Abstract
BACKGROUND
The benefits and safety of the treatment of mild chronic hypertension (blood pressure, <160/100 mm Hg) during pregnancy are uncertain. Data are needed on whether a strategy of targeting a blood pressure of less than 140/90 mm Hg reduces the incidence of adverse pregnancy outcomes without compromising fetal growth.
METHODS
In this open-label, multicenter, randomized trial, we assigned pregnant women with mild chronic hypertension and singleton fetuses at a gestational age of less than 23 weeks to receive antihypertensive medications recommended for use in pregnancy (active-treatment group) or to receive no such treatment unless severe hypertension (systolic pressure, ≥160 mm Hg; or diastolic pressure, ≥105 mm Hg) developed (control group). The primary outcome was a composite of preeclampsia with severe features, medically indicated preterm birth at less than 35 weeks’ gestation, placental abruption, or fetal or neonatal death. The safety outcome was small-for-gestational-age birth weight below the 10th percentile for gestational age. Secondary outcomes included composites of serious neonatal or maternal complications, preeclampsia, and preterm birth.
RESULTS
A total of 2408 women were enrolled in the trial. The incidence of a primary-outcome event was lower in the active-treatment group than in the control group (30.2% vs. 37.0%), for an adjusted risk ratio of 0.82 (95% confidence interval [CI], 0.74 to 0.92; P<0.001). The percentage of small-for-gestational-age birth weights below the 10th percentile was 11.2% in the active-treatment group and 10.4% in the control group (adjusted risk ratio, 1.04; 95% CI, 0.82 to 1.31; P = 0.76). The incidence of serious maternal complications was 2.1% and 2.8%, respectively (risk ratio, 0.75; 95% CI, 0.45 to 1.26), and the incidence of severe neonatal complications was 2.0% and 2.6% (risk ratio, 0.77; 95% CI, 0.45 to 1.30). The incidence of any preeclampsia in the two groups was 24.4% and 31.1%, respectively (risk ratio, 0.79; 95% CI, 0.69 to 0.89), and the incidence of preterm birth was 27.5% and 31.4% (risk ratio, 0.87; 95% CI, 0.77 to 0.99).
CONCLUSIONS
In pregnant women with mild chronic hypertension, a strategy of targeting a blood pressure of less than 140/90 mm Hg was associated with better pregnancy outcomes than a strategy of reserving treatment only for severe hypertension, with no increase in the risk of small-for-gestational-age birth weight. (Funded by the National Heart, Lung, and Blood Institute; CHAP ClinicalTrials.gov number, NCT02299414.)
IN THE UNITED STATES, CHRONIC HYPERtension develops in 2% or more of pregnancies.1,2 This condition disproportionately affects Black women and is associated with three to five times the risk of preeclampsia, placental abruption, preterm birth or small-for-gestational-age birth weight, or perinatal death.1,3,4 The condition is also associated with 5 to 10 times the risk of maternal death, heart failure, stroke, pulmonary edema, or acute kidney injury.1,3,4
Antihypertensive treatment is the standard of care for nonpregnant patients with a blood pressure of 140/90 mm Hg or higher, but treatment during pregnancy is controversial.3–6 Antihypertensive treatment during pregnancy reduces the frequency of severe hypertension (blood pressure, ≥160/110 mm Hg) but has not been shown to improve maternal, fetal, or neonatal outcomes and has been associated with an increased risk of small-for-gestational-age birth weight.7–11 Thus, treatment recommendations for pregnant women with chronic hypertension vary among international organizations.4,12,13 There is consensus to treat pregnant women with severe hypertension, but for women with mild chronic hypertension (which is typically defined as a blood pressure of <160/110 mm Hg), it is unclear whether to withhold antihypertensive medication until the increase in blood pressure is severe or to continue the patient’s previously established therapy.4,12,13
To evaluate the benefits and safety of pharmacologic antihypertensive therapy during pregnancy, we designed a randomized trial involving women with mild chronic hypertension, a condition that is estimated to affect 70 to 80% of pregnant women with chronic hypertension. Our preliminary data suggested a stepwise increase in adverse pregnancy outcomes with increasing blood pressure above 140/90 mm Hg during the first half of pregnancy.14 We hypothesized that a strategy of treating mild chronic hypertension during pregnancy with a blood-pressure goal of less than 140/90 mm Hg would result in a lower incidence of adverse maternal and perinatal outcomes than a strategy of withholding treatment until the blood pressure was 160/105 mm Hg or higher (a more conservative cutoff for severe hypertension that we used in the trial).
METHODS
TRIAL DESIGN AND OVERSIGHT
The investigator-initiated Chronic Hypertension and Pregnancy (CHAP) project was a multicenter, pragmatic, open-label, randomized, controlled trial conducted at more than 70 recruiting sites in the United States. The trial was conducted on the basis of a cooperative agreement with the CHAP Trial Consortium, which included both clinical and data coordinating centers. The trial protocol (available with the full text of this article at NEJM.org) was approved by a protocol review committee appointed by the National Heart, Lung, and Blood Institute (NHLBI) and by the institutional review board at each trial center. The trial was overseen by a steering committee and an independent data and safety monitoring board appointed by the NHLBI. All the authors assume responsibility for the accuracy and completeness of the data and for the fidelity of the trial to the protocol.
