Abstract
Over 2 years have passed since the start of the COVID-19 pandemic, which has claimed millions of lives. Unlike the early days of the pandemic, when management decisions were based on extrapolations from in vitro data, case reports and case series, clinicians are now equipped with an armamentarium of therapies based on high-quality evidence. These treatments are spread across seven main therapeutic categories: anti-inflammatory agents, antivirals, antithrombotics, therapies for acute hypoxaemic respiratory failure, anti-SARS-CoV-2 (neutralizing) antibody therapies, modulators of the renin–angiotensin–aldosterone system and vitamins. For each of these treatments, the patient population characteristics and clinical settings in which they were studied are important considerations. Although few direct comparisons have been performed, the evidence base and magnitude of benefit for anti-inflammatory and antiviral agents clearly outweigh those of other therapeutic approaches such as vitamins. The emergence of novel variants has further complicated the interpretation of much of the available evidence, particularly for antibody therapies. Importantly, patients with acute and chronic kidney disease were under-represented in many of the COVID-19 clinical trials, and outcomes in this population might differ from those reported in the general population. Here, we examine the clinical evidence for these therapies through a kidney medicine lens.
Subject terms: Molecularly targeted therapy, End-stage renal disease, Organ transplantation, SARS-CoV-2
The COVID-19 pandemic was met with large-scale efforts to assess novel and repurposed therapeutic interventions that could reduce patient morbidity and mortality. Here, the authors discuss the different types of therapies available to treat COVID-19, including their relevance to patients with kidney failure and kidney transplant recipients.
Key points
Multiple effective and safe therapeutics for COVID-19 have been developed since the start of the pandemic in early 2020, with several agents now approved for use across the spectrum of disease severity.
COVID-19 therapeutics investigated to date include anti-inflammatory agents, antivirals, antithrombotics, therapies for acute hypoxaemic respiratory failure, anti-SARS-CoV-2 (neutralizing) antibody therapies, modulators of the renin–angiotensin–aldosterone system and vitamins.
Patients with underlying kidney disease represent a vulnerable population at high risk of adverse outcomes from COVID-19, yet they were excluded or under-represented in many COVID-19 clinical trials.
Additional data on the efficacy and safety of COVID-19 therapeutics in patients with chronic kidney disease are needed, as well as data on therapeutics for the prevention and treatment of COVID-19-associated acute kidney injury.
Introduction
Since the beginning of the COVID-19 pandemic in March 2020, SARS-CoV-2 has claimed the lives of millions of people worldwide, and the number of deaths continues to rise with the emergence of new variants1. During this time, therapeutics against COVID-19 have also advanced at an astounding pace, and clinicians are now equipped with an armamentarium of treatment options for patients across the spectrum of COVID-19 severity, ranging from mild to critical illness.
Therapeutic agents investigated during the pandemic include medications with diverse mechanisms of action, such as antiviral therapies that inhibit viral replication directly, recombinant neutralizing monoclonal antibodies that block viral entry into host cells, adjunct therapies that target the host immune response (for example, anti-inflammatory and antithrombotic therapies), and therapies targeting the renin–angiotensin–aldosterone system (RAAS) (Fig. 1). Numerous randomized clinical trials (RCTs) have tested the safety and efficacy of these agents (Fig. 2), which include both novel and repurposed drugs. Multiple therapies have obtained emergency-use authorization (EUA) and, in some cases, approval from regulatory agencies, including the FDA and the EMA (Table 1).
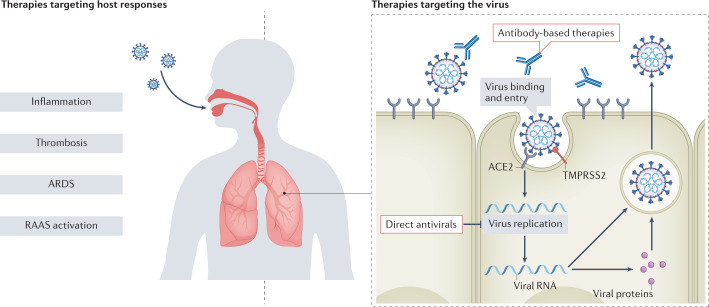
Fig. 1. Classes of therapies for COVID-19.
Therapies for COVID-19 can be broadly categorized as targeting the host response to infection (including inflammation, thrombosis, acute respiratory distress syndrome (ARDS) and renin–angiotensin–aldosterone system (RAAS) activation) or targeting the virus directly (including direct antivirals and antibody-based therapies). SARS-CoV-2 infection can lead to hyperinflammation characterized by abundant circulating levels of pro-inflammatory cytokines such as IL-6. Therapies targeting inflammation include immunosuppressive drugs such as glucocorticoids (for example, dexamethasone) and anti-IL-6 receptor antibodies (for example, tocilizumab). Several antithrombotic therapies have also been trialled to address the haemostatic and thrombotic complications associated with COVID-19, whereas different methods of oxygen delivery and intubation can be employed to treat patients with ARDS. COVID-19 can also disrupt RAAS homeostasis and drugs such as angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers are being investigated as potential therapies. Finally, therapies targeting SARS-CoV-2 directly include antivirals that disrupt viral replication and neutralizing antibody therapies that prevent virus entry into host cells.
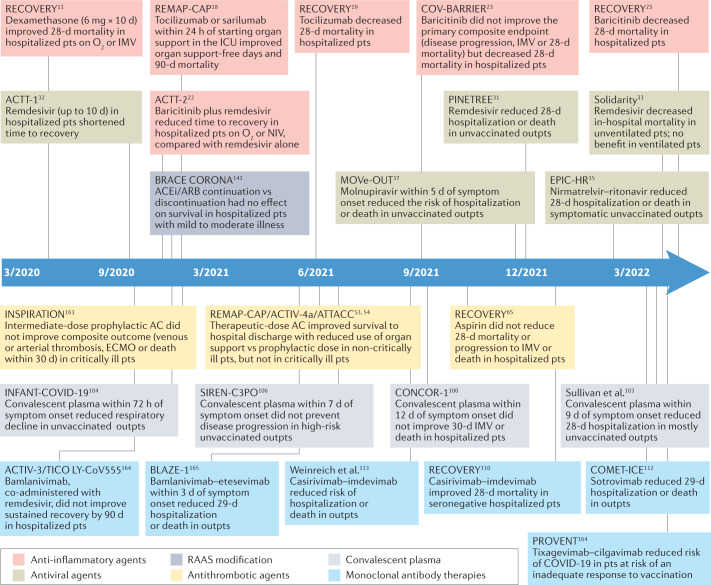
Fig. 2. Timeline of publication of pivotal phase III randomized clinical trials of COVID-19 therapies.
Timeline of publication and key features of pivotal phase III randomized clinical trials of COVID-19 therapies. These trials are categorized into six treatment categories: anti-inflammatory agents, antivirals, renin–angiotensin–aldosterone system (RAAS) modification, antithrombotic agents, convalescent plasma and monoclonal antibody therapies. AC, anticoagulation; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; d, days; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; IMV, invasive mechanical ventilation; NIV, noninvasive ventilation; outpts, outpatients; pts, patients. This timeline reflects published data as of 29 May 2022.
Table 1.
Authorized or approved therapeutics for COVID-19
| Drug | Setting | Patient population | Dosing regimen | Dose adjustment for kidney dysfunction | Date of FDA EUA or approval | Date of EMA authorization |
|---|---|---|---|---|---|---|
| Anti-inflammatory agents | ||||||
| Tocilizumab | Inpatient | Patients receiving corticosteroids and on supplemental oxygen, a ventilator or ECMO | 8 mg/kg i.v. once (max dose: 800 mg) | None | EUA, 24 June 2021a | 6 December 2021 |
| Baricitinib | Inpatient | Patients on supplemental oxygen, IMV or ECMO | 4 mg once daily |
eGFR ≥ 60: 4 mg daily; eGFR 30–59: 2 mg daily; eGFR 15–29: 1 mg daily; eGFR < 15: NR |
EUA, 19 November 2020; FDA approved, 10 May 2022 | Under review |
| Antiviral agents | ||||||
| Remdesivir | Inpatient and outpatient | Symptoms (mild to moderate) for < 7 days | 200 mg i.v. on day 1, then 100 mg i.v. daily from day 2 (3 days for non-hospitalized, 5 days or until discharge for hospitalized) | eGFR < 30: NR | EUA, 1 May 2020; FDA approved, 22 October 2020 | 3 July 2020 |
| Nirmatrelvir–ritonavir (Paxlovid) | Outpatient | Symptoms (mild to moderate) for < 5 days | 300 mg/100 mg oral twice daily for 5 days | eGFR 30–59: 150/100 mg twice daily for 5 days; eGFR < 30: NR | EUA, 22 December 2021 | 28 January 2022 |
| Molnupiravir | Outpatient | Symptoms (mild to moderate) for < 5 days | 800 mg orally twice daily for 5 days | None | EUA, 23 December 2021 | Under review |
| Antibody-based therapies | ||||||
| Convalescent plasma | Inpatient and outpatient | Hospitalized patients receiving supplemental oxygen, noninvasive ventilation or IMV, or ECMO | ~200 ml IV | None | EUA, 23 August 2020 | ND |
| Bamlanivimab/etesevimaba | Outpatient | Symptoms (mild to moderate) | 700 mg/1400 mg i.v. once | None | EUA, 9 February 2021 | Withdrawn from review 29 October 2021 |
| Casirivimab/imdevimaba | Outpatient | Symptoms (mild to moderate) for < 10 days | 600 mg/600 mg s.c. once | None | EUA, 21 November 2020 | 12 November 2021 |
| Sotrovimabb | Outpatient | Symptoms (mild to moderate) for < 7 days | 500 mg i.v. once | None | EUA, 26 May 2021 | 17 December 2021 |
| Bebtelovimab | Outpatient | Symptoms (mild to moderate) for < 7 days and at a high risk of severe illness | 175 mg i.v. once | None | EUA, 11 February 2022 | ND |
| Tixagevimab/cilgavimab (Evusheld) | Outpatient | Pre-exposure prophylaxis and with moderate to severe immune compromise due to a medical condition or immunosuppressive medication | 300 mg/300 mg i.m. once | None | EUA, 8 December 2021 | 25 March 2022 |
ECMO, extracorporeal membrane oxygenation; EUA, Emergency Use Authorization; eGFR, estimated glomerular filtration rate; i.m., intramuscular; IMV, invasive mechanical ventilation; i.v., intravenous; ND, not discussed; NR, not recommended; s.c., subcutaneous. aCurrently under priority review for FDA approval. bUse terminated in the USA owing to high frequency of the Omicron SARS-CoV-2 variant. cUse suspended in certain US states owing to lack of efficacy against the BA.2 variant.
In this Review, we discuss the therapeutic advances in the treatment of COVID-19 since the start of the pandemic. We also emphasize their relevance to patients with kidney disease, including chronic kidney disease (CKD) and kidney failure requiring kidney replacement therapy (KRT), as well as kidney transplant recipients, who are all at increased risk of adverse COVID-19 outcomes2,3. These patient populations were excluded or under-represented in many of the RCTs of novel therapies for COVID-19, presumably owing to concerns regarding altered pharmacokinetics and the potential for increased toxicity. We also discuss the role of these therapies in the prevention and treatment of COVID-19-associated acute kidney injury (AKI), which is a common and important complication of COVID-19 among hospitalized4 and critically ill patients5.
