Abstract
Data on the safety of COVID-19 vaccines in early pregnancy are limited. We conducted a national, population-based, matched cohort study assessing associations between COVID-19 vaccination and miscarriage prior to 20 weeks gestation and, separately, ectopic pregnancy. We identified women in Scotland vaccinated between 6 weeks preconception and 19 weeks 6 days gestation (for miscarriage; n = 18,780) or 2 weeks 6 days gestation (for ectopic; n = 10,570). Matched, unvaccinated women from the pre-pandemic and, separately, pandemic periods were used as controls. Here we show no association between vaccination and miscarriage (adjusted Odds Ratio [aOR], pre-pandemic controls = 1.02, 95% Confidence Interval [CI] = 0.96–1.09) or ectopic pregnancy (aOR = 1.13, 95% CI = 0.92–1.38). We undertook additional analyses examining confirmed SARS-CoV-2 infection as the exposure and similarly found no association with miscarriage or ectopic pregnancy. Our findings support current recommendations that vaccination remains the safest way for pregnant women to protect themselves and their babies from COVID-19.
Subject terms: Viral infection, Epidemiology, SARS-CoV-2
Data on the safety of COVD-19 vaccines in early pregnancy are limited. Here, the authors assess the rates of miscarriage and ectopic pregnancy following vaccination using electronic health record data from Scotland, and find no evidence of increased risks.
Introduction
COVID-19 vaccination is effective in reducing the risk of severe SARS-CoV-2 infection in pregnant women1,2, but high levels of vaccine hesitancy remain in pregnant populations. In England and Scotland, 59.5% and 63.0%, respectively, of women giving birth in January 2022 had received at least one prior dose of COVID-19 vaccination at any point, which was considerably lower than the coverage seen among the general population of reproductive age women at that time3–5.
Several factors are likely to contribute to COVID-19 vaccine hesitancy in pregnant women, including exclusion of pregnant women from the initial vaccine trials and hence inconsistent guidance early in the vaccination programme reflecting the lack of safety data at that time6. A growing body of evidence suggests that there is no association between COVID-19 vaccination in pregnancy and adverse late pregnancy outcomes, including preterm birth and stillbirth7. There is, in contrast, a paucity of data on early pregnancy outcomes. A recent systematic review identified only two studies, conducted in Norway and the US, comparing the risk of miscarriage between vaccinated and unvaccinated women7. Neither of these case-control studies found evidence of an association between COVID-19 vaccination and miscarriage. In the Norwegian population-based study utilizing routine records, women with first-trimester miscarriage had lower odds of having had a COVID-19 vaccination in the preceding 5 weeks compared with women with ongoing pregnancies (adjusted odds ratio [aOR] = 0.81, 95% CI = 0.69–0.95), and similar odds of having had a vaccination in the preceding 3 weeks (aOR = 0.91, 95% CI = 0.75–1.10)8. In the US study, women with miscarriage had similar odds of having COVID-19 vaccination in the previous 4 weeks compared with women with ongoing pregnancies (aOR = 1.02, 95% CI = 0.96–1.08)9. To our knowledge, there are no published data on the relationship between COVID-19 vaccination and ectopic pregnancy, the other major cause of early pregnancy loss.
There is an urgent need for robust evidence on the safety of COVID-19 vaccines in early pregnancy to inform decision-making among women, their healthcare providers, and policymakers. We use population-based pregnancy data from the COVID-19 in Pregnancy in Scotland (COPS) cohort10,11 to investigate whether there was any evidence for an association between COVID-19 vaccination between six weeks preconception and up to 19 weeks and 6 days (19 + 6) gestation and miscarriage, and up to 2 weeks and 6 days (2 + 6) and ectopic pregnancy. As a secondary objective, we examined whether there was any evidence for an association between SARS-CoV-2 infection and miscarriage or ectopic pregnancy.
Results
Study population
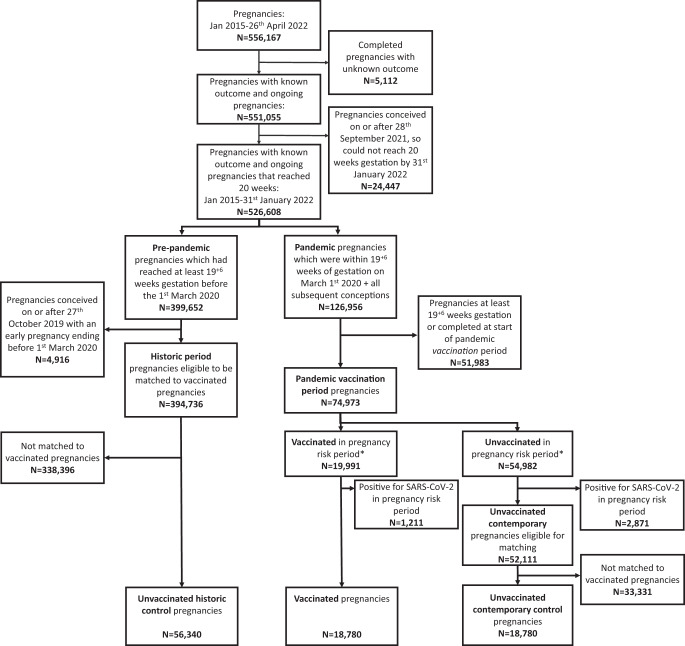
As of April 26, 2022, the COPS study database contained information on 556,167 pregnancies (in 361,606 women) of which 526,608 were eligible for inclusion: 399,652 from the historical pre-pandemic period and 126,956 from the contemporary pandemic period (Fig. 1).
Fig. 1. Selection of vaccinated and unvaccinated pregnancy cohorts for miscarriage outcome analysis.
Flow diagram showing the selection of pregnancies for the analysis of the association between COVID-19 vaccination and risk of miscarriage. *For miscarriage analysis, vaccination needs to be given between 6 weeks preconception and up to the earliest of (1) end of pregnancy or (2) 19 weeks 6 days gestation.
