Abstract
In Great Britain a recent independent scientific review for the government has concluded that the development of a cattle vaccine against Mycobacterium bovis holds the best long-term prospect for tuberculosis control in British herds. A sine qua non for vaccination is the development of a complementary diagnostic test to differentiate between vaccinated animals and those infected with M. bovis so that test-and-slaughter-based control strategies can continue alongside vaccination. In order to assess the feasibility of developing a differential diagnostic test for a live vaccine, we chose M. bovis BCG Pasteur as a model system. Recombinant forms of antigens which are expressed in M. bovis but not, or only at low levels, in BCG Pasteur (ESAT-6, MPB64, MPB70, and MPB83) were produced. These reagents were tested either alone or in combination by using peripheral blood mononuclear cells from M. bovis-infected, BCG-vaccinated, and Mycobacterium avium-sensitized calves. All four antigens induced in vitro proliferation and gamma interferon responses only in M. bovis-infected animals. A cocktail composed of ESAT-6, MPB64, and MPB83 identified infected animals but not those vaccinated with BCG. In addition, promiscuous T-cell epitopes of ESAT-6, MPB64, and MPB83 were formulated into a peptide cocktail. In T-cell assays with this peptide cocktail, infected animals were identified with frequencies similar to those obtained in assays with the protein cocktail, while BCG-vaccinated or M. avium-sensitized animals did not respond. In summary, our results suggest that peptide and protein cocktails can be designed to discriminate between M. bovis infection and BCG vaccination.
Bovine tuberculosis (TB) is caused by Mycobacterium bovis, a bacterium closely related to Mycobacterium tuberculosis, the major cause of human TB. M. bovis can infect a wide range of animal species and was the cause of approximately 2,000 human deaths per annum (6%) in England and Wales in the 1930s and 1940s (12). Infected cattle can spread the bacteria by aerosol and through infected milk. However, the introduction of pasteurization of milk and the test and slaughter of tuberculous cattle has dramatically reduced the transmission from cattle to humans, and in 1995 only 32 (1%) of the 3,200 isolates from patients with TB in Great Britain (GB) were identified as M. bovis (12). However, bovine TB has severe implications for animal welfare and animal health, since it causes reduced productivity and premature death in cattle and affected farms suffer severe economic losses. Therefore, a compulsory eradication program based on tuberculin testing and slaughter of reactor animals began in GB in 1950, and by 1960 the program had been successfully implemented throughout the whole of GB. Despite these measures, the number of cases of herd breakdowns reported has been steadily rising since 1988, mainly in the southwest of England but also in the rest of England and Wales. A recent independent scientific panel set up to review the control of bovine TB in GB (18) has concluded that the development of a cattle vaccine against M. bovis holds the best long-term prospect for TB control in British herds. A sine qua non for cattle vaccination is the development of a complementary diagnostic test to differentiate between vaccinated animals and those infected with M. bovis so that test-and-slaughter-based control strategies can continue alongside vaccination.
Despite the unpredictable and widely diverging efficacies of vaccination with M. bovis BCG (2, 9, 31, 37), it is still the only realistic vaccine candidate which could be applied to the field situation in the short term. Encouraging results with BCG have been reported from New Zealand, where a significant level of protection has been observed in vaccinated cattle subsequently experimentally challenged with M. bovis (3, 4). However, vaccination with BCG compromises tuberculin purified protein derivative (PPD) specificity (2, 14, 16). Thus, the development of cattle vaccines based on BCG (or attenuated M. bovis strains) will require the identification of specific, defined antigens that allow the discrimination of M. bovis-infected animals from vaccinated animals (differential diagnosis). Such antigens are ESAT-6 and MPB64, which due to a gene deletion are not expressed in BCG Pasteur (10, 11, 26), as well as MPB70 and MPB83, which are expressed at only low levels in BCG Pasteur and are serodominant in infected cattle (15, 40). It has been demonstrated that both ESAT-6 and MPB64, when used as skin test reagents, discriminated between infected and BCG-vaccinated guinea pigs (5). In addition, ESAT-6 was found to discriminate between cattle infected with TB and cattle sensitized by environmental mycobacteria (27). An alternative approach to using recombinant proteins is the application of synthetic peptides derived from antigens such as those described above. Synthetic peptides would have the advantages of lower production costs and easier standardization and quality control and carry no risk of infection since they are fully chemically synthesized.
To assess the feasibility of developing differential diagnostic tests for a live vaccine, we tested recombinant forms of ESAT-6, MPB64, MPB70, and MPB83 alone or in combination using peripheral blood mononuclear cells (PBMC) from M. bovis-infected, BCG-vaccinated, and Mycobacterium avium-sensitized calves. In addition, a cocktail composed of synthetic peptides encompassing “promiscuous peptides” derived from ESAT-6, MPB64, MPB70, and MPB83 was also assessed. Our results provide proof of the principle that both protein-based and suitably formulated peptide-based cocktails can be employed to specifically diagnose M. bovis-infected field reactor cattle without being compromised by BCG vaccination, thus satisfying the definition of reagents for differential diagnosis.
MATERIALS AND METHODS
Cattle.
Six- to 12-month-old calves of various breeds were obtained from herds free of bovine TB and kept in the Animal Services Unit. Four different groups of cattle were used in this study.
(i) M. bovis infection.
Calves were infected with an M. bovis field strain from GB (AF 2122/97) by intratracheal instillation of 104 CFU as described earlier (3, 4).
(ii) BCG vaccination.
Calves were vaccinated with BCG Pasteur by subcutaneous injection of 106 CFU into the side of the neck (3, 4), followed 8 weeks later by a booster injection with the same route and dose.
(iii) M. avium-biased cattle and other controls.
Several animals with stronger responses to avian PPD (PPD-A) than to mammalian PPD (PPD-M) were included as examples of sensitization with environmental mycobacteria. Blood was also obtained from animals from herds free of TB as negative controls.
(iv) Field samples.
Blood samples were obtained from cattle of mixed breeds from farms in Gloucestershire and Worcestershire, United Kingdom, that had been designated tuberculin test reactors following skin testing with the single intradermal comparative tuberculin test (SICTT). The skin tests were performed as specified previously (6a). All samples were transported by courier to our laboratory within 8 h of sampling and processed immediately upon arrival.
Antigens.
ESAT-6, MPB64, MPB70, and MPB83 were expressed in Escherichia coli by using the plasmid vector pET21d and purified by Ni affinity chromatography according to standard methodologies recommended by the manufacturer (Novagen). Ag85abc complex purified from BCG culture filtrate was kindly provided by K. Huygen, Brussels, Belgium. Synthetic peptides (16 residues long and overlapping by 8 residues) were synthesized by solid-phase peptide synthesis as described earlier (36). Peptide purity and sequence fidelity were confirmed by analytical reverse-phase high-pressure liquid chromatography and by electron-spray mass spectrometry, respectively. Proteins were used in cultures singly or in a cocktail of ESAT-6, MPB64, and MPB83, at 5 to 10 μg/ml, and peptides were used at 1 to 25 μg/ml. The sequences of peptides used in the peptide cocktail are given in Table 1. PPD-M and PPD-A tuberculins were obtained from the Tuberculin Production Unit at the Veterinary Laboratories Agency—Weybridge and used in culture at 10 μg/ml.