ELIGIBILITY AND BLOOD-PRESSURE MEASUREMENT
The complete inclusion and exclusion criteria are provided in the protocol and in the Supplementary Appendix, available at NEJM.org. Pregnant women with a known or new diagnosis of chronic hypertension and a viable singleton fetus before 23 weeks’ gestation were eligible. New chronic hypertension was defined as a systolic blood pressure of 140 mm Hg or higher, a diastolic blood pressure of 90 mm Hg or higher, or both on at least two occasions at least 4 hours apart before 20 weeks’ gestation in patients without chronic hypertension. Known chronic hypertension was confirmed by a documented elevation in blood pressure and previous or current antihypertensive therapy, including lifestyle measures.
Blood-pressure levels that were required for randomization depended on whether the patient had currently been prescribed and had adhered to an antihypertensive medical regimen. If the patient had not received an antihypertensive drug within 24 hours before measurement, a systolic pressure of 140 to 159 mm Hg or a diastolic pressure of 90 to 104 mm Hg was required. If the patient had been receiving antihypertensive therapy, a systolic pressure of less than 160 mm Hg and a diastolic pressure of less than 105 mm Hg was required. Patients with a systolic blood pressure of less than 140 mm Hg and a diastolic pressure of less than 90 mm Hg were also eligible to participate. Gestational age was determined according to the criteria of the American College of Obstetricians and Gynecologists (ACOG).15 The results of ultrasonography were required before randomization.
Included in the exclusion criteria were severe hypertension or a blood-pressure level warranting antihypertensive treatment with more than one medication (indicating the risk of severe hypertension), known secondary hypertension, multiple fetuses, prespecified high-risk coexisting illnesses or complications that may warrant treatment at a lower blood-pressure level, obstetric conditions that increase fetal risk, and contraindications to first-line antihypertensive drugs recommended for use in pregnant women.
INTERVENTIONS AND PROCEDURES
A protocol for accurate, reproducible, and pragmatic measurement of blood pressure during clinic visits was used for screening and enrollment and to guide any adjustments to medications. Blood pressure was measured with an automated device (Omron HEM-907) at randomization for ancillary research purposes; clinical caregivers were unaware of these measurements unless they had been used as the patient’s blood pressure for clinical management (clinic blood pressure). Research staff members were trained and certified to implement this protocol, with regular orientation and guidance also provided to clinical staff. The clinic blood-pressure levels and other documented levels (e.g., during urgent care or hospital admissions) were used to adjudicate trial outcomes, including preeclampsia. (Details regarding all trial interventions and procedures are provided in the Supplementary Appendix.)
Patients were randomly assigned to a blood-pressure goal of less than 140/90 mm Hg (active treatment) or to standard (control) treatment, in which antihypertensive therapy was withheld or stopped at randomization unless severe hypertension (systolic pressure, ≥160 mm Hg; or diastolic pressure, ≥105 mm Hg) developed. If severe hypertension was identified in the control group, the target blood pressure for treatment was less than 160/105 mm Hg.
Trial-group assignments were implemented regardless of whether the patients were currently taking an antihypertensive medication. A Web-based randomization program was generated with the use of SAS software, version 9.4 (SAS Institute), and assignments were stratified according to site, with variable block sizes of 2, 4, and 6 to conceal the trial-group assignments. All the patients provided written informed consent.
Patients in the active-treatment group were prescribed a first-line antihypertensive drug for pregnancy (labetalol or extended-release nifedipine, supplied by trial investigators) or other medication such as amlodipine or methyldopa if preferred by the patient. The dose was escalated to the maximum recommended dose that was not associated with unacceptable side effects before the initiation of a second medication (preferably, nifedipine or labetalol if the other medication was started first) to achieve the target blood pressure. The control group received similar antihypertensive medications only if severe hypertension developed.
During clinic visits, patients were asked about their adherence to their blood-pressure regimen before any dose adjustments. Pill counts were performed at the time of each refill. Other assessments — including frequency of clinic visits, ultrasonographic analysis, and fetal surveillance and timing of delivery — were performed according to usual practices at each trial site.
OUTCOMES
The primary outcome was a composite of preeclampsia with severe features occurring up to 2 weeks after birth, medically indicated preterm birth before 35 weeks’ gestation (i.e., because of maternal or fetal illness, not spontaneous labor or membrane rupture), placental abruption, or fetal or neonatal death. Preeclampsia was defined according to ACOG criteria.3 Of note, a blood pressure of 160/100 mm Hg or greater in the absence of signs and symptoms of preeclampsia, proteinuria, or laboratory abnormalities was not sufficient to diagnose preeclampsia with severe features.3
The primary outcome was assessed in five prespecified subgroups according to hypertension treatment status at baseline (newly diagnosed, diagnosed and receiving medication, or diagnosed but not receiving medication), race or ethnic group, diabetes status, gestational age at enrollment (<14 weeks vs. ≥14 weeks), and body-mass index (the weight in kilograms divided by the square of the height in meters) according to three categories (<30, 30 to <40, or ≥40).
The primary safety outcome was poor fetal growth, which was defined as a birth weight measuring less than the 10th percentile for gestational age and infant sex according to the Duryea population standard.16 Also assessed was a small-for-gestational-age birth weight measuring less than the 5th percentile.
Major secondary outcomes included a composite of maternal death or serious complications (heart failure, stroke, or encephalopathy; myocardial infarction or angina; pulmonary edema; admission to an intensive care unit [ICU] or intubation; or renal failure), any preterm birth (<37 weeks’ gestation), and a composite of serious neonatal complications (bronchopulmonary dysplasia, retinopathy of prematurity, necrotizing enterocolitis, or intraventricular hemorrhage of grade 3 or 4). Other maternal outcomes included preeclampsia and worsening chronic hypertension (severe hypertension without preeclampsia), mean clinic blood-pressure levels, cesarean delivery, and blood transfusion. Additional newborn outcomes included neonatal ICU (NICU) admission, length of hospital stay, birth weight of less than 2500 g, hypoglycemia, bradycardia, hypotension, ponderal index, head circumference, and placental weight. (Details regarding all primary and secondary outcome definitions are provided in the Supplementary Appendix.)