Therapies targeting inflammation
From the early stages of the COVID-19 pandemic, hyperinflammation has been proposed to have an important role in the pathophysiology of severe COVID-19 (ref. 6). Compared with healthy individuals, hospitalized patients with COVID-19 have elevated circulating concentrations of acute phase reactants (for example, ferritin)7, pro-inflammatory cytokines (for example, IL-6)8 and markers of coagulation and fibrinolysis (for example, D-dimer)9. Moreover, higher levels of these markers have been consistently associated with an increased risk of death in patients with COVID-19 (refs. 8,9). Although subsequent studies found that the severity of inflammation in COVID-19 might be similar to that observed in patients with sepsis or acute respiratory distress syndrome (ARDS) but without COVID-19 (ref. 10), the association between inflammatory markers and outcome severity prompted a series of trials of interventions that targeted inflammatory pathways.
Dexamethasone
The Randomized Evaluation of COVID-19 Therapy (RECOVERY) group conducted the largest RCT to date of glucocorticoids in patients with COVID-19 (ref. 11). RECOVERY randomly assigned 6,425 hospitalized adult patients with COVID-19 to receive oral or intravenous (i.v.) dexamethasone 6 mg daily for up to 10 days, or usual care. The primary outcome of mortality at 28 days was lower in the dexamethasone group (22.9% versus 25.7%; rate ratio (RR) 0.83, 95% CI 0.75–0.93). Pre-specified analyses of the primary outcome revealed significant heterogeneity according to the level of oxygen support (P < 0.001) — the mortality benefit with dexamethasone was greatest in patients receiving invasive mechanical ventilation (IMV) at randomization (RR, 0.64, 95% CI 0.51–0.81), whereas a trend towards harm was observed in patients not receiving oxygen at randomization (RR 1.19, 95% CI 0.92–1.55). These findings were corroborated by a meta-analysis conducted by the WHO Rapid Evidence Appraisal for COVID-19 (REACT) Working Group, which pooled data from seven RCTs of glucocorticoids in 1,703 critically ill patients with COVID-19 and found the odds ratio (OR) for 28-day mortality to be 0.66 (95% CI 0.53–0.82)12.
Tocilizumab
Tocilizumab is a humanized monoclonal antibody against the IL-6 receptor. Early observational data suggested a survival benefit in hospitalized patients with COVID-19 treated with tocilizumab13,14, particularly if administered early in the disease course. These data conflict with those of initial RCTs15,16, which were largely negative but were also underpowered to rule out a significant clinical benefit17. Subsequently, two large pragmatic RCTs demonstrated a mortality benefit.
The Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) randomly assigned 755 critically ill adult patients with COVID-19 to receive tocilizumab or usual care18. Tocilizumab was administered at a median of 1.2 days following hospital admission. In-hospital mortality was considerably lower in patients assigned to tocilizumab (28% versus 36%; median adjusted OR 1.64, 95% credible interval 1.14–2.35), as was 90-day mortality.
The RECOVERY group conducted the largest RCT of tocilizumab in COVID-19 to date19. A total of 4,116 adult patients hospitalized at 131 sites in the UK were randomly assigned to receive tocilizumab or usual care. Tocilizumab was administered at a median of 2 days following hospital admission. Mortality at 28 days was lower in patients assigned to tocilizumab (31% versus 35%; RR 0.85, 95% CI 0.76–0.94).
Some investigators have raised concerns regarding the lack of blinding in these large pragmatic trials20 but the results seem to be consistent across studies. Further, the findings were concordant with the results of the WHO REACT Working Group’s meta-analysis, which included 10,930 adult patients with COVID-19 from 27 RCTs that assessed the efficacy of IL-6 antagonists. In this meta-analysis, the OR for 28-day mortality with IL-6 antagonists was 0.86 (95% CI 0.79–0.95)21. Tocilizumab received FDA EUA for COVID-19 on 24 June 2021 and is currently under priority review by the FDA for approval (Table 1); this treatment was approved by the EMA on 6 December 2021 (Table 1).
Baricitinib
Baricitinib is an oral Janus kinase 1 (JAK1) and JAK2 inhibitor with anti-inflammatory properties. An RCT conducted in 1,033 hospitalized adults found that treatment with baricitinib (4 mg daily, up to 14 days) plus the antiviral remdesivir (discussed in more detail below) was superior to remdesivir alone in reducing time to recovery22. In a subsequent phase III RCT, 1,525 adult patients hospitalized at 101 centres across 12 countries were assigned to baricitinib (4 mg daily) or placebo for up to 14 days23. Although the primary composite outcome (the proportion who progressed to high-flow oxygen, noninvasive ventilation (NIV), IMV or death by day 28) was similar between groups, 28-day mortality was notably lower in the baricitinib group than in the placebo group (8% versus 13%; hazard ratio (HR) 0.57, 95% CI 0.41–0.78). Importantly, patients receiving IMV were excluded from this study. However, a subsequent smaller RCT conducted by the same group in 101 critically ill adults with COVID-19 receiving IMV or extracorporeal membrane oxygenation (ECMO) reported a 28-day mortality benefit with baricitinib versus placebo (39% versus 58%; HR 0.54, 95% CI 0.31–0.96)24. In the largest RCT of baricitinib to date, the RECOVERY group randomly assigned 8,156 hospitalized adults to receive baricitinib 4 mg daily until either 10 days or hospital discharge, versus usual care. Patients who received baricitinib had lower 28-day mortality than those receiving usual care (age-adjusted RR 0.87, 95% CI 0.77–0.99)25. Baricitinib was approved by the FDA in May 2022 and is currently under review by the EMA (Table 1).
Relevance to patients with kidney disease
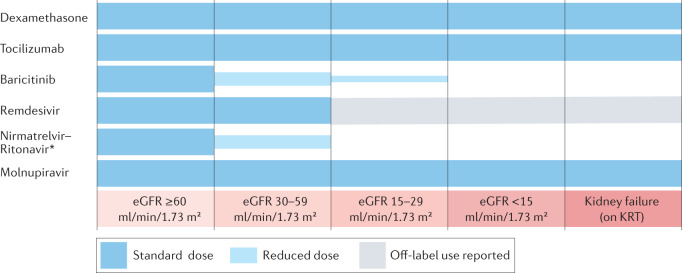
The largest RCTs that assessed dexamethasone and tocilizumab in COVID-19 included patients with AKI, CKD and kidney failure requiring KRT, as well as kidney transplant recipients. No dose adjustments are required for the use of these agents in patients with kidney dysfunction (Fig. 3). By contrast, the baricitinib dose is reduced to 2 mg daily in patients with estimated glomerular filtration rate (eGFR) 30–59 ml/min/1.73 m2, and to 1 mg daily in patients with eGFR 15–29 ml/min/1.73 m2 (Fig. 3). Whether the magnitude of benefit of anti-inflammatory therapies in patients with COVID-19 differs according to the level of kidney function at baseline has not been assessed.
Fig. 3. Anti-inflammatory and antiviral agents for COVID-19 including dose adjustment for kidney function impairment.
The immunosuppressive therapies dexamethasone and tocilizumab can be used without dose adjustments in patients with kidney disease, including those with kidney failure. However, the anti-inflammatory agent baricitinib must be administered at reduced doses in patients with estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2. In the case of antivirals used to treat SARS-CoV-2 infection, molnupiravir can be used without dose adjustments and remdesivir, although currently not recommended for use in patients with eGFR < 30 ml/min/1.73 m2, has been reportedly used in patients across the spectrum of kidney dysfunction, including in patients with kidney failure who require kidney replacement therapy (KRT). By contrast, nirmatrelvir–ritonavir is contraindicated in patients with eGFR <30 ml/min/1.73 m2 and, given its potential to increase exposure to calcineurin inhibitors (CNIs) and mammalian target of rapamycin (mTOR) inhibitors, must be used with extreme caution in recipients of solid organ transplants, and only if CNI and/or mTOR levels can be monitored closely. The asterisk indicates that in patients with eGFR 30–59 ml/min/1.73 m2, the dose of nirmatrelvir is reduced by 50% but the ritonavir dose remains unchanged.
The use of anti-inflammatory agents such as tocilizumab or dexamethasone could potentially increase the risk of secondary infection, especially in patients with kidney failure or in kidney transplant recipients. Of note, although the aforementioned RCTs of tocilizumab did not report an increased risk of secondary infection18, solid organ transplant recipients might have been under-represented in these trials.
Finally, whether glucocorticoids, IL-6 antagonists and other anti-inflammatory therapies reduce the risk of AKI in patients with COVID-19 has not been rigorously examined. However, the effect of dexamethasone and tocilizumab on the most severe form of AKI (that is, AKI requiring KRT) was assessed by the RECOVERY group trials of these agents and, in both cases, treatment reduced the incidence of KRT considerably11,19.
Antiviral therapies
At the outset of the COVID-19 pandemic, no antiviral agents were licensed for the treatment of this disease. Consequently, numerous repurposed therapies with in vitro antiviral activity entered clinical use and were tested in clinical trials. However, many widely used agents such as hydroxychloroquine, lopinavir or ritonavir, and ivermectin were shown to be ineffective COVID-19 therapies when studied in adequately powered RCTs26–28; more effective antivirals are now available.
Remdesivir
Remdesivir is a prodrug nucleoside analogue — its active metabolite reduces genome replication by inhibiting RNA-dependent RNA polymerase — and has antiviral activity against many RNA viruses in vitro, including SARS-CoV-2 (refs. 29,30). Remdesivir has been studied in patients with COVID-19 in both the outpatient and inpatient settings. In the PINETREE study, a 3-day course of i.v. remdesivir among unvaccinated outpatients with COVID-19 at a high risk of disease progression reduced 28-day hospitalization or death by 87% compared with placebo (0.7% versus 5.3%; HR 0.13, 95% CI 0.03–0.59)31. By contrast, remdesivir’s magnitude of benefit was modest among hospitalized patients. The pivotal ACTT-1 trial, which enrolled 1,062 patients, showed that the time to recovery was shorter with remdesivir than with placebo (10 days versus 15 days), and there was a trend towards lower 29-day mortality (11.4% versus 15.2%; HR 0.73, 95% CI 0.52–1.03)32. The WHO Solidarity trial, which is the largest RCT of remdesivir to date, randomly assigned 8,275 hospitalized patients to remdesivir or no study drug. Overall, treatment with remdesivir did not affect hospital mortality compared with control (14.5% versus 15.6%; RR 0.91, 95% CI 0.82–1.02), although it reduced hospital mortality modestly in patients who were not ventilated at study entry (11.9% versus 13.5%; RR 0.86, 95% CI 0.76–0.98); there was no benefit in patients who were already ventilated at the start of treatment33. Remdesivir was approved by the FDA on 22 October 2020 and by the EMA on 3 July 2020 (Table 1).
