Abstract
Abstract
Plastics are an integral but largely inconspicuous part of daily human routines. The present review paper uses cross-disciplinary scientific literature to examine and assess the possible effects of nanoplastics (NPs) concerning microplastics (MPs) on human health and summarizes crucial areas for future research. Although research on the nature and consequences of MPs has seen a substantial rise, only limited studies have concentrated on the atmospheric nanosized polymeric particles. However, due to the intrinsic technological complications in separating and computing them, their existence has been difficult to determine correctly. There is a consensus that these are not only existing in the environment but can get directly released or as the outcome of weathering of larger fragments, and it is believed to be that combustion can be the tertiary source of polymeric particles. NPs can have harmful consequences on human health, and their exposure may happen via ingestion, inhalation, or absorption by the skin. The atmospheric fallout of micro (nano) plastics may be responsible for contaminating the environment. Apart from this, different drivers affect the concentration of micro (nano) plastics in every environment compartment like wind, water currents, vectors, soil erosion, run-off, etc. Their high specific surface for the sorption of organic pollutions and toxic heavy metals and possible transfer between organisms at different nutrient levels make the study of NPs an urgent priority. These NPs could potentially cause physical damage by the particles themselves and biological stress by NPs alone or by leaching additives. However, there is minimal understanding of the occurrence, distribution, abundance, and fate of NPs in the environment, partially due to the lack of suitable techniques for separating and identifying NPs from complex environmental matrices.
Highlights
Micro (nano) plastics generated may reach the soil, water, and atmospheric compartments.
Atmospheric currents serve as a way to transport, leading to micro (nano) plastics pollution.
Exposure to micro (nano) plastics may happen via ingestion, inhalation, or absorption by the skin.
Nanoplastics may be environmentally more harmful than other plastic particles; the focus should be on defining the exact size range.
Visual classification of micro (nano) plastics is poor in reliability and may also contribute to microplastics being misidentified.
Graphical abstract
Keywords: Combustion, Polymeric particles, Toxicology, Occupational health, Additives, Research needs
Introduction
Plastic is a material that gives tremendous benefits to society. Plastics are (mainly) synthetic (manufactured) materials created from polymers, which are lengthy molecules constructed around carbon atom chains, with hydrogen, oxygen, sulfur, and nitrogen often filling in the spaces. They are low-cost, lightweight, robust, durable, corrosion-resistant, and thermally and electrically insulating materials. Polymers’ adaptability and diversity of features are employed to create a wide range of goods that provide medical and scientific advancements, energy savings, and a variety of other social advantages (Andrady and Neal 2009). These plastic polymers contain different functional groups, have different physical and chemical characteristics, and have different impacts on biota (Bolan et al. 2020; Palansooriya et al. 2020).
Even the same plastic polymers from the same geographical region might have different chemical compositions depending on how long the particles have been exposed to the environmental weathering (Cole et al. 2015). As it will be explained more in detail, the exposure of plastic derivatives in the environment promotes physical, chemical, and biological degradation processes leading to the accumulation of small plastic fragments in ecosystems, i.e., freshwater (Eerkes-Medrano et al. 2015), sediments (Yang et al. 2021a, b), soil (Li et al. 2020) , air (Prata 2018), and foodstuff (Kwon et al. 2020).
Our community is deeply concerned by precise shreds of evidence of ubiquitous large quantities of plastics everywhere, and shocking photographs are disseminated fast by social media everywhere. The term microplastics (MPs) has been known for a while, but nanoplastics (NPs) are a relatively new concept. Scientists have begun to use the term NPs to distinguish these polymers from MPs in recent years. This difference was made as a result of growing concern about the human health impacts of NPs. Although micro and NPs are perhaps less well known by many citizens, it has been shown in many studies (Dris et al. 2015, 2016, 2017; Rezaei et al. 2019; Su et al. 2020; Wang et al. 2020a, b) that there should be an enormous amount of polymeric particles they cannot see and, thus, they could be the hidden threat. Lozano et al. (2021) discovered that the effects of micro (nano) plastics on terrestrial systems (plant growth, microbial activity, soil biota) differed depending on the polymer type polypropylene (PP), polyurethane (PUR), polyethylene terephthalate (PET), polyethylene (PE), polystyrene (PS), and polycarbonate (PC), as well as the form. Fibers, for example, boosted soil aeration and porosity while films reduced soil bulk density. On the other hand, foam and fragments improved soil aeration and porosity. As a result, micro (nano) plastic physicochemical features play a significant role in their toxicity (Lambert et al. 2017). The oxidative stress displayed by Eisenia fetida when exposed to high-density PE and PP micro (nano) plastics was size-dependent, and the worms showed inflammation and neurotoxicity when treated with the micro (nano) plastics, according to Li et al. (2021). Environmentally degraded PS micro (nano) plastics, according to Hüffer et al. (2018), can produce polymeric surface oxidation, which increases the probability of interactions with biota or sorption of other organic pollutants. Micro (nano) plastics’ physical and chemical interactions with the environment are heavily influenced by environmental degradation. This environmental degradation process is influenced by factors in the background, such as ultraviolet radiation and mechanical or biological disintegration. A study by Hüffer et al. (2018) found that the polymer surface of PS micro (nano) plastics could become oxidized over time in the environment, making them more likely to interact with living things or adsorb organic contaminants. Castan et al. (2021) evaluated the partitioning and diffusion coefficients for PE and tire-wear micro (nano) plastics bound contaminant migration (agrochemicals or pesticides such as hexachlorobenzene, endrin, aldrin, dichlorodiphenyltrichloroethane, transnonachlor, and additives such as polychlorinated biphenyls) in soil, and found rapid desorption. They were skeptical about micro (nano) plastics’ ability to assist pollutant migration or mobility. However, more detailed information on their physical and chemical properties and toxicity are mentioned in the section (human exposure and harm).
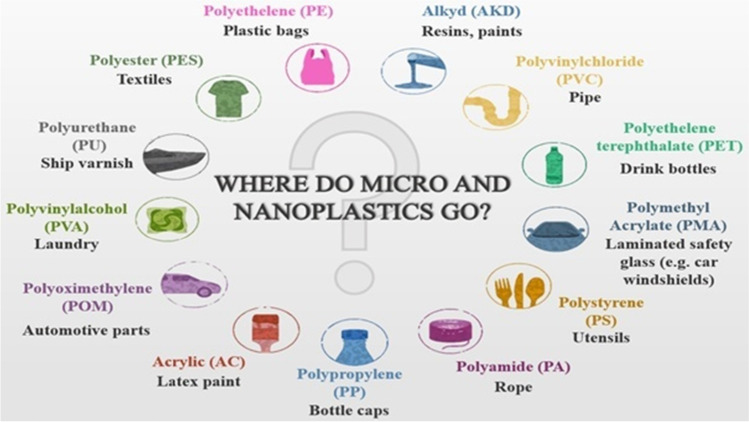
Society demands actions, but decisions are hugely difficult because decision-makers face many questions for which science still has no definite answers. Challenges remain for scientific measurement and test methods to better understand and monitor plastics’ origins, pathways, fate, and behavior. It is estimated that 4.8 to 12.7 million metric tons of mismanaged plastic waste enter the oceans from coastal countries each year (Jambeck et al. 2015). Currently, global demand surpasses 320 million tons per year, of which almost 40% is consumed as single-use packaging, contributing to plastic waste (Plastics-The Facts 2016). Rising production has brought with it increased production of plastic waste. Ten percent of the plastic produced worldwide was projected to end up as waste, and merely around 3% was recycled in 2016 (Thompson 2015; Wirnkora et al. 2019). Since plastic products started to reach the market on a large scale in the 1950s, plastic’s global production dramatically increased from 0.5 million tons per year in 1960 to 348 million tons in 2017 (Plastics Europe 2018; Wirnkora et al. 2019). A considerable amount of the plastic generated each year is lost to and persists in the marine environment, with a projected accumulative potential of 250 million tons by 2025 Jambeck et al. (2015), while as per Enyoh et al. (2019), in 2050, nearly 67.8 million metric tons would be left as waste in the natural setting or landfill. Presently, the forms of airborne MPs being studied include atmospheric fallout, deposition, dust, snow, and suspended MPs. Research on airborne or indoor MPs has only been performed in a few cities or regions like Paris-France, France, China, Turkey, Germany, Iran, Shanghai-China, London-England, California-USA, Sydney-Australia, Spain, Vietnam, the UK, and Poland (Table 1). Most studies focused on physical characteristics (size, color, shape), airborne MPs composition, and abundance. As per the author’s knowledge to date, only a few studies have examined the presence of MPs in the atmosphere, while none of these studies have mentioned atmospheric NPs (Table 1): primarily, these studies have focused either on fibers or fragments, but significantly less have examined the foam, spherule, film, granule, and microbead shaped MPs. Limited studies have focused on the different types of microplastic colors like transparent, blue, red, black, yellow, purple, green, dark blue, white, blue, brown, grey, white transparent, yellow-orange, red-pink, blue-green, black-grey, grey-brown, white-reddish (Table 1). The common polymer types of MPs examined until now include PE, PET, PP, PS, Polyvinyl chloride; PVC, Nylon; PA, Acrylonitrile butadiene styrene; ABS, Polyester; PES, Polyacrylonitrile; PAN, Poly(N-methyl acrylamide); PAA, Rayon; RY, Ethylene–vinyl acetate; EVA, Epoxy resin; EP, Alkyd resin; ALK, Polyamide 66; PA-66, Polyamide 6; PA-6, PUR and Poly(methacrylate)Acrylic; PMMA, etc. Current studies have focused on the micrometer size of plastics, but none have focused on the nanometer size. The maximum size found in the literature was > 1000 μm (Abbasi et al. 2019), while the minimum was 5 μm (Jenner et al. 2021; Szewc et al. 2021). Therefore, all of the studies conducted so far have focused on plastics at a micro-scale, but there is a gap of knowledge as regards the nano-size range of plastics. The size range shown in (Table 1) is the outcome of the literature done so far. The main aim of showing this size range was to highlight whether researchers focus on a micro or nano-size range of plastics. As the size decreases, the harmful impacts of micro (nano) plastics increase. Studies have discussed that micro (nano) plastics can infiltrate the lung depending on their aerodynamic diameter once inhaled. Especially MPs with an aerodynamic diameter < 10 μm have the potential to penetrate the lower respiratory tract (Foord et al. 1978; Prata 2018; Jacob et al. 2020). There is evidence that plastic fibers penetrate the deep human lung (Pauly et al. 1998). In addition, it has been shown that MPs particles can penetrate the lungs’ surface lining layer and are taken up by endothelial cells (Goodman et al. 2021). Even a recent study (Leslie et al. 2022) has shown the presence of MPs in the human blood. So it gets clear that at present, works are mainly focusing on the MPs than NPs (Table 1); the reason can be that MPs definition is more widely used and accepted than NPs, and presently the MPs is a hot and virgin topic maybe in future the concerns over NPs will, due to their size differentiation and harmful impacts. Furthermore, most instruments presently used for detecting polymeric particles have detection limits mainly up to the size of micrometers, or the authors are mainly focusing on the size of MPs as there are no standardized methods for nano and MPs; however, different authors have applied various procedures for MPs. In general, NPs have a limited size range compared with MPs, and the lack of technology to identify such small particles on a wide scale can also be the reason for less research. Another explanation might be because NPs come in various forms and chemical compositions, making it difficult to anticipate whether they would aggregate, sediment, or dissolve and how they will accumulate and move through the environment.
Table 1.
The abundance of indoor and outdoor airborne MPs in some regions and countries
| Location | Concentration | Shape/form | Size | Color | Polymer type | Analytical techniques | Reference |
|---|---|---|---|---|---|---|---|
| Paris France | (29—280 particles m−2 day−1 total atmospheric fallout) and (260—320 × 103 particles m−3 wastewater) | Fibers | 100–5000 μm | * | * | Stereomicroscope | (Dris et al. 2015) |
| France | 2 and 355 particles/m2/day | Fibers | 50–600 μm | * | * | Stereomicroscope and FTIR | (Dris et al. 2016) |
| China | Non-fibrous and fibers 175–313 particles/m2/day | Fiber, foam, fragment, and film | * | * |
PE, PP, and PS |
Digital microscope, FTIR, SEM | (Cai et al. 2017) |
| France | Indoor (1.0–60.0) fibers/m3, Outdoor (0.3–1.5) fibers/m3 | Fibers | 50–4850 μm | * | * | Stereomicroscope and FTIR | (Dris et al. 2017) |
| Turkey | 8 MPs per 30 mints | Fibers and fragments | * | DB, W, BL, TP, PP, and BR | PA 66, PA 6, PUR, PE, PP, and PES | Light microscope and FTIR | (Tunahan Kaya et al. 2018) |
| China | 1.7 and 16.2 particles m−3 |
Synthetic fragments, fibers, and nonsynthetic particles of protein and cellulose |
* | * | PES, PE, PA, PS, and PUR |
micro FTIR and FPA IS |
(Vianello et al. 2019) |
| Germany | PE and PP were 0.03 to 3.3 mg/g | * | * | * | PP and PE | Pyr-GC–MS | (Dierkes et al. 2019) |
| Germany | 136.5 and 512 MPs per m2/day | Fragments and fibers | (300–63 Fragments) (9300–63 fibers μm) | * |
PE, EVAC PTFE and PVA |
Fluorescence microscope and micro Raman spectroscopy | (Klein & Fischer 2019) |
| China |
PET 1550–120,000 mg/kg indoor and 212–9020 mg/kg outdoor and PC 4.6 mg/kg indoor and 2.0 mg/kg outdoor |
Fibers and granules | * | * | PET and PC |
LC–MS/MS, optical microscopy and micro FTIR, |
(Liu et al. 2019a) |
| China | 0–4.18 n/m3 | Fiber, fragments and granules | 23.07–9555 μm | BR, GN, YL, and GR | PET, PE, PES, PAN, PAA, RY, EVA, EP, and ALK | Stereomicroscope and micro FTIR, | (Liu et al. 2019b) |
| Iran | 900 MPs and 250 micro rubbers per 15 g sample |
Fiber, film, fragment or spherule |
(< 100 to > 1000 μm) | WT, YO, RP, BG or BGY | * |
Binocular microscopy, polarized light microscopy and fluorescence microscopy |
(Abbasi et al. 2019) |
| China |
54 granules g−1 of MPs in Sipunculus nudus |
* | * | * |
PE, PS, PVC and PET |
Fluorescent microscope | (Lv et al. 2019) |
| Shanghai. China | SAMPs ranged from 0 to 2 n/m3 | Fibrous, fragment, and microbead | * | GR, BL, GN, TP, PP, and BR |
PET, EP, PE, ALK, RY, PP, PA, and PS |
FTIR | (Liu et al. 2019c) |
| France |
Average daily particle deposition of 365 m–2 day |
Fragment, films, and fibers | * | * | PS, PE, PP, and PET | Visual microscopy inspection and micro Raman | (Allen et al. 2019) |
| London England | 575–1008 MPs/m2/day | Fibrous, non-fibrous, and synthetic | 20–25 μm (fibrous). 75 μm and 100 μm (non-fibrous) | * | PAN, PET, PA, PUR, PP, PS, PE, and PVC | Fluorescence, stereo microscope & FTIR | (Wright et al. 2019b) |
| China |
Dormitory (9.9 × 103), office (1.8 × 103) and corridor (1.5 × 103) MPs/m2/day |
Fibers | * | TP, BL, RD, BK, YL, PP and GN |
PES, RY, PMMA, Cellophane, PP, PS, and PA |
micro FTIR and stereomicroscope | (Zhang et al. 2020d) |
| California United states | Indoor (fibers 3.3 ± 2.9 and 12.6 ± 8.0 fragments m–3) outdoor air (0.6 ± 0.6 fibers and 5.6 ± 3.2 fragments m–3) | Fragments and fibers | Indoor fragments (58.6 ± 55) & fibers (641 ± 810.7) outdoor fragments (104.8 ± 64.9) & fibers (616 ± 536.7) μm | * | PE, PET, PVC, PS, PC, acrylic, and resin |
Fluorescent microscopy, micro Raman spectroscopy and FTIR |
(Gaston et al. 2020) |
| 12 different countries |
PET 38–120,000 μg/g & PC < 0.11–1700 μg/g |
* | * | * | PET and PC | HPLC–MS/MS | (Zhang et al. 2020a) |
| China | MPs 136–2060 items/kg, PET 536–660 mg/kg, and PC 83.9–196 mg/kg | Granule, fragment, fiber, and film, | 13 μm–5 mm | * | PC and PET | LC–MS/MS | (Duan et al. 2020) |
| Sydney Australia | MPs deposition rate 22–6169 fibers/m2/day | Fibers, fragments and films | 50 – 600 μm | BK, GN, BL, RD, GR, BR,TP | PE, PES, PA, PAA, PVC and PS | Stereomicroscope, fluorescent microscope and micro FTIR | (Soltani et al. 2021) |
| Spain |
1.5 and 13.9 MPs m−3 above rural and urban areas |
Fibers and fragments | 10–70 μm | W, BK, GN, TP, RD, WR, |
PES, PA, acrylic PUR, PS, BR and polyolefin, RY |
Stereomicroscope, and micro FTIR | (González-Pleiter et al. 2021) |
| Vietnam | 71–917 items m−2 day−1 | Fibers and fragments | 300–5000 μm | * | * | Stereomicroscope, and FTIR | (Truong et al. 2021) |
| United kingdom | 902–14,551 particles m−2 day−1 | Fibers film, spheres, foam and fragments | 5–5000 μm | * | PET, PA, acrylates, PP, PAN, PE, PMMA | Stereomicroscope, and micro FTIR | (Jenner et al. 2021) |
| Poland | 0–30 MPs m−2 day−1 | Fibres, fragments and film | 5–5000 μm | * |
PES, PP, PE, PVC, EPM and PVA |
Digital microscope and micro FTIR | (Szewc et al. 2021) |
* data is not available, while the abbreviations are mentioned at the top of the paper
The term plasticle is a shortened version of the expression “plastic particles” first introduced by Crawford & Quinn (2017). Ultraviolet radiation penetration catalyzes the photo-oxidation of plastic, causing it to become brittle. Degraded plastic particles into micro (0.1–1000 μm) in conjunction with wind, wave movement, and abrasion (Cózar et al. 2014) and potentially nanosized (≤ 0.1 μm) (Lambert and Wagner 2016a) particles, classified herein as micro and NPs, correspondingly. The size of plastic is a significant feature defining the item’s relationship with biota and its environmental fate (Besseling et al. 2017; Hüffer et al. 2017; Bhat et al. 2022). Under its degradation mechanisms, given where plastic is made, used, and disposed of, micro (nano) plastics may be assumed to be released and occur on land in our immediate environment (Narmadha et al. 2020; Zhang et al. 2021; Rezaei et al. 2022). MPs (≤ 5 mm) have gained considerable attention (Arthur et al. 2009; Verschoor 2015; Bergmann et al. 2015), and additional size definitions have been formulated, such as < 10 mm Graham & Thompson, (2009), 2–6 mm Cole et al. (2011), < 2 mm Ryan et al. (2009) even though attempts are being made to redefine them as ≤ 1 mm in size, as suggested by Claessens et al. (2011); Hartmann et al. (2019). MPs and the considerable smaller particles commonly stated to as NPs can be directly emitted into the environment (Koelmans et al. 2015; Hernandez et al. 2017) or can be produced once bigger plastic objects disintegrate and break under the influence of different environmental stressors (Gewert et al. 2015; Gigault et al. 2016). The exact mechanisms of fragmentation are unclear and currently under investigation (Song et al. 2017; Weinstein et al. 2016). Cózar et al. (2014) observed that the size distribution of floating plastic debris points at critical size-selective sinks removing millimeter-sized fragments of floating plastic on a large scale. This sink may involve a combination of fast nano-fragmentation of the microplastic into particles of microns or smaller, their transference to the ocean interior by food webs and ballasting processes, and processes yet to be discovered. Resolving the fate of the missing plastic debris is of fundamental importance to determine the nature and significance of the impacts of plastic pollution in the ocean. This hypothesis is further strengthened by different experimental modeling and field studies (Koelmans et al. 2017; Lambert and Wagner 2016a; Song et al. 2017; Ter Halle et al. 2017). In nearly all-natural ecosystems, MPs have been researched and identified globally, yet no lower-size boundaries or sub-groups have been formally established. Nonetheless, the expression nanoplastic is commonly employed but differently interpreted. Here, we principally admit the formal characterization of a nanomaterial given by the EU (2011/696/EU) Koelmans et al. (2015); Mattsson et al. (2018), conferring to which at minimum 50% of the particles should ensure at least one measurement lesser than 100 nm. Other studies define nanoplastic as plastic particles < 1 μm Da Costa et al. (2016); Koelmans et al. (2015); Lambert and Wagner (2016a); Ter Halle et al. (2017) or even < 20 μm Wagner et al. (2014). To the best of our knowledge, no current definition is available in the literature defending and explaining the word’s relevancy (NPs). Taking the nanomaterials (Hartmann et al. 2019) into consideration and the definition suggested by Da Costa et al. (2016); Koelmans et al. (2015); Lambert and Wagner (2016a); Ter Halle et al. (2017), in general, we can say that NPs are lesser than < 1 μm. Based on recent studies concerning atmospheric NPs, we propose the following definition: NPs are particles ranging between 300 and 1000 nm resulting from the degradation of plastic items and could be formed during the break-down of aged MPs, the manufacturing process, or even during the use of the object. The proposed definition of NPs was based on the analytical techniques used recently in the identification of NPs like Raman microscopes have been combined with particle-detection applications to simplify particle recognition (Frère et al. 2016; Schymanski et al. 2018) and successfully used Raman spectral imaging (Cole et al. 2013); identification of particulates depends upon the instrument parameters (Opilik et al. 2013,2013). In contrast to MPs, defining NPs is very important as they can pose a greater risk since they can quickly penetrate cells and tissues and get easily inhaled and ingested. The difficulty in separating and identifying NPs and their abundance in the atmosphere has been largely ignored to date; even their fate and transport are not well known. As a result, NPs’ physical appearance and health threat could be underestimated.
