Abstract

The long-lived radionuclides tritium and carbon-14 have been used for many years in pharmaceutical research and development for making key efficacy and toxicological decisions. Early discovery utilizes radiolabels for compound selection through radioligand binding assays and autoradiography. In preclinical safety evaluation, the use of labeled compounds for adsorption, distribution, metabolism, and excretion studies is often preferred for the added detection sensitivity. As the drug substance proceeds to the clinic, human metabolism studies are reliant on the use of labeled materials to fulfill required regulatory applications.
Keywords: Binding Assay, Autoradiography, C-14, Tritium, Absorption, Distribution, Metabolism and Excretion (ADME), Positron emission tomography (PET)
The use of
long-lived radioactive
compounds in drug discovery and development can provide highly valuable
data due to their ultrahigh sensitivity and ease of detection.1 Diverse radiolabel probes have been used in early
discovery, lead optimization, and candidate selection2 across multiple therapeutic areas throughout the pharmaceutical
industry, as is well documented.3 At AbbVie,
compounds labeled with tritium (3H) are widely used in
early discovery for receptor binding, in vitro hepatocellular
activity, and autoradiography (ARG). As the drug substances advance
to the preclinical stage, the identification and quantification of
metabolites across species is necessary. This is typically accomplished
using radiolabeled compounds in quantitative whole-body autoradiography
(QWBA)4 and in vivo adsorption,
distribution, metabolism, and excretion (ADME) studies.5 Advancement to late-stage clinical trials often
requires human radiolabeled studies to properly identify any unique
circulating metabolites.6
The choice of 3H versus 14C label is often dictated by the stage of a compound in development and the purpose of the study being conducted. The higher specific activity of tritium (∼29 Ci/mmol) compared to 14C (∼62 mCi/mmol) makes it ideal for studies in which the molar concentration is very low, such as ARG or receptor occupancy screens. Tritium is also the preferred isotope for early ADME studies due to the relatively low cost (often ∼100-fold less expensive than 14C per synthesis) and synthetic ease.7 Another advantage is that tritium can often be incorporated by late-stage synthesis through metal-mediated hydrogen/tritium exchange reactions.8 However, tritium labels are more susceptible to hydrolysis and exchange,9 especially during in vivo experiments, which can hamper mass balance and excretion studies.10 For these reasons, 14C is the preferred isotope for late-stage developmental studies in animals and humans and for studies that can take several months to complete, such as environmental fate.9
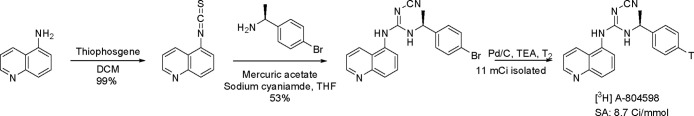
Radioligand binding assays can often be used to study receptor dynamics and pharmacokinetic and pharmacodynamic characteristics, in addition to identifying novel chemical structures to define ligand activity/selectivity in normal and diseased tissues.11 High specific activity radiolabeled compounds with high affinity and low non-specific binding are used for IC50 measurements and kinetic experiments to help quantify terms such as receptor occupancy (Bmax) and equilibrium dissociation constant (KD).12 An example from AbbVie was the use of radioligand binding for the discovery of a potent selective antagonist of P2X7 receptors and its utility for screening of possible new molecular entities (NMEs).13 The P2X7 receptors belong to the ATP family, localized in peripheral macrophages and glial cells of the central nervous system. Activation of P2X7 receptors enhances the calcium concentration, releasing the cytokine IL-1β, causing tissue inflammation/damage. We developed a structurally novel radioligand, [3H]A-804598, which was prepared in three steps as shown in Scheme 1.
Scheme 1. Preparation of Structurally Novel Radioligand [3H]A-804598.
With the high affinity for the receptor in rat (IC50 = 10 nm), mouse (IC50 = 9 nm) and human (IC50 = 11 nm), [3H]A-804598 enabled additional pharmacological studies to elucidate the functional role of P2X7 receptors in rodents to aid in the advancement of NMEs.
