Abstract
Monoamine oxidase B (MAO-B) metabolizes monoamines such as dopamine regarding neural transmission and controls its level in the mammalian’s brain. When MAO-B metabolizes dopamine abnormally, normal neurotransmission does not occur, and central nervous system disorders such as Parkinson’s disease may develop. Although several MAO inhibitors have been developed, most of them have no selectivity between monoamine oxidase A (MAO-A) and MAO-B, or they work irreversibly against the enzyme. This report describes the first case of screening of N-arylated heliamine derivatives to develop novel MAO-B selective inhibitors that can be synthesized concisely by microwave-assisted Pd nanoparticle-catalyzed Buchwald–Hartwig amination. We discovered that the derivatives 4h, 4i, and 4j display inhibitory activity against MAO-B with IC50 values of 1.55, 13.5, and 5.08 μM, respectively.
Keywords: monoamine oxidase, heliamine derivatives, MAO-B, Buchwald−Hartwig amination
Monoamine oxidase (MAO) is found in the outer mitochondrial membrane and metabolizes monoamines ingested from drugs or foods.1−5 MAO has two subtypes: MAO-A and MAO-B. These two subtypes have over 70% homogeneity in terms of amino acid sequence.6 Meanwhile, these can be differentiated by their key amino acids for substrate selectivity. MAO-A display selective affinity for hydroxylated amines such as noradrenaline and serotonin due to isoleucine (Ile) as the key amino acid, whereas MAO-B shows selectivity for nonhydroxylated amines such as benzylamine and beta-phenylethylamine by phenylalanine (Phe).6 In the mammalian brain, MAO controls neurotransmission by degradation of neurotransmitters such as dopamine. Of these two types, abnormal metabolism of dopamine by MAO-B can lead to serious central nervous disorders such as Parkinson’s disease due to improper neurotransmission.5 Therefore, several MAO inhibitors have been developed.
In the early development of MAO inhibitors, phenelzine (I), tranylcypromine (II), isocarboxazid (III), and iproniazid (IV), which are nonselective for MAO-A and MAO-B and act irreversibly by forming covalent bonds with amino acid residues of proteins, and MAO-A and -B nonselective, reversible caroxazone (V) was approved (Figure 1a, b). Pargyline (VI), selegiline (VII), and rasagiline (VIII) (MAO-B selective), which act selectively and irreversibly on MAO-B were also marketed (Figure 1c). However, nonselective MAO inhibitors have been withdrawn from the market and their use has been restricted due to the risk of fatal hypertension caused by the inhibition of MAO-mediated metabolism of tyramine, which is commonly found in aged cheese and wine.7 In addition, irreversible MAO inhibitors form covalent bonds with proteins so that the drugs continue to exert their medicinal effect until the enzyme is metabolized completely. As a result, side effects such as hepatic dysfunction, sexual dysfunction, and pregnancy damage have been considered problematic. Recently, safinamide (IX) has been marketed as a selective and reversible MAO-B inhibitor (Figure 1d). Nowadays, the research of drug design based on the safinamide skeleton has been advanced well.8 On the flip side, the designing based on other skeleton compounds is also important and perfomed.9,10
Figure 1.

Marketed monoamine oxidase (MAO) inhibitors.
(R)-Salsolinol (1) is a brain monoamine with a tetrahydroisoquinoline (THIQ) skeleton that is biosynthesized from l-tyrosine.111 is known that is metabolized by MAO-B12 and inhibits 48% of MAO activity at 2 mM in rat-stem.13 In other words, it is considered that can acquire high affinity and inhibitory activity for MAO-B by the functionalization of compounds with the THIQ skeleton. Indeed, heliamine (2) which has the THIQ skeleton as 1, is not MAO inhibitor (IC50 for murine MAO-A: 100 μM; MAO-B: > 1000 μM)14 but N-functionalized heliamine 2′ has some human MAO inhibitory activity (IC50 for human MAO-A: 8.1 ± 0.01 μM; MAO-B: 13.8 ± 0.16 μM)15 (Figure 2). Thus, it can be considered that the MAO inhibitory activity of salosolinol analogues tends to increase by the functionalization of amine moiety.
Figure 2.
Salsolinol analogues.
