Abstract
Recent in vitro studies have shown that interleukin 4 (IL-4) induces and gamma interferon (IFN-γ) inhibits collagen production. To define the TH1(IFN-γ) and TH2(IL-4) cytokine profiles in systemic sclerosis (Sscl), a disease characterized by widespread fibrosis, we investigated IL-4 and IFN-γ transcripts in peripheral blood mononuclear cells and plasma protein levels in 13 patients with Sscl. Two previously identified IL-4 transcripts, a full-length transcript and an alternatively spliced (truncated) transcript (designated IL-4δ2), were identified in patients and normal controls. Significantly increased levels of total IL-4 transcripts (full-length plus IL-4δ2 transcripts) were found in patients with Sscl in comparison to those found in healthy controls (P = 0.003), and this increase was primarily due to an increase in the level of the alternatively spliced IL-4δ2 form. The IL-4δ2/full-length-IL-4 transcript ratio was significantly increased in Sscl patients (P < 0.0001, versus healthy controls). Sequencing analysis revealed that the frequency of IL-4 clones carrying the IL-4δ2 transcript was also substantially increased in patients with Sscl. Plasma IL-4 protein levels were increased in Sscl patients compared to those in healthy controls (P = 0.001) and correlated with total IL-4 transcript levels. The up-regulation of the fibrogenic IL-4 (a TH2 cytokine) in Sscl suggests a pathogenic role for IL-4 in this disease.
The pathologic hallmark of systemic sclerosis (Sscl) (scleroderma) is widespread excessive fibrosis and endothelial cell injury, but the pathogenic mechanisms leading to these changes are not known (23). There is evidence of fibroblast, monocyte (19), endothelial cell (8), and eosinophil (14) activation in Sscl. Accumulating evidence suggests that T cells may also be involved in the disease process. There is a cellular infiltration of the skin, consisting of T cells, macrophages, and mast cells, early in the course of the disease (16, 31). T cells expressing activation antigens such as the interleukin 2 (IL-2) receptor (CD25), HLA-DR, and CD29 are increased in peripheral blood (11). Increased frequency of hypoxanthine guanine phosphoribosyl transferase gene-mutated T cells (36) and elevated adenosine deaminase activity (33) suggestive of T-cell activation were also found in the peripheral blood of patients with Sscl. T cells with the memory phenotype (CD45RO+) are found in lung biopsies from Sscl patients with lung involvement (42). T cells are also necessary for production of the anti-topoisomerase I autoantibodies (formerly Scl 70) (21) which are characteristic of Sscl. Finally, chronic graft-versus-host disease, which is a T-cell-mediated disease, exhibits clinical similarities with scleroderma and is associated with anti-topoisomerase I autoantibodies (6).
Cytokines produced by T cells are major determinants of the immune response. The two polar types of T-cell cytokines, TH1(IFN-γ) and TH2(IL-4, IL-5), are associated with the different outcomes of bacterial infections and autoimmune diseases in animal models (26). TH1 cytokines are associated with effective cell-mediated immune responses, whereas TH2 cytokines assist in antibody production (26). Recent in vitro studies have shown that IL-4 induces fibroblasts to produce collagen (10, 28, 34) and other extracellular matrix macromolecules (28, 41), whereas gamma interferon (IFN-γ) inhibits collagen production (18, 34). Two species of IL-4 transcript were reported, a full-length IL-4 with all four exons and a truncated one with exon 2 alternatively spliced out (IL-4δ2) (37). In this study, we analyzed IL-4 and IFN-γ cytokine protein and transcript levels in peripheral blood from patients with Sscl. We report the presence of increased levels of IL-4 transcripts (IL-4δ2) and IL-4 protein in Sscl patients. These results suggest that IL-4 may be involved in the pathogenesis of Sscl.
MATERIALS AND METHODS
Patients.
Thirteen consecutive patients with Sscl, attending the Rheumatology Clinic of Temple University Hospital, were included in the study. All patients were females (mean age ± standard deviation [SD], 50.3 ± 13.8 years). Five patients had diffuse Sscl and eight patients had limited disease as defined previously (23). In addition, 15 healthy controls (14 females and 1 male) (mean age ± SD, 41.1 ± 8 years) were used for comparison. The study was approved by the Institutional Review Board of Temple University Hospital, and all patients and healthy controls provided informed consent.
Peripheral blood.
Peripheral blood specimens (30 ml) were collected in heparinized tubes. Peripheral blood mononuclear cells (PBMC) were isolated by centrifugation on a Ficoll-Hypaque cushion. PBMC and plasma were kept at −80°C until used.
IL-4 and IFN-γ protein assays.
