Abstract
Objectives
Laryngotracheal reconstruction (LTR) is a complex operation used to treat subglottic stenosis. The use of simulator models is a valuable tool in surgical trainee education, particularly for operations such as LTR that are less common outside high‐volume centers. Three‐dimensional (3D) printing of the human airway may provide an effective and more accessible alternative to porcine cadaveric models. The objective of this study is to compare the educational value of a 3D‐printed model and a porcine cadaveric model as LTR simulation methods.
Methods
Simulated LTR procedures were completed by 12 otolaryngology residents and a faculty physician on the cadaveric model and the 3D‐printed simulator model. Both models were evaluated by fellowship‐trained pediatric otolaryngologists to establish construct validity. Pre‐procedure surveys of participants evaluated confidence and attitude toward models and post‐procedure surveys evaluated confidence, overall impressions, relevance, content validity, and face validity.
Results
Participants reported a similar mean increase in confidence after performing LTR on the 3D‐printed model (14%) and cadaveric model (11%). Participants rated both models similarly for utility as an overall training tool and in teaching surgical planning and improving operative techniques. However, participants found the 3D‐printed model more useful for teaching anatomy (p = .047).
Conclusion
3D‐printed models have practical benefits over cadaveric models; they do not decompose and can be custom made to model a disease state such as subglottic stenosis. Participants reported a similar mean increase in confidence after using either simulation. The 3D‐printed model is a promising simulation candidate as it compares well to an animal model and has the advantage of being more anatomically true to pediatric patients.
Level of Evidence: Level 2.
Keywords: airway reconstruction, airway stenosis, pediatric airway, resident education, surgical simulation
Three‐dimensional (3D) printing of the human airway may provide an effective and more accessible alternative to cadaveric models. This study compares the educational value of a 3D‐printed model and a porcine cadaveric model as LTR simulation methods. Results showed that both models can be effective in resident education and the practical benefits of 3D‐printed models are significant.
1. INTRODUCTION
Laryngotracheal reconstruction (LTR) is a complex operation used to treat subglottic and tracheal stenosis. The most common etiology of pediatric airway stenosis is prolonged endotracheal intubation. Airway management options for subglottic and tracheal stenosis were historically limited to tracheostomy until the development of open laryngeal approaches in the 1900s. The term “laryngotracheal reconstruction” was first used in 1953 by John Conley. 1 LTR is a complex surgical operation that, in the modern era, is performed mainly by fellowship trained pediatric otolaryngologists at dedicated children's hospitals.
The use of simulator models is a valuable tool in resident trainee education, particularly in highly technical surgical specialties such as otolaryngology. 2 , 3 As discussed, LTR is a highly complex operation that is relatively uncommonly performed at locations outside high‐volume LTR hospitals. To close the gap in education between higher volume and lower volume centers, simulation may be a useful tool. Currently, validated and commercially available animal models for LTR simulation training include suckling‐pig and lamb. 4 , 5 The rabbit model has been well studied, but not validated as a simulation tool. 4 A systematic review of available animal models in 2019 concluded that while a rabbit airway is an appropriate size for a neonate or younger child's airway, differences in laryngeal anatomy made LTR simulation difficult. Despite the larger size, sheep, pig, and goat larynges had more similar anatomy to human laryngotracheal complexes. 5
Three‐dimensional (3D) printing of the human airway has been developed to provide an effective and more accessible alternative to animal or cadaveric airway models. 6 The aim of this study is to directly compare a more traditional animal model to a 3D‐printed model for the simulation of laryngotracheal reconstruction.
2. MATERIALS AND METHODS
This prospective cohort study was deemed exempt by the Indiana University Institutional Review Board. All participants provided informed consent.
2.1. Participants
Simulated LTR procedures were completed by 12 otolaryngology residents and a faculty physician on the porcine cadaveric model and the 3D‐printed simulator model (n = 13). Resident trainees ranged from PGY 1 to PGY 5 levels, with 2 or more residents at each level of training. The faculty participant completed each model, while resident participants were randomized and matched by PGY level to complete either the 3D‐printed model (n = 7) or the porcine cadaveric model (n = 7).