Buchwald–Hartwig amination or a Cu-catalyzed carbon–nitrogen bond-forming reaction on the nitrogen of THIQ compounds with aryl halides 3 has been reported.16−192 could be also converted to N-arylated derivatives 4 by these metal-catalyzed coupling reactions (Scheme 1a).20−22 However, there is no report of the MAO-B inhibitory activity of N-arylated heliamine derivatives. Therefore, we expected that the N-arylated heliamine with a proper functional group on the aromatic ring could provide the MAO-B selective inhibitory activity. We carried out design (computational), synthesis (Buchwald–Hartwig amination), and MAO-B inhibitory activity and physical property evaluation.
Scheme 1. Metal-Catalyzed C–N Coupling Reaction to THIQ Skeleton and Conception for Development of Novel MAO-B Inhibitor.
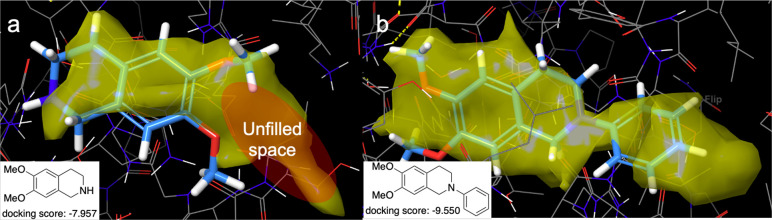
Initially, we investigated the docking study of N-arylated heliamine derivatives with hMAO-B (PDB ID: 2V5Z).23 When N-phenylheliamine (4a) was subjected to docking study as the first model molecule, it fit into the active site region of hMAO-B (yellow region) without a large space compared with 2 (Figure 3). It is interesting that 2 and 4a fit into the active site in different directions. In addition, 4a’s docking score (lower score is better) was also better than 2 (2: −7.957, 4a: −9.550). As a result, it is considered that the N-arylated heliamine structure is suitable for filling the active site. Docking studies of the other 11 heliamine derivatives with substituted aryl or heteroaryl, having an electron-donating group (EDG) or an electron-withdrawing group (EWG), were also conducted (Table 1), so that a designed molecule has a better docking score. As a result, most derivatives except o-methylester-substituted derivative 4l showed a good docking score. Especially, 4-benzyl alcohol derivative 4d (docking score: −10.264) or EWG substituted derivatives 4-formylphenyl derivative 4h (docking score: −10.140), 4-fluorophenyl derivative 4i (docking score: −10.100), 3,5-difluorophenyl derivative 4j (docking score: −10.467), and 3,5-bistrifluoromethylphenyl derivative 4k (docking score: −10.675) had better docking scores. We performed the same docking study for hMAO-A and the results are shown in Table 2.
Figure 3.
Docking study of (a) 2 and (b) 4a with hMAO-B (PDB ID: 2V5Z).
Table 1. Docking Score of N-Arylated Heliamine Derivatives 4 with hMAO-B (PDB ID: 2V5Z).

Table 2. Docking Score of N-Arylated Heliamine Derivatives 4 with hMAO-A (PDB ID: 2Z5Y).


Docking images of derivatives 4 having a good docking score are shown in Figure 4. All derivatives are well fitted into the active site (yellow region). Derivatives 4d, 4h, and 4i formed π―π interaction with nearby amino acid residue such as Tyr 326. It can be considered that the docking score became better by this interaction and proper structure matching. Even though derivatives 4j and 4k with fluorine functional group disubstituted aryl did not form such a π―π interaction, the docking scores were better than others. Therefore, N-arylated heliamines were then synthesized, and we predicted that derivatives with good docking scores have high potency for hMAO-B.
Figure 4.
Docking study of derivatives having good docking score with hMAO-B (PDB ID: 2V5Z): (a) 4d, (b) 4h, (c) 4i, (d) 4j, and (e) 4k.
We reported palladium nanoparticles-catalyzed product selective ligand-free Buchwald–Hartwig amination using continuously irradiation type microwave (cont. MW) in 2021.24 Regioisomer via an aryne intermediate was obtained by high temperature and strong basic conditions in case of conventional heating. In contrast, our methodology prevented aryne formation and gave only a desired coupling product by activation of metal nanoparticles catalyst or/and a compound having dipole moment by cont. MW and copper plate as microwave absorption amplifiers (Scheme 2).