Plasma IL-4 and IFN-γ protein levels were measured by a sandwich enzyme-linked immunosorbent assay (ELISA) (Coulter/Immunotech, Westbrook, Maine). Briefly, plasma samples in duplicate were incubated for 2 h at room temperature in plate wells coated with either anti-IL-4 or -IFN-γ monoclonal antibodies (MAb), respectively. After being washed, the plates were incubated with biotinylated anti-IL-4 MAb or biotinylated anti-IFN-γ MAb and streptavidin-peroxidase conjugate for 30 min and then washed again. Finally, after incubation with a chromogenic substrate (tetramethylbenzidine), the reaction was stopped with 2 N sulfuric acid, and color intensity was measured at 450 nm with an ELISA reader (Molecular Devices).
RT-PCR.
Total RNA was prepared by using RNAzol B according to the supplier’s instructions (Tel-Test, Friedwood, Tex.). cDNA was synthesized from 3 μg of RNA with SuperScript II reverse transcriptase (RT) and with oligo(dT) as a primer (GIBCO/BRL) at 42°C for 50 min in a 20-μl reaction mixture. Reaction mixtures were then heated at 70°C for 15 min to inactivate RT and incubated with RNase H at 37°C for 20 min to remove RNA. Finally, cDNA was diluted 1:3 and kept at −30°C until used. IL-4, IFN-γ, and β-actin (housekeeping gene) cDNAs were amplified in a 480 Perkin-Elmer thermocycler with the following sequence-specific primers (2, 32): IL-4, 5′-CTTCCCCCTCTGTTCTTCCT and 3′-TTCCTGTCGAGCCGTTTCAG; IFN-γ, 5′-AGTTATATCTTGGCTTTTCA and 3′-ACCGAATAATTAGTCAGCTT; β-actin, 5′-GTGGGGCGCCCCAGGCACCA and 3′-CTCCTTAATGTCACGCACGATTTC. These primers span at least one intron so that a combination of the cDNA and any contaminating genomic DNA would result in a product of different size. In particular, IL-4 primers amplify part of exon 1, exon 2, and part of exon 3 of IL-4 mRNA (2). Amplification of cDNA (50 ng of RNA equivalents) was carried out in a reaction mixture containing 50 mM Tris HCl (pH 8.0), 100 mM NaCl, 0.1 mM EDTA, 1 mM dithiothreitol, 1.5 mM MgCl2 (1.0 mM for IL-2 and IFN-γ), 50% glycerol, 1% Triton X-100, and 2.5 U of Taq DNA polymerase (Promega, Madison, Wis.) for 35 cycles, with each cycle at 94°C for 45 s, 55°C (65°C for β-actin) for 45 s, and 72°C for 90 s, with a final extension of 7 min at 72°C. For IL-4 amplification, a two-phase PCR was carried out at 94°C for 45 s and 67°C for 2.5 min. PCR products were visualized on ethidium bromide-stained 1.8% agarose gels after electrophoresis.
Quantitation of IL-4 and IFN-γ transcripts.
Full-length IL-4 and IFN-γ transcripts were quantitated with a competitive PCR designated MIMIC PCR (Clontech, Palo Alto, Calif.). In this method, one set of primers amplifies both the target cDNA and a competitive DNA fragment (MIMIC) in the same tube. A series of PCRs were carried out with a constant amount of target cDNA and twofold dilutions of the corresponding MIMIC of a known molar concentration, and PCR products were analyzed by electrophoresis in ethidium bromide-stained gels, as described previously (32). IL-4 transcripts were also measured by densitometry of ethidium bromide-stained gels with a scanner and Sigmagel software. PCR products of β-actin MIMIC of a known molar concentration were electrophoresed along with IL-4 transcript products as internal controls.
IL-4 mRNA cloning and sequencing.
IL-4 PCR products were purified from the Tris-acetate-EDTA low-melting-point agarose gel with NaI (GeneClean kit; Bio 101, Vista, Calif.). PCR products were ligated into the pCR2.1 vector with overhanging single 3′ deoxythymidine residues, following the supplier’s instructions (Invitrogen, Carlsbad, Calif.). The pCR2.1 vector was used to transform one-shot INVαF cells (Invitrogen), which were subsequently grown overnight in agar plates containing X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside). White microbial colonies were cultured in Terrific broth, and miniplasmid preparations were made by the alkaline lysis method. Plasmids were purified with the Wizard DNA cleanup system (Promega), and plasmids bearing IL-4 inserts were identified by PCR and agarose gel electrophoresis. IL-4 inserts were subjected to ThermoSequenase dye terminator sequencing PCR (Amersham, Cleveland, Ohio), and the PCR fragments were purified with Centricep spin columns (Princeton Separations, Adelphia, N.J.) and sequenced with the automated 373A DNA sequencer (Applied Biosystems, Perkin-Elmer).
Statistical analysis.