Nirmatrelvir–ritonavir
Nirmatrelvir–ritonavir (also known as Paxlovid) is a combination therapy consisting of nirmatrelvir, an oral 3C-like protease inhibitor that is active against the main viral protease that cleaves SARS-CoV-2 polyproteins during viral replication, and ritonavir, a strong cytochrome P450 3A4 (CYP3A4) inhibitor and pharmacokinetic boosting agent34. In the EPIC-HR trial, which enrolled 2,246 unvaccinated adult outpatients with COVID-19 at a high risk of disease progression, nirmatrelvir–ritonavir reduced the risk of 28-day hospitalization or death by 89% compared with placebo (0.7% versus 6.5%; difference of −6.32 percentage points, 95% CI −9.04 to −3.59)35. Nirmatrelvir–ritonavir received EUA from the FDA on 22 December 2021, and approval from the EMA on 28 January 2022 (Table 1).
Molnupiravir
Molnupiravir is an oral pro-drug of β-d-N4-hydroxycytidine (NHC), which is a cytidine analogue that has broad-spectrum antiviral activity against SARS-CoV-2 in vitro36. NHC is incorporated into new RNA strands of the SARS-CoV-2 genome as they are synthesized, causing an accumulation of deleterious mutations that is termed lethal mutagenesis. In the MOVe-OUT trial, which enrolled 1,433 adult outpatients with mild-to-moderate COVID-19, molnupiravir reduced 29-day hospitalization or death by approximately one-third compared with placebo (6.8% versus 9.7%; HR 0.69, 95% CI 0.48–1.01), which was an effect of lesser magnitude than that observed with other antivirals37.
Relevance to patients with kidney disease
Most RCTs of remdesivir excluded patients with an eGFR <30 ml/min/1.73 m2 (refs. 31–33). Remdesivir is formulated with sulfobutylether β-cyclodextrin sodium, which is a solubility enhancer that is eliminated by the kidney and is nephrotoxic in rats at high doses38. However, whether the doses of sulfobutylether β-cyclodextrin sodium administered during short courses of remdesivir represent a substantial safety concern is unclear, and remdesivir use in patients with severely impaired kidney function has been reported39–43. Nonetheless, remdesivir is not currently recommended in patients with eGFR <30 ml/min/1.73 m2 (Fig. 3), although a phase III RCT is ongoing to evaluate its efficacy and safety in that population44.
The EPIC-HR trial of nirmatrelvir–ritonavir described above excluded patients with an eGFR <45 ml/min/1.73 m2 (ref. 35). Nonetheless, nirmatrelvir–ritonavir can be used in patients with moderate CKD — a 50% dose reduction of nirmatrelvir is recommended in patients with an eGFR 30–59 ml/min/1.73 m2 (ref. 45) (Fig. 3). Nirmatrelvir–ritonavir is contraindicated in patients with eGFR <30 ml/min/1.73 m2, as well as in those with severe hepatic impairment. Importantly, ritonavir is a potent inhibitor of CYP3A and would therefore increase exposure to calcineurin inhibitors and mammalian target of rapamycin inhibitors, which are immunosuppressive drugs commonly used to prevent graft rejection in kidney transplant recipients. Accordingly, nirmatrelvir–ritonavir should be used with extreme caution in recipients of kidney and other solid organ transplants, and only if calcineurin inhibitors and/or mammalian target of rapamycin levels can be monitored closely46.
The MOVe-OUT trial of molnupiravir excluded patients with eGFR <30 ml/min/1.73 m2, and only 5.9% of participants had CKD37. However, because NHC is metabolized through an endogenous pyrimidine pathway47, the EUA for molnupiravir does not restrict its use in patients with advanced CKD or kidney failure requiring KRT, and no dose adjustment is required for patients with kidney disease (Fig. 3).
In summary, antiviral therapy now has an established role in preventing disease progression when used for early treatment of symptomatic patients at risk of poor outcomes (for example, unvaccinated patients and those with comorbidities such as CKD). Whether the remdesivir, nirmatrelvir–ritonavir and molnupiravir RCT data can be extrapolated to vaccinated populations, patients infected with new SARS-CoV-2 variants or patients with advanced CKD is unclear. Notably, all three drugs have limitations: remdesivir requires i.v. infusion, nirmatrelvir–ritonavir has important interactions with other drugs that might restrict or complicate its use, and the efficacy of molnupiravir is modest. Evidence directly relevant to patients with CKD or kidney failure and kidney transplant recipients is scarce and eagerly awaited.
Antithrombotic therapies
Hospitalized patients with COVID-19 experience relatively high rates of morbid and potentially fatal haemostatic and thrombotic complications, particularly venous thromboembolism (VTE)48,49. These complications result from a state of dysfunction in the thromboinflammatory coagulation system, sometimes referred to as ‘COVID-19-associated coagulopathy’50. The pathogenesis of this coagulopathy is incompletely understood and seems to be multifactorial. Contributing factors include endothelial injury (induced by the virus and/or the anti-viral immune response), a hypercoagulable state induced by elevated levels of acute-phase reactant coagulation factors (particularly fibrinogen and factor VIII) and other pro-inflammatory mechanisms, including the formation of neutrophil extracellular traps49–52. Consequently, empirical use of antithrombotic therapies, including empirical administration of anticoagulant or antiplatelet agents in the absence of a standard clinical indication, and empirical dose escalation to a dose higher than that otherwise indicated in patients who would typically receive prophylactic dosing, has been under investigation in patients with COVID-19 since the early days of the pandemic.
Importantly, the use of antithrombotic therapies requires careful balancing of thrombotic and bleeding risks. In most patient populations, major bleeding is associated with a higher mortality risk than VTE, including in patients with COVID-19 (ref. 48). The risks of both bleeding and thrombosis escalate with worsening illness, but not always in equal proportions, and the relative contribution of thrombotic injury to morbidity might depend on the phase of illness53,54. The potential benefit of empirical use or dose-escalation of anticoagulation or antiplatelet therapy in COVID-19 has been evaluated across different phases of illness (that is, in critically ill inpatients, non-critically ill inpatients, and outpatients) and at varying dose levels, particularly for anticoagulation (prophylactic, intermediate and therapeutic doses)55 (Table 2); several clinical trials are ongoing. Patients with COVID-19 and an existing indication for therapeutic anticoagulation (for example, VTE) or antiplatelet therapy (for example, coronary artery disease) should receive antithrombotic therapy as indicated by American Society of Hematology guidelines56.
Table 2.
Summary of evidence from major RCTs evaluating the use of empirical antithrombotic therapy in COVID-19
| Disease severity | Therapy | RCT | Summary of current evidence and recommendations |
|---|---|---|---|
| Inpatient, critically ill (severe illness) | Anticoagulation (heparin-based) |
ACTIV-4a–ATTACC– REMAP-CAPa, 54 INSPIRATION163 |
Critically ill patients with COVID-19 should receive pharmacological thromboprophylaxis with unfractionated or low molecular weight heparin Multiple RCTs show no benefit from intermediate- or therapeutic-dose anticoagulation over standard prophylactic-dose thromboprophylaxis, and potential for harm with dose escalation owing to higher bleeding rates Prophylactic dosing should be adjusted for weight and eGFR |
| Aspirin or P2Y12 inhibitorb |
RECOVERY65 REMAP-CAP67 |
No clear benefit, and higher rates of major bleeding with empirical use of antiplatelet agents in critically ill patients with COVID-19 | |
| Inpatient, not critically ill (moderate illness) | Heparin-based anticoagulation or rivaroxaban |
REMAP-CAP– ACTIV-4a–ATTACC53 |
Moderately ill patients with COVID-19 should receive at least pharmacological thromboprophylaxis Empirical therapeutic-dose anticoagulation might benefit certain individuals with high thrombosis and low bleeding risk, but data are conflicting |
| Aspirin | RECOVERY65 | No clear benefit for empirical use of aspirin in moderately ill patients with COVID-19 | |
| Outpatient, no hospitalization | Apixaban | ACTIV-4B66 | No clear benefit for empirical use of apixaban in outpatients with COVID-19 |
| Aspirin | ACTIV-4B66 | No clear benefit for empirical use of aspirin in outpatients with COVID-19 | |
| Outpatient, following hospital discharge | Anticoagulation (apixaban, rivaroxaban, or enoxaparin) | MICHELLE63 | Possible benefit for prophylactic-dose rivaroxaban post-discharge in certain patients hospitalized for COVID-19 who have high thrombosis and low bleeding risk |
eGFR, estimated glomerular filtration rate; RCT, randomized controlled trial. aATTACC–ACTIV-4a–REMAP-CAP was a multiplatform trial of anticoagulation in hospitalized patients with COVID-19. bClopidogrel, prasugrel or ticagrelor.
At present, the accumulated body of evidence suggests that antithrombotic management of patients with COVID-19 should not differ substantially from that of other populations with similar degrees of illness. However, where no contraindications exist, all hospitalized patients with COVID-19 should receive pharmacological thromboprophylaxis (in contrast to mechanical thromboprophylaxis alone)56, and empirical therapeutic anticoagulation can be considered in non-critically ill hospitalized patients, particularly in those with a high thrombosis risk (that is, patients with elevated D-dimer levels) and a low bleeding risk57,58. Importantly, although two large RCTs reported a benefit for therapeutic anticoagulation in non-critically ill hospitalized patients53,59, two others did not report a beneficial effect60,61. However, of the two trials that did not find a benefit, one was stopped early owing to feasibility (although data showed that therapeutic anticoagulation significantly reduced 28-day mortality compared with prophylactic anticoagulation)60, and the other investigated rivaroxaban, which is a factor Xa inhibitor61 (whereas other RCTs evaluated the use of unfractionated or low-molecular-weight heparin (LMWH)). Finally, patients at high risk of VTE might benefit from post-discharge pharmacological thromboprophylaxis62,63, which also applies to patients who were hospitalized without COVID-19 (ref. 64).
In contrast to therapeutic anticoagulation, empirical antiplatelet therapy does not seem to be beneficial at any stage of COVID-19 (refs. 65–67), including the RECOVERY trial of aspirin versus usual care in 14,892 hospitalized patients65, which is the largest trial of any agent in patients with COVID-19 to date. One trial found a reduction in 90-day mortality with antiplatelet therapy (aspirin or a P2Y12 inhibitor) in critically ill patients with COVID-19 (ref. 67), although this was a secondary end point and additional trials are ongoing to address this question68. Conversely, several trials have reported increased rates of major bleeding in patients treated with empirical antiplatelet versus no antiplatelet therapy, and in patients treated with therapeutic- versus prophylactic-dose anticoagulation therapy53,54,61,67. Importantly, given the substantial contribution of inflammation to COVID-19-associated coagulopathy, the development of anti-inflammatory therapies to treat COVID-19 might attenuate or nullify any potential benefit of escalated anticoagulation, leaving only elevated bleeding risk. This risk is particularly important to consider when assessing trials of antithrombotic therapies conducted before the routine use of anti-inflammatory therapies.