2.2. Simulation and surveys
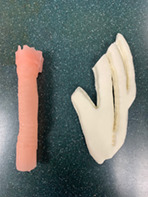
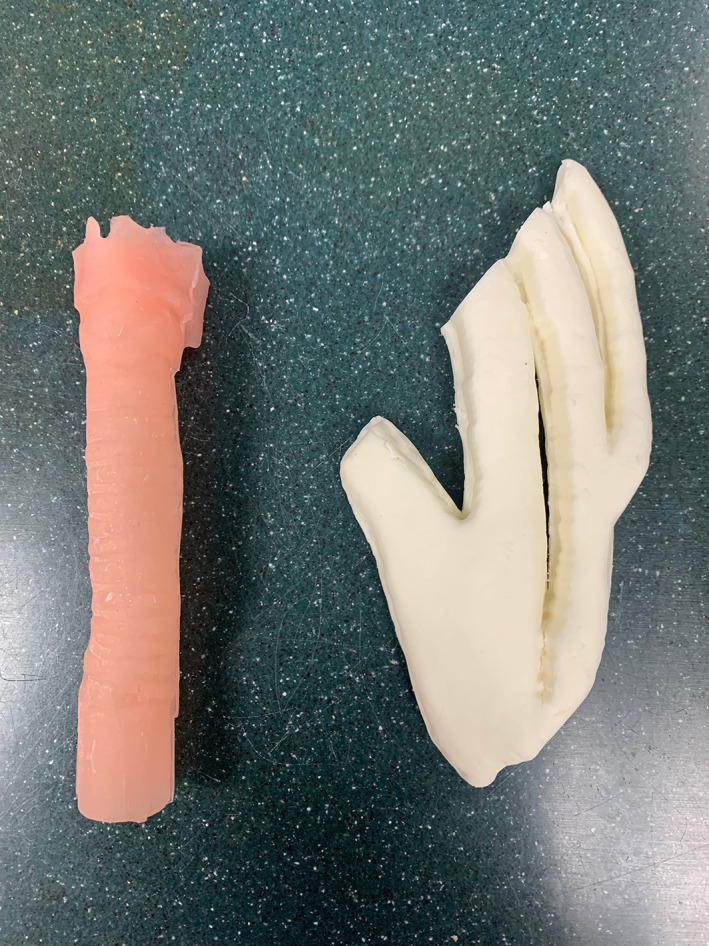
The 3D printed model in this study consisted of two parts, a pediatric sized laryngotracheal complex and a pediatric sized rib cartilage (Figure 1). Each airway model had grade III subglottic stenosis (Figure 2). Model manufacturing has been previously described. 6
FIGURE 1.
3D‐printed model of trachea and rib cartilage
FIGURE 2.
Porcine trachea and rib cartilage
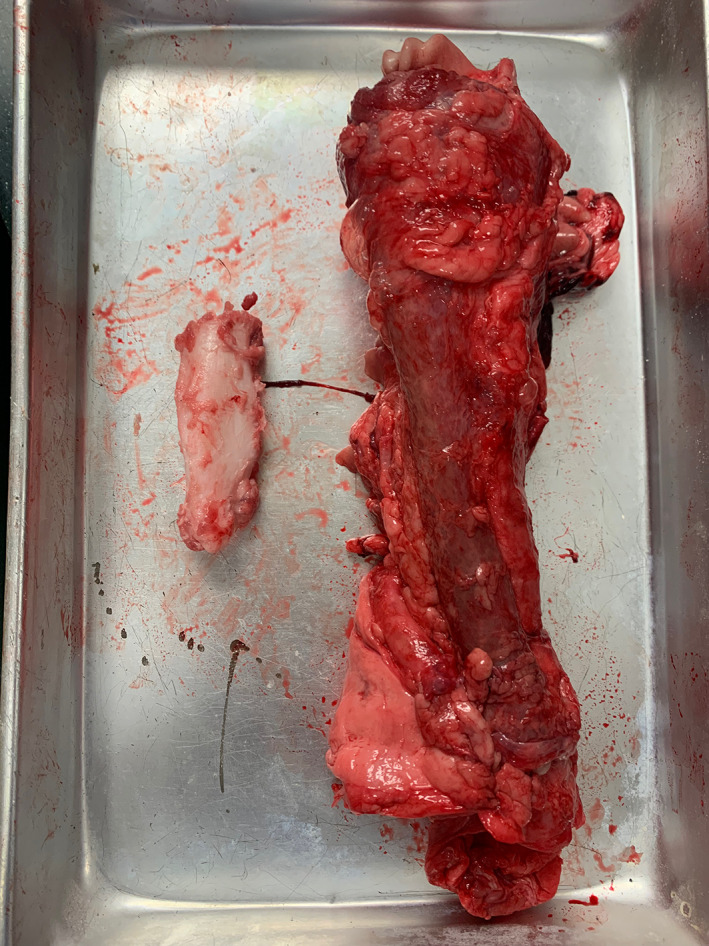
A porcine cadaveric model was used in this study, which consisted of a pig larynx and trachea and cartilaginous rib (Figure 3). The porcine models did not have simulated subglottic stenosis.
FIGURE 3.