Association between COVID-19 vaccination and miscarriage
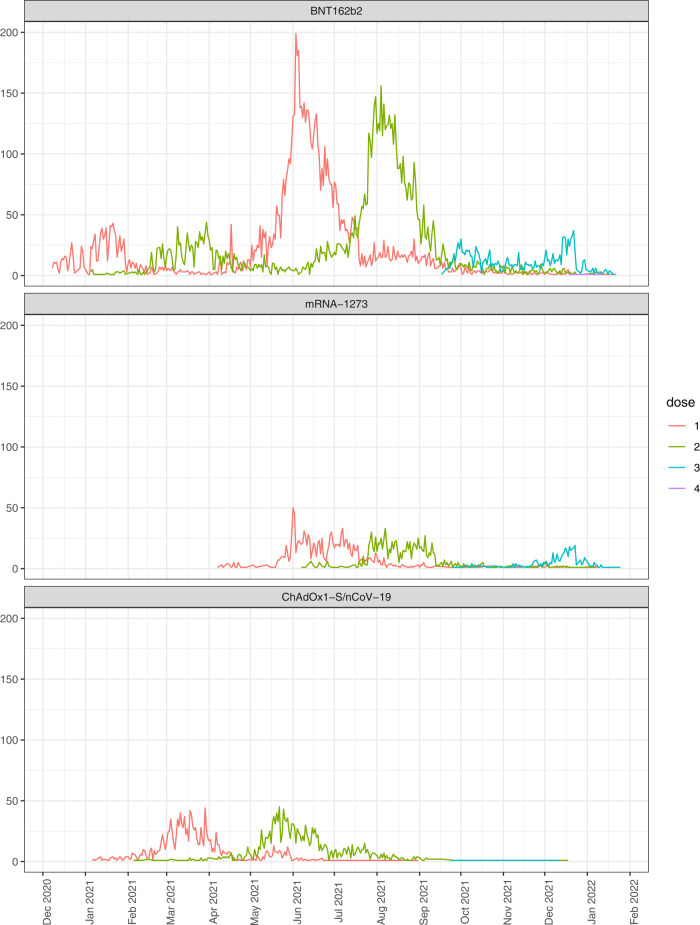
A total of 18,780 pregnant women received COVID-19 vaccination between 6 weeks preconception and 19 + 6 weeks gestation (or the end of pregnancy if earlier) (Fig. 1). The characteristics of the vaccinated cohort, and the matched historical and contemporary control groups, are provided in Table 1. Compared to controls, vaccinated women were more likely to be from the least deprived areas. Pfizer-BioNTech BNT162b2 was the most frequently received vaccine type, and around a quarter of the vaccinated women received two or more doses during the exposure period. Reflecting the roll out of the national vaccination programme in Scotland (Supplementary Fig. 1), most included vaccinations occurred in May to August 2021 (Fig. 2).
Table 1.
Key characteristics of vaccinated and control groups included in the vaccination and miscarriage analyses
| Cohort characteristics | Vaccinated | Unvaccinated (historical) | Unvaccinated (contemporary) | |
|---|---|---|---|---|
| Number of pregnancies | 18,780 | 56,340 | 18,780 | |
| Median age (min–max) | 31 (14–51) | 31 (14–51) | 31 (14–54) | |
| Deprivation (SIMD quintile) | 1 (most deprived) | 3501 (18.6%) | 12,258 (21.8%) | 4406 (23.5%) |
| 2 | 3503 (18.7%) | 11,031 (19.6%) | 3803 (20.3%) | |
| 3 | 3509 (18.7%) | 10,018 (17.8%) | 3457 (18.4%) | |
| 4 | 4171 (22.2%) | 11,657 (20.7%) | 3743 (19.9%) | |
| 5 (least deprived) | 3952 (21.0%) | 10,807 (19.2%) | 3241 (17.3%) | |
| Unknown | 144 (0.8%) | 569 (1.0%) | 130 (0.7%) | |
| Ethnicity | White | 15,897 (84.6%) | 37,156 (65.9%) | 15,672 (83.5%) |
| South Asian | 708 (3.8%) | 1274 (2.3%) | 633 (3.4%) | |
| Black/Caribbean/African | 234 (1.2%) | 676 (1.2%) | 375 (2.0%) | |
| Other/mixed ethnicity | 596 (3.2%) | 1410 (2.5%) | 683 (3.6%) | |
| Unknown | 1345 (7.2%) | 15,824 (28.1%) | 1417 (7.5%) | |
| Urban/rural status | Large urban areas | 6801 (36.2%) | 21,263 (37.7%) | 6999 (37.3%) |
| Other urban areas | 5974 (31.8%) | 20,171 (35.8%) | 6561 (34.9%) | |
| Accessible small towns | 1439 (7.7%) | 4512 (8.0%) | 1390 (7.4%) | |
| Remote small towns | 550 (2.9%) | 1597 (2.8%) | 572 (3.0%) | |
| Accessible rural areas | 1984 (10.6%) | 5611 (10.0%) | 2088 (11.1%) | |
| Remote rural areas | 831 (4.4%) | 2532 (4.5%) | 767 (4.1%) | |
| Unknown | 1201 (6.4%) | 654 (1.2%) | 403 (2.1%) | |
| Clinical vulnerability | Extremely vulnerable | 228 (1.2%) | 769 (1.4%) | 154 (0.8%) |
| Vulnerable | 4819 (25.7%) | 15,917 (28.3%) | 4868 (25.9%) | |
| Not vulnerable | 13,733 (73.1%) | 39,654 (70.4%) | 13,758 (73.3%) | |
| Exposure (vaccination) | ||||
| Gestation at first vaccination | Preconception | 8816 (46.9%) | – | – |
| 2 + 0–5 + 6 weeks | 4173 (22.2%) | – | – | |
| 6 + 0–10 + 6 weeks | 1311 (7.0%) | – | – | |
| 11 + 0–15 + 6 weeks | 2468 (13.1%) | – | – | |
| 16 + 0–19 + 6 weeks | 2012 (10.7%) | – | – | |
| Number of vaccinationsa | 1 | 13,792 (73.4%) | – | – |
| 2+ | 4988 (26.6%) | – | – | |
| Dose number at first vaccination | Dose 1 | 10,974 (58.4%) | – | – |
| Dose 2 | 6052 (32.2%) | – | – | |
| Dose 3 | 1754 (9.3%) | – | – | |
| Type of vaccinationa | BNT162b2 | 13,675 (72.8%) | – | – |
| mRNA-1273 | 2260 (12.0%) | – | – | |
| ChAdOx1-S/nCoV-19 | 2612 (13.9%) | – | – | |
| 1+ different type | 233 (1.2%) | – | – | |
| Outcome (miscarriage) | ||||
| Number of miscarriages ≤ 19 + 6 weeks | 1716 | 5566 | 1878 | |
| Gestation at miscarriage | ≤10 + 6 | 1302 (75.9%) | 5113 (91.9%) | 1313 (69.9%) |
| 11 + 0–13 + 6 | 300 (17.5%) | 295 (5.3%) | 412 (21.9%) | |
| ≥14 + 0 | 114 (6.6%) | 158 (2.8%) | 153 (8.1%) | |
| Imputed gestation for miscarriage | Yes | 975 (56.8%) | 4593 (82.5%) | 927 (49.4%) |
| No—from ANC booking | 576 (33.6%) | 308 (5.5%) | 721 (38.4%) | |
| No—from end of pregnancy record | 165 (9.6%) | 665 (11.9%) | 230 (12.2%) | |
| Interval to miscarriageb | <2 weeks | 84 (4.9%) | 223 (4.0%) | 101 (5.4%) |
| 2–5 weeks | 258 (15.0%) | 657 (11.8%) | 262 (14.0%) | |
| 6–9 weeks | 536 (31.2%) | 1902 (34.2%) | 586 (31.2%) | |
| ≥10 weeks | 838 (48.8%) | 2784 (50.0%) | 929 (49.5%) |
SIMD Scottish Index of multiple deprivation.
aBetween 6 weeks preconception and the earliest of either: end of pregnancy or 19 + 6 weeks gestation.
bInterval between vaccination (or gestation of matching) and miscarriage.