Patients were followed until 6 weeks after birth. An outcome adjudication committee conducted blinded reviews of patients suspected of having had the primary or selected secondary outcomes.
STATISTICAL ANALYSIS
The data and safety monitoring board approved a final sample size of 2404 (1202 per group), which was reduced from the originally planned enrollment of 4700 patients, as sufficient to detect a relative reduction of 33% in the incidence of the composite primary-outcome events. In these calculations, we assumed a baseline incidence of primary-outcome events of 16% in the control group, 10% nonadherence to the trial regimen or crossover, and 5% loss to follow-up, with 85% power and a two-sided alpha level of 0.05. A blinded reassessment of the sample size that was performed after 800 patients had completed the trial revealed that the incidence of the primary outcome was at least 30%. Thus, we determined that the enrollment of 2404 patients would suffice to detect relative effect sizes of 25% or more. This sample size would provide more than 80% power to detect a relative difference of 35% or more in the incidence of small-for-gestational-age birth weight, assuming a baseline incidence as low as 10%.
The primary analyses were performed in the intention-to-treat population. When the primary composite or birth-weight outcomes were undetermined (e.g., withdrawal from the trial before delivery), multiple imputation methods with five replicates were used. Details regarding these analyses are provided in the Supplementary Appendix.17 Multivariable log-binomial models were applied to each replicated set, and assessments of treatment effect were pooled. Adjusted risk ratios, 95% confidence intervals, and tests of statistical significance were calculated. Complete-case analyses were also conducted among all the patients with available data regarding the primary outcome and small-for-gestational-age birth weight; risk ratios and 95% confidence intervals were calculated. We also determined the number of patients who would need to be treated to prevent one primary-outcome event and the 95% confidence interval.
We replicated the primary-outcome analyses using logistic regression to estimate odds ratios according to the prespecified statistical plan. In addition, we conducted per-protocol analyses (in which crossovers were included in the group as treated) and survival analyses to account for the time that patients had been enrolled in the trial; both analyses included patients who had been lost to follow-up.
We performed one planned interim analysis of the primary outcome using a Lan–DeMets alpha spending function that approximated O’Brien–Fleming boundaries. The alpha level for the final primary analysis was therefore 0.0492; the safety outcome was evaluated at a 0.05 significance level. There was no prespecified plan to adjust for multiple testing. Results for secondary outcomes are reported with 95% confidence intervals without adjustment for multiplicity and thus should not be used to infer definitive effects.
RESULTS
CHARACTERISTICS OF THE PATIENTS
From September 2015 through March 2021, a total of 29,772 women underwent screening; 2419 women subsequently underwent randomization at 61 sites (Fig. S1 in the Supplementary Appendix). Major reasons for exclusion were a systolic blood pressure of less than 140 mm Hg and a diastolic blood pressure of less than 90 mm Hg in patients who either had not been prescribed antihypertensive treatment or had nonadherence to the prescribed regimen (in 39% of excluded patients) and advanced gestational age (in 32%). Ten patients were withdrawn immediately after randomization before any recording of data, and one withdrew consent. Therefore, the final sample size for analysis was 2408, with 1208 patients assigned to receive active treatment and 1200 to receive standard (control) treatment.
The characteristics of the patients were well balanced at baseline in the two groups (Table 1). A majority (56%) had known chronic hypertension and were receiving medication, 22% had known chronic hypertension and were not receiving medication, and 22% had newly diagnosed chronic hypertension. Labetalol and nifedipine were the most frequently used antihypertensive drugs before randomization (Table S2). Non-Hispanic Black women made up 48% of the patient population, Hispanic women 20%, and non-Hispanic White women 28%; 16% of the patients had diabetes mellitus, and 41% had a gestational age of less than 14 weeks.
Table 1.
Characteristics of the Patients at Baseline.*
| Characteristic | Active Treatment (N = 1208) | Control (N = 1200) |
|---|---|---|
| Age — yr | 32.3±5.6 | 32.3±5.8 |
| Race or ethnic group — no. (%)† | ||
| Non-Hispanic White | 347 (28.7) | 326 (27.2) |
| Non-Hispanic Black | 574 (47.5) | 570 (47.5) |
| Hispanic | 238 (19.7) | 250 (20.8) |
| Other | 49 (4.1) | 54 (4.5) |
| Mother’s type of insurance — no. (%) | ||
| Government-assisted insurance or Medicaid | 673 (55.7) | 656 (54.7) |
| Private insurance | 459 (38.0) | 463 (38.6) |
| None | 60 (5.0) | 65 (5.4) |
| Missing data | 16 (1.3) | 16 (1.3) |
| Type of chronic hypertension — no. (%) | ||
| Newly diagnosed | 263 (21.8) | 258 (21.5) |
| Diagnosed and receiving medication | 677 (56.0) | 681 (56.8) |
| Diagnosed and not receiving medication | 268 (22.2) | 261 (21.8) |
| Blood pressure — mm Hg | ||
| Systolic | 134.3±12.7 | 133.7±12.4 |
| Diastolic | 83.9±9.5 | 83.4±9.6 |
| Previous pregnancy — no. (%) | 1007 (83.4) | 989 (82.4) |
| Body-mass index‡ | ||
| Mean | 37.7±10.0 | 37.5±9.6 |
| Distribution — no. (%) | ||
| <30 | 295 (24.4) | 259 (21.6) |
| 30 to <40 | 460 (38.1) | 517 (43.1) |
| ≥40 | 434 (35.9) | 402 (33.5) |
| Gestational age <14 wk — no. (%) | 496 (41.1) | 481 (40.1) |
| Coexisting illness or lifestyle factor — no. (%) | ||
| Diabetes mellitus | 191 (15.8) | 189 (15.8) |
| Current smoker | 92 (7.6) | 82 (6.8) |
| Aspirin use | 539 (44.6) | 536 (44.7) |
Plus–minus values are means ±SD. Patients in the active-treatment group received antihypertensive treatment with a blood-pressure goal of less than 140/90 mm Hg; those in the control group received standard treatment (no antihypertensive therapy) unless severe hypertension developed (systolic pressure, ≥160 mm Hg; or diastolic pressure, ≥105 mm Hg). Data are shown for all the patients who underwent randomization, regardless of adherence to group assignment.