Scheme 2. Palladium Nanoparticle-Catalyzed Product Selective Ligand-Free Buchwald–Hartwig Amination Assisted by Continuous Irradiation Type Microwave.

SGlPd: Sulfur-modified Glass-supported Palladium (Pd), cont. MW: continuous irradiation type microwave.
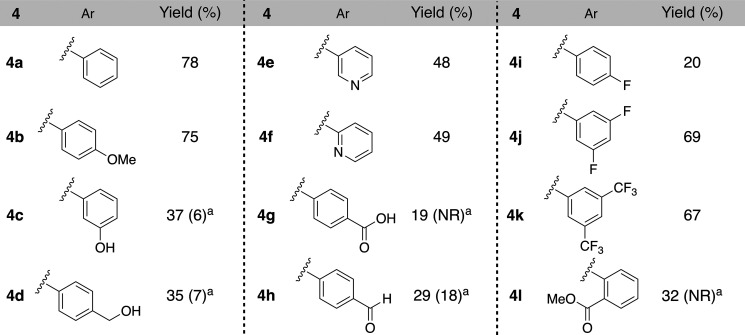
In the Buchwald–Hartwig amination using a homogeneous metal catalyst, the ligand should be changed to proceed with the reaction depending on substrates., whereas our methodology could synthesize comparative wide range C–N coupling products without optimization of the ligand, which allows the comprehensive synthesis of various derivatives to build a drug discovery library. The optimized reaction conditions of Buchwald–Hartwig amination in our previous work were applied to synthesize N-arylated heliamine derivatives (Table 3). As a result, all reactions could proceed well, even though some derivatives with benzoic acid or fluorophenyl were obtained in low yields. Noticeably, these derivatives were not obtained or were obtained in lower yields than the reaction using homogeneous Pd catalyst; therefore, our methodology is probably suitable for library synthesis, as expected.
Table 3. Synthesis of N-arylated Heliamine Derivatives 4.
The yields in parentheses are from results by the reaction using homogeneous palladium catalyst; reaction conditions: 2 (2.0 equiv), 3 (0.5 mmol), Pd2(dba)3 (4 mol %), (±)-BINAP (8 mol %), NaOtBu (2.0 equiv), toluene (1.5 mL), 100 °C, 30 min, then reflux, 10 h, NR is no reaction.
The hMAO-B was exposed to 10 μM of synthesized derivatives in dimethyl sulfoxide (DMSO) to evaluate inhibitory activity (Figure 5a). Derivatives 4h, 4i, and 4j with good docking scores exhibited strong inhibitory activity for hMAO-B. the IC50 values of 4h, 4i, and 4j were calculated as 1.55, 13.5, and 5.08 μM, respectively (Figure 5b). Safinamide and lazabemide, which are MAO-B selective inhibitors, were also evaluated as positive controls. These evaluations were performed three times.
Figure 5.
(a) Inhibitory effect of N-arylated heliamine derivatives on hMAO-B activity. (b) Inhibition curve of derivatives having high potency at (a) 4h, 4i, and 4j. All results were expressed as a percentage of control with no inhibitors or derivatives; the x-axis in b is a log scale.
The selectivity for hMAO-B of three derivatives (4h–4j) with strong inhibitory activity was investigated. When the derivatives were exposed to hMAO-A and inhibitory activity was evaluated in the same procedure, the IC50 values of all derivatives were above 100 μM, indicating that they were selective for hMAO-B. In addition, both hMAO-B inhibitory activity and selectivity of these three derivatives improved compared to N-alkylated heliamine 2′,25 which was enumerated in the introduction (Table 4). We considered the cause of hMAO-B selectivity of derivatives via a docking study of derivative 4h as a model molecule and hMAO-A (PDB ID: 2Z5Y)26 and hMAO-B (PDB ID: 2V5Z). Obviously, the structure of 4h was too big for the MAO-A active site (yellow region) and did not fit into the hMAO-A structure (Figure 6a). Conversely, 4h well fits into hMAO-B active site and forms a π―π interaction with nearby amino acid residues such as Tyr326. Thus, these derivatives showed a strong inhibitory activity against hMAO-B.