Fig. 2. COVID-19 vaccination between 6 weeks preconception and 19 weeks and 6 days gestation over time.
Line graph showing the total number of women vaccinated between 6 weeks preconception and up to 19 weeks and 6 days gestation by calendar time, dose number and type of vaccine.
By 19 + 6 weeks gestation, 9.1% (1716/18,780) of pregnancies in the vaccinated cohort ended in miscarriage, compared to 9.9% (5566/56,340) in historical controls and 10.0% (1878/18,780) in contemporary controls (Table 2). Our primary analyses (using historical controls) found no evidence that women vaccinated in pregnancy had higher odds of miscarriage in either the model accounting only for matching factors (OR = 0.98, 95% CI = 0.93–1.04) or adjusted analyses (aOR = 1.02, 95% CI = 0.96–1.09) (Table 2). Results of supplementary analyses (using contemporary controls) were similar (OR = 0.91, 95% CI = 0.85–0.98; aOR = 0.96, 95% CI = 0.88–1.04) (Table 2).
Table 2.
Odds ratios for the association between COVID-19 vaccination and miscarriage from multinomial logistic regression models
| Number of pregnancies | Number (%) of ongoing pregnancies | Number (%) of miscarriages | Odds ratio (95% CI)a | p-value | Adjusted odds ratio (95% CI)b | p-value | |
|---|---|---|---|---|---|---|---|
| Primary analysis (historical controls) | |||||||
| Unvaccinated | 56,340 | 44,669 (79.3%) | 5566 (9.9%) | 1 | 1 | ||
| Vaccinated | 18,780 | 14,119 (75.2%) | 1716 (9.1%) | 0.98 (0.93–1.04) | 0.55 | 1.02 (0.96–1.09) | 0.49 |
| BNT162b2 subgroup analysis (historical controls) | |||||||
| Unvaccinated | 41,025 | 32,958 (80.3%) | 3804 (9.3%) | 1 | 1 | ||
| Vaccinated | 13,675 | 10,485 (76.7%) | 1147 (8.4%) | 0.96 (0.89–1.03) | 0.22 | 1.00 (0.93–1.08) | 0.90 |
| mRNA-1273 subgroup analysis (historical controls) | |||||||
| Unvaccinated | 6780 | 5646 (83.3%) | 511 (7.5%) | 1 | 1 | ||
| Vaccinated | 2260 | 1744 (77.2%) | 162 (7.2%) | 1.06 (0.88–1.29) | 0.52 | 1.07 (0.87–1.33) | 0.51 |
| ChAdOx1-S/nCoV-19 subgroup analysis (historical controls) | |||||||
| Unvaccinated | 7836 | 5564 (71.0%) | 1152 (14.7%) | 1 | 1 | ||
| Vaccinated | 2612 | 1659 (63.5%) | 406 (15.5%) | 1.17 (1.03–1.33) | 0.01 | 1.17 (1.03–1.34) | 0.02 |
| Supplementary analysis (contemporary controls) | |||||||
| Unvaccinated | 18,780 | 14,162 (75.4%) | 1878 (10.0%) | 1 | 1 | ||
| Vaccinated | 18,780 | 14,119 (75.2%) | 1716 (9.1%) | 0.91 (0.85–0.98) | 0.01 | 0.96 (0.88–1.04) | 0.35 |
| BNT162b2 subgroup analysis (contemporary controls) | |||||||
| Unvaccinated | 13,675 | 10,494 (76.7%) | 1258 (9.2%) | 1 | 1 | ||
| Vaccinated | 13,675 | 10,485 (76.7%) | 1147 (8.4%) | 0.91 (0.83–0.99) | 0.03 | 0.99 (0.89–1.09) | 0.81 |
| mRNA-1273 subgroup analysis (contemporary controls) | |||||||
| Unvaccinated | 2260 | 1794 (79.4%) | 198 (8.8%) | 1 | 1 | ||
| Vaccinated | 2260 | 1744 (77.2%) | 162 (7.2%) | 0.85 (0.68–1.07) | 0.17 | 1.04 (0.76–1.43) | 0.79 |
| ChAdOx1-S/nCoV-19 subgroup analysis (contemporary controls) | |||||||
| Unvaccinated | 2612 | 1731 (66.3%) | 389 (14.9%) | 1 | 1 | ||
| Vaccinated | 2612 | 1659 (63.5%) | 406 (15.5%) | 1.08 (0.92–1.27) | 0.32 | 0.92 (0.76–1.11) | 0.41 |
All analyses exclude women with confirmed SARS-CoV-2 infection between 6 weeks preconception and the earliest of either: end of pregnancy or the end of the exposure period at 19 + 6 weeks gestation.
CI confidence interval.
aAdjusting for matching factors: maternal age and gestational age at the date of vaccination of index vaccinated pregnancy (and season of conception for primary and primary subgroup analysis).
bAdditionally adjusting for deprivation, urban/rural status, and clinical vulnerability (and ethnicity and season of conception in Supplementary analyses).
Compared to women receiving an mRNA vaccine (Pfizer-BioNTech BNT162b2 or Moderna mRNA-1273), women receiving Oxford/AstraZeneca ChAdOx1-S/nCoV-19 were more likely to be from the most deprived areas, and substantially more likely to categorized as clinically vulnerable or extremely vulnerable (Supplementary Table 1). In subgroup analyses, we found no evidence that women receiving either BNT162b2 or mRNA-1273 vaccines had higher odds of miscarriage compared to historical controls (aOR = 1.00, 95% CI = 0.93–1.08 and aOR = 1.07, 95% CI = 0.87–1.33, respectively) or contemporary controls (aOR = 0.99, 95% CI = 0.89–1.09 and aOR = 1.04, 95% CI = 0.76–1.43, respectively). We did find higher odds of miscarriage among women receiving ChAdOx1-S/nCoV-19 vaccine when compared to historical controls (aOR = 1.17, 95% CI = 1.03–1.34) but not contemporary controls (aOR = 0.92, 95% CI = 0.76–1.11) (Table 2).
