Abstract
Cognitive reappraisal is adaptive for decreasing symptoms of depression; however, a gap in the research is understanding the childhood processes that contribute to cognitive reappraisal in adolescence. This study examined executive function and frontal electroencephalogram (EEG) asymmetry during late childhood as predictors of adolescent cognitive reappraisal and depressive symptoms. Data were from 123 participants in late childhood (age 10) and adolescence (age 14.5). A moderated mediation model was fit to the data to examine frontal EEG asymmetry as a moderator in the relation between late childhood inhibitory control and adolescent cognitive reappraisal as well as adolescent cognitive reappraisal and adolescent depressive symptoms. Results indicated lower inhibitory control was associated with lower cognitive reappraisal when children had right frontal EEG asymmetry. Lower cognitive reappraisal in turn was associated with higher depressive symptoms for children with right frontal EEG asymmetry. Working memory and cognitive flexibility were also examined but were not significant indicators. Results suggest the potential for targeting inhibitory control and cognitive reappraisal to diminish depressive symptoms particularly among adolescents with right frontal EEG asymmetry.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10802-022-00983-5.
Keywords: Adolescence, Late childhood, Cognitive reappraisal, Depressive symptoms, Inhibitory control, Frontal EEG asymmetry
Introduction
Emotion regulation continues to develop through adolescence, with strategies becoming more complex by implementing greater cognitive abilities as children develop (Pons et al., 2004). Two strategies that have received much attention in the literature are antecedent and response-focused strategies associated with the process model of emotion regulation (Gross & Thompson, 2007; Gross, 1998a). Antecedent focused strategies modify the emotional input by engaging in cognitive reframing, such as cognitive reappraisal (CR), which is believed to be adaptive to individual functioning (Gross, 1998b; McRae, 2016). Response-focused strategies, like expressive suppression, modify the behavioral response following an emotion and have been considered maladaptive (Gross, 1998b).
The process model has been used extensively in adulthood research; more recently the process model has been applied to childhood and adolescent research (Chen et al., 2019; Parsafar & Davis, 2019). We incorporated the process model to examine whether executive function and frontal electroencephalogram (EEG) asymmetry (FA) may be important for CR and depressive symptoms among adolescents.
Executive Function and Depressive Symptoms
Executive functions are higher order cognitive processes, including inhibition, working memory, and cognitive flexibility, linked to the prefrontal cortex and associated with goal directed behavior (Diamond, 2013). In adults, executive function impairment is associated with depression (Cotrena et al., 2016; Fossati et al., 2002). As well, adult patients diagnosed with depressive disorder show marked deficits in executive function tasks when compared to control groups (Alves et al., 2014). After adults show improvements in depression, they subsequently show improvements in executive function (Biringer et al., 2005). The association between executive function and depression symptoms in childhood and adolescence show similar associations. Lower executive function is observed among clinically referred adolescents with depressive disorder (Holler et al., 2014). Adolescents of parents diagnosed with a depressive disorder, and thus at risk of developing depression themselves, have fewer depressive symptoms when they have better inhibitory control and cognitive flexibility (Davidovich et al., 2016). Emotion regulation may serve as a mechanism in the association between executive function and depressive symptoms. For instance, adults with attentional control deficits are more likely to engage in rumination as an emotion regulation strategy, which is subsequently associated with increasing levels of depression (DeJong et al., 2019).
Cognitive Reappraisal as a Mediator
Research indicates that adolescent emotion regulation strategies are important for minimizing adolescent depression (Shapero et al., 2019). CR may be one emotion regulation strategy that may help to mitigate these effects in adolescence. CR involves changes to thoughts about an emotional event by using positive reframing to alter the emotional experience and change the emotional impact (Lazarus & Alfert, 1964). Among adolescents, less adaptive use of CR is linked to greater clinical levels of anxiety and depression (Dryman & Heimberg, 2018; Young et al., 2019). Similarly, CR offsets the association between depressive symptoms and emotional responses to stress in adolescents (Shapero et al., 2019). In adults, being unable to inhibit negative emotions is linked to lower CR use compared to those who are more capable of inhibiting negative emotion; less use of CR is consequently linked to more depression (Joormann & Gotlib, 2010).
In children and adolescents, research indicates some association between executive function and depression (Vilgis et al., 2015), but there is little research examining potential mechanisms of this association in these age groups. Some research indicates that maladaptive and adaptive emotion regulation strategies mediate the association between executive function and depression in adolescents. Adolescents with greater executive function impairment show more maladaptive and less adaptive emotion regulation, which in turn is associated with depressive symptoms. Adolescents with more adaptive emotion regulation show fewer depressive symptoms (Wante et al., 2017). Among young adults, both CR and expressive suppression mediate the relation between executive function and depressive symptoms, with CR being associated with lower depressive symptoms (Hui et al., 2021). Both of these studies collected executive function through questionnaires, all variables were collected during one time-point, and the studies were conducted with clinically referred samples. Our study extends previous research by examining these relations longitudinally and using task-specific executive function measures among a community sample.
Executive function during late childhood may be an important factor that contributes to CR ability during adolescence which then impacts depressive symptoms. In adults the research examining associations between executive function, CR, and maladaptive outcomes is well-established, but this research is minimal in childhood and adolescence (Cotrena et al., 2016; Opitz et al., 2012; Schmeichel & Tang, 2015). Research examining associations between executive function and CR in 9- to 12-year-old children indicates that executive function ability precedes CR (Andrés et al., 2016). Specifically, when adolescents self-report better executive function, they indicate higher CR. Other research shows that adolescents who indicate greater reliance on emotion suppression, rather than CR, report more difficulties with working memory (Lantrip et al., 2016). Among adulthood research which examines executive function and CR links, the specific executive function measures which are associated to CR ability vary; thus, creating executive function composite scores may not be appropriate. Hence, our study examines executive function factors separately (i.e., inhibition, cognitive flexibility, and working memory), similar to previous adulthood research (Hendricks & Buchanan, 2016; McRae et al., 2012; Schmeichel & Tang, 2014). Further, we focused on executive function during late childhood as this is a time when maturation of neural circuits occurs and children become more capable of engaging multiple executive function, this is also a time when we see children’s ability to understand that changing thoughts can change emotions, a key component of CR (Best & Miller, 2010; Nagy et al., 2004; Pons et al., 2004).
Frontal Asymmetry as a Moderator
FA is measured through the use of EEG, which measures electrical activity from the scalp. FA provides information on individuals’ trait and state neurological correlates in emotion focused contexts. FA is the increased activation of either the right or left frontal hemisphere in relation to the other hemisphere and is associated with the behavioral expression and regulation of emotions (Fox, 1994; Fox et al., 1996). The approach/withdrawal model of resting state EEG proposes that greater left FA is associated with approach behavioral responses and positive affect, whereas greater right FA is associated with withdrawal behavioral responses and negative affect (Fox, 1994). We focused on trait FA as a moderator in our model because examining trait FA (i.e., during rest or baseline) provides information regarding the individual’s capacity to engage and recruit cognitive and affective processes specific to the individual (Reznik & Allen, 2018). In addition, greater right FA during baseline or resting state is linked with depression in adults and their children (e.g., Thibodeau et al., 2006).
Research examining direct links between FA and CR indicate that both state and trait left FA is associated with greater CR ability (Choi et al., 2016; Papousek et al., 2017; Wang et al., 2015). In a sample of 54 adolescents instructed to engage in a CR task, adolescents who engaged habitual CR showed greater left FA during the emotional tasks (Yang et al., 2021). Adulthood research that has examined the link between FA and CR indicates that left FA during resting state is greater among habitual cognitive reappraisers who are male, but not female. When individuals were instructed to engage CR, they displayed greater task left FA regardless of sex (Choi et al., 2016). In a study of adults ages 18 to 35, those who had a higher capacity for generating CR displayed greater left FA during the CR generation task (Papousek et al., 2017). Although FA has not been examined as a moderator between executive function and CR, executive function informs us of children’s ability to adequately face cognitive challenges. If children are incapable of recruiting adequate cognitive processes for successful emotion regulation strategies, then left FA may provide children with the capacity to engage brain electrical processes necessary for the successful implementation of CR for diminishing depressive symptoms in adolescence (Sudikoff et al., 2015).