Results were analyzed by the Student’s t test when data had a normal distribution and by the Mann-Whitney test when data did not have a normal distribution. Correlation between protein and transcript levels was assessed by the Spearman test with Prism software.
RESULTS
Plasma IL-4 and IFN-γ proteins.
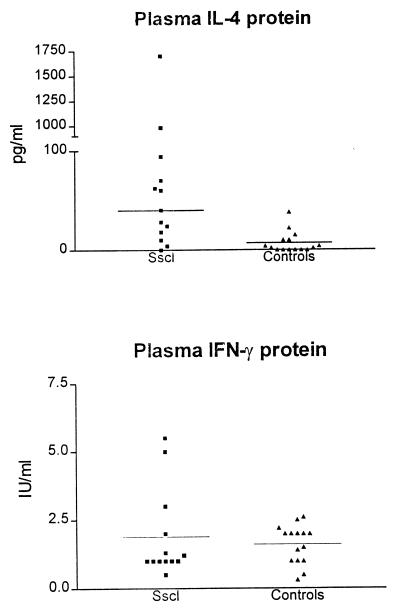
IL-4 protein levels were higher in patients with Sscl (median, 40 pg/ml; interquartile range, 18 to 62 pg/ml) than in healthy controls (7.1 ± 0.8 pg/ml [mean ± SD]) (Mann-Whitney test; two-tailed P = 0.001) (Fig. 1). Ten of 13 patients with Sscl had plasma IL-4 protein levels more than 2 SDs above the mean of those of healthy controls. Two patients had values of IL-4 protein (1,700 and 980 pg/ml) severalfold higher than the median (40 pg/ml). One of these two patients had rapidly progressing diffuse Sscl, and the other had experienced the recent onset of Sscl. There were not any significant differences in plasma IL-4 levels between patients with diffuse (n = 5) and limited (n = 8) Sscl (Student’s t test; P = 0.8). Plasma IFN-γ protein levels in patients with Sscl were not significantly different from those in healthy controls (Student’s t test; P = 0.5) (Fig. 1).
FIG. 1.
Plasma IL-4 and IFN-γ protein levels in patients with Sscl and healthy controls. Both cytokines were measured by sandwich ELISA. Horizontal lines indicate median values. IL-4 levels were higher in patients with Sscl than in healthy controls (Mann-Whitney test; two-tailed P = 0.001). P value for IFN-γ is 0.5.
IL-4 and IFN-γ transcripts.
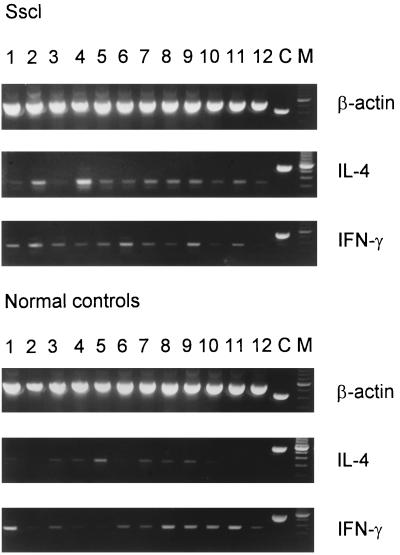
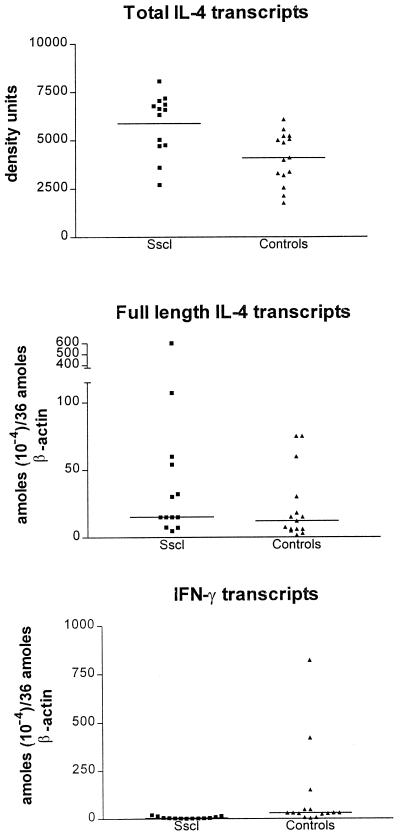
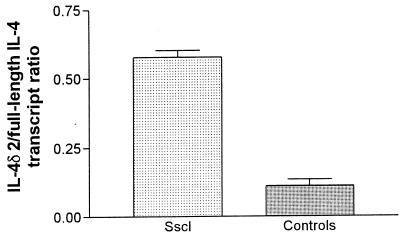
IL-4 and IFN-γ transcripts were detected in all patients and healthy donors. Representative IL-4 and IFN-γ results are shown in Fig. 2. Amplification of IL-4 transcripts resulted in two bands, one band of the expected size of 317 bp, which corresponds to the full-length IL-4 transcript, and a second band of 269 bp, which corresponds to the alternatively spliced IL-4 transcript, previously designated IL-4δ2 (37). Total IL-4 (full-length IL-4 plus IL-4δ2) transcript levels, measured by densitometry, were significantly higher in patients with Sscl than in healthy controls (Student’s t test; two-tailed P = 0.003) (Fig. 3). Levels of full-length IL-4 transcripts, measured by MIMIC PCR, were not significantly higher in Sscl than in healthy controls (nonparametric Mann-Whitney U test; one-tailed P = 0.07 and two-tailed P = 0.15) (Fig. 3). After logarithmic transformation to assume a normal distribution, differences in the levels of full-length IL-4 transcripts between the two groups were not significant (Student’s t test; two-tailed P = 0.11). Similarly, levels of full-length IL-4 transcripts, measured by densitometry, were not significantly higher in patients with Sscl than in healthy controls (data not shown). The IL4δ2/full-length-IL-4 transcript ratio, measured by densitometry, was significantly higher in patients with Sscl (mean, 0.577; range, 0.430 to 0.656) than in healthy controls (mean, 0.109; range, 0 to 0.209) (Mann-Whitney test; P < 0.001) (Fig. 4), suggesting that the IL-4 transcript increase was primarily