Abstract
Patients with inborn errors of immunity (IEI) in Argentina were encouraged to receive licensed Sputnik, AstraZeneca, Sinopharm, Moderna, and Pfizer vaccines, even though most of the data of humoral and cellular responses combination on available vaccines comes from trials conducted in healthy individuals. We aimed to evaluate the safety and immunogenicity of the different vaccines in IEI patients in Argentina.
The study cohort included adults and pediatric IEI patients (n = 118) and age-matched healthy controls (HC) (n = 37). B cell response was evaluated by measuring IgG anti-spike/receptor binding domain (S/RBD) and anti-nucleocapsid(N) antibodies by ELISA. Neutralization antibodies were also assessed with an alpha-S protein-expressing pseudo-virus assay. The T cell response was analyzed by IFN-γ secretion on S- or N-stimulated PBMC by ELISPOT and the frequency of S-specific circulating T follicular-helper cells (TFH) was evaluated by flow cytometry.
No moderate/severe vaccine-associated adverse events were observed. Anti-S/RBD titers showed significant differences in both pediatric and adult IEI patients versus the age-matched HC cohort (p < 0.05). Neutralizing antibodies were also significantly lower in the patient cohort than in age-matched HC (p < 0.01). Positive S-specific IFN-γ response was observed in 84.5% of IEI patients and 82.1% presented S-specific TFH cells. Moderna vaccines, which were mainly administered in the pediatric population, elicited a stronger humoral response in IEI patients, both in antibody titer and neutralization capacity, but the cellular immune response was similar between vaccine platforms. No difference in humoral response was observed between vaccinated patients with and without previous SARS-CoV-2 infection.
In conclusion, COVID-19 vaccines showed safety in IEI patients and, although immunogenicity was lower than HC, they showed specific anti-S/RBD IgG, neutralizing antibody titers, and T cell-dependent cellular immunity with IFN-γ secreting cells. These findings may guide the recommendation for a vaccination with all the available vaccines in IEI patients to prevent COVID-19 disease.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10875-022-01382-7.
Keywords: SARS-CoV-2, antibody response, T cell response, COVID-19, inborn errors of immunity, vaccination
Introduction
In December 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in the Hubei province of China and wreaked havoc in the world by causing the highly transmissible infectious disease coronavirus disease-2019 (COVID-19) [1]. The disease clinical spectrum is variable, ranging from asymptomatic to severe respiratory distress syndrome [2, 3]. The current death toll reported is 6.47 million people worldwide [4] but is likely underestimated and will probably continue to rise until there is the universal deployment of effective vaccines and therapeutics [5–8].
While data supporting the effectiveness and safety of the newly developed anti-SARS-CoV-2 vaccines is accumulating, initial studies enrolled mostly healthy volunteers. Studying the response to vaccination of patients with inborn errors of immunity (IEI) or immunocompromised is of particular interest for several reasons. On the one hand, the immune response following COVID-19 vaccination may differ in people who are moderately or severely immunocompromised at the time of vaccination. Therefore, analysis of the immune response to vaccines may be critical for guidance recommendations to prevent COVID-19 disease. Then, most reports showed increased morbidity and mortality of COVID-19 in patients with IEI [9–12], and with some IEI, such as those with impaired type I IFN responses, combined immunodeficiencies or immune dysregulation disorders, are predisposed to suffer severe COVID-19 having significantly worsened disease [13–15]. Patients with IEI could therefore benefit from a more “aggressive” immunization effort. On the other hand, IEI patients are characterized by reduced vaccine response, depending on the type of immune disorder [16]. Thus, in IEI patients, the underlying immune abnormality might impair the ability to respond to vaccination and to develop anti-SARS-CoV-2 protective immunity, thus leading to questions regarding the benefit of the vaccination approach.
In view of this, characterizing the immune response of IEI patients following SARS-CoV-2 vaccination is crucial, both for understanding their degree of protection and for formulating an optimal immunization scheme. Moreover, data gathered from analyses of the immune response of IEI patients to the anti-COVID-19 vaccine could be relevant to other patient populations, especially those with secondary and acquired immunodeficiency.
To date, there are limited studies that evaluate humoral and cellular immune response combinations in different vaccine platforms or with pediatric data in IEI patients. In addition, very few studies analyzed the frequency of follicular T helper cells in vaccinated people [17–26]. Hence, more studies about immunogenicity and safety in patients considering other platforms in patients with IEI are needed.
Currently, there are five vaccines licensed for emergency use in Argentina to prevent SARS-CoV-2 infection or severe infection and death related to COVID-19 [27]. Due to the accelerated transmission of COVID-19 and the incidence of the disease in our country and the rest of the world, it is vital to evaluate the effectiveness and impact of the vaccines applied in our territory in patients with IEI. In this work, we describe the safety and immunogenicity of the Gam-COVID-Vac (Sputnik-V), AZD1222 or COVISHIELD (AstraZeneca), BBIBP-CorV (Sinopharm), and mRNA-1273 (Moderna) vaccines in a cohort of IEI patients. Information on vaccine-associated adverse events was collected after each vaccine dose. We evaluated the B cell response by measuring IgG anti-Spike(S)/RBD and anti-nucleocapsid(N) antibodies by ELISA and neutralization antibodies with an alpha-S protein-expressing pseudo-virus assay. The IFN-γ production was evaluated on S or N-stimulated PBMC by ELISPOT, and the frequency of S-specific circulating T follicular-helper cells (TFH) was analyzed by flow cytometry.
Materials and Methods
Study Design and Patients
A total of 132 patients with IEIs (85 adults and 47 pediatrics) were enrolled sequentially between June and November 2021, according to the national vaccine strategy, first adult patients and then pediatric patients. This observational study included patients aged 12–78 years affected by IEI, according to IUIS phenotypic classification criteria [28]. Fourteen patients were excluded from the study because only the pre-vaccination sample was obtained. The final cohort included 118 patients (pre-vaccination sample (T0) n = 77, post first vaccination dose (T1) n = 87, post second vaccination dose (T2) n = 108), 79 adult patients (AP), and 39 pediatric patients (PP). The mean age of our adult patient cohort was 39.3 years (range 19–78 years), with 34 females and 45 males. The mean age of our pediatric patient cohort was 14.8 years (range 12–18 years), with 16 females and 23 males.
Adult (n = 27) and pediatric (n = 12) subjects without compromised immune systems or comorbidities also participated in the study. The blood samples from adults and plasma samples from pediatric subjects were analyzed as healthy control samples. The healthy adult donors were healthcare workers from the “Academia Nacional de Medicina” and healthy controls from the “Biobanco de Enfermedades Infecciosas,” while the healthy pediatric donors were adolescent children of healthcare workers, including with the consent of their parents. The mean age of healthy adult donors was 41.8 years (range 26–82 years) with 12 females and 15 males, and 14.4 years (range 12–17 years) for pediatric donors with 5 females and 7 males.
Blood samples from patients and healthy adult donors were collected prior to vaccination (T0), 28 days (+ / − 3 days) after the first (T1) and the second (T2) doses. Plasma was isolated and PBMCs were obtained by Ficoll-Hypaque density gradient centrifugation (GE) and cryopreserved in liquid nitrogen in fetal bovine serum (FBS, Serendipia). Serums from healthy pediatric samples were collected at T2. Plasma and serum were preserved at – 80 °C.
Safety profiles of the vaccines were recorded in participants who received at least 1 vaccine dose. Adverse events were registered from the first dose to 1 month after the last dose, and serious adverse events from the first dose until 6 months after the last dose. We considered local reactions the following: pain at the injection site, redness, and swelling. Systemic events were fever, fatigue, headache, chills, vomiting, diarrhea, new or worsening muscle pain, and new or worsening joint pain. Local and systemic reactions were classified in severity according to the following criteria: mild, awareness of a symptom and the symptom is easily tolerated; moderated, discomfort enough to cause interference with usual activity; severe, incapacity, unable to perform usual activities; requires absenteeism or bed resting [29].