Furthermore, we are unaware of any research that has examined whether FA moderates CR outcomes, although FA has been used extensively in emotion regulation research as a moderator of emotional outcomes (Reznik & Allen, 2018). Research in children who are at an increased risk of depression indicates that left FA and CR diminish depressive outcomes (Kudinova et al., 2018; Lopez-Duran et al., 2012). Cognitive reappraisal and FA may also interact to predict differences in depressive symptoms. When comparing emotion regulation and FA among a sample of maltreated and nonmaltreated children, results indicated that among the nonmaltreated group, resilience scores increased as a function of emotion regulation and not FA. Among the maltreated group only, trait FA had a direct effect on resilience scores after accounting for emotion regulation; specifically, children with trait left FA showed higher resilience scores than children with right FA (Curtis & Cicchetti, 2007). Hence, trait left FA may serve as a protective factor for adolescent depressive symptoms.
Current Study
We examined individual difference factors that have previously been associated with both CR and depressive symptoms in the adulthood literature and examined them in a sample of community adolescents. We tested our model through moderated mediation and hypothesized that FA would moderate the association between executive function and CR, such that greater executive function (i.e., inhibitory control, working memory, cognitive flexibility) would be associated with greater CR ability only for adolescents with left FA. Higher CR ability would in turn be associated with lower depressive symptoms for adolescents with left FA. Individual executive function factor differences were not hypothesized due to previous research having mixed findings (Andrés et al., 2016; McRae et al., 2012; Tabibnia et al., 2011).
Method
Participants
Participants were cohort 1 and cohort 2 of a longitudinal study examining the integration of emotion and cognition across early development. Participants were recruited during infancy using flyers, word of mouth, and mailing lists. The two cohorts participating in the current study represent half of the original sample. Cohort 3 was recruited by a university research lab in another state and ended the longitudinal study in middle childhood, with no adolescent follow-up visit. The current study examines data from cohort 1 and cohort 2, from the late childhood and adolescent visits.
In late childhood, cohort 1 visited the research lab during 2013 (n = 81) and cohort 2 visited the lab in 2016 (n = 80). In addition, 31 questionnaires were completed and mailed back to us for cohort 1 (n = 11) and cohort 2 (n = 20) from families who were unable to visit the research lab. Thus, the late childhood sample consisted of 192 children (range 9 – 12 years; M = 9.92, SD = 0.74) and their mothers. The sample was 85% White, 4% Hispanic, 9% Multi-racial/other, 1.5% Asian, and 0.5% Black. Only participants who contributed data during the late childhood visit were recruited for the adolescent visit.
The adolescent visit consisted of 78 in-lab participants and 45 questionnaire-only participants, for a total of 123 adolescents (51% girls) and their mothers. Due to COVID-19, in-lab visits for tasks and questionnaires (mid-August 2019 through mid-March 2020) were halted and data collection resumed online (late August through early October 2020) with questionnaires. There were 79 dyads who completed data pre-COVID-19, with 78 in-lab and one questionnaire-only family. During COVID-19, 44 questionnaire-only participants provided data. Adolescent age range (range 12 – 18 years; M = 14.64, SD = 1.94) at the time of participation was wide due to the mean 3-year age difference between cohorts 1 and 2. Thus, the adolescent visit consisted of cohort 1 (n = 57; range 15 – 18 years; M = 16.6, SD = 0.72) and cohort 2 (n = 66; range 12 – 14.5 years; M = 12.95, SD = 0.63). For the combined adolescent participants, 88% identified as White/Caucasian, 6% Multi-racial/other, 5% Hispanic, and 1% Asian. Mothers were highly educated (85% had a college degree or advanced degree).
We examined whether differences emerged on late childhood FA, late childhood executive function measures, sex, and maternal education for those participants who did and did not contribute data during adolescence. ANOVA revealed no significant differences for FA, executive function measures, and maternal education (all p’s > 0.30). To further examine whether differences in sex were observed for those adolescents who came into the research lab during the adolescent visit, a chi square test was conducted, no significant differences emerged (p = 0.14).
Procedures
Families visited the research lab (or completed and mailed in questionnaire packets) during late childhood and during adolescence. For both visits, participants entered the research lab with their parent, parents provided their written consent, and children/adolescents provided their written assent prior to starting the study. For both visits, mothers observed from an adjacent room while completing questionnaires. Institutional Review Board approval was obtained from the Virginia Tech IRB, protocol number 12-947 titled “Psychobiology of Cognitive Development in Middle Childhood” for the age 9 visit and by the Biomedical Research Alliance of New York (BRANY) IRB protocol numbers 19-030-568/19-352 titled “Psychobiology of Cognitive Development in Early Adolescence” for the adolescent visit.
During the late childhood visit, children were compensated with a $20 gift card and mothers with a $75 gift card for in-lab visits. Families who only completed questionnaires received two $20 gift cards, one for the child and one for the parent. During the adolescent visit, in-lab participants were compensated $50 cash for the adolescent and $50 cash for the mother. For the questionnaire only families, adolescent and the mother received a $20 gift card.
Measures Collected During Late Childhood
Frontal EEG Asymmetry
Baseline EEG was collected during a two-minute video (opening scene from The Lion King). Children were capped using a stretch cap (Electro-Cap, Inc.; Eaton, OH; E1-series cap) with electrodes in a modified 10/20 system pattern. EEG recordings were collected from 26 left, right, and midline scalp sites evenly distributed across the scalp. After the cap was positioned, abrasive gel was placed and gently rubbed at each electrode site. Conductive gel was then added at each electrode site. Electrode impedances were measured and accepted below 10Ω. EEG electrical activity was amplified from each lead using separate James Long Company Bioamps and bandpassed from 0.1 to 100 Hz. EEG signal was digitized on-line at 512 samples per second for each channel so that the data would not be affected by aliasing. The acquisition software was Snapshot-Snapstream (HEM Data Corp., Southfield, MI) and EEG Analysis software developed by the James Long Company (Caroga Lake, NY) was used for EEG processing. The data were re-referenced via software to an average reference configuration and then artifact scored for eye movements and gross motor artifact. Artifacted epochs were eliminated from all subsequent analyses. The EEG data was analyzed using a discrete Fourier Transform (DFT) using a Hanning window of 1-s width and 50% overlap. Power was computed for the 8-10 Hz frequency band. This frequency band has been used by others publishing FA research with children in late childhood (Forbes et al., 2008; Vuga et al., 2008). Data were log (ln) transformed to normalize EEG values. FA values were created by subtracting ln EEG power in the left hemisphere (F3) from ln EEG power in the right hemisphere (F4). Because of the inverse association between power values and activation, positive values indicate greater relative left frontal activation compared to the right (i.e., left FA) and negative values indicate greater relative right frontal activation compared to the left (i.e., right FA).
Working Memory
Working memory was measured through the Backward digit span task. In this task, the experimenter read a series of random single-digit numbers to participants. Children were asked to repeat out loud the numbers in reverse order, with practice trial of two sets of two digits. After children passed the practice trial, the test trials were collected. Test trials included two different three-digit sequences, increasing one single-digit for both trials in the sequence until participants failed to correctly repeat the digits in both trials of the sequence. The variable of interest was the last correct trial as an indicator of backward digit span score.
Cognitive Flexibility
Cognitive flexibility was measured using the Wisconsin Card Sorting Test (WCST; Heaton & Staff, 2003). For this computerized task, children were instructed to match a card of 64 total cards to one of four key cards. Images on the cards varied from shape, color, and quantity, children were asked to sort the cards according to one of three rules (e.g., by shape, by color, or by number) that they had to determine based on feedback from the computer. The sorting rules changed several times throughout the task, with computer feedback informing children of their errors. The age-standardized percentile score associated with conceptual level was the measure of interest.