due to an increase in the IL-4δ2 transcript level. The densitometry method was employed because the low levels of IL-4δ2 transcripts required large amounts of cDNA to give quantitative data by MIMIC PCR. IFN-γ transcript levels, measured by MIMIC PCR, were significantly lower in patients with Sscl than in healthy controls (Mann-Whitney test; two-tailed P = 0.0004) (Fig. 3). In both methods of quantitation (MIMIC PCR and densitometry), IL-4 and IFN-γ transcripts were normalized for β-actin as a measure of PBMC equivalents.
FIG. 2.
RT-PCR results for IL-4 and IFN-γ in PBMC from patients with Sscl and healthy controls. Representative transcripts are shown. C, corresponding MIMIC; M, 100-bp ladder.
FIG. 3.
PBMC IL-4 and IFN-γ transcript levels in patients with Sscl and healthy controls. Total IL-4 transcripts were semiquantitated by densitometry, as described in Materials and Methods. Full-length-IL-4 and IFN-γ transcripts were quantitated by MIMIC PCR. Horizontal lines indicate median values. Results were normalized for β-actin as a measure of PBMC equivalents. Total IL-4 transcript levels were higher in patients with Sscl than in healthy controls (Student’s t test; two-tailed P = 0.003). P values for full-length IL-4 and IFN-γ were 0.07 and 0.0004, respectively.
FIG. 4.
IL-4δ2/full-length-IL-4 transcript ratio in PBMC from patients with Sscl and healthy controls. The ratio was calculated by densitometry, as described in Materials and Methods, and it was significantly higher in patients with Sscl than in healthy controls (Mann-Whitney test; P < 0.0001). Error bars represent SDs.
Plasma IL-4 protein levels correlated with total IL-4 (full-length IL-4 plus IL-4δ2) transcript levels (Spearman correlation; r = 0.6; P = 0.01). Full-length IL-4 transcript levels alone did not correlate significantly with plasma IL-4 protein levels (Spearman correlation; r = 0.35; P = 0.06). There was also no correlation between IL-4δ2 transcript and plasma IL-4 protein levels.
Cloning and sequencing of IL-4 transcripts.
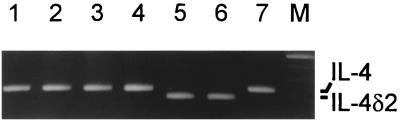
To accurately ascertain the proportions of full-length IL-4 and alternatively spliced IL-4δ2 transcripts in PBMC from patients with Sscl and healthy donors, we cloned the PCR-amplified transcripts into the pCR2.1 vector. Sequencing analysis of 49 IL-4 clones from one patient with Sscl and 47 IL-4 clones from a healthy donor revealed IL-4δ2 transcripts in 22.4 and 4.2% of the clones, respectively. Furthermore, IL-4 clones from an additional five patients and five normal donors were analyzed by PCR and gel electrophoresis to determine whether they contained full-length or IL-4δ2 transcripts. Representative results are shown in Fig. 5. The frequency of IL-4 clones carrying the truncated IL-4δ2 transcript was significantly higher in patients with Sscl (range, 19.4 to 58.1%) than in healthy donors (range, 0 to 15.4%) (Mann-Whitney U test; P = 0.004). IL-4δ2/total IL-4 transcript ratios for individual patients with Sscl were 8/27 (29.6%), 18/31 (58.1%), 7/36 (19.4%), 9/39 (23%), and 17/37 (45.9%). IL-4δ2/total IL-4 transcript ratios for individual healthy donors were 2/23 (8.7%), 1/24 (4.2%), 2/35 (5.7%), 4/26 (15.4%), and 0/37 (0%).