TABLE 1.
Components of the diagnostic peptide cocktail
| Peptide | Protein | Positiona | Amino acid sequence |
|---|---|---|---|
| P1 | ESAT-6 | 1–16 | MTEQQWNFAGIEAAAS |
| P2 | ESAT-6 | 17–32 | AIQGNVTSIHSLLDEG |
| P3 | ESAT-6 | 57–72 | KWDATATELNNALQNL |
| P4 | MPB83 | 195–214 | GLVCGGVHTANATVYMIDTV |
| P5 | MPB70 | 1–16 | MKVKNTIAATSFAAAG |
| P6 | MPB64 | 89–104 | APYELNITSATYQSAI |
| P7 | MPB64 | 105–120 | PPRGTQAVVLKVYQNA |
Numbers indicate positions of the peptides within the protein sequences.
Lymphocyte transformation assay (LTA).
PBMC were isolated from heparanized blood by Histopaque-1077 (Sigma, Poole, United Kingdom) gradient centrifugation and cultured in RPMI 1640 supplemented with 5% CPSR-1 (Sigma), nonessential amino acids (Sigma), 5 × 10−5 M 2-mercaptoethanol, 100 U of penicillin per ml, and 100 mg of streptomycin sulfate per ml. PBMC (2 × 105/well in 0.2-ml aliquots) were cultured in triplicate for 6 days in flat-bottom 96-well microtiter plates in the presence of antigen and radiolabelled during the last 16 to 20 h of culture with 37 kBq of [3H]thymidine (Amersham, Amersham, United Kingdom) per well, harvested onto glass fiber filters, and counted in a beta counter. Positive responses were defined as a stimulation index (SI), i.e., radioactivity with antigen/radioactivity without antigen, of ≥3 together with a signal strength of ≥1,000 cpm (13, 35, 41).
IFN-γ and interleukin-2 (IL-2) assays.
Whole-blood cultures were performed in 96-well plates in 0.25-ml/well aliquots by mixing heparinized blood with an equal volume of antigen-containing solution. Supernatants were harvested after 24 h of culture, and gamma interferon (IFN-γ) responses were determined by using the BOVIGAM enzyme-linked immunosorbent assay (ELISA) kit (CSL, Melbourne, Australia) (44). In vitro IFN-γ responses to PPD-M and PPD-A were interpreted as set out in the instructions accompanying the BOVIGAM ELISA kit. Results obtained with recombinant proteins, peptides, and diagnostic cocktails were deemed positive when the IFN-γ SI (ISI), i.e., optical density at 450 nm (OD450) with antigen/OD450 without antigen, was ≥2.0 (21, 22).
IL-2 production in the same supernatants was determined by the ability to sustain proliferation of lymphoblasts generated by stimulation with concanavalin A (ConA) (6). Briefly, ConA-induced lymphoblasts (5 μg of ConA/ml) were cocultured with the test supernatants for 24 h and then labelled for 24 h with 37 kBq of [3H]thymidine/well, harvested onto glass fiber filters, and counted in a beta counter. A standard of recombinant IL-2 (provided by R. Collins, Institute for Animal Health, Compton, United Kingdom) was included in each assay.
RESULTS
Recognition of recombinant mycobacterial antigens.
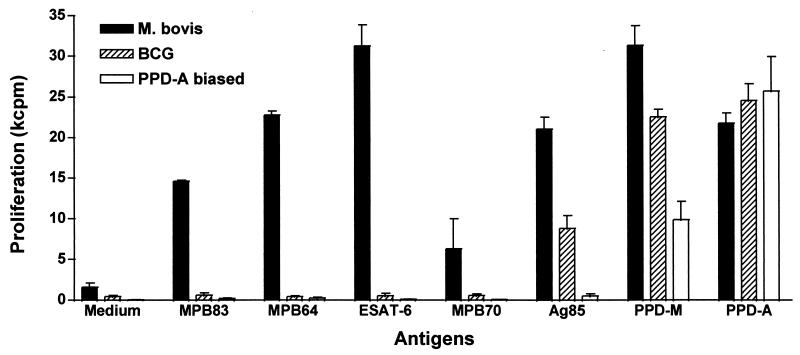
Recombinant forms of the M. bovis antigens ESAT-6, MPB64, MPB70, and MPB83 were expressed in E. coli and purified as described in Materials and Methods. These recombinant antigens were then tested for their ability to induce T-cell proliferation and IFN-γ secretion in PBMC from field reactor cattle (SICTT positive), M. bovis-infected animals, BCG-vaccinated cattle, or cattle naturally sensitized with environmental mycobacteria (defined by stronger PPD-A responses than PPD-M responses). Native Ag85 complex antigens purified from BCG culture filtrate were included as a control because they are strongly expressed in both M. bovis and BCG (39). The results of a representative experiment are shown in Fig. 1. Strong proliferative responses were observed in PBMC isolated from M. bovis field reactors stimulated with ESAT-6, MPB64, and MPB83, as well as in those cultures stimulated with the Ag85 complex antigens (shown for one representative field reactor in Fig. 1). Stimulation with MPB70 gave rise to weaker yet still significant responses.
FIG. 1.
Recognition of mycobacterial proteins. PBMC isolated from one SICTT-positive field reactor (M. bovis), three BCG-vaccinated cattle (BCG), and one animal with PPD-A-biased responses (PPD-A biased) were incubated with recombinant mycobacterial proteins and avian and mammalian tuberculin at 10 μg/ml for 6 days. Results are expressed as means ± standard errors.
In contrast, PBMC from BCG-vaccinated or from PPD-A-biased responders did not recognize these antigens, thus confirming that ESAT-6, MPB64, MPB70, and MPB83 might be suitable antigens to differentiate between M. bovis infection and BCG vaccination. BCG-vaccinated animals reacted with Ag85 complex antigens, while M. avium reactor cattle did not (Fig. 1). Similarly, PBMC from field reactor cattle produced IFN-γ in response to ESAT-6, MPB64, and MPB83, while BCG-vaccinated or PPD-A-biased reactor cattle did not (data not shown).
To define responder frequencies for ESAT-6, MPB64, and MPB83, a cohort of 18 field reactors was tested with these antigens. Proliferative responses were induced in 66, 33, and 50% of the animals, respectively (Table 2). These results confirm that only a subset of animals will respond to any given antigen, as described earlier (7, 8). In this context, although ESAT-6 was the dominant antigen, MPB83 and MPB64 were recognized by some animals that did not recognize ESAT-6 (Table 2).
TABLE 2.