Relevance to patients with kidney disease
In general, the aforementioned conclusions regarding antithrombotic therapies apply to patients with advanced kidney disease, with slight modifications. Patients with CKD or kidney failure requiring KRT are at an increased risk of both bleeding69 and clotting70 at baseline compared with patients with normal kidney function. These risks must be considered when evaluating non-critically ill patients for empirical therapeutic-dose anticoagulation and when evaluating hospitalized patients for post-discharge pharmacological thromboprophylaxis. Most major trials of anticoagulation in hospitalized patients with COVID-19 enrolled patients with advanced kidney disease, but dose-reduced LMWH or mandated the use of unfractionated heparin rather than LMWH in these patients. Although LMWH is usually preferred over unfractionated heparin, given its much lower risk of heparin-induced thrombocytopenia71 and ease of administration, LMWH must be used with caution and at lower doses in patients with advanced kidney disease owing to the potential increased risk of bleeding given that LMWH is excreted in the kidney72. Finally, although empirical dose escalation of prophylactic anticoagulation does not seem to be beneficial in critically ill patients with COVID-19 (ref. 54), patients receiving continuous KRT who experience recurrent clotting of the circuit filter might benefit from therapeutic-dose anticoagulation to prevent further clotting73. Of note, some small studies suggest that argatroban, which is a direct thrombin inhibitor, might be useful to prevent circuit filter thrombosis in these patients74,75 but high-level evidence to support its use is lacking. Importantly, in any critically ill patient with multi-organ system failure, acquired antithrombin deficiency might render heparin ineffective. This effect can be countered with either antithrombin repletion or a switch to a direct thrombin inhibitor, which would act independently of antithrombin76.
Finally, whether therapeutic anticoagulation can attenuate the risk of COVID-19-associated AKI is unknown. The pathophysiology of AKI in COVID-19 is complex and involves inflammation, endothelial injury, and microvascular thrombosis77. Although acute tubular injury is the most common finding at autopsy in patients with COVID-19-associated AKI, platelet-rich peritubular fibrin microthrombi in the kidney microcirculation have also been reported78. Therapeutic anticoagulation with heparin might mitigate microvascular thrombosis and attenuate ischaemia–reperfusion injury79 thereby preventing AKI, but supporting clinical trial data are lacking.
Therapies for acute respiratory failure
COVID-19 might cause critical illness through a variety of mechanisms, but acute hypoxaemic respiratory failure remains the leading cause7. In addition to decisions regarding the use of antivirals, immunomodulators and anticoagulants, which might benefit patients across the spectrum of illness, the treatment of COVID-19-induced respiratory failure requires special consideration of the method of oxygen delivery, timing of intubation, and the use of prone positioning or of adjuvant therapies for refractory hypoxaemia.
Oxygen delivery
Three noninvasive methods of oxygen delivery can be used in patients who require more oxygen than can be delivered by a standard nasal cannula — conventional oxygen therapy (usually a mask with an oxygen reservoir, termed a ‘non-rebreather mask’), a high-flow nasal cannula (HFNC), which delivers warmed and humidified oxygen through large-bore nasal cannulas at flow rates that exceed the patient’s peak inspiratory flow rate, or NIV, whereby a tight-fitting mask connected to a noninvasive ventilation machine provides continuous positive airway pressure or bilevel positive airway pressure.
Initially, concerns surrounding the use of HFNC or NIV included a potential increase in the risk to health-care workers owing to aerosolization of SARS-CoV-2 and potential exacerbation of lung injury owing to increased transpulmonary pressures from vigorous respiratory effort (so-called patient self-induced lung injury), which might be avoided with intubation and sedation80,81. During the first wave of COVID-19, these concerns led to the intubation of patients who had only moderate hypoxaemia82. Earlier intubation, however, potentially exposes patients to other risks, such as ventilator-induced lung injury and sedation-associated delirium. Accordingly, some studies showed that the outcomes of hospitalized patients improved as the use of HFNC and NIV became more liberal, and the approach to intubation became more conservative83. Furthermore, the availability of highly effective vaccines and research suggesting that HFNC and NIV do not significantly increase aerosolization of virus particles have allayed concerns regarding the risk to health care workers84.
Although the use of HFNC and NIV are now more common in the treatment of COVID-19 than earlier in the pandemic, their relative effectiveness remains unclear. The RECOVERY-RS trial compared available methods for oxygen delivery by randomly assigning 1,237 hospitalized adults with COVID-19-related acute hypoxaemic respiratory failure to conventional oxygen therapy, HFNC or NIV (specifically, continuous positive airway pressure). The study found that NIV led to lower rates of intubation than conventional oxygen delivery or HFNC, but mortality was not significantly different among the three groups. However, the interpretation of the study is complicated by a high rate of crossover between groups85.
Prone positioning
Prone positioning is one of the few interventions shown to reduce mortality among patients intubated for non-COVID-19 ARDS86. However, adoption of proning in patients with COVID-19 ARDS has been incomplete based on ongoing concerns regarding its effectiveness, resource utilization and the risk of dislodging support devices; reports show wide inter-hospital variation in the use of proning7. No RCT has evaluated proning in patients intubated owing to COVID-19 but observational studies suggest that early proning of these patients is associated with reduced mortality87.
Since the early days of the pandemic, some experts have proposed extending the use of proning to non-intubated spontaneously breathing patients (that is, awake proning). However, results of trials evaluating the use of awake proning have been mixed — some showed benefit88, whereas others showed no effect89 or even potential harm90.
Adjunctive therapy for hypoxemia
In the years leading up to the COVID-19 pandemic, the use of neuromuscular blockade in ARDS had been hotly debated. The Reevaluation of Systemic Early Neuromuscular Blockade (ROSE) trial, reported in 2019, showed that 48 h of neuromuscular blockade in patients with a partial pressure of arterial oxygen (PaO2) to fraction of inspired oxygen (FiO2) ratio <150 mmHg did not improve 90-day mortality91. No trials have specifically evaluated neuromuscular blockade in patients with COVID-19 but, based on the results of the ROSE trial, no major medical society has recommended the routine use of neuromuscular blockade. However, many patients with COVID-19 develop refractory hypoxaemia despite maximal ventilatory support, and both neuromuscular blockade and inhaled pulmonary vasodilators are routinely used in this setting despite a lack of evidence that they affect clinical outcomes in either COVID-19 or non-COVID-19 ARDS.
ECMO
For patients with refractory hypoxaemia despite maximal ventilatory support, proning, neuromuscular blockade, and inhaled pulmonary vasodilators, ECMO might be a life-saving therapy92,93. The ECMO to Rescue Lung Injury in Severe ARDS (EOLIA) trial, published in 2018, suggested a mortality benefit in patients with non-COVID-19 ARDS with severe hypoxaemia94, and the use of ECMO increased dramatically during the COVID-19 pandemic.
No RCTs have evaluated the efficacy of ECMO in patients with COVID-19 ARDS. However, two target trial emulation analyses, as well as a pseudo-randomized study that used a period of resource limitation as a natural experiment, suggested a robust mortality benefit from ECMO in patients with COVID-19 (refs. 92,93,95). In one target trial analysis, which used data from 55 geographically diverse sites from across the USA, patients with severe hypoxaemia (defined as PaO2 to FiO2 ratio <100 mmHg while receiving IMV) in whom ECMO was initiated within 7 days following intensive care unit admission, had a considerably lower mortality than similarly ill patients at ECMO-capable centres who were not treated with ECMO (34.6% versus 47.4%; HR 0.55, 95% CI 0.41–0.74)92. Similar findings were observed in a larger subsequent study, which used data from 7,345 adults admitted to intensive care units in 30 countries93. In the pseudo-randomized study, mortality among patients who qualified for ECMO but did not receive it owing to resource limitations was ~90%, compared with 43% among patients for whom those resources were available95. Although the data from these observational studies are compelling, they should nonetheless be interpreted cautiously, as they might have been affected by residual confounding (for example, disease severity and mortality risk might have affected treatment selection).
Relevance to patients with kidney disease
ARDS is independently associated with a higher risk of AKI in critically ill patients, and the combination of both syndromes portends a particularly poor prognosis in both COVID-19 and non-COVID-19 settings7,96. Crosstalk between injured lungs and kidneys has been well recognized in both animal models and human studies, and multiple mechanisms have been proposed to explain this pathophysiology (reviewed previously97).
Neutralizing antibody therapies
Antibodies have a key role in the adaptive immune response and are crucial to protecting the host from pathogens. Passive administration of pathogen-specific polyclonal or monoclonal antibodies (mAbs) has been used to control viral infections, with the goal of neutralizing a target virus by targeting it for elimination and preventing its entry into host cells, thereby eliminating the infection-associated disease98. Pathogen-specific neutralizing antibodies can either be transferred from patients who have recovered from a viral infection (that is, convalescent plasma) or synthesized as recombinant neutralizing mAbs through established molecular engineering techniques.
The use of convalescent plasma was rapidly implemented in the early phase of the pandemic in the absence of alternative treatment options99; since then, several studies have investigated the efficacy of this therapy. Multicentre, open-label RCTs repeatedly demonstrated a lack of benefit for convalescent plasma in hospitalized patients with COVID-19 (refs. 100–102). In non-hospitalized and mostly unvaccinated adults, a double-blind RCT of convalescent plasma versus control plasma administered within 8 days of symptom onset reduced hospitalization (2.9% versus 6.3%; absolute risk reduction, 3.4 percentage points, 95% CI 1.0–5.8)103. Similarly, in non-hospitalized unvaccinated older adults with mild COVID-19 symptoms, a double-blind RCT of convalescent plasma versus placebo administered within 72 hours of symptom onset reduced the progression of COVID-19 (ref. 104). By contrast, two RCTs in adult patients with mild or moderate COVID-19 symptoms for less than 1 week105,106 or failed to demonstrate any benefit from the administration of convalescent plasma. Lack of efficacy of convalescent plasma for adult patients with mild to moderate COVID-19 was further confirmed by a patient-level meta-analysis of 782 patients from two RCTs107. The emergence of new variants of SARS-CoV-2 added further uncertainty to the potential benefit of convalescent plasma therapy. Currently, the use of convalescent plasma collected prior to the Omicron (B1.1.529/BA1 and BA2) surge is not recommended by the FDA108 and has not been considered by the EMA.
Recombinant neutralizing mAbs have been the focus of a large number of studies109. These neutralizing mAbs are developed to target the receptor-binding spike protein of SARS-CoV-2, which mediates viral entry into host cells by binding to angiotensin-converting enzyme 2 (ACE2). Five mAb agents are currently available in the USA under EUA: bamlanivimab plus etesevimab, casirivimab plus imdevimab, sotrovimab, bebtelovimab, and tixagevimab plus cilgavimab (Table 1). One large RCT found that casirivimab plus imdevimab reduced 28-day mortality in hospitalized patients who were seronegative at baseline compared with standard of care (24% versus 30%; RR 0.79, 95% CI 0.69–0.91)110. Furthermore, several RCTs in non-hospitalized adults from high-risk groups with mild or moderate COVID-19 demonstrated that early treatment with recombinant neutralizing mAbs reduced the risk of hospitalization or death, particularly in patients who were seronegative111–113. Recombinant mAbs are highly effective at preventing hospitalization when given early but they are highly susceptible to loss of neutralizing activity as novel virus variants emerge114. Currently, in the USA, only bebtelovimab is recommended in non-hospitalized adult patients with COVID-19 at risk of disease progression when antiviral treatment is unavailable or contraindicated108.