FIG. 5.
IL-4 clones with full-length IL-4 and spliced IL-4δ2 transcripts. Representative PCR products were visualized after gel electrophoresis. M, 100-bp ladder.
DISCUSSION
In this study we found increased levels of alternatively spliced (truncated) IL-4δ2 transcripts in PBMC from patients with Sscl. The levels of total IL-4 transcripts were increased, and this increase was mainly due to an increase in the level of the alternatively spliced IL-4δ2 form. Plasma IL-4 protein levels were also significantly elevated in patients with Sscl versus those in healthy donors, in agreement with the reports of others (15, 25). These findings are important because IL-4 is emerging as a major fibrogenic cytokine. First, it induces collagen production by fibroblasts in vitro (20, 28). Second, overexpression of IL-4 in transgenic mice under the control of the insulin promoter in pancreatic Langerhans cells results in local fibrosis (24). Third, administration of anti-IL-4 antibody prevents graft-versus-host disease in mice, with a concomitant decrease in liver fibrosis (39), and markedly reduces hepatic fibrosis in schistosoma-infected mice (7). Finally, IL-4 induces production of transforming growth factor β (38), which is a fibrogenic cytokine (40). In our study, 10 of 13 patients with Sscl exhibited increased levels of plasma IL-4 protein. This finding that IL-4 protein levels were not increased in all patients with Sscl may reflect the natural course of the disease. Sscl first has a fibrotic indurating skin phase, which may be associated with increased IL-4 levels, and later has an atrophic skin-softening phase (23). It would be useful to see in a large number of patients with Sscl if plasma IL-4 protein levels correlate with the duration, the extent, or the rate of progression of the disease.
There was no difference in plasma IFN-γ protein levels between patients with Sscl and controls. IFN-γ (a TH1 cytokine) is emerging as an antifibrotic cytokine. It inhibits collagen synthesis by human and mouse fibroblasts in vitro (18, 34). IFN-γ also inhibits fibrosis induced by ethylene copolymer (13) in mice and exhibits some beneficial effects in patients with Sscl (12, 27). Finally, IFN-γ inhibits transforming growth factor β production (40). Given the reciprocal effect of IL-4 (a TH2 cytokine) and IFN-γ (a TH1 cytokine) on immune response and on fibrosis (35), our findings suggest that IL-4 is a major pathogenic factor in Sscl.
The presence of increased levels of IL-4δ2 transcripts in Sscl is interesting, since the functional properties of this variant have not been fully investigated. Deletion of the 16-amino-acid-long exon 2 product, which occurs in IL-4δ2, is expected to affect one of the two postulated sites of binding of IL-4 to its receptor (20, 29). One group reported that IL-4δ2 protein inhibits IL-4-induced T-cell proliferation (4) but induces collagen production by fibroblasts (5). The increased levels of IL-4δ2 transcripts in Sscl may reflect the activation status of T cells in Sscl. However, activation of normal T cells with OKT3 MAb (1) or phorbol myristate acetate and ionomycin (our unpublished results) up-regulates both transcripts, although the full-length IL-4 is by far the predominant transcript. IL-4δ2 transcript levels were rarely found to be increased in healthy donors (1). In our study, total IL-4 transcript levels in PBMC were significantly higher in Sscl patients than in healthy donors and correlated with plasma protein levels. Within the limitations of the methodology used, this finding indicates that T cells are most likely the source of increased plasma IL-4 protein in Sscl. T cells have not received much attention as participants in the pathogenesis of Sscl thus far. However, there is evidence of T-cell activation in Sscl (11, 33, 36, 42). An oligoclonal expansion of T cells, as implied by the restricted T-cell receptor β-chain junctional region length distribution, was found in PBMC and bronchoalveolar lavage samples, from Sscl patients with lung disease (44), which suggests an antigen-driven immune process. T cells also provide help to B cells for autoantibody production in Sscl (21). Our study showing the up-regulation of IL-4 in Sscl, along with the fibrogenic effect of IL-4 on fibroblasts and the up-regulation of adhesion molecules by IL-4 on endothelial cells (17), is consistent with the concept that TH2 cells are involved in the pathogenesis of Sscl. In accordance with this concept, a recent study, based on the presence of fetal cells in women with Sscl, has proposed that Sscl is caused by fetal antimaternal graft-versus-host reactions (3). Based on our results and those of others, we hypothesize that Sscl is an antigen-driven, immune-mediated disease of type TH2. An inciting antigen induces IL-6 and probably other cytokines by antigen-presenting cells and promotes the development of TH2 cells (30). TH2 cells, in turn, attract and activate fibroblasts, activate endothelial cells (17), and induce B-cell autoantibody production (9).
