The effects of blood transfusion on the functions of the immune system have been studied in humans and laboratory animals for more than 25 years. It is becoming customary to refer to these effects on the immune system as “immunomodulatory,” as they may involve augmentation as well as suppression of elements of the immune response. Clinically, the results of these actions have been recognized in prolonged graft survival in solid organ transplants (30), increased tumor recurrence after surgical resection (5, 15), increased postoperative infection rates (5, 16, 19) and other postoperative complications (43), decreased recurrence of Crohn’s disease (34), and increased progression of human immunodeficiency virus type 1 infection in patients who have received allogeneic blood (38). These effects may be abrogated by the use of syngeneic or autologous blood or leukodepleted allogeneic blood components (22, 24, 45). Most researchers feel that these effects are mediated by leukocytes (WBCs) present in the blood components and are related to the expression of class I and class II HLA antigens on the WBCs. Contributions by plasma, platelets, or erythrocytes (RBCs) have not been ruled out.
Laboratory studies on the effects of allogeneic transfusion on specific immunological functions, involving mostly mice but also some human subjects, have demonstrated decreased interleukin 2 (IL-2) secretion (46), decreased natural killer cell activity (18), decreased delayed-type hypersensitivity responses (27), decreased CD4/CD8 ratios (21), and decreased macrophage function (44). Acceptance of the concept of transfusion-induced immunomodulation is far from universal, and much controversy exists. Some human studies and meta-analyses suggest that the above clinical effects are not due to allogeneic transfusion. Differences in conclusions about the immunomodulatory effect in humans may reflect differences in study type, transfusion dose, blood component preparation, degree of correction for confounding variables, and the indications and thresholds for transfusion. This reviewer believes that a transfusion-induced immunomodulatory effect exists and will discuss a number of hypotheses, both new and old, which attempt to explain our current understanding of the immunologic basis for the effects described.
It is helpful to think of these immunomodulatory effects on the immune system as either antigen specific or nonspecific. Clinically, antigen-specific effects include tolerance to graft HLA antigens, leading to prolonged graft survival. This effect was first seen in the improved graft survival in kidney transplant recipients who had received multiple transfusions prior to kidney transplant (30). Both animals and humans can be shown to become hyporesponsive to specific HLA antigens when some or all of those antigens are shared by the blood donor and graft donor (42). This finding led to the practice of donor-specific transfusions, that is, transfusing blood from a graft donor to the recipient prior to organ donation in order to induce tolerance to the graft. This practice is now done more rarely, as there is the chance of transfusion sensitizing the recipient to donor antigens and because strong antirejection drugs such as cyclosporine and tacrolimus have reduced the contribution of the “transfusion effect” on graft survival to a minimal, though still measurable, level (29). Nonspecific effects are semiarbitrarily defined as those associated with more global, and less easily discriminated, changes in the immune response and include decreased macrophage function, decreased CD4/CD8 ratio, and decreased IL-2 secretion. Clinical consequences due to these nonspecific effects include increased tumor recurrence and increased postoperative infection rates. As it is very possible that a common mechanism leads to both specific and nonspecific immunomodulatory effects, this discussion will examine first the better-described, antigen-specific effects and then the possibilities that those antigen-specific effects result in more generalized, nonspecific effects.
ANTIGEN-SPECIFIC IMMUNOMODULATORY EFFECTS
An early theory for the mechanism of antigen-specific suppression proposed clonal deletion of T cells and B cells sensitized to blood or tissue donor antigens by an outside toxic agent. According to this proposal, T cells and B cells directed against histocompatibility antigens proliferate in response to the antigens present on WBCs in the transfused blood. When the organ transplant is performed, these cells rapidly proliferate in the presence of the cytotoxic immunosuppressant drugs given at the time of transplant, leading to destruction of the expanding clones (41). While this may occur to some extent, it certainly is not complete, as weak in vitro cytotoxic responses against donor antigens can be found in recipients of successful transplants (35, 36). Also, prolonged graft survival can be seen in previously transfused animals despite the omission of immunosuppressive drugs (8). Rather than destruction by cytotoxic drugs, another explanation for clonal deletion is activation-induced apoptosis of the previously activated T cells. Repeated stimulation of T cells with antigen, in high concentrations, leads to increased expression and activation of the Fas receptor, leading to T-cell apoptosis. This Fas activation is mediated by IL-2 (1). While it is possible that activation of the Fas pathway has some bearing on graft tolerance, this pathway is more likely critical for controlling an active immune response. Clonal deletion from Fas activation cannot explain why small doses of antigen may be tolerogenic, and many researchers have found decreases in IL-2 secretion from lymphocytes posttransfusion.