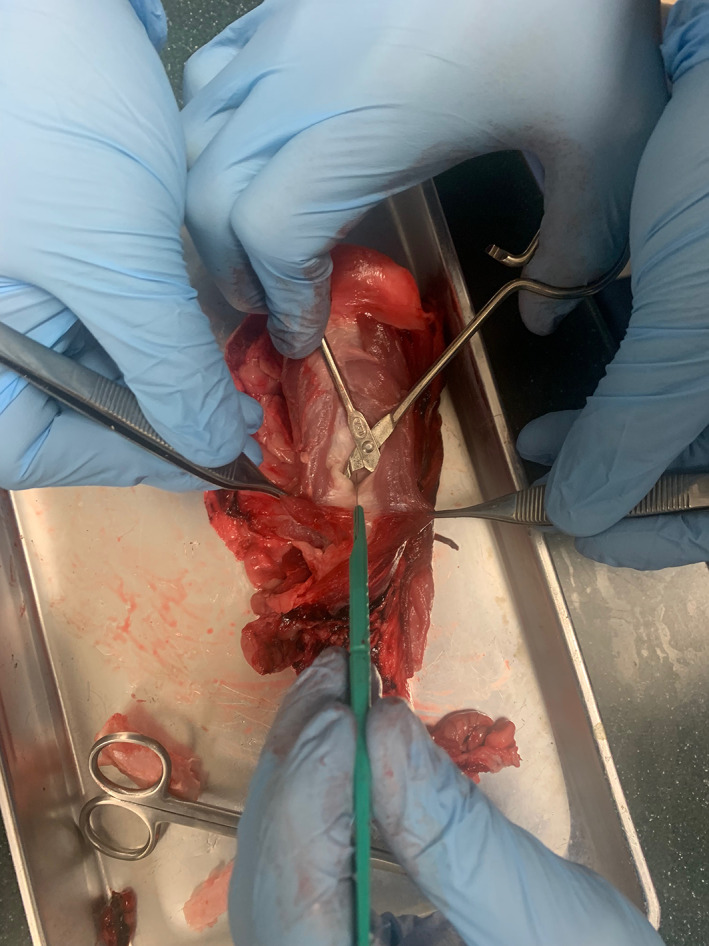
Simulation of anterior approach to airway reconstruction on porcine model
Prior to beginning the simulation, participants were instructed to watch a 10‐min instructional video from the University of Michigan with specific steps of performing LTR. 6 Participants were instructed to perform anterior airway grafting only (Figures 4 and 5).
FIGURE 4.
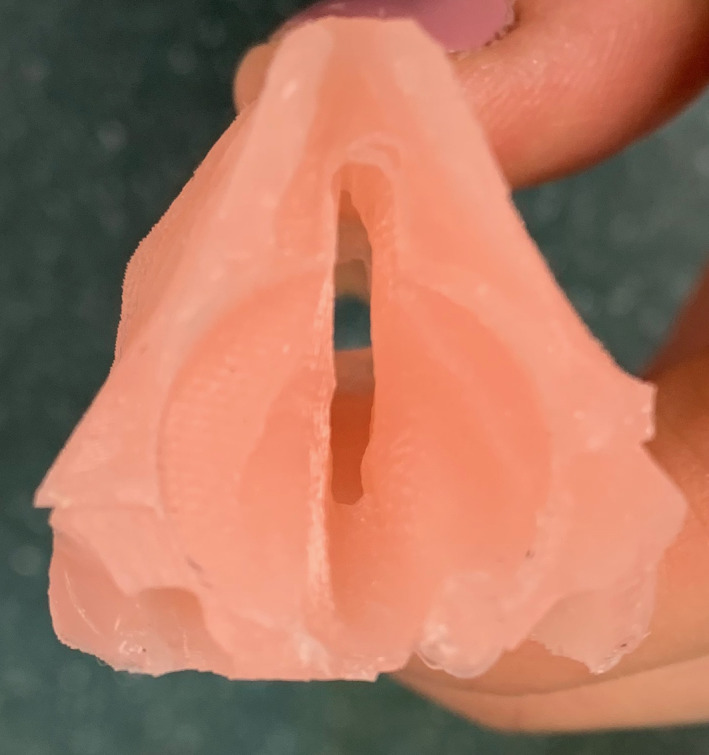
Cross‐sectional view of 3D‐printed model demonstrating subglottic stenosis
FIGURE 5.