Race or ethnic group was reported by the patients or was abstracted from records.
Data regarding body-mass index were missing for 19 patients in the active-treatment group and for 22 in the control group.
MEDICATION ADHERENCE
The patients in the active-treatment group were assigned to receive labetalol (61.7%) or nifedipine (35.6%); 2.7% received other medications (Table S3). Of 15,010 total clinic visits, 7717 were attended by patients in the active-treatment group. At 86% of these visits, the patients reported taking their assigned medications. At the last antenatal visit, more patients in the active-treatment group than in the control group reported taking medications (88.9% vs. 24.4%) (Table S4). The mean blood-pressure level during the period between randomization and delivery was lower in the active-treatment group than in the control group (systolic pressure, 129.5 mm Hg vs. 132.6 mm Hg, for a difference of −3.1; and diastolic pressure, 79.1 mm Hg vs. 81.5 mm Hg, for a difference of −2.3 mm Hg) (Fig. 1).
Figure 1. Mean Blood Pressure after Randomization.
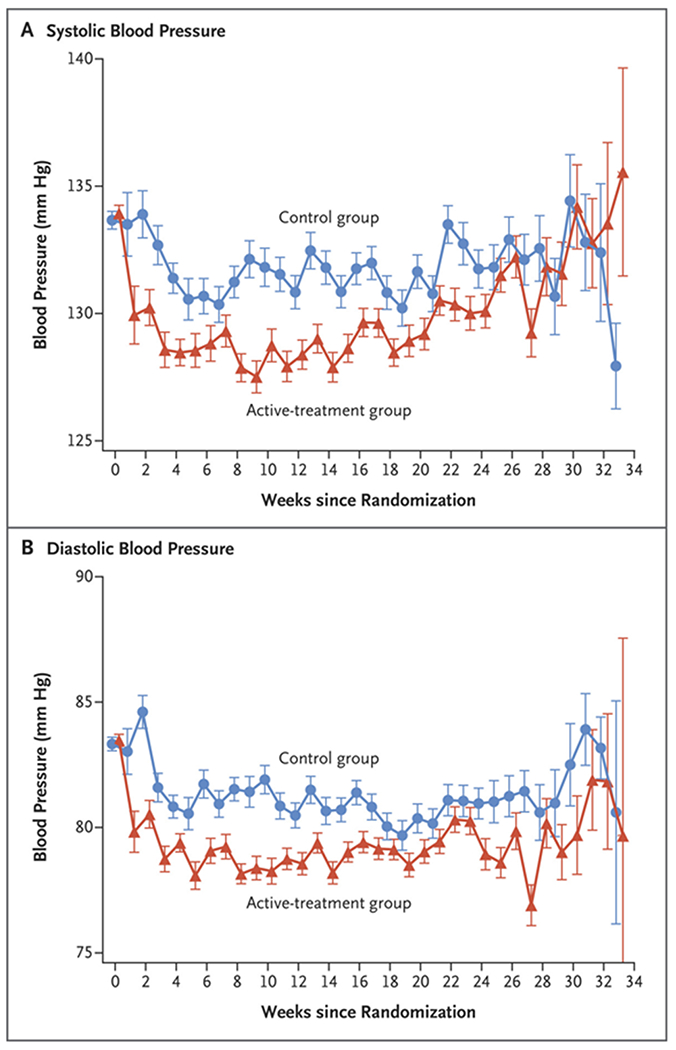
Between randomization and delivery, the overall mean blood-pressure level was lower in the active-treatment group than in the control group, both for systolic pressure (129.5 mm Hg vs. 132.6 mm Hg) and for diastolic pressure (79.1 mm Hg vs. 81.5 mm Hg). I bars indicate standard errors.
PRIMARY OUTCOME
A total of 83 patients were lost to follow-up: 38 (3.1%) in the active-treatment group and 45 (3.8%) in the control group. In the complete-case analysis, a primary-outcome event occurred in 353 of 1170 patients (30.2%) in the active-treatment group and in 427 of 1155 (37.0%) in the control group (risk ratio, 0.82; 95% confidence interval [CI], 0.73 to 0.92; P<0.001). After imputation, the adjusted risk ratio for a primary-outcome event was 0.82 (95% CI, 0.74 to 0.92; P<0.001) (Table 2). The number of patients who would need to be treated to prevent one primary-outcome event was 14.7 (95% CI, 9.4 to 33.7).
Table 2.