Another possible mechanism for antigen-specific suppression is the production of anti-idiotype antibodies (12, 36, 37). In this case, the transfusion stimulates a primary immune response against the allogeneic antigens. The T-cell receptors (TCRs) and antibodies produced are themselves “new antigens,” and the host produces an array of antibodies against these new antigens. After multiple transfusions, a network of anti-idiotype antibodies may be present and reacting against multiple determinants of the initial responding TCRs and antibodies. When a graft is performed, these anti-idiotypic antibodies dampen the rejection response by potentially interfering with the TCRs and the antigen-major histocompatibility complex (MHC) complex. Although strong proof that such antibodies are acting to control the immune response is lacking, renal transplant patients with detectable anti-idiotype antibodies have been shown to have greater graft survival (12, 36).
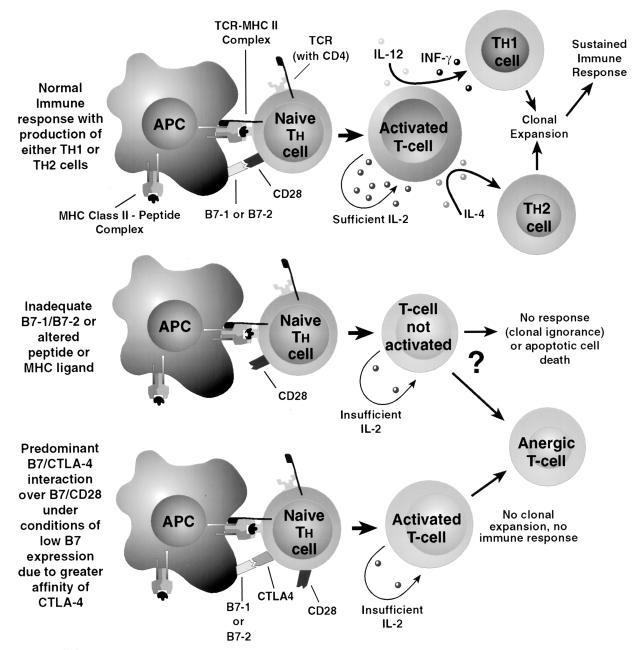
A more promising idea concerns transfusion-inducing lymphocyte anergy or unresponsiveness toward graft antigens. In order for T-helper cells (CD4+) to activate and undergo clonal expansion, their TCR must recognize the correct foreign antigen associated with its own HLA class II MHC antigen on an antigen-presenting cell (APC). This recognition sets a number of cellular events in motion, including production by the T cell of adequate IL-2, necessary to sustain T-cell activation and allow a full immune response with T-cell clonal expansion, B-cell activation, and the eventual development of functional memory T cells. In order to generate enough IL-2, this recognition must be accompanied by additional costimulatory signals, mainly stimulation of CD28 on the T cell by the B7-1/B7-2 molecule on the APC. The specificity of the antigen-MHC complex with the TCR and the duration of contact between the APC and the T cell is also thought to determine the T-cell response, again through the magnitude of IL-2 production by the T cell. T cells recognizing the antigen-class II MHC complex on the APC but not receiving the costimulatory signal become partially activated, expressing IL-2 receptor and CD69 but reduced IL-2 production. Without sustained IL-2 stimulation, they enter an anergic state in which they are functionally unresponsive to further exposure to that antigen, even when properly presented with the appropriate costimulator (1, 25) (Fig. 1). Interestingly, the major effect of the antirejection drugs cyclosporine and tacrolimus is to suppress the production of IL-2 by T cells. This may explain the reduced contribution of the “transfusion effect” on organ graft survival since the advent of these drugs. B7-1 and B7-2 molecules are absent or present in only very small amounts on resting APCs. Increased expression of these costimulators can be induced by a variety of cytokines (IL-12, IL-1, tumor necrosis factor alpha [TNF-α]) and bacterial antigens which may be found during an inflammatory response or by administering the antigen with an adjuvant.
FIG. 1.
The normal immune response is outlined in the upper portion of the figure, with the naive T cell receiving the proper antigen-MHC type II signal and proper costimulation, leading to production of an activated T cell producing enough IL-2 to develop and clonally expand toward a Th1 or Th2 type under the influence of IL-12/INF-γ or IL-4, respectively. Under conditions of inadequate B7 costimulation or altered peptide ligand-TCR binding, insufficient IL-2 is generated to sustain T-cell proliferation and either no response, T-cell death, or T-cell anergy is the possible outcome. Inadequate B7 costimulation may also lead to preferential binding to CTLA-4 over CD28 on the naive T cell, also leading to T-cell anergy.