Interleukin (IL-23) is indicated in several immunological diseases, such as psoriasis and arthritis.14 Despite a huge advancement in this field, the pharmacokinetics of IL-23 and other cytokines are not well understood, primarily due to their ultra-low endogenous levels.15,16 At AbbVie, we synthesized radiolabeled human recombinant IL-23 by reductive methylation on the lysine residues using [14C]formaldehyde (Scheme 2). The average incorporation of [14C] labels was 23 methyl groups, for a specific activity of 1249 mCi/mmol. The radiolabeled IL-23 was intravenously dosed to cynomolgus monkeys, and their serum samples were analyzed for the disappearance of [14C]IL-23 by accelerated mass spectrometry (AMS).17 The measured pharmacokinetic parameters are shown in Table 1. This data enabled the development of a model to predict the suppression of free IL-23 by therapeutic antibodies.
Scheme 2. Synthesis of Radiolabeled Human Recombinant IL-23.

Table 1. Measured Pharmacokinetic Parameters for Serum Samples with Radiolabeled IL-23.
| subject | T1/2 (h) | Cmax (pg/mL) | AUC (pg h/mL) | CL (L/h) | Vss (L) |
|---|---|---|---|---|---|
| 1 | 4.3 | 18.1 | 42.8 | 0.374 | 1.36 |
| 2 | 5.6 | 20.5 | 47.8 | 0.334 | 1.7 |
| 3 | 4.6 | 24.4 | 46.1 | 0.347 | 1.22 |
| mean | 4.8 | 21.0 | 45.5 | 0.352 | 1.43 |
| SD | 0.7 | 3.2 | 2.6 | 0.020 | 0.25 |
ARG is a powerful method for understanding the localized binding in vivo (after administration to an animal) and in vitro (on specific tissues) of a variety of compounds. In addition, ARG can provide information for dosimetry calculations required for adsorption, metabolism, and excretion in human (hAME) studies. For the early development of positron emission tomography (PET) tracers, ARG is often used with high specific activity tritium labeling to assess binding affinity and specificity for the area of concern.18 Tritium-labeled compounds with a half-life of >12 years can be used for a longer timespan than short-lived positron-emitting isotopes, such as 18F (half-life of 110 min), and the low energy of the beta radiation from tritium requires minimal engineering controls for safe handling. Similarly, quantitative whole-body autoradiography (QWBA or in vivo ARG) can give insight into the distribution. After an intravenous or oral dose, the single animal, typically a rodent, is euthanized at a specified time point. The body is then cryogenically frozen and thin cross-section slices are evaluated by autoradioluminography techniques to quantify the radioactivity present (3H or 14C). This not only gives a snapshot of the whole-body distribution but can also alert the toxicologist to any potential safety concerns.19 Rodent QWBA is routinely used as the defining safety study before any clinical studies, such as human metabolism and/or absolute bioavailability, that are often necessary for drug development before entering Phase III trials.
ADME studies are critical in both early discovery and development phases for NMEs. Recent advances in LC/MS and MS/MS technologies have increased their sensitivity, and they are often the first line in detecting parent and metabolites from in vitro studies and preclinical in vivo animal experiments.20 However, these methods can still suffer from limitations, depending on the chemical structure and the quantities of metabolite formed. The use of radioactive labeled compounds, primarily those with 3H and 14C, is still considered the most quantitative method for ADME studies and is often required by regulatory authorities in drug development.21
Metabolism is the predominate mode of clearance for a majority of small-molecule NMEs.22 Of the drugs that were primarily cleared by metabolism, the cytochrome P450 enzymes were heavily involved, which can often indicate drug–drug interactions.23 Therefore, the importance of understanding this risk early in the drug discovery timeline can be essential to differentiating compounds before advancement into development. Comprehensive profiling using radiolabeled NMEs in human liver systems, such as hepatocytes, can quantitatively capture the major metabolic pathways.
As drug substances advance further in the clinic, regulatory agencies have several requirements for the characterization of metabolites and elimination pathways.24 Typically, by this stage, several studies have been performed in the appropriate animal models. However, humans can often have distinct metabolism pathways, and a better understanding of this before a large-scale clinical trial is needed. At AbbVie, we have conducted several hAME studies in the past decade; one such example is venetoclax. Venetoclax is a well-known B-cell lymphoma-2 (BCl-2) inhibitor and is approved for the treatment of chronic lymphocytic leukemia (CLL). Radiolabeled [14C]venetoclax was prepared in five steps via a synthetic route developed at AbbVie25 (Scheme 3) for a single-dose study involving oral administration to four human volunteers at a dose of 100 μCi/200 mg. Parent [14C]venetoclax plus a major metabolite M27 were found to be the major components present in circulation.21 The M27 metabolite, at 12% of the total drug-related material, was disproportionate in humans, caused by the enzymatic oxidation on the dimethylcyclohexyl ring followed by cyclization, primarily formed by cytochrome P450 (CYP3A4) (Scheme 4).