Primary and Safety Outcomes.
| Outcome | Imputation Analysis (N = 2408)* | Complete-Case Analysis (N = 2325)† | ||||
|---|---|---|---|---|---|---|
| Adjusted Risk Ratio (95% CI) | P Value | Active Treatment | Control | Risk Ratio (95% CI) | P Value | |
| no./total no. (%) | ||||||
| Primary composite outcome | 0.82 (0.74–0.92) | <0.001 | 353/1170 (30.2) | 427/1155 (37.0) | 0.82 (0.73–0.92) | <0.001 |
|
| ||||||
| Preeclampsia with severe features | 0.80 (0.70–0.92) | 272/1170 (23.3) | 336/1155 (29.1) | 0.80 (0.70–0.92) | ||
|
| ||||||
| Medically indicated preterm birth at <35 wk | 0.73 (0.60–0.89) | 143/1170 (12.2) | 193/1155 (16.7) | 0.73 (0.60–0.89) | ||
|
| ||||||
| Placental abruption | 0.88 (0.49–1.59) | 20/1170 (1.7) | 22/1155 (1.9) | 0.90 (0.49–1.64) | ||
|
| ||||||
| Fetal or neonatal death at <28 days | 0.81 (0.54–1.22) | 41/1170 (3.5) | 50/1155 (4.3) | 0.81 (0.54–1.21) | ||
|
| ||||||
| Safety outcome | ||||||
|
| ||||||
| Small for gestational age | ||||||
|
| ||||||
| <10th percentile | 1.04 (0.82–1.31) | 0.76 | 128/1146 (11.2) | 117/1124 (10.4) | 1.07 (0.85–1.36) | 0.56 |
|
| ||||||
| <5th percentile | 0.89 (0.62–1.26) | 0.51 | 58/1146 (5.1) | 62/1124 (5.5) | 0.92 (0.65–1.30) | 0.63 |
Shown are the results of multiple imputation analysis performed with the use of multivariable log-binomial regression models to calculate adjusted risk ratios. The missing values were modeled within treatment group with the use of baseline characteristics that included diabetes status (yes or no), treatment status at enrollment (receiving or not receiving blood-pressure medication), age, body-mass index, and elevated blood pressure (≥150 mm Hg systolic or ≥100 mm Hg diastolic) at the first visit.
Complete-case analysis of the primary outcome included 2325 patients with sufficient data (1170 in the active-treatment group and 1155 in the control group). Complete-case analysis of the safety outcome included 2270 patients with sufficient data (1146 in the active-treatment group and 1124 in the control group); included in this analysis were assessments of data obtained during delivery.
With respect to the components of the primary outcome, preeclampsia with severe features occurred in 272 patients (23.3%) in the active-treatment group and in 336 (29.1%) in the control group; medically indicated preterm birth before 35 weeks’ gestation occurred in 143 patients (12.2%) and in 193 (16.7%), respectively. For these two components, the adjusted risk ratios as calculated by imputation — 0.80 (95% CI, 0.70 to 0.92) and 0.73 (95% CI, 0.60 to 0.89), respectively — were the same as the risk ratios for the complete-case analysis.
The safety outcome of newborns with a birth weight that was under the 10th percentile for their gestational age occurred in 128 of 1146 infants (11.2%) with mothers in the active-treatment group and in 117 of 1124 (10.4%) with mothers in the control group (risk ratio, 1.07; 95% CI, 0.85 to 1.36; P=0.56). In the imputation analysis of this comparison, the between-group difference was also not significant (adjusted risk ratio, 1.04; 95% CI, 0.82 to 1.31; P = 0.76). Similar results were reported for newborns with a birth weight that was under the 5th percentile for their gestational age, with values of 5.1% and 5.5%, respectively (risk ratio, 0.92; 95% CI, 0.65 to 1.30; P = 0.63).
SUBGROUP ANALYSES
Results of prespecified subgroup analyses for the primary outcome are shown in Figure 2. In all the subgroups, the 95% confidence intervals of the treatment effect on the primary outcome were consistent with the overall results, but the risk ratios for newly diagnosed hypertension and for a body-mass index of 40 or more were close to 1.00.
Figure 2. Risk of the Primary Outcome in Prespecified Subgroups.