Patients with COVID-19 were grouped according to severity categories following NIH guidelines [30].
SARS-CoV-2 Antibody ELISA
Quantitative SARS-CoV-2 spike-specific IgG antibodies titers were measured by using the ELISA test COVIDAR (Laboratorio Lemos S.R.L, Buenos Aires, Argentina), as previously described [31]. Assay plates were coated with a mixture of the spike (S) protein and receptor binding domain (RBD). Antibody concentrations of SARS-CoV-2 S protein expressed as international units/mL (IU/mL) were determined by constructing a calibration curve with serial dilutions of the standard included in the immunoassay kit (400 IU/mL, reactive human serum adjusted to WHO First International Standard for human immunoglobulin against SARS-CoV-2, NIBSC Code 20/136, version 2.0 of 12/17/2020). Each sample was properly diluted to fit an OD of 450 nm within the linear range of the calibration curve. Antibody concentrations were obtained by interpolating the OD 450 nm value for each sample into the calibration curve.
Qualitative SARS-CoV-2 nucleocapsid(N)-specific IgG antibodies were measured with a validated in-house ELISA protocol. Briefly, NUNC-Maxisorp® microplates (Thermo Fisher Scientific) were sensitized with 100 µL of SARS-CoV-2 nucleocapsid N recombinant protein (0.5 µg/mL in carbonate buffer pH 9.5) overnight at 4 °C. After blocking with 3% equine serum for 1 h at 37 °C, the plates were incubated with 100 µL of patient serum (1:50) for 60 min at 37 °C, followed by 100 µL of mouse anti-human IgG conjugated to horseradish peroxidase (1:40,000, Merck) for 30 min at 37 °C. Finally, the color development was performed by adding a TMB solution (Thermo Fisher Scientific) for 5–15 min at 37 °C, and the enzymatic reaction was stopped with 2 N sulfuric acid. Optical density (OD) reading was performed at 450 nm with a 630 nm correction, using an ELISA BIORAD microplate reader. The 0.5 OD cut-off value of the assay was obtained using a panel of 200 negative pre-pandemic sera. The sensitivity and specificity were calculated with 85 sera from patients with laboratory-based COVID-19 diagnosis (PCR and positive IgG anti-S/RBD carried out with commercial tests), resulting in 96.5% sensitivity and 96.9% specificity values.
Pseudotyped Lentivirus Neutralization Assay
The neutralization activity of serum was determined by the decrease of GFP expression in infected HEK-293 T [32]. Briefly, SARS-CoV-2 S-pseudotyped lentivirus was produced by co-transfection of HEK-293 T cells with plasmids bearing the S protein, a lentivirus backbone (VRC5602, NIH) and a GFP reporter gene (Addgene plasmid #11,619). We use the same pseudotyped lentivirus batch and the same operator to perform all the experiments on five separate days to avoid differences in patient and control neutralization measurements.
Neutralization assays were performed on HEK-293 T cells transiently transfected 24 h before transduction with ACE2 and TMPRSS2 protease genes. The heat-inactivated serum in samples containing RBD-specific IgG was serially diluted and incubated for 2 h with an equal volume of titrated pseudotyped lentivirus and was then added to HEK-293 T.
Pseudovirus infectivity was scored 48 h later, and images were obtained using an inverted fluorescence microscope (Olympus IX-71) and analyzed with the Micro-Manager Open Source Microscopy Software. Plasma antibody neutralization titers were calculated by a nonlinear regression curve to fit using GraphPad Prism software Inc. (La Jolla, CA, USA). Half maximal inhibitory concentration (IC50) and eighty inhibitory concentration (IC80), corresponding to the serum antibody dilution causing a 50 and 80% reduction of GFP positive cells compared to control “virus only” treated cells, was determined using the same software according to Ferrara and Temperton [33].
ELISpot Assay
Evaluation of IFN-γ secreting cells was detected by using Human IFN-γ ELISPOT Pair (BD Biosciences). The assay was performed as previously published [34]. Briefly, 2.5 × 105 PBMCs, from patients or healthy controls were culture on 96-well plates (MultiScreen IP plates; Millipore) coated with anti-human IFN-γ monoclonal antibody (BD Biosciences) and stimulated with SARS-CoV-2 S and N protein (kindly provided by Dr. J. Caramelo and Dra. A Gamarnik, Leloir Institute, Argentina) (10 µg/mL). Glycerol (0.2%) and PHA (10 µg/mL, Sigma) were used as negative and positive controls, respectively. After 16 h of stimulation, biotinylated anti-human IFN-γ monoclonal antibody, streptavidin-peroxidase, and AEC (3-amino-9-ethylcarbazole) substrate reagent set (BD Biosciences) were used to detect spots.
Scanning of plates was done on an ImmunoSpot reader and quantified with the ImmunoSpot software (Cellular Technology Ltd.). Based on control groups of pre-vaccinated individuals (n = 10) and mildly affected convalescent individuals (n = 10), we set the threshold for positive cellular response at 6 SFU (spot forming units) per 2.5 × 105 PBMCs (data not shown). The response ratio was defined as the number of SFU obtained in the stimulated condition relativized to SFU in the unstimulated condition. Samples were considered for analysis if the negative control was below the positive cellular response threshold and the positive control surpassed 100 SFU per 2.5 × 105 PBMCs [34].
Flow Cytometry Anti-Spike T cell Receptor Staining
PBMCs were thawed in RPMI 2% SFB with 25 U/mL of DNAse I (Roche) medium and washed with 10% SFB DNAse RPMI medium. PMBC, 1 × 106 cells, were stimulated overnight with SARS-CoV-2 S protein (10 µg/mL), medium 0.2% glycerol (negative control), and PHA (10 µg/mL, positive control), as was previously described [35].
PBMCs were stained with a mixture of antibodies at 4 °C for 20 min. The antibodies used were: FITC mouse anti-human CD4, PE mouse anti-human PD1, PerCP-Cy5.5 mouse anti-human CXCR5, and APC mouse anti-human CD154. All antibodies were obtained from BioLegend.
Samples were loaded onto a FACSAria II flow cytometer (BD) after antibody staining and cell fixation. Gating of populations positive for any marker was based on fluorescence minus one (FMO) control of each marker. Data were analyzed using FlowJo v.10.0.
Data Analysis
Statistical significance was determined by nonparametric tests: Mann–Whitney test, column statistics followed by Wilcoxon’s signed rank test, Kruskal–Wallis test, one-way ANOVA followed by Dunn’s multiple comparison test, or Spearman correlation test. In all cases, p < 0.05 was considered statistically significant. All data analyses were performed using GraphPad 9.1.2 Prism Software (San Diego, CA, USA).
Results
Study Design and Patients
The study cohort included 118 patients with IEIs (79 adults and 39 pediatrics). According to IUIS phenotypic classification criteria, 96 out of 118 patients had predominantly antibody deficiencies: 59 common variable immunodeficiencies (CVIDs), 12 specific antibody deficiencies (SADs), 9 selective IgA deficiencies, 8 hypogammaglobulinemia, 2 hyper IgM syndromes (HIGM), and 6 X-linked agammaglobulinemias (XLA). The other 22 out of 118 patients had: 1 auto-inflammatory disorder, 8 combined immunodeficiencies (CIDs), 2 C3 deficiencies, 2 congenital defects of phagocyte, 2 defects in intrinsic and innate immunity, 4 immune dysregulation disorders, and 3 primary immunodeficiencies and Down syndrome. Clinical features of IEI patients are shown in Table 1 and Table S1.
Table 1.