Immune responses against recombinant mycobacterial proteins in field animals that were positive by the single comparative tuberculin testa
| Animal | Skin test thickness (mm)b | In vitro proliferative
responsec
|
||||
|---|---|---|---|---|---|---|
| PPD-Md | PPD-A | ESAT-6e | MPB64 | MPB83 | ||
| 1 | 36 | 54.2 (27.8) | 54.7 (28.0) | 2.6 (1.3) | 38.4 (19.7) | 15.2 (7.8) |
| 2 | 31 | 233.0 (37.5) | 223.0 (30.0) | 260.8 (41.9) | 2.8 (0.5) | 102.0 (16.5) |
| 3 | 30 | 95.6 (38.5) | 85.1 (34.2) | 30.1 (12.1) | 3.6 (1.4) | 21.7 (8.7) |
| 4 | 22 | 161.0 (35.6) | 116.5 (25.8) | 199.0 (44.0) | 1.0 (0.2) | 2.1 (0.5) |
| 5 | 18 | 466.1 (66.7) | 66.5 (9.5) | 351.7 (50.3) | 3.8 (0.5) | 18.2 (2.6) |
| 6 | 14 | 65.0 (14.2) | 7.2 (1.6) | 4.3 (0.9) | 0.8 (0.2) | 1.8 (0.4) |
| 7 | 10 | 23.6 (13.8) | 19.2 (11.2) | 0.5 (0.3) | 1.9 (1.1) | 0.8 (0.4) |
| 8 | 9 | 119.4 (35.4) | 74.2 (20.5) | 29.4 (8.1) | 27.0 (7.5) | 1.6 (0.4) |
| 9 | 8 | 116.9 (35.4) | 198.1 (60.0) | 2.8 (0.8) | 2.3 (0.7) | 1.5 (0.5) |
| 10 | 8 | 25.1 (8.6) | 18.3 (6.3) | 8.8 (3.0) | 1.4 (0.5) | 8.2 (2.8) |
| 11 | 7 | 92.9 (45.8) | 64.5 (31.8) | 7.7 (3.8) | 11.5 (5.6) | 5.9 (2.9) |
| 12 | 7 | 233.0 (37.5) | 223.0 (36.0) | 260.8 (41.9) | 2.8 (0.5) | 102.0 (16.5) |
| 13 | 7 | 68.7 (14.3) | 34.0 (7.1) | 3.4 (0.7) | 1.9 (0.4) | 2.5 (0.5) |
| 14 | 6 | 25.5 (30.4) | 9.7 (11.5) | 12.6 (15.0) | 4.7 (5.6) | 13.4 (16.0) |
| 15 | 6 | 219.1 (28.7) | 18.5 (2.4) | 67.7 (8.9) | 0.8 (0.1) | 18.6 (2.4) |
| 16 | 5 | 3.5 (0.1) | 8.0 (0.3) | 2.1 (0.1) | 1.3 (0.1) | 1.5 (0.1) |
| 17 | 5 | 57.1 (14.6) | 8.1 (2.1) | 3.2 (0.8) | 4.6 (1.2) | 0.9 (0.2) |
| 18 | 3 | 377.0 (56.6) | 321.0 (48.2) | 55.7 (8.2) | 0.9 (0.1) | 1.0 (0.2) |
PBMC from field reactors were isolated and cultured within 8 h of blood sampling. Antigen concentrations were 10 μg/ml for PPD-M, PPD-A, MPB83, and MPB64 and 5 μg/ml for ESAT-5.
Skin test measurements at 72 h are thickness induced with PPD-A substracted from thickness induced with PPD-M.
Results are expressed as SIs (defined as radioactivity with antigen/radioactivity without antigen), with results for the individual antigen (in kilocounts per minute) shown in parentheses.
If the SI for PPD-M was greater than that for PPD-A, the result was deemed positive; positive responses are underlined (responder frequency was 83% (15 of 18).
For recombinant proteins and cocktails, if the SI was ≥3 and the radioactivity with antigen was ≥1,000 cpm, a response was deemed positive; positive responses are in bold. Responder frequencies were as follows: ESAT-6, 66% (12 of 18); MPB64, 33% (6 of 18); and MPB83, 50% (9 of 18).
Definition of diagnostic cocktails composed of either recombinant proteins or promiscuous synthetic peptides of ESAT-6, MPB64, and MPB83.
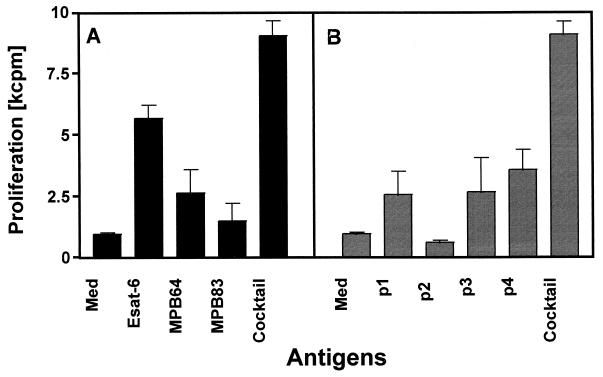
We next investigated whether diagnostic cocktails of pooled recombinant proteins or synthetic peptides representing their immunodominant epitopes would give rise to elevated responder frequencies as well as increased signal strength. A protein cocktail composed of ESAT-6, MPB64, and MPB83 was tested in parallel with a peptide cocktail containing seven promiscuous peptides derived from ESAT-6, MPB64, MPB70, and MPB83 (Table 1). These peptides were identified in a separate investigation which showed that they were recognized “promiscuously” by T cells from more than 50% of the M. bovis-infected cattle tested (data not shown and reference 36a). First, these diagnostic cocktails were tested in a small number of field reactors. The PBMC responses induced by cocktails were compared with those induced by their individual components. Results of a representative experiment which demonstrated that both protein and peptide cocktails induced stronger proliferative responses than any of their constituents alone are shown in Fig. 2. In addition, cocktails also compensated for the lack of recognition of individual protein or peptide constituents.
FIG. 2.
Proliferative responses induced by protein (A) and peptide (B) cocktails. PBMC isolated from a SICTT-positive reactor were incubated with recombinant ESAT-6, MPB64, or MPB83 individually (10 μg/ml) or a pool of all three (5 μg/ml each). PBMC from the same reactor animal were incubated with peptides p1 to p4 individually (10 μg/ml) or a pool of all four peptides (10 μg/ml each). Results are expressed as means ± standard errors.
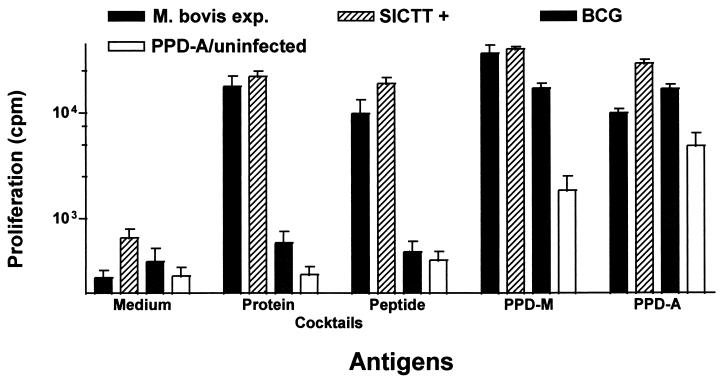
Both cocktails were further tested with PBMC from calves experimentally infected with M. bovis (2 animals), SICTT-positive field reactors (3 animals), animals vaccinated with BCG (7 animals), or PPD-A-biased responders (13 animals). Proliferative immune responses were induced in all field reactors and experimentally infected calves tested in this experiment. In contrast, only one of the BCG-vaccinated animals and 1 of the 13 PPD-A-biased responders or tuberculin-negative cattle had a weak reaction to the protein cocktail. Furthermore, none of the vaccinated or M. avium-sensitized, SICTT-negative animals reacted with the peptide cocktail (Fig. 3), whereas 6 of the 7 BCG-vaccinated cattle responded strongly to both bovine and avian tuberculin. Similar response patterns were observed when antigen-induced IFN-γ was measured in culture supernatants (data not shown).
