SUMMARY
OBJECTIVE:
Epicardial adipose tissue is a special form of visceral fat surrounding the heart. It is associated with cardiac and metabolic diseases. Epicardial adipose tissue is associated with risk factors for heart failure with preserved ejection fraction, such as obesity, metabolic syndrome, hypertension, and diabetes. In this study, we examined the importance of Epicardial adipose tissue as a predictor of heart failure with preserved ejection fraction.
METHODS:
Patients who were admitted to the Dicle University Medicine Faculty Heart Hospital between November 2013 and August 2014 were recruited for the study. The heart failure group consisted of 30 patients who were admitted to the cardiac intensive care unit, and the control group consisted of 30 patients who were admitted to cardiology polyclinics. We care about patients’ demographic and clinical features to be similar. Heart failure was diagnosed according to the European Cardiology Society 2012 heart failure guidelines. Epicardial adipose tissue was measured with a transthoracic parasternal long axis with an echocardiography device (GE Vivid S6). We compared the Epicardial adipose tissue measurements between the two groups.
RESULTS:
Epicardial adipose tissue was higher in patients with heart failure with preserved ejection fraction than in the control group (9.21±0.82 and 7.13±1.39 mm, respectively; p<0.001). Echocardiographic findings associated with left ventricular hypertrophy were intact ventricular septum (13.03±0.57 and 12.11±2.22 mm, respectively; p=0.013) and left ventricular mass index (131.13±18.00 and 117.90±20.30 g/m2, respectively; p=0.010). Findings associated with left ventricular diastolic dysfunction were as follows: left atrial volume index (60.71±21.53 and 44.92±9.93 mL/m2, respectively; p<0.001) and E/è (13.87±3.88 and 10.12±2.44, respectively; p<0.001) were higher in patients with heart failure with preserved ejection fraction than in the control group. Body mass index was not a significant indicator of obesity (p=0.097), but waist circumference was a significant indicator of visceral obesity (p<0.001). Logistic regression analyses indicated that Epicardial adipose tissue, age, left atrial volume index, left ventricular mass index, waist circumference, and E/é were significant in the Heart failure group; Epicardial adipose tissue was significant (p=0.012), and waist circumference significance was borderline (p=0.045).
CONCLUSIONS:
Epicardial adipose tissue was higher in patients with HF than in the control group, and Epicardial adipose tissue was a predictor of heart failure with preserved ejection fraction. In patients with heart failure with preserved ejection fraction, increased Epicardial adipose tissue means that Epicardial adipose tissue can be used as a biomarker of inflammation in the pathophysiology of heart failure with preserved ejection fraction.
KEYWORDS: Adipose tissue, Diastolic heart failure, Comorbidities, Obesity, Inflammation
INTRODUCTION
Heart failure is a common disease. It is seen in approximately 1–2% of the adult population in developed countries, and this rate rises to 10% in individuals aged 70 and over. Heart failure with preserved ejection fraction (HFpEF) constitutes approximately half of these cases 1,2 . HFpEF is associated with significant morbidity and mortality, and, so far, no treatment has been clearly demonstrated to improve the outcomes of HFpEF (in contrast to the efficacy of treatment for heart failure with reduced ejection fraction [HFrEF]) 3 . In recent years, many studies have been performed with the goal of reducing the morbidity and mortality associated with HFpEF. The EMPEROR-Preserved trial 4 is a popular study on this subject. In that study, empagliflozin reduced the combined risk of cardiovascular death and hospitalization for heart failure in patients with HFpEF, regardless of the presence or absence of diabetes. In addition, another study evaluated the efficacy of spironolactone treatment for HFpEF. Treatment with spironolactone did not significantly reduce the incidence of the primary composite outcome of death from cardiovascular causes, aborted cardiac arrest, and hospitalization in patients with HFpEF 5 .
Epicardial adipose tissue (EAT) is a special form of visceral adipose tissue stored around the heart. EAT has been shown to be associated with cardiac diseases and metabolic diseases 6 . The relationships between EAT and obesity, metabolic syndrome, diabetes, hypertension, coronary artery disease, and atherosclerosis have been the subjects of many studies 6–8 .