Atmospheric micro (nano) plastics are currently a relatively new topic; although publications on micro (nano) plastics in water or soil are numerous, this is not the case for their presence in the air. The keyword search “microplastics” and “nanoplastics” on the Web of Science showed 764 articles up to September 2021. While the keyword “microplastics” “nanoplastics” and “soil” shows 92 articles, plus the “microplastics” “nanoplastics” and “water” shows 370 articles; However, the “microplastics” “nanoplastics” and “atmosphere” show just 8 articles. So it gets clear that researchers are focusing on water and soil micro (nano) plastics; however, there is a need to check virgin atmospheric micro (nano) plastic contamination. Apart from being a novel topic, atmospheric micro (nano) plastics are an essential part of the plastic cycle and can harm human health, as discussed in the below headings. This review aims to provide an overview of current NPs specific knowledge, its relation to MPs, and human health effects. The most relevant sources of NPs, experimental and analytical capabilities, and insights into their fate and transport once released into the atmosphere will also be discussed. Furthermore, we summarize the overview of the implications on human health in detail, and lastly, we provide recommendations for future studies. We used previous work from environmental studies on MPs and engineered nanoparticles for NPs to predict the potential sources and health impacts of NPs.
Sources of micro (nano) plastics in the atmosphere
Increasing levels of plastic debris are among the most prominent environmental issues faced by people worldwide. Moreover, plastic gradually loses mechanical toughness through biotic and abiotic degradation pathways. Ultimately, it emits particles known as nano and MPs to the broader environment. Micro (nano) plastics are the most numerous debris reported in every compartment of the environment (Dris et al. 2016; Ivar et al. 2013; Jambeck et al. 2015; Lusher et al. 2015; Paytan et al. 2009), and contamination by these particulates can present a hazard for both terrestrial and aquatic organisms. Micro (nano) plastics are either primary, developed for different applications such as personal care products (facial and hand cleaners), cosmetic products, and air blast cleaning at a microscopic scale, or secondary, resulting from the breakdown of larger plastic debris and litter, mostly due to the impact of ultraviolet rays or mechanical abrasion (O’Brine and Thompson 2010). Textiles are one source of atmospheric MPs, and each clothing could release about 1900 fibers each wash (Browne et al. 2011). Synthetic clothes should be given special consideration. During their life cycle, especially during laundry procedures, they release millimeter and sub-millimeter-sized fibers and be considered secondary MPs. Therefore, synthetic textiles releasing micro (nano) plastics might be accountable for environmental issues in outdoor and indoor environments (Bhat et al. 2021). These MPs cover a wide and continuous variety of sizes and shapes, including 1-D (one larger dimension) fibers, 2-D (flat particle) fragments, and 3-D spherules (Dris et al. 2015). In the coming years, the nanoplastic presence in indoor environments and the outdoor atmosphere is likely to be of growing importance. Precise attention should be paid to the sources of NPs in every compartment of the environment exposed to severe anthropogenic impacts, from rural to urban areas.
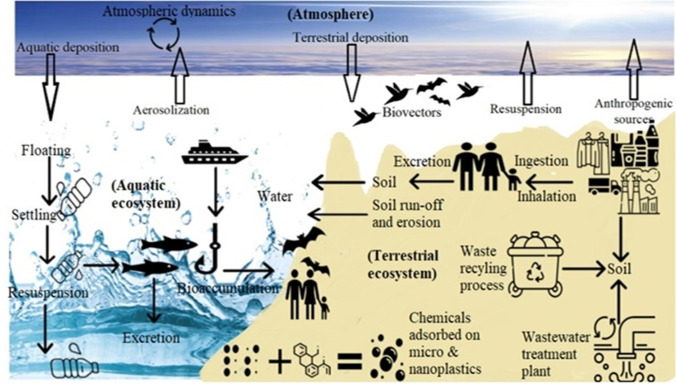
Micro (nano) plastics generated may reach the three necessary compartments of our environment (atmosphere, soil, and water) during their life cycle. Potential sources of polymeric particles in the atmosphere are industrial pollution, particle resuspension, and other anthropogenic factors (traffic, construction, urban infrastructure, etc.). The atmospheric fallout of micro (nano) plastics may be responsible for contaminating the environment. For terrestrial and marine ecosystems, airborne micro (nano) plastics could be a source of pollution. However, a complex shift of particles’ can happen among compartments: micro (nano) plastics from the water or soil may become airborne. In contrast, these compartments may be polluted by micro (nano) plastics from the atmosphere. Oceans, for example, can get ambient aerosols (Paytan et al. 2009); however, aerosols up to a few micrometers are also produced by sea spray (O’Dowd and De Leeuw 2007). The nanoplastic cycle in the environment could also involve this process (Fig. 1), where airborne NPs contaminate marine environments, and low-dimensional and low-density marine NPs become airborne in the marine spray; the behavior of these polymeric particles in these compartments is very less known. NPs are also increasingly being manufactured. Some products containing NPs are paints, adhesives, drug delivery vehicles, and electronics (Mattsson et al. 2018). 3D printing, for instance, can release polymeric nanoparticles (Stephens et al. 2013). The size reduction can cause specific particle characteristics both deliberately and due to environmental degradation, affecting their potential toxicity. A significant source of NPs responsible for contaminating ecosystems and respiratory pathologies in organisms may be the atmospheric compartment. The biogeochemical cycle of micro (nano) plastics is a novel way of conceptualizing micro (nano) plastic contamination in the environment (Fig. 1).
Fig. 1.
Conceptual model of the biogeochemical cycle of micro (nano) plastics
The micro (nano) plastic cycle is very complex and complicated compared with other biogeochemical cycles like water, carbon, nitrogen, phosphorus, and sulfur because it involves the interaction of micro (nano) plastics between the atmosphere, water, soil, and sediments, etc. (Fig. 1). Apart from this, different drivers affect the concentration of micro (nano) plastics in every environment compartment like wind, water currents, vectors, soil erosion, run-off, etc. From Fig. 1, it is clear that there is a clear transition of micro (nano) plastics between the three critical compartments of the environment (air, soil, and water). In comparison, in the environment, plasticizer chemicals can leach out. Many micro (nano) plastics would be suspended as dust in the air and transported. The deposition of particles into land and aquatic ecosystems will be facilitated by weather events, such as heavy rainfall. For a significant proportion of micro (nano) plastics, the ocean is commonly considered to represent a sink. The terrestrial and freshwater habitats serve as essential sources and routes for micro (nano) plastics to the sea. It remains possible that atmospheric currents serve as a way for particulate transport, leading to micro (nano) plastics pollution on land and within aquatic environments because of their lightweight existence and prevalent dispersion capacity (Evangeliou et al. 2020; Abbasi et al. 2021; Zhang et al. 2021). As particles join waterways, they would be exposed to similar distribution activities that mobilize other deposits, such as sand and silt, in the channels. Simply stated, the quicker the river flows, the higher energy it possesses, and so a larger number of particles can be drawn and transported. Generally, in the case of micro (nano) plastics, rivers are probably to have a source-regulated concerning transport, ensuring rivers can transport all plastics supplied to them. Regardless of the buoyancy of different plastics, wherever river potency decreases, micro (nano) plastics are likely to settle along with sinking sediment particles in slow-moving parts of water. Moreover, this sediment deposition might support micro (nano) plastics particles’ interment, whether micro (nano) plastics are concurrently deposited or are previously existing inside the residue. It is also possible that several particles would still be preserved within sediments during their path in the freshwater environment. It may be proposed that trapping and absorption of micro (nano) plastics into deposits could contribute to interment and long-term preservation within the substrate within lakes where sediment deposition degrees are high. At the same time, environmental boundaries are the minimum constrained, primarily affected by atmospheric passage directions rather than the uni-directional currents usually occurring on land and within water bodies.
Other newly discussed sources of atmospheric NPs could be from emissions during the recycling of polymeric materials. Moreover, there is no or little information regarding the combustion process as a polymeric particle emission source into the atmosphere. Incineration is commonly regarded as a method of permanently eliminating plastic trash. Still, there might be unburned material in the bottom ash, which is the solid waste from incinerators. The disposal and management of municipal solid waste incinerator bottom ash and fly ash is an unavoidable issue. Synthetic fibers were found in the unburned material from the bottom ash (Chimenos et al. 1999), that plastics and MPs may still be present in the bottom ash and might be transferred into the environment through reuse or dumping. Recently, MPs have been found in incineration plants’ bottom ash (Shen et al. 2021). In fly ash, bottom ash, and soil, MPs were found in abundance at 23, 171, and 86 particles/kg (Shen et al. 2021). Hu et al. (2021) assessed that high temperatures intensify the aging of plastics and facilitate microplastic fragmentation. Yang et al. (2021b) extracted the MPs from bottom ash in 12 mass-burn incinerators. Bottom ash was found to be an overlooked MPs source, with an abundance of 1.9–565 number/kg, implying that every metric tonne of trash produced 360 to 102,000 microplastic particles after incineration. PP and PS were the predominant types of MPs. MPs with a diameter of 50 μm to 1 mm accounted for 74% of the total. Granules, fragments, film, and fibers constituted 43%, 34%, 18%, and 5% of the total MPs. Additional research is needed to determine if incineration can finally eradicate micro (nano) plastics and assess the amount of micro (nano) plastics carried to the environment by bottom ash. The appropriate treatment of the bottom ash is required, and more research into the environmental behavior of micro (nano) plastics. Moreover, there is very little research on the release of MPs after fires. Hu et al. (2021) showed that the chemical composition and relative stiffness of heat-treated plastic surfaces changed, significantly enhancing the generation of MPs under external forces; over (2.1 ± 0.2) × 105 items/kg abundance of MPs released from PP, which were burned at 250 °C in air and trampled by a person. It was demonstrated that emissions from plastic waste recycling processes had affected the ambient environment (Huang et al. 2013; Hahladakis et al. 2018), a process that may also deposit NPs in the air. Fallout can also lead to skin or food deposition, causing cutaneous and gastrointestinal vulnerability with uncertain implications on human health. The sources show that micro (nano) plastics are ubiquitous in every environment compartment. Although micro (nano) plastics have been verified in these compartments, there is still a vast gap in experimental and analytical methods, and none of the standard procedures has been implemented until now for atmospheric polymeric particles.
Human exposure and harm
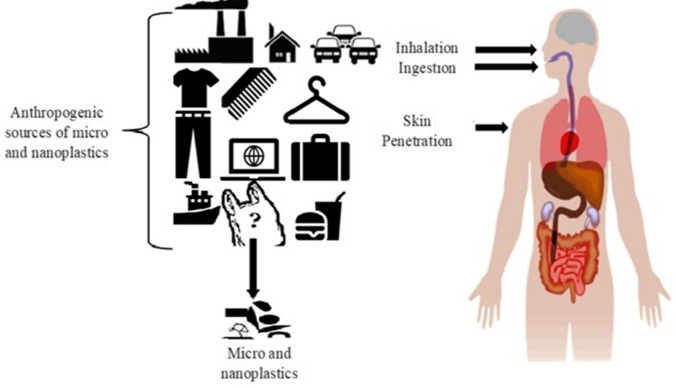
To illustrate the danger to human health exposure to NPs in the environment, we should primarily comprehend exposure. Consequently, we must recognize possible sources, concentrations, and aspects engaged in dispersing outdoor and indoor airborne NPs. These polymeric particles can likewise exist in unknown fractions of particulate matter and are of environmental concern. Concerns regarding the possible hazards that NPs bring to human health have been highlighted; however, the study is needed to answer numerous issues to determine the practical risks. For instance, we have no idea how many NPs are in the environment. This data is required to calculate the probability of occurrence, which is crucial for risk assessment. The level of certainty in the evaluations is an additional element to consider. Exposure to these NPs may happen via ingestion, oral inhalation, or absorption by the skin associated with the usage of plastic products or accidental means (Fig. 2). Inhalation is likely related to nanoplastic-containing aerosol exposure scenarios (Prata 2018). NPs uptake is expected to occur whenever we inhale the air, which is an integral part of the breathing cycle. Still, the inhaled concentration of NPs will depend on the number of NPs present in the inhaled air. On the other hand, through the use of personal care items such as NPs containing skincare and cleaning products or polluted water or air, possible contact with the skin may occur. As per the existing knowledge, nanoplastic particles’ ingestion is probably the key entry route because nanoplastic particles can be ingested by consuming seafood or drinking contaminated water (Koelmans et al. 2019; Zuccarello et al. 2019; Nicole 2021). Besides, under experimental conditions, nanoplastic uptake and accumulation and the trophic transfer of NPs within aquatic species have been shown, thereby strengthening the likelihood of NPs accumulating in the food chain (Fig. 1) and therefore resulting in human exposure (Cedervall et al. 2012; Mattsson et al. 2015; Eraslan et al. 2021). These NPs can get easily ingested by a wide range of species because of their smaller size. Polymeric particles also have adverse effects on organisms, and the possibility of their translocation, bioaccumulation, and trophic accumulation is currently being debated (Wright et al. 2013). Many materials in our daily life are made of micro (nano) plastics. Dris et al. (2016) detected the human-made fibers present in the atmospheric fallout, suggesting that the atmospheric phase contains fibers that lead to human exposure. This exposure raises concern. Pauly et al. (1998) observed human lungs with a microscope. It was shown that 87% of the studied lungs (n = 114) contained fibers. Cellulosic and plastic fibers were both observed. Moreover, the same study revealed that 97% of malignant lung specimens had fibers. The fibers’ length was mainly around 50 μm but could reach a length longer than 250 μm. So there is a high risk of inhalation of micro (nano) plastics from the atmosphere. Small polymeric particles and fibers could be breathed in and may settle in the lungs of adults and children (Churg and Brauer 2000; Atis et al. 2005; Prata 2018). A recent study using human breathing thermal manikin showed that MPs up to 272 might be consumed from inhaling indoor air over 24 h (Vianello et al. 2019). Other routes of exposure to atmospheric NPs might happen via the skin and exposed food from atmospheric fallout deposition. Nevertheless, distant from the possible risks posed to human health, possible risks are too posed to the environment. MPs have been proposed as one of ten emerging issues in UNEP Year Book 2014 and have been identified as an essential factor leading to biodiversity loss (Gall and Thompson 2015) and pose a potential threat to human health and activities (Eerkes-Medrano et al. 2015; Carbery et al. 2018; Bhat et al. 2022). Recent COVID-19 research on polluted exteriors and aerosols indicates that people can acquire coronavirus via the air and subsequently handling polluted substances. The virus was evident in aerosols for up to three hours, copper for up to four hours, cardboard for 24 h, and plastic and stainless steel for two to three days (Van Doremalen et al. 2020). Recently Kayalar et al. (2021) evaluated the possible presence of SARS-CoV-2 on ambient particulate matter taken from hospital gardens and urban sides. This has increased concern regarding the potential danger of airborne disease through coronavirus and flu virus through polymeric particles (NPs and MPs) in a contaminated atmosphere. There is a chance that nano and MPs can be the passive carriers of coronavirus like the way they carry the other microorganisms, persistent organic pollutants, polycyclic aromatic hydrocarbons, and bisphenol A. Detailed information about how nano and MPs carry these contaminants will be discussed in section (enhanced toxicity via adsorption). However, more research is needed in this field.
Fig. 2.
Sources and human exposure pathways to micro (nano) plastics (plasticles) in the environment
Micro (nano) plastics might be more environmentally harmful than large plastic waste owing to the greater specific surface area, the capability to adsorb more pollutants (Turner & Holmes, 2015), and easier access to organisms (Hurley et al. 2017). NPs are by-products of plastic materials that stem from different environmental stress factors. Therefore, human health risk assessment associated with NPs daily intake represents an essential topic for public health. As we know, the main human intake routes are inhalation, ingestion, and skin perfusions (Fig. 2). There is no confirmation concerning the uptake of ambient NPs through contact or inhalation, though several studies have suggested the concept. The capacity for airborne NPs to be inhaled depends on the size of the airborne NPs, deciding if they can penetrate the respiratory system. Thus, after entering from the nostrils or mouth, a particle can be inhalable (i.e., capable of remaining accumulated in the upper airways) and respirable (i.e., capable of moving and accumulating in the deep lung). Although environmental exposure has not been investigated, it is believed that industrial workers are more prone to the diseases caused by airborne NPs. In contrast to common exposure to low environmental concentrations, workers in certain positions may be exposed to high concentrations of NPs, resulting in occupational diseases; due to this reason, we also highlighted the occupational routes of nano and MPs.
Plastic-laden toxicity: monomers and additives
Micro (nano) plastics may contain endogenous chemical additives because they are added during the production of plastic products to achieve the desired properties for the intended use. Due to incomplete polymerization, these monomers and additives integrated into plastics can drift from the matrix, resulting in escape from internalized (ingested, inhaled) particles (Cole et al. 2011) or discharge from home items into the indoor air and dust, as had been shown for phthalates and bisphenol A (Rudel et al. 2003), so micro (nano) plastics can therefore be a source of pollutants. The process of desorption is assumed to be associated with the micro (nano) plastics’ physical structure, the contaminants’ polarity (Liu et al. 2018), the retention time of the particles in the air, and the chemistry of the atmosphere. Purposefully mixing chemical plastic additives (e.g., bisphenol A, phthalates, and flame retardants) during their manufacture are of growing concern as they may be emitted into the atmosphere from the material, such as phthalates and BPA, might perform as endocrine disruptors, interacting with endogenetic hormones (Cole et al. 2011), and can participate in the pathological processes of few lung illnesses (Ventrice et al. 2013; Xie et al. 2016). A common component of PC plastic and epoxy resins is bisphenol-A. It is employed to increase different plastic items' elasticity, transparency, and toughness as an additive. During the manufacture and use of plastic goods, bisphenol A can be emitted and is present both in aqueous and terrestrial ecosystems and in animals/organisms. In food, gasoline, ethanol, and different water types (tap water, boil tap water, MilliQ water, deionized water, and mineral water), bisphenol A levels vary from 0.05 μg L−1 to 1.6 mg L−1 (Wong et al. 2007; Koni and Per 2008; Biedermann-Brem and Gro 2009; Maia et al. 2009). Most of this was emitted from consumer plastic items, like plastic bottles and food packaging. Lomonaco et al. (2020) found that aromatic compounds were produced from PS, while carboxylic acids, lactones, and ketones were emitted mainly from polyolefins. By contrast, PET had a negligible release profile, which primarily included very modest quantities of ketones and aromatics. In addition, Lomonaco et al. (2020) highlighted that the most common MPs, that can be found as floating debris or as particulate matter that pollutes beaches practically all over the world, i.e., polyolefins and PS, emit toxic and harmful compounds (e.g., acrolein, benzene, propanal, methyl vinyl ketone, and methyl propenyl ketone). In addition to this phenomenon, Hüffer and Hofmann (2016) explained seven contaminants (n-hexane, cyclohexane, benzene, toluene, chlorobenzene, ethyl benzoate, and naphthalene) could get adsorbed on four MPs like PA, PE, PVC, and PS. So, it becomes clear that these nano and MPs represent an unrecognized source of harmful trace gases, increasing their toxicity.