Table 4. Comparison of Inhibitory Activity of Derivatives 4h–4j for hMAO-A and hMAO-B.
Data from ref (15).
Figure 6.
Docking study of 4h with hMAO-A and hMAO-B for selectivity investigation.
The viabilities (percentage of control) after 24 h of exposure of 10 μM N-arylated heliamine derivatives were as follows: 4h, 99.0 ± 3.6%; 4i, 104 ± 11.2%; 4j, 97.3 ± 4.9% (mean ± S.D.) (Figure 7). These compounds had no cytotoxic effect on human neuroblastoma cells.
Figure 7.
Cell viability after 24 h exposure of N-arylated heliamine derivatives, 4h, 4i, and 4j, on SH-SY5Y cells. The viability of each compound as a percentage of control is expressed as mean ± SD (n = 3).
Subsequently, synthesized derivatives were subjected to physical property evaluation: solubility in phosphate-buffered saline (PBS) solution, Caco2 cell permeability, and stability in human and mouse liver microsomes (Table 5). Derivatives having comparatively lower Log D values (2.5–3.7, predicted Log P value considering changes in lipophilicity due to molecular dissociation) such as 4c, 4d, 4e, 4f, and 4g had good solubility in PBS solution. In addition, these derivatives demonstrated good permeability. On the contrary, higher Log D values of 4a, 4b, 4h, 4i, 4j, and 4k (3.7–5.7) resulted in modest or low solubility and permeability. Especially, it is considerable that 4k with a good docking score had low hMAO-B inhibitory activity due to low solubility. In addition, derivatives with low Log D values also had good stability in liver microsomes, while those with high Log D values had poor stability. These results indicate that introducing a polar functional group into the derivative skeleton is necessary to set the Log D value from 1 to 3 and improve the physical property results.27
Table 5. Physical Properties of N-Arylated Heliamine Derivatives 4.
We have designed a series of N-arylated derivatives of heliamine using Glide, a protein-small molecule docking simulation function of Maestro, and synthesized them using our novel Buchwald–Hartwig reaction methodology. We also tested the synthesized derivatives for their inhibitory activity against hMAO-B and found high inhibitory activity in three derivatives: 4-formylphenyl 4h (IC50 = 1.55 μM), 4-fluorophenyl 4i (IC50 = 13.5 μM), and 3,5-difluorophenyl 4j (IC50 = 5.08 μM), while the mother compound heliamine’s IC50 for hMAO-B is larger than 100 μM. In comparison with MAO-A inhibitory activity, these three derivatives were selective for MAO-B. However, the physical properties of these derivatives might not be so good due to the high Log D value (modest lipophilicity). Therefore, installing of the polar functional group with a proton donor and/or acceptor is probably necessary for further development.
Acknowledgments
This work was partially supported by a Grant-in-Aid from the JSPS KAKENHI for Precisely Designed Catalysts with Customized Scaffolding (Grant JP 15KT0063), by the Platform Project for Supporting Drug Discovery and Life Science Research (Basis for Supporting Innovative Drug Discovery and Life Science Research (BINDS)) from the AMED under Grant JP21am0101084 (Support Number 3296), by Fujimorii Science and Technology Foundation and Sasagawa Science Research Foundation of The Japan Science Society.
Glossary
Abbreviations
- BINAP
2,2′-bis(diphenylphosphino)-1,1′-binaphthyl
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.2c00228.
