SUMMARY
OBJECTIVE:
We aimed to determine whether vitamin C has a protective effect on cisplatin-induced neuropathy in rats.
METHODS:
In total, 24 rats were included in the study of which 8 rats (no drug administered) were categorized as the control group. The remaining 16 rats were given a total dose of 20 mg/kg cisplatin to induce neuropathy. These drug-administered rats (16 rats) were randomly divided into two groups, namely, group-1 (n=8): cisplatin+saline and group-2 (n=8): cisplatin+vitamin C (500 mg/kg/day). All rats were tested for motor function and electromyographic activity 3 days after cisplatin. Motor performance was evaluated by an inclined-plane test. Compound muscle action potential was evaluated. Plasma malondialdehyde, glutathione, tumor necrosis factor-α, interleukin 6, and sciatic nerve HSP 70 levels were measured. Axon diameter and nerve growth factor expression levels were analyzed.
RESULTS:
Plasma malondialdehyde, tumor necrosis factor-α, and interleukin 6 levels were higher in the cisplatin+saline group than control group (p<0.001). But vitamin C significantly reduced malondialdehyde and inflammatory cytokine levels when compared with the cisplatin+saline group (p<0.001). Glutathione levels were lower in both cisplatin+saline and cisplatin+vitamin C groups than control group, but vitamin C significantly ameliorated the glutathione levels (p<0.05). Sciatic heat shock protein-70 levels were significantly higher in the cisplatin+vitamin C group than cisplatin+saline group. Compound muscle action potential amplitude and inclined plane test scores were significantly improved in the vitamin C group (p<0.05). Axon diameter and nerve growth factor expression ameliorated with vitamin C (p<0.05).
CONCLUSIONS:
We demonstrated the ameliorated effects of vitamin C on cisplatin-induced neuropathy through increased heat shock protein-70, nerve growth factor levels, and reduced inflammatory and oxidant effects. The results are promising to improve the neurotoxic effects of cisplatin in cancer patients.
KEYWORDS: Cisplatin, Drug related side effects and adverse reactions, Ascorbic acid, Heat shock proteins
INTRODUCTION
Cisplatin is a platinum-based antineoplastic drug used for the treatment of lung, testicular, ovarian, bladder, head-neck, cervical, and endometrial cancers 1 . The biological activity and toxicity of cisplatin are due to the metal ion compounds binding the proteins, nucleic acids, and other cellular components 2 . Cisplatin binds DNA and inhibits replication, cell cycle, and DNA repair 3 . DNA damage, oxidative stress, free oxygen radicals, and inflammatory cytokines lead to cisplatin-induced cytotoxicity 4 .
Dose-related major side effects of cisplatin are nephrotoxicity and neurotoxicity 5 . Common neurological side effects are paresthesia, dysesthesia, loss of tendon reflexes, hearing loss, visual disturbance, muscle weakness, loss of vibration, tremor, and ataxia 6 . Mitochondrial dysfunction, oxidative stress, and alterations of dorsal root ganglia are proposed mechanisms for neurotoxicity 7 .
Vitamin C [ascorbic acid (AA)] is a water-soluble antioxidant with various functions. It acts as a cofactor for the enzymes 8 and enhances the activities of antioxidant enzymes, such as glutathione peroxidase (GPx), catalase (CAT), and superoxide dismutase (SOD) 9 . Vitamin C protects against oxidative damage 10 and enhances immunity by supporting the innate and adaptive immune system 11 . This study was performed to investigate the neuroprotective effects of vitamin C against cisplatin neurotoxicity via antioxidant and anti-inflammatory effects.
METHODS
Animals
In total, 24 adult female Wistar rats, weighing 200–210 g, were used in the study. Animals were housed in cages and maintained under standard conditions with 12-h light/dark cycles at room temperature (22±2°C). They were fed with a standard pellet diet and tap water ad libitum throughout the study. The protocol employed in the study was approved by the Institutional Animal Care and Ethical Committee of the University of Science (Ethical Number: 04210523). All chemicals were obtained from Sigma-Aldrich Inc. unless otherwise noted.