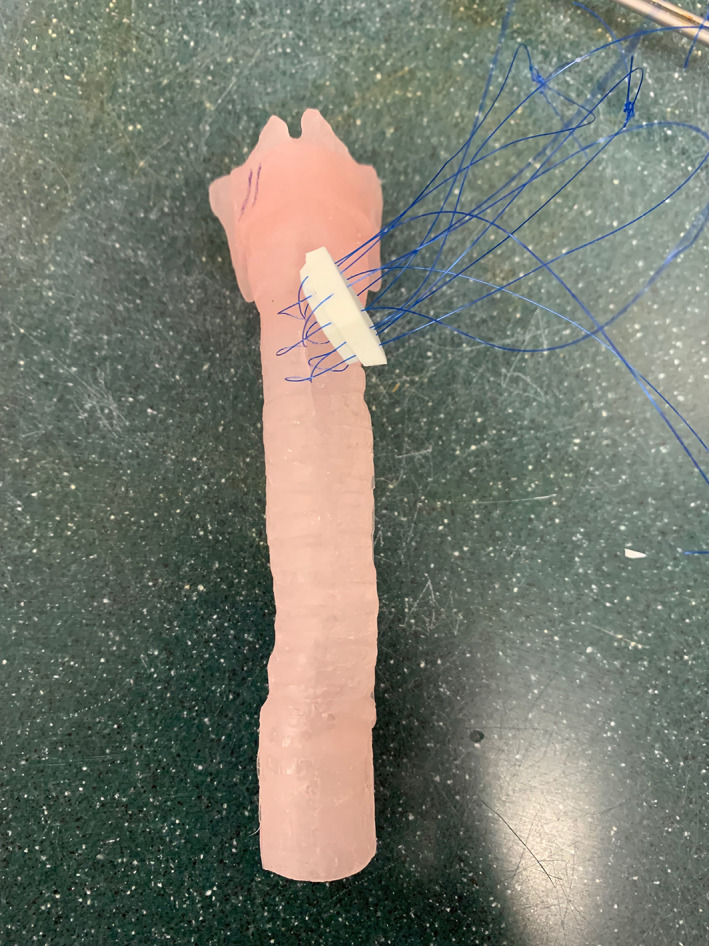
Simulation of anterior approach to airway reconstruction on 3D‐printed model
Each participant completed a pre‐procedure and post‐procedure survey. Pre‐procedure surveys of participants evaluated experience with LTR, perceived expertise in performing LTR, confidence performing LTR and attitudes toward simulators.
Post‐procedure surveys evaluated perceived expertise in performing LTR, confidence performing LTR, value of the simulator, face validity, global content validity, task specific content validity, relevance of the model to practice. Participants were also given an option to report on the pros and cons of the model they used.
2.3. Face and content validity
All participants evaluated face and content validity in their post‐procedure surveys. Face validity measures the realism of the simulation scenario. 7 Face validity was evaluated using a modified six‐item Likert scale questionnaire.
Content validity measures whether the learning objectives of the simulation were achieved. 7 Global content validity was evaluated using a five‐item Likert questionnaire. Task specific content validity was evaluated with a six‐item Likert questionnaire. 2 , 7
2.4. Construct and concurrent validity
Construct validity measures how effectively a test measures what it intends to measure. 8 Concurrent validity measures how well two tests agree, meaning it may be used to compare a new evaluation tool to the “gold standard” tool. 8
In this study, construct validity was measured by comparing senior resident performance to junior resident performance on the simulation. De‐identified models were evaluated by two fellowship‐trained pediatric otolaryngologists for construct and concurrent validity. The following criteria were rated on an integer scale from 1 to 5: overall rating, anterior airway incision, fashioning anterior graft, integration of graft into airway, and patency of airway. Interrater reliability was calculated using percent agreement and kappa statistic.
All statistical analysis, with exception of 2‐way ANOVA, was performed in Excel. Two‐way ANOVA was performed with an online calculator.
3. RESULTS
Simulated LTR procedures were completed by 12 otolaryngology residents and a faculty physician on the porcine cadaveric model and the 3D‐printed simulator model (n = 13). Resident trainees ranged from PGY 1 to PGY 5 levels, with 2 or more residents at each level of training. Six otolaryngology residents and the faculty physician completed the 3D‐printed model (n = 7) and porcine cadaveric model (n = 7). Of the resident participants, 66.7% (8/12) reported participating in 0 LTRs in the last year, while 33.3% (4/12) reported participating in 1–5 LTRs in the last year. Participants reported prior education about LTR through a variety of teaching methods: lectures, readings, online modules, videos, small group discussion procedural observation, surgical participation in cases. No participants had previously participated in simulation or animal dissection for LTR.
All participants rated their pre‐simulation confidence levels in performing LTR, means of 25% in the porcine cadaveric model and 20.83% in the 3D printed, without significant difference (Table 1). On an integer scale of 1–5, participants also rated their surgical expertise in performing an LTR overall, making the anterior airway incision, fashioning the anterior graft, and integrating the anterior graft into the airway. No significant difference between groups was found on their surgical expertise prior to simulation (Table 2).