Analytical data of selected compounds and 1H NMR, 13C NMR, and 19F NMR spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Kumar B.; Sheetal S.; Mantha A. K.; Kumar V. Recent developments on the structure-activity relationship studies of MAO inhibitors and their role in different neurological disorders. RSC Adv. 2016, 6, 42660–42683. 10.1039/C6RA00302H. [DOI] [Google Scholar]
- Finberg J. M. P.; Rabey J. M. Inhibitors of MAO-A and MAO-B in psychiatry and neurology. Front. Pharmacol. 2016, 7, 340–354. 10.3389/fphar.2016.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay R. Molecular aspects of monoamine oxidase B. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 69, 81–89. 10.1016/j.pnpbp.2016.02.005. [DOI] [PubMed] [Google Scholar]
- Tipton K. F. 90 years of monoamine oxidase: some progress and some confusion. J. Neural Transm. 2018, 125, 1519–1551. 10.1007/s00702-018-1881-5. [DOI] [PubMed] [Google Scholar]
- Hong R.; Li X. Discovery of monoamine oxidase inhibitors by medicinal chemistry approaches. MedChemComm 2019, 10, 10–25. 10.1039/C8MD00446C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsugeno Y.; Ito A. A Key Amino Acid Resonsible for Substrate Selectivity of Monoamine Oxidase A and B. J. Biol. Chem. 1997, 272, 14033–14036. 10.1074/jbc.272.22.14033. [DOI] [PubMed] [Google Scholar]
- Wimbiscus M.; Kostenko O.; Malone D. MAO inhibitors: Risks, benefits, and lore. Cleve. Clin. J. Med. 2010, 77, 859–882. 10.3949/ccjm.77a.09103. [DOI] [PubMed] [Google Scholar]
- Jin C.-F.; Wang Z.-Z; Chen K.-Z.; Xu T.-F.; Hao G.-F. Computational Fragment-Based Design Facilitates Discovery of Potent and Selective Monoamine Oxidase-B (MAO-B) Inhibitor. J. Med. Chem. 2020, 63, 15021–15036. 10.1021/acs.jmedchem.0c01663. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Enriquez F.; Costas-Lago M. C.; Besada P.; Alonso-Pena M.; Torres-Teran I.; Vina D.; Fontenla J. A.; Sturlese M.; Moro S.; Quezada E.; Teran C. Novel coumarin-pyridazine hydrids as selective MAO-B inhibitors for the Parkinson’s disease therapy. Bioorg. Chem. 2020, 104, 104203–104213. 10.1016/j.bioorg.2020.104203. [DOI] [PubMed] [Google Scholar]
- Delogu L. G.; Kumar A.; Gatto G.; Bustelo F.; Saavedra M. L.; Rodríguez-Franco I. M.; Laguna R.; Viña D. Synthesis and in vitro study of nitro and methoxy-2-phenylbenzofurans as human monoamine oxidase inhibitors. Bioorg. Chem. 2021, 107, 104616–104626. 10.1016/j.bioorg.2020.104616. [DOI] [PubMed] [Google Scholar]
- Kurnik-Łucka M.; Panula P.; Bugajski A.; Gil K. Salsolinol: an unintelligible and double-faced molecule-lessons learned from in vivo and in vitro experiments. Neurotox. Res. 2018, 33, 485–514. 10.1007/s12640-017-9818-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thull U.; Kneubuhler S.; Gaillard P.; Carrupt P.-A.; Testa B.; Altomare C.; Carotti A.; Jenner P.; St. P. McNaught K. Inhibition of monoamine oxidase by isoquinoline derivatives: Qualitative and 3D-quantitative structure-activity relationships. Biochem. Pharmacol. 1995, 50, 869–877. 10.1016/0006-2952(95)00220-T. [DOI] [PubMed] [Google Scholar]
- Yamanaka Y. Effect of salsolinol on rat brain and liver monoamine oxidase. Japan. J. Pharmacol. 1971, 21, 833–836. 10.1016/S0021-5198(19)36185-2. [DOI] [PubMed] [Google Scholar]
- Meller E.; Friedman E.; Schweitzer J. W.; Friedhoff A. J. Tetrahydro-β-carbolines: specific inhibitors of type A monoamine oxidase in rat brain. J. Neurochem. 1977, 28, 995–1000. 10.1111/j.1471-4159.1977.tb10661.x. [DOI] [PubMed] [Google Scholar]
- Zhu G.; Wang K.; Shi J.; Zhang P.; Yang D.; Fan X.; Zhang Z.; Liu W.; Sang Z. The development of 2-acetylphenol-donepezil hybrids as multifunctional agents for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. Lett. 2019, 29, 126625–126631. 10.1016/j.bmcl.2019.126625. [DOI] [PubMed] [Google Scholar]
