SUMMARY
OBJECTIVE:
This study aimed to analyze the association between self-reported symptoms and seroprevalence against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the population of Mato Grosso.
METHODS:
A household-based survey was conducted on 4,206 adults from 10 municipalities of Mato Grosso, in the Brazilian Midwest, who were selected by cluster sampling in three stages. Questionnaires were applied between September and October 2020, and chemiluminescence was used for the quantitative determination of immunoglobulin G (IgG) antibodies against the S1 and S2 proteins of SARS-CoV-2.
RESULTS:
Approximately half (47.0%) of individuals with SARS-CoV-2 antibodies (12.5%) reported having no symptoms. The most prevalent symptoms among individuals with antibodies were body pain (37.0%), fever (32.9%), and smell and taste change (28.7%). The search for a basic health unit was predominant (45.0%) as the first service, and only 5.3% reported being hospitalized.
CONCLUSION:
A high proportion of asymptomatic cases of coronavirus disease 2019 (COVID-19) was identified in the general population, even among older adults and individuals with comorbidities.
KEYWORDS: Coronavirus infections, SARS-CoV-2, COVID-19
INTRODUCTION
The new coronavirus identified in Wuhan, China, has spread around the world in late 2019 and is currently responsible for the most effective pandemic of the last century 1–3 . Due to the high transmissibility 4 , associated with various forms of contagion, such as direct contact with saliva, aerosol, feces, and urine, fomites, and contaminated personal objects that have contact with the mucosa 5–8 , its expansion was devastating in populations, totaling 243,354,428 cases and 4,943,926 deaths globally as on October 2021, with 21,723,559 reported cases and 605,457 deaths in Brazil. The state of Mato Grosso had the highest mortality rate from the disease, leading the ranking from April to September 2021 8 .
The clinical forms of the coronavirus disease 2019 (COVID-19) are essential because they generate anxiety mainly due to the relationship with the transmission. The incubation time after infection ranges from 2 to 14 days 2 and clinical manifestations can range from mild cases, characterized by mild clinical symptoms, without radiographic findings of pneumonia; common cases, where fever is associated with respiratory symptoms and radiographic manifestations of pneumonia; severe cases, which can lead to respiratory distress and hypoxia; and finally, critical cases, with respiratory failure and the need for mechanical ventilation, shock, and other complications, requiring treatment in intensive care units 9 . Mortality is more prevalent in older adults over 80 years of age 10 and people with comorbidities, such as heart disease, hypertension, diabetes, chronic respiratory diseases, and neoplasms 10 .
Thus, symptomatic infections are more prevalent in older individuals, and most young people and children can be asymptomatic carriers 11 . A systematic review 11 described 25 nonspecific symptoms and the most common symptoms are fever, cough, fatigue, and myalgia, which can lead to limitations in the assertive differential diagnosis with other acute febrile illnesses endemic in the region. International studies point to the risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) affecting respiratory, neurological, and gastrointestinal systems 2,5 .
The number of secondary infections observed in an affected individual with COVID-19 can range from 2–2.5% 12,13 , and Xavier et al. 14 highlighted that the spread of the virus occurs more commonly by asymptomatic or symptomatic individuals with mild/moderate conditions. However, the limitation of diagnostic tests hinders the assessment of the actual proportion of asymptomatic among infected cases. The national seroprevalence household-based survey of COVID-19 in Brazil (EPICOVID) showed a prevalence of 12.1% asymptomatic among the positive cases 15 . Thus, it is essential to carry out studies that describe the seroprevalence of SARS-CoV-2 in populations and investigate its clinical characteristics.
In this context, we aimed to analyze the relationship between self-reported symptoms and the seroprevalence of SARS-CoV-2 in the Mato Grosso population by a population-based epidemiological survey.