Patients’ characteristics, IEI diagnosis, age, and type of COVID-19 vaccines received
| Population | Sex: male:female | Age (y), mean + / − SD | Diagnosis IUIS, n | Vaccines | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| mRNA | SputnikV | Sinopharm | AstraZeneca | Adeno/Adeno | Adeno/mRNA | ||||||
| Adults (n = 79) | 45:34 | 39.3 ± 16.8 | Predominantly antibody deficiencies | XLA | 4 | 3 | 1 | ||||
| CVID | 49 | 2 | 18 | 4 | 12 | 3 | 10 | ||||
| Hyper IgM syndrome | 2 | 2 | |||||||||
| Hypogammaglobulinemia | 6 | 2 | 1 | 2 | 1 | ||||||
| SAD | 2 | 1 | 1 | ||||||||
| Selective IgA Deficiency | 4 | 1 | 1 | 1 | 1 | ||||||
| Combined immunodeficiency | 4 | 3 | 1 | ||||||||
| Defects in intrinsic and innate immunity | 1 | 1 | |||||||||
| Disease of immune Dysregulation | 2 | 1 | 1 | ||||||||
| Others | 5 | 2 | 1 | 1 | 1 | ||||||
| Pediatrics (n = 39) | 23:16 | 14.8 ± 1.8 | Predominantly antibody deficiencies | XLA | 2 | 2 | |||||
| CVID | 10 | 10 | |||||||||
| Hypogammaglobulinemia | 2 | 2 | |||||||||
| SAD | 10 | 10 | |||||||||
| Selective IgA Deficiency | 5 | 5 | |||||||||
| Combined immunodeficiency | 4 | 4 | |||||||||
| Defects in intrinsic and innate immunity | 1 | 1 | |||||||||
| Disease of immune Dysregulation | 2 | 2 | |||||||||
| Others | 3 | 3 | |||||||||
CVID, common variable immunodeficiency; XLA, X-linked agammaglobulinemia; SAD, specific antibody defect
Participants received the initial vaccine dose between January 2021 and November 2021. Adult patients (AP) received adenoviral vector vaccines (22 AstraZeneca, 27 Sputnik V, and 4 a combination of Sputnik V and AstraZeneca), inactivated vaccines (11 received Sinopharm), mRNA vaccines (4 received Moderna), and combination of different vaccine platforms (10 Sputnik and Moderna; 1 AstraZeneca and Pfizer). In the pediatric patients (PP) cohort, 39 reported vaccinations with mRNA vaccines (37 with Moderna, 1 with Pfizer, and 1 with a combination of Pfizer with Moderna) (Table 1). The median interval between vaccine doses was 59.4 days (range = 21–168 days).
During the study, 76 IEI patients received immunoglobulin-replacement therapy, 32 patients were treated with subcutaneous immunoglobulin, and 44 patients with intravenous immunoglobulins. The immunoglobulin batch preparations used in Argentina during the development of this study were free of anti-SARS-CoV-2 antibodies (data provided by manufacturers).
Symptomatic and PCR-confirmed COVID-19 was observed in 18 out of 118 patients before vaccination (Table 2 and Table S1), whereas 5 out of 118 patients got SARS-CoV-2 infection between the first and the second doses (data not shown).
Table 2.
Patients and clinical status of SARs-CoV-2 infection prior and following COVID-19 vaccination
| Population | Pre-vaccination SARs-CoV-2 infection | Post-vaccination SARs-CoV-2 infection | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | Mild | Moderate | Severe | Died | n (%) | Mild | Moderate | Severe | Died | |
| Adults (n = 79) | 16 (20.3) | 10 | 0 | 6 | 0 | 30 (38.0) | 26 | 2 | 0 | 2 |
| Pediatrics (n = 39) | 2 (5.1) | 2 | 0 | 0 | 0 | 16 (41.0) | 16 | 0 | 0 | 0 |
The healthy control (HC) cohort consists of 27 adults and 12 adolescents. Participants received vaccines between January 2021 and September 2021. Healthy adults (HA) donors received adenoviral vector vaccines (8 with AstraZeneca and 6 with Sputnik V), inactivated vaccines (8 with Sinopharm), and a combination of different vaccine platforms (5 with Sputnik and Moderna). For healthy pediatrics (HP), all donors reported vaccination with mRNA vaccines (12 with Moderna).
Safety Parameters
No moderate or severe adverse events were observed in IEI patients following vaccination. Regarding the PP cohort, only three pediatric patients (P6, P13, and P39) presented erythema, heat, and redness with Moderna at the vaccine application site, or moderated myalgia (Table S1). On the other hand, in the AP cohort, the most commonly reported adverse events were fever, myalgias, and pain at the site of injection. We observed that 9 out of 22 AP vaccinated with two doses of AstraZeneca presented mild systemic and/or local side effects, whereas only 3 out of 27 AP vaccinated with two doses of SputnikV presented mild local side effects. The combination of adenoviral vaccines with mRNA vaccines in AP resulted in 4 out of 11 with mild systemic and/or local side effects, whereas the combination of SputnikV/AstraZeneca presented in 2 out of 4 patients with mild systemic and/or local side effects (Table S1).
Specific Humoral Immune Response
IgG Antibody Responses
To determine the antibody response, we analyzed the presence of serum IgG anti-S/RBD antibodies in samples from IEI patients and HC (79 AP and 27 HA; 39 PP and 12 HP). Seroconversion capacity was analyzed by comparing antibody titers at different times pre- and post-vaccination: T0, T1, and T2. The specific IgG anti-N antibodies were determined at T2 to evaluate infection, and so patients that received Sinopharm (n = 11) were only analyzed in T0 pre-vaccination sample.
The analysis of samples at T0 showed that 12 out of 77 patients analyzed (15.6%) were positive for anti-S/RBD IgG (Table S1, Fig. 1a). According to the available data on COVID-19 compatible symptoms and PCR-confirmed laboratory tests (Table 2 and Table S1), not all IEI patients who had breakthrough COVID infection had antibodies. Only 8 from the 11 samples from patients with COVID-19 history analyzed at T0 were seropositive at baseline. The remaining 4 seropositive patients were asymptomatic or non-laboratory-confirmed diagnosed (Table S2).
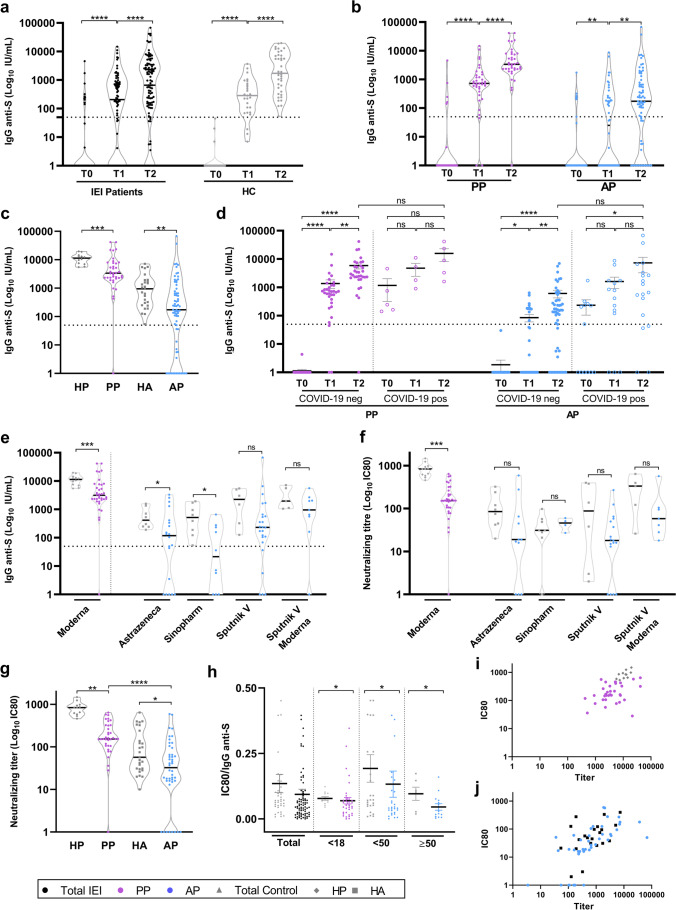
Fig. 1.