There is a possibility for continuous movement of inherent chemicals through a concentration grade to the surface of micro (nano) plastics once they start to break. These contaminants may be emitted upon assimilation and pass to the nearby tissues. If micro (nano) plastics can accumulate, they may display a source of chemicals to tissues and fluids if some additive leftover is leached. As per Linares et al. (2015), unreacted plastic monomers, additives, dyes, and pigments may have health consequences, like reproductive toxicity, carcinogenicity, and mutagenicity leached or volatilized and accumulated in plastics. For instance, house-settled dust contamination by polybrominated diphenyl ethers (Fromme et al. 2014; Rauert et al. 2014; Linares et al. 2015) or phthalates (Sukiene et al. 2017) is well known globally, likely due to fibrous microplastic emissions arising from the usage of plastic home textiles. Evolving research evidence supports the plausibility of human exposure to micro (nano) plastics. It is unclear if micro (nano) plastics and their related chemicals are transmitted by diet and/or inhalation to humans.
Nanoparticles as a matrix for the toxicity of nanoplastics
Analogies among the toxicity of nanoparticles and NPs should be simplified to address chemical toxicity. NPs are nanoparticles with different performance and harmfulness because of their small size and particles < 100 nm (Yildirimer et al. 2011; Elsaesser and Howard 2012). The consumption of macromolecules from body fluids to the nanoplastic surface can lead to the development of a corona that affects their performance and translocation (Gallagher et al. 2015). The toxicity of NPs from other nanoparticles cannot be easily extrapolated because NPs are inert and possess different surface and physicochemical features (Bouwmeester et al. 2015; Bhat et al. 2022). NPs can be contrasted with different insoluble nanoparticles, like gold and titanium oxide (Bouwmeester et al. 2015), together seen to undergo translocation (De Jong et al. 2008; Shi et al. 2013). Still, though titanium oxide has slight proof of a cancer-causing influence (Hext et al. 2005), besides merely causing mild swelling of the lungs in rats with improvement past three weeks (Grassian et al. 2007), a greater occurrence of cancer is associated with inhalable plastic particles (Vobecky et al. 1984; Marsh et al. 1994; Acquavela et al. 1998; Mastrangelo et al. 2002); in addition, exposure in mice causes granulomatous lesions having slow improvement past 12 weeks (Agarwal et al. 1978). This illustrates the complexity of contrasting NPs toxicity directly with other nanoparticles. Still, several statements may be formulated concerning the performance of NPs. Similar behavior is predictable in the brain, which might be influenced by nanoparticle accumulation in the olfactory mucosa and translocation through the olfactory nerve (Oberdörster et al. 2004), passing the blood–brain obstacle via passive diffusion means (particularly minute hydrophobic molecules) or endocytosis facilitated by the carrier, neuron depolarization can be affected (Hoet et al. 2004; Yang et al. 2010). While small hydrophobic particles, NPs might be in a position to pass the blood–brain barrier. PE has also been reported to reduce acetylcholinesterase activity in fish, likely influencing neurotransmission (1–5 mm) (Oliveira et al. 2013).
NPs have been extensively studied as persistent pollutants in marine and freshwater environments (Stroh et al. 2004; Mazurais et al. 2015). Reported evidence indicates that the ecological toxicological effects of NPs on aquatic organisms are complex. However, the pollution status and ecological toxicological impact of NPs in soil and the atmospheric environment remain unclear, with the atmospheric behavior being particularly poorly understood. Characteristics of NPs such as minimal diameter (˃100 nm), high tissue affinity, and high adsorption capacity make them prone to uptake by cells, and ready cellular internalization and accumulation can lead to health effects including genotoxicity, inflammation, and even carcinogenicity (Forte et al. 2016). However, the toxicity of NPs to lung cells remains mostly unexplored. Potential interaction between NPs and human skin happens via exposure to a polluted atmosphere or usage of individual maintenance items. Here is certain uncertainty as to whether the NPs will infiltrate human skin. The stratum corneum serves as a substantial barricade to the skin. The hydrophobic features make it difficult for NPs to enter via skin in water (Lehner et al. 2019). PS NPs with dimensions varying from 20 to 200 nm were merely able to penetrate the stratum corneum to a depth of 2–3 μm (Campbell et al. 2012). Yet, using different constituents in individual maintenance items can support the entry of NPs. Fresh perspectives on this subject can be given by extrapolating findings from other nanoparticles. Evidence has revealed that skincare lotion ingredients (i.e., glycerol, urea plus alpha hydroxyl acids) improve access to excised human skin of quantum dot nanoparticles (20.9 nm) (Jatana et al. 2016). Even though NPs and different nanoparticles (e.g., quantum dot, metal oxide nanoparticles) can vary in surface chemistry, the entry of NPs is highly reliant on particle size; subsequently, nanoparticles’ findings can provide valuable evidence on the entry of NPs of the same dimensions. Skin penetration might typically lead to the human intake of NPs; however, additional direct evidence is required. In general, we can say that the toxicity and behavior of NPs may or may not be similar to different nanoparticles. Therefore, generalization is not feasible, and the impacts of NPs of various dimensions, polymers, and surface features need to be studied to explain their toxicity in a better way. If nanoparticle information is extrapolated to NPs, it must be done with care as NPs have, unlike surface chemistry. Despite the numerous concerns regarding the potential adverse effects of plastic NPs, the establishment of baseline observations and long-term monitoring programs are still in their early days. Determining NPs contamination levels in targeted organisms is crucial as it will establish a temporal and spatial comparison and enable the assessment of real environmental and human health risks.
Enhanced toxicity via adsorption
Micro (nano) plastics can also act as passive samplers. These have a large surface-to-volume ratio as high as 4.37 m2/g (Teuten et al. 2007), making them a suitable sorbent for toxic chemicals adsorption in the environment. However, the attachment is dependent on plastic types and surface structures, which control the affinities to pollutants (Wirnkor et al. 2019). Toxic chemicals such as heavy metals and hydrophobic organic chemicals are attached by physical interaction. Therefore, desorption of the bound chemicals can occur quickly post-ingestion or during transport to a new environment by wind (Allen et al. 2019). The plausible mechanism by which toxic chemicals get attached to nano and microplastic surfaces includes hydrophobic adsorption through formed biofilm and plastic additives (Wirnkor et al. 2019). MPs may adsorb and accumulate hydrophobic organic pollutants like polycyclic aromatic hydrocarbons, organochlorine pesticides, and polychlorinated biphenyls to a great extent due to their hydrophobic surface (Bakir et al. 2014; Mato et al. 2001; Ogata et al. 2009). Heavy metals like lead, cadmium, nickel, zinc, and pathogens are also accumulated (Holmes et al. 2012; Rochman et al. 2014; Baldwin et al. 2016). Shen et al. (2021) found that different types of heavy metals like chromium, copper, zinc, and lead were absorbed on the surfaces of MPs. Due to the more stable molecular chain structure in a rubbery fraction of PE and PP, PE and PP have demonstrated greater sorption affinity for polycyclic aromatic hydrocarbons and dichlorodiphenyltrichloroethane than PS and PVC (Bakir et al. 2014; Wang and Wang 2018). In addition to plastic compounds, micro (nano) plastics can also discharge environmental pollutants because of their broad surface area and hydrophobicity. Weathering might intensify the uptake of pollutants by increasing polarity and surface area (Teuten et al. 2009; Wang et al. 2016). Nevertheless, the surface oxidation occurring due to the aging procedure might raise metals' affinity (Andrady 2011), reducing the interaction of hydrophobic compounds (Teuten et al. 2009). Therefore, old plastics are likely to consume diverse affinities for adsorption than their virgin equivalents. In addition, polycyclic aromatic hydrocarbons had also been found in MPs (Ogata et al. 2009; Frias et al. 2010; Bakir et al. 2012). Micro (nano) plastics tends to serve as a vector between organisms and the environmental compartment. Queries about polycyclic aromatic hydrocarbons related to MPs toxicity, monomers, and additives have previously been assessed by Horton et al. (2017) and Teuten et al. (2007).
Organic contaminants had been found in particulate matter in the atmosphere (Schnelle-Kreis et al. 2001; Van Vaeck and Van Cauwenberghe 1978). Polycyclic aromatic hydrocarbons can volatilize, reach the atmosphere where they may be able to travel long distances, or break into subdivisions and aerosols, depending on temperature and physicochemical properties (Jones and De Voogt 1999). Micro (nano) plastics might also be exposed to polycyclic aromatic hydrocarbons in the atmosphere. However, adsorption depends on the time that micro (nano) plastics are suspended, and the amount of micro (nano) plastics inhaled is potentially harmful to organisms. However, airborne exposure to novel persistent organic pollutants pellets for almost six days does not lead to substantial adsorption, implying that atmospheric persistent organic pollutants adsorption might be minimal (Mato et al. 2001). Meanwhile, atmospheric micro (nano) plastics remain extremely subjected to UV rays, and weathering phenomena can also occur, which implies that more research is needed. Atmospheric micro (nano) plastics are secondary to manufacturing procedures, which too carry chemicals and, at the same time, expose the workers’ lungs to all pollutants. As for other air pollutants, it is difficult to classify each agent’s role in pathophysiology, and toxicity can often result from a dynamic collaboration (additive, collusive, or adversary) among contaminant mixtures. Additionally, an investigation should attempt to explain the part of every particle, particularly in the work-related illnesses mentioned.
NPs on their surfaces can carry organisms. Due to their extreme surface area, special populations of microorganisms had been identified applying water MPs as surfaces (Oberbeckmann et al. 2015); these involve possibly disease-causing species like Vibrio spp. (Kirstein et al. 2016). NPs might serve as carriers for these species, move them over long distances, and expose organisms’ guts once consumed. Though NPs can be bare to fecal–oral pathogens in water ecosystems (like NPs bare to wastewater), airborne NPs transportation is possibly rare; it depends on the atmospheric encounter and adsorption of the microorganism to the particle’s surface. However, urban aerosols comprise different microorganisms, and particles may serve as a barrier to protect them against harmful UV rays (Brodie et al. 2007). In fact, microbes tend to be found in 4–20 μm aerodynamic equivalent diameter aerosols (Noble et al. 1963). Micro (nano) plastics might also serve as a barrier in the atmosphere to transport and protect microorganisms. Microorganisms can be carried directly into the human lung by sticking to the surface of micro (nano) plastics, evading protection mechanisms and likely causing toxicity, particularly in compromised parts previously affected by particle exposure. However, as earlier findings on aquatic systems have shown, the function of micro (nano) plastics as carriers is restricted once contrasted to additional paths, like water or, in this case, air. In occupational exposure, exclusions can happen infrequently.
Occupational routes of airborne nanoplastics
Exposure to airborne NPs may be higher in occupational settings than in the home, resulting in long-term health effects from these pollutants. Workers exposed to higher levels of airborne polymeric particles have been proven to be at greater risk of developing occupational illnesses. The implications of airborne micro (nano) plastics can be demonstrated by three industries: synthetic textile industry (Cortez Pimentel et al. 1975); flock industry (Eschenbacher et al. 1999; Kern et al. 2000; Barroso et al. 2002; Atis et al. 2005); vinyl chloride (VC); and PVC industry (Xu et al. 2004).
In the synthetic textile industries, there is a high concentration of fibers composed of PA, PES, PUR, polyolefin, acrylic, and vinyl-type polymers. Cortez Pimentel et al. (1975) evaluated that synthetic fiber dust from synthetic textile industries is often comprised of PES, PA, PUR, acrylic, polyolefin, and vinyl-kind polymers. Most studies have related synthetic fiber inhalation to respirational issues (Valic and Zuskin 1977; Goldberg and Thériault 1994; Zuskin et al. 1998). Chronic experience with irritants and persistent synthetic fibers can also contribute to cancer (Marsh et al. 1994; Mastrangelo et al. 2002). Synthetic fibers, additionally, appear to comprise lesser or comparable harmfulness than organic textile fiber inhalation. Several carbon-based fibers (e.g., hemp) can remain accountable for higher incidents of dyspnea than synthetic fibers; also, the inherent tendency to interact with the chemical of biological functions in humans might be due to their high biological activity (Valic and Zuskin 1977). So, between organic and synthetic fibers, cancer risks might be identical. The most significant primary MPs sources are assumed to be synthetic textiles, synthetic rubber tire erosion, and city dust, and wind transport is assessed to be accountable for 7% of the ocean’s pollution (Boucher and Friot 2017). Plastic particles from clothes and house furniture can be other sources of airborne MPs (Bhat et al. 2021; Dris et al. 2016, 2017; Liebezeit & Liebezeit 2015), building constituents, landfills, waste incineration (Dris et al. 2016), industrial discharges, particle resuspension, traffic-emitted particles (Dris et al. 2015), synthetic components (e.g., PS peat) used in horticultural soils, waste slurry consumed as manure (Liebezeit and Liebezeit 2015) and probably tumble dryer discharge. Besides, scientific investigations have been capable of detecting synthetic fibers from exterior surfaces and vehicle seats (Grieve and Biermann 1997; Roux and Margot 1997) and worn T-shirts (Marnane et al. 2006). Indeed, the foremost source of atmospheric nano and MPs is assumed to be synthetic clothing, fiber material, and amount reliant on vogue and period. Consequently, in both indoor and outdoor surroundings, synthetic materials might be accountable for environmental exposure.
The flock industry involves fiber coatings to enhance quality and add value to almost any surface in several ways like aesthetic, decorative, durability, insulation, texture, comfort, etc. Velvet-like or fleece-like materials are manufactured from 0.2 to 5.0 mm pulverized or cut fibers (flock) and are mainly made of PA, PES, PE, and PP, used in adhesive layered things (Eschenbacher et al. 1999; Kern et al. 2000; Barroso et al. 2002; Atis et al. 2005). Throughout the cutting process, these synthetic fibers often produce inhalable particles (Kern et al. 2000; Atis et al. 2005), which result in occupational lung disease: flock disease (Eschenbacher et al. 1999; Kern et al. 2000; Washko et al. 2000). A properly examined flock industry possessed an extraordinarily elevated occupational lung disease occurrence in Rhode Island (Kern et al. 1998). Further research showed insufficient ventilation that caused increasing concentrations of inhalable dust and the incidence of systemic and respiratory symptoms in 64.7% of manufacturing and maintenance employees, relative to working hours per week (Washko et al. 2000). The unusual respiratory symptoms in flock employees with a high prevalence frequently recover after exiting the work place and get worse when returning to the work (Eschenbacher et al. 1999; Kern et al. 2000).
Workers at PVC plants are frequently exposed to both VC and PVC (a white powder able to produce inhalable dust), which can have serious health consequences. PVC is formed by VC polymerization as white dust that might appear as an inhalable powder (Xu et al. 2004). On the other hand, several works disclose the link among experience to PVC powder and VC monomers to undifferentiated restrictive lung disease used in several activities (Cordasco et al. 1980; Ng et al. 1991; Mastrangelo et al. 2003; Xu et al. 2004). PVC dust, VC monomers, and thermal disintegration of items can result in toxicity (Antti-Poika et al. 1986; Lee et al. 1989; Ng et al. 1991). A worker exposed to PVC powder for 25 years experienced chronic morning cough, fatigue, dyspnea, radiological diffuse micronodular opacities, a minor decrease in vital ability, diffuse macrophage infiltration, and pneumoconiosis-diagnosed collagen formation (Arnaud et al. 1978). The occupational diseases mentioned tend to be consequence of the noxiousness of plastic units or leachates after inhalation. In human beings, the reaction to breathed particles can be specified as instant respiratory consequences (asthma-like), interstitial lung disease and granulomas with fiber inclusions (extrinsic allergic alveolitis, chronic pneumonia), inflammatory and fibrotic changes in the bronchial and peribronchial tissue (chronic bronchitis), and interalveolar septa lesions (pneumothorax) dependent on variations in person metabolism and vulnerability (Cortez Pimentel et al. 1975; Beckett 2000). In the synthetic textile, flock, and VC or PVC industries, occupational diseases frequently occur in employees with related indistinguishable airway and interstitial lung diseases, possibly because of the nuisance and persistent character of plastic particles (Valic and Zuskin 1977; Marsh et al. 1994; Mastrangelo et al. 2002). However, during a particular event of insufficient ventilation in an air spray unit, the staff’s constant breathing of polyacrylate nanoparticles revealed similar conditions causing two deaths due to breathing collapse (Song et al. 2009).
Micro (nano) plastic toxicity: animal experiments and in vitro toxicity tests
The hazards of micro (nano) plastics to human health can be deduced from animal and laboratory studies. Toxicology investigations of micro (nano) plastics in aquatic animals first relied on a well-known model organism, Danio rerio (Lu et al. 2016). In these investigations, micro (nano) plastics are consumed by lab-grown Danio rerio during a seven-day exposure period, and hepatotoxicity is determined using fluorescent-tagged PS micro (nano) plastics (20 µm, 5 µm, and 70 µm). When micro (nano) plastics are consumed, they can form pathogenic biofilms in the host's gut, disturbing or interacting with the gut microbiota (Li et al. 2022). For example, Xie et al. (2021) exposed Danio rerio to 10–1000 µg L−1 of MPs (8 µm, PS) and NPs (80 nm, PS) for 24 h and found that PS NPs caused more gut inflammation and gut-microbial community disturbance than MPs. The freshwater amphipod crustacean Hyalella azteca demonstrated impaired development and energy levels after exposure to PE and PP MNPs (fibers and particles) for 10 to 42 days (Au et al. 2015). Surprisingly, micro (nano) plastic fibers were more hazardous than particles. PMMA micro (nano) plastics might cause oxidative muscle stress and inhibit the growth of Atlantic gilt-head bream, Sparus aurata, within 96 h of exposure (Balasch et al. 2021). Microplastic-related illnesses have also been documented in secondary organs. In vitro, human red blood cell interactions with PS Microplastic resulted in blood cell aggregates and adhesion to endothelial cells (Barshtein et al. 2016). Granular histiocytosis induced by wear particles from the artificial joints, including MPs, was detected in the lymph nodes of patients having total hip arthroplasty (Hicks et al. 1996).
Sampling, sample preparation and analysis
Presently there are some protocols to detect MPs (Filella 2015); however, there is a lack of reliability in sampling, sample pre-treatment, analysis, and testifying results. The NPs analysis is more comprehensive, and procedures are not being developed. One of the main tasks is the pre-concentration of samples necessary for the current detection limit. The assemblage of sample materials/instruments and procedures is diverse depending on the air's location, whether indoor or outdoor.