Inhibitory Control
We measured inhibitory control through the Number Stroop (Ruffman et al., 2001). Three conditions were administered. During the letter or control condition, trials consisted of a string of letters on the computer screen. Children were instructed to count the number of letters as quickly and accurately as possible and press the corresponding keyboard number. For the incongruent condition, children were presented with a series of numbers and asked to select the number that corresponded with the total number of items on the screen (e.g., 5555 would be 4). In the mixed condition, children were presented with either strings of letters or strings of numbers and asked to count the items in the string. The measure of interest was reaction time in the mixed condition, with faster reaction times (i.e., lower values) indicative of better inhibitory control.
Measures Collected During Adolescence
Cognitive Reappraisal
Adolescents were asked to complete the Emotion Regulation Questionnaire (ERQ; Gross & John, 2003). The ERQ is a 10-item self-report questionnaire that asks respondents to rate their use of different emotion regulation strategies from 1 (strongly disagree) to 7 (strongly agree) with two subscales, cognitive reappraisal (CR) and expressive suppression. The CR subscale was used. Cronbach’s alpha for the CR subscale was 0.75.
Depressive Symptoms
Adolescents self-reported depressive symptoms on the Revised Children’s Anxiety and Depression Scale (RCADS). The questionnaire consists of 47 items, asking participants to rate how often certain things happen to them from 1 (never) to 4 (always). The RCADS consists of these subscales: separation anxiety disorder, social phobia, generalized anxiety disorder, panic disorder, obsessive compulsive disorder, and low mood (major depressive disorder). The low mood subscale raw score was used. Cronbach’s alpha was 0.96.
Pubertal Status
Adolescents self-reported pubertal status via the Pubertal Developmental Scale (PDS; Petersen et al., 1988). Participants rate growth on height, pubic hair, and skin changes. Both sexes then responded to two additional questions which asked boys to rate voice and facial hair changes, girls rated breast growth and onset of menarche. Ratings were reported using a 4-point Likert scale of 1 (has not yet begun) to 4 (seems completed), menarche was rated as a dichotomous 1 – “Yes” or 2 – “No”. A response of Yes was recoded as 4, No was recoded as 1. The pubertal status score was averaged across the five corresponding items for both boys and girls. Cronbach’s alpha for girls is 0.89 and for boys 0.94.
Analysis Plan
Analyses were conducted using MPlus version 8.3 (Muthén & Muthén, 1998-2017) with maximum likelihood estimation method. Data were missing completely at random using Little’s missing completely at random (MCAR) test on study variables: χ2 = 39.26, df = 27, p = 0.06. To account for missing data, we used full information maximum likelihood estimation (FIML). Moderated mediation analysis was examined using 10000 bootstrap samples and 95% confidence intervals (CI); a CI that does not include zero indicates statistical significance of the parameter. We followed codes developed by Stride et al. (2015) to examine moderated mediation analyses using Model 58. Fit indices of root mean square error of approximation (RMSEA; McDonald & Ho, 2002), confirmatory fit index (CFI), standardized root mean square residual (SRMR), chi square (χ2) were used to determine the fit of the model to the data for each hypothesis. The following cut offs were used; RMSEA < 0.08, SRMR < 0.08, CFI ≥ 0.95 (Hu & Bentler, 1999). We probed interaction terms if p values were 0.10 and lower given the difficulties in detecting interactions in non-experimental studies (McClelland & Judd, 1993; Whisman & McClelland, 2005). The index of moderated mediation was not used as it cannot be used when the indirect effect is not linear, such as when the predictor’s effect on the mediator and the mediator’s effect on the outcomes are both moderated by the same continuous variable (Edwards & Lambert, 2007; Hayes, 2015). All predictor and moderator variables were centered in order to minimize multicollinearity among the main effect and interaction effect variables.
Power Analysis
Power analyses were conducted to determine the necessary sample size to detect an effect. A-priori sample size calculator for multiple regression was used (Soper, 2021). For a moderated mediation model with 6 predictors, the minimum sample size needed to detect a medium effect (f2 = 0.15) would be n = 97 with 1 – β = 0.80, α = 0.05.
Results
Outliers were handled through Winsorization, such that values 3 SD above or below the mean were replaced by the next closest value. This technique was applied to one outlier for FA. Descriptive statistics for all variables of interest at each visit are shown in Table 1.
Table 1.
Correlations and descriptive statistics among variables
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| 1. Frontal Asymmetry | 1.00 | |||||
| 2. Inhibitory Control Number Stroop Test (ms) | 0.08 | 1.00 | ||||
| 3. Working Memory Backward Digit Span Test | -0.13 | -0.36** | 1.00 | |||
| 4. Cognitive Flexibility WCST | -0.17 | -0.15 | 0.13 | 1.00 | ||
| 5. Adolescent Cognitive Reappraisal (ERQ) | 0.05 | -0.23** | 0.06 | 0.06 | 1.00 | |
| 6. Adolescent Depressive Symptoms (RCADS) | -0.16 | 0.02 | -0.04 | 0.14 | -0.28** | 1.00 |
| N | 110 | 110 | 113 | 109 | 122 | 122 |
| Mean | -0.008 | 2234.91 | 4.12 | 55.71 | 4.53 | 17.81 |
| SD | 0.18 | 570.46 | 0.77 | 35.73 | 1.01 | 5.75 |
| Range | -0.48 – 0.43 | 1234.18 – 4074.92 | 2 – 6 | 2 – 99 | 1.83 – 7 | 10 – 39 |
| Skewness | -0.01 | 0.60 | -0.25 | -0.21 | -0.11 | 0.90 |
| Kurtosis | 0.10 | -0.01 | 0.26 | -1.61 | 0.27 | 0.93 |
RCADS is raw score
ms milliseconds, WCST Wisconsin card sorting test, ERQ emotion regulation questionnaire, RCADS revised - children’s anxiety and depression scale
*p < 0.05; **p < 0.01
To determine whether differences in questionnaire responses were evident due to COVID-19, t-tests were conducted with a pre- and during-COVID-19 grouping variable for the questionnaire responses. No significant differences emerged. Pre- and during-Covid-19 means on all questionnaires are presented in Table 2. Sex, cohort (as a proxy for age), and pubertal status differences in adolescent CR and adolescent depressive symptoms were examined as well (see Table 2). T-tests revealed that adolescent depressive symptoms were significantly different when cohort and pubertal status was considered (p < 0.05), but not sex (p = 0.19). Therefore, age and pubertal status were included as covariates for all analyses.
Table 2.
Sex, Cohort, and COVID Differences on Cognitive Reappraisal and Depressive symptoms
| N | Mean | SD | t-test | ||
|---|---|---|---|---|---|
| Adolescent Cognitive Reappraisal | Female | 59 | 4.50 | 0.98 | t(120) = -0.32, p = 0.75 |
| Male | 63 | 4.56 | 1.04 |
| Adolescent Cognitive Reappraisal | Cohort 1 | 56 | 4.54 | 1.00 | t(120) = 0.03, p = 0.98 |
| Cohort 2 | 66 | 4.53 | 1.02 |
| Adolescent Cognitive Reappraisal | Pre COVID -19 | 79 | 4.57 | 1.02 | t(120) = 0.61, p = 0.54 |
| During COVID -19 | 43 | 4.46 | 1.00 |
| Adolescent Depressive Symptoms | Female | 59 | 17.10 | 4.44 | t(120) = -1.32, p = 0.19 |
| Male | 63 | 18.48 | 6.70 |
| Adolescent Depressive Symptoms | Cohort 1 | 56 | 19.16 | 5.68 | t(120) = 2.44, p = 0.02 |
| Cohort 2 | 66 | 16.67 | 5.59 |
| Adolescent Depressive Symptoms | Pre COVID -19 | 79 | 17.37 | 6.02 | t(120) = -1.16, p = 0.25 |
| During COVID -19 | 43 | 18.63 | 5.18 |
Associations between task specific executive function and CR are mixed among the adulthood research, with some findings indicating only associations among some executive function factors and not others, although all executive function factors have been significantly linked to CR and depression in previous research (Andrés et al., 2016; Davidovich et al., 2016; Holler et al., 2014; McRae et al., 2012; Tabibnia et al., 2011; see Schmeichel & Tang, 2014, 2015 for comprehensive reviews). Therefore, we chose to address the previous research findings by examining each of the three executive function factors in separate models. For each model, we tested our hypothesis that the executive function component would predict CR, with FA as a moderator of that association. CR in turn would be related to depressive symptoms, with FA moderating that association.