Scheme 3. Preparation of Radiolabeled [14C]Venetoclax.
Scheme 4. Preparation of M27 Metabolite.
Metabolite M27 was found not to have clinically relevant on- or off-target pharmacologic activities.
Radiolabeled compounds play a vital role in drug discovery and development. In the lead optimization process, radioligand binding can assist in the characterization of dynamics, pharmacokinetics, and pharmacodynamics at the receptor site and facilitate the identification of NMEs. Likewise, ARG studies offer an efficient way to quantify these compounds in complex matrices. Selecting a compound candidate for development also requires having a strong understanding of the metabolism. Radiolabeling studies are considered the most quantitative methods for both in vitro and in vivo ADME studies. Finally, radiolabeled human metabolism studies are required for the clinical development of compounds.
Acknowledgments
The authors thank Eric Soli and William Lambert for their review of and comments on this manuscript.
The authors declare the following competing financial interest(s): All authors are employees of AbbVie and may own stock. The design, study conduct, and financial support for this research were provided by AbbVie. AbbVie participated in the interpretation of data, review, and approval of the publication.
Special Issue
Published as part of the ACS Medicinal Chemistry Letters virtual special issue “New Enabling Drug Discovery Technologies - Recent Progress”.
References
- Elmore C. S. The Use of Isotopically Labeled Compounds in Drug Discovery. Annu. Rep. Med. Chem. 2009, 44, 515–534. 10.1016/S0065-7743(09)04425-X. [DOI] [Google Scholar]
- Atzrodt J.; Derdau V.; Kerr W. J.; Reid M. Deuterium- and Tritium-Labelled Compounds: Applications in the Life Sciences. Angew. Chem. In.t Ed. Engl. 2018, 57 (7), 1758–1784. 10.1002/anie.201704146. [DOI] [PubMed] [Google Scholar]
- Elmore C. S.; Bragg R. A. Isotope Chemistry; A Useful Tool in the Drug Discovery Arsenal. Bioorg. Med. Chem. Lett. 2015, 25 (2), 167–71. 10.1016/j.bmcl.2014.11.051. [DOI] [PubMed] [Google Scholar]
- McEwen A.; Henson C. Quantitative Whole-body Autoradiography: Past, Present and Future. Bioanalysis 2015, 7, 557–568. 10.4155/bio.15.9. [DOI] [PubMed] [Google Scholar]
- Penner N.; Xu L.; Prakash C. Radiolabeled Absorption, Distribution, Metabolism, and Excretion Studies in Drug Development: Why, When, and How?. Chem. Res. Toxicol. 2012, 25, 513–531. 10.1021/tx300050f. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan S.; Reed A.; Du J. Radiosynthesis of (3) H- and (14) C-labeled Veliparib. J. Labelled Comp. Radiopharm. 2021, 64 (9), 356–362. 10.1002/jlcr.3928. [DOI] [PubMed] [Google Scholar]
- Krauser J. A. A Perspective on Tritium Versus Carbon-14: Ensuring Optimal Label Selection in Pharmaceutical Research and Development. J. Labelled Comp. Radiopharm. 2013, 56, 441–446. 10.1002/jlcr.3085. [DOI] [PubMed] [Google Scholar]
- Heys J. R. Organoiridium Complexes for Hydrogen Isoptope Exchange Labeling. J. Labelled Comp. Radiopharm. 2007, 50, 770–778. 10.1002/jlcr.1428. [DOI] [Google Scholar]