FIG. 3.
Proliferative responses induced by protein and peptide cocktails. PBMC isolated from 3 SICTT-positive field reactor cattle (SICTT+), 2 cattle experimentally infected with M. bovis (M. bovis exp.), 7 BCG-vaccinated cattle (BCG), and 13 cattle with either PPD-A-biased responses or negative SICTT responses (PPD-A/uninfected) were incubated with either the protein cocktail (5 μg/ml each) or the peptide cocktail described in Table 1 (10 μg/ml each). Results are expressed as mean proliferative responses ± standard errors.
Assessment of the diagnostic cocktails in field reactors.
Having demonstrated that both the protein and peptide diagnostic cocktails described above were not recognized by PMBC from BCG-vaccinated cattle, we next set out to quantify their ability to identify infected animals by testing a cohort of 35 SICTT-positive field animals. The blood samples were obtained within 7 to 28 days after skin testing from six farms in Gloucestershire and Worcestershire, transported to our laboratory within 6 to 8 h, and processed immediately upon arrival. Parameters tested were PBMC LTA responses and whole-blood IFN-γ production. The results of the LTAs can be summarized as follows (Table 3): 29 (82.8%) of the 35 SICTT-positive field reactors were identified on the basis of stronger responses to PPD-M than to PPD-A. Encouragingly, T cells from 74.2% (27 of 35) of these cattle recognized the protein cocktail, while T cells from 71.4% (25 of 33) reacted with the peptide cocktail, thus achieving sensitivity levels only slightly inferior to that for PPD. When IFN-γ production was measured in culture supernatants of whole-blood cultures and the criteria set out in the BOVIGAM ELISA kit were used to score for positivity, 82.8% of the SICTT-positive field reactors were identified, whereas the protein cocktail identified 68.6% (24 of 35) and the peptide cocktail identified 55.2% (16 of 29) of these cattle (Table 4).
TABLE 3.
Proliferative responses against diagnostic protein and peptide cocktails in field animals that were positive by the single comparative tuberculin testa
| Animal | Skin test thickness (mm)b | In vitro proliferative
responsec
|
|||
|---|---|---|---|---|---|
| PPD-Md | PPD-A | Cocktaile
|
|||
| Protein | Peptide | ||||
| 1 | 36 | 54.2 (27.8) | 54.7 (28.0) | 55.4 (28.4) | 8.3 (4.2) |
| 2 | 31 | 233.0 (37.5) | 223.0 (30.0) | 277.8 (44.7) | 156.0 (25.1) |
| 3 | 30 | 95.6 (38.5) | 85.1 (34.2) | 43.0 (17.3) | 42.2 (16.9) |
| 4 | 24 | 132.1 (40.8) | 49.5 (15.3) | 35.7 (11.0) | 1.1 (0.3) |
| 5 | 22 | 161.0 (35.6) | 116.5 (25.8) | 202.5 (44.7) | 158.4 (35.0) |
| 6 | 18 | 466.1 (66.7) | 66.5 (9.5) | 235.0 (33.6) | 181.7 (25.9) |
| 7 | 14 | 65.0 (14.2) | 7.2 (1.6) | 4.0 (0.9) | 1.8 (0.4) |
| 8 | 13 | 224.0 (30.7) | 91.8 (12.5) | 142.5 (19.3) | 48.4 (6.6) |
| 9 | 12 | 110.7 (54.5) | 42.5 (20.9) | 73.5 (36.2) | 26.2 (12.9) |
| 10 | 10 | 23.6 (13.8) | 19.2 (11.2) | 0.9 (0.5) | 2.1 (1.2) |
| 11 | 10 | 55.2 (35.7) | 23.7 (15.3) | 31.9 (20.7) | 18.8 (12.2) |
| 12 | 10 | 165.2 (30.0) | 165.2 (30.0) | 126.6 (23.0) | 39.6 (7.2) |
| 13 | 9 | 119.4 (35.4) | 74.2 (20.5) | 34.4 (9.5) | 15.0 (4.1) |
| 14 | 9 | 375.0 (36.4) | 438.0 (42.5) | 48.7 (4.7) | 45.2 (4.4) |
| 15 | 9 | 605.1 (35.7) | 191.5 (11.3) | 596.9 (35.2) | 451.4 (26.6) |
| 16 | 8 | 116.9 (35.4) | 198.1 (60.0) | 3.3 (1.0) | 5.6 (1.7) |
| 17 | 8 | 25.1 (8.6) | 18.3 (6.3) | 16.5 (5.6) | 10.0 (3.4) |
| 18 | 8 | 147.5 (40.1) | 114.4 (31.1) | 130.7 (35.5) | 123.6 (33.6) |
| 19 | 7 | 92.9 (45.8) | 64.5 (31.8) | 13.8 (6.8) | 3.7 (1.8) |
| 20 | 7 | 233.0 (37.5) | 223.0 (36.0) | 277.8 (44.7) | 156.0 (25.1) |
| 21 | 7 | 68.7 (14.3) | 34.0 (7.1) | 4.2 (0.9) | 8.9 (1.9) |
| 22 | 7 | 18.8 (29.2) | 12.3 (19.0) | 7.8 (12.0) | 4.4 (6.7) |
| 23 | 7 | 6.0 (5.7) | 4.7 (4.4) | 0.6 (0.5) | 1.2 (1.1) |
| 24 | 7 | 40.3 (9.8) | 33.3 (8.1) | 35.9 (8.7) | 11.7 (2.8) |
| 25 | 7 | 29.5 (2.2) | 7.9 (0.6) | 6.1 (0.4) | 1.7 (0.1) |
| 26 | 6 | 25.5 (30.4) | 9.7 (11.5) | 17.3 (20.6) | 10.9 (13.0) |
| 27 | 6 | 219.1 (28.7) | 18.5 (2.4) | 82.6 (10.8) | 19.7 (2.6) |
| 28 | 6 | 53.1 (5.8) | 13.9 (1.5) | 19.1 (2.1) | 1.3 (0.1) |
| 29 | 6 | 477.0 (52.4) | 477.0 (52.4) | 4.8 (0.5) | 2.2 (0.2) |
| 30 | 6 | 177.5 (9.9) | 31.9 (1.8) | 95.7 (5.4) | 16.0 (1.0) |
| 31 | 6 | 197.7 (31.2) | 31.0 (4.9) | 103.3 (16.3) | 47.5 (7.5) |
| 32 | 5 | 3.5 (0.1) | 8.0 (0.3) | 1.8 (0.1) | 2.1 (0.1) |
| 33 | 5 | 57.1 (14.6) | 8.1 (2.1) | 30.4 (7.8) | 3.8 (0.9) |
| 34 | 5 | 279.3 (31.5) | 33.9 (3.8) | 1.5 (0.1) | 2.0 (0.2) |
| 35 | 3 | 377.0 (56.6) | 321.0 (48.2) | 76.6 (3.8) | 144.6 (21.7) |
PBMC from field reactors were isolated and cultured within 8 h of blood sampling. For antigen concentrations; see Materials and Methods.