As EAT is associated with obesity, metabolic syndrome, diabetes, and hypertension, which constitute risk factors for HFpEF, this study was conducted to investigate the importance of EAT in the early diagnosis and treatment of HFpEF.
METHODS
Study population
The study included a total of 60 patients who required outpatient follow-up due to mild symptoms and signs of heart failure or who required coronary intensive care unit hospitalization due to severe symptoms and signs of heart failure between November 2013 and August 2014. Patients who declined to participate or whose information could not be obtained were excluded from the study. Patients with severe valvular disease, HFrEF, chronic lung diseases, anemia, chronic liver disease, chronic renal failure, malignancy, a history of severe trauma before a month, or a history of surgery were also excluded.
The patients included in the study were examined according to the 2012 heart failure guidelines of the European Society of Cardiology (9). Routine tests, such as hemogram and biochemistry, as well as N-terminal pro-brain natriuretic peptide (NT-proBNP), electrocardiography (ECG), and echocardiography tests, were performed to diagnose HFpEF. Approval for this study was obtained from the University Ethics Committee (approval date: 05/08/2014; number: 313). Information about the study was provided both orally and in written form to the patients or their trustees. All patients were informed about the study in accordance with the ethical principles of human research, as reported in the second Helsinki Declaration, and their written informed consent was obtained.
Definitions and laboratory parameters
The following demographic and clinical data were recorded for all patients: age; gender; body mass index (BMI) (kg/m2); waist circumference (cm); smoking status; dietary approaches to stop hypertension; known hypertension treated with antihypertensive drugs, or two times blood pressure measurement above 140/90 mmHg; diabetes mellitus (known diabetes treated with diet or medication, or fasting serum glucose level above 126 mg/dL); heart valve diseases (natural or prosthetic valve diseases, including mild-moderate stenosis and/or regurgitation); history of ischemic heart disease (history of myocardial infarction, percutaneous coronary intervention, coronary artery bypass surgery, or diagnosis of coronary artery disease by invasive or noninvasive tests); atrial fibrillation (AF); and history of previous ischemic stroke or transient ischemic attack.
Blood samples were taken from all patients, and the following analyses were performed: hemogram; WBC (white blood cell), HGB (hemoglobin), HCT (hematocrit), PLT (platelet), biochemical parameters; fasting blood sugar, urea, creatinine, ALT (alanine aminotransferase), AST (aspartate aminotransferase), and lipid profile; total cholesterol, LDL (low-density lipoprotein), HDL (high-density lipoprotein), triglyceride, and NT-proBNP were studied. In addition, GFR (glomerular filtration rate) was calculated with the Cockroft-Gault formula.
Electrocardiography and echocardiography
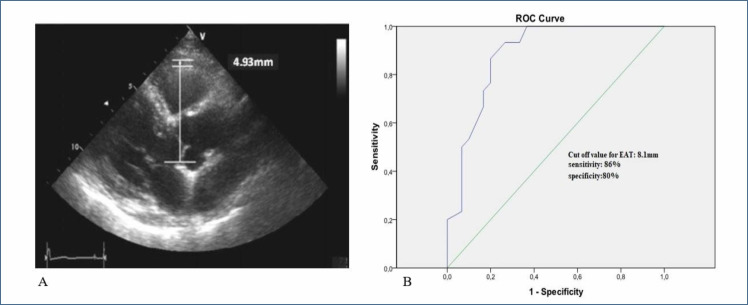
The 12-lead ECG recordings were taken with a 12-channel ECG device at a speed of 25 mm/s and a calibration of 10 mm/mV. According to the European Society of Echocardiography 9 , transthoracic echocardiographic evaluation was performed using a standard two-dimensional and M-mode recording echocardiography device (Vivid S6 General Electric Vingmed ultrasound Horten, Norway) using a 2.5–3.25 MHz transducer. Left ventricular ejection fraction (LVEF>50%), left ventricular mass index (LVMI over 95 g/m2 in women, 115 g/m2 in men), left ventricular end-diastolic volume index (LVEDVI>97 mL/m2), left atrial volume index (LAVI>34 mL/m2), diastolic dysfunction grades (stages 1, 2, 3, and 4), diastolic parameters (E, A, Dt, and E/é), and tricuspid annular systolic excursion (TAPSE) as an indicator of right ventricular systolic function, systolic pulmonary arterial pressure and interventricular septum (IVS), left ventricular systolic and diastolic diameters, left atrium diameter, and right ventricle and right atrium diameter were evaluated. EAT was measured above the free wall of the right ventricle from the parasternal long axis view in the left lateral position by echocardiography (Figure 1A).