Experimental Procedure
In total, 24 rats were included in the study. Eight rats were considered as the control group, and they were not administered any drug. The remaining 16 rats were given cisplatin at a dose of 2.5 mg/kg/day twice a week for 4 weeks (total dose of 20 mg/kg) to induce neuropathy 12 . The rats administered cisplatin were divided into 2 groups. Group 1 rats (n=8) were given 1 mL/kg/day 0.9% NaCl (saline) intraperitoneally daily for 4 weeks, and Group 2 rats (n=8) were given 500 mg/kg/day vitamin C (Redox-C Ampul, 500 mg/5 mL, Bayer) intraperitoneally daily for 4 weeks 13 . Two of the rats receiving cisplatin+saline died during the study. No death was reported in rats receiving cisplatin+vitamin C. At the end of the study, all rats were tested for motor function and electromyography (EMG). Following EMG recordings, at the end of the study, all animals were sacrificed (cervical dislocation) with anesthesia (100 mg/kg, Ketasol, Richter Pharma AG, Austria)/xylazine (50 mg/kg, Rompun, Bayer, Germany), and their blood samples were collected by cardiac puncture for biochemical analysis. They were centrifuged at 3000 rpm for 10 min at room temperature and stored at −20°C until assay.
Measurement of lipid peroxidation
Lipid peroxidation was determined in plasma samples by measuring malondialdehyde (MDA) levels. Briefly, trichloroacetic acid and thiobarbituric acid reactive substances (TBARS) and reagents were added to the plasma samples and then mixed and incubated at 100°C for 60 min. After cooling on ice, the samples were centrifuged at 3000 rpm for 20 min, and the absorbance of the supernatant was read at 535 nm. MDA levels were expressed as nM, and tetraethoxypropane was used for calibration.
Measurement of tissue glutathione levels
Glutathione (GSH) content in plasma samples was measured spectrophotometrically according to Ellman's method. In this method, thiols interact with 5,5’-dithiobis-(2-nitrobenzoic acid) (DTNB) and form a colored anion with a maximum peak at 412 nm. GSH levels were calculated from the standard calibration curve and expressed as μM.
Measurement of plasma tumor necrosis factor-α and interleukin 6 levels
Plasma tumor necrosis factor-α (TNF-α) and interleukin 6 (IL-6) levels were measured using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Biosciences).
Sciatic nerve biochemical analysis
After decapitation, sciatic nerves were rapidly removed and stored at 20°C until biochemical analyses were performed. For tissue analysis, whole nerve tissues were homogenized with a glass homogenizer in phosphate-buffered saline (PBS) that was five times the volume of the obtained tissue (pH 7.4) and centrifuged for 15 min. The supernatant was collected, and the total protein concentration homogenates were determined according to Bradford's method using bovine serum albumin as the standard. The sciatic nerve levels of heat shock protein-70 (HSP-70) in the tissue supernatants were measured using the commercially available rat ELISA kits. All samples obtained from animals were measured in duplicate according to the manufacturer's guideline.
Electrophysiological recordings
Electrophysiological recordings were performed 3 days after cisplatin. EMG was recorded under anesthesia (100 mg/kg, Ketasol, Richter Pharma AG, Austria)/xylazine (10 mg/kg, Rompun, Bayer, Germany). EMG was obtained three times from the right sciatic nerve stimulated supramaximally (intensity 10 V, duration 0.05 ms, frequency 1 Hz, in the range of 0.5–5000 Hz, 40 kHz/s with a sampling rate) by a bipolar subcutaneous needle stimulation electrode (BIOPAC Systems, Santa Barbara, CA) from the sciatic notch. Compound muscle action potentials (CMAPs) were recorded from 2–3 interosseous muscles by unipolar platinum electrodes. Data were evaluated using 3.6.7 software (BIOPAC Systems, Inc) with distal latency and amplitude of CMAP as the parameters. During the EMG recordings, the rectal temperatures of the rats were monitored by a rectal probe (HP Viridia 24-C; Hewlett-Packard Company, Palo Alto, CA), and the temperature of each rat was kept at approximately 36–37°C by a heating pad.
Assessment of motor function
The motor performances of the rats were evaluated by an inclined-plane test according to the method described by Rivlin and Tator. Briefly, the rat was placed oblique to the long axis of an inclined plane. The initial angle of the inclined plane was 10°. The inclined angle slowly increased, and the maximum angle of the plate on which the rat preserved its position for 5 s without falling was recorded as motor score. The inclined plane angle was measured three times in each rat to find an average value.