The route of administration is significant in determining what sort of response is elicited by T cells to a given antigen. Antigens administered in the skin are processed by tissue dendritic cells which intrinsically express B7 costimulators on their surface, with presentation in the peripheral lymph nodes usually resulting in a full immune response by T cells against that antigen. Antigens introduced intravascularly, such as allogeneic blood, or by the oral route in some animal models, may be presented by splenic and liver macrophages or by blood monocytes or B cells, which do not constitutively express, or which express only small amounts of, the B7 costimulators on their surface. These so-called “nonprofessional” APCs may thus induce unresponsive T cells due to inadequate IL-2 production resulting from the lack of costimulatory interactions (26).
One hypothesis is that leukocytes in the transfused unit can act as APCs, especially if type II MHC antigens are shared with the recipient. Leukocytes present in stored blood have been found to maintain their class II MHC antigens but lose the ability to costimulate (25, 26). If these nonprofessional APCs are exposed to antigen in the presence of adjuvant or inflammation, with subsequent generation of IL-1, IL-12, and TNF-α, then B7 costimulatory molecules are expressed in higher numbers and the T cells are pushed toward a full immune response. This model has undergone some changes, prompted by more recent discoveries about the function of the CTLA-4 molecule found on T cells which, like CD 28, uses B7-1 and B7-2 molecules on APCs as ligands. This molecule was initially thought to stimulate the T-cell immune response, acting synergistically with CD28, but now it appears to have an important role in inhibiting T-cell responses. This modified model proposes that if there is no expression of B7 molecules on the APC surface, then there is no response from the T cell and the T cell is left functionally naive. CTLA-4 is not found on naive T cells but quickly is expressed upon activation of the T cells. CTLA-4 is in competition with CD28 for the B7 ligand, and with low levels of expression of B7 on the APC, the CTLA-4 molecule wins that competition due to its higher binding affinity (2). With a preponderance of CTLA-4 binding, the T cell enters an anergic state or at least becomes much harder to stimulate to produce IL-2. Continued antigen presentation in the presence of low levels of B7 on the APC may induce the generation of anergic memory T cells (2, 31).
There has also been described induction of anergy in naive T cells exposed to peptide ligands on the MHC complex which vary only slightly from the true ligand for that TCR. In this case, the signal via the TCR is insufficient and, despite the presence of the correct costimulators, T-cell activation is not perpetuated and anergy results (1). This may be due to an inadequate, or curtailed, signal from the TCR after interaction, but with inexact binding, between the antigen-MHC complex and the TCR. Thus far, these altered antigen-MHC complex effects have been studied only with alterations of the peptide moiety. Interestingly, since the advent of molecular methods of HLA typing, many serologic HLA phenotypes have been found to be made up of multiple genotypic variants, many differing by one amino acid. Since TCR recognition involves contact with both peptide and class II MHC residues, it seems possible that minimal differences between MHC molecules presenting an otherwise recognizable foreign peptide may also cause this incomplete TCR signalling.
The existence of suppressor T cells is extrapolated from the fact that tolerance to certain antigens can be transferred to naive subjects via splenic lymphocytes, a process sometimes referred to as “infectious tolerance” (1, 10). This effect is mediated by the production of soluble suppressive factors. The nature of these factors is not clear, but a number of possibilities have been proposed; these include secreted TCRs which competitively inhibit TCRs on T cells (7) and generally inhibitory cytokines such as IL-10 and transforming growth factor β (TGF-β). These effects and factors are poorly characterized due to difficulties in isolating and cloning these T-suppressor cells. Some investigators doubt the existence of a pure suppressor cell, though a number of T-cell types do demonstrate some suppressive activity; for example, Th1 cells suppress the development of Th2 cells and vice versa. Recently another T-helper subset has been described in studies on oral tolerance and has been referred to as Th3. These cells apparently secrete neither Th1 nor Th2 cytokines but secrete TGF-β instead (17). Due to the possibility that several cell types, or several mechanisms, which suppress the immune response exist, I will refer to these cells as “suppressive” T cells rather than as the more specific “T-suppressor” cells.
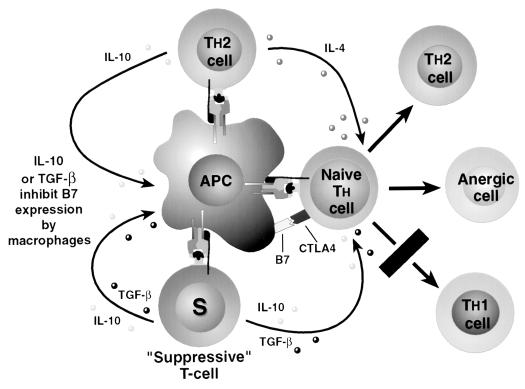
Complementing the hypothesis of suppressive T cells is the concept of “bystander suppression,” also referred to as the action of “veto cells.” This idea involves active suppression of immune response induction to a polypeptide with multiple antigenic epitopes after tolerance is induced to only one epitope. To demonstrate bystander suppression, an isolated epitope from a polypeptide is given in a tolerogenic fashion. After tolerance to that epitope is established, attempting immunization with a second isolated epitope results in a strong immune response; however, if the native polypeptide is administered, the immune response to the second epitope is reduced or absent (23). Bystander suppression has also been described when antigens are administered simultaneously. If the subject is tolerant to one of the antigens, suppression of the immune response toward the second novel antigen is seen if the two antigens are given as a single injection (23, 40). The theory of how bystander suppression is accomplished involves simultaneous presentation of the tolerant antigen and the novel antigen and the same APC such that the tolerant T cell and the naive T cell are in close proximity. The tolerant T cell then releases factors, potentially inhibitory cytokines such as IL-10 and TGF-β, which regulate the response of the naive T cell toward an anergic or possibly Th2 cell type. The tolerant T cell could possibly be a Th2 cell itself, releasing IL-10 and IL-4 into the microenvironment and thereby biasing the naive T cell toward a Th2 differentiation (Fig. 2).