Figure 1. (A) Measurement of epicardial adipose tissue from the anterior wall of the right ventricle in the parasternal long axis by transthoracic echocardiography. (B) Receiver operating characteristic of Epicardial adipose tissue to determine sensitivity and specificity.
Statistical analysis
Data were analyzed using the SPSS for Windows version 25.0 (Armonk, NY: IBM Corp.). The Kolmogorov-Smirnov test was used to confirm the normality of the distribution of continuous variables. Continuous variables were defined as mean±standard deviation or median (interquartile range); categorical variables were given as percentages. Parametric continuous variables were compared using the Student's t-test, and nonparametric continuous variables were compared using the Mann-Whitney U test. Chi-square test and Fisher's exact test were used to compare categorical variables. Univariate and multivariate logistic regression was performed to examine the association between EAT and HFpEF after adjusting for all confounders. The variables resulting from the univariate analysis with a p-value <0.05 were entered as covariates in the multivariate regression model. The level of significance was interpreted using the “p”-value. A p-value of <0.05 was considered significant.
RESULTS
A total of 60 patients were included in our study; 30 of them were HFpEF patients and 30 were in the control group. The mean age of the individuals was 71.60±6.70 years in the heart failure group and 63.70±9.02 years in the control group (p<0.001). In all, 54 (90%) of the participants were female, and 6 (10%) were male. Clinical characteristics, medications, and demographic data of both groups are shown in Table 1. The age, waist circumference, New York Heart Association (NYHA) class, AF incidence, use of diuretics, beta blockers, and statins were significantly higher in the HFpEF group compared to the control group (Table 1). The antiaggregants, anticoagulants, digoxin, and spironolactone were not compared between groups due to not being applicable in the control group (Table 1).
Table 1. Demographic, clinical characteristics, laboratory findings, and echocardiography parameters of patients.
| Demographic and clinical characteristics | Heart failure N=30 | Control N=30 | p-value | ||
|---|---|---|---|---|---|
| Age mean (year) | 71.60±6.70 | 63.70±9.02 | <0.001 | ||
| Sex | Female (%) | 26 (87) | 28 (93) | 0.335 | |
| Male (%) | 4 (13) | 2 (7) | |||
| BMI (kg/m2) | 32.79±4.72 | 30.86±4.01 | 0.097 | ||
| Waist circumference (cm) | 103.33±8.95 | 93.06±10.2 | <0.001 | ||
| Smoking (%) | 3 (10) | 4 (13) | 0.5 | ||
| Diet (%) | 8 (27) | 14 (47) | 0.090 | ||
| NYHA | Class 1 (%) | 0 | 27 (90) | <0.001 | |
| Class 2 (%) | 2 (6) | 3 (10) | |||
| Class 3 (%) | 14 (47) | 0 | |||
| Class 4 (%) | 14 (47) | 0 | |||
| Ischemic heart disease (%) | 18 (60) | 8 (27) | 0.009 | ||
| Hypertension (%) | 28 (93) | 30 (100) | 0.246 | ||
| Diabetes mellitus (%) | 13 (43) | 10 (33) | 0.298 | ||
| Stroke (%) | 1 (3) | 4 (13) | 0.161 | ||
| Atrial fibrillation (%) | 10 (33) | 3 (10) | 0.028 | ||
| Diuretics | 27 (90) | 16 (53) | 0.002 | ||
| ACE/ARB | 25 (83) | 20 (67) | 0.136 | ||
| Beta Blockers | 22 (73) | 12 (40) | 0.009 | ||