Skin test measurements at 72 h are thickness induced with PPD-A subtracted from thickness induced with PPD-M.
Results are expressed as SIs (defined as radioactivity with antigen/radioactivity without antigen) with results for the individual antigen shown (in kilocounts per minute) in parentheses.
If the SI for PPD-M was greater than that for PPD-A, the result was deemed positive; positive responses are underlined (responder frequency was 82.8% [29 of 35]).
For protein and peptide cocktails, if the SI was ≥3 and the radioactivity with antigen was ≥1,000 cpm, a response was deemed positive; positive responses are in bold. Responder frequencies were as follows: protein cocktail, 74.2% (27 of 35), and peptide cocktail, 71.4% (25 of 35).
TABLE 4.
IFN-γ responses against diagnostic protein and peptide cocktails in field animals that were positive by the single comparative tuberculin testa
| Animal | Skin test thickness (mm)b | In vitro IFN-γ
responsec
|
|||
|---|---|---|---|---|---|
| PPD-Md | PPD-A | Cocktaile
|
|||
| Protein | Peptide | ||||
| 1 | 36 | 14.9 (2.12) | 5.1 (0.62) | 5.8 (0.72) | 2.4 (0.21) |
| 2 | 31 | 80.3 (3.33) | 22.3 (0.89) | 63.5 (2.62) | 34.4 (1.40) |
| 3 | 30 | 35.6 (2.14) | 16.9 (0.98) | 14.5 (0.84) | 10.0 (0.56) |
| 4 | 24 | 35.2 (2.90) | 6.1 (0.43) | 4.6 (0.30) | 1.6 (0.05) |
| 5 | 22 | 26.9 (2.88) | 9.0 (0.92) | 21.3 (2.33) | 16.4 (1.77) |
| 6 | 18 | 13.7 (0.83) | 1.4 (0.02) | 16.3 (1.01) | 9.9 (0.58) |
| 7 | 14 | 7.2 (0.50) | 3.0 (0.16) | 3.5 (0.20) | 2.4 (0.11) |
| 8 | 13 | 16.8 (2.91) | 6.3 (0.98) | 6.7 (1.054) | 5.1 (0.75) |
| 9 | 12 | 40.9 (2.87) | 11.3 (0.74) | 18.2 (1.31) | ND |
| 10 | 10 | 6.6 (0.41) | 5.7 (0.35) | 0.9 (−0.01) | 1.1 (0.01) |
| 11 | 10 | 8.3 (1.54) | 3.2 (0.47) | 5.6 (0.97) | 3.9 (0.63) |
| 12 | 10 | 25.9 (2.52) | 14.3 (1.16) | 6.5 (0.56) | 3.2 (0.23) |
| 13 | 9 | 6.4 (0.53) | 2.6 (0.16) | 3.2 (0.21) | 1.5 (0.08) |
| 14 | 9 | 12.3 (1.11) | 6.4 (0.55) | 4.0 (0.31) | 3.8 (0.28) |
| 15 | 9 | 32.8 (2.22) | 26.2 (1.76) | 6.1 (0.36) | 4.4 (0.24) |
| 16 | 8 | 7.5 (0.62) | 9.5 (0.81) | 2.0 (0.12) | 1.0 (−0.01) |
| 17 | 8 | 2.9 (0.11) | 2.3 (0.07) | 1.1 (0.01) | 1.5 (0.03) |
| 18 | 8 | 21.0 (1.58) | 3.8 (0.21) | 10.5 (0.75) | ND |
| 19 | 7 | 4.0 (0.24) | 4.9 (0.31) | 0.9 (−0.01) | 0.7 (−0.02) |
| 20 | 7 | 1.9 (0.05) | 1.3 (0.02) | 1.6 (0.04) | 1.6 (0.04) |
| 21 | 7 | 2.5 (0.15) | 2.3 (0.12) | 1.1 (0.01) | 0.9 (−0.01) |
| 22 | 7 | 9.5 (2.04) | 3.2 (0.52) | 3.7 (0.84) | ND |
| 23 | 7 | 1.8 (0.14) | 1.2 (0.04) | 1.3 (0.04) | 0.63 (−0.06) |
| 24 | 7 | 8.8 (0.76) | 3.9 (0.28) | 5.0 (0.38) | ND |
| 25 | 7 | 9.9 (0.86) | 1.4 (0.05) | 16.9 (1.56) | ND |
| 26 | 6 | 5.0 (1.08) | 2.3 (0.34) | 4.1 (0.83) | 2.4 (0.38) |
| 27 | 6 | 2.9 (0.24) | 1.6 (0.07) | 1.4 (0.05) | 1.1 (0.01) |
| 28 | 6 | 3.7 (0.27) | 1.5 (0.05) | 1.6 (0.06) | 2.1 (0.11) |
| 29 | 6 | 18.2 (2.04) | 15.7 (1.74) | 1.6 (0.06) | 1.6 (0.06) |
| 30 | 6 | 2.0 (0.38) | 0.7 (−0.1) | 1.0 (0.01) | 0.6 (−0.14) |
| 31 | 6 | 4.7 (0.54) | 1.0 (0.01) | 4.0 (0.44) | ND |
| 32 | 5 | 4.5 (0.76) | 3.8 (0.61) | 3.1 (0.45) | 2.2 (0.25) |
| 33 | 5 | 2.3 (0.14) | 1.4 (0.04) | 2.0 (0.11) | 2.0 (0.11) |
| 34 | 5 | 5.1 (0.69) | 1.56 (0.27) | 0.7 (−0.05) | 0.6 (−0.07) |
| 35 | 3 | 4.8 (0.33) | 4.2 (0.29) | 3.0 (0.18) | 3.4 (0.21) |
Antigens were cultured in whole-blood cultures for 24 h. For antigen concentrations see the legend for Fig. 3.
Skin test measurements at 72 h are thickness induced with PPD-A subtracted from thickness induced with PPD-B.
Results are expressed as ISIs (defined as OD450 with antigen/OD450 without antigen), with ΔOD450s in parentheses.
A result was deemed positive if the ΔOD450 for PPD-M minus the ΔOD450 for PPD-A was ≥0.05, as set out in the the instructions accompanying the BOVIGAM ELISA kit; positive responses are underlined.
Positive results (defined as an ISI of ≥2) are in bold. ND, not determined.
IL-2 as a surrogate for proliferation.
