Abstract
Introduction
The present study aimed to investigate the antifungal activity of silver nanoparticles (SNPs) against agents of suspected rhino orbital mucormycosis.
Methods
Thirty-two strains were isolated from endoscopy-guided nasal swab and/or tissue biopsy after debridement/surgery on Sabouraud dextrose agar without cyclohexamide. Antifungal activity was conducted according to Clinical and Laboratory Standards Institute’s (CLSI) guidelines, M38-A2. The average size of silver nanoparticle was less than 10 nm.
Results
Minimum inhibitory concentration (MIC) of nanoparticles of all strains was in the concentration range of 1–64 μg/ml and minimal fungicidal concentration (MFC) at 16–512 μg/ml.
Conclusion
The SNPs revealed significant antifungal activity against agents of invasive mycosis.
Keywords: Mucormycosis, Silver nanoparticles, Aspergillosis, Minimum inhibitory concentration
Introduction
COVID-19-associated mucormycosis emerged as a major challenge during the second wave of the pandemic (Mehta and Pandey 2020). The most common presentations of invasive fungal infection post COVID was rhino orbito cerebral (ROCM) and pulmonary (El-Kholy et al. 2021). Mucormycosis is an opportunistic infection affecting patients especially with poorly controlled diabetes whereas aspergillosis can develop in immunocompetent patients as well (Ibrahim et al. 2012). Mucormycosis and invasive aspergillosis both are fulminant diseases as the fungi are angioinvasive, and rapidly destructive. Mucormycosis and aspergillosis coinfection in the same host is also a known entity. The most frequently isolated species causing rhino-orbital and rhino-orbital-cerebral mucormycosis and aspergillosis are Rhizopus arrhizus and Aspergillus flavus respectively. High-dose liposomal amphotericin B along with surgical debridement is recommended as the first-line treatment.
Nanomaterials with unique physical and chemical properties, including a small size and a high surface area-to-volume ratio, allow for an increased ability to surpass most physiological barriers to therapeutic targets and to interact with pathogen membranes and cell walls (Li et al. 2013). Several nanomaterials have shown anti-microbial activity against E. coli, P. aeruginosa, B. subtilis, S. aureus, and C. albicans through multiple mechanisms including the interruption of transmembrane electron transfer, disruption of cell envelop oxidization of cell components, or production of reactive oxygen species (ROS) (Dakal et al. 2016).
Silver as a nanomaterial has been used for application with antifungal and antimicrobial agents. Silver nanoparticles are well-known for their biocidal properties, including antibacterial, antifungal, antiviral, and anticancer activities (Dakal et al. 2016). As nanosilver has shown potential antifungal effect, it may be evaluated for its role in the treatment of mucormycosis (Gajbhiye et al. 2009). The objectives of the present study were to evaluate the antifungal activity of silver nanoparticles (SNPs) against agents of mucormycosis and aspergillosis isolated from patients of rhino orbital mucormycosis during the second wave of COVID-19.
Material and methods
Study design
This prospective observational study was conducted for a period of 6 months on all isolates from confirmed patients of rhino orbital mucormycosis presenting to a tertiary care hospital in Faridabad, India, from May 2021 to October 2021 during the second wave of Covid 19. The study was conducted at the Department of Microbiology, ESIC Medical College and Hospital, Faridabad and approved by Institutional Ethics Committee (134 X/11/13/2021).
Clinical specimen
Endoscopy-guided nasal swab and/or tissue biopsy after debridement/surgery were collected from patients suspected of having rhino orbital mucormycosis and were sent to the laboratory for direct KOH mount, culture, and histopathology.
Specimen processing
Tissue specimens were cultured on Sabouraud dextrose agar without cyclohexamide at 37 ℃ and 25 ℃ for 3 weeks. Fungal growth was identified on the basis of colony morphology and microscopic appearance on lactophenol stain by slide culture technique.
Quality control
Aspergillus flavus ATCC 204304 and Aspergillus fumigatus ATCC 204305 were used as quality control strains. Antifungal activity of Ag-NPs was investigated according to Clinical and Laboratory Standards Institute’s (CLSI) guidelines M38-A2 (CLSI 2008).