METHODS
A household-based epidemiological survey was carried out in pole municipalities of the socioeconomic regions of Mato Grosso, a Brazilian Midwestern state, and the main cities include Cuiabá, Várzea Grande, Cáceres, Rondonópolis, Barra do Garças, Tangará da Serra, Alta Floresta, Água Boa, Juína, and Sinop. The state of Mato Grosso has an extension of 903,207.047 km2, with 3,567,234 inhabitants as estimated in 2021. According to the last 2010 Census, the population density was 3.36 inhabitants/km2 and the Human Development Index (HDI) was 0.725 16 .
A three-stage cross-sectional cluster sampling was adopted as follows: census sector (selected with probability proportional to the number of permanent households according to the 2010 Census data); household (selected from a systematic sampling); resident above the age of 18 years (one randomly selected resident). The sample was estimated as 4,530 individuals, proportionally distributed by population size of the municipalities (25,000-65,000 inhabitants; 65,000–150,000 inhabitants; 150,000–300,000 inhabitants; >300,000 inhabitants; >300,000 inhabitants). Further details about the sampling design and the draw of households are available in the study by Oliveira et al 17 .
A resident aged above 18 years was randomly selected to answer the questionnaire and submitted to blood sample collection in the residence during data collection from September 16 to October 15, 2020 by professionals after undergoing the training to standardize interviews and blood collection. The questionnaire was applied using the Epi InfoTM software, version 7.2, on smartphones (U.S. Department of Health & Human Services – Washington – USA).
The biological samples were submitted to centrifugation in each municipality to obtain the serum, which, in turn, was cryopreserved at −20°C and transported to the Central Public Health Laboratory of Mato Grosso (Laboratório Central de Saúde Pública de Mato Grosso, LACEN-MT). The laboratory analysis was conducted using a commercial kit imported by Diasorin (MS Registry: 103.398.40-56) from the Italian company Liaison under batch 354020 and validity till December 15, 2020, through chemiluminescence for the quantitative determination of immunoglobulin G (IgG) antibodies against the S1 and S2 proteins of SARS-CoV-2, with the supplier's report of 97.4% sensitivity (percentage of positive hits) and 98.5% specificity (percentage of negative hits). The authors also performed an internal validation, besides following the LACEN-MT biosafety protocols at all testing stages. This test was chosen after accessing the available commercial kits and internal testing to measure the quality.
In this study, the presence of self-reported symptoms since the onset of the pandemic was evaluated through the question “Did you have signs and symptoms potentially related to COVID-19 since March 2020 (symptoms such as fever, headache, body pain, cough, sore throat, and smell and taste alteration)?” The questionnaire was applied before receiving the test result so that respondents were blind to their serological status. The following symptoms were evaluated: smell or taste change, fever, sore throat, cough, difficulty in breathing, palpitation, tremors or chills, body ache, diarrhea, and vomiting. Symptoms were classified as follows: asymptomatic for no symptoms reported; oligosymptomatic for those who reported one or two symptoms except smell or taste change; and symptomatic for those who reported two or more symptoms and smell and taste change. The number of symptoms and the proportion of asymptomatic individuals were analyzed by the variables such as gender, age group, ethnicity/skin color, schooling, and household income.
The classification of symptoms by search for health services and reported morbidity was also analyzed. Questions include “If you had symptoms, did you seek any health service?” with a yes/no answer, and “Which health service you sought first?” with Basic Health Unit, Polyclinic, UPA, Exclusive COVID-19 Unit, Private Emergency Care, Private Clinic, or Other as answer options. Previous comorbidities were considered: hypertension, diabetes, asthma or bronchitis, cancer (any type), chronic kidney disease, chronic lung disease, some heart diseases, and some mental disorders such as depression.
All analyses were performed using the Stata software version 12, which allows incorporating weighting factors and considers the complex design of the sample. All ethical aspects in the research were agreed as per Resolution N° 466/2012 of the National Health Council (Conselho Nacional de Saúde), and the project was approved by the Ethics Committee of the University of the State of Mato Grosso on April 23, 2020 (Opinion 3.986.293/2020). All participants signed an informed consent form and were treated at their homes following strict biosafety protocols.