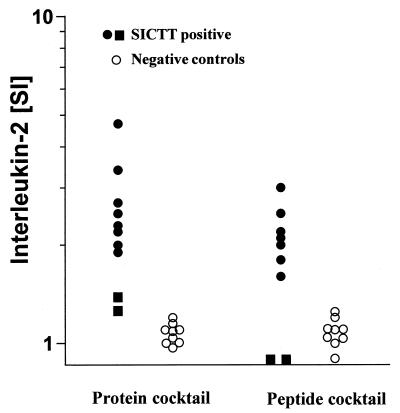
Since LTAs are labor-intensive and take 5 to 6 days to complete, a surrogate marker for proliferation amenable to an ELISA format and to whole-blood cultures would be highly desirable. We tested whether determination of IL-2 levels in culture supernatants from 24-h whole-blood cultures could serve as such a surrogate assay. IL-2 production was measured in culture supernatants obtained from cultures from 9 field reactors and from 10 control animals from farms with no recent history of TB. The results of this pilot experiment were compared to those obtained with PBMC LTAs and suggest that IL-2 production could indeed serve as a surrogate for proliferation (Fig. 4). Neither the protein nor the peptide cocktail induced IL-2 production in control animals, whereas IL-2 production was induced in cultures from field reactors whose lymphocytes recognized both cocktails in LTAs. Interestingly, IL-2 levels were comparable to those in the control group in supernatants from two animals whose PBMC did not proliferate following stimulation with either the peptide or the protein cocktail.
FIG. 4.
IL-2 production as a surrogate marker for proliferation. Whole-blood cultures in the presence of either the protein or the peptide cocktail were performed with samples obtained from 9 SICTT-positive field reactors and from 10 negative control animals from farms with no history of TB. Supernatants were collected after 24 h, and IL-2 production was determined. Filled circles, samples from cattle recognizing the cocktails in LTAs; filled squares, samples from cattle not responding to cocktails in LTAs.
DISCUSSION
The development of diagnostic reagents capable of differentiating between infection and vaccination is a prerequisite for the development of a vaccine against TB in cattle so that existing test-and-slaughter control strategies can continue alongside vaccination. Such differential diagnosis would also be of benefit to human vaccination regimes so that individuals who, despite vaccination, contract TB and require chemotherapy can be identified. BCG vaccination has been shown to compromise tuberculin skin testing in cattle (and in humans) and in vitro assays which utilize tuberculin, including the IFN-γ-based ELISA kit (42–44).
The general trend in the diagnosis of bovine TB in cattle points to a preference for blood-based tests since, in contrast to the tuberculin skin test, they require only a single farm visit, and it has been suggested that a blood-based test could be used in conjunction with the SICTT to reduce the time between consecutive tests in animals with inconclusive results (1). Such blood-based assays are also being developed for other species, including humans (20, 32, 33).
ESAT-6 and MPB64, both constituents of the diagnostic cocktails used in this study, have been tested previously in cattle. ESAT-6 has been shown to induce IFN-γ production in 85 to 90% of the field reactors tested in Northern Ireland (27, 28). Since the gene encoding ESAT-6 is not present in BCG or in most environmental mycobacteria, ESAT-6 is an obvious candidate antigen for differential diagnosis. This study confirms that ESAT-6 induces T-cell responses in the majority of field reactors tested, albeit with a lower frequency, 66%. This lower figure could be due to a difference in the genetic makeup of the two study populations or to differences in the levels of expression of ESAT-6 in some M. bovis strains in GB. A recent study (21) has defined the responder frequency for MPB64 as 50%, which is higher than the 33% observed in our study population. These differences in responder frequencies between different study populations highlight the necessity to evaluate the frequencies of responders to specific antigens in the target population. To our knowledge, frequencies of responders to MPB83 among cattle have not been determined before. We were encouraged that 50% of the field reactors tested recognized this protein, and it was therefore incorporated into our diagnostic cocktails.
The data presented in this study provides proof of the principle that differential diagnosis discriminating between vaccination and infection can be achieved. Our study has also shown that only a subset of infected animals will respond to a single antigen, reinforcing previous observations (7, 8) and demonstrating that it is unlikely that single antigens will be able to identify the vast majority of infected animals. We have also demonstrated that stronger responses can be induced with reagent pools than with their individual components. Recently, protein pools composed of mycobacterial antigens which provide improved specificity over tuberculin in a guinea pig model have been defined (23). A further advantage in using diagnostic antigen cocktails is that it reduces the possibility of selecting “escape mutants”, i.e., strains of tubercle bacilli lacking the diagnostic antigen. Such natural mutants have been described. For example, strains of M. tuberculosis which do not express the 19-kDa lipoprotein have been identified (19).
The use of synthetic peptides rather than recombinant antigens as diagnostic reagents has the added advantages of lower production costs, easier standardization, and lack of any risk of infection from the recombinant strain. However, due to the genetic diversity of the major histocompatibility complex (MHC), the use of peptides has in the past been deemed impractical, since it was believed that a large pool of epitopes would be required to achieve wide population coverage. Fortunately, it has been recognized over recent years that a significant proportion of MHC class II-restricted peptides are recognized by T cells in the context of multiple MHC alleles. This has been demonstrated to be particularly so in the recognition of mycobacterial antigens by murine, human (reviewed in reference 34), and bovine CD4+ T cells (29, 30). Moreover, rationally designed peptide cocktails have been shown to induce responses in PBMC from human TB patients and BCG-vaccinated donors with high sensitivity (17). The results presented in this study further confirm these results, since we were able to detect more than 70% of reactor cattle with a small pool of seven peptides.
The responder frequencies obtained for field samples by LTAs with the protein and peptide cocktails were comparable to those obtained with tuberculin (74.2 and 71.4% versus 82.8%, respectively). The same responder frequency was observed when responses to PPD-M were compared with those to PPD-A by using IFN-γ as a readout system. This sensitivity is within the range observed in different studies (between 76 and 98%) during the evaluation of the IFN-γ assay (38, 43, 44). The sensitivity of the IFN-γ assay, indicated by the results observed for the defined protein and peptide cocktails, was significantly lower than that indicated by results obtained in LTAs with the same antigens (68.6 and 55%, respectively). This could reflect the greater standardization of culture conditions for LTA, particularly with respect to cell numbers and culture conditions.
LTAs are highly labor-intensive, depend on specialized skills and equipment, and require several days for the results to become available. Thus, surrogate readout systems of proliferation amenable to whole-blood-based ELISAs are desirable. Determination of production of soluble IL-2 receptors has been used recently with good results (24, 25). In this context, our results demonstrating that the levels of IL-2 in 24-h whole-blood culture supernatants are in concordance with the results observed for the LTAs are highly encouraging.
In conclusion, we have demonstrated that diagnostic cocktails based on either recombinant protein or peptides derived from antigens expressed by M. bovis but not by the vaccine strain, in this case BCG Pasteur, can distinguish between vaccinated and infected individuals. We predict that the addition of other such antigens to the cocktails will increase their sensitivity further without compromising specificity. The development of such protein and peptide pools is now under investigation in our laboratory.
ACKNOWLEDGMENTS
We acknowledge the valuable assistance of the Veterinary Field Service Staff of the Ministry of Agriculture Fisheries and Food, particularly in the Gloucester and Worcester Animal Health Offices, in the collection of blood samples from field reactor cattle for use in this study. We also express our appreciation for the staff of the Animal Services Unit at Veterinary Laboratories Agency—Weybridge.