Histology and nerve growth factor immunohistochemistry
Sciatic nerve specimens were embedded in paraffin, sectioned at 5-μm thicknesses, and stained with hematoxylin and eosin. All sections were photographed with an Olympus C-5050 digital camera mounted on an Olympus BX51 microscope. The diameter of the sciatic nerve axons was measured using Image-Pro Express 1.4.5 (Media Cybernetics, Inc). Six sections from each animal were used for the measurement of axon diameter. For each section, we measured 100 axons.
For quantitative immunohistochemical examination, six sections from each animal were used. Sections were incubated with 10% H2O2 for 30 min to eliminate endogenous peroxidase activity and then blocked with goat serum (Invitrogen) for 1 h at room temperature. Subsequently, sections were incubated with primary antibodies (Santacruz Biotechnology;1/100) against nerve growth factor (NGF). Antibody detection was carried out with the Histostain-Plus Bulk kit (Invitrogen) against rabbit immunoglobulin G, and 3,3′-diaminobenzidine (DAB) was used to visualize the final product. Two blinded observers counted the total immune-positive Schwann cells under a light microscope at 100× magnification. Data were expressed as mean ± standard error of the means (SEM).
Statistical analysis
Statistical evaluation was performed using SPSS version 15.0 for Windows. The groups of parametric variables were compared using the Student's t-test and analysis of variance. Also, the groups of nonparametric variables were compared using the Mann-Whitney U test. In addition, the Shapiro-Wilk test was used for parametric-non-parametric differentiation. Results are presented as mean±SEM. A p<0.05 was considered statistically significant.
RESULTS
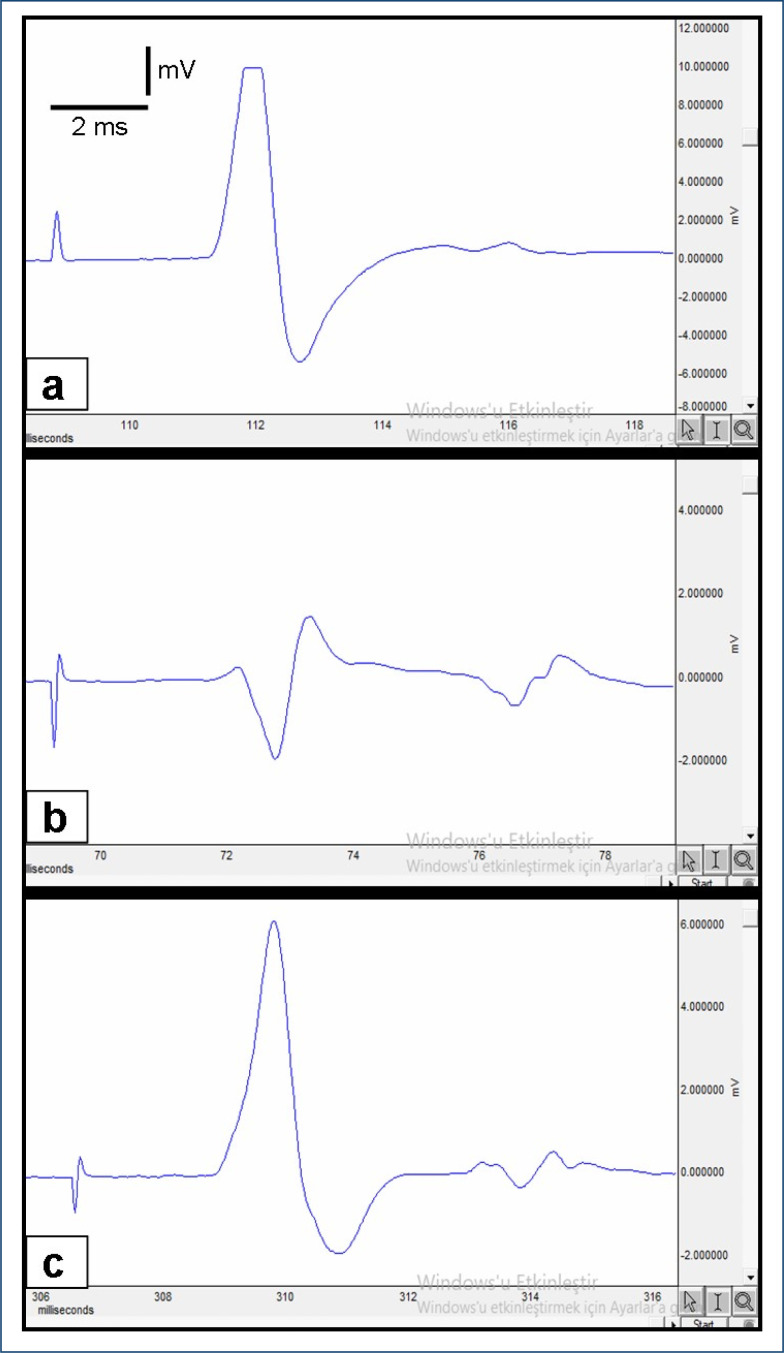
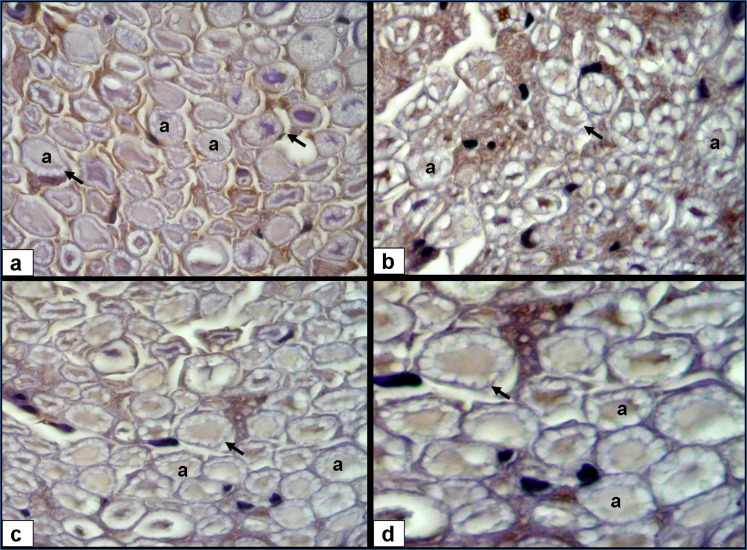