Association between COVID-19 vaccination and ectopic pregnancy
A total of 10,570 pregnant women received COVID-19 vaccination between six weeks preconception and 2 + 6 weeks gestation (Supplementary Fig. 2). Characteristics of the vaccinated cohort, and matched control groups, are provided in Supplementary Table 2. By 19 + 6 weeks gestation, 1.2% of pregnancies (126/10,570) in the vaccinated cohort had ended in ectopic pregnancy, compared to 1.2% of pregnancies (379/31,710) in historical controls and 1.1% (336/31,710) in contemporary controls (Table 3). We found no evidence that women vaccinated in pregnancy had higher odds of ectopic pregnancy in primary or supplementary analyses (aOR = 1.13, 95% CI = 0.92–1.38 and aOR = 1.12, 95% CI = 0.90–1.40 respectively) (Table 3). Similarly, in subgroup analyses, we found no evidence of higher odds of ectopic pregnancy in women receiving any specific vaccine type (Supplementary Table 3 and Table 3).
Table 3.
Odds ratios for the association between COVID-19 vaccination and ectopic pregnancy from multinomial logistic regression models
| Number of pregnancies | Number (%) of ongoing pregnancies | Number (%) of ectopic pregnancies | Odds ratio (95% CI)a | p-value | Adjusted odds ratio (95% CI)b | p-value | |
|---|---|---|---|---|---|---|---|
| Primary analysis (historical controls) | |||||||
| Unvaccinated | 31,710 | 23,438 (73.9%) | 379 (1.2%) | 1 | 1 | ||
| Vaccinated | 10,570 | 7328 (69.3%) | 126 (1.2%) | 1.05 (0.86–1.29) | 0.61 | 1.13 (0.92–1.38) | 0.25 |
| BNT162b2 subgroup analysis (historical controls) | |||||||
| Unvaccinated | 22,020 | 16,315 (74.1%) | 271 (1.2%) | 1 | 1 | ||
| Vaccinated | 7340 | 5124 (69.8%) | 87 (1.2%) | 1.02 (0.80–1.30) | 0.90 | 1.11 (0.87–1.42) | 0.39 |
| mRNA-1273 subgroup analysis (historical controls) | |||||||
| Unvaccinated | 3120 | 2405 (77.1%) | 22 (0.7%) | 1 | 1 | ||
| Vaccinated | 1040 | 705 (67.8%) | 13 (1.3%) | 1.99 (1.00–3.98) | 0.05 | 2.07 (0.98–4.38) | 0.06 |
| ChAdOx1-S/nCoV-19 subgroup analysis (historical controls) | |||||||
| Unvaccinated | 5862 | 4189 (71.5%) | 76 (1.3%) | 1 | 1 | ||
| Vaccinated | 1954 | 1264 (64.7%) | 26 (1.3%) | 1.12 (0.72–1.76) | 0.61 | 1.16 (0.74–1.83) | 0.52 |
| Supplementary analysis (contemporary controls) | |||||||
| Unvaccinated | 31,710 | 22,901 (72.2%) | 336 (1.1%) | 1 | 1 | ||
| Vaccinated | 10,570 | 7,328 (69.3%) | 126 (1.2%) | 1.17 (0.95–1.43) | 0.14 | 1.12 (0.90–1.40) | 0.31 |
| BNT162b2 subgroup analysis (contemporary controls) | |||||||
| Unvaccinated | 22,020 | 15,957 (72.5%) | 238 (1.1%) | 1 | 1 | ||
| Vaccinated | 7340 | 5124 (69.8%) | 87 (1.2%) | 1.13 (0.89–1.45) | 0.32 | 1.12 (0.86–1.46) | 0.41 |
| mRNA-1273 subgroup analysis (contemporary controls) | |||||||
| Unvaccinated | 3120 | 2308 (74.0%) | 26 (0.8%) | 1 | 1 | ||
| Vaccinated | 1040 | 705 (67.8%) | 13 (1.3%) | 1.62 (0.83–3.18) | 0.16 | 1.28 (0.55–2.99) | 0.57 |
| ChAdOx1-S/nCoV-19 subgroup analysis (contemporary controls) | |||||||
| Unvaccinated | 5862 | 4117 (70.2%) | 63 (1.1%) | 1 | 1 | ||
| Vaccinated | 1954 | 1264 (64.7%) | 26 (1.3%) | 1.34 (0.84–2.13) | 0.21 | 1.15 (0.69–1.91) | 0.60 |
All analyses exclude women with confirmed SARS-CoV-2 infection between 6 weeks preconception and the earliest of either: end of pregnancy or the end of the exposure period at 2 + 6 weeks gestation.
CI confidence interval.
aAdjusting for matching factors: maternal age and gestational age at the date of vaccination of index vaccinated pregnancy (and season of conception for primary and primary subgroup analysis).
bAdditionally adjusting for deprivation, urban/rural status, and clinical vulnerability (and ethnicity and season of conception in supplementary analyses).
Association between SARS-CoV-2 infection and miscarriage
A total of 3025 pregnant women had confirmed SARS-CoV-2 infection between 6 weeks preconception and 19 + 6 weeks gestation (Supplementary Fig. 3). Characteristics of this infected cohort, and matched control groups, are provided in Supplementary Table 4. We found no evidence that women infected in pregnancy had higher odds of miscarriage in primary or supplementary analyses (aOR = 1.12, 95% CI = 0.94–1.34 and aOR = 1.00, 95% CI = 0.84–1.20, respectively) (Table 4).
Table 4.
Odds ratios for the association between SARS-CoV-2 infection and miscarriage from multinomial logistic regression models
| Number of pregnancies | Number (%) of ongoing pregnancies | Number (%) of miscarriages | Odds ratio (95% CI)a | p-value | Adjusted odds ratio (95% CI)b | p-value | |
|---|---|---|---|---|---|---|---|
| Primary analysis (historical controls) | |||||||
| Uninfected | 9075 | 7734 (85.2%) | 600 (6.6%) | 1 | 1 | ||
| Infected | 3025 | 2426 (80.2%) | 204 (6.7%) | 1.13 (0.95–1.34) | 0.16 | 1.12 (0.94–1.34) | 0.22 |
| Supplementary analysis (contemporary controls) | |||||||
| Uninfected | 9075 | 7495 (82.6%) | 584 (6.4%) | 1 | 1 | ||
| Infected | 3025 | 2426 (80.2%) | 204 (6.7%) | 1.10 (0.93–1.30) | 0.28 | 1.00 (0.84–1.20) | 0.96 |
All analyses exclude women who received COVID-19 vaccination between 6 weeks preconception and the earliest of either: end of pregnancy or the end of the exposure period at 19 + 6 weeks gestation.
CI confidence interval.
aAdjusting for matching factors: maternal age and gestational age at the date of infection of index infected pregnancy (and season of conception for primary analysis).
bAdditionally adjusting for deprivation, urban/rural status, and clinical vulnerability (and ethnicity and season of conception in supplementary analysis).
Association between SARS-CoV-2 infection and ectopic pregnancy
A total of 915 pregnant women had confirmed SARS-CoV-2 infection between 6 weeks preconception and 2 + 6 weeks gestation (Supplementary Fig. 4). Characteristics of this infected cohort and matched control groups, are provided in Supplementary Table 5. We found no evidence that women infected in pregnancy had higher odds of ectopic pregnancy in primary or supplementary analyses (aOR = 0.76, 95% CI = 0.34–1.69 and aOR = 0.78, 95% CI = 0.34–1.79, respectively) (Table 5).