RESULTS
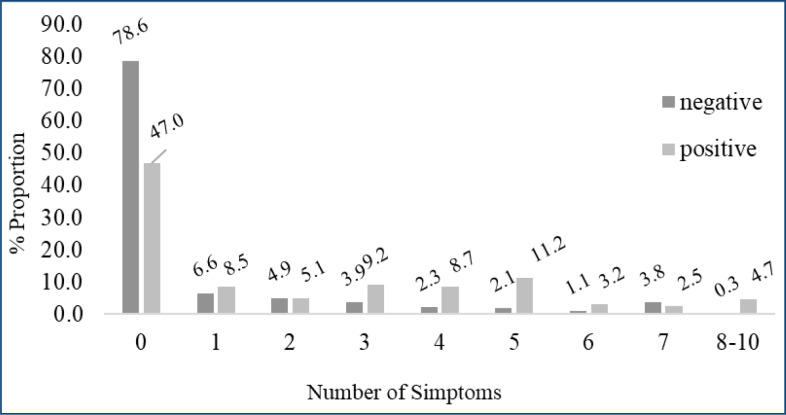
The mean age of the 4,206 (92.8% of the initial sample) analyzed individuals was 46.2 years (SD 16.3), and 53.7% of them were men. The COVID-19 prevalence was estimated as 12.5% (95%CI 10.5–14.7), and 47.0% of the positive cases were asymptomatic. The mean number of symptoms was lower and a higher proportion of asymptomatic was found among the youngest (18–29 years), with no significant differences observed according to gender, ethnicity/skin color, schooling, and household income (Table 1). Approximately half of the individuals in whom antibodies to SARS-CoV-2 were detected reported having no symptoms (47.0%) (Figure 1).
Table 1. Number of symptoms and proportion of asymptomatic patients according to sociodemographic characteristics among patients with antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), Mato Grosso, 2020.
| General | Number of symptoms | 95%CI | p-value | % of asymptomatic | 95%CI | p-value | |
|---|---|---|---|---|---|---|---|
| 47.0 | 37.2–57.0 | ||||||
| Sex | 0.14 | 0.11 | |||||
| Female | 2.58 | 2.03–3.13 | 38.0 | 27.6–49.5 | |||
| Male | 1.83 | 1.08–2.59 | 54.0 | 38.9–68.4 | |||
| Age group (years) | 0.01 | 0.03 | |||||
| 18–29 | 1.03 | 0.38–1.69 | 67.8 | 47.0–83.3 | |||
| 30–49 | 2.64 | 2.00–3.27 | 35.8 | 24.0–49.6 | |||
| 50–59 | 2.05 | 1.40–2.71 | 49.1 | 36.3–62.2 | |||
| 60 or above | 2.25 | 1.20–3.29 | 50.6 | 32.8–68.3 | |||
| Race/skin colob | 0.92 | 0.92 | |||||
| White | 2.23 | 1.04–3.43 | 50.7 | 27.2–73.9 | |||
| Brown | 2.24 | 1.67–2.82 | 46.4 | 34.8–58.3 | |||
| Black | 1.77 | 0.90–2.67 | 47.0 | 23.5–72.0 | |||
| Schooling | 0.60 | 0.16 | |||||
| Until complete elementary school | 1.80 | 1.21–2.38 | 56.8 | 43.7–68.9 | |||
| Incomplete and complete high school | 1.96 | 1.47–2.45 | 47.1 | 35.4–59.0 | |||
| Undergraduate or more | 3.45 | 2.09–4.81 | 30.5 | 11.7–59.3 | |||
| Family income | 0.36 | 0.40 | |||||
| Less than one minimum wage (less than R$1,045.00) | 1.59 | 0.78–2.40 | 41.7 | 24.2–61.8 | |||
| From one to less than three minimum wages (from R$1,045.00–R$3,134.99) | 1.95 | 1.41–2.47 | 49.8 | 38.9–60.8 | |||
| Three or more minimum wages (BRL 3,135.00 or more) | 2.60 | 1.79–3.41 | 41.2 | 26.8–73.2 | |||
CI: confidence interval; R$: Brazilian official currency (Reais, R$); BRL: projection of the value in Brazilian Reais.