The present study, along with others (25), may have significant clinical implications for Sscl, for which the currently available treatments are largely ineffective. Manipulation of cytokines to neutralize or decrease IL-4 production and boost IFN-γ production may be a new strategy for the treatment of Sscl. Anti-IL-4 antibody or soluble IL-4 receptors alone or in combination with IFN-γ (or IL-12) are attractive candidate agents in this respect (22, 26). In mice with schistosoma infection, administration of IL-12 or anti-IL-4 antibody markedly reduced tissue fibrosis and IL-4 production (7, 43). The validity of these assumptions needs to be tested in patients with early-stage Sscl and very high plasma protein IL-4 levels.
ACKNOWLEDGMENTS
We thank J. Gaughan (Department of Physiology [Biostatistics], Temple University School of Medicine) for help with the statistical analysis.
This work was supported by grants T32 AI07101 and PO1 AI40160 from the National Institutes of Health.
REFERENCES
- 1.Alms W J, Atamas S P, Yurovsky V V, White B. Generation of a variant of human interleukin-4 by alternative splicing. Mol Immunol. 1996;33:361–370. doi: 10.1016/0161-5890(95)00154-9. [DOI] [PubMed] [Google Scholar]
- 2.Arai N, Nomura D, Villaret D, DeWaal Malefijt R, Seiki M, Yoshida M, Minoshima S, Fukuyama R, Maekawa M, Kudoh J, Shimuzu N, Yokota K, Abe E, Yokota T, Takebe Y, Arai K. Complete nucleotide sequence of the chromosomal gene for human IL-4 and its expression. J Immunol. 1989;142:274–282. [PubMed] [Google Scholar]
- 3.Artlett C M, Smith J B, Jimenez S A. Identification of fetal DNA and cells in skin lesions from women with systemic sclerosis. N Engl J Med. 1998;338:1186–1191. doi: 10.1056/NEJM199804233381704. [DOI] [PubMed] [Google Scholar]
- 4.Atamas S P, Choi J, Yurovsky V V, White B. An alternative splice variant of human IL-4, IL-4δ2, inhibits IL-4-stimulated T cell proliferation. J Immunol. 1996;156:435–441. [PubMed] [Google Scholar]
- 5.Atamas S P, Yurovsky V V, Wigley F M, Wise R, Bleecker E, White B. Complete and alternative spliced mRNA for interleukin-4 in pulmonary lymphocytes: systemic sclerosis alveolitis versus asthma. Arthritis Rheum. 1997;40(Suppl.):S39. [Google Scholar]
- 6.Bell S A, Faust H, Mittermuller J, Kolb H-J, Meurer M. Specificity of antinuclear antibodies in scleroderma-like chronic graft-versus-host disease: clinical correlation and histocompatibility locus antigen association. Br J Dermatol. 1996;134:848–854. [PubMed] [Google Scholar]
- 7.Cheever A W, Williams M E, Wynn T A, Finkelman F D, Seder R A, Cox T M, Hieny S, Caspar P, Sher A. Anti-IL-4 treatment of schistosoma mansoni-infected mice inhibits development of T cells and non-B, non-T cells expressing Th2 cytokines while decreasing egg-induced hepatic fibrosis. J Immunol. 1994;153:753–759. [PubMed] [Google Scholar]
- 8.Claman H N, Giorno R C, Seibold J R. Endothelial and fibroblast activation in scleroderma. The myth of uninvolved skin. Arthritis Rheum. 1991;34:1495–1501. doi: 10.1002/art.1780341204. [DOI] [PubMed] [Google Scholar]
- 9.Erb K J, Ruger B, von Brevern M, Ryffel B, Schimpl A, Rivett K. Constitutive expression of interleukin (IL)-4 in vivo causes autoimmune-type disorders in mice. J Exp Med. 1997;185:329–339. doi: 10.1084/jem.185.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fertin C, Nicolas J F, Gillery P, Kalis B, Banchereau J, Maquart F X. Interleukin-4 stimulates collagen synthesis by normal and scleroderma fibroblasts in dermal equivalents. Cell Mol Biol. 1991;37:823–829. [PubMed] [Google Scholar]
- 11.Fiocco U, Rosada M, Cozzi L, Ortolani C, de Silvestro G, Ruffatti A, Cozzi E, Gallo C. Early phenotypic activation of circulating helper memory T cells in scleroderma: correlation with disease activity. Ann Rheum Dis. 1993;52:272–277. doi: 10.1136/ard.52.4.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freundlich B, Jimenez S A, Steen V, Medsger T A, Jr, Szkolnicki M, Jaffe H. Treatment of systemic sclerosis with recombinant interferon-γ. Arthritis Rheum. 1992;35:1134–1142. doi: 10.1002/art.1780351005. [DOI] [PubMed] [Google Scholar]