TABLE 1.
Ratings of confidence by resident participants before and after using both simulator models
| Please rate your confidence in performing an LTR before/after using the simulator (n = 12) | ||||||||
|---|---|---|---|---|---|---|---|---|
| None (0%) | Limited, and I would need a lot of coaching (25%) | Some, but I would need more practice (50%) | Moderate, I could perform this surgery with some oversight (75%) | Confident, I can perform the surgery independently (100%) | Mean | 95% confidence Interval (CI) | p‐value | |
| Porcine model (PRE, n = 6) | 1 | 4 | 1 | 0 | 0 | 25% | 11.8%–38.2% | .73 |
| Porcine model (POST, n = 6) | 0 | 3 | 3 | 0 | 0 | 37.5% | 26.1%–48.94% | |
| 3D‐printed model (PRE, n = 6) | 3 | 1 | 2 | 0 | 0 | 20.8% | 0.28%–41.4% | 1 |
| 3D‐printed model (POST, n = 6) | 0 | 3 | 3 | 0 | 0 | 37.5% | 26.1%–48.94% | |
TABLE 2.
Ratings of surgical expertise (on a Likert scale 1–5) by resident participants in performing an LTR before and after using the simulator
| Porcine model (PRE, n = 6) | 3D‐printed model (PRE, n = 6) | Porcine model (POST, n = 6) | 3D‐printed model (POST, n = 6) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | p‐value | Mean | 95% CI | Mean | 95% CI | p‐value | |
| Overall expertise | 1.67 | 1.23–2.10 | 1.5 | 0.80–2.20 | .69 | 2.42 | 1.53–2.80 | 2 | 1.47–2.53 | .69 |
| Anterior airway incision expertise | 1.67 | 1.23–2.10 | 1.67 | 0.98–2.35 | 1 | 2.57 | 1.90–2.77 | 2 | 1.47–2.53 | .34 |
| Fashioning anterior graft expertise | 1.5 | 1.04–1.96 | 1.33 | 0.90–1.76 | .60 | 2.71 | 1.80–3.20 | 2 | 1.47–2.53 | .27 |
| Integration of graft into airway expertise | 1.5 | 1.04–1.96 | 1.67 | 0.98–2.35 | .69 | 2.42 | 1.54–2.80 | 2.17 | 1.53–2.80 | 1 |
After performing LTR simulation, participants were asked to again rate their confidence and surgical expertise by the same criteria (Table 2). Confidence in performing LTR increased to 37.5% in both the porcine and 3D‐printed model groups. The increase in confidence was not statistically different between groups (Table 1). Participants were also asked to rate the relevance of the model to their practice. There was no significant difference between the groups (Table 3).
TABLE 3.
Please rate the relevance of this simulator to your practice
| No relevance (1) | Slightly relevant (2) | Moderately relevant (3) | Relevant (4) | Very relevant (5) | Mean | 95% CI | p‐value | |
|---|---|---|---|---|---|---|---|---|
| Porcine model | 0 | 1 | 2 | 4 | 0 | 3.42 | 2.77–4.09 | .09 |
| 3D‐printed model | 0 | 0 | 1 | 4 | 2 | 4.14 | 3.57–4.72 |
Note: p‐value calculated using unpaired two tailed t‐test.
3.1. Face validity
Face validity was assessed by all participants (n = 13) and results are displayed in Table 4. Face validity is a subjective type of validity, and generally used in the early stages of development. 8 No significant difference was seen in realism of suturing, depth perception or instrument application between groups. Anatomical appearance, graft cartilage, airway cartilage realism all favored the porcine model (p = .031, .0005, and .0005, respectively).
TABLE 4.
Face validity (rated on a Likert scale 1–5) by all participants
| Mean rating (95% CI) | ≥Agree, % | p‐value for mean ratings, unpaired two‐tailed t‐test | |
|---|---|---|---|
| Appearance of anatomical structures is realistic | |||
| Porcine model (n = 7) | 4.286 (3.87–4.69) | 100 | .031 |
| 3D‐model (n = 7) | 3.429 (2.77–4.09) | 57.1 | |
| The cartilage graft tissue feels realistic | |||
| Porcine model |
4.571 (4.12–5.02) |
100 | .0005 |
| 3D‐model | 2.571 (1.76–4.09) | 14.3 | |
| The airway cartilage feels realistic | |||
| Porcine model |
4.429 (3.98–4.88) |
100 | .0005 |
| 3D‐model | 2.429 (1.61–3.24) | 14.3 | |
| Suturing feels realistic | |||
| Porcine model | 4.143 (3.25–5.04) | 85.7 | .403 |
| 3D‐model | 3.714 (3.08–4.35) | 57.1 | |
| Depth perception is realistic | |||
| Porcine model | 4.286 (3.65–4.92) | 85.7 | .290 |
| 3D‐model | 3.857 (3.28–4.43) | 71.4 | |
| Instrument application is realistic | |||
| Porcine model | 4.142 (3.57–4.72) | 85.7 | .354 |
| 3D‐model | 3.714 (2.92–4.51) | 71.4 | |
Bolded values were statistically significant.