Yellow (n=31) and indigenous (n=3) were excluded.
Bold indicates statistically significant values.
Figure 1. Proportion of serological test for antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) according to the number of symptoms. Mato Grosso, 2020.
All 10 symptoms evaluated were significantly more prevalent among individuals with antibodies against SARS-CoV-2, and the most prevalent symptoms were body pain (37.0%), fever (32.9%), smell and taste change (28.7%), and sore throat (25.0%). The most significant differences in prevalence of symptoms between the antibody-positive and antibody-negative groups were loss of smell/taste, palpitation, tremor or chills, and difficulty in breathing (Table 2).
Table 2. Proportion of symptoms and prevalence ratio (PR), according to the presence of antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), Mato Grosso, 2020.
| Test results | Prevalence Ratio | ||||
|---|---|---|---|---|---|
| Negative | Positive | PR | 95%CI | ||
| Change in smell or taste | 3.71 | 28.70 | 7.56 | 5.05 | 11.32 |
| Fever | 8.78 | 32.92 | 3.75 | 2.54 | 5.54 |
| Sore throat | 10.10 | 25.20 | 2.49 | 1.64 | 4.94 |
| Cough | 8.04 | 28.74 | 3.42 | 2.36 | 4.94 |
| Difficulty in breathing | 4.29 | 16.99 | 3.96 | 2.47 | 6.35 |
| Palpitation | 1.80 | 9.33 | 5.09 | 3.06 | 8.45 |
| Shiver or chills | 2.02 | 11.30 | 5.58 | 3.18 | 9.77 |
| Body ache | 11.92 | 37.04 | 3.11 | 2.18 | 4.43 |
| Diarrhea | 5.53 | 18.57 | 3.35 | 1.81 | 6.22 |
| Vomiting | 1.96 | 6.02 | 3.06 | 1.56 | 6.02 |
CI: confidence interval; PR: prevalence ratio
Approximately half (51.3%) of the patients with symptoms reported having sought some health service, which was higher among the symptomatic (71.2%) when compared to the oligosymptomatic (28.8%). Approximately 45% reported seeking a basic health unit as the first service, and 5.3% reported having been hospitalized, with no significant difference observed between those classified as oligosymptomatic and symptomatic. Approximately half of the patients who reported having hypertension, diabetes, asthma, or bronchitis and some mental illnesses were classified as symptomatic, followed by 47.4% of those who reported heart disease and 31.6% of those who reported kidney disease (Table 3).
Table 3. Search for health services by patients who presented symptoms similar to COVID-19 and classification of symptoms according to the presence of comorbidities among patients with antibodies to COVID-19, Mato Grosso, 2020.
| % weighted | Asymptomatic | Oligosymptomatic | Symptomatic | |||||
|---|---|---|---|---|---|---|---|---|
| % | 95%CI | % | 95%CI | % | 95%CI | |||
| Did you seek health services? | ||||||||
| Yes | 51.3 | – | – | 28.8 | 19.8–39.8 | 71.2 | 60.1–81.1 | |
| No | 48.6 | – | – | 55.5 | 45.2–64.4 | 45.5 | 35.5–54.8 | |
| Which health service did you seek first? | ||||||||
| Basic Health Unit | 45.0 | – | – | 32.0 | 18.0–50.1 | 68.0 | 49.8–82.0 | |
| Polyclinic | 4.1 | – | – | 18.2 | 7.1–39.3 | 81.8 | 60.4–93.0 | |
| UPA | 13.7 | – | – | 16.8 | 8.1–31.5 | 83.2 | 68.5–91.8 | |
| Exclusive unit for COVID-19 | 13.3 | – | – | 29.5 | 10.0–61.2 | 70.5 | 38.8–90.0 | |
| Private emergency care | 6.2 | – | – | 29.9 | 15.6–49.7 | 70.1 | 50.3–84.4 | |
| Private clinic | 5.2 | – | – | 29.3 | 9.0–64.3 | 70.7 | 36.5–91.0 | |
| Other | 12.4 | – | – | 37.8 | 21.5–57.3 | 62.2 | 42.7–78.5 | |
| Admitted at health service | ||||||||
| Yes | 5.6 | – | – | 8.4 | 1.7–32.1 | 91.6 | 67.9–98.3 | |
| No | 94.4 | – | – | 23.0 | 14.2–35.0 | 77.0 | 65.0–87.8 | |
| Comorbidities | ||||||||