Humoral response in patients before and after immunization. a, b Anti-S/RBD IgG levels measured by ELISA at T0 (pre-vaccination), T1 (post first vaccine dose), and T2 (post second vaccine dose) in all IEI and HC (a) or in PP and AP populations (b). c Comparison of anti-S/RBD IgG levels between IEI and HC at T2. d Longitudinal anti-S/RBD IgG levels in IEI patients with or without a history of COVID-19 infection. e Anti-S/RBD IgG levels for the different vaccines in PP, AP, HP, and HA at T2. f Neutralizing titer levels for the different vaccines in PP, AP, HP, and HA at T2. g Comparison of neutralizing titer levels between IEI and HC at T2. h Ratio of neutralizing titer IC80 and IgG anti-S/RBD antibodies for IEI patients and HC according to age. i, j Correlation between neutralizing titer IC80 and anti-S/RBD IgG at T2 for pediatrics (i) and adults (j). AP = adult patients (blue circles), PP = pediatric patients (purple circles), HC = healthy controls, HA = healthy adults’ controls (gray squares), and HP = healthy pediatric controls (gray diamonds). Black circles represent the analysis of the total IEI population (AP + PP). IC80 = eighty inhibitory concentrations corresponding to the serum antibody dilution causing an 80% reduction of GFP positive cells compared to control virus-only treated cells. Dotted line threshold at 50 IU/mL in anti-Spike/RBD antibody titers graphs. Statical analysis was performed using Kruskal–Wallis test followed by Dunn’s multiple comparison test (a, b, and d), Mann–Whitney test (c, e, f, g, and h), and Spearman’s correlation (i and j). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
The analysis of post-vaccination serum samples in IEI patients showed that 65.5% seroconverted at T1 and 80.6% at T2, whereas HC showed 88.9% seroconverted at T1 and 100% at T2 (Fig. 1a).
We investigated the seroconversion rate with age and found significant differences among PP (12–18 years old) and AP (older than 18 years old) but not between two AP groups (18–50 years old and older than 50 years old) (Fig. S1). Regarding variation in antibody response between genders observed in previous works [36], we found no significant differences between males and females (Fig. S2). Based on these findings, we grouped patients as PP (39/118) for pediatrics aged between 12 and 18 years old and AP (79/118) for adults aged between 19 and 78 years old.
Our findings showed that 46.0% of AP were positive at T1 compared to 91.9% of positivity at T1 for PP. Regarding T2, 71.4% of AP with IEI were seroconverted, whereas 97.4% of PP were positive (Fig. 1b). We also compared IEI patients with HC at T2 and we found a significantly higher titer for control samples, both pediatrics and adults, than for IEI patients (p < 0.005 and p < 0.05, respectively) (Fig. 1c).
The absence of seroconversion in IEI patients was found in 2.6% (1/39) of PP and in 28.6% (20/70) of AP. Concerning the former, the patient was vaccinated with Moderna and had XLA. For the latter, 3 had XLA, 13 had CVID, 2 patients had HIGM, 1 had an autoinflammatory syndrome, 1 had CID, and 1 presented selective IgA deficiency. (Table S2) Regarding CVID without seroconversion, 12 out of 13 patients presented less than 200 CD19 + B cells/uL (data not shown). Considering the vaccines received, our findings showed that 6 out of 10 AP vaccinated with 2 doses of Sinopharm did not seroconvert (2 had XLA), whereas antibodies resulted also negative in 5 out of 24 AP vaccinated with Sputnik V, 8 out of 20 AP with AstraZeneca (1 XLA), 1 out of 2 AP with a combination of Sputnik V/AstraZeneca vaccines, 1 out of 9 AP with a combination of adenoviral and mRNA vaccines Sputnik V/Moderna, and none of the 4 that received Moderna vaccines (Fig. 1e and Table S2).
We also considered in the antibody analysis the COVID-19 infection before vaccination by comparing the antibody levels between patients without COVID-19 symptoms reported and negative for anti-S/RBD antibody at baseline with respect to patients who reported symptomatic COVID-19, qPCR positive test (pre- or inter-vaccine doses) and were positive for anti-S/RBD antibodies at T0. We did not observe significant differences in IgG anti-S/RBD titers at T2 between patients with or without COVID-19 history (Fig. 1d), as previously reported [24, 37, 38]. Regarding patients with COVID-19, unlike what was previously reported in HC [39], we observed an increment of anti-S/RBD antibodies after the first and second doses of SARS-CoV2 vaccines. The increase of antibody titers in IEI patients, regardless of previous COVID-19 diagnosis, suggested the importance of subsequent boosts for this group of patients. Those patients that suffered from COVID-19 and did not develop anti-S/RBD specific IgG after vaccination had XLA (A3) or CVID diagnosis (A22, A25, and A33). The latter had a severe COVID-19 (Table S1). These CVID patient presented low CD19 + B cell count (A22: 141 CD19 + B cells/µL; A25: 170 CD19 + B cells/µL; A33: 75 CD19 + B cells/µL). Previous reports showed that patients with low CD19 + B cells have weak anti-S IgG responses after vaccination [18].
We also analyzed N-specific IgG in T2 samples from patients not vaccinated with Sinopharm to estimate the previous COVID-19. We found that 38.8% (38/98) of the samples were positive for anti-N IgG antibodies, and 8 out of the 38 positive sera also had IgG anti-S/RBD antibodies at baseline (Fig. S3a, Table S2).
In addition, we measured anti-N IgG antibodies in pre-vaccination samples from those patients who received Sinopharm. Only 1 sample was positive for anti-N antibodies. He showed neither COVID-19-compatible symptoms nor positive anti-S/RBD IgG (Table S2). This emphasizes that in this population of patients with IEI, with a broad spectrum of antibody responses, a negative antibody response for S/RBD is not indicative of the absence of asymptomatic SARS-CoV-2 infection history, nor is a negative response of antibodies against N. In other words, added to the clinic and PCR of symptomatic patients, for asymptomatic IEI patients, the sum of responses may be closer to the reality of contact with SARS-CoV-2.
We next compared the antibody levels between patients without COVID-19 symptoms reported, negative for anti-S/RBD antibody at baseline and negative for anti-N antibody after two doses of vaccines with respect to patients that were symptomatic, qPCR positive for SARS-CoV-2 (pre- or inter-vaccine doses) and seroconverted (positive prior to vaccination anti-S/RBD antibodies and/or positive for anti-N antibodies after vaccination). We did not find a significant difference in the specific IgG titers in IEI patients exposed and not exposed to the virus (Fig. S3b).
Regarding the type of vaccine, we observed less titers for all the vaccines applied in patients with IEI with respect to the HC group. In particular, we found significant differences in PP vaccinated with Moderna concerning the HP group (p < 0.005, Fig. 1e) and in AP vaccinated with AstraZeneca or Sinopharm with respect to the HA group (p < 0.05, Fig. 1e).
Neutralizing Antibodies
To further assess the functionality of antibodies elicited following vaccination, we evaluated the serum neutralization capacity of a pseudovirus expressing Wuhan RBD to infect ACE2-transfected Vero cells. Serum-containing anti-S/RBD IgG from PP (36) and AP (42) at T2 were analyzed for the IC80. Furthermore, 27 samples from fully vaccinated HA and 12 from fully vaccinated HP were assessed as controls. Our findings showed neutralizing capacity in 81.0% (35/42) of sera from AP. The negative sera were from 5 patients with CVID and 2 with hypogammaglobulinemia (Table S2, Fig. 1g).
Regarding the PP cohort, 35 out of 36 sera (97.2%) had neutralizing capacity; only 1 patient was negative and had a CD25 deficiency. Interestingly, one XLA pediatric patient (P1) was positive for anti-S/RBD IgG and also had neutralizing antibodies (Table S2, Fig. 1g).
Remarkably, the comparison of the IC80 neutralization capacity among IEI patients and HC revealed that control subjects had higher IC80 titers than IEI patients (PP p < 0.01 and AP p < 0.05, Fig. 1g). Furthermore, IC80 was significantly higher in PP than in AP (Fig. 1g).