3.2. Content validity
Both global and task specific content validity were evaluated by all participants (n = 13, Table 5). No significant difference was seen between groups with the exception of usefulness if teaching anatomy, which favored the 3D printed model (p = .047).
TABLE 5.
Content validity rated by all participants
| Mean rating (95% CI) | ≥Agree, % | p‐value for mean ratings, unpaired two‐tailed t‐test | |
|---|---|---|---|
| This model is useful for teaching anatomy | |||
| Porcine model (n = 7) | 3.286 (2.49–4.09) | 25 | .047 |
| 3D‐model (n = 7) | 4.143 (3.83–4.86) | 75 | |
| This model is useful for teaching surgical planning | |||
| Porcine model | 4.143 (3.83–4.46) | 87.5 | .271 |
| 3D‐model | 4.429 (3.98–4.88) | 75 | |
| This model is useful for improving operative techniques | |||
| Porcine model | 4.429 (3.98–4.88) | 87.5 | .611 |
| 3D‐model | 4.286 (3.88–4.69) | 75 | |
| This model is useful for improving hand eye coordination | |||
| Porcine model | 4.286 (3.65–4.92) | 75 | .690 |
| 3D‐model | 4.429 (3.98–4.88) | 75 | |
| This model is useful as an overall training tool | |||
| Porcine model | 4.286 (3.88–4.69) | 87.5 | 1.000 |
| 3D‐model | 4.286 (3.88–4.69) | 75 | |
| This model helps to develop skills needed for LTR | |||
| Porcine model | 4.286 (3.88–4.69) | 87.5 | 1.000 |
| 3D‐model | 4.286 (3.88–4.69) | 75 | |
| This model helps develop dexterity, accuracy, and precision with instruments | |||
| Porcine model | 4.000 (3.32–4.68) | 62.5 | .268 |
| 3D‐model | 4.429 (3.98–4.88) | 75 | |
| This model helps to develop fundamentals of anterior airway incision | |||
| Porcine model | 4.143 (3.83–4.68) | 87.5 | .730 |
| 3D‐model | 4.000 (3.16–4.84) | 62.5 | |
| This model helps to develop fundamentals of anterior graft fashioning | |||
| Porcine model | 4.571 (4.12–5.02) | 87.5 | .218 |
| 3D‐model | 4.143 (3.57–4.72) | 75 | |
| This model helps to develop fundamentals of anterior graft integration into airway | |||
| Porcine model | 4.286 (3.88–4.69) | 87.5 | .290 |
| 3D‐model | 3.857 (3.10–4.61) | 75 | |
| Use of this model will increase resident competency when used to train residents prior to their first LTR | |||
| Porcine model | 4.571 (4.12–5.01) | 87.5 | .317 |
| 3D‐model | 4.290 (3.88–4.69) | 75 | |
Bolded values were statistically significant.
3.3. Construct validity
Each model was evaluated by two independent faculty raters on an integer scale of 1–5 in the categories of overall LTR, anterior airway incision, fashioning anterior graft, integration of graft into airway, and patency of airway. Interrater reliability was measured by percent agreement and kappa statistic.
For the porcine cadaver model, percent agreement on average was 82.1%. Notably, airway incision had the lowest interrater agreement of 57.1% and all other categories was greater than 80%. Kappa statistic was also calculated with average value of 0.777, again with the lowest value being for the airway incision category at 0.464. For the 3D‐printed model, percent agreement on average was 45.7%. All categories had an interrater agreement of <60%. Kappa statistic was also calculated with average value of 0.321, with no values above 0.500.
Two‐way ANOVA was performed to analyze the effect of training year (junior resident vs. Senior resident vs. attending physician) and task (overall, incision, fashioning, integration [and only in the case of 3D printed] patency of airway) on score received by faculty graders. Simple main effect analysis revealed the training year did have a statistically significant effect on score received for both porcine and 3D printed models (p = .002 and p = .0009, respectively). Simple main effect analysis showed that task did not have a statistically significant effect on score received for both porcine and 3D printed models (p = .876 and p = .340, respectively).