The NIH COVID-19 treatment guideline panel recommends pre-exposure prophylaxis with tixagevimab plus cilgavimab (Evusheld) in moderately to severely immunocompromised adults without active SARS-CoV-2 infection108. These specific recombinant neutralizing mAbs carry a modification of the fragment crystallizable (Fc) region that prolongs their half-life and hence their potential protective effect for up to 6 months. A double-blind RCT conducted in 5,197 patients at a high risk of severe outcomes demonstrated that tixagevimab plus cilgavimab reduced the incidence of COVID-19 infection (0.2%) compared with placebo (1.0%) (relative risk reduction 76.7%; 95% CI 46.0–90.0)115. Of note, tixagevimab plus cilgavimab have neutralizing activity against Omicron sub-variants BA1.1.529 (refs. 114,115).
Overall, based on these pharmacological, epidemiological and clinical trial data, recommendations for the use of convalescent plasma and recombinant neutralizing mAbs for COVID-19 treatment or prevention are necessarily fluid, as they require frequent updates depending on the resistance patterns of prevalent virus variants and the results of ongoing research.
Relevance to patients with kidney disease
Kidney transplant recipients and patients with CKD or kidney failure requiring KRT are at a high risk of progression to severe illness and death from COVID-19 (refs. 2,116) and could theoretically benefit from early treatment with convalescent plasma or recombinant neutralizing mAbs. Convalescent plasma has been examined in these populations and, although several observational studies suggested some benefit117–120, clinical trial data to support the routine use of convalescent plasma in patients with COVID-19 with underlying kidney disease are lacking. Similarly, no high-grade evidence supports the use of convalescent plasma as pre-exposure prophylaxis in these populations.
Of note, small observational studies suggested that early treatment with recombinant neutralizing mAbs might be beneficial in patients with kidney failure requiring KRT or in kidney transplant recipients with mild COVID-19 (refs. 121,122). Additionally, data from several observational studies suggested that kidney transplant recipients might benefit from pre-exposure prophylaxis with tixagevimab plus cilgavimab123,124. Importantly, given that mAbs are metabolized via target-mediated elimination, no dose adjustment is required in patients with kidney impairment (Table 1). However, conclusive data from RCTs conducted in this patient population are lacking and the existing studies were conducted before the emergence of the Omicron variant, which was resistant to most neutralizing mAbs125–127.
Therapies targeting the RAAS
Interest in the effects of SARS-CoV-2 on the host RAAS emerged early in the pandemic128. The RAAS has key roles in the regulation of vascular tone, electrolyte balance, inflammation, thrombosis and response to injury, and might be dysregulated in COVID-19 (ref. 129). Moreover, ACE2, which converts angiotensin II into angiotensin (1–7), is the functional host receptor for SARS-CoV-2 (ref. 130). ACE2 is abundantly expressed on the surface of nasopharyngeal and lung alveolar epithelial cells, and throughout the vascular endothelium; soluble ACE2 is also present in the circulation131. Angiotensin II increases vascular tone, upregulates inflammation, increases capillary permeability and activates clotting and profibrotic responses, whereas angiotensin (1–7) provides counter-regulatory functions to angiotensin II132.
COVID-19 might disrupt ACE2 homeostasis via several mechanisms, and lead to a maladaptive increase in angiotensin II and a reduction of protective angiotensin (1–7). These mechanisms include direct binding to virus particles, which might reduce the bioavailability of ACE2, as well as COVID-19-mediated destruction of alveolar epithelial cells, which are a primary source of ACE2 (ref. 133). These observations led to the hypothesis that attenuating angiotensin II activity and augmenting angiotensin (1–7) might be beneficial in COVID-19. Therapies being evaluated in RCTs include those that inhibit angiotensin II production (ACE inhibitors), inhibit angiotensin II binding to the angiotensin type I receptor (angiotensin II receptor blockers (ARBs)), accelerate conversion of angiotensin II to angiotensin (1–7) (recombinant ACE2) or activate angiotensin (1–7) signalling (investigational drugs, including TRV-027 and TXA-127). Recombinant ACE2 might confer the additional advantage of serving as a decoy for SARS-CoV-2, potentially limiting viral invasion134.
These hypotheses are supported by observations that ACE inhibitors or ARBs mitigate the severity of acute lung injury in experimental models of COVID-19 (ref. 135), as well as models of acute lung injury induced by sepsis, chemical aspiration, SARS-CoV-1 or ventilation136. Observational studies suggested that the use of ACE inhibitors and ARBs is associated with improved outcomes in pneumonia137, influenza138 and sepsis139. Among patients with COVID-19, a meta-analysis of 52 observational studies found that antecedent ACE inhibitor or ARB use was associated with improved survival140. By contrast, an RCT in 659 hypertensive adults hospitalized for mild or moderate COVID-19 who were already taking an ACE inhibitor or an ARB prior to hospitalization found that continuation of ACE inhibitors and ARBs resulted in a similar number of hospital-free days compared with discontinuation141. However, whether initiating RAAS modulators in the setting of acute COVID-19 is beneficial is unclear and is being evaluated in several RCTs. Notably, as with other COVID-19 treatments, the risk–benefit balance might depend on the stage and/or severity of COVID-19, as the potential for these agents to cause or worsen hypotension, hyperkalaemia and/or kidney impairment might increase with greater illness severity. The benefits of these therapies remain uncertain owing to the limited number of published RCTs and their modest sample sizes142,143. Therefore, initiating these treatments in patients with COVID-19 who do not have accepted clinical indications is not recommended outside of RCT settings.
Vitamin supplements
In addition to the COVID-19 therapeutics discussed above, the potential benefits of several vitamin and nutritional supplements — including vitamin D, nicotinamide and vitamin C — have been investigated.
Biologically active vitamin D (1,25-dihydroxyvitamin D (1,25D)) exerts immunomodulatory effects by binding to the cytoplasmic vitamin D receptor (VDR) that is expressed in T, B, and antigen-presenting cells. The 1,25D–VDR complex translocates to the nucleus, where it binds to DNA sequence elements in vitamin D-responsive genes144. Over 200 vitamin D target genes have been identified, including genes that encode antimicrobial peptides (for example, cathelicidin) with broad activity against a range of bacteria and viruses145.
This established biological process, in combination with the epidemiological data linking vitamin D deficiency to adverse health outcomes across multiple clinical settings, generated enthusiasm for a potential therapeutic role for vitamin D long before the COVID-19 pandemic. However, two RCTs found that administration of high-dose vitamin D3 did not reduce hospital length of stay or mortality compared with placebo in non-COVID-19 critically ill patients with vitamin D deficiency146,147. In patients with COVID-19, an RCT conducted in 240 hospitalized adults with moderate to severe illness found that a single dose of vitamin D3 (200,000 IU) had no effect on the length of hospital stay compared with placebo148. Of note, the study had several important limitations, including being underpowered149. The Vitamin D for COVID Trial (VIVID) is an ongoing phase III RCT that will enrol 1,880 outpatients with newly diagnosed COVID-19 to assess whether a 28-day course of vitamin D3 improves outcomes compared with placebo150.
Nicotinamide is a vitamin B3 analogue that attenuates AKI in preclinical models through its effects on mitochondrial oxidation and prostaglandin production151, and also attenuated AKI in a pilot RCT in adults undergoing cardiac surgery152. Several RCTs are currently ongoing to investigate nicotinamide for AKI prevention in various non-COVID-19 settings, including in patients undergoing aortic aneurysm repair153 and those with septic shock154. In patients with COVID-19, data are more limited, although a prospective cohort study conducted in 201 hospitalized adults with COVID-19 found that administration of nicotinamide was associated with a lower risk of KRT or death compared with control individuals (HR 0.64, 95% CI 0.40–1.00)155. Ongoing RCTs of nicotinamide in patients with COVID-19 are investigating several outcomes, including AKI156 and cognitive function157.
Administration of vitamin C (ascorbic acid) has been investigated in several non-COVID-19 clinical settings, including in critically ill patients with sepsis or septic shock, given its antioxidant, anti-inflammatory and free radical scavenging properties. However, a meta-analysis of 11 RCTs concluded that use of high-dose i.v. vitamin C does not improve survival in patients with sepsis158. Furthermore, the 2022 LOVIT trial found that administration of i.v. vitamin C in critically ill patients with sepsis who were receiving vasopressor therapy increased the risk of death or persistent organ dysfunction at 28 days compared with placebo159. In the setting of COVID-19, an open-label 2 × 2 factorial RCT tested the efficacy of a 10-day course of oral vitamin C (8,000 mg) and zinc gluconate (50 mg) in non-hospitalized adults. The study was stopped early for futility after enrolling 214 patients160. Moreover, a pilot trial conducted in China randomly assigned 56 critically ill patients with COVID-19 to receive 24 g vitamin C daily for 7 days or placebo but the study was terminated early and found no effect of vitamin C on mortality or duration of IMV161. In summary, despite the interest in several vitamin supplements for the treatment of COVID-19, data to support their efficacy are currently lacking, although several RCTs are ongoing.
Conclusions
Multiple effective and safe therapeutics for COVID-19 have been developed during the past 2 years, with several agents now having been approved for use across the spectrum of disease severity. The extraordinarily rapid pace of drug development for this disease represents an incredible feat for the global scientific community. In addition to the development of direct antiviral agents and recombinant neutralizing mAbs with activity against SARS-CoV-2, anti-inflammatory, immunomodulatory, and antithrombotic agents that target the host immune response to COVID-19 have also been introduced and have a crucial role in preventing severe illness and death. Indeed, although few direct comparisons have been performed, anti-inflammatory therapies appear to be among the most effective in improving outcomes, at least among hospitalized patients. Efforts to develop effective therapies to treat emerging novel variants of SARS-CoV-2 are also underway. In combination with mass vaccination campaigns, the development of highly efficacious therapies to treat COVID-19 has undoubtedly contributed to the lower rates of hospitalization, critical illness and death that are observed today compared with earlier stages of the pandemic162.
Despite these impressive therapeutic advances for the general population, patients with kidney disease, including CKD or kidney failure, and kidney transplant recipients, are often excluded or under-represented in clinical trials. This patient population is immunocompromised and often has multiple comorbidities, such as hypertension, diabetes mellitus, coronary artery disease and cancer, and is therefore at a high risk of developing severe COVID-19 and death. Accordingly, greater efforts are needed to include these high-risk populations in RCTs of COVID-19 therapeutics. Additionally, more studies are needed to understand the impact of novel and repurposed agents on the prevention and treatment of COVID-19-associated AKI, which is linked to high mortality5. Many of the larger RCTs investigating COVID-19 therapeutics failed to collect or report data on AKI, or only recorded whether patients received KRT (Table 3), which represents a missed opportunity.
Table 3.