The work performed was jointly funded by the Ministry of Agriculture Fisheries and Food, Great Britain, and EU contract AIR CT 92-0697.
REFERENCES
- 1.Anonymous. New Zealand: Bovigam™, it is official. Vet Diagn Newsl. 1997;2:1–3. [Google Scholar]
- 2.Berggren S A. Field experiment with BCG vaccine in Malawi. Br Vet J. 1981;137:88–94. doi: 10.1016/s0007-1935(17)31792-x. [DOI] [PubMed] [Google Scholar]
- 3.Buddle B M, de Lisle G W, Pfeffer A, Aldwell F E. Immunological responses and protection against Mycobacterium bovis in calves vaccinated with a low dose of BCG. Vaccine. 1995;13:1123–1130. doi: 10.1016/0264-410x(94)00055-r. [DOI] [PubMed] [Google Scholar]
- 4.Buddle B M, Keen D, Thomson A, Jowett G, McCarthy A R, Heslop J, De Lisle G W, Stanford J L, Aldwell F E. Protection of cattle from bovine tuberculosis by vaccination with BCG by the respiratory or subcutaneous route, but not by vaccination with killed Mycobacterium vaccae. Res Vet Sci. 1995;59:10–16. doi: 10.1016/0034-5288(95)90023-3. [DOI] [PubMed] [Google Scholar]
- 5.Elhay M J, Oettinger T, Andersen P. Delayed-type hypersensitivity responses to ESAT-6 and MPT64 from Mycobacterium tuberculosis in the guinea pig. Infect Immun. 1998;66:3454–3456. doi: 10.1128/iai.66.7.3454-3456.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emery D L, Dufty J H, Wood P R. An analysis of cellular proliferation, and synthesis of lymphokines and specific antibody in vitro by leucocytes from immunised cattle. Vet Immunol Immunopathol. 1988;18:67–80. doi: 10.1016/0165-2427(88)90037-2. [DOI] [PubMed] [Google Scholar]
- 6a.European Economic Community. EEC directive 80/219 EEC, amending directive 64/432 annexe B. Official Journal. 1980;L047:25–32. [Google Scholar]
- 7.Fifis T, Corner L A, Rothel J S, Wood P R. Cellular and humoral immune responses of cattle to purified Mycobacterium bovis antigens. Scand J Immunol. 1994;39:267–274. doi: 10.1111/j.1365-3083.1994.tb03370.x. [DOI] [PubMed] [Google Scholar]
- 8.Fifis T, Rothel J S, Wood P R. Soluble Mycobacterium bovis protein antigens: studies on their purification and immunological evaluation. Vet Microbiol. 1994;40:65–81. doi: 10.1016/0378-1135(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 9.Francis J. Bovine tuberculosis. London, England: Staples Press; 1947. [Google Scholar]
- 10.Haga S, Yamaguchi R, Nagai S, Matsuo K, Yamazaki A, Nakamura R M. Delayed-type hypersensitivity to a recombinant mycobacterial antigen, MPB64, in guinea pigs sensitized to Mycobacterium tuberculosis or Mycobacterium bovis BCG. J Leukoc Biol. 1995;57:221–225. doi: 10.1002/jlb.57.2.221. [DOI] [PubMed] [Google Scholar]
- 11.Harboe M, Oettinger T, Wiker H G, Rosenkrands I, Andersen P. Evidence for occurrence of the ESAT-6 protein in Mycobacterium tuberculosis and virulent Mycobacterium bovis and for its absence in Mycobacterium bovis BCG. Infect Immun. 1996;64:16–22. doi: 10.1128/iai.64.1.16-22.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardie R M, Watson J M. Mycobacterium bovis in England and Wales: past, present and future. Epidemiol Infect. 1992;109:23–33. [PMC free article] [PubMed] [Google Scholar]
- 13.Harris D P, Vordermeier H M, Friscia G, Roman E, Surcel H M, Pasvol G, Moreno C, Ivanyi J. Genetically permissive recognition of adjacent epitopes from the 19-kDa antigen of Mycobacterium tuberculosis by human and murine T cells. J Immunol. 1993;150:5041–5050. [PubMed] [Google Scholar]
- 14.Hart P D, Sutherland I, Thomas J. The immunity conferred by effective BCG and vole bacillus vaccines, in relation to individual variations in induced tuberculin sensitivity and to technical variations in the vaccines. Tubercle. 1967;48:201–210. [Google Scholar]
- 15.Hewinson R G, Michell S L, Russell W P, McAdam R A, Jacobs W J. Molecular characterization of MPT83: a seroreactive antigen of Mycobacterium tuberculosis with homology to MPT70. Scand J Immunol. 1996;43:490–499. doi: 10.1046/j.1365-3083.1996.d01-78.x. [DOI] [PubMed] [Google Scholar]
- 16.Hubrig T, Kruger W. Untersuchungen zur Tuberkuloseschutzimpfung bei Rindern. Monatsh Vetmed. 1958;13:513–519. [Google Scholar]
- 17.Jurcevic S, Hills A, Pasvol G, Davidson R N, Ivanyi J, Wilkinson R J. T cell responses to a mixture of Mycobacterium tuberculosis peptides with complementary HLA-DR binding profiles. Clin Exp Immunol. 1996;105:416–421. doi: 10.1046/j.1365-2249.1996.d01-791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krebs J R. Bovine tuberculosis in cattle and badgers. London, England: Ministry of Agriculture, Fisheries and Food Publications; 1997. [Google Scholar]
- 19.Lathigra R, Zhang Y, Hill M, Garcia M J, Jackett P S, Ivanyi J. Lack of production of the 19-kDa glycolipoprotein in certain strains of Mycobacterium tuberculosis. Res Microbiol. 1996;147:237–249. doi: 10.1016/0923-2508(96)81384-2. [DOI] [PubMed] [Google Scholar]
- 20.Liebana E, Aranaz A, Urquia J J, Mateos A, Dominguez L. Evaluation of the gamma-interferon assay for eradication of tuberculosis in a goat herd. Aust Vet J. 1998;76:50–53. doi: 10.1111/j.1751-0813.1998.tb15686.x. [DOI] [PubMed] [Google Scholar]
- 21.Lightbody K A, Girvin R M, Mackie D P, Neill S D, Pollock J M. T-cell recognition of mycobacterial proteins MPB70 and MPB64 in cattle immunized with antigen and infected with Mycobacterium bovis. Scand J Immunol. 1998;48:44–51. doi: 10.1046/j.1365-3083.1998.00349.x. [DOI] [PubMed] [Google Scholar]