| Hypertension or high blood pressure | 23.8 | 39.8 | 23.3–58.9 | 9.8 | 5.0–19.5 | 50.4 | 29.6–71.1 | |
| Diabetes or blood sugar | 7.2 | 36.2 | 18.2–59.3 | 13.4 | 41.2–35.7 | 50.4 | 30.3–70.4 | |
| Asthma or bronchitis | 1.9 | 43.7 | 23.9–65.7 | 6.3 | 0.8–35.7 | 50.0 | 28.6–71.3 | |
| Chronic kidney disease | 0.7 | 22.6 | 4.1–66.3 | 45.8 | 9.0–87.9 | 31.6 | 7.6–72.1 | |
| Chronic lung disease | 0.2 | 97.4 | 74.8–99.8 | – | – | 2.6 | 0.2–25.2 | |
| Some heart diseases | 0.2 | 52.6 | 24.8–78.6 | – | – | 47.4 | 21.2–75.1 | |
| Some mental disorders | 0.3 | 21.9 | 7.0–51.0 | 23.1 | 8.7–48.4 | 55.0 | 37.9–70.9 | |
CI: confidence interval; Results not shown for cancer due to the low prevalence in the study population (1.3 and 1.1%, respectively).
DISCUSSION
A high proportion of asymptomatic cases were found among individuals with the presence of antibodies to SARS-CoV-2 in the state of Mato Grosso, even among those who reported comorbidities. Furthermore, less than half of those who reported symptoms compatible with COVID-19 sought health services. These results show the importance of asymptomatic carriers in the transmission of COVID-19, as pointed out above, 17–20 also highlighting the critical proportion of people who did not perform diagnosis and isolation measures to prevent transmission between their contacts even with symptoms similar to the disease.
The prevalence of asymptomatic cases was higher than that observed in a nationwide seroprevalence survey conducted in sentinel cities in 26 Brazilian states and the Federal District from May to June 2020 (12.1%) 15 and lower than that observed in systematic review where median asymptomatic prevalence in men and women was 61.1 and 55.7%, respectively 21 . This household-based survey showed no differences between asymptomatic and symptomatic individuals regarding gender, ethnicity/skin color, education, and household income. However, there were higher proportions of asymptomatic young people from Mato Grosso or who had a lower number of symptoms. This result is significant as young people make up most of the active workforce and are more socially active, increasing the likelihood of disease transmission without noticing the infection 20 .
Undocumented infected cases are estimated as primary source for the geographic spread of COVID 17,22 and the proportion of asymptomatic is considerable, reaching rates ranging from 9.2–69%. However, the range of symptoms produced by COVID-19 is knowingly broad, varying by clinical form of the disease, and may manifest in different systems, with a predominance in the respiratory, neurological, and gastrointestinal systems 2,5,11 . In Mato Grosso, the most prevalent symptoms among individuals with antibodies against SARS-CoV-2 were myalgia, fever, sore throat, and smell and taste change.
Myalgia and fever are described as one of the initial symptoms of COVID-19 12 and were perceived by the population, though they are characterized as nonspecific symptoms of acute febrile illnesses 23 , which can often coexist in endemic areas, such as Mato Grosso, and cause confusion in the clinical suspicion. However, other diseases such as hantavirus, malaria, dengue, chikungunya, zika, and influenza must be considered for the differential diagnosis by health professionals in this region 24 .
The pain of guaranteeing that it refers more specifically to respiratory infections is also a nonspecific symptom. However, it is easily perceived by the discomfort it causes in the deglutition. Ageusia and anosmia are described by two-thirds of European patients with COVID-19, and are critical symptoms that guide early diagnosis 25 .