Table 5.
Odds ratios for the association between SARS-CoV-2 infection and ectopic pregnancy from multinomial logistic regression models
| Number of pregnancies | Number (%) of ongoing pregnancies | Number (%) of ectopic pregnancies | Odds ratio (95% CI)a | p-value | Adjusted Odds ratio (95% CI)b | p-value | |
|---|---|---|---|---|---|---|---|
| Primary analysis (historical controls) | |||||||
| Uninfected | 2745 | 1960 (71.4%) | 33 (1.2%) | 1 | 1 | ||
| Infected | 915 | 579 (63.3%) | 8 (0.9%) | 0.80 (0.37–1.75) | 0.58 | 0.76 (0.34–1.69) | 0.50 |
| Supplementary analysis (contemporary controls) | |||||||
| Uninfected | 2745 | 1901 (69.3%) | 32 (1.2%) | 1 | 1 | ||
| Infected | 915 | 579 (63.3%) | 8 (0.9%) | 0.82 (0.37–1.78) | 0.61 | 0.78 (0.34–1.79) | 0.56 |
All analyses exclude women who received COVID-19 vaccination between 6 weeks preconception and the earliest of either: end of pregnancy or the end of the exposure period at 2 + 6 weeks gestation.
CI confidence interval.
aAdjusting for matching factors: maternal age and gestational age at the date of infection of index infected pregnancy (and season of conception for primary analysis).
bAdditionally adjusting for deprivation, urban/rural status, and clinical vulnerability (and ethnicity and season of conception in supplementary analysis).
Discussion
In our population-based, matched cohort study using routine health data from Scotland, COVID-19 vaccination between 6 weeks preconception to 19 weeks and 6 days gestation was not associated with an increased risk of miscarriage; and COVID-19 vaccination between 6 weeks preconception to 2 weeks and 6 days gestation was not associated with ectopic pregnancy. Results were similar regardless of whether we used historical or contemporary unvaccinated controls. Our results relating to miscarriage are in line with the limited existing evidence including studies that compared the risk in a vaccinated and unvaccinated group with adjustment for key confounders8,9, as well as studies that compared the risk in women vaccinated early in pregnancy to previously published historical rates of miscarriage12,13. Our results relating to an ectopic pregnancy are novel, as no comparable published evidence is available.
We conducted pre-specified subgroup analyses, exploring the association between specific vaccine types and early pregnancy outcomes. Different vaccine types were offered at different times, and to women in different risk groups, during the roll out of the vaccination programme in Scotland in line with evolving policy (see Supplementary Fig. 1)14. The majority of women vaccinated in pregnancy received an mRNA vaccine. We found no association between mRNA vaccines and miscarriage. The ChAdOx1-S/nCoV-19 vaccine was mainly given to women in clinical risk groups (in addition to pregnancy per se, for example, women with pre-existing cardiac or respiratory disease) early in the vaccination programme15. We found an association between vaccination with ChAdOx1-S/nCoV-19 and miscarriage when compared with historical controls but not when compared with contemporary controls. Caution is required in the interpretation of these results, particularly those using historical controls. Pregnant women who received ChAdOx1-S/nCoV-19 were much more likely than their matched controls (or women who received an mRNA vaccine) to be classed as clinically vulnerable or clinically extremely vulnerable, reflecting the particular nature of the group receiving this vaccine. Whilst our marker of clinical vulnerability reflects a range of conditions conferring additional vulnerability to severe COVID-19 disease (see Supplementary Table 6), it may not fully account for conditions associated with an increased risk of miscarriage. In addition, regardless of the year of pregnancy, clinical vulnerability status was ascertained from pandemic-era records, which may lead to over-ascertainment of clinical vulnerability in historical controls. This means there is a particular risk of residual confounding affecting the results of the ChAdOx1-S/nCoV-19 subgroup analyses, especially those using historical controls. We found no unusual patterns in the gestation at miscarriage, or the lag between vaccination and miscarriage, for any vaccine types. An analysis of pregnancy outcomes in 121 women who became pregnant whilst participating in clinical trials of ChAdOx1/nCoV-19 found that the rate of miscarriage was no higher in the ChAdOx1/nCoV-19 group than in the control group, with an adjusted risk ratio of 0.84 (95% CI = 0.24–2.90)16. The only other study exploring COVID-19 vaccine type and miscarriage that included ChAdOx1-S/nCoV-19, also did not show any evidence of an association between ChAdOx1-S/nCoV-19 and miscarriage (aOR 0.84 in vaccinated women compared to unvaccinated women; 95% CI = 0.48–1.48)8. On balance, the evidence relating to ChAdOx1-S/nCoV-19 and miscarriage is reassuring. However, replication of analyses in settings where this vaccine is used more widely in women across different clinical risk profiles would be beneficial.
We found no evidence for an association between SARS-CoV-2 infection and either miscarriage or ectopic pregnancy, but there is imprecision in our estimates due to the relatively small number of women with SARS-CoV-2 infection in the preconception period or early pregnancy. Early in the pandemic, Baud and colleagues reported a case in which a second-trimester miscarriage appeared to be linked with placental infection with SARS-CoV-217, but since then the emerging evidence on infection and miscarriage has been conflicting. Two small hospital-based studies conducted early in the pandemic found no evidence for an association between SARS-CoV-2 infection and the risk of miscarriage18,19. More recently, a study from the United Kingdom (UK), in which a sample of women who conceived during the COVID-19 pandemic were asked to self-report if they had a SARS-CoV-2 diagnosis or miscarriage in the first 13 weeks of pregnancy, found an increased risk of miscarriage but with wide confidence intervals and was subject to recall bias (adjusted risk ratio = 1.7, 95% CI = 1.0–3.0)20. In our study, we restricted the analysis to only the period when testing was widely available in the community but we cannot rule out that some women that had SARS-CoV-2 infection were misclassified as uninfected if they did not test or report positive test results. The relationship between SARS-CoV-2 infection and miscarriage and ectopic pregnancy warrants further investigation.
The strengths of our study include the use of population-based data covering a whole country and the use of routinely recorded data. Our study design accounted for key biases that tend to impact studies of vaccination in pregnancy, notably immortal time bias and cohort truncation bias21. To avoid immortal time bias, we matched on the gestational age at exposure, thereby ensuring that our exposed and control groups were balanced with respect to the underlying risk of early pregnancy outcomes at different gestational ages. We also repeated the analyses using different comparison groups to test the robustness of our results.