Plasma MDA level as an indicator of lipid peroxidation was significantly higher in the cisplatin+saline group (p<0.001). TNF-α and IL-6 levels were also higher in the cisplatin+saline group when compared with the control group (p<0.001). But vitamin C significantly reduced MDA, TNF-α, and IL-6 levels (p<0.001). GSH levels were lower in both cisplatin+saline and cisplatin+vitamin C groups than control group, but vitamin C significantly ameliorated the GSH levels (p<0.05). Sciatic nerve HSP-70 levels were significantly higher in the cisplatin+vitamin C group than cisplatin+saline group (p<0.001). The effects of vitamin C on MDA, TNF-α, IL-6, GSH, and sciatic nerve HSP-70 levels are detailed in Table 1. CMAP amplitude and inclined plane score were significantly lower in the cisplatin+saline group than control group (p<0.05) (Figure 1). EMG and inclined plane scores were significantly improved by vitamin C (p<0.05) (Table 1). In the cisplatin+saline group, the myelin sheath was degenerated and the NGF expression was decreased (Figure 2). Axon diameter and NGF immunoexpression were ameliorated with vitamin C (p<0.05) (Table 1).
Table 1. The effects of vitamin C on plasma malondialdehyde, tumor necrosis factor-α, IL-6, glutathione, sciatic nerve heat shock protein-70 levels, electromyographic records, inclined-plane test, nerve growth factor expression levels, and axon diameters.
| Normal control | Cisplatin+saline | Cisplatin+500 mg/kg vitamin C | |
|---|---|---|---|
| MDA (nM) | 53.5±4.9 | 126.1±10.2** | 92.9±8.1## |
| TNF-α (pg/mL) | 19.4±1.1 | 81.5±4.3** | 45.8±7.06# |
| IL-6 (pg/mL) | 8.3±0.9 | 545.7±30.4** | 165.1±28.2## |
| GSH (μM) | 10.1±3.2 | 6.2±0.5* | 8.1±9.7# |
| Sciatic nerve HSP-70 (μg/mg protein) | 7.7±1.4 | 10.8±1.1* | 36.7±5.9## |
| CMAP latency (ms) | 2.18±0.04 | 2.79±0.08* | 2.81±0.03 |
| CMAP amplitude (mV) | 13.6 ± 0.3 | 4.1 ± 0.4* | 6.9±0.5# |
| Inclined plane score (°) | 92.4±1.8 | 72.6±2.5* | 90.1±3.1# |
| NGF expression (%) | 71.8±9.2 | 36.3±12.5** | 53.2±7.7## |
| Axon diameter, μm | 3.28±0.32 | 2.04±0.11* | 2.95±0.23# |
Results were presented as mean±standard error of the means. Statistical analyses were performed by one-way ANOVA test.