| CCB | 9 (30) | 5 (17) | 0.222 | ||
| Statins | 11 (37) | 4 (13) | 0.037 | ||
| Antiaggregants | 21 (70) | 10 (33) | 0.004 | ||
| Anticoagulants | 6 (20) | 0 | N/A | ||
| Digoxin | 5 (17) | 0 | N/A | ||
| Spironolactone | 4 (13) | 0 | N/A | ||
| Laboratory findings of patients | |||||
| White blood cell count (×103 μl) | 8.11±2.49 | 10.95±3.053 | 0.362 | ||
| Hemoglobin (g/dL) | 11.21±1.16 | 12.81±0.95 | <0.001 | ||
| Platelets (×103 μL) | 248±82.91 | 268±101.06 | 0.420 | ||
| NT-proBNP (pg/dL) | 542.26±361.33 | 74.93±19.35 | <0.001 | ||
| GFR (ml/min/1.73 m2) | 61.12±24.68 | 76.30±22.32 | 0.015 | ||
| Creatine (mg/dL) | 1.20±0.55 | 0.91±0.43 | 0.028 | ||
| ALT (U/L) | 24.13±30.22 | 17.56±5.85 | 0.247 | ||
| AST (U/L) | 24.43±11.57 | 22.90±8.86 | 0.567 | ||
| Total cholesterol (mg/dL) | 177.10±57.56 | 198.26±44.02 | 0.115 | ||
| LDL (mg/dL) | 132.37±105.52 | 116.03±38.21 | 0.429 | ||
| HDL (mg/dL) | 43.83±40.76 | 45.43±29.37 | 0.862 | ||
| Triglyceride (mg/dL) | 159.80±90.48 | 167.50±68.31 | 0.711 | ||
| Echocardiography parameters of patients | |||||
| LVEF (%) | 58.73±4.11 | 60.96±3.70 | 0.031 | ||
| EAT (mm) | 9.21±0.82 | 7.13±1.39 | <0.001 | ||
| LA (mm) | 40.30±10.92 | 35.57±6.50 | 0.047 | ||
| IVS (mm) | 13.03±0.57 | 12.11±2.22 | 0.013 | ||
| LVMI (g/m2) | 131.13±18.00 | 117.90±20.30 | 0.010 | ||
| LVEDVI (mL/m2) | 44.39±10.42 | 40.20±6.24 | 0.064 | ||
| LAVI (mL/m2) | 60.71±21.53 | 44.92±9.93 | <0.001 | ||
| E/é | 13.87±3.88 | 10.12±2.44 | <0.001 | ||
| Diastolic dysfunction Stage 1 (%) | 11 (58) | 19 (70) | 0.531 | ||
| Stage 2 (%) | 8 (42) | 8 (30) | |||
| TAPSE (cm) | 2.71±3.30 | 2.74±0.37 | 0.961 | ||
| SPAP (mmHg) | 32.66±6.67 | 26.20±3.75 | <0.001 | ||
Data are expressed as mean±standard deviation (SD) or frequencies (percentages) as appropriate. BMI: body mass index; NYHA: New York Heart Association; ACE/ARB: angiotensin converting enzyme inhibitors/angiotensin receptor blockers; CCB: calcium channel blockers; NT-proBNP: n-terminal pro-brain natriuretic peptide; GFR: glomerular filtration rate; ALT: alanine aminotransferase; AST: aspartate aminotransferase; LDL: low-density lipoprotein; HDL: high-density lipoprotein; LVEF: left ventricular ejection fraction; EAT: epicardial adipose tissue; LA: left atrium; IVS: interventricular septum; LVMI: left ventricular mass index (LVMI over 95 g/m2 in women, 115 g/m2 in men); LVEDVI: left ventricular end-diastolic volume index; LAVI: left atrial volume index (LAVI>34 mL/m2); TAPSE: tricuspid annular systolic excursion; SPAP: systolic pulmonary arterial pressure. The statistically significant p-values has shown with bold characters.
The echocardiographic findings of the patients are shown in Table 1. Left atrial diameter, IVS thickness, LVMI, LAVI, E/é, and systolic pulmonary arterial pressure (SPAP) were found to be statistically significantly higher in the heart failure group than in the control group, but the ejection fraction was found to be lower (Table 1).
Laboratory parameters such as HGB, NT-proBNP, GFR, and creatinine were statistically different between the two groups. There was no difference in other parameters. While NT-proBNP was found to be higher in the HF group, HGB, creatine, and GFR were found to be lower (Table 1).