4. DISCUSSION
Simulation training for residents has been shown to improve patient outcomes. 9 While simulation does not replace clinical training, it can provide trainees with increased confidence. 7 While otolaryngology resident education has become increasingly standardized, certain operations such as LTR are done in higher volumes at certain centers. Simulation can be highly beneficial to improve resident confidence in performing a less common, high risk airway procedure.
This is the first published study, to the authors' knowledge, to directly compare a 3D printed model to an animal model for simulation of laryngotracheal reconstruction. Both models have been validated independently 4 , 6 and each has distinct pros and cons. Participants had similar increases in confidence and expertise after performing either simulation in this study (0.60 and 0.21, respectively), and found both models similarly relevant to their practice.
With any new tool, establishing validity is an important step prior to wider adoption. This study measured face, content, and construct validity. Face validity slightly favored the porcine model, although both models had good face validity overall (Table 3). Global and task specific validity was high for both models (Table 4). The final measure of validity in this study was construct validity, which was established as high for both models.
3D‐printed models have many practical benefits. They do not decompose, which means they can be saved for future use/reference. Progress may be tracked and preserved on a 3D‐printed model. In terms of accessibility, cost per 3D‐printed model is approximately $2.60 USD 6 while cost per porcine cadaver model ranges from $6–8 USD when purchased from online distributors. 10 , 11 Since 3D‐printed models do not decompose or require special storage, they can be shipped from a facility that 3D prints the models and stored easily. In contrast, the porcine model requires special packaging and shipping materials, as well as storage in a refrigerator or freezer to stay fresh. The lower cost and more straightforward storage of 3D‐printed models are a major benefit to this model. There is also an ethical concern with using animal models regarding loss of animal life when alternative options exist.
Another benefit is that a 3D‐printed model can be customized to replicate specific airway scenarios and grades of subglottic stenosis to allow for improved preoperative planning. 6 , 12 A significant drawback of using the porcine model for simulation is that it cannot be easily customized to simulate subglottic or tracheal stenosis. Faculty raters were able to evaluate patency of the airway after the 3D printed model was completed (Figure 6), but this was not possible in the porcine model. Participants rated the 3D‐printed model more useful for teaching anatomy than the porcine model (mean 4.14 vs. 3.29, p = .047), which may be related to the absence of airway stenosis in the porcine model.
FIGURE 6.

Cross‐sectional view of 3D‐printed model after simulated anterior grafting
Drawbacks of 3D‐printed models are mainly the realism of the model. The laryngotracheal complex model does not have surrounding soft tissue and synthetic material is not a perfect substitute for skin, subcutaneous fat, muscle, and cartilage. Porcine cadavers have the distinct advantage of having actual skin, adipose tissue, muscle, and cartilage. An anterior approach to the trachea may be better simulated due to the overlying muscle and adipose tissue on the airway cartilage (Figure 4). This was reflected in participants rating the porcine model higher in the face validity categories of anatomic structures appearing realistic (p = .03), cartilage graft appearing realistic (p = .0005) and airway cartilage appearing realistic (p = .0005).
A major disadvantage of the porcine cadaveric model is that it has larger dimensions in comparison to the 3D printed‐model, more consistent with adult tracheal proportions. In the future, a suckling‐pig trachea could be utilized rather than an adult pig, to simulate the trachea of a 5–10 year old. 4 On average, the adult pig trachea used in this simulation was approximately 1–2 cm in diameter with a larynx of 3–4 cm in height. This was reflected in participants finding the 3D printed model more useful for teaching anatomy (p = .03).
4.1. Limitations
This was a single institution study at a relatively low volume LTR center. Given that the author's institution is the only otolaryngology residency training program in the state, recruiting additional resident trainees to complete the simulations was a challenge, resulting in a smaller sample size. It was thought to be low yield to have medical students or non‐otolaryngology residents complete the simulation. Ultimately, this resulted in the study being under‐powered. A future study would aim to be multi‐institutional to recruit more participants and provide greater statistical power to the study. Another limitation of this being a single institution study is that only 33% of residents in the study had participated in an LTR within the last year. Utility of simulation may or may not vary for residents training at a higher volume center, comparison studies would be useful to elucidate this.
Faculty ratings for the tasks had good interrater reliability for the porcine model, but relatively worse reliability for the 3D‐printed model. Given that the porcine model is a more established simulation and teaching format, it may have been more familiar for evaluation. The porcine model also could not be evaluated for airway patency as there was no pre‐existing subglottic stenosis. For future studies, rating criteria should be more clearly established for both models, but particularly the 3D‐printed model.