Cognitive Flexibility
The model with cognitive flexibility (WCST) demonstrated a poor fit (χ2 = 70.10, df = 9, N = 123, p < 0.001; CFI = 0.00; RMSEA = 0.24; SRMR = 0.12). Modification indices indicated adding a correlation between pubertal status and age; after this correlation was included, model fit improved (χ2 = 4.82, df = 4, N = 123, p = 0.31; CFI = 0.96; RMSEA = 0.04; SRMR = 0.03). Age was not a significant indicator of adolescent depressive symptoms and thus was removed from the model (χ2 = 4.15, df = 3, N = 123, p = 0.25; CFI = 0.95; RMSEA = 0.06; SRMR = 0.03). Modification indices further indicated adding a correlation between FA and CR; the resulting model fit was indicative of a good fitting model (χ2 = 1.93, df = 2, N = 123, p = 0.38; CFI = 1.00; RMSEA = 0.00; SRMR = 0.02).
Within the model, higher CR was associated with lower depressive symptoms, b = -1.82 (β = -0.32, SE = 0.08, p < 0.001, 95% CI [-2.73, -0.87]). In addition, higher pubertal status was associated with higher depressive symptoms b = 1.73 (β = 0.25, SE = 0.09, p < 0.01, 95% CI [0.54, 3.11]; Supplement Fig. S1). No other paths were significant.
Working Memory
The model with working memory (backward digit span test) initially demonstrated a poor fit (χ2 = 59.51, df = 9, N = 123, p = 0.00; CFI = 0.00; RMSEA = 0.21; SRMR = 0.11). Modification indices indicated adding a correlation between pubertal status and age; after this correlation was included, model fit improved (χ2 = 4.85, df = 4, N = 123, p = 0.30; CFI = 0.97; RMSEA = 0.04; SRMR = 0.03). Age was not a significant indicator of adolescent depressive symptoms and thus was removed (χ2 = 4.39, df = 3, N = 123, p = 0.22; CFI = 0.95; RMSEA = 0.06; SRMR = 0.03). Modification indices indicated adding a correlation between FA and CR; the resulting model fit was indicative of a good fitting model (χ2 = 1.25, df = 2, N = 123, p = 0.54; CFI = 1.00; RMSEA = 0.00; SRMR = 0.02).
Within the model, higher CR was associated with lower depressive symptoms, b = -1.81 (β = -0.32, SE = 0.08, p < 0.001, 95% CI [-2.75, -0.88]; Supplement Fig. S2). The interaction between CR and FA was not significant, b = 5.65 (β = 0.17, SE = 0.10, p = 0.08, 95% CI [-0.52, 12.06]), however, we further probed this interaction effect, considering the known difficulties in detecting interaction effects in non-experimental studies (McClelland & Judd, 1993; Whisman & McClelland, 2005). Simple slopes analysis revealed the effect of CR was significant for right FA, b = -2.60, SE = 0.74, 95% CI [-4.00, -1.10] but not for left FA, b = -1.02, SE = 0.56, 95% CI [-2.12, 0.06] (see Supplement Fig. S3). In addition, higher pubertal status was associated with higher depressive symptoms b = 1.91 (β = 0.27, SE = 0.09, p < 0.01, 95% CI [0.69, 3.34]). No other paths were significant.
Inhibitory Control
We first analyzed the final model with the number Stroop task (inhibitory control), but the model did not converge, likely due to high variance within the task. We log-transformed the number Stroop task to minimize variance. After controlling for age and pubertal status, the model with inhibitory control (number Stroop task) was analyzed and demonstrated poor fit (i.e., χ2 = 67.71, df = 49, N = 123, p < 0.001; CFI = 0.00; RMSEA = 0.23; SRMR = 0.12). Modification indices indicated adding a correlation between pubertal status and age; after this correlation was included, model fit improved (χ2 = 6.72, df = 4, N = 123, p = 0.16; CFI = 0.91; RMSEA = 0.07; SRMR = 0.04). Within the model, age was not a significant indicator of depressive symptoms; thus, the path was trimmed for parsimony. Resulting model fit had room for improvement (χ2 = 5.21, df = 3, N = 123, p = 0.16; CFI = 0.93; RMSEA = 0.08; SRMR = 0.04). Modification indices indicated adding a correlation between CR and the interaction variable. The final model was analyzed and demonstrated good fit (χ2 = 1.81, df = 2, N = 123, p = 0.40; CFI = 1.00; RMSEA = 0.00; SRMR = 0.02).
Mediating Effect of Cognitive Reappraisal
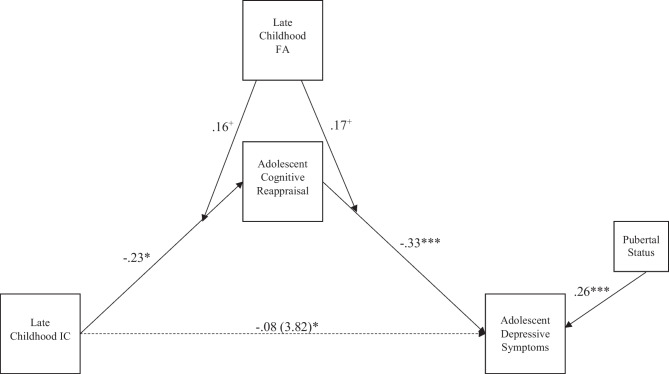
Moderated mediation analysis was examined using 10000 bootstrap samples and 95% confidence intervals (CI). As shown in Table 3 and summarized in Fig. 1, the direct association between inhibitory control and depressive symptoms was not significant. Instead, inhibitory control was directly associated with CR, b = -0.90 (β = -0.23, SE = 0.09, p = 0.01, 95% CI [-0.39, -0.04]), which in turn was significantly associated with depressive symptoms, b = -1.90 (β = -0.33, SE = 0.08, p < 0.001, 95% CI [-0.48, -0.17]). Worse late childhood inhibitory control (i.e., slower reaction time) was associated with lower adolescent CR, and lower CR was associated with higher depressive symptoms. Additionally, higher pubertal status was associated with higher depressive symptoms, b = 1.87 (β = 0.26, SE = 0.08, p = 0.002, 95% CI [0.63, 3.27]).
Table 3.
Moderated mediation results
| Cognitive Reappraisal (M) | Depressive Symptoms (Y) | |
|---|---|---|
| Predictors | Coeff. (SE) | Coeff. (SE) |
| Pubertal Status | – | 1.87 (0.65)** |
| IC | -0.90 (0.37)* | -1.84 (2.08) |
| IC X FA | 3.69 (2.06) + | – |
| CR | – | -1.90 (0.49)*** |
| CR X FA | – | 5.69 (3.26) + |
| IC CR Depressive Symptoms | ||
|---|---|---|
| Conditional indirect effects | Coeff. (SE) | 95% CI |
| Low (Right FA) | 3.82 (1.88) | 0.99, 8.41 |
| High (Left FA) | 0.42 (0.70) | -0.46, 2.44 |
Coefficients are unstandardized; parentheses indicate the standard errors
IC inhibitory control, FA frontal EEG asymmetry, CR cognitive reappraisal
*** p < 0.001, **p < 0.01, *p < 0.05, +p < 0.10
Fig. 1.