Besides ageusia and anosmia, palpitation, tremor/chills, and difficulty in breathing were the most prevalent symptoms among SARS-CoV-2-reactive agents in Mato Grosso. The perception of these additional symptoms may be associated with the fact that they are found in more persistent clinical forms and may predict a clinical evolution to the moderate and severe phases, 9 which drives the search by health services.
The high prevalence of asymptomatic individuals was noteworthy, even among individuals with comorbidities associated with more severe COVID-19. In addition, in our study, 2.9% reported hospitalization due to COVID-19, approximately half of the individuals reported some symptoms, and 45% who sought care accessed primary health care (PHC). In Brazil, which has a robust health system operating universally and characterized by an extensive PHC network, access to these services is facilitated by its high capillarization throughout the national territory and within its reach of expressive segments of the population 26 .
Among the limitations of this study is its cross-sectional design, besides self-reported responses about symptoms, which may be subjected to recall bias.
CONCLUSION
A population-based study with a highly accurate diagnostic strategy for identifying antibodies against SARS-CoV-2 showed a high proportion of asymptomatic cases, even among older adults and individuals with comorbidities. The low proportion of individuals who sought health services when they presented symptoms also points to the possible number of cases that were not notified and, therefore, were not considered in the epidemiological surveillance case control and contact monitoring strategies. These results show the difficulty in controlling the transmission of the disease in Mato Grosso.
ACKNOWLEDGMENT
The authors are extremely grateful to all those who agreed to participate in the research and those who worked in home visits, collection of biological samples, carrying out diagnostic tests, guiding the population, and delivering the results to the 4,306 people visited, activities that were carried out by the health professionals from the municipalities of Várzea Grande, Cáceres, Rondonópolis, Barra do Garças, Tangará da Serra, Alta Floresta, Água Boa, Juína and Sinop in partnership with academics in the health area of the University of the State of Mato Grosso, the Federal University of Mato Grosso, the Multiprofessional Residency in Health at the Federal University of Rondonópolis, and the Biomedicine at the University of Cuiabá.
REFERENCES
- 1.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li X, Wang W, Zhao X, Zai J, Zhao Q, Li Y, et al. Transmission dynamics and evolutionary history of 2019-nCoV. J Med Virol. 2020;92(5):501–511. doi: 10.1002/jmv.25701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang J, Zhao S, Liu M, Zhao Z, Xu Y, Wang P, et al. ACE2 expression by colonic epithelial cells is associated with viral infection, immunity and energy metabolism. MedRxiv. 2020 doi: 10.1101/2020.02.05.20020545. 20020545. [DOI] [Google Scholar]
- 4.Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Fang J, Zhu Y, Chen L, Ding F, Zhou R, et al. Clinical characteristics of non-critically ill patients with novel coronavirus infection (COVID-19) in a Fangcang Hospital. Clin Microbiol Infect. 2020;26(8):1063–1068. doi: 10.1016/j.cmi.2020.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158(6):1831.e3–1833.e3. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brasil . Centro de operações de emergência em Saúde Pública – COVID-19. Brasília: Ministério da Saúde; 2021. [[cited on Out 29, 2021]]. Ministério da Saúde. Boletim epidemiológico Especial n°86. Doença pelo novo Coronavírus COVID-19. Semana Epidemiológica 42 17/10 a 23/10/2021. Available from: https://www.gov.br/saude/pt-br/media/pdf/2021/outubro/29/boletim_epidemiologico_covid_86-final-_29out.pdf . [Google Scholar]