Preparation of culture media for microbroth dilution
RPMI powder (HiMedia, Mumbai) was dissolved in distilled water and sodium bicarbonate (2 g/l) and added to the medium. Then the medium was filtered, distributed and transferred to tubes and stored at 4 °C. Before using the medium, 1 ml glutamine was added to 100 ml medium.
Preparation of suspension of fungi
Fungal isolates were checked for purity and identified to the species level by studying detailed morphology by slide culture technique. Isolates which were not in their pure form were excluded from the study. Aspergillus and Rhizopus conidial inoculum suspensions were prepared from well-sporulated cultures (typically 3 days old) grown on potato dextrose agar and adjusted spectrophotometrically to a turbidity that ranged from 0.4 to 0.7 McFarland standards at 530 nm corresponding to 1.5 × 108 CFU/ml. For the M38-A2 method, the suspension was then diluted twice with RPMI 1640 broth to achieve the density needed for the final inoculum density (0.4 × 104 to 5 × 104 CFU/ml) in duplicate wells.
Silver nanoparticles
The average size of silver nanoparticle was less than 10 nm. The procured SNPs were prepared using green nanotechnology (Ahmad et al. 2019).
Preparation of SNP serial dilutions
The silver nanoparticles (SNPs) were procured from Jagsonpal Pharmaceuticals in a concentration of 1000 ppm. One part per million of SNP equals 1 µg/mL using which following dilutions were made: 8 µg/mL, 16 µg/mL, 32 µg/mL,64 µg/mL, 128 µg/mL, 256 µg/mL, and 512 µg/mL (Espinel-Ingroff et al. 2002).
Determination of MIC
Growth (SNP-free) and fungus-free controls were included along with aforementioned dilutions of SNP in microtitre plates. The plates were incubated at 35 °C and examined for the MICs after 48 h. MIC endpoints were defined as 50% reduction in growth compared to the drug-free wells. MICs were recorded for each isolate.
Determination of MFC
The minimum fungicidal concentration was determined according to the protocol described by Espinel-Ingroff et al. (Espinel-Ingroff et al. 2002). Twenty microliters of each well with complete inhibition of fungal growth was withdrawn and cultured in plates with Sabouraud dextrose agar for 72 h at 30 °C. The MFC was defined as the lowest drug dilution that yielded fewer than three colonies or complete absence of growth.
Statistical analysis
Microsoft excel was used to enter data and create line graph.
Results
A total of 48 patients were diagnosed with rhinoorbital mucormycosis during a 6-month period. Patients were diagnosed on the basis of history, clinical examination, radiological investigation, histopathology, direct KOH mount examination, and fungal culture. Thirty-two fungal isolates were retrieved from the confirmed cases. Rhizopus arrhizus was isolated alone from five patients and Rhizopus arrhizus and Aspergillus spp. as coinfection infection in seven cases. However, Aspergillus alone was isolated from 12 patients. Fusarium spp. was isolated from one patient of suspected invasive rhino orbital sinusitis. In 16 cases, fungal pathogen could not be isolated on culture.
Minimum inhibitory concentration of SNP
Table 1 shows antifungal activity of SNPs on 32 isolates of Rhizopus arrhizus, A. niger, A. fumigatus, A. flavus, and F. oxysporum. After 48 h of incubation, R. arrhizus had MIC range from < 8 to 64 µg/ml, A. flavus had MIC range from < 8 to 64 µg/ml, A. fumigatus had MIC range from 8 to 32 µg/ml, A. niger MIC of < 8 µg/ml, and F. oxysporum MIC as 32 µg/ml. MIC 50 and MIC 90 of Aspergillus spp. was 16 µg/ml and 64 µg/ml respectively whereas MIC 50 and MIC 90 of Rhizopus arrhizus was 16 µg/ml each.
Table 1.