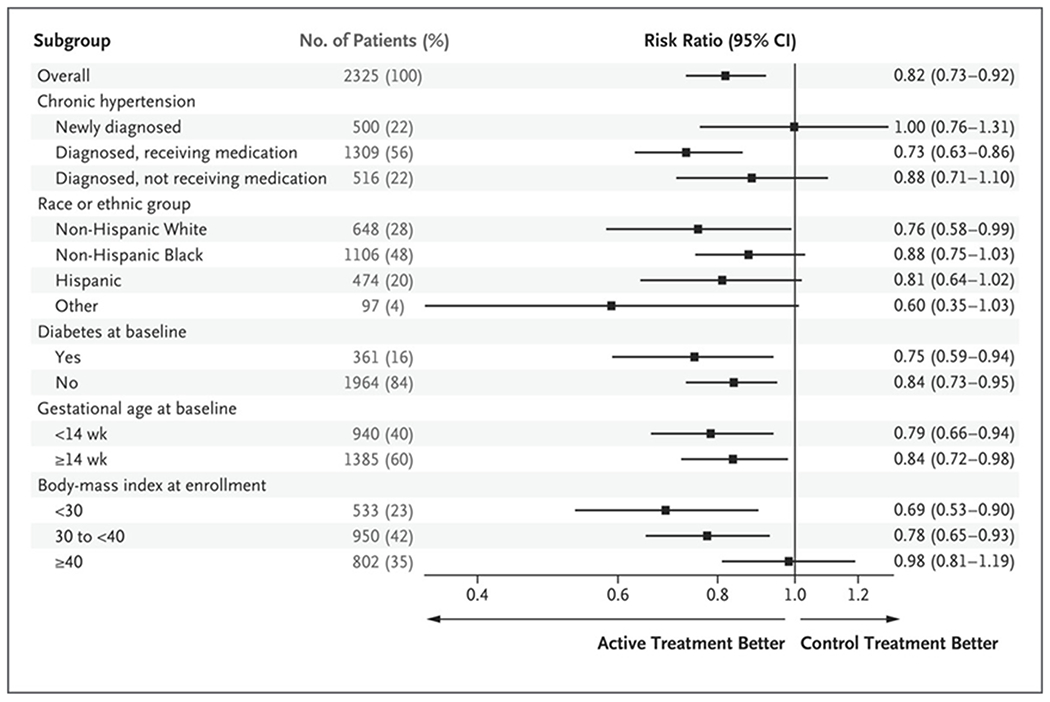
The primary outcome was a composite of preeclampsia with severe features occurring up to 2 weeks after birth, medically indicated preterm birth before 35 weeks’ gestation, placental abruption, or fetal or neonatal death.
SECONDARY MATERNAL AND NEONATAL OUTCOMES
The incidence of the maternal composite outcome was low and did not differ substantially between the two treatment groups (Table 3). Severe maternal hypertension occurred in 436 of 1208 patients (36.1%) in the active-treatment group and in 531 of 1200 patients (44.2%) in the control group. Preeclampsia, with or without severe features, occurred in 295 patients (24.4%) and 373 (31.1%), respectively.
Table 3.
Maternal Outcomes.*
| Outcome | Active Treatment (N = 1208) | Control (N = 1200) | Treatment Effect (95% CI)† |
|---|---|---|---|
| Composite cardiovascular complications — no. (%) | 25 (2.1) | 33 (2.8) | 0.75 (0.45 to 1.26) |
| Maternal death | 1 (0.1) | 2 (0.2) | 0.50 (0.05 to 5.47) |
| Heart failure | 1 (0.1) | 1 (0.1) | 0.99 (0.06 to 15.9) |
| Stroke | 0 | 0 | NA |
| Myocardial infarction or angina | 0 | 0 | NA |
| Pulmonary edema | 5 (0.4) | 11 (0.9) | 0.45 (0.16 to 1.30) |
| ICU admission or intubation | 12 (1.0) | 16 (1.3) | 0.75 (0.35 to 1.57) |
| Encephalopathy | 1 (0.1) | 0 | 1.00 (1.00 to 1.00) |
| Renal failure | 9 (0.8) | 14 (1.2) | 0.64 (0.28 to 1.47) |
| Severe hypertension — no. (%) | 436 (36.1) | 531 (44.3) | 0.82 (0.74 to 0.90) |
| Any preeclampsia — no. (%) | 295 (24.4) | 373 (31.1) | 0.79 (0.69 to 0.89) |
| Severe hypertension plus proteinuria | 189 (15.7) | 215 (17.9) | 0.87 (0.73 to 1.04) |
| Eclampsia | 0 | 1 (0.1) | NA |
| HELLP | 0 | 3 (0.3) | NA |
| Hypertension plus end-organ dysfunction | 136 (11.3) | 181 (15.1) | 0.75 (0.61 to 0.92) |
| Nonsevere preeclampsia | 23 (1.9) | 37 (3.1) | 0.62 (0.37 to 1.03) |
| Worsening chronic hypertension — no. (%) | 132 (10.9) | 156 (13.0) | 0.84 (0.68 to 1.04) |
| Mean blood pressure during prenatal visits — mm Hg‡ | |||
| Systolic | 129.5±10.0 | 132.6±10.1 | −3.11 (−3.95 to 2.28) |
| Diastolic | 79.1±7.4 | 81.5±8.0 | −2.33 (−2.97 to 0.04) |
| Gestational age at delivery — wk§ | 36.6±4.3 | 36.3±5.1 | 0.24 (−0.15 to 0.62) |
| Cesarean delivery — no. (%) | 592 (49.0) | 582 (48.5) | 1.01 (0.93 to 1.10) |
| Any blood transfusion — no. (%) | 46 (3.8) | 53 (4.4) | 0.86 (0.59 to 1.27) |
Plus–minus values are means αSD. Data are shown for all the patients who underwent randomization, regardless of adherence to group assignment. HELLP denotes hemolysis, elevated liver enzymes, and low platelets, ICU intensive care unit, and NA not applicable.
The treatment effect is a risk ratio unless otherwise indicated.
The treatment effect for blood pressure during prenatal visits is the between-group difference. Data in this category were missing for 71 patients in the active-treatment group and for 79 in the control group.
The treatment effect for mean gestational age at delivery is the between-group difference. Data in this category were missing for 27 patients in the active-treatment group and for 36 in the control group.
Among neonatal outcomes, preterm birth before 37 weeks’ gestation occurred in 332 of 1208 infants (27.5%) in the active-treatment group and in 377 of 1200 (31.4%) in the control group. Low birth weight (<2500 g) occurred in 232 infants (19.2%) and 277 (23.1%), respectively (Table 4). The frequencies of outcomes of severe neonatal complications and NICU admission did not appear to differ substantially between the two groups. Reported adverse events are provided in Table S5.
Table 4.