- Organization for Economic Co-operation and Development . OECD Guidelines for the Testing of Chemicals, Test No. 308: Aerobic and Anaerobic Transformation in Aquatic Sediment Systems, Apr 24, 2002. 10.1787/9789264070523-en [DOI] [Google Scholar]
- Shaffer C. L.; Gunduz M.; Thornburgh B. A.; Fate G. D. Using a Tritiated Compound to Elucidate Its Preclinical Metabolic and Excretory Pathways in Vivo: Exploring Tritium Exchange Risk. Drug Metab. Dispos. 2006, 34, 1615–1623. 10.1124/dmd.106.010934. [DOI] [PubMed] [Google Scholar]
- a Louis J. V.; Lu Y.; Pieschl R.; Tian Y.; Hong Y.; Dandapani K.; Naidu S.; Vikramadithyan R. K.; Dzierba C.; Sarvasiddhi S. K.; Nara S. J.; Bronson J.; Macor J. E.; Albright C.; Kostich W.; Li Y. W. [(3)H]BMT-046091 a Potent and Selective Radioligand to Determine AAK1 Distribution and Target Engagement. Neuropharmacology 2017, 118, 167–174. 10.1016/j.neuropharm.2017.03.015. [DOI] [PubMed] [Google Scholar]; b Hu E.; Ma J.; Biorn C.; Lester-Zeiner D.; Cho R.; Rumfelt S.; Kunz R. K.; Nixey T.; Michelsen K.; Miller S.; Shi J.; Wong J.; Hill Della Puppa G.; Able J.; Talreja S.; Hwang D. R.; Hitchcock S. A.; Porter A.; Immke D.; Allen J. R.; Treanor J.; Chen H. Rapid Identification of a Novel Small Molecule Phosphodiesterase 10A (PDE10A) Tracer. J. Med. Chem. 2012, 55 (10), 4776–87. 10.1021/jm3002372. [DOI] [PubMed] [Google Scholar]; c Colabufo N. A.; Abate C.; Contino M.; Inglese C.; Ferorelli S.; Berardi F.; Perrone R. Tritium Radiolabelling of PB28, a Potent Sigma-2 Receptor Ligand: Pharmacokinetic and Pharmacodynamic Characterization. Bioorg. Med. Chem. Lett. 2008, 18 (6), 2183–7. 10.1016/j.bmcl.2007.12.056. [DOI] [PubMed] [Google Scholar]
- Hulme E. C.; Trevethick M. A. Ligand Binding Assays at Equilibrium: Validation and Interpretation. Br. J. Pharmacol. 2010, 161 (6), 1219–37. 10.1111/j.1476-5381.2009.00604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly-Roberts D. L.; Namovic M. T.; Surber B.; Vaidyanathan S. X.; Perez-Medrano A.; Wang Y.; Carroll W. A.; Jarvis M. F. [3H]A-804598 ([3H]2-Cyano-1-[(1S)-1-phenylethyl]-3-quinolin-5-ylguanidine) is a Novel, Potent, and Selective Antagonist Radioligand for P2X7 Receptors. Neuropharmacology 2009, 56 (1), 223–9. 10.1016/j.neuropharm.2008.06.012. [DOI] [PubMed] [Google Scholar]
- A Croxford A. L.; Kulig P.; Becher B. IL-12 and IL-23 in Health and Disease. Cytokine & Growth Factors Rev. 2014, 25, 415. 10.1016/j.cytogfr.2014.07.017. [DOI] [PubMed] [Google Scholar]; b Yago T.; Nanke Y.; Kawamoto M.; Kobashigawa T.; Yamanaka H.; Kotake S. IL-23 and Th17 Disease in Inflammatory Arthritis. J. Clin Med. 2017, 6, 81. 10.3390/jcm6090081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi M.; Lahdou I.; Opelz G.; Mehrabi A.; Zeier M.; Schnitzler P.; Daniel V. IL-23 Plasma Level Is Strongly Associated with CMV Status and Reactivation of CMV in Renal Transplant Recipients. BMC Immunol 2016, 17, 35. 10.1186/s12865-016-0175-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. Y.; Chen X. Y.; Li J.; Zhang H. Y.; Liu J.; Sun L. D. MiR-200a Expression in CD4+ T Cells Correlates with the Expression of Th17/Treg Cells and Relevant Cytokines in Psoriasis Vulgaris: A Case Control Study. Biomed Pharmacother 2017, 93, 1158. 10.1016/j.biopha.2017.06.055. [DOI] [PubMed] [Google Scholar]