Sample collection
A sampling pump may be used for indoor air sample processing (Dris et al. 2016, 2017; Gaston et al. 2020; Prata et al. 2020). Likely, a vacuum pump or vacuum cleaner usually used by households and dirt obtained in cleaner bags can be sampled for deposited MPs indoors, directly sweeping the floor with a PA brush or by hog bristle brushes (Liu et al. 2019a; Zhang et al. 2020a) or the deposition of MPs can be carried out directly by exposing filters or petri dishes (Dris et al. 2017; Zhang et al. 2020d). Two sample collection approaches were typically used for outdoor atmospheric microplastic analysis, including a wet deposition sampler, particulate fallout collector, or ambient filter sampler. These tools are placed at a precise height in the outdoor atmosphere (ground, aerial, or upper) (Abbasi et al. 2019; Cai et al. 2017; Dris et al. 2015, 2016, 2017; Gaston et al. 2020; Klein & Fischer 2019; Liu et al. 2019b, c; Prata et al. 2020; Tunahan Kaya et al. 2018; Wright et al. 2020) over some time. It is crucial to investigate the amount of airborne MPs in a wet and dry atmospheric deposition to estimate a total load of MPs input into the environment.
Filtration substrate
Standard filters used for detecting MPs in air samples include glass fiber GF/A Whatman filters (1.6 µm) (Dris et al. 2015), quartz fiber GF/A Whatman filter (1.6 µm, 47 mm) (Dris et al. 2016, 2017), glass microfiber filters Whatman GF/B (1.0 μm) (Cai et al. 2017), 0.2 μm pore size alumina-based membrane filters; silver membrane filters (1.2 μm pore size) (Wright et al. 2019a, b), GF/A glass microfiber filter (Whatman), with a 1.6-μm pore size and 90 and 47 mm diameter (Gaston et al. 2020; Liu et al. 2019b, c), polytetrafluoroethylene (PTFE) filter papers (46.2 mm in diameter and 2 mm pore size); S&S filter papers (2 mm pore size) (Abbasi et al. 2019), 5 μm cellulose filter membranes (Whatman GF/B) (Zhang et al. 2020d).
Sample preparation
Settled microplastic in indoor dust tends to adhere to dust particles. Consequently, they are isolated depending on their density. Synthetic polymers are floated by density separation using inorganic salts such as zinc chloride (ZnCl2), sodium hydroxide (NaOH), sodium iodide (NaI), sodium polytungstate (Na6O39W12, SPT), and lithium metatungstate (Li2O13W4-24). When separated, they are then additionally treated (post-treatment) using relatively vigorous chemicals such as potassium hydroxide (KOH), hydrogen peroxide (H2O2), sodium hypochlorite solution (NaClO), perchloric acid (HClO4), and nitric acid (HNO3) to remove natural debris or unnecessary material on the microplastic surface. These chemicals can chemically degrade, or morphology affect nano (microplastics), so their usage will be dependent on the presence of particular nano (microplastics).
Analytical techniques
The undesirable substances can be organic and inorganic, like biofilm and dust. It can hinder analysis and create a significant issue in detecting synthetic polymers with MPs. If not eliminated, these non-analytes can have the same chemical and physical properties that closely match the analyte of interest. The final examination, before concluding the findings, involves the usage of various spectroscopic or spectrometric methods. The classical procedures used for recognition and measurement comprise the usage of a naked eye or microscope for visualization; however, the standard techniques include Fourier-transform infrared spectroscopy (FTIR), Raman and Scanning Electron Microscopy/Energy Dispersive X-Ray Spectroscopy (SEM–EDS), while the sophisticated techniques include Liquid chromatography-mass spectrometry (LC–MS/MS), high-performance liquid chromatography-mass spectrometry (HPLC–MS/MS), thermal desorption/pyrolysis, Pyrolysis–gas chromatography-mass spectrometry (Pyr-GCMS), thermal desorption gas chromatography with mass spectrometric detection, and thermogravimetric analysis-Fourier transform infrared-gas chromatography–mass spectrometry for a compositional profile. The experimental theory and its utility for microplastic research are thoroughly reviewed (Abbasi et al. 2019; Allen et al. 2019; Duan et al. 2020; Shim et al. 2017; Zhang et al. 2020b, c). Analytical methods are frequently used in parallel, one extracting and one computing, one detecting and the other verifying. For example, it is not recommended to recognize microplastic size < 500 μm through visual recognition alone. Consequently, a follow-up procedure for clarification, such as micro FTIR and micro Raman, has been suggested by Hidalgo-ruz et al. (2012); Shim et al. (2017).
Fourier-transform infrared spectroscopy
FTIR is generally perceived as the most suitable analytical tool for microplastic analysis (Rocha-santos and Duarte 2015). The detection of small particles can be carried out by Focal Plane Array (FPA)-micro FTIR thus far, considered the most promising approach for small microplastic particles. It avoids the pre-sorting of MPs and provides unbiased data by the analysts Loder et al. (2015); Primpke et al. (2017). Since NPs have physical, optical, and chemical constraints, conventional optical and chemical analysis procedures used to detect and characterize MPs are not appropriate for NPs. Examples include FTIR and Raman microspectroscopy, which have spatial resolutions that are insufficient for studying NPs because of their small sizes. Identifying MPs on its own entails a high risk of providing analytical findings that are both falsely positive and falsely negative. Still, this risk increases considerably when NPs must be detected. This is a significant factor in the lack of information and understanding of secondary NPs and how they are formed. To estimate the processes of the creation of the NPs, it is critical to better understand the degradation mechanisms of various plastics at advanced stages of environmental degradation.
Light scattering techniques
Several techniques, like laser light scattering (e.g., dynamic light scattering, laser diffraction, nanoparticle tracking analysis, multi-angle light scattering) and electron microscopy (e.g., SEM, transmission electron microscopy) can be used to quantify the amount, size, and particle size distribution of NPs (Mattsson et al. 2018; Schwaferts et al. 2019). However, none of these methods can chemically identify the NPs (Mattsson et al. 2018). Dynamic light scattering, which operates in the range of 1 nm–3 m (Schwaferts et al. 2019), is one of the most extensively utilized methods for size and particle size distribution characterization. In polydisperse samples, the method may underestimate the size of bigger particles since it relies on theoretical models based on spheres (Schwaferts et al. 2019). Nanoparticle tracking analysis is a more sensitive technique for analyzing secondary NPs produced by fragmentation than dynamic light scattering because it is less susceptible to disruptions from bigger particles in polydisperse samples (Ekvall et al. 2019; Schwaferts et al. 2019). Nanoparticle tracking analysis relies on the scattering of laser light captured by a microscope and a digital camera, as well as subsequent software processing, to provide information on individual particle hydrodynamic diameters in the low nanometer to low micrometer size range (Xu 2015). Lambert et al. utilized nanoparticle tracking analysis to identify generated NPs during weathering of several plastics, including polylactic acid, PP, PS, PE, and PET (Lambert and Wagner 2016a, b), as well as a rubber latex material (Lambert et al. 2013), while Ekvall et al. (2019) used nanoparticle tracking analysis to monitor the creation of NPs from mechanical breakdown of everyday PS products. Because it permits solid particles distributed in liquid media to be characterized across a wide size range (10 nm–10 mm) via static light scattering, laser diffraction may be useful for NPs (and MPs) (Xu 2015; Schwaferts et al. 2019). It is far more challenging to comprehend the scattering patterns caused by non-spherical particles. Multi-angle light scattering takes advantage of laser light scattering at various inclinations.
Raman spectroscopy
Raman spectroscopy is a non-destructive spectroscopic method that enables the observation of low-frequency modes in a structure, like rotational and vibrational interactions. It is a basic technique that produces a structural fingerprint that has been effectively used to classify microplastic particles in various environmental matrices. Infrared spectroscopy is analogous to Raman spectroscopy, specifically FTIR, since it facilitates the accurate detection of polymers based on their infrared spectrum. FTIR and Raman techniques might be regarded as complementary methods, as molecular vibrations that are Raman inactive are infrared active and vice-versa. Raman microscopes were combined with particle recognition applications to simplify component identification to minimize operator bias (Frère et al. 2016; Schymanski et al. 2018). The software will distinguish particles between 300 nm and ≥ 30 mm (Opilik et al. 2013). However, its adaptation to air samples is complicated. The elevated levels of particles in atmospheric samples will consequence in lengthy investigation times and undercounting particles adjoining each other depending on the sample density. Raman spectral imaging had been suggested as a substitute method for countering operative bias and optimizing spectroscopic study of adjacent particles. Raman microscopes that had effectively employed Raman spectral imaging to categorize polymeric particles from 400 nm (Cole et al. 2013), in simple spectral images comprising solitary the plastic particulate and the Raman substrate, to ≥ 50 μm, include confocal Raman spectroscopy, stimulated Raman scattering (Cheng and Xie 2013; Réhault et al. 2015; Zada et al. 2018), coherent anti-Stokes Raman microscopy (Cole et al. 2013), and structured line illumination Raman microscopy (Watanabe et al. 2015). The benefit of operating traditional Raman spectrometers is that they are commonly obtainable, instinctive to usage, and can recognize ≥ 2 μm MPs. Although Raman spectral imaging can detect virgin MPs that would enter the pulmonary alveoli if inhaled, it was only authenticated to identify virgin MPs > 4 μm in atmospheric particulate matter gathered on filters (Wright et al. 2019a). Although the boundaries of microplastic identification have been scientifically improved, there is still a need to refine Raman spectral imaging to classify respirable atmospheric MPs in dynamic spectral images, as PM2.5 has been correlated with an enhanced effect on well-being (Pope and Dockery 2006). The polymeric material of ambient particulate matter (filters can interfere with FTIR and Raman spectral imaging signals when filters are directly observed under them. Wright et al. (2019a) found that quartz, alumina, and PTFE filters were not appropriate for visual assessment of MPs and/or were not compatible with direct Raman spectral imaging in a sample. MPs were noticeable against cellulose, while particulate matter-contaminated filters (4 and 24 h) were burning throughout the study. The greater microplastic density was found for the silver membrane filter, and respirable MPs were also found in a 24-h sample of particulate matter. Filter composition should not have a prominent Raman spectrum or fluorescent background in the polymer bands of interest (Käppler et al. 2015). The silver membrane filter has little signal obstruction related to the aluminum membrane filters for micro-FTIR (Wright et al. 2019b).
Atomic force microscopy
Atomic force microscopy has been coupled with vibrational spectroscopy (like FTIR) to allow the chemical identification of individual submicron particles. It is possible to get chemical and topographical images of nanoparticles and infrared absorption spectra using this method, with a spatial resolution of around 100 nm (Dazzi et al. 2012; Huth et al. 2012). For polymer identification, Huth et al. (2012) showed that nano FTIR, based on scattering-type scanning near-field optical microscopy and a novel laser-based coherent-continuum infrared light source, can provide infrared spectra with a spatial resolution of 20 nm suitable for conventional FTIR databases. However, it has yet to be demonstrated that these methods can be used for the high-throughput chemical detection of many particles in environmental materials (Gillibert et al. 2019) used optical tweezers to catch dispersed (in liquid) particles and use Raman spectroscopy to analyze NPs down to the 50 nm range for chemical characterization. Micro Raman can compare particles with a lower diffraction limit of 250 nm compared to micro FTIR with a maximum diffraction limit of 10 μm and provide a more significant resolution analysis for microplastic particles (Araujo et al. 2018; Renner et al. 2018). The micro FTIR limit on the analysis of MPs is about 20 μm in a practical application. Käppler et al. (2016) showed the underestimation during micro FTIR analysis of approximately 35% of MPs < 20 μm compared to micro Raman. A comprehensive polymer library for identification and comparative study benefits from the micro FTIR analyses. As the uses of micro Raman grow, a library with similar resources is expected to be established, allowing the identification of the polymer type equal to the existing micro FTIR library. This means micro Raman now has the upper hand regarding the lower limit of the study of the particle size and the cheap cost associated with completing the analysis.
Scanning electron microscopy
Nanoplastic examination, detection, and quantification remain a challenging issue since it is not enough to describe these particles morphologically but also to acquire a comprehensive nanoscale chemical analysis. There are currently no guidelines developed for the identification and quantification of NPs in specific environmental samples. No papers identify the effective detection and characterization of NPs in the environment, as it is challenging to differentiate them from natural nanoparticles. Therefore, novel methods for properly identifying these particles in environmental samples are necessary to establish, and specific methods can transfer from other sciences, whereas original methodologies can be established that are fit for purpose. SEM–EDS is a commonly used method for characterizing engineered nanomaterials. It is the widely used surface analytical method that can achieve greater than 50 pm spatial resolution (5.0 × 10–5 μm) (Erni et al. 2009). A recent technique for detecting NPs in aquatic and complex matrices, such as in food, is asymmetric flow field-flow fractionation combined with multi-angle light scattering (Mintenig et al. 2018). The fractionation stage asymmetric flow field-flow fractionation allows synthetic polymers to be isolated, while multi-angle light scattering decides their size. However, at present, this technique also has some drawbacks, including poor recovery rates (~ 50% for 50 nm plastic particles (Mintenig et al. 2018) and increased light scattering background, which inhibits the detection of, for instance, PE, anticipated to be among the maximum ubiquitous NPs in the environment (Da Costa et al. 2016). Though not yet enhanced, the recovery rates presently achieved can be improved by upgrading the separation conditions in the asymmetric flow field-flow fractionation phase. As per Da Costa et al. (2019), these can involve pH and buffer levels, which directly affect the operation of this procedure. However, these are currently mere ideas, and they remain conceptual. Magnetic field-flow fractionation, gel electrophoresis, and size-exclusion chromatography are a few methods utilized to analyze designed nanoparticles that have been shown to be effective in the separation of NPs (Nguyen et al. 2019). Conclusion: there is a comprehensive critical evaluation of the many techniques for the extraction, separation, and preconcentration of NPs (Schwaferts et al. 2019).
Advantages and disadvantages of the analytical techniques
The advantages and limitations of the analytical techniques used for the analysis described above are summarized in Table 2. The most commonly used technique for particle size measurement is laser light scattering. As the laser moves through the suspension of NPs, the Brownian motion, which depends on the hydrodynamic diameter of NPs, will cause a variation in its strength. In order to analyze the surface morphology of NPs, electron microscopy is generally implemented. As the wavelength of electrons is far shorter than that of visible light, relative to optical microscopies (> 1 μm), electron microscopes have much higher resolutions (several nanometers) (Wang et al. 2020a). To concurrently analyze elemental distributions, electron microscopy can be paired with energy-dispersive X-ray spectrometers. Though various traditional methods for illustrating a nanoplate particle, such as FTIR and X-ray photoelectron spectroscopy, are not employed because of their size limitations, they still might offer abundant beneficial information for bulk nanoplastic samplings (Schwaferts et al. 2019). As it is clear multiple instruments have been used to analyze micro (nano) plastics. Still, it is difficult to find all these instruments in one laboratory or university as they are very costly. Here one of the critical questions is which instrument should be used first and last to get better results and save samples for an extended period. To our knowledge, a stereo or metal microscope should be used first to identify the presence or absence of micro (nano) plastics and to characterize them morphologically; FTIR and Raman should follow this. Using FTIR and Raman simultaneously can be a good option. Raman can identify most of the plastic particles that FTIR can not read as it has the upper hand concerning the lower limit of particle size analysis. All these instruments (stereo or metal microscope, FTIR, and Raman) are not harming the sample, which is the crucial advantage of these instruments and the sample can be used again for further analysis. There is a probability that intensity of Raman lazer can affect the sample if lazer intensity is high, so the lazer power should be within limits. This can followed by the usage of SEM–EDS and thermochemical techniques like (Pyr-GC–MS, LC–MS-MS, HPLC–MS Thermal desorption-GCMS, Thermogravimetric analysis-FTIR-GC–MS and time of flight secondary ion mass spectrometry) etc. all these instruments are affecting the sample and the sample can not be used again. Developing novel morphologically and proper experimental and analytical techniques can be crucial for finding an appropriate fate and transport of micro (nano) plastics.
Table 2.
Analytical techniques currently used to identify and quantify micro (nano) plastics in the atmosphere
| Techniques | Advantages | Limitations |
|---|---|---|
| Stereomicroscope |
Large numbers of MPs may be identified at a minimal cost; particle size, color, and form can be easily determined It is simple, straightforward, and quick |
Limitations of more than 500 µm; The examiner's subjectivity may cause significant inaccuracies There are no chemical confirmation results |
| FTIR |
FTIR techniques are trustworthy, fast, and nondestructive Micro-FTIR can study particles as small as 20 µm, while FPA-FTIR may detect thousands of particles in a single measurement |
The instruments are costly Samples must be IR active, and absorbance spectra from samples smaller than 20 µm may be challenging to interpret |
| Raman | A reliable technique, nondestructive and can detect particles down to 1 µm | Expensive, and there can be an interference by pigments during the analysis |
| SEM |
The high-resolution image of the samples can be produced (< 0.5 nm resolution) and can detect surface textures of micro (nano) plastics The chemical composition of samples can be identified by SEM–EDS |
It is a time-consuming and labor-intensive approach, and the samples must be prepared appropriately that is coating in a high vacuum for observation |
| Pyr-GC–MS |
Polymer types and additives may be examined in a single run Pyr-GC–MS is unaffected by sample shape, size, or color, and the procedure is reliable |
Time-consuming, expensive and destructive Morphological characterization of samples can not be detected Per run, only one particle with certain weight can be assessed, and its database is available only for selected polymers |
Fate and transport
In indoor and outdoor environments, the fate and dispersal of nano and MPs rely on many factors affecting human exposure. Factors that influence the behavior and transport of micro (nano) plastics in the atmosphere can also be similar to those of particulate matter, viz.: (a) vertical gradient of emission concentration (higher near-ground concentrations); (b) wind speed (increased wind speed contributes to reduced concentration); (c) the direction of the wind (parallel to perpendicular to obstacles); (d) the rainfall and (e) the temperature; (lesser temperatures intensifies nucleation and condensation causing in reduced atmospheric levels) (Kaur et al. 2007). In addition, wind variation induced by city topography (e.g., the distance among buildings), local meteorology, and thermal movement can contribute to the dispersal of air pollutants in outdoor urban environments (heat islands disturbing airflow) (Fernando et al. 2001). Precipitation, wind, local conditions, and particle size impact the particle’s residence time in the atmosphere and successive atmospheric fallout, causing bigger particles to be settled by gravity or afterward nucleation (Perrino 2010; Dris et al. 2015). Lower density polymers are lighter, and the wind will transport them, polluting terrestrial and aquatic habitats. Human exposure to atmospheric micro (nano) plastics is thus source-dependent and affected by meteorological and geographical aspects. In the outdoor atmosphere, the experience of lower levels of atmospheric MPs is anticipated because of mixing (Dris et al. 2017). But, the elimination of micro (nano) plastics may be decreased under unfavorable atmospheric settings (like low wind speed), causing higher exposure concentrations. The movement of this pollutant through the atmosphere can be influenced by the various shapes of micro (nano) plastics. Films, for example, may be very thin and smooth, thereby providing a wider surface area for atmospheric transport compared to fragments of a similar mass (Allen et al. 2019). The effect of shape on atmospheric transport is presently unfamiliar, and additional studies are needed. Precise microplastic shapes or sizes can be more likely to trigger physical injury to organisms, with smaller pointed particles more readily crossing membrane barriers than particles with regular exteriors or longer edges (Hidalgo-ruz et al. 2012).
The behavior of indoor airborne MPs depends on room separation, ventilation, and airflow, leading to elevated levels downwind in rooms (Alzona et al. 1979). Airborne nanoparticles (< 100 nm), like NPs, can quickly disperse among sections and stay airborne (Seaton et al. 2010). Meanwhile, maximum sources of fine particulate matter (Chang et al. 2006) and MPs (Dris et al. 2017) appear to be indoors, and humans spend almost 70–90% of their time indoors (Alzona et al. 1979); as a result, indoor exposure to airborne NPs tends to be further critical. Impacts on human health are probably also the result of workplace exposure and exposure at home. Ineffective environments in workplaces or homes with elevated concentrations of polymeric materials, like lack of effective ventilation, can lead to chronic exposure to high concentrations of NPs.