- 22.Lightbody K A, Girvin R M, Pollock D A, Mackie D P, Neill S D, Pollock J M. Recognition of a common mycobacterial T-cell epitope in MPB59 of Mycobacterium bovis. Immunology. 1998;93:314–322. doi: 10.1046/j.1365-2567.1998.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyashchenko K, Manca C, Colangeli R, Heijbel A, Williams A, Gennaro M L. Use of Mycobacterium tuberculosis complex-specific antigen cocktails for a skin test specific for tuberculosis. Infect Immun. 1998;66:3606–3610. doi: 10.1128/iai.66.8.3606-3610.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nuallain E M, Davis W C, Costello E, Pollock J M, Monaghan M L. Detection of Mycobacterium bovis infection in cattle using an immunoassay for bovine soluble interleukin-2 receptor-alpha (sIL-2R-alpha) produced by peripheral blood T-lymphocytes following incubation with tuberculin PPD. Vet Immunol Immunopathol. 1997;56:65–76. doi: 10.1016/s0165-2427(96)05734-0. [DOI] [PubMed] [Google Scholar]
- 25.Nuallain E M, Davis W C, Fisher A D, Monaghan M L. Development of a sandwich immunoassay for the detection of soluble bovine interleukin-2 receptor-alpha (sIL-2R-alpha) and its use to measure cell-mediated immunity in cattle. Vet Res Commun. 1997;21:19–28. doi: 10.1023/b:verc.0000009697.61013.6f. [DOI] [PubMed] [Google Scholar]
- 26.Philipp W J, Nair S, Guglielmi G, Lagranderie M, Gicquel B, Cole S T. Physical mapping of Mycobacterium bovis BCG Pasteur reveals differences from the genome map of Mycobacterium tuberculosis H37Rv and from M. bovis. Microbiology. 1996;142:3135–3145. doi: 10.1099/13500872-142-11-3135. [DOI] [PubMed] [Google Scholar]
- 27.Pollock J M, Andersen P. The potential of the ESAT-6 antigen secreted by virulent mycobacteria for specific diagnosis of tuberculosis. J Infect Dis. 1997;175:1251–1254. doi: 10.1086/593686. [DOI] [PubMed] [Google Scholar]
- 28.Pollock J M, Andersen P. Predominant recognition of the ESAT-6 protein in the first phase of infection with Mycobacterium bovis in cattle. Infect Immun. 1997;65:2587–2592. doi: 10.1128/iai.65.7.2587-2592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pollock J M, Douglas A J, Mackie D P, Neill S D. Identification of bovine T-cell epitopes for three Mycobacterium bovis antigens: MPB70, 19,000 MW and MPB57. Immunology. 1994;82:9–15. [PMC free article] [PubMed] [Google Scholar]
- 30.Pollock J M, Douglas A J, Mackie D P, Neill S D. Peptide mapping of bovine T-cell epitopes for the 38 kDa tuberculosis antigen. Scand J Immunol. 1995;41:85–93. doi: 10.1111/j.1365-3083.1995.tb03537.x. [DOI] [PubMed] [Google Scholar]
- 31.Rodrigues L C, Smith P G. Tuberculosis in developing countries and methods for its control. Trans R Soc Trop Med Hyg. 1990;84:739–744. doi: 10.1016/0035-9203(90)90172-b. [DOI] [PubMed] [Google Scholar]
- 32.Streeton J A, Desem N, Jones S L. Sensitivity and specificity of a gamma interferon blood test for tuberculosis infection. Int J Tuberc Lung Dis. 1998;2:443–450. [PubMed] [Google Scholar]
- 33.Villena V, Lopez-Encuentra A, Echave-Sustaeta J, Martin-Escribano P, Ortuno-de-Solo B, Estenoz-Alfaro J. Interferon-gamma in 388 immunocompromised and immunocompetent patients for diagnosing pleural tuberculosis. Eur Respir J. 1996;9:2635–2639. doi: 10.1183/09031936.96.09122635. [DOI] [PubMed] [Google Scholar]
- 34.Vordermeier H M. T-cell recognition of mycobacterial antigens. Eur Respir J Suppl. 1995;20:657s–667s. [PubMed] [Google Scholar]
- 35.Vordermeier H M, Harris D P, Friscia G, Roman E, Surcel H M, Moreno C, Pasvol G, Ivanyi J. T cell repertoire in tuberculosis: selective anergy to an immunodominant epitope of the 38-kDa antigen in patients with active disease. Eur J Immunol. 1992;22:2631–2637. doi: 10.1002/eji.1830221024. [DOI] [PubMed] [Google Scholar]
- 36.Vordermeier H M, Harris D P, Moreno C, Ivanyi J. Promiscuous T cell recognition of an H-2 IA-presented mycobacterial epitope. Eur J Immunol. 1994;24:2061–2067. doi: 10.1002/eji.1830240919. [DOI] [PubMed] [Google Scholar]
- 36a.Vordermeier, H. M., et al. Unpublished data.
- 37.Waddington F G, Ellwood D C. An experiment to challenge the resistance to tuberculosis in BCG vaccinated cattle in Malawi. Br Vet J. 1972;128:541–552. doi: 10.1016/s0007-1935(17)36683-6. [DOI] [PubMed] [Google Scholar]
- 38.Whipple D L, Bolin C A, Davis A J, Jarnagin J L, Johnson D C, Nabors R S, Payeur J B, Saari D A, Wilson A J, Wolf M M. Comparison of the sensitivity of the caudal fold skin test and a commercial gamma-interferon assay for diagnosis of bovine tuberculosis. Am J Vet Res. 1995;56:415–419. [PubMed] [Google Scholar]
- 39.Wiker H G, Harboe M. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol Rev. 1992;56:648–661. doi: 10.1128/mr.56.4.648-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wiker H G, Nagai S, Hewinson R G, Russell W P, Harboe M. Heterogenous expression of the related MPB70 and MPB83 proteins distinguish various substrains of Mycobacterium bovis BCG and Mycobacterium tuberculosis H37Rv. Scand J Immunol. 1996;43:374–380. doi: 10.1046/j.1365-3083.1996.d01-61.x. [DOI] [PubMed] [Google Scholar]
- 41.Wilkinson R J, Vordermeier H M, Wilkinson K A, Sjolund A, Moreno C, Pasvol G, Ivanyi J. Peptide specific response to M. tuberculosis: clinical spectrum, compartmentalization and effect of chemotherapy. J Infect Dis. 1998;178:760–768. doi: 10.1086/515336. [DOI] [PubMed] [Google Scholar]
- 42.Wood P R, Corner L A, Rothel J S, Baldock C, Jones S L, Cousins D B, McCormick B S, Francis B R, Creeper J, Tweddle N E. Field comparison of the interferon-gamma assay and the intradermal tuberculin test for the diagnosis of bovine tuberculosis. Aust Vet J. 1991;68:286–290. doi: 10.1111/j.1751-0813.1991.tb03254.x. [DOI] [PubMed] [Google Scholar]
- 43.Wood P R, Corner L A, Rothel J S, Ripper J L, Fifis T, McCormick B S, Francis B, Melville L, Small K, de Witte K, et al. A field evaluation of serological and cellular diagnostic tests for bovine tuberculosis. Vet Microbiol. 1992;31:71–79. doi: 10.1016/0378-1135(92)90142-g. [DOI] [PubMed] [Google Scholar]
- 44.Wood P R, Rothel J S. In vitro immunodiagnostic assays for bovine tuberculosis. Vet Microbiol. 1994;40:125–135. doi: 10.1016/0378-1135(94)90051-5. [DOI] [PubMed] [Google Scholar]