- Zhang T. T.; Ma J.; Durbin K. R.; Montavon T.; Lacy S. E.; Jenkins G. J.; Doktor S.; Kalvass J. C. Determination of IL-23 Pharmacokinetics by Highly Sensitive Accelerator Mass Spectrometry and Subsequent Modeling to Project IL-23 Suppression in Psoriasis Patients Treated with Anti-IL-23 Antibodies. AAPS J. 2019, 21 (5), 82. 10.1208/s12248-019-0352-8. [DOI] [PubMed] [Google Scholar]
- Skaddan M. B.; Wooten D. W.; Wilcox K. C.; Voorbach M. J.; Reuter D. R.; Jia Z. J.; Forster-Duke K. D.; Hickson J. A.; Vaidyanathan S.; Reed A. D.; Tovcimak A. E.; Quo Q.; Comley R. A.; Lee L.; Finnema S. J.; Mudd S. R.. [18F]BTK-1: A Novel Positron Emission Tomography Tracer for Imaging Bruton's Tyrosine Kinase. Molecular Imaging and Biology 2022, 10.1007/s11307-022-01733-1 [DOI] [PubMed] [Google Scholar]; b Lemoine L.; Saint-Aubert L.; Marutle A.; Antoni G.; Eriksson J. P; Ghetti B.; Okamura N.; Nennesmo I.; Gillberg P.-G.; Nordberg A. Visualization of Regional Tau Depositis Using 3H-THK5117 in Alzheimer Brain Tissue. Acta Neuropathol. Commun. 2015, 3, 40/1–40/11. 10.1186/s40478-015-0220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Solon E. G. Use of Radioactive Compounds and Autoradiography to Determine Drug Tissue Distribution. Chem. Res. Toxicol. 2012, 25, 543–555. 10.1021/tx200509f. [DOI] [PubMed] [Google Scholar]; b Solon E. G.; Balani S. K.; Lee F. W. Whole-body Autoradiography in Drug Discovery. Curr. Drug. Metab. 2002, 3, 451–462. 10.2174/1389200023337207. [DOI] [PubMed] [Google Scholar]
- Prakash C.; Shaffer C. L.; Nedderman A. Analytical Strategies for Identifying Drug Metabolites. Mass Spectrom. Rev. 2007, 26, 340–369. 10.1002/mas.20128. [DOI] [PubMed] [Google Scholar]
- FDA . Guidance for Industry: Safety Testing of Drug Metabolites, Federal Register E8-2827, Feb 15, 2008. https://www.federalregister.gov/d/E8-2827. [Google Scholar]
- a Isin E. M.; Elmore C. S.; Nilsson G. N.; Thompson R. A.; Weidolf L. Use of Radiolabeled Compounds in Drug Metabolism and Pharmacokinetics Studies. Chem. Res. Toxicol. 2012, 25, 532–542. 10.1021/tx2005212. [DOI] [PubMed] [Google Scholar]; b Kubinyi H. Drug Research: Myths, Hype and Reality. Nature Rev. Drug Discovery 2 2003, 2, 665–668. 10.1038/nrd1156. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J. Role of Cytochromes P450 in Chemical Toxicity and Oxidative Stress: Studies with CYP2E1. Mutat. Res. 2005, 569 (1–2), 101–110. 10.1016/j.mrfmmm.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Ku Y.-Y.; Chan V. S.; Christesen A.; Grieme T.; Mulhern M.; Pu Y.-M.; Wendt M. D. Devlopment of a Convergent Large-scale Synthesis for Venetoclax, a First-in-Class BCL-2 Selective Inhibitor. J. Org. Chem. 2019, 84, 4814–4829. 10.1021/acs.joc.8b02750. [DOI] [PubMed] [Google Scholar]
- Liu H.; Michmerhuizen M. J.; Lao Y.; Wan K.; Salem A. H.; Sawicki J.; Serby M.; Vaidyanathan S.; Wong S. L.; Agarwal S.; Dunbar M.; Sydor J.; de Morais S. M.; Lee A. J. Metabolism and Disposition of a Novel B-Cell Lymphoma-2 Inhibitor Venetoclax in Humans and Characterization of Its Unusual Metabolites. Drug Metab. Dispos. 2017, 45 (3), 294–305. 10.1124/dmd.116.071613. [DOI] [PubMed] [Google Scholar]