FIG. 2.
Actions of “suppressive” T cells. Th2 cells may act as suppressors of the Th1 immune response by locally supplying IL-4 during T-cell activation, biasing naive T cells toward a Th2-type differentiation. “Bystander suppression” may also be present, where specifically suppressive T cells, such as the Th3 cells described in the gut, may inhibit, through local cytokine actions, activation of naive T cells being presented a novel antigen by the same or adjacent APCs presenting the suppressed antigen to the “suppressive” T cell.
NONSPECIFIC IMMUNOMULATORY EFFECTS
The mechanism of nonspecific immunomodulation has centered on the observed, apparently nonspecific, decrease in NK cell function, decrease in cytotoxic lymphocyte activity, decrease in delayed-type hypersensitivity, and decrease in macrophage function and/or activation. Since WBC reduction does appear to reduce both specific and nonspecific immunomodulatory effects, it is likely that the two phenomena are linked (24, 45). The first two specific mechanisms described, clonal deletion and anti-idiotype antibodies, show no evident connection to the nonspecific effects since they primarily involve antigen-MHC-TCR interaction on only those T cells expressing that individual antigen’s TCR. It is possible that anti-idiotype networks might generate cross-reacting antibodies which could influence other cell interactions, but no evidence for this exists. These mechanisms may still be involved in the total host response, but since no clear link to the nonspecific immunomodulatory effects can be seen they will not enter into this part of the discussion.
Induction of Th2 response, clonal anergy, and bystander suppression are more easily linked to nonspecific immunomodulation. Studies in both mice and humans have demonstrated a reduction in IL-2 production in stimulated lymphocytes after transfusion (46). Recently, a preponderance of Th2-type cytokines (IL-4, IL-5, IL-10) over Th1 cytokines (gamma interferon, IL-2) in posttransfusion stimulated lymphocytes was found in both mice and humans (3, 13, 22). The Th2 cytokine pattern is associated with decreased cytotoxic cell functions and inhibition of macrophage activation. This pattern potentially accounts for the increased tumor recurrence, increased infection rates, more rapid progression of viral disease, and reduction in inflammatory bowel disease episodes ascribed to allogeneic transfusion. There are a few possible reasons behind this Th2 response. Antigen presentation intravascularly or orally tends to stimulate a Th2-type response (1). This may be because of a disparate response of splenic, gut, or liver macrophages compared to subcutaneous antigen presentation, which involves skin dendritic cells and usually elicits a strong Th1 response (1, 3, 33). This response may also be shifted to the Th1 response by the presence of systemic inflammation, or by the use of adjuvants, through the actions of gamma interferon, IL-1, IL-2, and TNF-α, leading to increased production of IL-12 and increased surface expression of B7-1 and B7-2 on APCs. Without IL-12 or in the presence of IL-4 and with few costimulator molecules on the APC, the response could shift to the Th2 type or to T-cell anergy through the actions of CTLA-4.
Release of TGF-β and prostaglandin E2 from macrophages has been found to be increased after transfusion (4, 13). Both of these molecules are inhibitory to the cellular immune response and are known to inhibit IL-2 production and target cell response (1). Oral tolerance studies have postulated the existence of a third T-helper subset, Th3, which is inhibitory toward the response of both Th1 and Th2 cells through the production of high amounts of TGF-β. These cells proliferate poorly and are produced during tolerance induction with low levels of orally administered antigen, and their production is enhanced by IL-4 and positively self-regulated by TGF-β (17). Thus, a strong Th2 response to a specific antigen may potentially increase production of TGF-β, further reducing the immune response in a nonspecific manner. Whether these Th3 cells or functionally similar cells exist outside of the gut is uncertain. Prostaglandin E2 production by liver macrophages is enhanced by allogeneic blood introduced into the portal circulation in a mouse model (32, 33). Prostaglandin E2 has been shown to inhibit the Th1 response (4).