There are some limitations that need to be considered. Firstly, we could not include early miscarriages where the woman did not seek healthcare advice. However, as UK guidance is to attend for assessment in any case of suspected pregnancy loss, and we included data from both community and secondary healthcare settings, we anticipate these numbers are small. Secondly, we had to rely on imputed gestation at end of pregnancy for a high proportion of pregnancies ending in early loss, which may have led to misclassification of vaccination or infection status (due to inaccurate assignment of the relevant exposure period) for some pregnancies. Imprecise information on gestation at pregnancy loss is a challenge faced by all studies examining early pregnancy losses, as many losses occur before a dating ultrasound scan is performed and because embryo/foetal demise frequently occurs sometime before the onset of symptoms of pregnancy loss. Thirdly, it was not possible to conduct subgroup analyses by the number of doses when looking at early pregnancy outcomes due to the relatively short exposure window for vaccination. Finally, there remains potential for residual confounding in our analyses. In addition to the issues relating to the measurement of clinical vulnerability discussed above, we were not able to adjust for body mass index (BMI) or smoking; or include diabetes in clinical vulnerability scores, due to substantial differential levels of missing data for these covariates between early pregnancy losses (with high levels of missing data), and those pregnancies where pregnancies continued past the second trimester and a birth record was generated (low levels of missing data).
In conclusion, this study adds robust population-based evidence on the safety of COVID-19 vaccinations for women who are planning to become pregnant or who are in the early stages of pregnancy. Overall, our analyses found no evidence of an increased risk for miscarriage or ectopic pregnancy after COVID-19 vaccination, supporting current recommendations that vaccination remains the safest way for pregnant women to protect themselves and their babies from COVID-19.
Methods
We conducted a population-based, matched cohort study following a study protocol and statistical analysis plan22, and reported results according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines23.
Study population
Data were obtained from the dynamic, population-based COVID-19 in Pregnancy in Scotland (COPS) cohort24, which included all completed and ongoing pregnancies in Scotland from January 1, 2015, onwards. This cohort has been described in detail elsewhere10,11. In brief, information on pregnancies, including estimated date of conception and, for completed pregnancies, the pregnancy outcome and gestational age at end of pregnancy were extracted for all women aged 11–55 at conception from antenatal care (ANC) booking records; General Practitioner (GP) records; general acute hospital discharge records (Scottish Morbidity Record (SMR) 01); maternity hospital discharge records (SMR 02); statutory termination of pregnancy notifications (AAS); National Records of Scotland (NRS) statutory live birth registrations; NHS Scotland live birth notifications; and NRS statutory stillbirth registrations. National data on COVID-19 vaccination (including date and type of vaccination) and confirmed SARS-CoV-2 infections were incorporated into the COPS study cohort using unique identifiers.
We used the COPS database as updated on April 26, 2022. We included pregnancies with an estimated conception date up to September 28, 2021, and identified outcomes occurring up to January 31, 2022, hence all included pregnancies could be observed up to 20 weeks gestation (or end of pregnancy if earlier). We excluded the small number of completed pregnancies that had unknown pregnancy outcome (N = 5112, 0.9% of all pregnancies included in COPS database).
COVID-19 vaccination and early pregnancy outcomes
Exposure
The COVID-19 vaccination programme started in Scotland on December 8, 2020. A timeline of the programme as relevant to pregnant women is provided in Supplementary Figure 1. Our primary exposure was receipt of a COVID-19 vaccine, including any vaccine type available in Scotland (ChAdOx1-S/nCoV-19 [Oxford/AstraZeneca], BNT162b2 [Pfizer-Biontech], or mRNA-1273 [Moderna]) and any dose (first, second, etc.). Women were included if they were vaccinated from 6 weeks before conception to the end of the outcome-specific exposure period, defined as 19 weeks and 6 days gestation (19 + 6 weeks) for miscarriage and 2 + 6 weeks for ectopic pregnancy. A shorter exposure period was used for ectopic pregnancies as implantation has occurred by 2 + 6 weeks, hence pregnancy location cannot be influenced by subsequent exposures.
Outcomes
All pregnancies were categorized as completed or ongoing at 19 + 6 weeks. Completed pregnancies were further categorized by the outcome as miscarriage (including small numbers of molar pregnancies and live births at a non-viable gestational age); ectopic pregnancy; or termination of pregnancy. The outcomes of interest (miscarriage and ectopic pregnancy) were ascertained through ICD-10 or Read Coded Clinical Terms diagnostic codes recorded on hospital discharge or GP records, respectively25.
Some source records (notably GP and SMR01 general (non-maternity) hospital discharge records) do not contain information on gestation, and gestation may be missing on other record types. Where gestation at end of pregnancy was unknown, it was assumed to be 10 + 0 weeks for miscarriages and 8 + 0 weeks for ectopic pregnancies. The exception was miscarriages identified through SMR02 maternity hospital discharge records: these records tend to relate to more advanced pregnancies hence 12 + 0 weeks was assumed.
Matching to unvaccinated controls
In our primary analyses, we matched vaccinated women to unvaccinated historical (pre-pandemic period) controls. This avoided potential bias from undiagnosed SARS-CoV-2 infection and ensured a sufficient pool of potential controls26. Different pregnancies from a single woman could be included in the matched cohort. If a vaccinated woman also had a pregnancy in the pre-pandemic period, that pregnancy was a valid control, as long as the woman was not matched to herself. Potential controls had an estimated conception date from January 1, 2015, up to October 27, 2019, hence all control pregnancies could be observed up to 20 weeks gestation prior to the start of the pandemic on March 1, 2020. Each vaccinated woman was matched to three historical controls by maternal age at conception (±one year), the season of conception (January–March, April–June, July–September, October–December), and gestation (in completed weeks) at first vaccination in pregnancy (e.g. if a woman was vaccinated at 15 weeks gestation, she would be matched to unvaccinated women with an ongoing pregnancy at 15 weeks gestation).
Analyses using historical controls are vulnerable to bias if there are secular trends in the outcomes of interest. We, therefore, conducted supplementary analyses matching vaccinated women to unvaccinated contemporary (pandemic vaccination period) controls. Controls were considered unvaccinated if they did not receive any COVID-19 vaccination between 6 weeks of preconception and the end of the outcome-specific exposure period. Vaccinated women were matched to contemporary controls by maternal age at conception (±1 year, except at tail ends of distribution with women aged <20 or >40 matched within these age groups) and gestation (in completed weeks) at first vaccination in pregnancy. Each vaccinated woman was matched to one control in the miscarriage analysis (due to a limited pool of controls), and three controls in the ectopic analysis.
As our aim was to assess the safety of vaccination per se (rather than identifying any impact of vaccination on pregnancy outcomes mediated through reduced risk of SARS-CoV-2 infection), in all analyses we excluded pregnancies if the woman had confirmed SARS-CoV-2 infection between 6 weeks preconception and the earliest of: (1) the end of pregnancy or (2) the end of the outcome-specific exposure period. This exclusion was applied before the matched cohorts were selected.