p<0.05,
p<0.001 (different from control group);
p<0.05,
p<0.001 (different from cisplatin and saline group).
MDA: malondialdehyde; TNF-α: tumor necrosis factor-α; GSH: glutathione; HSP-70: heat shock protein-70; CMAP: compound muscle action potential; NGF: nerve growth factor.
Figure 1. Compound muscle action potential recorded from: A) normal group, B) cisplatin+saline group, and C) cisplatin+vitamin C group.
Figure 2. NGF immunohistochemistry; A) normal group; axon (a), myelin sheath (arrow) (3,3′-diaminobenzidine, 40×); B) cisplatin+saline group, decreased axon diameter and NGF expression, degenerated myelin sheath (3,3′-diaminobenzidine, 40×); C) cisplatin+vitamin C group, increased axon diameter and NGF expression (3,3′-diaminobenzidine, 40×); and D) improved myelin sheath (3,3′-diaminobenzidine, 100×).
DISCUSSION
Cisplatin is one of the most effective drugs used in adult and pediatric cancer therapy. Cisplatin accumulates in the nucleus and forms adducts with nuclear DNA (nDNA), which leads to cytotoxicity in dividing cells 4 . Cisplatin also forms adducts with mitochondrial DNA (mtDNA), and after cisplatin therapy, intracellular reactive oxygen species (ROS) levels increase in normal cells 14 . In cisplatin-induced nephrotoxicity, ROS induce kidney damage by decreasing the antioxidant levels and intracellular GSH levels 15 . Excessive ROS production and deterioration of the oxidative/antioxidative balance are also important mechanisms in cisplatin-induced axonal injury. Several studies reported an association between cisplatin cytotoxicity and increased lipid peroxidation and decreased GSH levels. Vitamin C shows antioxidant effects by decreasing ROS and inhibiting lipid peroxidation 16 . Antioxidants such as resveratrol, curcumin, and vitamin E reduce toxicity by suppressing lipid peroxidation and anti-inflammatory effects 17–19 .
In our study, we measured plasma MDA levels as an indicator of oxidative stress. MDA formed as a result of polyunsaturated fatty acid peroxidation 20 . According to our results, plasma MDA levels increased due to cisplatin exposure, but vitamin C significantly reduced MDA probably by suppressing lipid peroxidation.
Akman et al. clearly demonstrated the protective effect of oxytocin (OT) in cisplatin-induced neurotoxicity 21 . The neuroprotective effect seems to be associated with antioxidant and anti-inflammatory (decrease in TNF-α levels) activities of OT. Vitamin C also decreased the levels of proinflammatory mediators. In our recent study, vitamin C decreased the levels of proinflammatory cytokines such as TNF-α and IL-6.
Heat shock proteins increased due to high temperature and severe stress. Albokhadaim et al reported that vitamin C shows antioxidant effects through HSPs in the liver and heart 22 . Silistre et al found the neuroprotective effects of ascorbic acid by increasing HSP-70 in rodent sepsis model 23 . In our study, sciatic nerve HSP-70 levels were statistically significantly higher in the cisplatin+vitamin C group than cisplatin +saline group (p<0.001). According to all of these findings, we may suggest that one of the neuroprotective mechanisms of vitamin C is an increase in HSP 70 levels.