The concept of generalized bystander suppression, possibly mediated by TGF-β, is attractive as a reason for nonspecific immunomodulation. Transfused antigens invoking an antigen-specific tolerance would be presented by APCs along with potential pathogen or tumor antigens. Those tolerized T cells may then locally inhibit an immune response by T cells specific for the other antigen. Bystander suppression has been shown to be regionally localized when antigens are given by inoculation; in effect, both the tolerant antigen and the test antigen must be injected into tissue which drains to the same lymph nodes (40). This may be less of a factor in transfused antigens, as they should be distributed to all tissues regardless of where the new antigen enters the immune system.
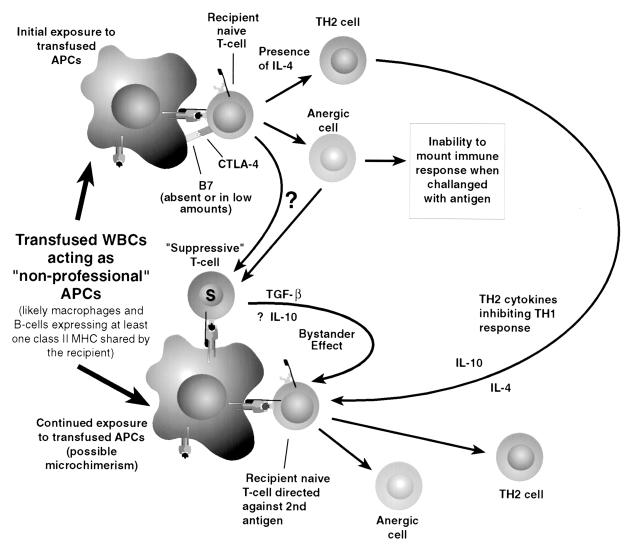
Continued tolerance appears to require continued exposure to the tolerized antigen (10). In solid organ transplants this is no problem, but in blood transfusion the effects would be expected to be transient, with the effect dwindling as the foreign antigens are removed through destruction of the donor WBCs and turnover of the other constituents of the transfused blood. Continued exposure to WBC antigens has been proposed through the apparent long-term survival of donor lymphocytes and macrophages in some recipients, referred to as microchimerism. Microchimerism has been demonstrated by nested PCR techniques to look for specific donor sequences. Circulating foreign mononuclear cells have been found as many as 29 years later in solid organ transplant recipients (39). Recently, caution regarding low specificity with nested PCR to detect microchimerism was expressed after one study detected frequent false-positive bands by the technique (9). It is possible that long-term WBC microchimerism is an effect of tolerance rather than its cause. Despite many advances in the understanding of tolerance induction and modulation of the immune response, the complex orchestration of the immune response remains enigmatic. The functions and interactions of various cell types and their cytokines are constantly being redefined, usually in the direction of increasing complexity. One summary of possible events after the transfusion of allogeneic APCs is shown in Fig. 3.
FIG. 3.
Possible effects on the host immune system of donor WBCs are presented here, showing both early, antigen-specific immunosuppression and later, nonspecific suppression by Th2 suppression of the Th1 response or “bystander suppression” potentially mediated through the actions of donor APCs persisting as a result of microchimerism.
CLINICAL DIRECTIONS
With this information come strategies for abrogating transfusion-induced immunomodulation, to avoid the problem of unwanted immunosuppression, and for enhancement of the effect to promote tolerance induction in organ graft recipients. Avoidance of transfusion-induced immunomodulation is best accomplished through the avoidance of transfusion or the use of autologous blood, as these measures also reduce the other complications of transfusion such as RBC incompatibility or viral disease. Reduction of WBCs in blood components has been shown for years to reduce immunosuppressive effects, and with modern leukocyte depletion filters, the residual WBC counts are even lower (24). There is no established minimum WBC load for ablating the immunomodulatory effect (6), and even modest reductions may be beneficial. Negating this immunomodulatory effect has a potentially significant impact on the public health, as even low estimates of mortality from increased postoperative infection rates and cancer recurrence attributable to allogeneic transfusion make the risk of transfusion-induced immunomodulation significantly greater than any other transfusion risk (11).
Enhancement of tolerance induction and immunomodulation is being examined with a variety of cellular blood products from the organ donor and the combination and timing of various immunosuppressive drug regimens combined with donor-specific transfusions. Transfusions of WBCs from the spouses of women with spontaneous recurrent abortions have been shown to reduce miscarriages and show a suppression of cell-mediated immunity in those patients (14). Discrepancy in HLA class II antigens between mother and fetus have been thought to mediate improvement of rheumatoid arthritis in women with the disease (28). The effects of UV-B-irradiated blood products are presently being investigated, and though the WBCs present in them appear to be unable to act as APCs, there have been reports of sensitization to HLA antigens. One report notes induction of graft tolerance in mice with an increase in the Th2 cytokine pattern with UV-B-irradiated spleen cells (20). Thus, UV-B-irradiated components may be useful for inducing tolerance in graft recipients while reducing the risk of sensitization.