In the multivariate regression analysis, EAT was an independent predictor of HFpEF (OR 3.890; 95%CI 1.345–11.252; p=0.012), together with waist circumference (OR 1.117; 95%CI 1.002–1.245; p=0.045, Table 2). At a cutoff value of 8.1 mm, the EAT thickness predicted HFpEF with a sensitivity of 86% and a specificity of 80% (Figure 1B).
Table 2. Univariate and multivariable logistic regression analysis to determine independent predictors of Heart failure with preserved ejection fraction.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| OR | 95%CI | p-value | OR | 95%CI | p-value | |
| Age | 1.127 | 1.048–1.213 | 0.001 | 1.082 | 0.970–1.207 | 0.157 |
| EAT | 4.523 | 2.148–9.523 | <0.001 | 3.890 | 1.345–11.252 | 0.012 |
| LVMI | 1.038 | 1.007–1.070 | 0.016 | 0.993 | 0.944–1.046 | 0.799 |
| Waist circumference | 1.118 | 1.047–1.195 | 0.001 | 1.117 | 1.002–1.245 | 0.045 |
| LAVI | 1.089 | 1.030–1.151 | 0.003 | 1.033 | 0.943–1.131 | 0.488 |
| E/é | 1.450 | 1.180–1.782 | <0.001 | 1.132 | 0.895–1.431 | 0.302 |
EAT: epicardial adipose tissue; LVMI: left ventricular mass index; LAVI: left atrial volume index; OR: odds ratio; CI: confidence interval. The statistically significant p-values has shown with bold characters.
DISCUSSION
In this study, we examined the relationship between EAT and HFpEF. There are two main findings in this study. First, EAT was significantly higher in the HFpEF group. Second, parameters leading to HFpEF by causing left ventricular concentric remodeling – IVS and LVMI – were found to be increased in the HF group, and parameters associated with diastolic dysfunction – LA diameter, LAVI, E/é, and AF – were also found to be increased in the HF group. Furthermore, EAT was an independent predictor of HFpEF together with waist circumference.
Heart failure is a common disease and is seen in approximately 1–2% of the adult population in developed countries, and this rate increases up to 10% in individuals aged 70 and over. HFpEF constitutes approximately half of these patients 1 . In studies conducted so far, no treatment has been proved to reduce mortality due to HFpEF. Therefore, it is more important to predict HFpEF.
Structural and functional changes associated with LV concentric remodeling and LV diastolic dysfunction are the basic pathophysiological mechanisms in HFpEF 10,11 . Comorbid diseases and especially obesity produce a systemic pro-inflammatory state. Inflammation causes coronary microvascular endothelial cells to produce free oxygen radicals and reduces the bioavailability of nitric oxide (NO) in cardiomyocytes. Protein kinase G (PKG) activity in cardiomyocytes is reduced due to damaged NO bioavailability. Decreased PKG activity causes hypophosphorylation of the giant cytoskeletal protein titin and triggers LV concentric remodeling and cardiomyocyte stiffness. Cardiomyocyte stiffness and collagen deposition by myofibroblasts cause diastolic dysfunction, which is the main functional impairment in HFpEF. Ather et al. 12 found the prevalence of comorbid diseases that cause systemic inflammatory status to be higher in the HFrEF group than in the HFpEF group.
In visceral obesity, macrophages infiltrate the adipose tissue, release pro-inflammatory cytokines, and cause a systemic inflammatory state 13 . Jelic et al. 14 found that obstructive sleep apnea disease and obesity cause endothelial dysfunction, inflammation, and oxidative stress. Similarly, the relationship between obesity and HFpEF was investigated in the I-PRESERVE study, and more clinical adverse outcomes were found in patients with a high BMI 15 . Kalogeropoulos et al. 16 investigated the relationship between heart failure and inflammation in elderly patients and concluded that systemic inflammatory conditions caused by comorbid diseases may be a predictor of HFpEF.