- 13.Granstein R D, Murphy G F, Margolis R J, Byrne M H, Amento E P. Gamma-interferon inhibits collagen synthesis in vivo in the mouse. J Clin Investig. 1987;79:1254–1258. doi: 10.1172/JCI112945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gustafsson R, Fredens K, Nettlbladt O, Hallgren R. Eosinophil activation in systemic sclerosis. Arthritis Rheum. 1991;34:414–422. doi: 10.1002/art.1780340406. [DOI] [PubMed] [Google Scholar]
- 15.Hasegawa M, Fujimoto M, Kikuchi K, Takehara K. Elevated serum levels of interleukin-4 (IL-4), IL-10, and IL-13 in patients with systemic sclerosis. J Rheumatol. 1997;24:328–332. [PubMed] [Google Scholar]
- 16.Hawkins R A, Claman H N, Clark R A F, Steigerwald J C. Increased dermal mast cell population in progressive systemic sclerosis: a link in chronic fibrosis? Ann Intern Med. 1985;102:182–186. doi: 10.7326/0003-4819-102-2-182. [DOI] [PubMed] [Google Scholar]
- 17.Iademarco M F, Barks J L, Dean D. Regulation of vascular cell adhesion molecule-1 expression by IL-4 and TNF-alpha in cultured endothelial cells. J Clin Investig. 1995;95:264–271. doi: 10.1172/JCI117650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jimenez S A, Freundlich B, Rosenbloom J. Selective inhibition of human diploid fibroblast collagen synthesis by interferons. J Clin Investig. 1984;74:1112–1116. doi: 10.1172/JCI111480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kantor T V, Friberg D, Medsger T A, Jr, Buckinham R B, Whiteside T L. Cytokine production and serum levels in systemic sclerosis. Clin Immunol Immunopathol. 1992;65:278–285. doi: 10.1016/0090-1229(92)90158-k. [DOI] [PubMed] [Google Scholar]
- 20.Kruse N B, Shen J, Arnold S, Tony H P, Muller T, Sebald W. Two distinct functional sites of human interleukin-4 are identified by variants impaired in either receptor binding or receptor activation. EMBO J. 1993;12:5121–5126. doi: 10.1002/j.1460-2075.1993.tb06207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuwana M, Medsger T A, Jr, Wright T M. T and B collaboration is essential for the autoantibody response to DNA topoisomerase I in systemic sclerosis. J Immunol. 1995;155:2703–2714. [PubMed] [Google Scholar]
- 22.Manetti J R, Parronchi P, Giudizi G, Piccinni M-P, Maggi E, Trinchieri G, Romagnani S. Natural killer cell stimulatory factor (interleukin-12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993;177:1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medsger T A., Jr . Systemic sclerosis (scleroderma), localized forms of scleroderma, and calcinosis. In: McCarty D J, Koopman W J, editors. Arthritis and allied conditions. Philadelphia, Pa: Lea & Febiger; 1993. pp. 1253–1292. [Google Scholar]
- 24.Mueller R, Krahl T, Sarvetnick N. Tissue-specific expression on interleukin-4 induces extracellular matrix accumulation and extravasation of B cells. Lab Investig. 1997;76:117–128. [PubMed] [Google Scholar]
- 25.Needleman B W, Wigley F M, Stair R W. Interleukin-1, interleukin-2, interleukin-4, interleukin-6, tumor necrosis factor α, and interferon-γ levels in sera from patients with scleroderma. Arthritis Rheum. 1992;35:67–72. doi: 10.1002/art.1780350111. [DOI] [PubMed] [Google Scholar]
- 26.Paul W E, Seder R A. Lymphocyte responses and cytokines. Cell. 1994;76:241–251. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 27.Polisson R P, Gilkeson G S, Pyun E H, Pisetsky D S, Smith E A, Simon L S. A multicenter trial of recombinant human interferon gamma in patients with systemic sclerosis: effects on cutaneous fibrosis and interleukin-2 receptor levels. J Rheumatol. 1996;23:654–658. [PubMed] [Google Scholar]
- 28.Postlethwaite A E, Holness M A, Katai H, Raghow R. Human fibroblasts synthesize elevated levels of extracellular matrix proteins in response to interleukin 4. J Clin Investig. 1992;90:1479–1485. doi: 10.1172/JCI116015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Powers R, Garrett D S, Marsh C J, Frieden E A, Gronenborn A M, Clore G M. Three-dimensional solution structure of human interleukin-4 by multidimensional heteronuclear magnetic resonance spectroscopy. Science. 1992;256:1673–1677. doi: 10.1126/science.256.5064.1673. [DOI] [PubMed] [Google Scholar]