- 9.Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 10.Dowd JB, Andriano L, Brazel DM, Rotondi V, Block P, Ding X, et al. Demographic science aids in understanding the spread and fatality rates of COVID-19. MedRXiv. 2020 doi: 10.1101/2020.03.15.20036293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sousa AR, Neto, Carvalho ARB, Oliveira EMN, Magalhães RLB, Moura MEB, Freitas DRJ. Symptomatic manifestations of the disease caused by coronavirus (COVID-19) in adults: systematic review. Rev Gaucha Enferm. 2021;42(spe):e20200205. doi: 10.1590/1983-1447.2021.20200205. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization . Coronavirus disease 2019(COVID-19) situation report-67. Geneva: WHO; 2020. [Google Scholar]
- 13.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xavier AR, Silva JS, Almeida JPC, Conceição JFF, Lacerda GS, Kanaan S, et al. COVID-19: clinical and laboratory manifestations in novel coronavirus infection. J Bras Patol Med Lab. 2020;56:e3232020. doi: 10.5935/1676-2444.20200049. [DOI] [Google Scholar]
- 15.Menezes AMB, Victora CG, Hartwig FP, Silveira MF, Horta BL, Barros AJD, et al. High prevalence of symptoms among Brazilian subjects with antibodies against SARS-CoV-2. Sci Rep. 2021;11:13279–13279. doi: 10.1038/s41598-021-92775-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Instituto Brasileiro de Geografia e Estatística . Cidades e estados. Rio de Janeiro: IBGE; 2021. [[cited on Ago. 20, 2021]]. Available from: https://www.ibge.gov.br/cidades-e-estados/mt.html . [Google Scholar]
- 17.Oliveira EC, Terças-Trettel ACP, Andrade ACS, Muraro AP, Santos ESD, Espinosa MM, et al. Prevalência de anticorpos contra SARS-CoV-2 em Mato Grosso, Brasil: pesquisa de base populacional [Prevalence of SARS-CoV-2 antibodies in the State of Mato Grosso, Brazil: a population-based survey] Cad Saude Publica. 2022;38(5):e00093021. doi: 10.1590/0102-311XPT093021. [DOI] [PubMed] [Google Scholar]
- 18.Kronbichler A, Kresse D, Yoon S, Lee KH, Effenberger M, Shin JI. Asymptomatic patients as a source of COVID-19 infections: A systematic review and meta-analysis. Int J Infect Dis. 2020;98:180–186. doi: 10.1016/j.ijid.2020.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu YC, Liao CH, Chang CF, Chou CC, Lin YR. A locally transmitted case of SARS-CoV-2 infection in Taiwan. N Engl J Med. 2020;382:1070–1072. doi: 10.1056/NEJMc2001573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382(10):970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergeri I, Whelan M, Ware H, Subissi L, Nardone A, Lewis HC, et al. medRxiv. Preprint; Global epidemiology of SARS-CoV-2 infection: a systematic review and meta-analysis of standardized population-based seroprevalence studies, Jan 2020-Oct 2021. [DOI] [Google Scholar]
- 22.Johansson MA, Quandelacy TM, Kada S, Prasad PV, Steele M, Brooks JT, et al. SARS-CoV-2 Transmission from people without COVID-19 symptoms. JAMA Netw Open. 2021;4(1):e2035057. doi: 10.1001/jamanetworkopen.2020.35057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coura JR, editor. Dinâmica das doenças infecciosas e parasitárias. Rio de Janeiro: Guanabara Koogan; 2015. pp. 1175–2045. [Google Scholar]
- 24.Terças-Trettel ACP, Oliveira EC, Fontes CJF, Melo AVG, Oliveira RC, Guterres A, et al. Malaria and Hantavirus pulmonary syndrome in gold mining in the Amazon Region, Brazil. Int J Environ Res Public Health. 2019;16(10):1852–1852. doi: 10.3390/ijerph16101852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luers JC, Rokohl AC, Loreck N, Wawer Matos PA, Augustin M, Dewald F, et al. Olfactory and Gustatory Dysfunction in Coronavirus Disease 2019 (COVID-19) Clin Infect Dis. 2020;71(16):2262–2264. doi: 10.1093/cid/ciaa525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarti TD, Lazarini WS, Fontenelle LF, Almeida APSC. Qual o papel da Atenção Primária à Saúde diante da pandemia provocada pela COVID-19? Epidemiol Serv Saúde. 2020;29(2):e2020166. doi: 10.5123/S1679-49742020000200024. [DOI] [PubMed] [Google Scholar]