We next analyzed the neutralizing capacity in vaccinated individuals according to previous COVID-19 status (patients without COVID-19 symptoms, negative for anti-S/RBD antibody at T0 and negative for anti-N antibody at T2 versus patients that were symptomatic, qPCR positive for SARS-CoV-2 and positive for anti-S/RBD antibodies at T0 and/or positive for anti-N antibodies at T2). Vaccinated subjects naïve to COVID-19 showed no significant difference in IC80 compared with COVID-19-positive patients, either in the PP or AP cohorts (Fig. S3c).
Finally, we examined the quality of the specific humoral immune response elicited following vaccination. Our findings showed that IEI patients had a significantly lower ratio than HC (p < 0.05, Fig. 1h), and the neutralizing ability capacity correlated with the antibody titer in both adults and minors (PP p = 0.026; HP p = 0.010; AP p < 0.0001; HA p = 0.045, Fig. 1i and j).
This reduced functional capacity was observed regardless of the vaccine received. Nevertheless, a broader difference was observed in PP administered with Moderna than in HP (p < 0.005, Fig. 1f).
T Cell-Mediated Immune Responses
IFN-γ Cellular Response
To get further insight on cellular immune response that was elicited in IEI patients, we investigated whether vaccines triggered a SARS-CoV-2-specific T cell response. We assessed the IFN-γ secretion by ELISPOT in S or N-stimulated PBMC from 23 PP, 35 AP, and 26 HA at T2 (Fig. 2a). The S-protein stimulation rendered 82.9 (29/35) and 87.0% (20/23) of positivity in AP and PP, respectively. The spot count did not significantly differ between AP and PP cohorts (Fig. 2b). Our findings revealed that 22/29 (75.9%) AP and 19/20 (95.0%) PP had detectable anti-S/RBD specific antibodies after full vaccination (Table S2).
Fig. 2.
IFN-γ production by ELISPOT after the second dose of vaccination. a Images of wells (IFN-γ SFU) of control/medium-, S-, N-, and PHA-stimulated PBMC from IEI patients and HC. b Number of SFU relative to the control of HA, PP, and AP stimulated with S protein at T2. c Number of SFU relative to the control of each IEI patient after stimulation with S protein at T2, stratified by diagnosis. d, Number of SFU relative to the control of each IEI patient after stimulation with S protein at T2, segregated by vaccine type. SFU = spot forming units, CVID = common variable immunodeficiency, CID = combined immunodeficiency, XLA = X-linked agammaglobulinemia, and Ab. def. = antibody deficiency. Each dot represents a different individual analyzed. AP (n = 35), PP (n = 23), and HA (n = 9). SFU = spot forming units and AU = arbitrary units. The dotted line in 6 was set as the threshold. Statistical analysis was performed by using Kruskal–Wallis test, followed by Dunn’s multiple comparison test (b) and Mann–Whitney test (d). P < 0.05, **P < 0.01, *P < 0.001, ***P < 0.0001
Eight out of the 30 AP that were positive for S-stimulated T cell response did not seroconvert after the second vaccination: 5 had CVID, 2 were XLA patients, and 1 had T cell deficiency (Table S2). Likewise, 1 PP with XLA that showed positive S-induced IFN-γ did not seroconvert (Table S2). Overall, 3 patients with XLA did not mount a specific humoral immune response but elicited cellular immunity with the secretion of IFN-γ.
The analysis of the S-induced IFN-γ secretion in HA showed that 25/26 of them were positive. For both, PP and AP with IEI, we observed a lesser IFN-γ response to S-protein with respect to HA but without significant differences. This assessment could not be carried out in HP due to the lack of PBMC samples (Fig. 2b).
Regarding patients with the absence of IFN-γ induction upon COVID-19 vaccination, 5 AP had CVID while the 3 PP had SAD, hypogammaglobulinemia and CD4 T cell lymphopenia, and CVID (Fig. 2c). Two out of these 5 AP (A30 and A34) showed the presence of antibodies at T2, being only 1 (A34) of them positive for neutralization antibodies. About the 3 PP, 100% developed antibodies (Table S2).
Twenty-six samples had detectable cellular responses to N-protein stimulation. Among them, 14 were also positive for anti-N antibodies, and the other 2 negatives for anti-N had reported COVID-19 positive. Overall, ten patients without a history of SARS-CoV-2 infection, anti-S/RBD positive at baseline and/or anti-N antibodies after two doses of vaccination, presented a cellular response to N-protein in T2, three of them with negative anti-N antibodies (close to the cut off value) (Table S2 and Fig. S4).
We did not observe a relationship between IFN-γ-secreting T cells and vaccines. Nevertheless, unlike the humoral response that was stronger in PP than in AP with IEI, the PP vaccinated with mRNA vaccine presented a similar T cell response than AP vaccinated with mRNA vaccines, adenoviral vector vaccines, or inactivated vaccines (Fig. 2d and Table S2).
We next compared the IFN-γ secretion considering COVID-19 infection (patients without COVID-19 symptoms reported, negative for anti-S/RBD antibody at T0 and negative for anti-N antibody at T2 with respect to patients that were symptomatic, qPCR positive for SARS-CoV-2 and positive for anti-S/RBD antibodies at T0 and/or positive for anti-N antibodies at T2). Although a trend was observed for a higher IFN-γ secretion in COVID-19-positive patients, only AP showed a significant increase (Fig. S3d).
In sum, only 4 AP (A7, A11, A12, and A44) did not develop a humoral immune response nor cellular immune response against COVID-19 vaccines, and 1 AP (A30) did not develop a cellular response nor neutralization antibody, all of them having CVID and vaccinated with adenoviral vaccines (Table S2).
Circulating T Follicular Helper Cells (TFH)
We finally assessed the frequency of TFH cells at T0 and T2 based on previous reports identifying the circulating TFH cells as representative of the germinal center with a critical role in T-dependent B cell maturation and antibody production [40, 41]. The gating strategy used to identify the PD-1+CXCR5+CD4+ circulating TFH cells is shown in Fig. 3a.
Fig. 3.
Circulating total and specific TFH cells in IEI patients. a Gating strategy used to identify TFH cells by multiparametric flow cytometry. b Frequency of total circulating CD4 + CXCR5 + PD1 + TFH cells at T0 and T2 for PP and AP. c Frequency of total circulating CD4 + CXCR5 + PD1 + TFH cells at T2 in HC and IEI patients. d Spike specific CD4 + CXCR5 + PD1 + CD154 + TFH cells at T0 and T2 for PP and AP after 24 h of stimulation with S protein. e, f Spike-specific CD4 + CXCR5 + PD1 + CD154 + TFH-cells relative to control at T0 and T2 for AP and PP (e) and segregated for vaccine type (f). AP (n = 29), PP (n = 27), and HA (n = 15). Statistical analysis was performed by using Wilcoxon signed rank test (b, e, and f), Mann–Whitney test (c), and Friedman’s test (d). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
The frequency of total circulating TFH cells analysis showed no significant differences between pre-vaccination and post-vaccination samples from IEI patients (Fig. 3b). As shown by others, we found that patients with IEI had a significantly reduced frequency of total circulating TFH cells compared to HC [42–44]. Our findings showed an 8.9% (range 3.2–18.1%) vs 3.1% (range 0.7–7.5%) frequency of total TFH cells for HC and IEI patients, respectively. (p < 0.0001) (Fig. 3c).
To further examine the virus-specific TFH cells, PBMCs from 29 AP and 27 PP were stimulated with the S protein or PHA as a positive control. The S-specific TFH cell population was analyzed according to the cell surface expression of CD154 (Fig. 3a and 3d). We only observed a significantly higher expression of CD154 in S-stimulated TFH cells with respect to the control condition in T2 and not in T0 (Fig. 3d). We found that 82.8% (24/29) of the AP and 81.5% (22/27) of the PP with IEI presented an increase of cell frequency at T2 (p < 0.0001, Fig. 3e).