MIC and MFC of SNP against 32 strains of Rhizopus, Aspergillus, and Fusarium
| Strains | < 8 µg/ml | 8 µg/ml | 16 µg/ml | 32 µg/ml | 64 µg/ml | 128 µg/ml | 256 µg/ml | 512 µg/ml | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | |
|
Aspergillus sp n = 19 |
1 | 0 | 6 | 0 | 5 | 0 | 5 | 2 | 1 | 3 | 1 | 6 | 0 | 7 | 0 | 1 |
| Aspergillus flavus (n = 13) | 1 | 0 | 2 | 0 | 4 | 0 | 4 | 1 | 1 | 2 | 1 | 5 | 0 | 4 | 0 | 1 |
| Aspergillus fumigatus (n = 4) | 0 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 0 |
| Aspergillus niger (n = 2) | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
|
Rhizopus arrhizus n = 12 |
2 | 0 | 3 | 0 | 6 | 1 | 0 | 4 | 1 | 2 | 0 | 4 | 0 | 0 | 0 | 1 |
|
Fusarium oxysporum n = 1 |
0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
The minimum fungicidal concentration
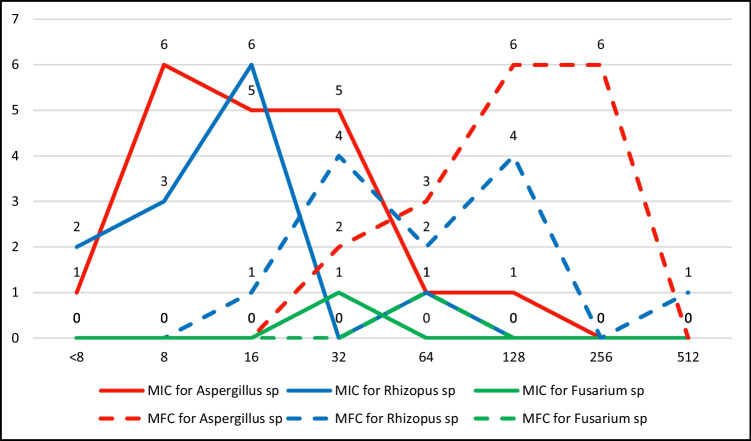
Figure 1 shows line graph of MIC and MFC of SNPs against filamentous fungi. R. arrhizus had MFC range from 16 to 512 µg/ml, A. flavus had MIC range from 32 to 256 µg/ml, A. fumigatus had MFC range from 64 to 256 µg/ml, A. niger MFC of 32–256 µg/ml, and F. oxysporum MFC as 64 µg/ml.
Fig. 1.
Line graph showing MIC and MFC of SNP against 32 strains of filamentous fungi
Discussion
Liposomal amphotericin B in initial dose of 5 mg/kg body wt (10 mg/kg body wt in case of CNS involvement) is the treatment of choice for invasive mucormycosis. Amphotericin B is associated with renal insufficiency, hypokalemia, hypomagnesemia, hypocalcemia, and hypophosphatemia. With limited armor of antifungals available, the need for alternate agents is on the rise. SNPs have been shown to be anti-mycotic activity against pathogenic yeast and dermatophytes and non-toxic to human keratinocytes (Rónavári et al. 2018).
Earlier studies on SNPs have revealed significant antifungal activity against amphotericin B-resistant C. glabrata strains. Results of antifungal activity against resistant C. glabrata strains after exposure to Ag-NPs with inhibitory action at a 0.125–0.5 μg/ml concentration (Lotfali et al. 2021).
The mechanism of inhibitory effect of SNPs on microorganisms is that they are capable of changing membrane and cell wall structure in the resistant strains, possibly through pore formation on the membrane and cell wall (Dakal et al. 2016). Some studies have reported that the positive charge on the Ag+ ion is crucial for its antimicrobial activity through the electrostatic attractions between the negatively charged cell membrane of microorganisms and the positively charged nanoparticles (Vijayakumar et al. 2013). SNPs have shown to have antifungal therapeutic potential in various studies (Monteiro et al. 2012).
Several applications of SNPs are established in literature. Silver nanoparticles (AgNPs) are utilized in the dental prosthesis matrixes in the field of dental medicine at low concentrations and have been able to selectively destroy cellular membranes (Matteis et al. 2019). Administration of topical formulations of nanosilver particles in combination with current drugs has been used for treating vaginal candidiasis and preventing the disease recurrence (Monteiro et al. 2012).