Summarized model fitting results of associations among late childhood IC (inhibitory control), adolescent cognitive reappraisal, and adolescent depressive symptoms moderated by late childhood FA (frontal EEG asymmetry). Standardized estimates presented. Parentheses indicate the unstandardized indirect effect estimate of late childhood IC on adolescent depressive symptoms. **p < 0.01, *p < 0.05, +p < 0.10
Moderated Mediation Effects
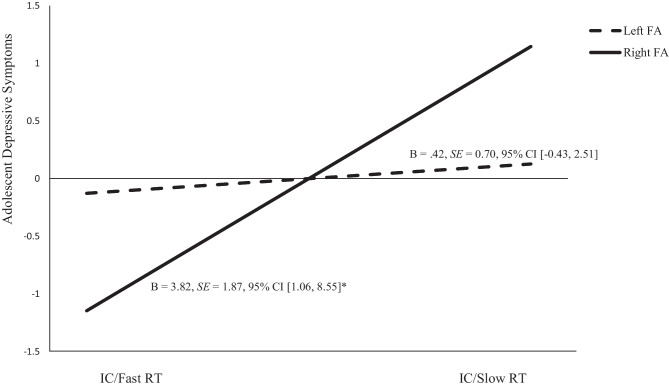
The interaction effect between FA and inhibitory control on adolescent CR was not statistically significant at p < 0.05 (b = 3.69, β = 0.16, SE = 0.09, p = 0.08, 95% CI [-0.66, 7.56]; see Table 3 and Fig. 2). However, we further probed this interaction effect, given the known difficulties in detecting interaction effects in non-experimental studies (McClelland & Judd, 1993; Whisman & McClelland, 2005). Simple slopes analysis revealed the direct effect of inhibitory control on the mediator (i.e., CR) was conditional, such that it was significant only at lower levels of FA (i.e., right FA), b = -1.42, SE = 0.44, 95% CI [-2.31, -0.55] and not at higher levels of FA (i.e., left FA), b = -0.38, SE = 0.49, 95% CI [-1.37, 0.57]. Figure 2 provides a visual representation of the interaction between inhibitory control and FA on the mediator (i.e., CR).
Fig. 2.
CR (cognitive reappraisal) as a function of IC (inhibitory control) and FA (frontal EEG asymmetry). Slow reaction time (RT) is indicative of worse IC. Unstandardized estimates are presented
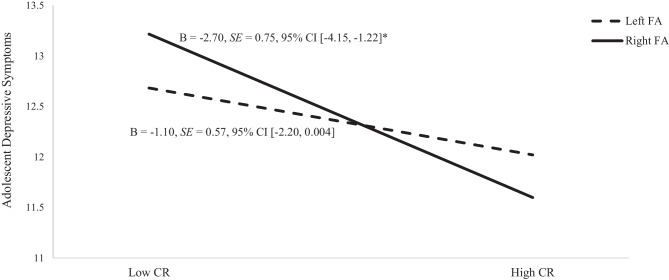
Results indicated the moderation effect of FA on the association between the mediator (i.e., CR) and depressive symptoms was not significant, b = 5.69 (β = 0.17, SE = 0.10, p = 0.08, 95% CI [-0.70, 11.78]; Fig. 3), however we further probed this interaction effect given the known difficulties in detecting interaction effects in non-experimental studies (McClelland & Judd, 1993; Whisman & McClelland, 2005). Simple slopes analysis revealed that the effect of CR on depressive symptoms was dependent upon the levels of FA, such that the CR-depressive symptoms link was significantly only at lower levels of FA (i.e., right FA), b = -2.70, SE = 0.75, 95% CI [-4.15, -1.22] but not at higher levels of FA (i.e., left FA), b = -1.10, SE = 0.57, 95% CI [2.20, 0.004].
Fig. 3.
Depressive Symptoms as a function of CR (cognitive reappraisal) and FA (frontal EEG asymmetry) for the model with inhibitory control. Unstandardized estimates are presented
The conditional indirect effect of inhibitory control on depressive symptoms via CR was found for adolescents with lower levels of FA (i.e., right FA; see Table 3 and Fig. 4), b = 3.82, SE = 1.87, 95% CI [1.06, 8.55]. That is, for those with right FA during late childhood, adolescent CR mediated the relation between late childhood inhibitory control and depressive symptoms, such that worse late childhood inhibitory control (i.e., slower reaction time) was associated with lower CR, and lower CR was in turn associated with higher depressive symptoms among adolescents, after controlling for pubertal status. Better late childhood inhibitory control (i.e., faster reaction time) was associated with higher CR, which was in turn associated with lower adolescent depressive symptoms, after controlling for pubertal status (see Table 3 and Fig. 4).
Fig. 4.
Indirect effect of IC (inhibitory control) on adolescent depressive symptoms through CR (cognitive reappraisal) for right FA (frontal EEG asymmetry) and left FA. Slow RT (reaction time) is indicative of worse IC. Unstandardized estimates are presented. Values for adolescent depressive symptoms are standardized
Discussion
We examined whether adolescent CR mediates the association between executive function and adolescent depressive symptoms, and whether the indirect effect is further conditional on FA during late childhood. Our findings indicate that not all executive functions emerge as significant in this association; only the model with middle childhood inhibitory control emerged as significant. Cognitive flexibility and working memory during middle childhood were not significant predictors of adolescent CR or depressive symptoms. This was surprising, given previous research which finds that cognitive flexibility and working memory are also associated with CR ability and depressive symptoms in adolescence and adulthood (Andrés et al., 2016; Davidovich et al., 2016; Holler et al., 2014; McRae et al., 2012). Because our study is one of the first to examine these relations longitudinally, it may be that when considering the development of CR, inhibitory control during middle childhood plays an important role, but when considering simultaneous executive function, all three executive function factors may be important for concurrent CR. We also did not find direct effects between the executive function measures during late childhood and depressive symptoms in adolescence, it may be that concurrent EF is more strongly associated directly with depressive symptoms in adolescence as opposed to indirect longitudinal relations (Han et al., 2016; Steinberger & Barch, 2021). Future research should continue to examine the developmental trajectory of more advanced cognitive strategies of emotion regulation as they relate to cognitive processes and subsequent depressive symptoms.
We further extend previous research by examining indirect effects as being conditional on trait FA, which is associated with a tendency toward depressive symptoms (Thibodeau et al., 2006). We probed the interaction of FA within our model when the interaction term was statistically significant at p < 0.10 given that interaction terms are known to be difficult to detect using non-experimental data. Nevertheless, the relatively small interaction effects indicate the need for further replication. These findings are interesting nonetheless, considering that among adolescents, depressive symptoms have been increasing over the last few decades, research indicates worrisome increases among adolescents born between 1990 and 2000 (Keyes et al., 2019). One mechanism that may help to decrease depressive symptoms among adolescents is developing their emotion regulation strategies, specifically CR. Our results indicate that when children have better inhibitory control this is associated with lower adolescent depressive symptoms through CR. For adolescents with right FA, targeting inhibitory control can be one way to reduce the risk for depressive symptoms (Thibodeau et al., 2006).
Within our sample we did not find any significant sex differences. This finding was surprising given previous research findings which indicate that girls are at an increased risk of developing depression, a drastic increase in depression is visible during the adolescent years, and is more chronic for girls (Breslau et al., 2017; Essau et al., 2010). In our sample, adolescents who self-reported higher pubertal status also self-reported higher depressive symptoms (Keyes et al., 2019). Our sample was not diverse, with the majority of participants being White and having highly educated parents. Recent literature that examines cultural, cognitive, and biological factors indicates these factors put girls at an increased risk of developing depression compared to boys (Gupta et al., 2013; Hamilton et al., 2015; Hyde et al., 2008; Shorey et al., 2022). Although we did not find sex differences within our sample, future research should continue to examine these associations further while also considering external factors which may differentially impact depressive outcomes for girls and boys.
For our sample, only the executive function of inhibitory control was a significant predictor of adolescent depressive symptoms mediated by CR. In particular, this association was significant when children displayed right FA during late childhood; specifically, better inhibitory control (i.e., faster reaction time) predicted higher adolescent CR. When children had worse inhibitory control (i.e., slower reaction time) and right FA during late childhood, they self-reported lower CR as adolescents. For those with right FA during late childhood, lower CR was then linked to a higher number of depressive symptoms in adolescence. Previous adulthood research indicates differences regarding the specific executive function variables that are linked to CR ability (McRae et al., 2012; Tabibnia et al., 2011). For our study, cognitive flexibility and working memory were not significantly associated with CR ability. We focused on habitual CR rather than task-specific CR, which was the focus of much of the previous work with adults. We may have found differences regarding direct links of working memory and cognitive flexibility if we had also used task-specific CR (Schmeichel & Tang, 2015) because asking adolescents to learn a new emotion regulation strategy and implement it during emotion elicitation may require other executive function, in addition to inhibitory control. By focusing on trait FA, we were able to examine FA as an individual difference factor that provides adolescents with the capability to engage CR and protect against depressive symptoms.