Major COVID-19 RCTs that assessed AKI outcomes
| Trial name | No. of patients | Treatment arms | Patient population | Definition of AKI | AKI outcome |
|---|---|---|---|---|---|
| Anti-inflammatory therapies | |||||
| RECOVERY (dexamethasone)11 | 6,425 | Dexamethasone versus usual care | Hospitalized adults | Receipt of KRT | RR 0.61 (95% CI 0.48–0.76) |
| RECOVERY (tocilizumab)19 | 4,116 | Tocilizumab versus usual care | Hospitalized adults | Receipt of KRT | RR 0.72 (95% CI 0.58–0.90) |
| RECOVERY (baricitinib)25 | 8,156 | Baricitinib versus usual care | Hospitalized adults | Receipt of KRT | RR 0.78 (95% CI 0.59–1.03) |
| ACTT-2 (ref. 22) | 1,033 | Baricitinib + RDV versus placebo + RDV | Hospitalized adults | AKI or kidney failurea |
Baricitinib + RDV: 5/507 (1.0%) Placebo + RDV: 16/509 (3.1%) |
| Antiviral therapies | |||||
| ACTT-1 (ref. 32) | 1,048 | RDV versus placebo | Hospitalized adults | GFR decreased, AKI or failurea |
RDV: 14/532 (2.6%) Placebo: 17/516 (3.3%) |
| Antithrombotic therapies | |||||
| INSPIRATION163 | 562 | Intermediate- versus standard-dose anticoagulation | Critically ill adults | Receipt of KRT | OR 1.49; (95% CI 0.58–3.86) |
| RECOVERY (Aspirin)65 | 14,892 | Aspirin versus usual care | Hospitalized adults | Receipt of KRT | RR 0.99 (95% CI 0.84–1.17) |
| Anti-SARS-CoV-2 (neutralizing) antibody therapies | |||||
| CONCOR-1 (ref. 100) | 938 | Convalescent plasma versus standard of care | Hospitalized adults | Receipt of KRT | RR 0.83 (95% CI 0.31–2.27) |
| RECOVERY (casirivimab/imdevimab)110 | 9,785 | Casirivimab/imdevimab versus usual care | Hospitalized adults | Receipt of KRT | RR 1.04 (95% CI 0.86–1.28) |
| Therapies targeting the RAAS | |||||
| BRACE-CORONA141 | 659 | Discontinuing versus continuing ACEi/ARB | Hospitalized adults | Receipt of KRT | RR 2.0 (95% CI 0.80–5.37) |
ACEi, angiotensin-converting enzyme inhibitor; AKI, acute kidney injury; ARB, angiotensin II receptor blocker; GFR, glomerular filtration rate; KRT, kidney replacement therapy; RAAS, renin–angiotensin–aldosterone system; RCT, randomized controlled trial; RDV, remdesivir; RR, relative risk. aDefinitions not available.
Glossary
- Pseudo-randomized study
The use of naturally occurring variation in an exposure to identify its effect on an outcome of interest; also known as a natural experiment.
- Target trial emulation analyses
The application of principles from randomized clinical trials to observational studies.
Author contributions
All authors researched data for the article, made substantial contributions to discussions of the content and wrote, reviewed or edited the manuscript before submission.
Peer review
Peer review information
Nature Reviews Nephrology thanks L. Forni, R. Haynes and W. Karsan for their contribution to the peer review of this work.
Competing interests
H.A.-S. reports no disclosures relevant to the manuscript; universal disclosures include consultancy (Agios, Dova/Sobi, argenx, Rigel, Novartis, Forma, Moderna) and research funding (Agios, Dova/Sobi, Amgen). P.R.L. is an investigator in the REMAP-CAP ACE2 RAS Domain, which is investigating renin–angiotensin–system-modulating treatments for COVID-19, is supported by a Heart and Stroke Foundation of Canada National New Investigator Award, and has received unrelated consulting honoraria from Novartis, CorEvitas, and Brigham and Women’s Hospital (Boston, MA, USA), as well as unrelated royalties from McGraw-Hill Publishing. L.D.S. is inventor of a patent licensed to SQI Diagnostic and has received unrelated research funding from the Canadian Institutes of Health Research. M.E.S. has received research funding from Gilead Sciences awarded to her institution; additional, unrelated disclosures include research funding from AbbVie, Merck, EMD-Serono, Angion and serving as a scientific advisory board member for Travere and Mallinckrodt. The other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
2/6/2023
A Correction to this paper has been published: 10.1038/s41581-023-00686-0
References
- 1.Karim SSA, Karim QA. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet. 2021;398:2126–2128. doi: 10.1016/S0140-6736(21)02758-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flythe JE, et al. Characteristics and outcomes of individuals with pre-existing kidney disease and COVID-19 admitted to intensive care units in the United States. Am. J. Kidney Dis. 2021;77:190–203.e1. doi: 10.1053/j.ajkd.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Massie AB, et al. Quantifying excess deaths among solid organ transplant recipients in the COVID-19 era. Am. J. Transplant. 2022;22:2077–2082. doi: 10.1111/ajt.17036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan L, et al. AKI in hospitalized patients with COVID-19. J. Am. Soc. Nephrol. 2021;32:151–160. doi: 10.1681/ASN.2020050615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta S, et al. AKI treated with renal replacement therapy in critically ill patients with COVID-19. J. Am. Soc. Nephrol. 2021;32:161–176. doi: 10.1681/ASN.2020060897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta P, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta S, et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern. Med. 2020;180:1436–1447. doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Del Valle DM, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020;26:1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Short SAP, et al. d-dimer and death in critically ill patients with coronavirus disease 2019. Crit. Care Med. 2021;49:e500–e511. doi: 10.1097/CCM.0000000000004917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson JG, et al. Cytokine profile in plasma of severe COVID-19 does not differ from ARDS and sepsis. JCI Insight. 2020;5:140289. doi: 10.1172/jci.insight.140289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19. N. Engl. J. Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta S, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern. Med. 2021;181:41–51. doi: 10.1001/jamainternmed.2020.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guaraldi G, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2:e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stone JH, et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N. Engl. J. Med. 2020;383:2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salvarani C, et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern. Med. 2021;181:24–31. doi: 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leaf DE, Gupta S, Wang W. Tocilizumab in covid-19. N. Engl. J. Med. 2021;384:86–87. doi: 10.1056/NEJMc2032911. [DOI] [PubMed] [Google Scholar]
- 18.REMAP-CAP Investigators Interleukin-6 receptor antagonists in critically ill patients with covid-19. N. Engl. J. Med. 2021;384:1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Normand SLT. The RECOVERY platform. N. Engl. J. Med. 2021;384:757–758. doi: 10.1056/NEJMe2025674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA. 2021;326:499–518. doi: 10.1001/jama.2021.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalil AC, et al. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N. Engl. J. Med. 2021;384:795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marconi VC, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir. Med. 2021;9:1407–1418. doi: 10.1016/S2213-2600(21)00331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ely EW, et al. Efficacy and safety of baricitinib plus standard of care for the treatment of critically ill hospitalised adults with COVID-19 on invasive mechanical ventilation or extracorporeal membrane oxygenation: an exploratory, randomised, placebo-controlled trial. Lancet Respir. Med. 2022;10:327–336. doi: 10.1016/S2213-2600(22)00006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.RECOVERY Collaborative Group. Baricitinib in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial and updated meta-analysis. Lancet. 2022;400:359–368. doi: 10.1016/S0140-6736(22)01109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Axfors C, et al. Mortality outcomes with hydroxychloroquine and chloroquine in COVID-19 from an international collaborative meta-analysis of randomized trials. Nat. Commun. 2021;12:2349. doi: 10.1038/s41467-021-22446-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arabi YM, et al. Lopinavir-ritonavir and hydroxychloroquine for critically ill patients with COVID-19: REMAP-CAP randomized controlled trial. Intensive Care Med. 2021;47:867–886. doi: 10.1007/s00134-021-06448-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reis G, et al. Effect of early treatment with ivermectin among patients with Covid-19. N. Engl. J. Med. 2022;386:1721–1731. doi: 10.1056/NEJMoa2115869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheahan TP, et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl Med. 2017;9:eaal3653. doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gottlieb RL, et al. Early remdesivir to prevent progression to severe Covid-19 in outpatients. N. Engl. J. Med. 2022;386:305–315. doi: 10.1056/NEJMoa2116846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beigel JH, et al. Remdesivir for the treatment of Covid-19 — final report. N. Engl. J. Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.WHO Solidarity Trial Consortium. Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO Solidarity randomised trial and updated meta-analyses. Lancet. 2022;399:1941–1953. doi: 10.1016/S0140-6736(22)00519-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Owen DR, et al. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science. 2021;374:1586–1593. doi: 10.1126/science.abl4784. [DOI] [PubMed] [Google Scholar]
- 35.Hammond J, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N. Engl. J. Med. 2022;386:1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheahan TP, et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci. Transl Med. 2020;12:eabb5883. doi: 10.1126/scitranslmed.abb5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jayk Bernal A, et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N. Engl. J. Med. 2022;386:509–520. doi: 10.1056/NEJMoa2116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoover RK, et al. Clinical pharmacokinetics of sulfobutylether-β-cyclodextrin in patients with varying degrees of renal impairment. J. Clin. Pharmacol. 2018;58:814–822. doi: 10.1002/jcph.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Estiverne C, et al. Remdesivir in patients with estimated GFR <30 ml/min per 1.73 m2 or on renal replacement therapy. Kidney Int. Rep. 2021;6:835–838. doi: 10.1016/j.ekir.2020.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thakare S, et al. Safety of remdesivir in patients with acute kidney injury or CKD. Kidney Int. Rep. 2021;6:206–210. doi: 10.1016/j.ekir.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pettit NN, et al. Remdesivir use in the setting of severe renal impairment: a theoretical concern or real risk? Clin. Infect. Dis. 2021;73:e3990–e3995. doi: 10.1093/cid/ciaa1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sukeishi A, et al. Population pharmacokinetic modeling of GS-441524, the active metabolite of remdesivir, in Japanese COVID-19 patients with renal dysfunction. CPT Pharmacomet. Syst. Pharmacol. 2022;11:94–103. doi: 10.1002/psp4.12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seethapathy R, Zhao S, Long JD, Strohbehn IA, Sise ME. A propensity score-matched observational study of remdesivir in patients with COVID-19 and severe kidney disease. Kidney360. 2022;3:269–278. doi: 10.34067/KID.0006152021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT04745351 (2022).
- 45.US Food and Drug Administration. Fact sheet for healthcare providers: emergency use authorization for paxlovid. FDAhttps://www.fda.gov/media/155050/download (2022).