However, this is the first study of SNP on agents of mucormycosis and other invasive rhinoorbital mycosis. In a study on toxigenic species of Aspergillus species, the MIC 50 of AgNPs against Aspergillus flavus, has shown to be 8 μg/ml (Bocate et al. 2019). SNPs were evaluated for anti-fungal activity against agents of mucormycosis in the current study which showed that they prevented visible growth (MIC) at a concentration range of < 8–64 μg/ml and inhibited fungal growth at 16–512 μg/ml (Table 1). SNPs showed MIC at a concentration range of < 8–128 μg/ml and were fungicidal at 32–256 μg/ml for Aspergillus spp. (Table 1). All species of Aspergillus tested with SNP showed similar MIC pattern. Single isolate of Fusarium showed MIC and MFC of 32 and 64 µg/ml respectively. The MIC 50 and MIC 90 of Aspergillus spp. was 16 µg/ml and 64 µg/ml respectively. SNPs were observed to be inhibitory to Rhizopus arrhizus, at a range of < 8–64 µg/ml and fungicidal at 16 µg/ml, MIC 50 and MIC 90 of Rhizopus spp. 16 µg/ml each. Another study showed susceptibility of Aspergillus, Alternaria, and Fusarium isolated from fungal keratitis to Ag-NPs at 1 μg/ml (Xu et al. 2013). From our study, it is shown that SNPs are potential anti mycotic alternatives against agents of ROCM. A concentration of 16 µg/ml may be used for further in vivo studies for defining therapeutic limits.
ROCM is an acute, invasive, and often fatal mycosis despite aggressive treatment with systemic antifungal therapy and surgical debridement. Successful outcome of the cases depend on the early diagnosis and prompt surgical intervention. SNPs exhibit potent in vitro antifungal activity against agents of ROCM. This may be exploited in the treatment of fungal infections by local instillation by nasal spray or intraoperative instillation when used within therapeutic limits at an early stage of the disease.
Conclusions
SNPs have known antimicrobial action and is being used in local treatment of different diseases. Further studies may be needed to confirm the therapeutic range of SNPs against agents of mucormycosis where it works regardless of their antifungal resistance mechanisms.
Acknowledgements
Authors acknowledge Jagsonpal Pharmaceuticals for providing silver nanoparticles used in the study
Author contribution
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Dr Kuhu Chatterjee, Dr Juhi Taneja, Dr Shilpa Khullar, and Dr Anil Kumar Pandey. The first draft of the manuscript was written by Dr Juhi Taneja, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Ethics approval and consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of ESIC Medical College & Hospital Faridabad, India (IEC no.134 X/11/13/2021).
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Footnotes
The corresponding author is a member of Indian Society of Medical Mycologists (ISMM) and the laboratory participates in External Quality Assurance Scheme for Mycology.