Among adults, depression is linked to deficits in working memory, inhibitory control, and cognitive flexibility, but these associations are not well understood during adolescence (Fossati et al., 2002; Vilgis et al., 2015). Childhood and adolescent research indicates that symptoms of depression are associated with slower reaction times on inhibitory control tasks (Cataldo et al., 2005; Vilgis et al., 2015). This association is also evident in children and adolescents with deficits in working memory and cognitive flexibility (Vilgis et al., 2015). Our results indicate that only inhibitory control is a significant contributor to adolescent depressive symptoms when both CR and FA are considered in the model. The indirect effect of inhibitory control on depressive symptoms through CR indicates that children with right FA are better capable of engaging CR when they are more effective inhibitors, and their higher CR is protective against depression. Based on previous literature indicating right FA as a risk factor being associated with more depressive symptoms, our findings indicate this association requires a consideration of other protective factors (Thibodeau et al., 2006) or a more complex model of adolescent FA and depressive symptoms. Our results highlight inhibitory control and CR serve as protective factors in the development of depressive symptoms among children and adolescents with right FA.
Our study had some limitations. The sample demographics were limited to primarily White youth with educated parents. Because adolescents from diverse ethnic and socio-economic backgrounds are at an increased risk of depressive symptoms, it is imperative to ensure that future research is generalizable to a more diverse sample of adolescents (Merikangas et al., 2010; Wagstaff & Polo, 2012). For example, adolescents who perceive greater racial and ethnic discrimination are at an increased risk of more depression and internalizing symptoms (Benner et al., 2018). Less use of CR and greater expressive suppression among Latino and Asian heritage college students is associated with greater depressive symptoms (Juang et al., 2016). Adolescents from disadvantaged socioeconomic backgrounds are at an increased risk of stressors that negatively impact brain development, subsequently impacting cognitive and emotional abilities (Noble & Giebler, 2020; Noble et al., 2021). Therefore, incorporating a model focused on increasing these cognitive and affective regulatory skills in youth from disadvantaged backgrounds can have a substantial impact on subsequent development.
We examined habitual CR and not task-specific CR, as done in most of the adulthood research literature. Our findings may have been more aligned with adult research if we had used the same CR. Although we examined all three executive function factors associated with habitual CR, only one model indicated significant associations among the variables, the model with inhibitory control. Future research should continue to examine the association among various EFs and habitual and task-specific CR to further understand how and when interventions may be implemented to decrease symptoms of depression among adolescents (Luciana, 2016). A final limitation of our study was that both CR and depressive symptoms were collected concurrently, therefore we cannot make directional inferences between these two variables.
Nevertheless, our findings may be of help in developing early CR interventions for adolescent depression focused on inhibitory control. Overall, our findings have the potential to inform future interventions that can be targeted toward inhibitory control during late childhood, as well as during early childhood. Inhibitory control development is critical for emotion regulation from early childhood (Whedon et al., 2021). For children with right FA, targeting inhibitory control could reduce the risk for depression symptoms during adolescence (Blair & Diamond, 2008; Carlson & Wang, 2007). This can be an easy and cost-effective intervention that could substantially impact adolescent depressive symptoms (Moilanen et al., 2010). Teaching young children to regulate their emotions by implementing these strategies into school curricula can be beneficial for the long-term effects of diminishing the rise of depressive symptoms in adolescence (Hoffmann et al., 2020).
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to the families for their participation in our research.
Funding
This research was supported in part by grant HD049878 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) awarded to Martha Ann Bell. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the National Institutes of Health.
Data Availability
Data used in these analyses are available from the corresponding author upon reasonable request.
Compliance with Ethical Standards
Ethics Approval
This study was approved by the Virginia Tech IRB (late childhood protocol #12-947) and by the Biomedical Research Alliance of New York (BRANY) IRB (adolescent protocol-#19-030-568/19-352).
Consent to Participate
Informed consent and child assent was obtained from all participants in this study.
Conflict of Interest
The authors have no conflicts to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alves M, Yamamoto T, Arias-Carrion O, Rocha N, Nardi A, Machado S, Cardoso A. Executive function impairments in patients with depression. CNS & Neurological Disorders - Drug Targets. 2014;13(6):1026–1040. doi: 10.2174/1871527313666140612102321. [DOI] [PubMed] [Google Scholar]
- Andrés ML, Castañeiras C, Stelzer F, Canet L, Introzzi I. Executive functions and cognitive reappraisal ability: The relationship in children. Psicología Desde El Caribe. 2016;33(2):55–82. doi: 10.14482/psdc.33.2.7278. [DOI] [Google Scholar]
- Benner AD, Wang Y, Shen Y, Boyle AE, Polk R, Cheng Y-P. Racial/ethnic discrimination and well-being during adolescence: A meta-analytic review. American Psychologist. 2018;73(7):855–883. doi: 10.1037/amp0000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best JR, Miller PH. A developmental perspective on executive function. Child Development. 2010;81(6):1641–1660. doi: 10.1111/j.1467-8624.2010.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biringer E, Lundervold A, Stordal K, Mykletun A, Egeland J, Bottlender R, Lund A. Executive function improvement upon remission of recurrent unipolar depression. European Archives of Psychiatry and Clinical Neuroscience. 2005;255(6):373–380. doi: 10.1007/s00406-005-0577-7. [DOI] [PubMed] [Google Scholar]
- Blair C, Diamond A. Biological processes in prevention and intervention: The promotion of self-regulation as a means of preventing school failure. Development and Psychopathology. 2008;20(3):899–911. doi: 10.1017/S0954579408000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau J, Gilman SE, Stein BD, Ruder T, Gmelin T, Miller E. Sex differences in recent first-onset depression in an epidemiological sample of adolescents. Translational Psychiatry. 2017;7(5):1–6. doi: 10.1038/tp.2017.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SM, Wang TS. Inhibitory control and emotion regulation in preschool children. Cognitive Development. 2007;22(4):489–510. doi: 10.1016/j.cogdev.2007.08.002. [DOI] [Google Scholar]
- Cataldo MG, Nobile M, Lorusso ML, Battaglia M, Molteni M. Impulsivity in depressed children and adolescents: A comparison between behavioral and neuropsychological data. Psychiatry Research. 2005;136(2–3):123–133. doi: 10.1016/j.psychres.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Chen W, Zhang D, Liu J, Pan Y, Sang B. Parental attachment and depressive symptoms in Chinese adolescents: The mediation effect of emotion regulation. Australian Journal of Psychology. 2019;71(3):241–248. doi: 10.1111/ajpy.12239. [DOI] [Google Scholar]
- Choi D, Sekiya T, Minote N, Watanuki S. Relative left frontal activity in reappraisal and suppression of negative emotion: Evidence from frontal alpha asymmetry (FAA) International Journal of Psychophysiology. 2016;109:37–44. doi: 10.1016/j.ijpsycho.2016.09.018. [DOI] [PubMed] [Google Scholar]
- Cotrena C, Branco LD, Shansis FM, Fonseca RP. Executive function impairments in depression and bipolar disorder: Association with functional impairment and quality of life. Journal of Affective Disorders. 2016;190:744–753. doi: 10.1016/j.jad.2015.11.007. [DOI] [PubMed] [Google Scholar]
- Curtis WJ, Cicchetti D. Emotion and resilience: A multilevel investigation of hemispheric electroencephalogram asymmetry and emotion regulation in maltreated and nonmaltreated children. Development and Psychopathology. 2007;19(3):811–840. doi: 10.1017/S0954579407000405. [DOI] [PubMed] [Google Scholar]