The sensory neurons of the dorsal root ganglia (DRG) are the primary target of platinum-based chemotherapy. Mitochondrial dysfunction, oxidative stress, and DNA adducts lead to the apoptosis of DRG neurons and sensorial neuronopathy 23 . NGF is a member of the neurotrophin family. NGF is essential for the development and functional integrity of neurons 24 . NGF also acts as an antioxidant by inducing the expression of superoxide dismutase and catalase. NGF neutralizes the superoxide radicals and hydrogen peroxide 25 . Increased ROS levels induce the deficiency of nerve NGF levels. ROS production and related NGF decrease double the neurotoxic effects of cisplatin. NGF deficiency is also one of the major etiopathogenetic mechanisms of diabetic neuropathy. Obrosova et al. found a 1.5-fold decrease in the sciatic nerve NGF concentrations in diabetic rats 25 . In our study, neuronal NGF protein expression was statistically significantly lower in the cisplatin+saline group than control group (p<0.01). Also, the myelin sheath degenerated in the same group; 500 mg/kg of vitamin C ameliorated both axon diameter and NGF expressions in peripheral nervous tissues, including the sciatic nerve (p<0.05). Both oxidative stress and decreases in NGF concentration in the nerve were partially prevented by the antioxidant, i.e., vitamin C.
According to the efficacy of cisplatin in cancer treatment, peripheral neurotoxicity will continue to be one of the major dose-related side effects without any proven preventive treatment. Our experimental study demonstrated the ameliorated effects of vitamin C on cisplatin-induced neuropathy through increased HSP-70 and NGF levels and reduced inflammatory and oxidant effects. The results of this rat model study are promising to improve the adverse neurotoxic effects of cisplatin in cancer patients.
Footnotes
Funding: none.
REFERENCES
- 1.Rosenberg B. Fundamental studies with cisplatin. Cancer. 1985;55(10):2303–2316. doi: 10.1002/1097-0142(19850515)55:10. [DOI] [PubMed] [Google Scholar]
- 2.Howell SB, Safaei R, Larson CA, Sailor MJ. Copper transporters and the cellular pharmacology of the platinum-containing cancer drugs. Mol Pharmacol. 2010;77(6):887–894. doi: 10.1124/mol.109.063172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marullo R, Werner E, Degtyareva N, Moore B, Altavilla G, Ramalingam SS, et al. Cisplatin induces a mitochondrial-ROS response that contributes to cytotoxicity depending on mitochondrial redox status and bioenergetic functions. PLoS One. 2013;8(11):811–862. doi: 10.1371/journal.pone.0081162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sastry J, Kellie SJ. Severe neurotoxicity, ototoxicity and nephrotoxicity following high-dose cisplatin and amifostine. Pediatr Hematol Oncol. 2005;22(5):441–445. doi: 10.1080/08880010590964381. [DOI] [PubMed] [Google Scholar]
- 6.Mollman JE. Cisplatin neurotoxicity. N Engl J Med. 1990;322(2):126–127. doi: 10.1056/NEJM199001113220210. [DOI] [PubMed] [Google Scholar]
- 7.Staff NP, Cavaletti G, Islam B, Lustberg M, Psimaras D, Tamburin S. Platinum-induced peripheral neurotoxicity: from pathogenesis to treatment. J Peripher Nerv Syst. 2019;24:26–39. doi: 10.1111/jns.12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Padayatty SJ, Levine M. New insights into the physiology and pharmacology of vitamin C. CMAJ. 2001;164(3):353–355. [PMC free article] [PubMed] [Google Scholar]
- 9.Abdel-Daim MM, Abushouk AI, Donia T, Alarifi S, Alkahtani S, Aleya L, et al. The nephroprotective effects of allicin and ascorbic acid against cisplatin-induced toxicity in rats. Environ Sci Pollut Res. 2019;26:13502–13509. doi: 10.1007/s11356-019-04780-4. [DOI] [PubMed] [Google Scholar]
- 10.Howard PA, Meyers DG. Effect of vitamin C on plasma lipids. Annals of Pharmacotherapy. 1995;29(11):1129–1136. doi: 10.1177/106002809502901112. [DOI] [PubMed] [Google Scholar]