- 46.Salerno DM, et al. Early clinical experience with nirmatrelvir/ritonavir for the treatment of COVID-19 in solid organ transplant recipients. Am. J. Transplant. 2022;22:2083–2088. doi: 10.1111/ajt.17027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swanstrom R, Schinazi RF. Lethal mutagenesis as an antiviral strategy. Science. 2022;375:497–498. doi: 10.1126/science.abn0048. [DOI] [PubMed] [Google Scholar]
- 48.Al-Samkari H, et al. Thrombosis, bleeding, and the observational effect of early therapeutic anticoagulation on survival in critically ill patients with COVID-19. Ann. Intern. Med. 2021;174:622–632. doi: 10.7326/M20-6739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al-Samkari H, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136:489–500. doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iba T, Connors JM, Levy JH. The coagulopathy, endotheliopathy, and vasculitis of COVID-19. Inflamm. Res. 2020;69:1181–1189. doi: 10.1007/s00011-020-01401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goshua G, et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7:e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Al-Samkari H, Song F, Van Cott EM, Kuter DJ, Rosovsky R. Evaluation of the prothrombin fragment 1.2 in patients with coronavirus disease 2019 (COVID-19) Am. J. Hematol. 2020;95:1479–1485. doi: 10.1002/ajh.25962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.ATTACC Investigators, ACTIV-4a Investigators & REMAP-CAP Investigators Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19. N. Engl. J. Med. 2021;385:790–802. doi: 10.1056/NEJMoa2105911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.REMAP-CAP Investigators, ACTIV-4a Investigators & ATTACC Investigators Therapeutic anticoagulation with heparin in critically ill patients with Covid-19. N. Engl. J. Med. 2021;385:777–789. doi: 10.1056/NEJMoa2103417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Al-Samkari H. Finding the optimal thromboprophylaxis dose in patients with COVID-19. JAMA. 2021;325:1613–1615. doi: 10.1001/jama.2021.4295. [DOI] [PubMed] [Google Scholar]
- 56.Cuker A, et al. American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. Blood Adv. 2021;5:872–888. doi: 10.1182/bloodadvances.2020003763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.National Institutes of Health. COVID-19 treatment guidelines: antithrombotic therapy in patients with COVID-19. NIHhttps://www.covid19treatmentguidelines.nih.gov/therapies/antithrombotic-therapy/ (2022).
- 58.American Society of Hematology. ASH clinical practice guidelines on venous thromboembolism. ASHhttps://www.hematology.org/education/clinicians/guidelines-and-quality-care/clinical-practice-guidelines/venous-thromboembolism-guidelines/ash-guidelines-on-use-of-anticoagulation-in-patients-with-covid-19 (2022).
- 59.Spyropoulos AC, et al. Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19: the HEP-COVID randomized clinical trial. JAMA Intern. Med. 2021;181:1612–1620. doi: 10.1001/jamainternmed.2021.6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sholzberg M, et al. Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with covid-19 admitted to hospital: RAPID randomised clinical trial. BMJ. 2021;375:n2400. doi: 10.1136/bmj.n2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lopes RD, et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet. 2021;397:2253–2263. doi: 10.1016/S0140-6736(21)01203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Giannis D, et al. Postdischarge thromboembolic outcomes and mortality of hospitalized patients with COVID-19: the CORE-19 registry. Blood. 2021;137:2838–2847. doi: 10.1182/blood.2020010529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ramacciotti E, et al. Rivaroxaban versus no anticoagulation for post-discharge thromboprophylaxis after hospitalisation for COVID-19 (MICHELLE): an open-label, multicentre, randomised, controlled trial. Lancet. 2022;399:50–59. doi: 10.1016/S0140-6736(21)02392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spyropoulos AC, et al. Post-discharge prophylaxis with rivaroxaban reduces fatal and major thromboembolic events in medically ill patients. J. Am. Coll. Cardiol. 2020;75:3140–3147. doi: 10.1016/j.jacc.2020.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.RECOVERY Collaborative Group. Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2022;399:143–151. doi: 10.1016/S0140-6736(21)01825-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Connors JM, et al. Effect of antithrombotic therapy on clinical outcomes in outpatients with clinically stable symptomatic COVID-19: the ACTIV-4B randomized clinical trial. JAMA. 2021;326:1703–1712. doi: 10.1001/jama.2021.17272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.REMAP-CAP Writing Committee for the REMAP-CAP Investigators Effect of antiplatelet therapy on survival and organ support-free days in critically ill patients with COVID-19: a randomized clinical trial. JAMA. 2022;327:1247–1259. doi: 10.1001/jama.2022.2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT04505774 (2022).
- 69.Kaw D, Malhotra D. Platelet dysfunction and end-stage renal disease. Semin. Dial. 2006;19:317–322. doi: 10.1111/j.1525-139X.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- 70.Wattanakit K, Cushman M, Stehman-Breen C, Heckbert SR, Folsom AR. Chronic kidney disease increases risk for venous thromboembolism. J. Am. Soc. Nephrol. 2008;19:135–140. doi: 10.1681/ASN.2007030308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martel N, Lee J, Wells PS. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood. 2005;106:2710–2715. doi: 10.1182/blood-2005-04-1546. [DOI] [PubMed] [Google Scholar]
- 72.Garcia DA, Baglin TP, Weitz JI, Samama MM. Parenteral anticoagulants: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e24S–e43S. doi: 10.1378/chest.11-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Endres P, et al. Filter clotting with continuous renal replacement therapy in COVID-19. J. Thromb. Thrombolysis. 2021;51:966–970. doi: 10.1007/s11239-020-02301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arnold F, et al. Comparison of different anticoagulation strategies for renal replacement therapy in critically ill patients with COVID-19: a cohort study. BMC Nephrol. 2020;21:486. doi: 10.1186/s12882-020-02150-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seshadri M, Ahamed J, Laurence J. Intervention in COVID-19 linked hypercoagulable states characterized by circuit thrombosis utilizing a direct thrombin inhibitor. Thrombosis Update. 2020;1:100009. doi: 10.1016/j.tru.2020.100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rahbar E, Cotton BA, Wade CE, Cardenas JC. Acquired antithrombin deficiency is a risk factor for venous thromboembolism after major trauma. Thromb. Res. 2021;204:9–12. doi: 10.1016/j.thromres.2021.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Legrand M, et al. Pathophysiology of COVID-19-associated acute kidney injury. Nat. Rev. Nephrol. 2021;17:751–764. doi: 10.1038/s41581-021-00452-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rapkiewicz AV, et al. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: a case series. EClinicalMedicine. 2020;24:100434. doi: 10.1016/j.eclinm.2020.100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hisama N, et al. Anticoagulant pretreatment attenuates production of cytokine-induced neutrophil chemoattractant following ischemia-reperfusion of rat liver. Dig. Dis. Sci. 1996;41:1481–1486. doi: 10.1007/BF02088576. [DOI] [PubMed] [Google Scholar]
- 80.Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020;323:2329–2330. doi: 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- 81.Papoutsi E, Giannakoulis VG, Xourgia E, Routsi C, Kotanidou A. Siempos II. Effect of timing of intubation on clinical outcomes of critically ill patients with COVID-19: a systematic review and meta-analysis of non-randomized cohort studies. Crit. Care. 2021;25:121. doi: 10.1186/s13054-021-03540-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ziehr DR, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am. J. Respir. Crit. Care Med. 2020;201:1560–1564. doi: 10.1164/rccm.202004-1163LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Anesi GL, et al. Characteristics, outcomes, and trends of patients with COVID-19-related critical illness at a learning health system in the United States. Ann. Intern. Med. 2021;174:613–621. doi: 10.7326/M20-5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gaeckle NT, et al. Aerosol generation from the respiratory tract with various modes of oxygen delivery. Am. J. Respir. Crit. Care Med. 2020;202:1115–1124. doi: 10.1164/rccm.202006-2309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Perkins GD, et al. Effect of noninvasive respiratory strategies on intubation or mortality among patients with acute hypoxemic respiratory failure and COVID-19: the RECOVERY-RS randomized clinical trial. JAMA. 2022;327:546–558. doi: 10.1001/jama.2022.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guérin C, et al. Prone positioning in severe acute respiratory distress syndrome. N. Engl. J. Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 87.Mathews KS, et al. Prone positioning and survival in mechanically ventilated patients with coronavirus disease 2019-related respiratory failure. Crit. Care Med. 2021;49:1026–1037. doi: 10.1097/CCM.0000000000004938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ehrmann S, et al. Awake prone positioning for COVID-19 acute hypoxaemic respiratory failure: a randomised, controlled, multinational, open-label meta-trial. Lancet Respir. Med. 2021;9:1387–1395. doi: 10.1016/S2213-2600(21)00356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alhazzani AW, et al. Effect of awake prone positioning on endotracheal intubation in patients with COVID-19 and acute respiratory failure: a randomized clinical trial. JAMA. 2022;327:2104–2113. doi: 10.1001/jama.2022.7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Qian ET, et al. Assessment of awake prone positioning in hospitalized adults with COVID-19: a nonrandomized controlled trial. JAMA Intern. Med. 2022;182:612–621. doi: 10.1001/jamainternmed.2022.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.National Heart, Lung, and Blood Institute PETAL Clinical Trials Network. Moss M, et al. Early neuromuscular blockade in the acute respiratory distress syndrome. N. Engl. J. Med. 2019;380:1997–2008. doi: 10.1056/NEJMoa1901686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shaefi S, et al. Extracorporeal membrane oxygenation in patients with severe respiratory failure from COVID-19. Intensive Care Med. 2021;47:208–221. doi: 10.1007/s00134-020-06331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Urner M, et al. Venovenous extracorporeal membrane oxygenation in patients with acute covid-19 associated respiratory failure: comparative effectiveness study. BMJ. 2022;377:e068723. doi: 10.1136/bmj-2021-068723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Goligher EC, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome and posterior probability of mortality benefit in a post hoc Bayesian analysis of a randomized clinical trial. JAMA. 2018;320:2251–2259. doi: 10.1001/jama.2018.14276. [DOI] [PubMed] [Google Scholar]
- 95.Gannon WD, et al. Association between availability of ECMO and mortality in COVID-19 patients eligible for ECMO: a natural experiment. Am. J. Respir. Crit. Care Med. 2022;205:1354–1357. doi: 10.1164/rccm.202110-2399LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Darmon M, et al. Acute respiratory distress syndrome and risk of AKI among critically ill patients. Clin. J. Am. Soc. Nephrol. 2014;9:1347–1353. doi: 10.2215/CJN.08300813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Teixeira JP, Ambruso S, Griffin BR, Faubel S. Pulmonary consequences of acute kidney injury. Semin. Nephrol. 2019;39:3–16. doi: 10.1016/j.semnephrol.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 98.Pantaleo G, Correia B, Fenwick C, Joo VS, Perez L. Antibodies to combat viral infections: development strategies and progress. Nat. Rev. Drug Discov. 2022;21:676–696. doi: 10.1038/s41573-022-00495-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Janiaud P, et al. Association of convalescent plasma treatment with clinical outcomes in patients with COVID-19: a systematic review and meta-analysis. JAMA. 2021;325:1185–1195. doi: 10.1001/jama.2021.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bégin P, et al. Convalescent plasma for hospitalized patients with COVID-19: an open-label, randomized controlled trial. Nat. Med. 2021;27:2012–2024. doi: 10.1038/s41591-021-01488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.RECOVERY Collaborative Group. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial. Lancet. 2021;397:2049–2059. doi: 10.1016/S0140-6736(21)00897-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Writing Committee for the REMAP-CAP Investigators Effect of convalescent plasma on organ support-free days in critically ill patients with COVID-19: a randomized clinical trial. JAMA. 2021;326:1690–1702. doi: 10.1001/jama.2021.18178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sullivan DJ, et al. Early outpatient treatment for Covid-19 with convalescent plasma. N. Engl. J. Med. 2022;386:1700–1711. doi: 10.1056/NEJMoa2119657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Libster R, et al. Early high-titer plasma therapy to prevent severe Covid-19 in older adults. N. Engl. J. Med. 2021;384:610–618. doi: 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Alemany A, et al. High-titre methylene blue-treated convalescent plasma as an early treatment for outpatients with COVID-19: a randomised, placebo-controlled trial. Lancet Respir. Med. 2022;10:278–288. doi: 10.1016/S2213-2600(21)00545-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Korley FK, et al. Early convalescent plasma for high-risk outpatients with Covid-19. N. Engl. J. Med. 2021;385:1951–1960. doi: 10.1056/NEJMoa2103784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Millat-Martinez P, et al. Prospective individual patient data meta-analysis of two randomized trials on convalescent plasma for COVID-19 outpatients. Nat. Commun. 2022;13:2583. doi: 10.1038/s41467-022-29911-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.National Institutes of Health. Anti-SARS-CoV-2 antibody products summary recommendations: COVID-19 treatment guidelines. NIHhttps://www.covid19treatmentguidelines.nih.gov/therapies/anti-sars-cov-2-antibody-products/summary-recommendations/ (2022).