- Davidovich S, Collishaw S, Thapar AK, Harold G, Thapar A, Rice F. Do better executive functions buffer the effect of current parental depression on adolescent depressive symptoms? Journal of Affective Disorders. 2016;199:54–64. doi: 10.1016/j.jad.2016.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJong H, Fox E, Stein A. Does rumination mediate the relationship between attentional control and symptoms of depression? Journal of Behavior Therapy and Experimental Psychiatry. 2019;63:28–35. doi: 10.1016/j.jbtep.2018.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. Executive functions. Annual Review of Psychology. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryman MT, Heimberg RG. Emotion regulation in social anxiety and depression: A systematic review of expressive suppression and cognitive reappraisal. Clinical Psychology Review. 2018;65:17–42. doi: 10.1016/j.cpr.2018.07.004. [DOI] [PubMed] [Google Scholar]
- Edwards JR, Lambert LS. Methods for integrating moderation and mediation: A general analytical framework using moderated path analysis. Psychological Methods. 2007;12(1):1–22. doi: 10.1037/1082-989X.12.1.1. [DOI] [PubMed] [Google Scholar]
- Essau CA, Lewinsohn PM, Seeley JR, Sasagawa S. Gender differences in the developmental course of depression. Journal of Affective Disorders. 2010;127(1–3):185–190. doi: 10.1016/j.jad.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Shaw DS, Silk JS, Feng X, Cohn JF, Fox NA, Kovacs M. Children’s affect expression and frontal EEG asymmetry: Transactional associations with mothers’ depressive symptoms. Journal of Abnormal Child Psychology. 2008;36(2):207–221. doi: 10.1007/s10802-007-9171-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossati P, Ergis AM, Allilaire JF. Executive functioning in unipolar depression: A review. L’encephale. 2002;28(2):97–107. [PubMed] [Google Scholar]
- Fox NA. Dynamic cerebral processes underlying emotion regulation. Monographs of the Society for Research in Child Development. 1994;59(2–3):152–166. doi: 10.1111/j.1540-5834.1994.tb01282.x. [DOI] [PubMed] [Google Scholar]
- Fox NA, Schmidt LA, Calkins SD, Rubin KH, Coplan RJ. The role of frontal activation in the regulation and dysregulation of social behavior during the preschool years. Development and Psychopathology. 1996;8:89–102. doi: 10.1017/S0954579400006982. [DOI] [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: Divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology. 1998;74(1):224–237. doi: 10.1037/0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Gross JJ. The emerging field of emotion regulation: An integrative review. Review of General Psychology. 1998;2(5):271–299. doi: 10.1037/1089-2680.2.3.271. [DOI] [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003;85(2):348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Thompson RA. Emotion regulation: Conceptual foundations. In: Gross JJ, editor. Handbook of Emotion Regulation. The Guilford Press; 2007. pp. 3–24. [Google Scholar]
- Gupta A, Leong F, Valentine JC, Canada DD. A meta-analytic study: The relationship between acculturation and depression among Asian Americans. American Journal of Orthopsychiatry. 2013;83(2–3):372–385. doi: 10.1111/ajop.12018. [DOI] [PubMed] [Google Scholar]
- Hamilton JL, Stange JP, Abramson LY, Alloy LB. Stress and the development of cognitive vulnerabilities to depression explain sex differences in depressive symptoms during adolescence. Clinical Psychological Science. 2015;3(5):702–714. doi: 10.1177/2167702614545479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han G, Helm J, Iucha C, Zahn-Waxler C, Hastings PD, Klimes-Dougan B. Are executive functioning deficits concurrently and predictively associated with depressive and anxiety symptoms in adolescents? Journal of Clinical Child & Adolescent Psychology. 2016;45(1):44–58. doi: 10.1080/15374416.2015.1041592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. An index and test of linear moderated mediation. Multivariate Behavioral Research. 2015;50(1):1–22. doi: 10.1080/00273171.2014.962683. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Staff PAR. Wisconsin card sorting test-64: Computer version 2-research edition (WCST-64:CV2) Lutz, FL: Psychological Assessment Resources; 2003. [Google Scholar]
- Hendricks MA, Buchanan TW. Individual differences in cognitive control processes and their relationship to emotion regulation. Cognition and Emotion. 2016;30(5):912–924. doi: 10.1080/02699931.2015.1032893. [DOI] [PubMed] [Google Scholar]
- Hoffmann JD, Brackett MA, Bailey CS, Willner CJ. Teaching emotion regulation in schools: Translating research into practice with the RULER approach to social and emotional learning. Emotion. 2020;20(1):105–109. doi: 10.1037/emo0000649. [DOI] [PubMed] [Google Scholar]
- Holler K, Kavanaugh B, Cook NE. Executive functioning in adolescent depressive disorders. Journal of Child and Family Studies. 2014;23(8):1315–1324. doi: 10.1007/s10826-013-9789-z. [DOI] [Google Scholar]
- Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6(1):1–55. doi: 10.1080/10705519909540118. [DOI] [Google Scholar]
- Hui Q, Yao C, You X. The mechanism of executive dysfunction in depressive symptoms: The role of emotion regulation strategies. Current Psychology. 2021 doi: 10.1007/s12144-021-01528-7. [DOI] [Google Scholar]
- Hyde JS, Mezulis AH, Abramson LY. The ABCs of depression: Integrating affective, biological, and cognitive models to explain the emergence of the gender difference in depression. Psychological Review. 2008;115(2):291–313. doi: 10.1037/0033-295X.115.2.291. [DOI] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH. Emotion regulation in depression: Relation to cognitive inhibition. Cognition and Emotion. 2010;24(2):281–298. doi: 10.1080/02699930903407948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juang LP, Moffitt U, Kim SY, Lee RM, Soto JA, Hurley E, Weisskirch RS, Blozis SA, Castillo LG, Huynh Q-L, Whitbourne SK. Cognitive reappraisal and expressive suppression: Links to racial-ethnic discrimination and adjustment among Latino/a and Asian-heritage college students. Journal of Adolescence. 2016;53:21–33. doi: 10.1016/j.adolescence.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Gary D, O’Malley PM, Hamilton A, Schulenberg J. Recent increases in depressive symptoms among US adolescents: Trends from 1991 to 2018. Social Psychiatry and Psychiatric Epidemiology. 2019;54(8):987–996. doi: 10.1007/s00127-019-01697-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudinova AY, James K, Gibb BE. Cognitive reappraisal and depression in children with a parent history of depression. Journal of Abnormal Child Psychology. 2018;46(4):849–856. doi: 10.1007/s10802-017-0333-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantrip C, Isquith PK, Koven NS, Welsh K, Roth RM. Executive function and emotion regulation strategy use in adolescents. Applied Neuropsychology: Child. 2016;5(1):50–55. doi: 10.1080/21622965.2014.960567. [DOI] [PubMed] [Google Scholar]
- Lazarus RS, Alfert E. Short-circuiting of threat by experimentally altering cognitive appraisal. Journal of Abnormal and Social Psychology. 1964;69(2):195–205. doi: 10.1037/h0044635. [DOI] [PubMed] [Google Scholar]
- Lopez-Duran NL, Nusslock R, George C, Kovacs M. Frontal EEG asymmetry moderates the effects of stressful life events on internalizing symptoms in children at familial risk for depression. Psychophysiology. 2012;49(4):510–521. doi: 10.1111/j.1469-8986.2011.01332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M. Executive function in adolescence: A commentary on regulatory control and depression in adolescents: Findings from neuroimaging and neuropsychological research. Journal of Clinical Child and Adolescent Psychology. 2016;45(1):84–89. doi: 10.1080/15374416.2015.1123638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland GH, Judd CM. Statistical difficulties of detecting interactions and moderator effects. Psychological Bulletin. 1993;114(2):376–390. doi: 10.1037/0033-2909.114.2.376. [DOI] [PubMed] [Google Scholar]
- McDonald RP, Ho MHR. Principles and practice in reporting structural equation analyses. Psychological Methods. 2002;7(1):64–82. doi: 10.1037/1082-989X.7.1.64. [DOI] [PubMed] [Google Scholar]
- McRae K. Cognitive emotion regulation: A review of theory and scientific findings. Current Opinion in Behavioral Sciences. 2016;10:119–124. doi: 10.1016/j.cobeha.2016.06.004. [DOI] [Google Scholar]
- McRae K, Jacobs SE, Ray RD, John OP, Gross JJ. Individual differences in reappraisal ability: Links to reappraisal frequency, well-being, and cognitive control. Journal of Research in Personality. 2012;46(1):2–7. doi: 10.1016/j.jrp.2011.10.003. [DOI] [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, Benjet C, Georgiades K, Swendsen J. Lifetime prevalence of mental disorders in U.S. adolescents: Results from the national comorbidity survey replication-adolescent supplement (NCS-A) Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(10):980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moilanen KL, Shaw DS, Dishion TJ, Gardner F, Wilson M. Predictors of longitudinal growth in inhibitory control in early childhood. Social Development. 2010;19(2):326–347. doi: 10.1111/j.1467-9507.2009.00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén, L. K., & Muthén, B. O. (1998-2017). Mplus user’s guide (8th ed.). Los Angeles, CA: Muthén & Muthén.
- Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. Journal of Cognitive Neuroscience. 2004;16(7):1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- Noble KG, Giebler MA. The neuroscience of socioeconomic inequality. Current Opinion in Behavioral Sciences. 2020;36:23–28. doi: 10.1016/j.cobeha.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Hart ER, Sperber JF. Socioeconomic disparities and neuroplasticity: Moving toward adaptation, intersectionality, and inclusion. American Psychologist. 2021;76(9):1486–1495. doi: 10.1037/amp0000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz PC, Gross JJ, Urry HL. Selection, optimization, and compensation in the domain of emotion regulation: Applications to adolescence, older age, and major depressive disorder. Social and Personality Psychology Compass. 2012;6(2):142–155. doi: 10.1111/j.1751-9004.2011.00413.x. [DOI] [Google Scholar]
- Papousek I, Weiss EM, Perchtold CM, Weber H, de Assunção VL, Schulter G, Lackner HK, Fink A. The capacity for generating cognitive reappraisals is reflected in asymmetric activation of frontal brain regions. Brain Imaging and Behavior. 2017;11(2):577–590. doi: 10.1007/s11682-016-9537-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsafar P, Davis EL. Divergent effects of instructed and reported emotion regulation strategies on children’s memory for emotional information. Cognition and Emotion. 2019;33(8):1726–1735. doi: 10.1080/02699931.2019.1598937. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF015379. [DOI] [PubMed] [Google Scholar]
- Pons F, Harris PL, de Rosnay M. Emotion comprehension between 3 and 11 years: Developmental periods and hierarchical organization. European Journal of Developmental Psychology. 2004;1(2):127–152. doi: 10.1080/17405620344000022. [DOI] [Google Scholar]
- Reznik SJ, Allen JJB. Frontal asymmetry as a mediator and moderator of emotion: An updated review. Psychophysiology. 2018;55(1):e12965. doi: 10.1111/psyp.12965. [DOI] [PubMed] [Google Scholar]
- Ruffman T, Rustin C, Garnham W, Parkin AJ. Source monitoring and false memories in children: Relation to certainty and executive functioning. Journal of Experimental Child Psychology. 2001;80(2):95–111. doi: 10.1006/jecp.2001.2632. [DOI] [PubMed] [Google Scholar]
- Schmeichel BJ, Tang D. The relationship between individual differences in executive functioning and emotion regulation: A comprehensive review. In: Forgas JP, Harmon-Jones E, editors. The Control Within: Motivation and its regulation. Psychology Press; 2014. pp. 133–151. [Google Scholar]
- Schmeichel BJ, Tang D. Individual differences in executive functioning and their relationship to emotional processes and responses. Current Directions in Psychological Science. 2015;24(2):93–98. doi: 10.1177/0963721414555178. [DOI] [Google Scholar]
- Shapero BG, Stange JP, McArthur BA, Abramson LY, Alloy LB. Cognitive reappraisal attenuates the association between depressive symptoms and emotional response to stress during adolescence. Cognition and Emotion. 2019;33(3):524–535. doi: 10.1080/02699931.2018.1462148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorey S, Ng ED, Wong CHJ. Global prevalence of depression and elevated depressive symptoms among adolescents: A systematic review and meta-analysis. British Journal of Clinical Psychology. 2022;61(2):287–305. doi: 10.1111/bjc.12333. [DOI] [PubMed] [Google Scholar]
- Soper, D. S. (2021). A-priori sample size calculator for multiple regression [Software]. Retrieved February 10, 2021, from https://www.danielsoper.com/statcalc
- Steinberger DC, Barch DM. Investigating the link between depression, cognition, and motivation in late childhood. Child Psychiatry & Human Development. 2021 doi: 10.1007/s10578-021-01267-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stride C. B., Gardner S., Catley. N., & Thomas, F. (2015). Mplus code for mediation, moderation, and moderated mediation models. Retrieved March 13, 2021, from http://www.offbeat.group.shef.ac.uk/FIO/mplusmedmod.htm
- Sudikoff EL, Bertolin M, Lordo DN, Kaufman DAS. Relationships between executive function and emotional regulation in healthy children. Journal of Neurology and Psychology. 2015;S2(8):1–8. [Google Scholar]
- Tabibnia G, Monterosso JR, Baicy K, Aron AR, Poldrack RA, Chakrapani S, Lee B, London ED. Different forms of self-control share a neurocognitive substrate. Journal of Neuroscience. 2011;31(13):4805–4810. doi: 10.1523/JNEUROSCI.2859-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeau R, Jorgensen RS, Kim S. Depression, anxiety, and resting frontal EEG asymmetry: A meta-analytic review. Journal of Abnormal Psychology. 2006;115(4):715–729. doi: 10.1037/0021-843X.115.4.715. [DOI] [PubMed] [Google Scholar]
- Vilgis V, Silk TJ, Vance A. Executive function and attention in children and adolescents with depressive disorders: A systematic review. European Child and Adolescent Psychiatry. 2015;24(4):365–384. doi: 10.1007/s00787-015-0675-7. [DOI] [PubMed] [Google Scholar]
- Vuga M, Fox NA, Cohn JF, Kovacs M, George CJ. Long-term stability of electroencephalographic asymmetry and power in 3 to 9 year-old children. International Journal of Psychophysiology. 2008;67(1):70–77. doi: 10.1016/j.ijpsycho.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagstaff, A. E., & Polo, A. J. (2012). Ethnicity and adolescent depression: Prevalence, access to services, and promising interventions. The Prevention Researcher, 19(4), 8–10. Retrieved November 16, 2021, from https://link.gale.com/apps/doc/A319811842/HRCA?u=anon~779bf6a5&sid=googleScholar&xid=6ac13d3a
- Wang F, Wang C, Yin Q, Wang K, Li D, Mao M, Zhu C, Huang Y. Reappraisal writing relieves social anxiety and may be accompanied by changes in frontal alpha asymmetry. Frontiers in Psychology. 2015;6:1–11. doi: 10.3389/fpsyg.2015.01604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wante L, Mezulis A, Van Beveren M-L, Braet C. The mediating effect of adaptive and maladaptive emotion regulation strategies on executive functioning impairment and depressive symptoms among adolescents. Child Neuropsychology. 2017;23(8):935–953. doi: 10.1080/09297049.2016.1212986. [DOI] [PubMed] [Google Scholar]
- Whedon M, Perry NB, Curtis EB, Bell MA. Private speech and the development of self-regulation: The importance of temperamental anger. Early Childhood Research Quarterly. 2021;56:213–224. doi: 10.1016/j.ecresq.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whisman MA, McClelland GH. Designing, testing, and interpreting interactions and moderator effects in family research. Journal of Family Psychology. 2005;19(1):111–120. doi: 10.1037/0893-3200.19.1.111. [DOI] [PubMed] [Google Scholar]
- Yang M, Deng X, An S. The relationship between habitual use and real-time emotion regulation strategies in adolescents: Evidence from frontal EEG asymmetry. Neuropsychologia. 2021;162:108056. doi: 10.1016/j.neuropsychologia.2021.108056. [DOI] [PubMed] [Google Scholar]
- Young K, Sandman C, Craske M. Positive and negative emotion regulation in adolescence: Links to anxiety and depression. Brain Sciences. 2019;9(4):76. doi: 10.3390/brainsci9040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in these analyses are available from the corresponding author upon reasonable request.