Regarding vaccines, we observed that Moderna and AstraZeneca triggered a significant increase in the frequency of TFH. However, 2 out of the 3 AP vaccinated with Sputnik/Moderna combination were older than 50 years and had lesser vaccine response than children or younger adults (Fig. 3f).
The analysis of the impact of COVID-19 infection on circulating TFH frequency showed that the previous infection increased this S-specific cell population (p < 0.0001) (Fig. S3e).
The analysis of the TFH frequency in terms of IEI showed that from the 5 AP patients without higher expression of S-induced CD154 expression at T2, 2 had CVID (A5 and A31), 1 had HIGM (A54) and 2 had XLA (A3 and A4). Regarding PP without specific THF response, 1 had XLA (P2), 1 had SAD (P19), 1 had Selective IgA Deficiency (P25), 1 had CID (P33), and 1 had CD4 T cell lymphopenia and Down syndrome (P38) (Table S2 and Fig. S5).
We did not find a correlation between circulating S-specific TFH cells and antibody titer or neutralization IC80 (data not shown).
Post-Vaccination COVID-19 Disease
After the complete vaccine scheme with two doses almost all patients who presented symptomatic SARS-CoV-2 infection (30/118) had a mild or moderate presentation of COVID-19 without needing to be admitted to intensive care (Table 2 and Table S1). However, A4 and A11 presented critical COVID-19 disease and died (44 and 49 years old respectively, both males). One of them, A4, was a XLA patient without a specific humoral immune response and with chronic lung and cardiovascular disease as comorbidities. The other, A11, was a CVID patient without S-specific IFN-γ T cell response and antibody response and with chronic lung disease and bronchiectasis as comorbidities.
Discussion
Vaccines are the most effective intervention to combat and prevent contagious diseases and reduce mortality rates. The massive vaccination against SARS-CoV-2, with no precedents in the history of vaccinology, has been successful in controlling the most severe consequences of the pandemic and preventing severe illness and death. Vaccine safety and immunogenicity in patients with IEI, in whom adaptive immune responses triggered by vaccines are often restricted, has been a critical point of interest. Most of the studies have been carried out in healthy volunteers, whereas data regarding a combination of humoral and cellular response with different COVID-19 vaccine platforms in defined patient populations, such as IEI patients, are still being gathered [21–26].
In this study, we evaluated the safety, reactogenicity, and immunogenicity of different vaccines used in Argentina against COVID-19 in a cohort of patients aged between 12 and 78 years diagnosed with IEI, compared to HC. We evaluated the humoral and cellular immune response to different vaccine platforms (mRNA-, adenoviral-, and inactivated virus-based vaccines and the combination of adenoviral and mRNA vaccines) employed in homologous and heterologous schemes. No moderate or severe adverse events were observed in IEI patients following vaccination; only mild systemic and/or local reactions. As shown by others, severity does not appear to be increased in these patients.
Our data shows that vaccines in patients with IEI were able to induce a humoral response, which means specific IgG antibodies with neutralizing capacity; cellular response, with increased frequency of circulating specific TFH cells and IFN-γ-secreting cells; or both. In fact, anti-S/RBD IgG was detected in 97.4 (37/38) and 72.9% (51/70) of IEI pediatric and adult samples, respectively, at T2, whereas seroconversion was 100% in our HC cohort. A low proportion of patients showed seroconversion before vaccination, which means that these patients were exposed to the virus prior to vaccination. Our findings showed that the combination of immunity provided by infection and vaccines did not enhance the production of specific antibodies compared to vaccinated individuals, as reported by others in IEI patients [24] and healthy subjects [39].
We observed discrete differences in the antibody titer following the first and second doses between patients without SARS-CoV-2 exposure concerning patients with a previous history of COVID-19. As previously reported, we did not observe significant differences in IgG anti-S/RBD titers at T2 between patients with or without COVID-19 history. [24, 37, 38] Regardless of SARs-CoV-2 exposure, the antibody titers significantly increased overtime, remarking the importance of subsequent boosts for this group of patients. Furthermore, the IgG median titer was higher in age-matched healthy subjects than in PP (p < 0.005) and AP (p < 0.01).
While studies focused on seroconversion showed that 67–85% of IEI patients developed detectable anti-S IgG antibodies [18, 45, 46], other studies showed that neutralizing antibodies were detected at lower levels than in healthy controls [20, 47]. In our study, neutralizing antibodies were detected in 70 out of 78 patients with antibody production at T2, and it was significantly higher in pediatric than in adult IEI patients (p < 0.001) but lower than in age-matched HC (p < 0.01 and p < 0.05, respectively).
In addition, we observed significant differences in antibody response with the mRNA vaccine Moderna in both antibody titer and neutralization capacity (p < 0.01 and p < 0.005, respectively), compared with other vaccine platforms, as observed by others [46]. Furthermore, the frequency of specific TFH cells was higher in patients vaccinated with mRNA vaccines than in those that received the other vaccines. Nevertheless, no difference in INF-γ secreting cell response was observed when the different vaccines were compared. The mRNA vaccines were applied mostly in the pediatric population in Argentina. This strategy could explain why PP presented significant differences in humoral response compared with AP, with no differences in INF-γ secreting cell response.
Almost all patients with a diagnosis of XLA, due to a defect in BTK, evaluated in this study did not develop antibody responses against SARS-CoV-2 vaccines. Nevertheless, one pediatric patient (P3) presented a functional antibody response at T2, likely due to the variable expressivity of their BTK mutation [45].
Excluding XLA patients from our analysis of humoral response, 74.6% of AP and 100% of PP showed seroconversion after two vaccination doses. Among them, 83.3% of AP and 97.1% of PP were able to neutralize the SARS-CoV2 infection. Being TFH cells critical to induce high affinity neutralizing antibodies, we found that 82.1% in total IEI patients and 87.5% excluding XLA patients, presented S-specific circulating TFH cells.
Positive S-specific IFN-γ response was observed in 84.5% of IEI patients. Remarkably, all XLA patients evaluated developed specific IFN-γ-dependent cellular immune responses, which highlights that vaccination in this population, despite not generating antibody protection, may provide cellular protection. Other authors have also described that the T cell compartment is normal in these patients, characterized by the absence or very low frequency of peripheral mature B cells [48, 49].
Regarding prior COVID-19 infections and cellular response, although a trend was observed for a higher IFN-γ secretion in COVID-19-positive patients, only AP showed a significant increase.
Five patients (A7, A11, A12, A30, and A44) failed to develop a functional humoral response or cellular response, all of them adults with CVID diagnosis and vaccinated with adenoviral vaccines. Patient A30 was the only AP treated with methotrexate. The previous report showed that methotrexate reduces the immunogenicity of SARS-CoV-2 vaccination and recommended pausing the treatment for at least 10 days after vaccination [46].
Although the data collected in this and other studies on vaccination against SARS-CoV2 in patients with IEI, the long-term immunogenicity of both humoral and cellular responses remains to be studied, while there are in the literature some studies comparing third vaccine doses between adenoviral vector and mRNA vaccines [50]. Importantly, in our study, we did not find significant differences with respect to healthy controls in the post-vaccination cellular response, so all the vaccines evaluated were equally immunogenic for patients with IEI. While other studies demonstrated comparable cellular immune responses among IEI patients and healthy controls [20, 47], others reported a significantly lower magnitude of their T cell response [23, 26].