- 11.Carr AC, Maggini S. Vitamin C and immune function. Nutrients. 2017;9(11):1211–1211. doi: 10.3390/nu9111211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akdemir A, Zeybek B, Akman L, Ergenoglu AM, Yeniel AO, Erbas O, et al. Granulocyte-colony stimulating factor decreases the extent of ovarian damage caused by cisplatin in an experimental rat model. J Gynecol Oncol. 2014;25(4):328–333. doi: 10.3802/jgo.2014.25.4.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sunnetci Silistre E, Erbas O. The ameliorative effects of ascorbic acid on critical illness polyneuropathy in rodent sepsis model. J Pediatr Intensive Care. 2020;9(4):265–270. doi: 10.1055/s-0040-1710587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santos NAG, Catão CS, Martins NM, Curti C, Bianchi MLP, Santos AC. Cisplatin-induced nephrotoxicity is associated with oxidative stress, redox state unbalance, impairment of energetic metabolism and apoptosis in rat kidney mitochondria. Arch Toxicol. 2007;81:495–504. doi: 10.1007/s00204-006-0173-2. [DOI] [PubMed] [Google Scholar]
- 15.Chen MF, Yang CM, Su CM, Liao JW, Hu ML. Inhibitory effect of vitamin C in combination with vitamin K3 on tumor growth and metastasis of Lewis lung carcinoma xenografted in C57BL/6 mice. Nutr Cancer. 2011;63(7):1036–1043. doi: 10.1080/01635581.2011.597537. [DOI] [PubMed] [Google Scholar]
- 16.Apostolopoulou K, Konstantinou D, Alataki R, Papapostolou I, Zisimopoulos D, Kalaitzopoulou E, et al. Ischemia-Reperfusion Injury of Sciatic Nerve in Rats: Protective Role of Combination of Vitamin C with E and Tissue Plasminogen Activator. Neurochem Res. 2018;43(3):650–658. doi: 10.1007/s11064-017-2465-8. [DOI] [PubMed] [Google Scholar]
- 17.Valentovic MA, Ball JG, Brown JM, Terneus MV, McQuade E, Van Meter S, et al. Resveratrol attenuates cisplatin renal cortical cytotoxicity by modifying oxidative stress. Toxicol In Vitro. 2014;28(2):248–257. doi: 10.1016/j.tiv.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendonça LM, Silva Machado C, Teixeira CC, Freitas LA, Bianchi ML, Antunes LM. Curcumin reduces cisplatin-induced neurotoxicity in NGF-differentiated PC12 cells. Neurotoxicology. 2013;34:205–211. doi: 10.1016/j.neuro.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Pace A, Savarese A, Picardo M, Maresca V, Pacetti U, Del Monte G, et al. Neuroprotective effect of vitamin E supplementation in patients treated with cisplatin chemotherapy. J Clin Oncol. 2003;21(5):927–931. doi: 10.1200/JCO.2003.05.139. [DOI] [PubMed] [Google Scholar]
- 20.Davey MW, Stals E, Panis B, Keulemans J, Swennen RL. High-throughput determination of malondialdehyde in plant tissues. Anal Biochem. 2005;347(2):201–207. doi: 10.1016/j.ab.2005.09.041. [DOI] [PubMed] [Google Scholar]
- 21.Akman T, Akman L, Erbas O, Terek MC, Taskiran D, Ozsaran A. The preventive effect of oxytocin to cisplatin-induced neurotoxicity: an experimental rat model. Biomed Res Int. 2015;2015:167235–167235. doi: 10.1155/2015/167235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albokhadaim IF, Althnaian TA, El-Bahr SM. Gene expression of heat shock proteins/factors (HSP60, HSP70, HSP90, HSF-1, HSF-3) and antioxidant enzyme activities in heat stressed broilers treated with vitamin C. Pol J Vet Sci. 2019;22(3):565–572. doi: 10.24425/pjvs.2019.129965. [DOI] [PubMed] [Google Scholar]
- 23.Staff NP, Cavaletti G, Islam B, Lustberg M, Psimaras D, Tamburin S. Platinum-induced peripheral neurotoxicity: from pathogenesis to treatment. J Peripher Nerv Syst. 2019;24:S26–S39. doi: 10.1111/jns.12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aloe L, Bracci-Laudiero L, Bonini S, Manni L. The expanding role of nerve growth factor: from neurotrophic activity to immunologic diseases. Allergy. 1997;52(9):883–894. doi: 10.1111/j.1398-9995.1997.tb01247.x. [DOI] [PubMed] [Google Scholar]
- 25.Obrosova IG, Fathallah L, Stevens MJ. Taurine counteracts oxidative stress and nerve growth factor deficit in early experimental diabetic neuropathy. Exp Neurol. 2001;172(1):211–219. doi: 10.1006/exnr.2001.7789. [DOI] [PubMed] [Google Scholar]