SARS-CoV-2 infection and early pregnancy outcomes
We also compared early pregnancy outcomes in women with and without confirmed SARS-CoV-2 infection. Here our exposure of interest was confirmed SARS-CoV-2 infection from 6 weeks preconception to the end of the outcome-specific exposure period, between May 18, 2020 (the date from which widespread community viral testing was available in Scotland) and January 31, 2022. Confirmed infection was defined in line with national guidance27. Up to January 5, 2022, this was by positive SARS-CoV-2 reverse transcription (RT) polymerase chain reaction (PCR) test result. From January 6, 2022, by a positive SARS-CoV-2 RT-PCR test result or by a positive lateral flow device (LFD) test result (unless the positive LFD result was followed by a negative RT-PCR result within 48 h). The date of onset of SARS-CoV-2 was defined as the date the woman’s first positive sample was taken. Subsequent positive test results within 90 days of an index positive result were discounted, with any positive test taken >90 days following the first positive sample considered a second or subsequent infection. Taking a similar approach to the vaccination analysis, we matched infected women to uninfected historical controls on maternal age, the season of conception, and gestational age at infection for primary analyses and to uninfected contemporary controls on maternal age and gestational age at infection for supplementary analyses (with 3:1 matching possible for all groups due to sufficient controls). Historical controls were used for the primary analysis to avoid any misclassification of women with undiagnosed infection as uninfected. In all infection analyses, we excluded women who received COVID-19 vaccination between 6 weeks preconception and the earliest of (1) the end of pregnancy or (2) the end of the outcome-specific exposure period.
Statistical analyses
We undertook descriptive analyses of the characteristics of exposed and control groups; details of exposures; and pregnancy outcomes by 19 + 6 weeks gestation (see Supplementary Table 6 for details of available characteristics/confounder variables). Multinomial logistic regression was used to estimate the odds of each early pregnancy outcome (i.e. miscarriage, ectopic pregnancy, and termination) compared to ongoing pregnancy by 19 + 6 weeks gestation among vaccinated compared to unvaccinated pregnant women. A multinomial logistic regression allowed us to jointly model all the potential early pregnancy outcomes, rather than restricting to only specific outcomes post-exposure, which would have implications for the balance achieved by matching. Matching characteristics (maternal age, gestational age, and, in primary analyses only, the season of conception) were included in all models. Additional potential confounders (maternal deprivation level, urban/rural residence status, clinical vulnerability, and in supplementary analyses only, ethnicity and season of conception) were included in models providing adjusted odds ratios (aORs). In these analyses, we included a separate unknown/missing group for confounders where there were low levels of missing data, as well as conducting additional analyses removing individuals with missing data from models to assess whether the inclusion of these data were impacting our results. Ethnicity was not included in primary analyses due to the high level of missing data for historical controls. Other potential confounders of interest, specifically smoking status, body mass index, and presence of diabetes was not included in any analyses, due to high levels of missing data on these variables for women whose pregnancies ended in an early loss. The same analytical process was followed to assess the association between vaccination at up to 2 + 6 weeks gestation and ectopic pregnancy and between SARS-CoV-2 infection and each early pregnancy outcome.
Analyses were conducted in R 3.6.1.
Subgroup analyses
We undertook planned subgroup analyses stratifying our primary and supplemental vaccination analyses by type of vaccine (ChAdOx1-S/nCoV-19, BNT162b2, or mRNA-1273). We excluded pregnant women (and their matched controls) from these subgroup analyses if different vaccine types were received in the exposure period.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
Our thanks to the EAVE II Patient Advisory Group and Sands charity for their support. COPS is a sub-study of EAVE II, which is funded by the Medical Research Council (MR/R008345/1) with the support of BREATHE—The Health Data Research Hub for Respiratory Health [MC_PC_19004], which is funded through the UK Research and Innovation Industrial Strategy Challenge Fund and delivered through Health Data Research UK. Additional support has been provided through Public Health Scotland and Scottish Government DG Health and Social Care and the Data and Connectivity National Core Study, led by Health Data Research UK in partnership with the Office for National Statistics and funded by UK Research and Innovation. COPS has received additional funding from Tommy’s charity. S.J.S. is funded by a Wellcome Trust Clinical Career Development Fellowship (209560/Z/17/Z). S.V.K. acknowledges funding from an NRS Senior Clinical Fellowship (SCAF/15/02), the Medical Research Council (MC_UU_00022/2) and the Scottish Government Chief Scientist Office (SPHSU17). K.B. is funded by a Wellcome Senior Research Fellowship (220283/Z/20/Z).
Author contributions
R.W., S.J.S., C.R., and A.S. conceived the study. R.W., S.J.S., C.C., C.R., and A.S. designed the study. R.W., S.J.S., J.P., and C.C. drafted the protocol. J.C., C.D., J.D., L.E.M.H., L.H., A.G., L.L., T.M., E.M., and B.T. prepared the dataset for analysis. C.C., J.P., S.H., and B.T. performed data analysis. C.C., R.W., S.J.S., and A.S. prepared the first draft of the manuscript. F.A., B.A., K.B., C.L.G., S.V.K., C.Mc., J.M., M.O’.L, L.D.R., S.A.S., and C.R.S. provided critical input to drafts of the manuscript. R.W., S.J.S., C.C., and A.S. gave final approval for the version to be published. R.W. acts as a guarantor for the study.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Data availability
Aggregate data files on COVID-19 vaccinations and SARS-CoV-2 infections among pregnant women are available here: https://www.opendata.nhs.scot/organization/health_protection. Patient-level data underlying this article cannot be shared publicly due to data protection and confidentiality requirements. Public Health Scotland is the data holder for the data used in this study. Data can be made available to approved researchers for analysis after securing relevant permissions from the data holders via the Public Benefit and Privacy Panel. Enquiries regarding data availability should be directed to phs.edris@phs.scot.
Code availability
Metadata and code are available on GitHub at https://github.com/Public-Health-Scotland/COPS-public28.
Competing interests
A.S. and C.R. were members of the Scottish Government’s COVID-19 Advisory Group. A.S. and C.R. are members of the New and Emerging Respiratory Virus Threats Advisory Group (NERVTAG) risk stratification subgroup and the Scottish Government’s Committee on Pandemic Preparedness. C.R. is a member of the Scientific Pandemic Influenza Group on Modelling. A.S. is a member of AstraZeneca’s Thrombotic Thrombocytopenic Advisory Group. All roles are unremunerated. S.V.K. was co-chair of Scottish Government’s Expert Reference Group on Ethnicity and COVID-19. The remaining authors declare no competing interests.
Ethics
COPS has ethical approval from the National Research Ethics Service Committee, South East Scotland 02 (REC 12/SS/0201: SA 2) and information governance approval from the Public Benefit and Privacy Panel for Health and Social Care (2021-0116). All data were housed within Public Health Scotland and accessed only by approved researchers.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Sarah J. Stock, Rachael Wood.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-33937-y.