Altogether, our results support that COVID-19 vaccines have a favorable safety, immunogenicity, and efficacy profiles in pediatric and adult IEI patients, although with lower immunogenicity than in control subjects. In conclusion, COVID-19 continues to represent a risk for developing severe forms of the disease in immunocompromised patients, and vaccines against SARS-COV-2 proved to be an effective tool to induce a protective immune response, emphasizing the importance of vaccination. Our findings may guide the recommendation for COVID-19 vaccination with the different platforms in IEI patients to prevent COVID-19 disease and the need for subsequent boosts.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We acknowledge the support of the following agencies and organizations: National Council for Scientific and Technical Research (CONICET), National Agency for Scientific and Technological Promotion (ANPCyT), TAKEDA, Fundación IBYME, and Fundación de Ciencias Exactas y Naturales (FUNDACEN). We also acknowledge Manuel de la Mata and Federico Fuchs for the borrowed equipment, Julio Caramelo and Andrea Gamarnik for providing us with S and N proteins, the members of the Laboratorio de Agrobiotecnología for the use of equipment, Florencia Pignataro and other members of the Argentinian AntiCovid Consortium from the IB3 for producing coronavirus proteins and having made them available during our set-up of the trials for this work, to Federico Fuchs for the critical reading of this manuscript and to the Secretaries from “Centro de Inmunología Clínica” for all patients and their families and collected the informed consents, and to the technicians of Flow Cytometry Guillermo Piazza and María Eva Danti. Finally, thanks to all the patients, families, and donors for participating in this study.
Author Contribution
LE, AC, and MBA did most of the experiments and created the figures; JBF, JB, and MB contributed to the purification of patient samples, anti-S/RBD measurement by ELISA, IFN-gamma determination by ELISPOT, and Flow Cytometry experiments from patient samples; SV, MIPM, and II participated in the neutralization assays; ChC contributed with patient samples purification; MNB, PB, and MFQ contributed in ELISA and ELISPOT experiments in healthy donors; LT, MR, RC, GR, MF, and GD collaborated with anti-S/RBD and anti-N experiments by ELISA measured in patient samples; RP, IU, MVP, GS, IM, GV, ALL, DR, LRFM, MNB, PB, MFQ, and LB recruited patients or healthy donors and performed the clinical analysis; IU, LB, and MBA designed and supervised the research study; MBA, GD, MB, AC, IU, RP, and LB analyzed the data; MBA, LE, AC, MB, and GD wrote the paper. All authors contributed to the article and approved the submission.
Funding
This work was supported by grants and fellowships from the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), and TAKEDA.
Data Availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Declarations
Ethics Approval
The protocol was approved by the Ethics and Research Committees of the “Hospital de Agudos C. G. Durand,” “Hospital Interzonal Especializado Materno Infantil Victorio Tetamanti,” “Hospital Interzonal General de Agudos Dr. Oscar Alende,” and “Programa Interdisciplinario de Bioética de la Universidad Nacional de Mar del Plata,” according to the ethical standards of each institutional research committee and with the 1964 Helsinki declaration.
Consent to Participate
Participants, or their parents, signed informed consent.
Competing Interests
The authors declare no competing interests.
Footnotes
Lorenzo Erra and Ignacio L. Uriarte are co-first authors.
Liliana Bezrodnik and María Belén Almejun are co-last authors.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lorenzo Erra, Ignacio L. Uriarte, Liliana Bezrodnik, and María Belén Almejun equally contributed to this study.
References
- 1.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762–8. 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed]
- 2.Zhou P, Lou Yang X, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–3. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization (WHO). WHO coronavirus (COVID-19) dashboard [Internet]. Available from: https://covid19.who.int/. Accessed 20 Jun 2022.
- 5.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shields AM, Burns SO, Savic S, Richter AG, Anantharachagan A, Arumugakani G, et al. COVID-19 in patients with primary and secondary immunodeficiency: the United Kingdom experience. J Allergy Clin Immunol. 2021;147(3):870–875.e1. doi: 10.1016/j.jaci.2020.12.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goudouris ES, Pinto-Mariz F, Mendonça LO, Aranda CS, Guimarães RR, Kokron C, et al. Outcome of SARS-CoV-2 infection in 121 patients with inborn errors of immunity: a cross-sectional study. J Clin Immunol. 2021;41(7):1479–1489. doi: 10.1007/s10875-021-01066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castano-Jaramillo LM, Yamazaki-Nakashimada MA, O’Farrill-Romanillos PM, Muzquiz Zermeño D, Scheffler Mendoza SC, Venegas Montoya E, et al. COVID-19 in the context of inborn errors of immunity: a case series of 31 patients from Mexico. J Clin Immunol. 2021;41(7):1463–1478. doi: 10.1007/s10875-021-01077-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho en H, Mathew S, Peluso MJ, Cunningham-Rundles C. Clinical outcomes and features of COVID-19 in patients with primary immunodeficiencies in New York City. J Allergy Clin Immunol Pract. 2021;9(1):490–493. doi: 10.1016/j.jaip.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez L, Sang PC, Tian Y, Sang Y. Dysregulated interferon response underlying severe COVID-19. Viruses. 2020;12(12):1433. 10.3390/v12121433. [DOI] [PMC free article] [PubMed]
- 14.Meyts I, Bucciol G, Quinti I, Neven B, Fischer A, Seoane E, et al. Coronavirus disease 2019 in patients with inborn errors of immunity: an international study. J Allergy Clin Immunol. 2021;147(2):520–531. doi: 10.1016/j.jaci.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mogensen TH. Human genetics of SARS-CoV-2 infection and critical COVID-19. Clin Microbiol Infect. 2022. 10.1016/j.cmi.2022.02.022. [DOI] [PMC free article] [PubMed]
- 16.Sobh A, Bonilla FA. Vaccination in primary immunodeficiency disorders. J Allergy Clin Immunol Pract. 2016;4(6):1066–1075. doi: 10.1016/j.jaip.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Hagin D, Freund T, Navon M, Halperin T, Adir D, Marom R, et al. Immunogenicity of Pfizer-BioNTech COVID-19 vaccine in patients with inborn errors of immunity. J Allergy Clin Immunol. 2021;148(3):739–749. doi: 10.1016/j.jaci.2021.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delmonte OM, Bergerson JRE, Burbelo PD, Durkee-Shock JR, Dobbs K, Bosticardo M, et al. Antibody responses to the SARS-CoV-2 vaccine in individuals with various inborn errors of immunity. J Allergy Clin Immunol. 2021;148(5):1192–1197. doi: 10.1016/j.jaci.2021.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amodio D, Ruggiero A, Sgrulletti M, Pighi C, Cotugno N, Medri C, et al. Humoral and cellular response following vaccination with the BNT162b2 mRNA COVID-19 vaccine in patients affected by primary immunodeficiencies. Front Immunol. 2021;12(October):1–13. doi: 10.3389/fimmu.2021.727850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Leeuwen LPM, GeurtsvanKessel CH, Ellerbroek PM, de Bree GJ, Potjewijd J, Rutgers A, et al. Immunogenicity of the mRNA-1273 COVID-19 vaccine in adult patients with inborn errors of immunity. J Allergy Clin Immunol. 2022;149(6):1949–1957. doi: 10.1016/j.jaci.2022.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drzymalla E, Green RF, Knuth M, Khoury MJ, Dotson WD, Gundlapalli A. COVID-19-related health outcomes in people with primary immunodeficiency: a systematic review. Clin Immunol. 2022;243:109097. 10.1016/j.clim.2022.109097. [DOI] [PMC free article] [PubMed]
- 22.Gao Y, Cai C, Wullimann D, Niessl J, Rivera-Ballesteros O, Chen P, et al. Immunodeficiency syndromes differentially impact the functional profile of SARS-CoV-2-specific T cells elicited by mRNA vaccination. Immunity. 2022;55(9):1732–46.e5. 10.1016/j.immuni.2022.07.005. [DOI] [PMC free article] [PubMed]
- 23.Gupta S, Agrawal S, Sandoval A, Su H, Tran M, Demirdag Y. SARS-CoV-2-specific and functional cytotoxic CD8 cells in primary antibody deficiency: natural infection and response to vaccine. J Clin Immunol. 2022;42(5):914–22. 10.1007/s10875-022-01256-y. [DOI] [PMC free article] [PubMed]
- 24.Salinas AF, Mortari EP, Terreri S, Quintarelli C, Pulvirenti F, Di Cecca S, et al. SARS-CoV-2 vaccine induced atypical immune responses in antibody defects: everybody does their best. J Clin Immunol. 2021;41(8):1709–1722. doi: 10.1007/s10875-021-01133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quinti I, Locatelli F, Carsetti R. The immune response to SARS-CoV-2 vaccination: insights learned from adult patients with common variable immune deficiency. Front Immunol. 2022;12(January):1–7. doi: 10.3389/fimmu.2021.815404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arroyo-Sánchez D, Cabrera-Marante O, Laguna-Goya R, Almendro-Vázquez P, Carretero O, Gil-Etayo FJ, et al. Immunogenicity of anti-SARS-CoV-2 vaccines in common variable immunodeficiency. J Clin Immunol. 2022;42(2):240–252. doi: 10.1007/s10875-021-01174-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ministerio de Salud. SARS-CoV-2 vaccines approved in Argentina [Internet]. 2021. Available from: https://www.argentina.gob.ar/anmat/covid-19-acciones/vacunas. Accessed 23 Apr 2021.