Neonatal Outcomes.*
| Outcome | Active Treatment (N = 1208) | Control (N = 1200) | Treatment Effect (95% CI)† |
|---|---|---|---|
| Composite of severe neonatal complications – no. (%) | 24 (2.0) | 31 (2.6) | 0.77 (0.45 to 1.30) |
| Bronchopulmonary dysplasia | 8 (0.7) | 14 (1.2) | 0.57 (0.24 to 1.35) |
| Retinopathy of prematurity | 16 (1.3) | 20 (1.7) | 0.79 (0.41 to 1.53) |
| Necrotizing enterocolitis | 2 (0.2) | 2 (0.2) | 0.99 (0.14 to 7.06) |
| Intraventricular hemorrhage, grade 3 or 4 | 3 (0.3) | 4 (0.3) | 0.75 (0.17 to 3.32) |
| Preterm birth at <37 wk — no. (%) | 332 (27.5) | 377 (31.4) | 0.87 (0.77 to 0.99) |
| Birth weight <2500 g — no. (%) | 232 (19.2) | 277 (23.1) | 0.83 (0.71 to 0.97) |
| NICU admission — no. (%) | 368 (30.5) | 402 (33.5) | 0.91 (0.81 to 1.02) |
| Neonatal hospital stay | |||
| Mean no. of days‡ | 2.8±1.7 | 2.9±1.7 | −0.05 (−0.18 to 0.09)§ |
| ≥3 days — no. (%) | 590 (48.8) | 592 (49.3) | 0.98 (0.90 to 1.06) |
| Ponderal index — g/cm3¶ | 2.9±3.7 | 2.7±2.8 | 0.16 (−0.11 to 0.43)§ |
| Head circumference — cm‖ | 33.3±3.0 | 33.0±3.2 | 0.31 (0.05 to 0.56)§ |
| Placental weight — g** | 466.3±177.6 | 464.6±175.6 | 1.67 (−17.57 to 20.91)§ |
| Hypoglycemia — no. (%) | 191 (15.8) | 195 (16.3) | 0.97 (0.81 to 1.17) |
| Bradycardia — no. (%) | 31 (2.6) | 35 (2.9) | 0.88 (0.55 to 1.42) |
| Hypotension — no. (%) | 7 (0.6) | 16 (1.3) | 0.43 (0.18 to 1.05) |
| Any respiratory support — no. (%) | 219 (18.1) | 243 (20.3) | 0.90 (0.76 to 1.06) |
| Respiratory distress syndrome — no. (%) | 149 (12.3) | 171 (14.3) | 0.87 (0.71 to 1.06) |
| Transient tachypnea — no. (%) | 70 (5.8) | 63 (5.3) | 1.10 (0.79 to 1.54) |
| Seizures — no. (%) | 3 (0.3) | 1 (0.1) | 2.98 (0.31 to 28.6) |
| Hyperbilirubinemia — no. (%) | 266 (22.0) | 283 (23.6) | 0.93 (0.81 to 1.08) |
| Apgar score of <7 at 5 min — no. (%)†† | 68 (5.6) | 80 (6.7) | 0.84 (0.62 to 1.16) |
| Sepsis — no. (%) | |||
| Suspected or proven | 138 (11.4) | 163 (13.8) | 0.84 (0.68 to 1.04) |
| Proven | 21 (1.7) | 34 (2.8) | 0.61 (0.36 to 1.05) |
Plus–minus values are means αSD. Data are shown for all the patients who underwent randomization, regardless of adherence to group assignment. NICU denotes neonatal ICU.
The treatment effect is a risk ratio unless otherwise indicated.
Data regarding the mean number of days of hospital stay were missing for 38 infants of mothers in the active-treatment group and 53 in the control group.
The treatment effect in this category is the between-group difference.
Data regarding the ponderal index were missing for 71 infants of mothers in the active-treatment group and 83 in the control group.
Data regarding head circumference were missing for 84 infants of mothers in the active-treatment group and 97 in the control group.
Data regarding placental weight were missing for 549 infants of mothers in the active-treatment group and 562 in the control group.
The Apgar score ranges from 1 to 10, with higher values indicating a greater level of health of the neonate; a value of 7 or higher indicates normal health status.
ADDITIONAL ANALYSES
The results of several additional analyses were consistent with the primary results, including analyses with calculated odds ratios (Table S6), per-protocol analyses (Table S7), survival analyses (Fig. S2), and sensitivity analyses (Tables S8, S9, and S10).
DISCUSSION
In pregnant women with mild chronic hypertension, active treatment with a blood-pressure target of less than 140/90 mm Hg was associated with better pregnancy outcomes than a control strategy of no antihypertensive treatment unless the systolic blood pressure was 160 mm Hg or higher or the diastolic pressure was 105 mm Hg or higher. Women who received active treatment had a lower risk of one or more primary-outcome events of preeclampsia with severe features, medically indicated preterm birth at less than 35 weeks’ gestation, placental abruption, or fetal or neonatal death. The estimates of the components of the primary outcome and most secondary outcomes (including the composites of serious maternal or neonatal complications, preeclampsia, and preterm birth) were consistent with the results of the primary analysis. It was determined that 14 to 15 patients would need to receive active treatment to prevent one primary-outcome event. There were no significant between-group differences in the safety outcome of newborns who were under either the 10th percentile or the 5th percentile for gestational-age weight. The between-group difference in mean blood pressure after randomization was seemingly small.