Two leading scientific problems relating to atmospheric micro (nano) plastics must be better understood to upgrade model computations besides limiting the causes of atmospheric micro (nano) plastics. The primary problem includes atmospheric nano and MPs environmental features, fate, and behavior. A range of queries, e.g., concerning the distinctive sizes, shapes, and forms of atmospheric micro (nano) plastics and the association of these characteristics to meteorological constraints, the life cycle of micro (nano) plastics in the atmosphere, and the lengthiest transport distance or atmospheric micro (nano) plastics, must be worked out in both urban and remote areas. Another problem includes the part of atmospheric transport in the deposition of micro (nano) plastics. In order to investigate this problem, interactions among micro (nano) plastics and other chemicals (such as mercury or polycyclic aromatic hydrocarbons) in the atmosphere, atmospheric micro (nano) plastics fractionation processes (changes in dimensions, forms, colors, and constituents throughout dry and wet deposition procedures) and the degree of such atmospheric transport for nano and MPs must be investigated and examined. In precise, Bank & Hansson (2019) introduced a new model favoring the plastic cycle as the constant and dynamic drive of plastic products, including humans, among various abiotic and biotic environment compartments. Atmospheric transport, still, is emphasized as an imperative path and has a strong effect on the complexity of source-sink plastic contamination in different environments (together with the cryosphere) (Horton and Dixon 2017). The environmental fate of small-scale plastic particles is significant for the appraisal of their latent capacity for dangers, yet this is confused by how the particles’ size, shape, density, and charge continually change after some time.
Since it is clear that nano and microplastic pollution has exhibited a global distribution, including seas, lakes, rivers, and terrestrial environment, in recent years; however, little attention was paid to the atmospheric environment though the fact that plastic debris can escape as wind-blown debris, which play an essential role in the physical and chemical exchange process between the atmosphere, land surface, and the aquatic environment. In aquatic environments, MPs act as transporters of persistent organic pollutants (Bakir et al. 2014). The settling down of plastic particles is decreased relative to normally suspended solids due to their low density, and they can migrate along with the adsorbed pollution over long distances. The fibers in the atmosphere, including MPs, could be transported by wind to the aquatic environment (Free et al. 2014) or deposited on cities or agrosystems surfaces. After deposition, they could impact terrestrial organisms or be transported into the aquatic systems through the runoff. In addition to other reasons, such as the deterioration of agrarian PE sheets or the discharge of fibers from drying cloths outdoor, the wind-driven transportation of MPs from sludge-based manures might also give rise to atmospheric microplastic concentrations (Bouwmeester et al. 2015). This atmospheric micro (nano) plastic contamination has been studied in different regions like identification of MPs on alpine river floodplains (Scheurer and Bigalke 2018) and lake sediment (Hurley et al. 2018), which illustrates the terrestrial microplastic occurrence, and in megacities as aerosol pollution (Cai et al. 2017; Gasperi et al. 2018). MPs have recently been quantified at a comparable daily rate in a deposition at a remote, pristine mountain catchment (French Pyrenees) (365/m2/day) (Allen et al. 2019). It is believed that MPs had been carried by air mass trajectory for up to 95 km, hitting the sparsely populated areas (Allen et al. 2019). This was further verified by findings of MPs in distant (Arctic, Swiss Alps) and metropolitan (Bremen, Germany) snow. However, the approximate annual deposition was low (average 1.4–66 MPs/m2) (Bergmann et al. 2019). So it gets clear that atmospheric micro (nano) plastics can be transported by the wind far away from their sources, which implies that one person's micro (nano) plastics discharge can affect the other. For the first time, González-Pleiter et al. (2021) showed that MPs exist hundreds of meters above ground level. The findings revealed that MPs range from 1.5 MPs m−3 over rural areas to 13.9 MPs m−3 over urban areas. As per their findings, atmospheric transport and deposition simulations revealed that urban areas could be reservoirs of MPs that could potentially end up in distant areas. This study shed light on the long-range atmospheric transport of MPs, demonstrating how they can pose a global pollution problem. This was further supported by the presence of MPs in the Tibetan Plateau (Zhang et al. 2021). Little about aerial transportation of urban atmospheric nano and MPs are understood, yet the possible route to a virgin and pristine environment. In addition to growing our understanding of long-range micro (nano) plastics transport, it is similarly significant to study micro (nano) plastics pollution in urban areas. There, concentrations are probably to be greater and, consequently, exposures more significant than in remote places; but there is a lack of support on the degree of this event. The amount of polymeric particles in the atmosphere is expected to rise due to the legacy of plastic items contaminating the planet, as plastic replaces many materials still in use, such as glass in the bottling of mineral water and soft drinks as well as food packaging and storage containers. In remote regions, atmospheric transport is a significant way for the deposition of nano and MPs. But, the origins and fate of ambient nano and MPs remain poorly understood. More work is needed to investigate the micro (nano) plastics and know where they come from, where they end up and which mechanisms and factors lead to their transport and fallout. Under the fate and trasport of micro (nano) plastics it is essential to find the relation on NPs with atmospheric particulate matter.
Nano-plastics as atmospheric particulate matter.
Air is a highly significant matter for humans and animals’ existence. Nevertheless, it is dangerous to breathe toxic air, which can contribute to human and animal deaths. The challenge faced by air pollution is rising global significance, primarily because of the growing worldwide population, which plays a significant part in contaminating and causing substantial destruction of various habitats. Among several atmospheric contaminants, airborne micro (nano) plastics are recently recognized and are of current concern. Micro (nano) plastics are likely to be found in particulate matter, but their significance is not yet investigated as a fraction of particulate matter. Ambient particulate matter comprises a combination of airborne solids and liquids from natural or man-made sources like soil, sea, burning, and biosphere (Perrino 2010). Primary particulate matter is released into the air, whereas secondary particulate matter arises from the chemical switch of ambient gaseous precursors (like nucleation and condensation). Particulate matter is graded as PM10 (< 10 μm), PM2.5 (< 2.5 μm, fine particles), and ultrafine particles (< 0.1 μm) through the aerodynamic diameter. While the MPs are classified as smaller than 5 mm (Arthur et al. 2009; Verschoor 2015; Hartmann et al. 2016) and NPs (< 1000 nm) (Gigault et al. 2018; Hartmann et al. 2019), although there is still some debates about the size-boundary of NPs (1000 nm vs. 100 nm) between the two categories. The size classification of the micro (nano) plastics clarifies that their size range falls among the size range of particulate matter, so there are chances that micro (nano) plastics can probably get attached to particulate matter. Also, at lower concentrations of emissions, the population holds a significant amount of pollutants in their respiratory tracts, contributing to the growth of illness in vulnerable persons. Atmospheric pollution is positively correlated with death from lung cancer and cardiopulmonary disease, still when corrected for health risk factors that might indicate accumulated burden (Dockery et al. 1993). The high surface area and capability to enter indoors linked to particulate matter illness are particularly unsafe for ultrafine particles (Churg and Brauer 2000). Particulate matter can also influence climate, in addition to health, through the dispersion of solar rays and interference with cloud formation (Perrino 2010).
Organic compounds, accountable for 20–60% of the particulate matter mass (Perrino 2010), comprise a portion recognized as organic carbon consisting of a dynamic combination of various materials created by any mechanism that releases these particles into the air (e.g., tire and brake abrasion) (Seinfeld and Pankow 2003). Plastics are organic compounds (hydrocarbons) that can contain NPs as non-identified materials in the organic carbon fraction of ambient particulate matter. Subsequently, particulate matter morbidity and mortality might already include the environmental effects of airborne NPs. There are growing sources of ambient NPs (e.g., synthetic garments), contributing to a rise in environmental concentrations. Concerning these newly detected atmospheric particles, still, no monitoring and mitigation mechanisms were introduced. In the coming decades, NPs might become an increasingly significant fraction of particulate matter. The study must also concentrate on noval forms of identifying and computing atmospheric NPs as a part of particulate matter. Fate and transport of micro (nano) plastics make the field of the micro (nano) plastics open to find their exact effects on human health from their primary sources.
Conclusions
Polymers are one of the utmost essential materials of the twenty-first century, being found nearly everywhere and having a significant impact on our everyday lives in many respects. Undoubtedly, plastics have changed human existence. However, these products as a whole are often one of the greatest causes of environmental degradation that humanity is exposed to. NPs are probably the least known and characterized type of airborne plastics, but, conversely, they could potentially also be the most hazardous ones due not only to their capability to cross biological barriers but also due to their high surface area, which may have significant implications in the bioaccumulation and bioamplification mechanisms of other pollutants. Because of their widespread presence in the air and their lightweight and small size, they may be inhaled or ingested by humans, posing severe health risks. Atmospheric micro (nano) plastics can trigger airway and interstitial lung diseases, which are often interpreted as industrial diseases in the flock, synthetic textiles, and PVC industries due to processes such as dust overload, oxidative stress, translocation, and gene mutation. These are recognized measurable effects. It is evident from the examined literature that in micro (nano) plastics environmental research, there is some uncertainty and even unclear definitions. When evaluating the existence of MPs in diverse environmental matrices, scientists use multiple methods, and, to this day, environmentally isolated NPs remain indefinable. Because of the disintegration of bigger plastic particles, when secondary MPs are created, NPs are predicted to be produced when, for instance, MPs are exposed to the elements, and, in practice, experimental studies confirm this theoretical statement. However, while it is predicted that environmental NPs will far exceed mesoplastics and macroplastics by number, there are still no systematic, proven methodologies for the accurate determination of their existence and definitely for their determination of ecotoxicological effects/or whether they occur as stated in the literature. Developing compact methods for extracting/isolating NPs from environmental matrices will lead effectively to a deeper understanding of the prevalence of these materials. Therefore, NPs are not just novel contaminants but also possible new matrices and surfaces for adsorbing, absorbing, associating, and transferring other pollutants, microorganisms, and organic content. Therefore, the concentrations measured should be environmentally important, and studies shall also concentrate on identifying and detecting the processes causing certain results, which are still poorly known at the moment. Additionally, due to naturally present NPs, these findings must also be benchmarked against any observations reported. Based on experimental data and field observations, there is a clear knowledge gap concerning the information regarding the surface interactions of NPs in the natural environment and their fate and implications for organisms.
The removal of NPs from the environment is an enormous challenge and requires different approaches to modify daily routines that contribute to the use and release of plastic particles in the environment. This can be achieved through public education and economic stimulus that reward environmentally friendly actions and taxation of activities leading to plastic debris formations. Research on alternative materials and the intended use of plastic may stop a boomerang release-effect plastic-associated story.
Recommendations for further research
Based on this review, we have identified some critical knowledge gaps that need to be considered to understand the source, effect, fate, and transport of airborne NPs in a better way:
-
A.Sources, fate, and transport
- Factors for the distribution and deposition of airborne NPs should be determined.
- Examining the combustion process as an emission source for polymeric particles, including nano and MPs in the air.
- Identifying the sources of NPs in the air.
- Constituting and implementing receptor modeling for source apportionment of micro (nano) plastics.
- To validate long-range transport (> 100 km) of NPs in the atmosphere, further event-based studies would be required.
- Assess how environmental distribution and consequences of NPs are influenced by weathering and abiotic degradation.
- To what degree does the atmospheric NPs fallout lead to aquatic and terrestrial pollution?
-
B.Analytical methods and techniques
- Establish analytical methods for accurate quantification of micro and, more importantly, NPs that eliminate any modifications to the plastic particles examined during the samples’ preparation and analysis.
- Develop reference libraries and extend them constantly, allowing for method validation and comparison of inter-methods.
- Perform laboratory exposure experiments using animal testing.
- Create standard procedures or operational protocols (SOP) to evaluate atmospheric micro and NPs and create a standardized reporting unit for the analysis.
- Establish clear (eco)toxicity evaluation procedures, taking into account the physical and chemical properties of the samples examined and the contaminants likely leached and the contaminants absorbed/adsorbed, like persistent organic pollutants.
-
C.Relation with microplastics and particulate matter
- What proportion of NPs exposure do MPs comprise?
- What proportion of NPs exposure does particulate matter comprise?
-
D.Toxicology
- Is there evidence of NPs uptake in humans?
- What are the total exposure concentrations from dietary and airborne sources of NPs?
- Due to their particular chemical compositions/properties, do different biological reactions to NPs manifest?
- There is an immediate need for data about the effects of NPs on human health. Before this is decided, though, it is important to properly ascertain whether and, if so, how we are exposed. To this end, coordination between groups in ecology, toxicology, epidemiology, and air quality is needed to develop appropriate research projects involving unique monitoring strategies. When reporting on the existence of NPs, both length and diameter should be included because the diameter is critical for respirability. In contrast, length plays a key role in survival and toxicity.
- Due to the sizes of skin pores, NPs can enter through them. Investigations to study humans’ dermal sensitivity process to airborne NPs should also be undertaken to understand further how NPs infiltrate pores.
- Assess if NPs can accumulate in the human body, namely in tissues and/or specific organs, such as the lungs. Try to understand if there is an inflammatory response induced by NPs
-
E.Hydrophobicity and adsorptive nature of nanoplastics
- Identification and quantification of airborne pollutants adsorbed to atmospheric NPs.
- Do size, shape, polymer type, and hydrophobicity influence toxicity?
Abbreviations
- NPs
Nanoplastics
- MPs
Microplastics
- TP
Transparent
- BL
Blue
- RD
Red
- BK
Black
- YL
Yellow
- PP
Purple
- GN
Green
- DB
Dark blue
- W
White
- BL
Blue
- BR
Brown
- GR
Grey
- WT
White transparent
- WR
White-redish
- YO
Yellow-orange
- RP
Red-pink
- BG
Blue-green
- BGY
Black-grey
- PP
Polypropylene
- PS
Polystyrene
- PE
Polyethylene
- PA
Nylon
- PAN
Polyacrylonitrile
- PAA
Poly(N-methyl acrylamide)
- RY
Rayon
- EVA
Ethylene vinyl acetate
- EP
Epoxy resin
- ALK
Alkyd resin
- PUR
Polyurethane
- PTFE
Polytetrafluoroethylene
- PET
Polyethylene terephthalate
- PC
Polycarbonate
- PVC
Polyvinyl chloride
- PES
Polyester
- EVAC
Ethylene-vinyl acetate
- PVA
Polyvinyl acetate
- EP
Epoxy resin
- PA-66
Polyamide 66
- PA-6
Polyamide 6
- BR
Polybutadiene
- PUR
Polyurethane
- PMMA
Poly(methacrylate)acrylic
- EPM
Poly(ethylene-propylene)
- Pyr-GCMS
Pyrolysis gas chromatography mass spectrometry
- FTIR
Fourier transform infrared spectroscopy
- SEM-EDS
Scanning electron microscopy with energy-dispersive spectroscopy
- LC–MS/MS
Liquid chromatography-mass spectrometry
- HPLC–MS/MS
High-performance liquid chromatography-mass spectrometry
- FPA
Focal plane array
Funding
This study was supported by the Eskişehir Technical University Research Fund (project number 21DRP106) and we are thankfull to Presidency For Turks And Related Communities for providing PhD grant to Mansoor Ahmad Bhat.