- 28.Boufisha A, Jeddane L, Picard C, Al-Herz W, Ailal F, Chatila T, et al. Human inborn errors of immunity: 2019 update of the IUIS phenotypical classification. J Clin Immunol. 2020;40(1):66–81. doi: 10.1007/s10875-020-00758-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization, Mort M, Baleta A, Destefano F, Nsubuga JG, et al. Vaccine safety basics : learning manual. World Health Organization; 2013. https://apps.who.int/iris/handle/10665/340576.
- 30.COVID-19 treatment guidelines panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available from: https://www.covid19treatmentguidelines.nih.gov/. Accessed 1 Sept 2022. [PubMed]
- 31.Ojeda DS, Lopez Ledesma MMG, Pallarés HM, Costa Navarro GS, Sanchez L, Perazzi B, et al. Emergency response for evaluating SARS-CoV-2 immune status, seroprevalence and convalescent plasma in Argentina. Plos Pathog. 2021;17(1):1–18. doi: 10.1371/journal.ppat.1009161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Argentinian AntiCovid Consortium Covalent coupling of Spike’s receptor binding domain to a multimeric carrier produces a high immune response against SARS-CoV-2. Sci Rep. 2022;12(1):692. doi: 10.1038/s41598-021-03675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferrara F, Temperton N. Pseudotype neutralization assays: from laboratory bench to data analysis. Methods Protoc. 2018;1(1):8. 10.3390/mps1010008. [DOI] [PMC free article] [PubMed]
- 34.Giannone D, Vecchione MB, Czernikier A, Polo ML, Gonzalez Polo V, Cruces L, et al. SARS-CoV-2 humoral and cellular immune responses in COVID-19 convalescent individuals with HIV. J Infect. 2022;85(3):334–63. 10.1016/j.jinf.2022.05.026. [DOI] [PMC free article] [PubMed]
- 35.Zhang J, Wu Q, Liu Z, Wang Q, Wu J, Hu Y, et al. Spike-specific circulating T follicular helper cell and cross-neutralizing antibody responses in COVID-19-convalescent individuals. Nat Microbiol. 2021;6(1):51–58. doi: 10.1038/s41564-020-00824-5. [DOI] [PubMed] [Google Scholar]
- 36.Badano MN, Sabbione F, Keitelman I, Pereson M, Aloisi N, Colado A, et al. Humoral response to the BBIBP-CorV vaccine over time in healthcare workers with or without exposure to SARS-CoV-2. Mol Immunol. 2021;2022(143):94–99. doi: 10.1016/j.molimm.2022.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lo Sasso B, Giglio RV, Vidali M, Scazzone C, Bivona G, Gambino CM, et al. Evaluation of anti-SARS-CoV-2 s-RBD IgG antibodies after COVID-19 mRNA BNT162b2 vaccine. Diagnostics. 2021;11(7):1–9. doi: 10.3390/diagnostics11071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Narowski TM, Raphel K, Adams LE, Huang J, Vielot NA, Jadi R, et al. SARS-CoV-2 mRNA vaccine induces robust specific and cross-reactive IgG and unequal neutralizing antibodies in naive and previously infected people. Cell Rep. 2022;38(5):110336. doi: 10.1016/j.celrep.2022.110336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rossi AH, Ojeda DS, Varese A, Sanchez L, Gonzalez Lopez Ledesma MM, Mazzitelli I, et al. Sputnik V vaccine elicits seroconversion and neutralizing capacity to SARS-CoV-2 after a single dose. Cell Rep Med. 2021;2(8):100359. 10.1016/j.xcrm.2021.100359. [DOI] [PMC free article] [PubMed]
- 40.Schmitt N, Bentebibel S-E, Ueno H. Phenotype and functions of memory Tfh cells in human blood Tfh cells in lymphoid organs and in the blood. Trends Immunol. 2014;35(9):436–442. doi: 10.1016/j.it.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bentebibel SE, Khurana S, Schmitt N, Kurup P, Mueller C, Obermoser G, et al. ICOS + PD-1 + CXCR3 + T follicular helper cells contribute to the generation of high-avidity antibodies following influenza vaccination. Sci Rep. 2016;6:1–8. doi: 10.1038/srep26494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gerosa J, Lougaris V, Baronio M, Plebani A, Cicalese MP, Fousteri G. Beta2 integrins are required for follicular helper T cell differentiation in humans. Clin Immunol. 2017;1(180):60–62. doi: 10.1016/j.clim.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 43.Cicalese MP, Gerosa J, Baronio M, Montin D, Licciardi F, Soresina A, et al. Circulating follicular helper and follicular regulatory T cells are severely compromised in human CD40 deficiency: a case report. Front Immunol. 2018;9:1761. 10.3389/fimmu.2018.01761. [DOI] [PMC free article] [PubMed]
- 44.De Leo P, Gazzurelli L, Baronio M, Montin D, Di Cesare S, Giancotta C, et al. NFKB2 regulates human Tfh and Tfr pool formation and germinal center potential. Clin Immunol. 2020;210:108309. doi: 10.1016/j.clim.2019.108309. [DOI] [PubMed] [Google Scholar]
- 45.Bergman P, Blennow O, Hansson L, Mielke S, Nowak P, Chen P, et al. COVAXID-collaborator group (shown separately). Safety and efficacy of the mRNA BNT162b2 vaccine against SARS-CoV-2 in five groups of immunocompromised patients and healthy controls in a prospective open-label clinical trial. EBioMedicine. 2021;74:103705. 10.1016/j.ebiom.2021.103705. [DOI] [PMC free article] [PubMed]
- 46.Ponsford MJ, Evans K, Carne EM, Jolles S, Bramhall K, Grant L, et al. COVID-19 vaccine uptake and efficacy in a national immunodeficiency cohort. J Clin Immunol. 2022;42(4):728–731. doi: 10.1007/s10875-022-01223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shields AM, Faustini SE, Hill HJ, Al-Taei S, Tanner C, Ashford F, et al. COV-AD consortium. SARS-CoV-2 vaccine responses in individuals with antibody deficiency: findings from the COV-AD study. J Clin Immunol. 2022;42(5):923–34. 10.1007/s10875-022-01231-7. [DOI] [PMC free article] [PubMed]
- 48.Timmers E, de Weers M, Alt FW, Hendriks RW, Schuurman RKB. X-linked agammaglobulinemia. Clin Immunol Immunopathol. 1991;61(2):S83–93. doi: 10.1016/S0090-1229(05)80042-X. [DOI] [PubMed] [Google Scholar]
- 49.Oshiro TM, da Silva LT, Ortega MM, Perazzio SF, da Duarte AJ S, Carneiro-Sampaio M. Patient with agammaglobulinemia produces anti-SARS-CoV-2 reactive T-cells after CoronaVac vaccine. Clinics. 2021;2022(77):100007. doi: 10.1016/j.clinsp.2022.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shields AM, Faustini SE, Hill HJ, Al-Taei S, Tanner C, Ashford F, et al. Increased seroprevalence and improved antibody responses following third primary SARS-CoV-2 immunisation: an update from the COV-AD study. Front Immunol. 2022;13:912571. doi: 10.3389/fimmu.2022.912571. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its supplementary information files).