- 30.Rincon M, Anguita J, Nakamura T, Fikrig E, Flavell R A. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J Exp Med. 1997;185:461–469. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roumm A D, Whiteside T L, Medsger T A, Jr, Rodman G P. Lymphocytes in the skin of patients with progressive systemic sclerosis. Arthritis Rheum. 1984;27:645–653. doi: 10.1002/art.1780270607. [DOI] [PubMed] [Google Scholar]
- 32.Sakkas L I, Scanzello C, Johanson N, Burkholder J, Mitra A, Salgame P, Katsetos C D, Platsoucas C D. T cells and T-cell cytokine transcripts in the synovial membrane in patients with osteoarthritis. Clin Diagn Lab Immunol. 1998;5:430–437. doi: 10.1128/cdli.5.4.430-437.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sasaki T, Nakajima H. Serum adenosine deaminase activity in systemic sclerosis (scleroderma) and related disorders. J Am Acad Dermatol. 1992;27:411–414. doi: 10.1016/0190-9622(92)70209-x. [DOI] [PubMed] [Google Scholar]
- 34.Sempowski G D, Derdak S, Phipps R P. Interleukin-4 and interferon-gamma discordantly regulate collagen biosynthesis by functionally distinct lung fibroblast subsets. J Cell Physiol. 1996;167:290–296. doi: 10.1002/(SICI)1097-4652(199605)167:2<290::AID-JCP13>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 35.Serpier H, Gillery P, Salmon-Ehr V, Garnotel R, Georges N, Kalis B, Maquart F X. Antagonistic effects of interferon-gamma and interleukin-4 on fibroblast cultures. J Investig Dermatol. 1997;109:158–162. doi: 10.1111/1523-1747.ep12319207. [DOI] [PubMed] [Google Scholar]
- 36.Sfikakis P P, Tesar J, Theocharis S, Klipple G L, Tsokos G C. Increased frequency of in vivo hprt gene-mutated T cells in the peripheral blood of patients with systemic sclerosis. Ann Rheum Dis. 1994;53:122–127. doi: 10.1136/ard.53.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sorg R V, Enczmann J, Sorg U R, Schneider E M, Wernet P. Identification of an alternatively spliced transcript of human interleukin-4 lacking the sequence encoded by exon 2. Exp Hematol. 1993;21:560–563. [PubMed] [Google Scholar]
- 38.Strober W, Kelsall B, Fuss I, Marth T, Ludviksson B, Ehrhardt R, Neurath M. Reciprocal IFN-gamma and TGF-beta responses regulate the occurrence of mucosal inflammation. Immunol Today. 1997;18:61–64. doi: 10.1016/s0167-5699(97)01000-1. [DOI] [PubMed] [Google Scholar]
- 39.Ushiyama C, Hirano T, Miyajima H, Okumura K, Ovary Z, Hashimoto H. Anti-IL-4 antibody prevents graft-versus-host disease in mice after bone marrow transplantation. The IgE allotype is an important marker of graft-versus-host disease. J Immunol. 1995;154:2687–2696. [PubMed] [Google Scholar]
- 40.Varga J, Jimenez J A. Modulation of collagen gene expression: its relation to fibrosis in systemic sclerosis and other disorders. Ann Intern Med. 1995;122:60–62. doi: 10.7326/0003-4819-122-1-199501010-00010. [DOI] [PubMed] [Google Scholar]
- 41.Wegrowski Y, Paltot V, Gillery P, Kalis B, Randoux A, Maquart F X. Stimulation of sulphated glycosaminoglycan and decorin production in adult dermal fibroblasts by recombinant human interleukin-4. Biochem J. 1995;307:673–678. doi: 10.1042/bj3070673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wells A U, Lorimer S, Majumdar S, Harrison N K, Corrin B, Black C M, Jeffery P K, du Bois R M. Fibrosing alveolitis in systemic sclerosis: increase in memory T-cells in lung interstitium. Eur Respir J. 1995;8:266–271. doi: 10.1183/09031936.95.08020266. [DOI] [PubMed] [Google Scholar]
- 43.Wynn T A, Cheever A W, Jankovic D, Poindexter R W, Caspar P, Lewis F A, Sher A. An IL-12-based vaccination method for preventing fibrosis induced by schistosome infection. Nature. 1995;376:594–596. doi: 10.1038/376594a0. [DOI] [PubMed] [Google Scholar]
- 44.Yurovsky V V. The repertoire of T cell receptors in systemic sclerosis. Crit Rev Immunol. 1995;15:155–165. doi: 10.1615/critrevimmunol.v15.i2.30. [DOI] [PubMed] [Google Scholar]