- Hari P. D.; König B. Eosin Y catalyzed visible light oxidative C-C and C-P bond formation. Org. Lett. 2011, 13, 3852–3855. 10.1021/ol201376v. [DOI] [PubMed] [Google Scholar]
- Lou Y.; Han X.; Kuglstatter A.; Kondru K. R.; Sweeney K. Z.; Soth M.; McIntosh J.; Litman R.; Suh J.; Kocer B.; Davis D.; Park J.; Frauchiger S.; Dewdney N.; Zecic H.; Taygerly P. J.; Sarma K.; Hong J.; Hill J. R.; Gabriel T.; Goldstein M. D.; Owens D. T. Structure-based drug design of RN486, a potent and selective Bruton’s tyrosine kinase (BTK) inhibitor, for the treatment of rheumatoid arthritis. J. Med. Chem. 2015, 58, 512–516. 10.1021/jm500305p. [DOI] [PubMed] [Google Scholar]
- Mudithanapelli C.; Dhorma P. L.; Kim M. PIFA-Promoted, solvent-controlled selective functionalization of C(sp2)-H or C(sp3)-H: nitration via C-N bond cleavage of CH3NO2, cyanation, or oxygenation in water. Org. Lett. 2019, 21, 3098–3102. 10.1021/acs.orglett.9b00751. [DOI] [PubMed] [Google Scholar]
- Yang L.; Qiu Z.; Wu J.; Zhao J.; Shen T.; Huang X.; Liu Z.-Q. Molecular oxygen-mediated radical alkylation of C(sp3)-H bonds with boronic acids. Org. Lett. 2021, 23, 3207–3210. 10.1021/acs.orglett.1c00948. [DOI] [PubMed] [Google Scholar]
- Johnson A. J.; Luo J.; Zhang X.; Chen Y.-S.; Morton D. M.; Echeverría E.; Torres E. F.; Zhang J. Porphyrin-metalation-mediated tuning of photoredox catalytic properties in metal-organic frameworks. ACS Catal. 2015, 5, 5283–5291. 10.1021/acscatal.5b00941. [DOI] [Google Scholar]
- Xia Q.; Li Y.; Cheng L.; Liang X.; Cao C.; Dai P.; Deng H.; Zhang W.; Wang Q. Electron donor-acceptor complex-initiated photochemical cyanation for the preparation of α-amino nitriles. Org. Lett. 2020, 22, 9638–9643. 10.1021/acs.orglett.0c03703. [DOI] [PubMed] [Google Scholar]
- Ma P.; Liu Y.; Chen L.; Zhao X.; Yang B.; Zhang J. Photocatalyst- and additive-free decarboxylative alkylation of N-aryl tetrahydroisoquinolines induced by visible light. Org. Chem. Front. 2021, 8, 2473–2479. 10.1039/D1QO00261A. [DOI] [Google Scholar]
- Binda C.; Wang J.; Pisani L.; Caccia C.; Carotti A.; Salvati P.; Edmondson D. E.; Mattevi A. Structures of human monoamine oxidase B complexes with selective noncovalent inhibitors: safinamide and coumarin analogs. J. Med. Chem. 2007, 50, 5848–5852. 10.1021/jm070677y. [DOI] [PubMed] [Google Scholar]
- Yamada M.; Ohta R.; Harada K.; Takehara T.; Haneoka H.; Murakami Y.; Suzuki T.; Ohki Y.; Takahashi N.; Akiyama T.; Sirimangkalakitti N.; Sako M.; Murai K.; Arai M.; Arisawa M. Product selective reaction controlled by the combination of palladium nanoparticles, continuous microwave irradiation, and a co-existing solid; ligand-free Buchwald-Hartwig amination vs. aryne amination. Green Chem. 2021, 23, 8131–8137. 10.1039/D1GC01782A. [DOI] [Google Scholar]
- We believe that there is a conformational change on the protein side regarding the fact that the larger compound 2′ is active. It should be added that the discussion here is based on the fixed templates of hMAO-A (PDB ID: 2Z5Y) and hMAO-B (PDB ID: 2V5Z), and further structural information on the protein side is needed for further discussion.
- Son S.-Y.; Ma J.; Kondou Y.; Yoshimura M.; Yamashita E.; Tsukihara T. Structure of human monoamine oxidase A at 2.2-Å resolution: The control of opening the entry for substrates/inhibitors. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 5739–5744. 10.1073/pnas.0710626105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring M. J Lipophilicity in drug discovery. Expert Opin. Drug Dicov. 2010, 5, 235–248. 10.1517/17460441003605098. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.