REFERENCES
- 1.Abbas A K, Lichtman A H, Pober J S. Cellular and molecular immunology. 3rd ed. Philadelphia, Pa: W. B. Saunders; 1997. [Google Scholar]
- 2.Allison J P, Chambers C, Hurwitz A, Sullivan T, Boitel B, Fournier S, Brunner M, Krummel M. Immunological tolerance. Chichester, England: John Wiley and Sons; 1998. A role for CTLA-4-mediated inhibitory signals in peripheral T cell tolerance? pp. 92–102. [DOI] [PubMed] [Google Scholar]
- 3.Babcock G F, Alexander J W. The effects of blood transfusion on cytokine production by TH1 and TH2 lymphocytes in the mouse. Transplantation. 1996;61:465–468. doi: 10.1097/00007890-199602150-00026. [DOI] [PubMed] [Google Scholar]
- 4.Betz M, Fox B S. Prostaglandin E2 inhibits production of TH 1 lymphokines but not TH 2 lymphokines. J Immunol. 1991;146:108–113. [PubMed] [Google Scholar]
- 5.Blumberg N, Heal J M. Effects of transfusion on immune function—cancer recurrence and infection. Arch Pathol Lab Med. 1994;118:371–379. [PubMed] [Google Scholar]
- 6.Bordin J O, Heddle N M, Blajchman M A. Biologic effects of leukocytes present in transfused cellular blood products. Blood. 1994;84:1703–1721. [PubMed] [Google Scholar]
- 7.Brunson M E, Alexander J W. Mechanisms of transfusion induced immunosuppression. Transfusion. 1990;30:651–658. doi: 10.1046/j.1537-2995.1990.30790385527.x. [DOI] [PubMed] [Google Scholar]
- 8.Bucin D. Specific immune tolerance related to disparity in MHC class I region. Med Hypotheses. 1995;44:132–136. doi: 10.1016/0306-9877(95)90086-1. [DOI] [PubMed] [Google Scholar]
- 9.Carter A S, Bunce M, Cerundolo L, Welsh K I, Morris P J, Fuggle S V. Detection of microchimerism after allogeneic blood transfusion using nested polymerase chain reaction amplification with sequence specific primers (PCR-SSP): a cautionary tale. Blood. 1998;92:683–689. [PubMed] [Google Scholar]
- 10.Chen Z K, Cobbold S P, Waldmann H, Metcalfe S. Amplification of natural regulatory immune mechanisms for transplantation tolerance. Transplantation. 1996;62:1200–1206. doi: 10.1097/00007890-199611150-00002. [DOI] [PubMed] [Google Scholar]
- 11.Dzik S, Blajchman M A, Blumberg N, Kirkley S A, Heal J M, Wood K. Current research on the immunomodulatory effect of allogeneic blood transfusion. Vox Sang. 1996;70:187–194. doi: 10.1111/j.1423-0410.1996.tb01324.x. [DOI] [PubMed] [Google Scholar]
- 12.Forwell M A, Cocker J E, Peel M G, Tsakiris D J, Briggs J D, Junor B J, MacSween R N, Sandilands G P. Correlation between high molecular weight Fcγ-receptor blocking factors in serum and renal allograft survival. Transplantation. 1987;44:227–233. doi: 10.1097/00007890-198708000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Gafter U, Kalechman Y, Sredni B. Blood transfusion enhances production of T-helper-2 cytokines and transforming growth factor β in humans. Clin Sci. 1996;91:519–523. doi: 10.1042/cs0910519. [DOI] [PubMed] [Google Scholar]
- 14.Gafter U, Sredni B, Segal B, Kalechman Y. Suppressed cell-mediated immunity and monocyte and natural killer cell activity following allogeneic immunization of women with spontaneous recurrent abortion. J Clin Immunol. 1997;17:408–419. doi: 10.1023/a:1027372409361. [DOI] [PubMed] [Google Scholar]
- 15.Heiss M M, Mempel W, Delanoff C, Jauch K W, Gabka C, Mempel M, Dieterich H J, Eissner H J, Schildberg F W. Blood transfusion-modulated tumor recurrence: first results of a randomized study of autologous versus allogeneic blood transfusion in colorectal cancer surgery. J Clin Oncol. 1994;12:1859–1867. doi: 10.1200/JCO.1994.12.9.1859. [DOI] [PubMed] [Google Scholar]
- 16.Houbiers J G, van de Velde C J, van de Watering L M, Hermans J, Schreuder S, Bijnen A B, Pahlplatz P, Schattenkerk M E, Wobbes T, de Vries J E, Klementschitsch P, van de Maas A H, Brand A. Transfusion of red cells is associated with increased incidence of bacterial infection after colorectal surgery: a prospective study. Transfusion. 1997;37:126–134. doi: 10.1046/j.1537-2995.1997.37297203513.x. [DOI] [PubMed] [Google Scholar]