The fact that obesity causes a systemic inflammatory condition and there is a reciprocal relationship between obesity and HFpEF makes obesity an important issue to be investigated in patients with HFpEF. Obesity is also part of metabolic syndrome. BMI is used as an objective indicator of obesity. Metabolic syndrome, which is seen as a risk factor for cardiovascular diseases, is associated with visceral obesity. Abdominal fat is a part of visceral obesity and is determined by measuring waist circumference. Therefore, waist circumference is one of the diagnostic criteria for metabolic syndrome, and increased waist circumference is a risk factor for cardiovascular diseases. EAT forms another part of visceral obesity and can be measured using various imaging methods.
In studies on EAT, Corradi et al. 17 found a relationship between the amount of epicardial fat and ventricular mass. Iacobellis et al. 18 found a positive correlation between the amount of epicardial fat and ventricular myocardial mass. These two studies also support that obesity causes a systemic inflammatory state and causes LV concentric remodeling as a result of a series of pathophysiological mechanisms 19 . Mohammed et al. 20 found that comorbid diseases cause more myocardial structure and dysfunction than arterial hypertension.
Marchington et al. 21,22 could not find a relationship between EAT and the total amount of adipose tissue in other fat stores of the body in various animals. This finding is in line with echocardiographic, magnetic resonance imaging, and autopsy findings in humans, suggesting that the amount of epicardial fat is related to the amount of visceral fat rather than the amount of total fat 17,22–26 .
Considering that BMI is used as an obesity indicator and waist circumference is used for visceral obesity, in our study, BMI did not cause a significant difference between the two groups, and the waist circumference was significantly increased in the HF group, which was consistent with the literature 22,23 .
As with any study, certain design limitations are inherent. First, this was a single-center study and had a relatively small sample size. Second, heart function was assessed only by using transthoracic echocardiography. Other imaging methods such as cardiac computed tomography, 3D echocardiography, and heart catheterization that can quantitatively show EAT, left atrial functions, and filling pressures were not used. Finally, the study consisted predominantly of female population, so further studies with an equally distributed gender population are needed.
CONCLUSIONS
We found increased EAT in the heart failure group and determined EAT as a predictor for HFpEF. The EAT could be used as an easy and practical inflammatory indicator to identify HFpEF patients. Further multi-center and larger-scale investigations are warranted for clinical assessment of HFpEF.
Footnotes
Funding: none.
REFERENCES
- 1.Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur J Heart Fail. 2008;10(10):933–989. doi: 10.1016/j.ejheart.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93(9):1137–1146. doi: 10.1136/hrt.2003.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ilieșiu AM, Hodorogea AS. Treatment of Heart Failure with Preserved Ejection Fraction. Adv Exp Med Biol. 2018;1067:67–87. doi: 10.1007/5584_2018_149. [DOI] [PubMed] [Google Scholar]
- 4.Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med. 2021;385(16):1451–1461. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 5.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383–1392. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 6.Le Jemtel TH, Samson R, Ayinapudi K, Singh T, Oparil S. Epicardial adipose tissue and cardiovascular disease. Curr Hypertens Rep. 2019;21(5):36–36. doi: 10.1007/s11906-019-0939-6. [DOI] [PubMed] [Google Scholar]
- 7.Iacobellis G, Sharma AM. Epicardial adipose tissue as new cardio-metabolic risk marker and potential therapeutic target in the metabolic syndrome. Curr Pharm Des. 2007;13(21):2180–2184. doi: 10.2174/138161207781039670. [DOI] [PubMed] [Google Scholar]
- 8.Patel VB, Shah S, Verma S, Oudit GY. Epicardial adipose tissue as a metabolic transducer: role in heart failure and coronary artery disease. Heart Fail Rev. 2017;22(6):889–902. doi: 10.1007/s10741-017-9644-1. [DOI] [PubMed] [Google Scholar]