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abbasi S, Keshavarzi B, Moore F, et al. Distribution and potential health impacts of microplastics and microrubbers in air and street dusts from Asaluyeh County. Iran Environ Pollut. 2019;244:153–164. doi: 10.1016/j.envpol.2018.10.039. [DOI] [PubMed] [Google Scholar]
- Abbasi S, Turner A, Hoseini M, Amiri H. Microplastics in the Lut and Kavir Deserts. Iran Environ Sci Technol. 2021;55:5993–6000. doi: 10.1021/acs.est.1c00615. [DOI] [PubMed] [Google Scholar]
- Acquavela JF, Douglass S, Phillips SC. Evaluation of excess colorectal cancer incidence among workers involved in the manufacture of polypropylene. J Occup Med. 1998;30:438–442. doi: 10.1097/00043764-198805000-00012. [DOI] [PubMed] [Google Scholar]
- Agarwal DK, Kaw JL, Srivastava SP, Seth PK. Some biochemical and histopathological changes induced by polyvinyl chloride dust in rat lung. Environ Res. 1978;16:333–341. doi: 10.1016/0013-9351(78)90166-4. [DOI] [PubMed] [Google Scholar]
- Allen S, Allen D, Phoenix VR, et al. Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nat Geosci. 2019;12:339–344. doi: 10.1038/s41561-019-0335-5. [DOI] [Google Scholar]
- Alzona J, Cohen bL, Rudolph H, et al. Indoor-outdoor relationships for airborne particulate matter of outdoor origin. Atmos Environ. 1979;13:55–60. doi: 10.1016/0004-6981(79)90244-0. [DOI] [Google Scholar]
- Andrady AL. Microplastics in the marine environment. Mar Pollut Bull. 2011;62:1596–1605. doi: 10.1016/j.marpolbul.2011.05.030. [DOI] [PubMed] [Google Scholar]
- Andrady AL, Neal MA. Applications and societal benefits of plastics. Philos Trans R Soc B Biol Sci. 2009;364:1977–1984. doi: 10.1098/rstb.2008.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antti-Poika M, Nordman H, Nickels J, et al. Lung disease after exposure to polyvinyl chloride dust. Thorax. 1986;41:566–567. doi: 10.1136/thx.41.7.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo CF, Nolasco MM, Ribeiro AMP, Ribeiro-Claro PJA. Identification of microplastics using Raman spectroscopy: latest developments and future prospects. Water Res. 2018;142:426–440. doi: 10.1016/j.watres.2018.05.060. [DOI] [PubMed] [Google Scholar]
- Arnaud A, Santi PPDE, Garbe L, et al. Polyvinyl chloride pneumoconiosis. Thorax. 1978;33:19–25. doi: 10.1136/thx.33.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur C, Baker J, Bamford H (2009) Proceedings of the International Research Workshop on the Occurrence, Effects, and Fate of Microplastic Marine Debris
- Atis S, Tutluoglu B, Levent E, et al. The respiratory effects of occupational polypropylene flock exposure. Eur Respir J. 2005;25:110–117. doi: 10.1183/09031936.04.00138403. [DOI] [PubMed] [Google Scholar]
- Au SY, Bruce TF, Bridges WC, Klaine SJ. Responses of Hyalella azteca to acute and chronic microplastic exposures. Environ Toxicol Chem. 2015;34:2564–2572. doi: 10.1002/etc.3093. [DOI] [PubMed] [Google Scholar]
- Bakir A, Rowland SJ, Thompson RC. Competitive sorption of persistent organic pollutants onto microplastics in the marine environment. Mar Pollut Bull. 2012;64:2782–2789. doi: 10.1016/j.marpolbul.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Bakir A, Rowland SJ, Thompson RC. Transport of persistent organic pollutants by microplastics in estuarine conditions. Estuar Coast Shelf Sci. 2014;140:14–21. doi: 10.1016/j.ecss.2014.01.004. [DOI] [Google Scholar]
- Balasch JC, Brandts I, Barría C, et al. Short-term exposure to polymethylmethacrylate nanoplastics alters muscle antioxidant response, development and growth in Sparus aurata. Mar Pollut Bull. 2021;172:112918. doi: 10.1016/j.marpolbul.2021.112918. [DOI] [PubMed] [Google Scholar]
- Baldwin AK, Corsi SR, Mason SA. Plastic debris in 29 great lakes tributaries: relations to watershed attributes and hydrology. Environ Sci Technol. 2016;50:10377–10385. doi: 10.1021/acs.est.6b02917. [DOI] [PubMed] [Google Scholar]
- Bank MS, Hansson SV. The Plastic Cycle: A novel and holistic paradigm for the Anthropocene. Environ Sci Technol. 2019;53:7177–7179. doi: 10.1021/acs.est.9b02942. [DOI] [PubMed] [Google Scholar]
- Barroso E, Ibañez MD, Aranda FI, Romero S. Polyethylene flock-associated interstitial lung disease in a Spanish female. Eur Respir J. 2002;20:1610–1612. doi: 10.1183/09031936.02.00030102. [DOI] [PubMed] [Google Scholar]
- Barshtein G, Livshits L, Shvartsman LD, et al. Polystyrene nanoparticles activate erythrocyte aggregation and adhesion to endothelial cells. Cell Biochem Biophys. 2016;74:19–27. doi: 10.1007/s12013-015-0705-6. [DOI] [PubMed] [Google Scholar]
- Beckett WS. Occupational Respiratory Diseases. N Engl J Med. 2000;342:406–413. doi: 10.1056/NEJM200002103420607. [DOI] [PubMed] [Google Scholar]
- Bergmann M, Mützel S, Primpke S, Tekman MB. White and wonderful microplastics prevail in snow from the Alps to the Arctic. Sci Adv. 2019;5:1–10. doi: 10.1126/sciadv.aax1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann M, Gutow L, Klages M (2015) Marine anthropogenic litter, 1st edn. Springer Cham, p 1–447. 10.1007/978-3-319-16510-3
- Besseling E, Quik JTK, Sun M, Koelmans AA. Fate of nano- and microplastic in freshwater systems: a modeling study. Environ Pollut. 2017;220:540–548. doi: 10.1016/j.envpol.2016.10.001. [DOI] [PubMed] [Google Scholar]
- Bhat MA, Eraslan FN, Gedik K, Gaga EO (2021) Impact of Textile product emissions : toxicological considerations in assessing indoor air quality and human health. In: Malik JA, Marathe S (eds) Ecological and Health Effects of Building Materials, 1st edn. Springer Nature Switzerland, pp 505–541. 10.1007/978-3-030-76073-1_27
- Bhat MA, Gedik K, Gaga EO (2022) Environmental toxicity of emerging micro and nanoplastics: a lesson learned from nanomaterials. In: Dar AH, Nayik GA (eds) Nanotechnology Interventions in Food Packaging and Shelf Life, 1st edn. Taylor & Francis (CRC Press), pp 311–337. 10.1201/9781003207641-18
- Biedermann-Brem S, Gro K. Release of bisphenol A from polycarbonate baby bottles : water hardness as the most relevant factor. Eur Food Res Technol. 2009;228:679–684. doi: 10.1007/s00217-008-0978-8. [DOI] [Google Scholar]
- Bolan NS, Karthikeyan K, Bolan SS, et al. et al. An introduction to the chemistry and manufacture of plastics. In: Bola NS, Kirkham MB, Halsband C, et al.et al., editors. An introduction to the chemistry and manufacture of plastics. 1. Boca Raton: CRC Press; 2020. pp. 85–93. [Google Scholar]
- Boucher J, Friot D (2017) Primary microplastics in the oceans: a global evaluation of sources. IUCN, Gland, Switzerland. p 43. 10.2305/IUCN.CH.2017.01.en
- Bouwmeester H, Hollman PCH, Peters RJB. Potential health impact of environmentally released micro- and nanoplastics in the human food production chain: experiences from nanotoxicology. Environ Sci Technol. 2015;49:8932–8947. doi: 10.1021/acs.est.5b01090. [DOI] [PubMed] [Google Scholar]
- Brodie EL, DeSantis TZ, Moberg Parker JP, et al. Urban aerosols harbor diverse and dynamic bacterial populations. PNAS. 2007;104:299–304. doi: 10.1073/pnas.0608255104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne MA, Crump P, Niven SJ, et al. Accumulation of microplastic on shorelines woldwide: Sources and sinks. Environ Sci Technol. 2011;45:9175–9179. doi: 10.1021/es201811s. [DOI] [PubMed] [Google Scholar]
- Cai L, Wang J, Peng J, et al. Characteristic of microplastics in the atmospheric fallout from Dongguan city, China: preliminary research and first evidence. Environ Sci Pollut Res. 2017;24:24928–24935. doi: 10.1007/s11356-017-0116-x. [DOI] [PubMed] [Google Scholar]
- Campbell CSJ, Contreras-Rojas LR, Delgado-Charro MB, Guy RH. Objective assessment of nanoparticle disposition in mammalian skin after topical exposure. J Control Release. 2012;162:201–207. doi: 10.1016/j.jconrel.2012.06.024. [DOI] [PubMed] [Google Scholar]
- Carbery M, Connor WO, Thavamani P. Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health. Environ Int J. 2018;115:400–409. doi: 10.1016/j.envint.2018.03.007. [DOI] [PubMed] [Google Scholar]
- Castan S, Henkel C, Hüffer T, Hofmann T. Microplastics and nanoplastics barely enhance contaminant mobility in agricultural soils. Commun Earth Environ. 2021;2:1–9. doi: 10.1038/s43247-021-00267-8. [DOI] [Google Scholar]
- Cedervall T, Hansson LA, Lard M, et al. Food chain transport of nanoparticles affects behaviour and fat metabolism in fish. PLoS One. 2012;7:1–6. doi: 10.1371/journal.pone.0032254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TJ, Hsieh YF, Kao HM. Numerical investigation of airflow pattern and particulate matter transport in naturally ventilated multi-room buildings. Indoor Air. 2006;16:136–152. doi: 10.1111/j.1600-0668.2005.00410.x. [DOI] [PubMed] [Google Scholar]
- Cheng J-X, Xie XS (2013) Coherent Raman scattering microscopy, 1st ed. Taylor & Francis Group, London
- Chimenos JM, Segarra M, Fernández MA, Espiell F. Characterization of the bottom ash in municipal solid waste incinerator. J Hazard Mater. 1999;64:211–222. doi: 10.1016/S0304-3894(98)00246-5. [DOI] [Google Scholar]
- Churg A, Brauer M. Ambient atmospheric particles in the airways of human lungs. Ultrastruct Pathol. 2000;24:353–361. doi: 10.1080/019131200750060014. [DOI] [PubMed] [Google Scholar]
- Claessens M, De MS, Van LL, et al. Occurrence and distribution of microplastics in marine sediments along the Belgian coast. Mar Pollut Bull. 2011;62:2199–2204. doi: 10.1016/j.marpolbul.2011.06.030. [DOI] [PubMed] [Google Scholar]
- Cole M, Lindeque P, Halsband C, Galloway TS. Microplastics as contaminants in the marine environment: a review. Mar Pollut Bull. 2011;62:2588–2597. doi: 10.1016/j.marpolbul.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Cole M, Lindeque P, Fileman E, et al. Microplastic ingestion by zooplankton. Environ Sci Technol. 2013;47:6646–6655. doi: 10.1021/es400663f. [DOI] [PubMed] [Google Scholar]
- Cole M, Lindeque P, Fileman E, et al. The impact of polystyrene microplastics on feeding, function and fecundity in the marine copepod Calanus helgolandicus. Environ Sci Technol. 2015;49:1130–1137. doi: 10.1021/es504525u. [DOI] [PubMed] [Google Scholar]
- Cordasco EM, Demeter SL, Kerkay J, et al. Pulmonary manifestations of vinyl and polyvinyl chloride (interstitial lung disease) Newer Aspects Chest. 1980;78:828–834. doi: 10.1378/chest.78.6.828. [DOI] [PubMed] [Google Scholar]
- Cortez Pimentel J, Avila R, Galvao Lourenco A. Respiratory disease caused by synthetic fibres: a new occupational disease. Thorax. 1975;30:204–219. doi: 10.1136/thx.30.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cózar A, Echevarría F, González-gordillo JI, et al. Plastic debris in the open ocean. Proc Natl Acad Sci U S A. 2014;11:10239–10244. doi: 10.1073/pnas.1314705111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford CB, Quinn B (2017) Microplastics, standardisation and spatial distribution. In: Crawford CB, Quinn B (eds) Microplastic Pollutants, 1st edn. Elsevier, pp 101-130. 10.1016/B978-0-12-809406-8.00005-0
- Da Costa JP, Santos PSM, Duarte AC, Rocha-Santos T. (Nano)plastics in the environment - sources, fates and effects. Sci Total Environ. 2016;566–567:15–26. doi: 10.1016/j.scitotenv.2016.05.041. [DOI] [PubMed] [Google Scholar]
- Da Costa JP, Reis V, Paço A, et al. Micro(nano)plastics – analytical challenges towards risk evaluation. TrAC - Trends Anal Chem. 2019;111:173–184. doi: 10.1016/j.trac.2018.12.013. [DOI] [Google Scholar]
- Dazzi A, Prater CB, Hu Q, et al. AFM-IR: Combining atomic force microscopy and infrared spectroscopy for nanoscale chemical characterization. Appl Spectrosc. 2012;66:1365–1384. doi: 10.1366/12-06804. [DOI] [PubMed] [Google Scholar]
- De Jong WH, Hagens WI, Krystek P, et al. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials. 2008;29:1912–1919. doi: 10.1016/j.biomaterials.2007.12.037. [DOI] [PubMed] [Google Scholar]
- Dierkes G, Lauschke T, Becher S, et al. Quantification of microplastics in environmental samples via pressurized liquid extraction and pyrolysis-gas chromatography. Anal Bioanal Chem. 2019;411:6959–6968. doi: 10.1007/s00216-019-02066-9. [DOI] [PubMed] [Google Scholar]
- Dockery DW, Arden Pope C, II, Cy C, et al. An association between air pollution and mortality in six U.S. Cities N Engl J Med. 1993;329:1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- Dris R, Gasperi J, Rocher V, et al. Microplastic contamination in an urban area: a case study in Greater Paris. Environ Chem. 2015;12:592–599. doi: 10.1071/EN14167. [DOI] [Google Scholar]
- Dris R, Gasperi J, Saad M, et al. Synthetic fibers in atmospheric fallout: a source of microplastics in the environment? Mar Pollut Bull. 2016;104:290–293. doi: 10.1016/j.marpolbul.2016.01.006. [DOI] [PubMed] [Google Scholar]
- Dris R, Gasperi J, Mirande C, et al. A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environ Pollut. 2017;221:453–458. doi: 10.1016/j.envpol.2016.12.013. [DOI] [PubMed] [Google Scholar]
- Duan Z, Zhao S, Zhao L, et al. Microplastics in Yellow River Delta wetland: occurrence, characteristics, human influences, and marker. Environ Pollut. 2020;258:113232. doi: 10.1016/j.envpol.2019.113232. [DOI] [PubMed] [Google Scholar]
- Eerkes-Medrano D, Thompson RC, Aldridge DC. Microplastics in freshwater systems: a review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res. 2015;75:63–82. doi: 10.1016/j.watres.2015.02.012. [DOI] [PubMed] [Google Scholar]
- Ekvall MT, Lundqvist M, Kelpsiene E, et al. Nanoplastics formed during the mechanical breakdown of daily-use polystyrene products. Nanoscale Adv. 2019;1:1055–1061. doi: 10.1039/c8na00210j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsaesser A, Howard CV. Toxicology of nanoparticles. Adv Drug Deliv Rev. 2012;64:129–137. doi: 10.1016/j.addr.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Enyoh CE, Verla AW, Verla EN et al (2019) Airborne microplastics: a review study on method for analysis, occurrence, movement and risks. Environ Monit Assess 191. 10.1007/s10661-019-7842-0 [DOI] [PubMed]
- Eraslan FN, Bhat MA, Gaga EO, Gedik K (2021) Comprehensive analysis of research trends in volatile organic compounds emitted from building materials : a bibliometric analysis. In: Malik JA, Marathe S (eds) Ecological and Health Effects of Building Materials, 1st edn. Springer Nature Switzerland, pp 87–109. 10.1007/978-3-030-76073-1_6
- Erni R, Rossell MD, Kisielowski C, Dahmen U. Atomic-resolution imaging with a sub-50-pm electron probe. Phys Rev Lett. 2009;102:096101. doi: 10.1103/PhysRevLett.102.096101. [DOI] [PubMed] [Google Scholar]
- Eschenbacher WL, Kreiss K, Lougheed MD, et al. Clinical pathology workshop summary nylon flock – associated interstitial lung disease. Am J Respir Crit Care Med. 1999;159:2003–2008. doi: 10.1164/ajrccm.159.6.9808002. [DOI] [PubMed] [Google Scholar]
- Evangeliou N, Grythe H, Klimont Z et al (2020) Atmospheric transport is a major pathway of microplastics to remote regions. Nat Commun 11:3381. 10.1038/s41467-020-17201-9 [DOI] [PMC free article] [PubMed]
- Fernando HJS, Lee SM, Anderson J, et al. Urban fluid mechanics: air circulation and contaminant dispersion in cities. Environ Fluid Mech. 2001;1:107–164. doi: 10.1023/A:1011504001479. [DOI] [Google Scholar]
- Filella M. Questions of size and numbers in environmental research on microplastics: methodological and conceptual aspects. Environ Chem. 2015;12:527–538. doi: 10.1071/EN15012. [DOI] [Google Scholar]
- Foord N, Black A, Walsh M. Regional deposition of 2.5-7.5 μm diameter inhaled particles in healthy male non-smokers. J Aerosol Sci. 1978;9:343–357. doi: 10.1016/0021-8502(78)90037-X. [DOI] [Google Scholar]
- Forte M, Iachetta G, Tussellino M, et al. Polystyrene nanoparticles internalization in human gastric adenocarcinoma cells. Toxicol Vitr J. 2016;31:126–136. doi: 10.1016/j.tiv.2015.11.006. [DOI] [PubMed] [Google Scholar]
- Free CM, Jensen OP, Mason SA, et al. High-levels of microplastic pollution in a large, remote, mountain lake. Mar Pollut Bull. 2014;85:156–163. doi: 10.1016/j.marpolbul.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Frère L, Paul-Pont I, Moreau J, et al. A semi-automated Raman micro-spectroscopy method for morphological and chemical characterizations of microplastic litter. Mar Pollut Bull. 2016;113:461–468. doi: 10.1016/j.marpolbul.2016.10.051. [DOI] [PubMed] [Google Scholar]
- Frias JPGL, Sobral P, Ferreira AM. Organic pollutants in microplastics from two beaches of the Portuguese coast. Mar Pollut Bull. 2010;60:1988–1992. doi: 10.1016/j.marpolbul.2010.07.030. [DOI] [PubMed] [Google Scholar]
- Fromme H, Hilger B, Kopp E, et al. Polybrominated diphenyl ethers (PBDEs), hexabromocyclododecane (HBCD) and “novel” brominated flame retardants in house dust in Germany. Environ Int. 2014;64:61–68. doi: 10.1016/j.envint.2013.11.017. [DOI] [PubMed] [Google Scholar]
- Gall SC, Thompson RC. The impact of debris on marine life. Mar Pollut Bull. 2015;92:170–179. doi: 10.1016/j.marpolbul.2014.12.041. [DOI] [PubMed] [Google Scholar]
- Gallagher LG, Li W, Ray RM, et al. Occupational exposures and risk of stomach and esophageal cancers: update of a cohort of female textile workers in Shanghai, China. Am J Ind Med. 2015;58:267–275. doi: 10.1002/ajim.22412. [DOI] [PubMed] [Google Scholar]
- Gasperi J, Wright SL, Dris R, et al. Microplastics in air: are we breathing it in? Curr Opin Environ Sci Heal. 2018;1:1–5. doi: 10.1016/j.coesh.2017.10.002. [DOI] [Google Scholar]
- Gaston E, Woo M, Steele C, et al. Microplastics differ between indoor and outdoor air masses: insights from multiple microscopy methodologies. Appl Spectrosc. 2020;74(9):1079–1098. doi: 10.1177/0003702820920652. [DOI] [PubMed] [Google Scholar]
- Gewert B, Plassmann MM, Macleod M. Pathways for degradation of plastic polymers floating in the marine environment. Environ Sci Process Impacts. 2015;17:1513–1521. doi: 10.1039/c5em00207a. [DOI] [PubMed] [Google Scholar]
- Gigault J, Pedrono B, Maxit B, Ter Halle A. Marine plastic litter: the unanalyzed nano-fraction. Environ Sci Nano. 2016;3:346–350. doi: 10.1039/c6en00008h. [DOI] [Google Scholar]
- Gigault J, ter Halle A, Baudrimont M, et al. Current opinion: what is a nanoplastic? Environ Pollut. 2018;235:1030–1034. doi: 10.1016/j.envpol.2018.01.024. [DOI] [PubMed] [Google Scholar]
- Gillibert R, Balakrishnan G, Deshoules Q, et al. Raman tweezers for small microplastics and nanoplastics identification in seawater. Environ Sci Technol. 2019;53:9003–9013. doi: 10.1021/acs.est.9b03105. [DOI] [PubMed] [Google Scholar]
- Goldberg MS, Thériault G. Retrospective cohort study of workers of a synthetic textiles plant in quebec: I. General Mortality Am J Ind Med. 1994;25:889–907. doi: 10.1002/ajim.4700250612. [DOI] [PubMed] [Google Scholar]
- González-Pleiter M, Edo C, Aguilera Á, et al. Occurrence and transport of microplastics sampled within and above the planetary boundary layer. Sci Total Environ. 2021;761:143213. doi: 10.1016/j.scitotenv.2020.143213. [DOI] [PubMed] [Google Scholar]