The original online version of this article was revised due to a retrospective Open Access cancellation.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
11/3/2022
A Correction to this paper has been published: 10.1007/s10123-022-00285-2
References
- Ahmad S, Munir S, Zeb N, et al. Green nanotechnology: a review on green synthesis of silver nanoparticles—an ecofriendly approach. Int J Nanomedicine. 2019;14:5087–5107. doi: 10.2147/IJN.S200254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocate KP, Reis GF, de Souza PC, Oliveira Junior AG, Durán N, Nakazato G, Furlaneto MC, de Almeida RS, Panagio LA. Antifungal activity of silver nanoparticles and simvastatin against toxigenic species of Aspergillus. Int J Food Microbiol. 2019;16(291):79–86. doi: 10.1016/j.ijfoodmicro.2018.11.012. [DOI] [PubMed] [Google Scholar]
- CLSI (2008) Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard—second edition. CLSI document M38-A2. Vol. 28 No. 16. Clinical and Laboratory Standard Institute, Wayne
- Dakal TC, Kumar A, Majumdar RS, Yadav V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front Microbiol. 2016;7:1831. doi: 10.3389/fmicb.2016.01831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakal TC, Kumar A, Majumdar RS, Yadav V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front Microbiol. 2016;16(7):1831. doi: 10.3389/fmicb.2016.01831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis V, Cascione M, Toma CC, Albanese G, De Giorgi ML, Corsalini M, Rinaldi R. Silver nanoparticles addition in poly(methyl methacrylate) dental matrix: topographic and antimycotic studies. Int J Mol Sci. 2019;20(19):4691. doi: 10.3390/ijms20194691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Kholy NA, El-Fattah AMA, Khafagy YW (2021) Invasive fungal sinusitis in post COVID-19 patients: a new clinical entity. Laryngoscope. :10.1002/lary.29632 [DOI] [PMC free article] [PubMed]
- Espinel-Ingroff A, Chaturvedi V, Fothergill A, Rinaldi MG. Optimal testing conditions for determining MICs and minimum fungicidal concentrations of new and established antifungal agents for uncommon molds: NCCLS collaborative study. J Clin Microbiol. 2002;40(10):3776–3781. doi: 10.1128/JCM.40.10.3776-3781.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajbhiye M, Kesharwani J, Ingle A, Gade A, Rai M. Fungus-mediated synthesis of silver nanoparticles and their activity against pathogenic fungi in combination with fluconazole. Nanomedicine. 2009;5(4):382–386. doi: 10.1016/j.nano.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Ibrahim AS, Spellberg B, Walsh TJ, Kontoyiannis DP. Pathogenesis of mucormycosis. Clin Infect Dis. 2012;54(Suppl 1):S16–22. doi: 10.1093/cid/cir865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LH, Yen MY, Ho CC, Wu P, Wang CC, Maurya PK, Chen PS, Chen W, Hsieh WY, Chen HW. Non-cytotoxic nanomaterials enhance antimicrobial activities of cefmetazole against multidrug-resistant Neisseria gonorrhoeae. PLoS ONE. 2013;8(5):e64794. doi: 10.1371/journal.pone.0064794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotfali E, Toreyhi H, MakhdoomiSharabiani K, Fattahi A, Soheili A, Ghasemi R, Keymaram M, Rezaee Y, Iranpanah S. Comparison of antifungal properties of gold, silver, and selenium nanoparticles against amphotericin B-resistant Candida glabrata Clinical Isolates. Avicenna J Med Biotechnol. 2021;13(1):47–50. doi: 10.18502/ajmb.v13i1.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta S, Pandey A. Rhino-orbital mucormycosis associated with COVID-19. Cureus. 2020;12:e10726. doi: 10.7759/cureus.10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro DR, Silva S, Negri M, Gorup LF, de Camargo ER, Oliveira R, Barbosa DB, Henriques M. Silver nanoparticles: influence of stabilizing agent and diameter on antifungal activity against Candida albicans and Candida glabrata biofilms. Lett Appl Microbiol. 2012;54(5):383–391. doi: 10.1111/j.1472-765X.2012.03219.x. [DOI] [PubMed] [Google Scholar]
- Rónavári A, Igaz N, Gopisetty MK, Szerencsés B, Kovács D, Papp C, Vágvölgyi C, Boros IM, Kónya Z, Kiricsi M, Pfeiffer I. Biosynthesized silver and gold nanoparticles are potent antimycotics against opportunistic pathogenic yeasts and dermatophytes. Int J Nanomedicine. 2018;1(13):695–703. doi: 10.2147/IJN.S152010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar M, Noorlidah A, Ahmed ABA, Nancy FT, Priya K. Biosynthesis, characterisation and anti-bacterial effect of plant-mediated silver nanoparticles using Artemisia nilagirica. Ind Crops Prod. 2013;41:235–240. doi: 10.1016/j.indcrop.2012.04.017. [DOI] [Google Scholar]
- Xu Y, Gao C, Li X, He Y, Zhou L, Pang G, et al. In vitro antifungal activity of silver nanoparticles against ocular pathogenic filamentous fungi. J Ocular Pharmacol Therap. 2013;29:270–274. doi: 10.1089/jop.2012.0155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.