- 9.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33(14):1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 10.Zile MR, Gottdiener JS, Hetzel SJ, McMurray JJ, Komajda M, McKelvie R, et al. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation. 2011;124(23):2491–2501. doi: 10.1161/CIRCULATIONAHA.110.011031. [DOI] [PubMed] [Google Scholar]
- 11.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure––abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350(19):1953–1959. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 12.Ather S, Chan W, Bozkurt B, Aguilar D, Ramasubbu K, Zachariah AA, et al. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol. 2012;59(11):998–1005. doi: 10.1016/j.jacc.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taube A, Schlich R, Sell H, Eckardt K, Eckel J. Inflammation and metabolic dysfunction: links to cardiovascular diseases. Am J Physiol Heart Circ Physiol. 2012;302(11):H2148–H2165. doi: 10.1152/ajpheart.00907.2011. [DOI] [PubMed] [Google Scholar]
- 14.Jelic S, Lederer DJ, Adams T, Padeletti M, Colombo PC, Factor PH, et al. Vascular inflammation in obesity and sleep apnea. Circulation. 2010;121(8):1014–1021. doi: 10.1161/CIRCULATIONAHA.109.900357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haass M, Kitzman DW, Anand IS, Miller A, Zile MR, Massie BM, et al. Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: results from the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Circ Heart Fail. 2011;4(3):324–331. doi: 10.1161/CIRCHEARTFAILURE.110.959890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalogeropoulos A, Georgiopoulou V, Psaty BM, Rodondi N, Smith AL, Harrison DG, et al. Inflammatory markers and incident heart failure risk in older adults: the, health ABC (Health, Aging, and Body Composition) study. J Am Coll Cardiol. 2010;55(19):2129–2137. doi: 10.1016/j.jacc.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corradi D, Maestri R, Callegari S, Pastori P, Goldoni M, Luong TV, et al. The ventricular epicardial fat is related to the myocardial mass in normal, ischemic and hypertrophic hearts. Cardiovasc Pathol. 2004;13(6):313–316. doi: 10.1016/j.carpath.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Iacobellis G, Ribaudo MC, Zappaterreno A, Iannucci CV, Leonetti F. Relation between epicardial adipose tissue and left ventricular mass. Am J Cardiol. 2004;94(8):1084–1087. doi: 10.1016/j.amjcard.2004.06.075. [DOI] [PubMed] [Google Scholar]
- 19.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62(4):263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 20.Mohammed SF, Borlaug BA, Roger VL, Mirzoyev SA, Rodeheffer RJ, Chirinos JA, et al. Comorbidity and ventricular and vascular structure and function in heart failure with preserved ejection fraction: a community-based study. Circ Heart Fail. 2012;5(6):710–719. doi: 10.1161/CIRCHEARTFAILURE.112.968594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marchington JM, Pond CM. Site-specific properties of pericardial and epicardial adipose tissue: the effects of insulin and high-fat feeding on lipogenesis and the incorporation of fatty acids in vitro. Int J Obes. 1990;14(12):1013–1022. [PubMed] [Google Scholar]
- 22.Iacobellis G, Assael F, Ribaudo MC, Zappaterreno A, Alessi G, Di Mario U, et al. Epicardial fat from echocardiography: a new method for visceral adipose tissue prediction. Obes Res. 2003;11(2):304–310. doi: 10.1038/oby.2003.45. [DOI] [PubMed] [Google Scholar]
- 23.Iacobellis G, Ribaudo MC, Assael F, Vecci E, Tiberti C, Zappaterreno A, et al. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J Clin Endocrinol Metab. 2003;88(11):5163–5168. doi: 10.1210/jc.2003-030698. [DOI] [PubMed] [Google Scholar]
- 24.Iacobellis G, Leonetti F, Di Mario U. Images in cardiology: massive epicardial adipose tissue indicating severe visceral obesity. Clin Cardiol. 2003;26(5):237–237. doi: 10.1002/clc.4960260508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sironi AM, Gastaldelli A, Mari A, Ciociaro D, Positano V, Buzzigoli E, et al. Visceral fat in hypertension: influence on insulin resistance and beta-cell function. Hypertension. 2004;44(2):127–133. doi: 10.1161/01.HYP.0000137982.10191.0a. [DOI] [PubMed] [Google Scholar]
- 26.Olivetti G, Giordano G, Corradi D, Melissari M, Lagrasta C, Gambert SR, et al. Gender differences and aging: effects on the human heart. J Am Coll Cardiol. 1995;26(4):1068–1079. doi: 10.1016/0735-1097(95)00282-8. [DOI] [PubMed] [Google Scholar]