- 17.Inobe J, Slavin A J, Komagata Y, Chen Y, Liu L, Weiner H L. IL-4 is a differentiation factor for transforming growth factor-β secreting Th3 cells and oral administration of IL-4 enhances oral tolerance in experimental allergic encephalomyelitis. Eur J Immunol. 1998;28:2780–2790. doi: 10.1002/(SICI)1521-4141(199809)28:09<2780::AID-IMMU2780>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 18.Jensen L S, Andersen A J, Christiansen P M, Hokland P, Juhl C O, Madsen G, Mortensen J, Moller-Nielsen C, Hanberg-Sorensen F, Hokland P. Postoperative infection and natural killer cell function following blood transfusion in patients undergoing elective colorectal surgery. Br J Surg. 1992;79:513–516. doi: 10.1002/bjs.1800790613. [DOI] [PubMed] [Google Scholar]
- 19.Jensen L S, Kissmeyer-Nielsen P, Wolff B, Qvist N. Randomised comparison of leucocyte-depleted versus buffy-coat-poor blood transfusion and complications after colorectal surgery. Lancet. 1996;348:841–845. doi: 10.1016/S0140-6736(96)06168-5. [DOI] [PubMed] [Google Scholar]
- 20.Kao K J. Induction of humoral immune tolerance to major histocompatibility complex antigens by transfusions of UVB irradiated leukocytes. Blood. 1996;88:4375–4382. [PubMed] [Google Scholar]
- 21.Kaplan J, Sarnaik S, Gitlin J, Lusher J. Diminished helper/suppressor ratios and natural killer activity in recipients of repeated blood transfusions. Blood. 1984;64:308–310. [PubMed] [Google Scholar]
- 22.Kirkley S A, Cowles J, Pellegrini V D, Harris C M, Boyd A D, Blumberg N. Increased T helper 2 (Th2) type cytokine secretion in surgical patients receiving allogeneic transfusions. Transfus Med. 1998;8:195–204. doi: 10.1046/j.1365-3148.1998.00149.x. [DOI] [PubMed] [Google Scholar]
- 23.Liu L M, Weiner H L. T-cell response to orally administered antigens and its role in the treatment of autoimmune diseases. Chem Immunol. 1998;72:139–160. doi: 10.1159/000058708. [DOI] [PubMed] [Google Scholar]
- 24.Meryman H T. Transfusion-induced alloimmunization and immunosuppression and the effects of leukocyte depletion. Transfus Med Rev. 1989;3:180–193. doi: 10.1016/s0887-7963(89)70078-x. [DOI] [PubMed] [Google Scholar]
- 25.Mincheff M S, Meryman H T. Costimulatory signals necessary for induction of T cell proliferation. Transplantation. 1990;49:768–772. doi: 10.1097/00007890-199004000-00023. [DOI] [PubMed] [Google Scholar]
- 26.Mincheff M S, Meryman H T, Kapoor V, Alsop P, Wotzel M. Blood transfusion and immunomodulation: a possible mechanism. Vox Sang. 1993;65:18–24. doi: 10.1111/j.1423-0410.1993.tb04519.x. [DOI] [PubMed] [Google Scholar]
- 27.Neilsen H J, Hammer J H, Moesgaard F, Kehlet H. Comparison of the effects of SAG-M and whole blood transfusions on postoperative suppression of delayed hypersensitivity. Can J Surg. 1991;34:146–150. [PubMed] [Google Scholar]
- 28.Nelson J L, Hughes K A, Smith A G, Nisperos B B, Branchaud A M, Hansen J A. Maternal-fetal disparity in HLA class II alloantigens and the pregnancy induced amelioration of rheumatoid arthritis. N Engl J Med. 1993;329:466–471. doi: 10.1056/NEJM199308123290704. [DOI] [PubMed] [Google Scholar]
- 29.Opelz G. The role of HLA matching and blood transfusions in the cyclosporine era. Transplant Proc. 1989;21:603–612. [PubMed] [Google Scholar]
- 30.Opelz G, Sengar D P S, Mickey M R, Terasaki P I. Effect of blood transfusions on subsequent kidney transplants. Transplant Proc. 1973;5:253–259. [PubMed] [Google Scholar]
- 31.Pape K A, Khoruts A, Ingulli E, Mondino A, Merica R, Jenkins M K. Immunological tolerance. Chichester, England: John Wiley and Sons; 1998. Antigen-specific CD4+ T cells that survive after the induction of peripheral tolerance possess an intrinsic lymphokine production defect; pp. 103–119. [DOI] [PubMed] [Google Scholar]