- Goodman KE, Hare JT, Khamis ZI, et al. Exposure of human lung cells to polystyrene microplastics significantly retards cell proliferation and triggers morphological changes. Chem Res Toxicol. 2021;34:1069–1081. doi: 10.1021/acs.chemrestox.0c00486. [DOI] [PubMed] [Google Scholar]
- Graham ER, Thompson JT. Deposit- and suspension-feeding sea cucumbers (Echinodermata)ingest plastic fragments. J Exp Mar Bio Ecol. 2009;368:22–29. doi: 10.1016/j.jembe.2008.09.007. [DOI] [Google Scholar]
- Grassian VH, O’Shaughnessy PT, Adamcakova-Dodd A, et al. Inhalation exposure study of Titanium dioxide nanoparticles with a primary particle size of 2 to 5 nm. Environ Health Perspect. 2007;115:397–402. doi: 10.1289/ehp.9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve MC, Biermann T. The population of coloured textile fibres on outdoor surfaces. Sci Justice. 1997;37:231–239. doi: 10.1016/S1355-0306(97)72196-8. [DOI] [Google Scholar]
- Hahladakis JN, Velis CA, Weber R, et al. An overview of chemical additives present in plastics : migration, release, fate and environmental impact during their use, disposal and recycling. J Hazard Mater. 2018;344:179–199. doi: 10.1016/j.jhazmat.2017.10.014. [DOI] [PubMed] [Google Scholar]
- Hartmann NB, Hüffer T, Thompson RC, et al. Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. Environ Sci Technol. 2019;53:1039–1047. doi: 10.1021/acs.est.8b05297. [DOI] [PubMed] [Google Scholar]
- Hartmann NB, Rist S, Baun A (2016) Aquatic ecotoxicity testing of nanoplastics (and microplastics) - lessons learned from nanoecotoxicology. In SETAC Europe 26th Annual Meeting - abstract book (pp 43–44). SETAC Europe
- Hernandez LM, Youse N, Tufenkji N. Are there nanoplastics in your personal care products? Environ Sci Technol Lett. 2017;4:280–285. doi: 10.1021/acs.estlett.7b00187. [DOI] [Google Scholar]
- Hext PM, Tomenson JA, Thompson P. Titanium dioxide: inhalation toxicology and epidemiology. Ann Occup Hyg. 2005;49:461–472. doi: 10.1093/annhyg/mei012. [DOI] [PubMed] [Google Scholar]
- Hicks DG, Judkins AR, Sickel JZ, et al. Granular histiocytosis of pelvic lymph nodes following total hip arthroplasty: the presence of wear debris, cytokine production, and immunologically activated macrophages. J Bone Jt Surg Ser A. 1996;78:482–496. doi: 10.2106/00004623-199604000-00002. [DOI] [PubMed] [Google Scholar]
- Hidalgo-ruz V, Gutow L, Thompson RC, Thiel M. Microplastics in the marine environment: a review of the methods used for identification and quantification. Environ Sci Technol. 2012;46:3060–3075. doi: 10.1021/es2031505. [DOI] [PubMed] [Google Scholar]
- Hoet PHM, Brüske-Hohlfeld I, Salata OV. Nanoparticles - known and unknown health risks. J Nanobiotechnology. 2004;2:1–15. doi: 10.1186/1477-3155-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes LA, Turner A, Thompson RC. Adsorption of trace metals to plastic resin pellets in the marine environment. Environ Pollut. 2012;160:42–48. doi: 10.1016/j.envpol.2011.08.052. [DOI] [PubMed] [Google Scholar]
- Horton AA, Walton A, Spurgeon DJ, et al. Microplastics in freshwater and terrestrial environments: evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci Total Environ. 2017;586:127–141. doi: 10.1016/j.scitotenv.2017.01.190. [DOI] [PubMed] [Google Scholar]
- Horton AA, Dixon SJ (2017) Microplastics : an introduction to environmental transport processes. WIREs Water 5:e1268:1–10. 10.1002/wat2.1268
- Hu L, Fu J, Wang S, et al. Microplastics generated under simulated fire scenarios: characteristics, antimony leaching, and toxicity. Environ Pollut. 2021;269:115905. doi: 10.1016/j.envpol.2020.115905. [DOI] [PubMed] [Google Scholar]
- Huang DY, Zhou SG, Hong W, et al. Pollution characteristics of volatile organic compounds, polycyclic aromatic hydrocarbons and phthalate esters emitted from plastic wastes recycling granulation plants in Xingtan Town, South China. Atmos Environ. 2013;71:327–334. doi: 10.1016/j.atmosenv.2013.02.011. [DOI] [Google Scholar]
- Hüffer T, Hofmann T. Sorption of non-polar organic compounds by micro-sized plastic particles in aqueous solution. Environ Pollut. 2016;214:194–201. doi: 10.1016/j.envpol.2016.04.018. [DOI] [PubMed] [Google Scholar]
- Hüffer T, Praetorius A, Wagner S, et al. Microplastic exposure assessment in aquatic environments: learning from similarities and differences to engineered nanoparticles. Environ Sci Technol. 2017;51:2499–2507. doi: 10.1021/acs.est.6b04054. [DOI] [PubMed] [Google Scholar]
- Hüffer T, Weniger AK, Hofmann T. Sorption of organic compounds by aged polystyrene microplastic particles. Environ Pollut. 2018;236:218–225. doi: 10.1016/j.envpol.2018.01.022. [DOI] [PubMed] [Google Scholar]
- Hurley RR, Woodward JC, Rothwell JJ. Ingestion of microplastics by freshwater tubifex worms. Environ Sci Technol. 2017;51:12844–12851. doi: 10.1021/acs.est.7b03567. [DOI] [PubMed] [Google Scholar]
- Hurley R, Woodward J, Rothwell JJ. Microplastic contamination of river beds significantly reduced by catchment-wide flooding Rachel. Nat Geosci. 2018;11:251–257. doi: 10.1038/s41561-018-0080-1. [DOI] [Google Scholar]
- Huth F, Govyadinov A, Amarie S, et al. Nano-FTIR absorption spectroscopy of molecular fingerprints at 20 nm spatial resolution. Nano Lett. 2012;12:3973–3978. doi: 10.1021/nl301159v. [DOI] [PubMed] [Google Scholar]
- Ivar JA, Costa MF, Barletta M, et al. Pelagic microplastics around an archipelago of the Equatorial Atlantic. Mar Pollut Bull. 2013;75:305–309. doi: 10.1016/j.marpolbul.2013.07.040. [DOI] [PubMed] [Google Scholar]
- Jacob H, Besson M, Swarzenski PW, et al. Effects of virgin micro- and nanoplastics on fish: trends, meta-analysis, and perspectives. Environ Sci Technol. 2020;54:4733–4745. doi: 10.1021/acs.est.9b05995. [DOI] [PubMed] [Google Scholar]
- Jambeck JR, Geyer R, Wilcox C, et al (2015) Plastic waste inputs from land into the ocean. Science (80- ) 347:768–771 [DOI] [PubMed]
- Jatana S, Callahan LM, Pentland AP, DeLouise LA. Impact of cosmetic lotions on nanoparticle penetration through ex vivo C57BL/6 hairless mouse and human skin: a comparison study. Cosmetics. 2016;3:1–21. doi: 10.3390/cosmetics3010006.Impact. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner LC, Sadofsky LR, Danopoulos E, Rotchell JM. Household indoor microplastics within the Humber region (United Kingdom): quantification and chemical characterisation of particles present. Atmos Environ. 2021;259:118512. doi: 10.1016/j.atmosenv.2021.118512. [DOI] [Google Scholar]
- Jones KC, De Voogt P. Attribute Learning for Image / Video Understanding. Persistent Org Pollut State Sci. 1999;100:209–221. [Google Scholar]
- Käppler A, Windrich F, Löder MGJ, et al. Identification of microplastics by FTIR and Raman microscopy : a novel silicon filter substrate opens the important spectral range below 1300 cm − 1 for FTIR transmission measurements. Anal Bioanal Chem. 2015;407:6791–6801. doi: 10.1007/s00216-015-8850-8. [DOI] [PubMed] [Google Scholar]
- Käppler A, Fischer D, Oberbeckmann S, et al. Analysis of environmental microplastics by vibrational microspectroscopy: FTIR, Raman or both? Anal Bioanal Chem. 2016;408:8377–8391. doi: 10.1007/s00216-016-9956-3. [DOI] [PubMed] [Google Scholar]
- Kaur S, Nieuwenhuijsen MJ, Colvile RN. Fine particulate matter and carbon monoxide exposure concentrations in urban street transport microenvironments. Atmos Environ. 2007;41:4781–4810. doi: 10.1016/j.atmosenv.2007.02.002. [DOI] [Google Scholar]
- Kayalar Ö, Arı A, Babuççu G, et al (2021) Existence of SARS-CoV-2 RNA on ambient particulate matter samples: a nationwide study in Turkey. Sci Total Environ 789:147976. 10.1016/j.scitotenv.2021.147976 [DOI] [PMC free article] [PubMed]
- Kern DG, Crausman RS, Durand KTH, et al. Flock worker’s lung: chronic interstitial lung disease in the nylon flocking industry. Ann Intern Med. 1998;129:261–272. doi: 10.7326/0003-4819-129-4-199808150-00001. [DOI] [PubMed] [Google Scholar]
- Kern DG, Kuhn C, Wesley Ely E, et al. Flock worker’s lung: broadening the spectrum of clinicopathology, narrowing the spectrum of suspected etiologies. Chest. 2000;117:251–259. doi: 10.1378/chest.117.1.251. [DOI] [PubMed] [Google Scholar]
- Kirstein IV, Kirmizi S, Wichels A, et al. Dangerous hitchhikers? Evidence for potentially pathogenic Vibrio spp. on microplastic particles. Mar Environ Res. 2016;120:1–8. doi: 10.1016/j.marenvres.2016.07.004. [DOI] [PubMed] [Google Scholar]
- Klein M, Fischer EK. Microplastic abundance in atmospheric deposition within the Metropolitan area of Hamburg, Germany. Sci Total Environ. 2019;685:96–103. doi: 10.1016/j.scitotenv.2019.05.405. [DOI] [PubMed] [Google Scholar]
- Koelmans AA, Kooi M, Law KL, Van Sebille E. All is not lost: deriving a top-down mass budget of plastic at sea. Environ Res Lett. 2017;12:114028. doi: 10.1088/1748-9326/aa9500. [DOI] [Google Scholar]
- Koelmans AA, Mohamed Nor NH, Hermsen E, et al. Microplastics in freshwaters and drinking water: critical review and assessment of data quality. Water Res. 2019;155:410–422. doi: 10.1016/j.watres.2019.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelmans AA, Besseling E, Shim WJ (2015) Nanoplastics in the aquatic environment. critical review. In: Bergmann M, Gutow L, Klages M (eds) Marine Anthropogenic Litter, Ist edn. Springer, Cham. pp 325–340. 10.1007/978-3-319-16510-3_12
- Koni SB, Per G. Release of bisphenol A from polycarbonate baby bottles : mechanisms of formation and investigation of worst case scenarios. Eur Food Res Technol. 2008;227:1053–1060. doi: 10.1007/s00217-008-0819-9. [DOI] [Google Scholar]
- Kwon JH, Kim JW, Pham TD et al (2020) Microplastics in food: A review on analytical methods and challenges. International Journal of Environmental Research and Public Health 17(18):6710. 10.3390/ijerph17186710 [DOI] [PMC free article] [PubMed]
- Lambert S, Wagner M. Chemosphere characterisation of nanoplastics during the degradation of polystyrene. Chemosphere. 2016;145:265–268. doi: 10.1016/j.chemosphere.2015.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert S, Wagner M. Formation of microscopic particles during the degradation of different polymers. Chemosphere. 2016;161:510–517. doi: 10.1016/j.chemosphere.2016.07.042. [DOI] [PubMed] [Google Scholar]
- Lambert S, Sinclair CJ, Bradley EL, Boxall ABA. Effects of environmental conditions on latex degradation in aquatic systems. Sci Total Environ. 2013;447:225–234. doi: 10.1016/j.scitotenv.2012.12.067. [DOI] [PubMed] [Google Scholar]
- Lambert S, Scherer C, Wagner M. Ecotoxicity testing of microplastics: considering the heterogeneity of physicochemical properties. Integr Environ Assess Manag. 2017;13:470–475. doi: 10.1002/ieam.1901. [DOI] [PubMed] [Google Scholar]
- Lee HS, Yap J, Wang YT, et al. Occupational asthma due to unheated polyvinylchloride resin dust. Br J Ind Med. 1989;46:820–822. doi: 10.1136/oem.46.11.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner R, Weder C, Petri-Fink A, Rothen-Rutishauser B. Emergence of nanoplastic in the environment and possible impact on human health. Environ Sci Technol. 2019;53:1748–1765. doi: 10.1021/acs.est.8b05512. [DOI] [PubMed] [Google Scholar]
- Leslie HA, van Velzen MJM, Brandsma SH, et al. Discovery and quantification of plastic particle pollution in human blood. Environ Int. 2022;163:107199. doi: 10.1016/j.envint.2022.107199. [DOI] [PubMed] [Google Scholar]
- Li B, Song W, Cheng Y, et al. Ecotoxicological effects of different size ranges of industrial-grade polyethylene and polypropylene microplastics on earthworms Eisenia fetida. Sci Total Environ. 2021;783:147007. doi: 10.1016/j.scitotenv.2021.147007. [DOI] [PubMed] [Google Scholar]
- Li J, Song Y, Cai Y (2020) Focus topics on microplastics in soil: Analytical methods occurrence transport and ecological risks. Environmental Pollution 257113570-S0269749119335882 113570. 10.1016/j.envpol.2019.113570 [DOI] [PubMed]
- Li W, Chen X, Li M, et al. Microplastics as an aquatic pollutant affect gut microbiota within aquatic animals. J Hazard Mater. 2022;423:127094. doi: 10.1016/j.jhazmat.2021.127094. [DOI] [PubMed] [Google Scholar]
- Liebezeit G, Liebezeit E. Origin of synthetic particles in honeys. Pol J Food Nutr Sci. 2015;65:143–147. doi: 10.1515/pjfns-2015-0025. [DOI] [Google Scholar]
- Linares V, Bellés M, Domingo JL. Human exposure to PBDE and critical evaluation of health hazards. Arch Toxicol. 2015;89:335–356. doi: 10.1007/s00204-015-1457-1. [DOI] [PubMed] [Google Scholar]
- Liu C, Li J, Zhang Y, et al. Widespread distribution of PET and PC microplastics in dust in urban China and their estimated human exposure. Environ Int. 2019;128:116–124. doi: 10.1016/j.envint.2019.04.024. [DOI] [PubMed] [Google Scholar]
- Liu K, Wang X, Fang T, et al. Source and potential risk assessment of suspended atmospheric microplastics in Shanghai. Sci Total Environ. 2019;675:462–471. doi: 10.1016/j.scitotenv.2019.04.110. [DOI] [PubMed] [Google Scholar]
- Liu K, Wang X, Wei N, et al. Accurate quantification and transport estimation of suspended atmospheric microplastics in megacities: implications for human health. Environ Int. 2019;132:105127. doi: 10.1016/j.envint.2019.105127. [DOI] [PubMed] [Google Scholar]
- Liu X, Zheng M, Wang L et al (2018) Sorption behaviors of tris-(23-dibromopropyl) isocyanurate and hexabromocyclododecanes on polypropylene microplastics. Marine Pollution Bulletin 135581-586 S0025326X18305496. 10.1016/j.marpolbul.2018.07.061 [DOI] [PubMed]
- Loder MGJ, Kuczera M, Mintenig S, et al. Focal plane array detector-based micro-Fourier-transform infrared imaging for the analysis of microplastics in environmental samples. Environ Chem. 2015;12:563–581. doi: 10.1071/EN14205. [DOI] [Google Scholar]
- Lomonaco T, Manco E, Corti A, et al. Release of harmful volatile organic compounds (VOCs) from photo-degraded plastic debris: a neglected source of environmental pollution. J Hazard Mater. 2020;394:122596. doi: 10.1016/j.jhazmat.2020.122596. [DOI] [PubMed] [Google Scholar]
- Lozano YM, Lehnert T, Linck LT, et al. Microplastic shape, polymer type, and concentration affect soil properties and plant biomass. Front Plant Sci. 2021;12:1–14. doi: 10.3389/fpls.2021.616645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Zhang Y, Deng Y, et al. Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environ Sci Technol. 2016;50:4054–4060. doi: 10.1021/acs.est.6b00183. [DOI] [PubMed] [Google Scholar]
- Lusher AL, Tirelli V, Connor IO, Officer R (2015) Microplastics in Arctic polar waters : the first reported values of particles in surface and sub-surface samples. Nat Publ Gr 5:14947. 10.1038/srep14947 [DOI] [PMC free article] [PubMed]
- Lv L, Qu J, Yu Z, et al. A simple method for detecting and quantifying microplastics utilizing fluorescent dyes - Safranine T, fluorescein isophosphate, Nile red based on thermal expansion and contraction property. Environ Pollut. 2019;255:1–11. doi: 10.1016/j.envpol.2019.113283. [DOI] [PubMed] [Google Scholar]
- Maia J, Cruz JM, Sendón R, et al. Effect of detergents in the release of bisphenol A from polycarbonate baby bottles. Food Res Int. 2009;42:1410–1414. doi: 10.1016/j.foodres.2009.07.003. [DOI] [Google Scholar]
- Marnane RN, Elliot DA, Coulson SA. A pilot study to determine the background population of foreign fibre groups on a cotton / polyester T-shirt. Sci Justice. 2006;46:215–220. doi: 10.1016/S1355-0306(06)71601-X. [DOI] [PubMed] [Google Scholar]
- Marsh JP, Mossman BT, Driscoll KE, et al. Effects of aramid, a high strength synthetic fiber, on respiratory cells in vitro. Drug Chem Toxicol. 1994;17:75–92. doi: 10.3109/01480549409014303. [DOI] [PubMed] [Google Scholar]
- Mastrangelo G, Fedeli U, Fadda E, et al. Epidemiologic evidence of cancer risk in textile industry workers: a review and update. Toxicol Ind Health. 2002;18:171–181. doi: 10.1191/0748233702th139rr. [DOI] [PubMed] [Google Scholar]
- Mastrangelo G, Fedeli U, Fadda E, et al. Lung cancer risk in workers exposed to poly(vinyl chloride) dust: a nested case-referent study. Occup Environ Med. 2003;60:423–428. doi: 10.1136/oem.60.6.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mato Y, Isobe T, Takada H, et al. Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environ Sci Technol. 2001;35:318–324. doi: 10.1021/es0010498. [DOI] [PubMed] [Google Scholar]
- Mattsson K, Ekvall MT, Hansson LA, et al. Altered behavior, physiology, and metabolism in fish exposed to polystyrene nanoparticles. Environ Sci Technol. 2015;49:553–561. doi: 10.1021/es5053655. [DOI] [PubMed] [Google Scholar]
- Mattsson K, Jocic S, Doverbratt I, Hansson LA (2018) Nanoplastics in the aquatic environment. In: Zeng EY Microplastic Contamination in Aquatic Environments, 1st edn. Elsevier Inc, pp 379–399. 10.1016/B978-0-12-813747-5.00013-8
- Mazurais D, Ernande B, Quazuguel P, et al. Evaluation of the impact of polyethylene microbeads ingestion in European sea bass (Dicentrarchus labrax) larvae. Mar Environ Res J. 2015;112:78–85. doi: 10.1016/j.marenvres.2015.09.009. [DOI] [PubMed] [Google Scholar]
- Mintenig SM, Bäuerlein PS, Koelmans AA, et al. Closing the gap between small and smaller: towards a framework to analyse nano- and microplastics in aqueous environmental samples. Environ Sci Nano. 2018;5:1640–1649. doi: 10.1039/c8en00186c. [DOI] [Google Scholar]
- Narmadha VV, Jose J, Patil S, et al. Assessment of microplastics in roadside suspended dust from urban and rural environment of Nagpur, India. Int J Environ Res. 2020;14:629–640. doi: 10.1007/s41742-020-00283-0. [DOI] [Google Scholar]
- Ng TP, Lee HS, Low YM, et al. Pulmonary effects of polyvinyl chloride dust exposure on compounding workers Published by : the Scandinavian Journal of Work, Environment & Health, the Finnish Institute of Occupational Health, the Danish National Research Centre for the Working Enviro. Scand J Work Env Heal. 1991;17:53–59. doi: 10.5271/sjweh.1734. [DOI] [PubMed] [Google Scholar]
- Nguyen B, Claveau-Mallet D, Hernandez LM, et al. Separation and analysis of microplastics and nanoplastics in complex environmental samples. Acc Chem Res. 2019;52:858–866. doi: 10.1021/acs.accounts.8b00602. [DOI] [PubMed] [Google Scholar]
- Nicole W. Microplastics in seafood: how much are people eating? Environ Health Perspect. 2021;129:1–2. doi: 10.1289/EHP8936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble WC, Lidwell OM, Kingston D. The size distribution of airborne particles carrying micro-organisms. J Hyg (Lond) 1963;61:385–391. doi: 10.1017/S0022172400020994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brine T, Thompson RC. Degradation of plastic carrier bags in the marine environment. Mar Pollut Bull. 2010;60:2279–2283. doi: 10.1016/j.marpolbul.2010.08.005. [DOI] [PubMed] [Google Scholar]
- O’Dowd CD, De Leeuw G. Marine aerosol production: a review of the current knowledge. Philos Trans R Soc A Math Phys Eng Sci. 2007;365:1753–1774. doi: 10.1098/rsta.2007.2043. [DOI] [PubMed] [Google Scholar]
- Oberbeckmann S, Löder MGJ, Labrenz M. Marine microplastic-associated biofilms - a review. Environ Chem. 2015;12:551–562. doi: 10.1071/EN15069. [DOI] [Google Scholar]
