblanc JC, Jean J, Rivière G, et al. Dietary exposure to pesticide residues and associated health risks in infants and young children—results of the French infant total diet study. Environ Int. 2020;137:105529. doi: 10.1016/j.envint.2020.105529. [DOI] [PubMed] [Google Scholar]
- Pašková V, Hilscherová K, Bláha L. Teratogenicity and embryotoxicity in aquatic organisms after pesticide exposure and the role of oxidative stress. Rev Environ Contam Toxicol. 2011;211:25–61. doi: 10.1007/978-1-4419-8011-3_2. [DOI] [PubMed] [Google Scholar]
- Rahimi T, Rafati F, Sharifi H, Seyedi F. General and reproductive health outcomes among female greenhouse workers: a comparative study. BMC Womens Health. 2020;20:103. doi: 10.1186/s12905-020-00966-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramlau-Hansen CH, Thulstrup AM, Nohr EA, Bonde JP, Sørensen TI, Olsen J. Subfecundity in overweight and obese couples. Hum Reprod. 2007;22:1634–1637. doi: 10.1093/humrep/dem035. [DOI] [PubMed] [Google Scholar]
- Razi S, Rezaeian M, Dehkordi FG, Manshoori A, Goujani R, Vazirinejad R. Exposure to pistachio pesticides and stillbirth: a case-control study. Epidemiol Health. 2016;38:e2016016. doi: 10.4178/epih.e2016016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas-Huetos A, Bulló M, Salas-Salvadó J. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: a systematic review of observational studies. Hum Reprod Update. 2017;23:371–389. doi: 10.1093/humupd/dmx006. [DOI] [PubMed] [Google Scholar]
- Schisterman EF, Sjaarda LA, Clemons T, Carrell DT, Perkins NJ, Johnstone E, Lamb D, Chaney K, Van Voorhis BJ, Ryan G, et al. Effect of folic acid and zinc supplementation in men on semen quality and live birth among couples undergoing infertility treatment: a randomized clinical trial. JAMA. 2020;323:35–48. doi: 10.1001/jama.2019.18714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settimi L, Spinelli A, Lauria L, Miceli G, Pupp N, Angotzi G, Fedi A, Donati S, Miligi L, Osborn J, et al. Spontaneous abortion and maternal work in greenhouses. Am J Ind Med. 2008;51:290–295. doi: 10.1002/ajim.20556. [DOI] [PubMed] [Google Scholar]
- Sharma R, Biedenharn KR, Fedor JM, Agarwal A. Lifestyle factors and reproductive health: taking control of your fertility. Reprod Biol Endocrinol. 2013;11:66. doi: 10.1186/1477-7827-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma RK, Singh P, Setia A, Sharma AK. Insecticides and ovarian functions. Environ Mol Mutagen. 2020;61:369–392. doi: 10.1002/em.22355. [DOI] [PubMed] [Google Scholar]
- Shirangi A, Fritschi L, Holman CD. Maternal occupational exposures and risk of spontaneous abortion in veterinary practice. Occup Environ Med. 2008;65:719–725. doi: 10.1136/oem.2007.035246. [DOI] [PubMed] [Google Scholar]
- Sieke C, Michalski B, Kuhl T. Probabilistic dietary risk assessment of pesticide residues in foods for the German population based on food monitoring data from 2009 to 2014. J Expo Sci Environ Epidemiol. 2018;28:46–54. doi: 10.1038/jes.2017.7. [DOI] [PubMed] [Google Scholar]
- Skoracka K, Ratajczak AE, Rychter AM, Dobrowolska A, Krela-Kaźmierczak I. Female fertility and the nutritional approach: the most essential aspects. Adv Nutr. 2021;12:2372–2386. doi: 10.1093/advances/nmab068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmor A, Dunphy B. Female obesity and infertility. Best Pract Res Clin Obstet Gynaecol. 2015;29:498–506. doi: 10.1016/j.bpobgyn.2014.10.014. [DOI] [PubMed] [Google Scholar]
- Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- van der Steeg JW, Steures P, Eijkemans MJ, Habbema JD, Hompes PG, Burggraaff JM, Oosterhuis GJ, Bossuyt PM, van der Veen F, Mol BW. Obesity affects spontaneous pregnancy chances in subfertile, ovulatory women. Hum Reprod. 2008;23:324–328. doi: 10.1093/humrep/dem371. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev. 2012;33:378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickram S, Rohini K, Srinivasan S, Nancy Veenakumari D, Archana K, Anbarasu K, Jeyanthi P, Thanigaivel S, Gulothungan G, Rajendiran N, et al. Role of zinc (Zn) in human reproduction: a journey from initial spermatogenesis to childbirth. Int J Mol Sci. 2021;22:2188. doi: 10.3390/ijms22042188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitagliano A, Petre GC, Francini-Pesenti F, De Toni L, Di Nisio A, Grande G, Foresta C, Garolla A. Dietary supplements for female infertility: a critical review of their composition. Nutrients. 2021;13:3552. doi: 10.3390/nu13103552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk M, Jaklič H, Zorn B, Peterlin B. Association between male infertility and genetic variability at the PON1/2 and GSTM1/T1 gene loci. Reprod Biomed Online. 2011;23:105–110. doi: 10.1016/j.rbmo.2011.03.021. [DOI] [PubMed] [Google Scholar]
- Weis GCC, Assmann CE, Mostardeiro VB, Alves AO, da Rosa JR, Pillat MM, de Andrade CM, Schetinger MRC, Morsch VMM, da Cruz IBM, et al. Chlorpyrifos pesticide promotes oxidative stress and increases inflammatory states in BV-2 microglial cells: a role in neuroinflammation. Chemosphere. 2021;278:130417. doi: 10.1016/j.chemosphere.2021.130417. [DOI] [PubMed] [Google Scholar]
- Willis WO, de Peyster A, Molgaard CA, Walker C, MacKendrick T. Pregnancy outcome among women exposed to pesticides through work or residence in an agricultural area. J Occup Med. 1993;35:943–949. doi: 10.1097/00043764-199309000-00019. [DOI] [PubMed] [Google Scholar]
- Yang Q, Zheng J, Chen W, Chen X, Wen D, Chen W, Xiong X, Zhang Z. Association between preadmission metformin use and outcomes in intensive care unit patients with sepsis and type 2 diabetes: a cohort study. Front Med (lausanne) 2021;8:640785. doi: 10.3389/fmed.2021.640785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Dong T, Hu W, Wang X, Xu B, Lin Z, Hofer T, Stefanoff P, Chen Y, Wang X, et al. Association between exposure to a mixture of phenols, pesticides, and phthalates and obesity: comparison of three statistical models. Environ Int. 2019;123:325–336. doi: 10.1016/j.envint.2018.11.076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.