- 32.Perez R, Johnson J, Winkler J D, Rudich S, Carter L, Katznelson S, German J B. Kupffer cell-mediated lymphocyte apoptosis: a PGE2-dependent mechanism of portal venous transfusion-induced immunosuppression? J Surg Res. 1998;78:37–41. doi: 10.1006/jsre.1998.5394. [DOI] [PubMed] [Google Scholar]
- 33.Perez R V, Swanson C, Morgan M, Erickson K, Hubbard N E, German J B. Portal venous transfusion up regulates Kuppfer cell cyclooxygenase activity. Transplantation. 1997;64:135–139. doi: 10.1097/00007890-199707150-00023. [DOI] [PubMed] [Google Scholar]
- 34.Peters W R, Fry R D, Fleshman J W, Kodner I J. Multiple blood transfusions reduce the recurrence rate of Crohn’s disease. Dis Colon Rectum. 1989;32:749–753. doi: 10.1007/BF02562122. [DOI] [PubMed] [Google Scholar]
- 35.Pfeffer P F, Thorsby E, Hirschberg H. Cell-mediated cytotoxicity toward the donor in patients with well-functioning kidney grafts. Possible mechanism of specifically reduced cytotoxic response. Transplantation. 1983;35:546–551. doi: 10.1097/00007890-198306000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Reed E, Hardy M, Benvenisty A, Lattes C, Brensliver J, McCabe R, Reemstma K, King D W, Suciu-Foca N. Effect of anti-idiotypic antibodies to HLA on graft survival in renal allograft recipients. N Engl J Med. 1987;316:1450–1455. doi: 10.1056/NEJM198706043162305. [DOI] [PubMed] [Google Scholar]
- 37.Singal D P, Leber B, Harnish D G, Frame B, Joseph S, Blajchman M A. Molecular genetic basis for the antiidiotype antibody response associated with successful renal allograft survival in humans. Transplant Proc. 1991;23:1059–1061. [PubMed] [Google Scholar]
- 38.Sloand E, Kumar P, Klein H G, Merritt S, Sacher R. Transfusion of blood components to persons infected with human immunodeficiency virus type 1: relationship to opportunistic infection. Transfusion. 1994;34:48–53. doi: 10.1046/j.1537-2995.1994.34194098603.x. [DOI] [PubMed] [Google Scholar]
- 39.Starzl T E, Demetris A J, Trucco M, Zeevi A, Ramos H, Terasaki P, Rudert W A, Kocova M, Ricordi C, Ildstad S, Murase N. Chimerism and donor-specific non-reactivity 27 to 29 years after kidney allotransplantation. Transplantation. 1993;55:1272–1277. doi: 10.1097/00007890-199306000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teng Y, Gorczynski R M, Hozumi N. The function of TGF-β-mediated innocent bystander suppression associated with physiological self-tolerance in vivo. Cell Immunol. 1998;190:51–60. doi: 10.1006/cimm.1998.1389. [DOI] [PubMed] [Google Scholar]
- 41.Terasaki P I. The beneficial transfusion effect on kidney graft survival attributed to clonal deletion. Transplantation. 1984;37:119–125. doi: 10.1097/00007890-198402000-00001. [DOI] [PubMed] [Google Scholar]
- 42.Valente J F, Alexander J W. Immunobiology of renal transplantation. Surg Clin North Am. 1998;78:1–25. doi: 10.1016/s0039-6109(05)70631-9. [DOI] [PubMed] [Google Scholar]
- 43.van de Watering L M, Hermans J, Houbiers J G, van den Broek P J, Bouter H, Boer F, Harvey M S, Huysmans H A, Brand A. Beneficial effects of leukocyte depletion of transfused blood on postoperative complications in patients undergoing cardiac surgery: a randomized clinical trial. Circulation. 1998;97:562–568. doi: 10.1161/01.cir.97.6.562. [DOI] [PubMed] [Google Scholar]
- 44.Waymack J P, Gallon L, Barcelli U, Alexander J W. Effect of blood transfusions on macrophage function in a burned animal model. Curr Surg. 1986;43:305–307. [PubMed] [Google Scholar]
- 45.Wheatley T J, Veitch P S, Horsburgh M L, Bell P R F. Transfusion-induced immunosuppression: abrogation by leukodepletion. Transplant Proc. 1997;29:2962–2963. doi: 10.1016/s0041-1345(97)00746-x. [DOI] [PubMed] [Google Scholar]
- 46.Wood M L, Gottschalk R, Monaco A P. Effect of blood transfusion on IL-2 production. Transplantation. 1988;45:930–935. doi: 10.1097/00007890-198805000-00018. [DOI] [PubMed] [Google Scholar]