References
- 1.Dagan N, et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat. Med. 2021;27:1693–1695. doi: 10.1038/s41591-021-01490-8. [DOI] [PubMed] [Google Scholar]
- 2.Goldshtein I, et al. Association between BNT162b2 vaccination and incidence of SARS-CoV-2 infection in pregnant women. JAMA. 2021;326:728–735. doi: 10.1001/jama.2021.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stock, S. J. et al. SARS-CoV-2 infection and COVID-19 vaccination rates in pregnant women in Scotland. Nat. Med.28, 504–512 (2022). [DOI] [PMC free article] [PubMed]
- 4.UK Health Security Agency. COVID-19 Vaccine Surveillance Report—Week 19 (UK Health Security Agency, accessed 10 June 2022); https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1075115/COVID-19_vaccine_surveillance_report_12_May_2022_week_19.pdf (2022).
- 5.Public Health Scotland. COVID-19 Statistical Report—11 May 2022 (Public Health Scotland, accessed 10 June 2022); https://www.publichealthscotland.scot/publications/covid-19-statistical-report/covid-19-statistical-report-11-may-2022/ (2022).
- 6.Iacobucci G. Covid-19 and pregnancy: vaccine hesitancy and how to overcome it. BMJ. 2021;375:n2862. doi: 10.1136/bmj.n2862. [DOI] [PubMed] [Google Scholar]
- 7.Prasad S, et al. Systematic review and meta-analysis of the effectiveness and perinatal outcomes of COVID-19 vaccination in pregnancy. Nat. Commun. 2022;13:2414. doi: 10.1038/s41467-022-30052-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magnus MC, et al. Covid-19 vaccination during pregnancy and first-trimester miscarriage. N. Engl. J. Med. 2021;385:2008–2010. doi: 10.1056/NEJMc2114466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kharbanda EO, et al. Spontaneous abortion following COVID-19 vaccination during pregnancy. JAMA. 2021;326:1629–1631. doi: 10.1001/jama.2021.15494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stock, S. J. et al. Cohort profile: the COVID-19 in pregnancy in Scotland (COPS) dynamic cohort of pregnant women to assess effects of viral and vaccine exposures on pregnancy. Int. J. Epidemiol.51, e245–e255 (2022). [DOI] [PMC free article] [PubMed]
- 11.Stock SJ, et al. COVID-19 in Pregnancy in Scotland (COPS): protocol for an observational study using linked Scottish national data. BMJ Open. 2020;10:e042813. doi: 10.1136/bmjopen-2020-042813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Favre G, et al. COVID-19 mRNA vaccine in pregnancy: results of the Swiss COVI-PREG registry, an observational prospective cohort study. Lancet Reg. Health—Eur. 2022;18:100410. doi: 10.1016/j.lanepe.2022.100410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zauche LH, et al. Receipt of mRNA Covid-19 vaccines and risk of spontaneous abortion. N. Engl. J. Med. 2021;385:1533–1535. doi: 10.1056/NEJMc2113891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.GOV.UK. JCVI Issues New Advice on COVID-19 Vaccination for Pregnant Women (GOV.UK, accessed 17 June 2022); https://www.gov.uk/government/news/jcvi-issues-new-advice-on-covid-19-vaccination-for-pregnant-women (2021).
- 15.GOV.UK. COVID-19: the Green Book, Ch. 14a (GOV.UK, accessed 23 September 2022); https://www.gov.uk/government/publications/covid-19-the-green-book-chapter-14a (2022).
- 16.Hillson K, et al. Fertility rates and birth outcomes after ChAdOx1 nCoV-19 (AZD1222) vaccination. Lancet. 2021;398:1683–1684. doi: 10.1016/S0140-6736(21)02282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baud D, et al. Second-trimester miscarriage in a pregnant woman with SARS-CoV-2 infection. JAMA. 2020;323:2198–2200. doi: 10.1001/jama.2020.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cosma S, et al. Coronavirus disease 2019 and first-trimester spontaneous abortion: a case-control study of 225 pregnant patients. Am. J. Obstet. Gynecol. 2021;224:391.e1–.e7. doi: 10.1016/j.ajog.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.la Cour Freiesleben N, et al. SARS-CoV-2 in first trimester pregnancy: a cohort study. Hum. Reprod. 2021;36:40–47. doi: 10.1093/humrep/deaa311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balachandren, N. et al. SARS-CoV-2 infection in the first trimester and the risk of early miscarriage: a UK population-based prospective cohort study of 3041 pregnancies conceived during the pandemic. Hum. Reprod.37, 1126–1133 (2022). [DOI] [PMC free article] [PubMed]
- 21.Kharbanda EO, Vazquez-Benitez G. COVID-19 mRNA vaccines during pregnancy: new evidence to help address vaccine hesitancy. JAMA. 2022;327:1451–1453. doi: 10.1001/jama.2022.2459. [DOI] [PubMed] [Google Scholar]
- 22.Public Health Scotland. COPS-public Git Hubhttps://github.com/Public-Health-Scotland/COPS-public (Public Health Scotland, 2021).
- 23.von Elm E, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 24.The University of Edinburgh. Covid-19 in Pregnancy in Scotland—About COPS (The University of Edinburgh, accessed 16 June 2022); https://www.ed.ac.uk/usher/eave-ii/covid-19-in-pregnancy-in-scotland/about (2022).
- 25.Rouse CE, et al. Spontaneous abortion and ectopic pregnancy: Case definition & guidelines for data collection, analysis, and presentation of maternal immunization safety data. Vaccine. 2017;35:6563–6574. doi: 10.1016/j.vaccine.2017.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.European Medicines Agency. Guideline of the Exposure to Medicinal Products During Pregnancy: Need for Post-authorisation Data (European Medicines Agency, accessed 16 June 2022); https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/guideline-exposure-medicinal-products-during-pregnancy-need-post-authorisation-data_en.pdf (2005).
- 27.Public Health Scotland. Further Changes to COVID-19 Reporting (Public Health Scotland, accessed 16 June 2022); https://publichealthscotland.scot/news/2022/january/further-changes-to-covid-19-reporting/ (2022).
- 28.Calvert, C., Hillman, S., Pan, J. & Taylor, B. A Population-based Matched Cohort Study of Early Pregnancy Outcomes Following COVID-19 Vaccination and SARS-CoV-2 Infection.10.5281/zenodo.7156268 (2022). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Aggregate data files on COVID-19 vaccinations and SARS-CoV-2 infections among pregnant women are available here: https://www.opendata.nhs.scot/organization/health_protection. Patient-level data underlying this article cannot be shared publicly due to data protection and confidentiality requirements. Public Health Scotland is the data holder for the data used in this study. Data can be made available to approved researchers for analysis after securing relevant permissions from the data holders via the Public Benefit and Privacy Panel. Enquiries regarding data availability should be directed to phs.edris@phs.scot.
Metadata and code are available on GitHub at https://github.com/Public-Health-Scotland/COPS-public28.