Ultimately, the most useful validity measure for a test or simulation is predictive validity. Predictive validity is the extent to which performance on a test predicts actual performance, in this case whether better performance on the simulator predicts better performance in the operating room. 8 This would be the most clinically meaningful validity to establish and would be important to evaluate in further studies.
5. CONCLUSIONS
This study, to the authors' knowledge, is the first to directly compare 3D‐printed and animal models for simulation of LTR. Participants reported a similar mean increase in confidence after using either simulation. Each model was rated for face, content, and construct validity with good results. The porcine model was rated higher in anatomic and cartilage realism, but participants found both models overall useful for LTR simulation.
The 3D‐printed model is a promising simulation candidate as it compares well to an animal model and has the advantage of being more anatomically true to pediatric patients. In addition, 3D‐printed models have practical benefits over cadaveric models; they do not decompose, and can be custom made to model a disease state such as subglottic stenosis. 3D‐printed simulation models are a promising, accessible option in resident education.
FUNDING INFORMATION
No sources of financial support or external funding.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose. David Zopf is a cofounder of University of Michigan affiliated startup MakeMedical LLC, which provided no financial support for the research.
Falls M, Vincze J, Brown J, et al. Simulation of laryngotracheal reconstruction with 3D‐printed models and porcine cadaveric models. Laryngoscope Investigative Otolaryngology. 2022;7(5):1603‐1610. doi: 10.1002/lio2.884
[Correction added on Aug 24, 2022, after first online publication: Figure legends have been revised for Figures 2 through 4 in this version.]
Contributor Information
Megan Falls, Email: megfalls@iu.edu.
Jonathan Ting, Email: joting@iu.edu.
REFERENCES
- 1. Koempel JA, Cotton RT. History of pediatric laryngotracheal reconstruction. Otolaryngol Clin North Am. 2008;41(5):825‐835, vii. [DOI] [PubMed] [Google Scholar]
- 2. Alwani MM, Svenstrup TJ, Bandali EH, et al. Validity testing of a three‐dimensionally printed endoscopic sinonasal surgery simulator. Laryngoscope. 2020;130(12):2748‐2753. [DOI] [PubMed] [Google Scholar]
- 3. Musbahi O, Aydin A, Al Omran Y, Skilbeck CJ, Ahmed K. Current status of simulation in otolaryngology: a systematic review. J Surg Educ. 2017;74(2):203‐215. [DOI] [PubMed] [Google Scholar]
- 4. Okhovat S, Milner TD, Clement WA, Wynne DM, Kunanandam T. Validation of animal models for simulation training in pediatric laryngotracheal reconstruction. Ann Otol Rhinol Laryngol. 2020;129(1):46‐54. [DOI] [PubMed] [Google Scholar]
- 5. Milner TD, Okhovat S, Clement WA, Wynne DM, Kunanandam T. A systematic review of simulated laryngotracheal reconstruction animal models. Laryngoscope. 2019;129(1):235‐243. [DOI] [PubMed] [Google Scholar]
- 6. Reighard CL, Green K, Powell AR, Rooney DM, Zopf DA. Development of a high fidelity subglottic stenosis simulator for laryngotracheal reconstruction rehearsal using 3D printing. Int J Pediatr Otorhinolaryngol. 2019;124:134‐138. [DOI] [PubMed] [Google Scholar]
- 7. Hogan CJ, Winters R. The Current Role of Medical Simulation in Otolaryngology. StatPearls; 2021. [PubMed] [Google Scholar]
- 8. Gallagher AG, Ritter EM, Satava RM. Fundamental principles of validation, and reliability: rigorous science for the assessment of surgical education and training. Surg Endosc. 2003;17(10):1525‐1529. [DOI] [PubMed] [Google Scholar]
- 9. Cox T, Seymour N, Stefanidis D. Moving the needle: simulation's impact on patient outcomes. Surg Clin North Am. 2015;95(4):827‐838. [DOI] [PubMed] [Google Scholar]
- 10. Nebraska Scientific. Published 2018. Accessed March 05, 2022. https://www.nebraskascientific.com/fresh‐frozen‐specimens/4129‐pig‐larynx‐with‐trachea‐fresh‐frozen‐no‐chemicals.html
- 11. Nasco Education. Published 2021. Accessed March 05, 2022. https://www.enasco.com/p/Nasco‐Guard,‐Preserved‐Pig‐Organ‐‐‐Larynx%2BLS03612M
- 12. Richard Z, Jackson E, Jung JP, Kanotra SP. Feasibility and potential of three‐dimensional printing in laryngotracheal stenosis. J Laryngol Otol. 2019;133(6):530‐534. [DOI] [PubMed] [Google Scholar]