- Oberdörster G, Sharp Z, Atudorei V, et al. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol. 2004;16:437–445. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- Ogata Y, Takada H, Mizukawa K, et al. International Pellet Watch: Global monitoring of persistent organic pollutants (POPs) in coastal waters. 1. Initial phase data on PCBs, DDTs, and HCHs. Mar Pollut Bull. 2009;58:1437–1446. doi: 10.1016/j.marpolbul.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Oliveira M, Ribeiro A, Hylland K, Guilhermino L. Single and combined effects of microplastics and pyrene on juveniles (0+ group) of the common goby Pomatoschistus microps (Teleostei, Gobiidae) Ecol Indic. 2013;34:641–647. doi: 10.1016/j.ecolind.2013.06.019. [DOI] [Google Scholar]
- Opilik L, Schmid T, Zenobi R. Modern raman imaging: vibrational spectroscopy on the micrometer and nanometer scales. Annu Rev Anal Chem. 2013;6:379–398. doi: 10.1146/annurev-anchem-062012-092646. [DOI] [PubMed] [Google Scholar]
- Palansooriya KN, Wijesekara H, Bradney L, et al. et al. Characteristics of particulate plastics interrestrial ecosystems. In: Bola NS, Kirkham MB, Halsband C, et al.et al., editors. An introduction to the chemistry and manufacture of plastics. 1. Boca Raton: CRC Press; 2020. pp. 107–121. [Google Scholar]
- Pauly JL, Stegmeier J, Cheney T, et al. Inhaled cellulosic and plastic fibers found in human lung tissue ’. Cancer Epidemiol Biomarkers Prev. 1998;7:419–428. [PubMed] [Google Scholar]
- Paytan A, Mackey KRM, Chen Y, et al. Toxicity of atmospheric aerosols on marine phytoplankton. Proc Natl Acad Sci U S A. 2009;106:4601–4605. doi: 10.1073/pnas.0811486106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrino C (2010) Atmospheric particulate matter. Proceedings of a C.I.S.B. Minisymposium. 35–43. 10.1002/9780470999318.ch9
- Plastics – the Facts (2016) An analysis of European plastics production, demand and waste data; Plastics Europe. 1-35
- Plastics Europe (2018) Plastics—the facts 2018: an analysis of european plastics production. Demand and Waste Data. 1-45
- Pope CA, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc. 2006;56:709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- Prata JC. Airborne microplastics: consequences to human health? Environ Pollut. 2018;234:115–126. doi: 10.1016/j.envpol.2017.11.043. [DOI] [PubMed] [Google Scholar]
- Prata JC, Castro JL, da Costa JP, et al. An easy method for processing and identification of natural and synthetic microfibers and microplastics in indoor and outdoor air. MethodsX. 2020;7:1–9. doi: 10.1016/j.mex.2019.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primpke S, Lorenz C, Rascher-Friesenhausen R, Gerdts G. Analytical methods an automated approach for microplastics analysis using focal plane array ( FPA ) FTIR microscopy and image analysis. Anal Methods. 2017;9:1499–1511. doi: 10.1039/c6ay02476a. [DOI] [Google Scholar]
- Rauert C, Harrad S, Suzuki G, et al. Test chamber and forensic microscopy investigation of the transfer of brominated flame retardants into indoor dust via abrasion of source materials. Sci Total Environ. 2014;493:639–648. doi: 10.1016/j.scitotenv.2014.06.029. [DOI] [PubMed] [Google Scholar]
- Réhault J, Crisafi F, Kumar V, et al. Broadband stimulated Raman scattering with Fourier-transform detection. Opt Express. 2015;23:25235. doi: 10.1364/oe.23.025235. [DOI] [PubMed] [Google Scholar]
- Renner G, Schmidt TC, Schram J. Analytical methodologies for monitoring micro(nano)plastics: which are fit for purpose? Curr Opin Environ Sci Heal. 2018;1:55–61. doi: 10.1016/j.coesh.2017.11.001. [DOI] [Google Scholar]
- Rezaei M, Riksen MJPM, Sirjani E, et al. Wind erosion as a driver for transport of light density microplastics. Sci Total Environ. 2019;669:273–281. doi: 10.1016/j.scitotenv.2019.02.382. [DOI] [PubMed] [Google Scholar]
- Rezaei M, Abbasi S, Pourmahmood H, et al. Microplastics in agricultural soils from a semi-arid region and their transport by wind erosion. Environ Res. 2022;212:113213. doi: 10.1016/j.envres.2022.113213. [DOI] [PubMed] [Google Scholar]
- Rocha-santos T, Duarte AC. Trends in analytical chemistry a critical overview of the analytical approaches to the occurrence, the fate and the behavior of microplastics in the environment. Trends Anal Chem. 2015;65:47–53. doi: 10.1016/j.trac.2014.10.011. [DOI] [Google Scholar]
- Rochman CM, Hentschel BT, The SJ (2014) Long-term sorption of metals is similar among plastic types: implications for plastic debris in aquatic environments. PLoS One 9(1): e85433. 10.1371/journal.pone.0085433 [DOI] [PMC free article] [PubMed]
- Roux C, Margot P. The population of textile fibres on car seats. Sci Justice. 1997;37:25–30. doi: 10.1016/S1355-0306(97)72137-3. [DOI] [Google Scholar]
- Rudel RA, Camann DE, Spengler JD, et al. Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust. Environ Sci Technol. 2003;37:4543–4553. doi: 10.1021/es0264596. [DOI] [PubMed] [Google Scholar]
- Ryan PG, Moore CJ, Van Franeker JA, Moloney CL. Monitoring the abundance of plastic debris in the marine environment. Phil Trans Biol Sci. 2009;364:1999–2012. doi: 10.1098/rstb.2008.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheurer M, Bigalke M. Microplastics in Swiss floodplain soils. Environ Sci Technol. 2018;52:3591–3598. doi: 10.1021/acs.est.7b06003. [DOI] [PubMed] [Google Scholar]
- Schnelle-Kreis J, Gebefügi I, Welzl G et al (2001) Occurrence of particle-associated polycyclic aromatic compounds in ambient air of the city of Munich. Atmos Environ 35:S71–S81. 10.1016/s1352-2310(00)00557-4
- Schwaferts C, Niessner R, Elsner M, Ivleva NP. Methods for the analysis of submicrometer- and nanoplastic particles in the environment. TrAC Trends Anal Chem. 2019;112:52–65. doi: 10.1016/j.trac.2018.12.014. [DOI] [Google Scholar]
- Schymanski D, Goldbeck C, Humpf HU, Fürst P. Analysis of microplastics in water by micro-Raman spectroscopy: release of plastic particles from different packaging into mineral water. Water Res. 2018;129:154–162. doi: 10.1016/j.watres.2017.11.011. [DOI] [PubMed] [Google Scholar]
- Seaton A, Tran L, Aitken R, Donaldson K (2010) Nanoparticles, human health hazard and regulation. J R Soc Interface 7:S119–S129. 10.1098/rsif.2009.0252.focus [DOI] [PMC free article] [PubMed]
- Seinfeld JH, Pankow JF. Organic atmospheric particulate material. Annu Rev Phys Chem. 2003;54:121–140. doi: 10.1146/annurev.physchem.54.011002.103756. [DOI] [PubMed] [Google Scholar]
- Shen M, Hu T, Huang W, et al. Can incineration completely eliminate plastic wastes? An investigation of microplastics and heavy metals in the bottom ash and fly ash from an incineration plant. Sci Total Environ. 2021;779:146528. doi: 10.1016/j.scitotenv.2021.146528. [DOI] [PubMed] [Google Scholar]
- Shi H, Magaye R, Castranova V, Zhao J. Titanium dioxide nanoparticles: a review of current toxicological data. Part Fibre Toxicol. 2013;10:15. doi: 10.1186/1743-8977-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim WJ, Hong H, Eo S. Identification methods in microplastic analysis: a review. Anal Methods. 2017;9:1384–1391. doi: 10.1039/C6AY02558G. [DOI] [Google Scholar]
- Soltani NS, Taylor MP, Wilson SP. Quantification and exposure assessment of microplastics in Australian indoor house dust. Environ Pollut. 2021;283:117064. doi: 10.1016/j.envpol.2021.117064. [DOI] [PubMed] [Google Scholar]
- Song Y, Li X, Du X. Exposure to nanoparticles is related to pleural effusion, pulmonary fibrosis and granuloma. Eur Respir J. 2009;34:559–567. doi: 10.1183/09031936.00178308. [DOI] [PubMed] [Google Scholar]
- Song YK, Hong SH, Jang M, et al. Combined effects of UV exposure duration and mechanical abrasion on microplastic fragmentation by polymer type. Environ Sci Technol. 2017;51:4368–4376. doi: 10.1021/acs.est.6b06155. [DOI] [PubMed] [Google Scholar]
- Stephens B, Azimi P, El Orch Z, Ramos T. Ultrafine particle emissions from desktop 3D printers. Atmos Environ. 2013;79:334–339. doi: 10.1016/j.atmosenv.2013.06.050. [DOI] [Google Scholar]
- Stroh A, Zimmer C, Gutzeit C, et al. Iron oxide particles for molecular magnetic resonance imaging cause transient oxidative stress in rat macrophages. Free Radic Biol Med. 2004;36:976–984. doi: 10.1016/j.freeradbiomed.2004.01.016. [DOI] [PubMed] [Google Scholar]
- Su L, Sharp SM, Pettigrove VJ, et al. Superimposed microplastic pollution in a coastal metropolis. Water Res. 2020;168:115140. doi: 10.1016/j.watres.2019.115140. [DOI] [PubMed] [Google Scholar]
- Sukiene V, Von Goetz N, Gerecke AC, et al. Direct and air-mediated transfer of labeled SVOCs from indoor sources to dust. Environ Sci Technol. 2017;51:3269–3277. doi: 10.1021/acs.est.6b06051. [DOI] [PubMed] [Google Scholar]
- Szewc K, Graca B, Dołęga A. Atmospheric deposition of microplastics in the coastal zone: characteristics and relationship with meteorological factors. Sci Total Environ. 2021;761:143272. doi: 10.1016/j.scitotenv.2020.143272. [DOI] [PubMed] [Google Scholar]
- Ter Halle A, Jeanneau L, Martignac M, et al. Nanoplastic in the North Atlantic Subtropical Gyre. Environ Sci Technol. 2017;51:13689–13697. doi: 10.1021/acs.est.7b03667. [DOI] [PubMed] [Google Scholar]
- Teuten EL, Rowland SJ, Galloway TS, Galloway TS. Potential for plastics to transport hydrophobic contaminants. Environ Sci Technol. 2007;41:7759–7764. doi: 10.1021/es071737s. [DOI] [PubMed] [Google Scholar]
- Teuten EL, Saquing JM, Knappe DRU, et al. Transport and release of chemicals from plastics to the environment and to wildlife. Philos Trans R Soc B Biol Sci. 2009;364:2027–2045. doi: 10.1098/rstb.2008.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RC (2015) Microplastics in the marine environment : sources , consequences and solutions. In: Bergmann M, Gutow L, Klages M (eds) Marine nature conservation in Europe Stralsund: Federal Agency for Nature Conservation, pp 185–200. 10.1007/978-3-319-16510-3_7
- Truong TNS, Strady E, Kieu-Le TC, et al. Microplastic in atmospheric fallouts of a developing Southeast Asian megacity under tropical climate. Chemosphere. 2021;272:129874. doi: 10.1016/j.chemosphere.2021.129874. [DOI] [PubMed] [Google Scholar]
- Tunahan Kaya A, Yurtsever M, Çiftçi Bayraktar S (2018) Ubiquitous exposure to microfiber pollution in the air. Eur Phys J Plus 133:488. 10.1140/epjp/i2018-12372-7
- Turner A, Holmes LA. Adsorption of trace metals by microplastic pellets in fresh water. Environ Chem. 2015;12:600. doi: 10.1071/EN14143. [DOI] [Google Scholar]
- Valic F, Zuskin E. Respiratory-function changes in textile workers exposed to synthetic fibers. Arch Environ Heal Int J. 1977;32:283–287. doi: 10.1080/00039896.1977.10667296. [DOI] [PubMed] [Google Scholar]
- Van Doremalen, N., Bushmaker T, Morris DH, et al (2020) Aerosol and surface stability of SARS-CoV-2 as Compared with SARS-CoV-1. new engl J Med 382:1564–1567 [DOI] [PMC free article] [PubMed]
- Van Vaeck L, van Cauwenberghe K. Cascade impactor measurements of the size distribution of the major classes of organic pollutants in atmospheric particulate matter. Atmos Environ. 1978;12:2229–2239. doi: 10.1016/0004-6981(78)90179-8. [DOI] [Google Scholar]
- Ventrice P, Ventrice D, Russo E, De Sarro G. Phthalates: European regulation, chemistry, pharmacokinetic and related toxicity. Environ Toxicol Pharmacol. 2013;36:88–96. doi: 10.1016/j.etap.2013.03.014. [DOI] [PubMed] [Google Scholar]
- Verschoor AJ (2015) Towards a definition of microplastics Considerations for the specification of physico-chemical properties. RIVM Letter report 2015-0116
- Vianello A, Jensen RL, Liu L, Vollertsen J. Simulating human exposure to indoor airborne microplastics using a Breathing Thermal Manikin. Sci Rep. 2019;9:1–11. doi: 10.1038/s41598-019-45054-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vobecky J, Devroede G, Caro J. Risk of large-bowel cancer in synthetic fiber manufacture. Cancer. 1984;54:2537–2542. doi: 10.1002/1097-0142(19841201)54:11<2537::AID-CNCR2820541138>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Wagner M, Scherer C, Alvarez-Muñoz D, et al. Microplastics in freshwater ecosystems: what we know and what we need to know. Environ Sci Eur. 2014;26:1–9. doi: 10.1016/0163-8343(83)90040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Wang J. Different partition of polycyclic aromatic hydrocarbon on environmental particulates in freshwater: microplastics in comparison to natural sediment. Ecotoxicol Environ Saf. 2018;147:648–655. doi: 10.1016/j.ecoenv.2017.09.029. [DOI] [PubMed] [Google Scholar]
- Wang J, Tan Z, Peng J, et al. The behaviors of microplastics in the marine environment. Mar Environ Res. 2016;113:7–17. doi: 10.1016/j.marenvres.2015.10.014. [DOI] [PubMed] [Google Scholar]
- Wang L, Wu WM, Bolan NS, et al. Environmental fate, toxicity and risk management strategies of nanoplastics in the environment: current status and future perspectives. J Hazard Mater. 2020;401:123415. doi: 10.1016/j.jhazmat.2020.123415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Enyoh CE, Chowdhury T, Chowdhury AH. Analytical techniques, occurrence and health effects of micro and nano plastics deposited in street dust. Int J Environ Anal Chem. 2020;00:1–19. doi: 10.1080/03067319.2020.1811262. [DOI] [Google Scholar]
- Washko RM, Day B, Parker JE, Kreiss K. Epidemiologic investigation of respiratory morbidity at a nylon flock plant. Am J Ind Med. 2000;3(638):628–638. doi: 10.1002/1097-0274(200012)38:6<628::AID-AJIM3>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Palonpon AF, Smith NI, et al. Structured line illumination Raman microscopy. Nat Commun. 2015;6:1–6. doi: 10.1038/ncomms10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein JE, Crocker BK, Gray AD. From macroplastic to microplastic: degradation of high-density polyethylene, polypropylene, and polystyrene in a salt marsh habitat. Environ Toxicol Chem. 2016;35:1632–1640. doi: 10.1002/etc.3432. [DOI] [PubMed] [Google Scholar]
- Wirnkor A, Christian V, Enyoh E, et al. Microplastic – toxic chemical interaction : a review study on quantified levels, mechanism and implication. SN Appl Sci. 2019;1:1–30. doi: 10.1007/s42452-019-1352-0. [DOI] [Google Scholar]
- Wirnkora VA, Eberea EC, Ngozib VE. Microplastics, an emerging concern : a review of analytical techniques for detecting and quantifying microplatics. Anal Methods Environ Chem J. 2019;2:15–32. [Google Scholar]
- Wong KO, Leo LW, Seah HL. Dietary exposure assessment of infants to bisphenol A from the use of polycarbonate baby milk bottles. Food Addit Contam. 2007;22:280–288. doi: 10.1080/02652030500077502. [DOI] [PubMed] [Google Scholar]
- Wright SL, Thompson RC, Galloway TS. The physical impacts of microplastics on marine organisms: a review. Environ Pollut. 2013;178:483–492. doi: 10.1016/j.envpol.2013.02.031. [DOI] [PubMed] [Google Scholar]
- Wright SL, Levermore JM, Kelly FJ. Raman spectral imaging for the detection of inhalable microplastics in ambient particulate matter samples. Environ Sci Technol. 2019;53:8947–8956. doi: 10.1021/acs.est.8b06663. [DOI] [PubMed] [Google Scholar]
- Wright SL, Ulke J, Font A, et al. Atmospheric microplastic deposition in an urban environment and an evaluation of transport. Environ Int. 2019;136:105411. doi: 10.1016/j.envint.2019.105411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright SL, Ulke J, Font A et al (2020) Atmospheric microplastic deposition in an urban environment and an evaluation of transport. Environment International 136105411-S0160412019330351 105411. 10.1016/j.envint.2019.105411 [DOI] [PMC free article] [PubMed]
- Xie MY, Ni H, Zhao DS, et al. Exposure to bisphenol A and the development of asthma: a systematic review of cohort studies. Reprod Toxicol. 2016;65:224–229. doi: 10.1016/j.reprotox.2016.08.007. [DOI] [PubMed] [Google Scholar]
- Xie S, Zhou A, Wei T, et al. Nanoplastics induce more serious microbiota dysbiosis and inflammation in the gut of adult zebrafish than microplastics. Bull Environ Contam Toxicol. 2021;107:640–650. doi: 10.1007/s00128-021-03348-8. [DOI] [PubMed] [Google Scholar]
- Xu R. Light scattering: A review of particle characterization applications. Particuology. 2015;18:11–21. doi: 10.1016/j.partic.2014.05.002. [DOI] [Google Scholar]
- Xu H, Verbeken E, Vanhooren HM, et al. Pulmonary toxicity of polyvinyl chloride particles after a single intratracheal instillation in rats. Time course and comparison with silica. Toxicol Appl Pharmacol. 2004;194:111–121. doi: 10.1016/j.taap.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Yang Z, Liu ZW, Allaker RP, et al. A review of nanoparticle functionality and toxicity on the central nervous system. J R Soc Interface. 2010;7:S411–S422. doi: 10.1007/978-94-007-1787-9_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Zhang Y, Kang S, et al. Microplastics in freshwater sediment: a review on methods, occurrence, and sources. Sci Total Environ. 2021;754:141948. doi: 10.1016/j.scitotenv.2020.141948. [DOI] [PubMed] [Google Scholar]
- Yang Z, Lü F, Zhang H, et al. Is incineration the terminator of plastics and microplastics? J Hazard Mater. 2021;401:123429. doi: 10.1016/j.jhazmat.2020.123429. [DOI] [PubMed] [Google Scholar]
- Yildirimer L, Thanh NTK, Loizidou M, Seifalian AM. Toxicological considerations of clinically applicable nanoparticles. Nano Today. 2011;6:585–607. doi: 10.1016/j.nantod.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zada L, Leslie HA, Vethaak AD, et al. Fast microplastics identification with stimulated Raman scattering microscopy. J Raman Spectrosc. 2018;49:1136–1144. doi: 10.1002/jrs.5367. [DOI] [Google Scholar]
- Zhang J, Wang L, Kannan K. Microplastics in house dust from 12 countries and associated human exposure. Environ Int. 2020;134:105314. doi: 10.1016/j.envint.2019.105314. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Zhao Y, Du F et al (2020d) Microplastic fallout in different indoor environments. Environ Sci Technol 54(11):6530–6539. 10.1021/acs.est.0c00087 [DOI] [PubMed]
- Zhang X, Mell A, Li F, et al. Rapid fingerprinting of source and environmental microplastics using direct analysis in real time-high resolution mass spectrometry. Anal Chim Acta. 2020;1100:107–117. doi: 10.1016/j.aca.2019.12.005. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kang S, Allen S, et al. Atmospheric microplastics: a review on current status and perspectives. Earth-Science Rev. 2020;203:103118. doi: 10.1016/j.earscirev.2020.103118. [DOI] [Google Scholar]
- Zhang Y, Gao T, Kang S, et al. Microplastics in glaciers of the Tibetan Plateau: Evidence for the long-range transport of microplastics. Sci Total Environ. 2021;758:143634. doi: 10.1016/j.scitotenv.2020.143634. [DOI] [PubMed] [Google Scholar]
- Zuccarello P, Ferrante M, Cristaldi A, et al. Exposure to microplastics (<10 Μm) associated to plastic bottles mineral water consumption: the first quantitative study. Water Res. 2019;157:365–371. doi: 10.1016/j.watres.2019.03.091. [DOI] [PubMed] [Google Scholar]
- Zuskin E, Mustajbegovic J, Schachter EN, et al. Respiratory findings in synthetic textile workers. Am J Ind Med. 1998;33:263–273. doi: 10.1002/(SICI)1097-0274(199803)33:3<263::AID-AJIM8>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.