- 109.Taylor PC, et al. Neutralizing monoclonal antibodies for treatment of COVID-19. Nat. Rev. Immunol. 2021;21:382–393. doi: 10.1038/s41577-021-00542-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.RECOVERY Collaborative Group. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2022;399:665–676. doi: 10.1016/S0140-6736(22)00163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dougan M, et al. A randomized, placebo-controlled clinical trial of bamlanivimab and etesevimab together in high-risk ambulatory patients with COVID-19 and validation of the prognostic value of persistently high viral load. Clin. Infect. Dis. 2021;75:e440–e449. doi: 10.1093/cid/ciab912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gupta A, et al. Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2022;327:1236–1246. doi: 10.1001/jama.2022.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Weinreich DM, et al. REGEN-COV antibody combination and outcomes in outpatients with Covid-19. N. Engl. J. Med. 2021;385:e81. doi: 10.1056/NEJMoa2108163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Takashita E, et al. Efficacy of antibodies and antiviral drugs against Covid-19 Omicron variant. N. Engl. J. Med. 2022;386:995–998. doi: 10.1056/NEJMc2119407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Levin MJ, et al. Intramuscular AZD7442 (tixagevimab-cilgavimab) for prevention of Covid-19. N. Engl. J. Med. 2022;386:2188–2200. doi: 10.1056/NEJMoa2116620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Molnar MZ, et al. Outcomes of critically ill solid organ transplant patients with COVID-19 in the United States. Am. J. Transpl. 2020;20:3061–3071. doi: 10.1111/ajt.16280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cristelli MP, et al. Efficacy of convalescent plasma to treat mild to moderate COVID-19 in kidney transplant patients: a propensity score matching analysis. Transplantation. 2022;106:e92–e94. doi: 10.1097/TP.0000000000003962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kluger MA, et al. Convalescent plasma treatment for early post-kidney transplant acquired COVID-19. Transpl. Infect. Dis. 2021;23:e13685. doi: 10.1111/tid.13685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rodionov RN, et al. Potential benefit of convalescent plasma transfusions in immunocompromised patients with COVID-19. Lancet Microbe. 2021;2:e138. doi: 10.1016/S2666-5247(21)00030-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Thompson MA, et al. Association of convalescent plasma therapy with survival in patients with hematologic cancers and COVID-19. JAMA Oncol. 2021;7:1167–1175. doi: 10.1001/jamaoncol.2021.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Arikawa S, et al. Effectiveness of neutralizing antibody cocktail in hemodialysis patients: a case series of 20 patients treated with or without REGN-COV2. Clin. Exp. Nephrol. 2022;26:476–485. doi: 10.1007/s10157-021-02151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gueguen J, et al. Early administration of anti-SARS-CoV-2 monoclonal antibodies prevents severe Covid-19 in kidney transplant patients. Kidney Int. Rep. 2022;7:1241–1247. doi: 10.1016/j.ekir.2022.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bertrand D, et al. Efficacy of anti-SARS-CoV-2 monoclonal antibody prophylaxis and vaccination on the Omicron variant of COVID-19 in kidney transplant recipients. Kidney Int. 2022;102:440–442. doi: 10.1016/j.kint.2022.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Al Jurdi A, et al. Tixagevimab/cilgavimab pre-exposure prophylaxis is associated with lower breakthrough infection risk in vaccinated solid organ transplant recipients during the omicron wave. Am. J. Transplant. 2022 doi: 10.1111/ajt.17128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.US Food and Drug Administration. Fact sheet for health care providers emergency use authorization (EUA) of bamlanivimab and etesevimab. FDAhttps://www.fda.gov/media/145802/download (2020).
- 126.US Food and Drug Administration. Fact sheet for health care providers emergency use authorization (EUA) of REGEN-COV. FDAhttps://www.fda.gov/media/145611/download (2020).
- 127.US Food and Drug Administration. Fact sheet for healthcare providers emergency use authorization (EUA) of sotrovimab. FDAhttps://www.fda.gov/media/149534/download (2020).
- 128.Vaduganathan M, et al. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N. Engl. J. Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sfera A, et al. Intoxication with endogenous angiotensin II: a COVID-19 hypothesis. Front. Immunol. 2020;11:1472. doi: 10.3389/fimmu.2020.01472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hoffmann M, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hamming I, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jiang F, et al. Angiotensin-converting enzyme 2 and angiotensin 1-7: novel therapeutic targets. Nat. Rev. Cardiol. 2014;11:413–426. doi: 10.1038/nrcardio.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Imai Y, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rysz S, et al. COVID-19 pathophysiology may be driven by an imbalance in the renin-angiotensin-aldosterone system. Nat. Commun. 2021;12:2417. doi: 10.1038/s41467-021-22713-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lawler PR, Derde LPG, Mc Verry B, Russell JA, van de Veerdonk F. The renin-angiotensin system in acute lung injury. Crit. Care Med. 2022;50:1411–1415. doi: 10.1097/CCM.0000000000005567. [DOI] [PubMed] [Google Scholar]
- 137.Caldeira D, Alarcão J, Vaz-Carneiro A, Costa J. Risk of pneumonia associated with use of angiotensin converting enzyme inhibitors and angiotensin receptor blockers: systematic review and meta-analysis. BMJ. 2012;345:e4260. doi: 10.1136/bmj.e4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chung SC, Providencia R, Sofat R. Association between angiotensin blockade and incidence of influenza in the United Kingdom. N. Engl. J. Med. 2020;383:397–400. doi: 10.1056/NEJMc2005396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kim J, et al. Effect of antihypertensive medications on sepsis-related outcomes: a population-based cohort study. Crit. Care Med. 2019;47:e386–e393. doi: 10.1097/CCM.0000000000003654. [DOI] [PubMed] [Google Scholar]
- 140.Baral R, et al. Association between renin-angiotensin-aldosterone system inhibitors and clinical outcomes in patients with COVID-19: a systematic review and meta-analysis. JAMA Netw. Open. 2021;4:e213594. doi: 10.1001/jamanetworkopen.2021.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lopes RD, et al. Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: a randomized clinical trial. JAMA. 2021;325:254–264. doi: 10.1001/jama.2020.25864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Duarte M, et al. Telmisartan for treatment of Covid-19 patients: an open multicenter randomized clinical trial. EClinicalMedicine. 2021;37:100962. doi: 10.1016/j.eclinm.2021.100962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Puskarich MA, et al. A multi-center phase II randomized clinical trial of losartan on symptomatic outpatients with COVID-19. EClinicalMedicine. 2021;37:100957. doi: 10.1016/j.eclinm.2021.100957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ramagopalan SV, et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding: associations with disease and evolution. Genome Res. 2010;20:1352–1360. doi: 10.1101/gr.107920.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu PT, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 146.National Heart, Lung, and Blood Institute PETAL Clinical Trials Network. Ginde AA, et al. Early high-dose vitamin D3 for critically ill, vitamin D-deficient patients. N. Engl. J. Med. 2019;381:2529–2540. doi: 10.1056/NEJMoa1911124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Amrein K, et al. Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: the VITdAL-ICU randomized clinical trial. JAMA. 2014;312:1520–1530. doi: 10.1001/jama.2014.13204. [DOI] [PubMed] [Google Scholar]
- 148.Murai IH, et al. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial. JAMA. 2021;325:1053–1060. doi: 10.1001/jama.2020.26848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Leaf DE, Ginde AA. Vitamin D3 to treat COVID-19: different disease, same answer. JAMA. 2021;325:1047–1048. doi: 10.1001/jama.2020.26850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT04536298 (2022).
- 151.Tran MT, et al. PGC1α drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature. 2016;531:528–532. doi: 10.1038/nature17184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Poyan Mehr A, et al. De novo NAD+ biosynthetic impairment in acute kidney injury in humans. Nat. Med. 2018;24:1351–1359. doi: 10.1038/s41591-018-0138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT04342975 (2022).
- 154.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT04589546 (2021).
- 155.Raines NH, et al. Niacinamide may be associated with improved outcomes in COVID-19-related acute kidney injury: an observational study. Kidney360. 2021;2:33–41. doi: 10.34067/KID.0006452020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT05038488 (2021).
- 157.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT04809974 (2022).
- 158.Sato R, et al. Effect of IV high-dose vitamin c on mortality in patients with sepsis: a systematic review and meta-analysis of randomized controlled trials. Crit. Care Med. 2021;49:2121–2130. doi: 10.1097/CCM.0000000000005263. [DOI] [PubMed] [Google Scholar]
- 159.Lamontagne F, et al. Intravenous vitamin C in adults with sepsis in the intensive care unit. N. Engl. J. Med. 2022;386:2387–2398. doi: 10.1056/NEJMoa2200644. [DOI] [PubMed] [Google Scholar]
- 160.Thomas S, et al. Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory patients with SARS-CoV-2 infection: the COVID A to Z randomized clinical trial. JAMA Netw. Open. 2021;4:e210369. doi: 10.1001/jamanetworkopen.2021.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang J, et al. Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Ann. Intensive Care. 2021;11:5. doi: 10.1186/s13613-020-00792-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Johns Hopkins University & Medicine. Coronavirus Resource Center. JHUhttps://coronavirus.jhu.edu/ (2022).
- 163.INSPIRATION Investigators Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA. 2021;325:1620–1630. doi: 10.1001/jama.2021.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.ACTIV-3/TICO LY-CoV555 Study Group A neutralizing monoclonal antibody for hospitalized patients with Covid-19. N. Engl. J. Med. 2021;384:905–914. doi: 10.1056/NEJMoa2033130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dougan M, et al. Bamlanivimab plus etesevimab in mild or moderate Covid-19. N. Engl. J. Med. 2021;385:1382–1392. doi: 10.1056/NEJMoa2102685. [DOI] [PMC free article] [PubMed] [Google Scholar]