In this trial, we found that active treatment with antihypertensive drugs improved pregnancy outcomes without apparent harm. In prespecified subgroup analyses of the primary outcome, the point estimates for the risk ratio approximated 1.00 for patients with newly diagnosed chronic hypertension and for patients with a body-mass index of 40 or more, but the 95% confidence intervals were wide and consistent with the overall treatment effect. The trial was not powered to assess differences in treatment effects across subgroups. Additional evaluation of treatment effect in patients with newly diagnosed hypertension or a body-mass index of 40 or more may be informative. Our results suggest that the incidence of severe hypertension was lower among patients who received active treatment, which was consistent with the findings of previous trials and a systematic review of antihypertensive therapy for mild chronic hypertension in pregnancy.7,18,19 Earlier trials that focused on mild chronic hypertension were underpowered for pregnancy outcomes, although their point estimates were supportive of our findings.7,18 The Control of Hypertension in Pregnancy Study (CHIPS), which compared “tight versus less-tight” antihypertensive treatment in women with mild or severe chronic or pregnancy-associated hypertension who were enrolled at 14 to 33 weeks’ gestation, showed no between-group difference in the primary outcome of NICU admission or pregnancy loss. Our finding suggesting that there was no substantial between-group difference in NICU admission is consistent with that reported in CHIPS.19 In CHIPS, among the patients in the overall sample, the percentage of newborns who were under the 10th percentile for gestational-age weight was 16.1% in the group with less-tight blood-pressure control and 19.7% in the group with tight control (adjusted odds ratio, 0.78; 95% CI, 0.56 to 1.08); in the subgroup with chronic hypertension (75% of the trial population), the percentages were 13.9% and 19.7%, respectively (adjusted odds ratio, 0.66; 95% CI, 0.44 to 1.00).9,19 It is likely that differences between our findings and those of previous trials stem from the use of different entry criteria, treatment approaches, sample sizes, and choice of trial outcomes. In our trial, the mean between-group differences in both systolic blood pressure (3.1 mm Hg) and diastolic pressure (2.3 mm Hg) were unadjusted for the time after randomization and appear to mask larger differences in blood pressure over several weeks (Fig. 1). Moreover, similar reductions in blood pressure have been associated with improvements in cardiovascular end points.20,21 Better blood-pressure control in the active-treatment group may have contributed to our findings. The incidence of cesarean deliveries in our trial (49%) was consistent with the incidence that is expected among women with chronic hypertension.19 Aspirin use at baseline (in approximately 45% of the patients) did not appear to influence the treatment effect for the primary outcome in post hoc analysis; aspirin use was much higher at the time of delivery (76 to 77% in both groups).
Strengths of our trial include the large sample size, multiple trial centers, close oversight by an independent data and safety monitoring board, and the use of centralized blinded adjudication to confirm key outcomes. The trial population mirrored the age and racial and ethnic diversity of women with chronic hypertension who are giving birth in the United States, including a higher proportion of Black and Hispanic women22 (Table S11). A limitation of the trial was the open-label approach, which was judged to be appropriate in consideration of the ethical and logistic challenges of administering blinded treatments. The high ratio of 12 patients who were screened for each patient who underwent randomization may arouse concern about the generalizability of our findings. However, the characteristics of the screened population and the enrolled population were similar (Table S12), and more than 60% of the patients who were excluded were either above the gestational age for enrollment or had a history of known or suspected chronic hypertension but had a blood pressure below the entry threshold; the physiologic drop in blood pressure during pregnancy may have contributed to this factor. Of note, the trial protocol did not incorporate an analysis of blood-pressure measurements that were taken at home.
In our trial, a strategy of treating mild chronic hypertension resulted in a lower risk of adverse pregnancy outcomes than a strategy of reserving treatment unless hypertension became severe, without increasing the risk of low birth weight for gestational age. Our findings support the treatment of pregnant women with chronic hypertension with a blood-pressure target of less than 140/90 mm Hg, including the continuation of their established antihypertensive therapy. Studies of the long-term effect of antihypertensive treatment on cardiovascular and other outcomes in pregnant women with mild chronic hypertension and their offspring may further clarify the role of antihypertensive therapy.
Supplementary Material
Acknowledgments
Supported by the National Heart, Lung, and Blood Institute.
APPENDIX
The authors’ full names and academic degrees are as follows: Alan T. Tita, M.D., Ph.D., Jeff M. Szychowski, Ph.D., Kim Boggess, M.D., Lorraine Dugoff, M.D., Baha Sibai, M.D., Kirsten Lawrence, M.D., Brenna L. Hughes, M.D., Joseph Bell, M.D., Kjersti Aagaard, M.D., Ph.D., Rodney K. Edwards, M.D., Kelly Gibson, M.D., David M. Haas, M.D., Lauren Plante, M.D., Torri Metz, M.D., Brian Casey, M.D., Sean Esplin, M.D., Sherri Longo, M.D., Matthew Hoffman, M.D., George R. Saade, M.D., Kara K. Hoppe, D.O., Janelle Foroutan, M.D., Methodius Tuuli, M.D., Michelle Y. Owens, M.D., Hyagriv N. Simhan, M.D., Heather Frey, M.D., Todd Rosen, M.D., Anna Palatnik, M.D., Susan Baker, M.D., Phyllis August, M.D., M.P.H., Uma M. Reddy, M.D., Wendy Kinzler, M.D., Emily Su, M.D., Iris Krishna, M.D., Nicki Nguyen, M.D., Mary E. Norton, M.D., Daniel Skupski, M.D., Yasser Y. El-Sayed, M.D., Dotum Ogunyemi, M.D., Zorina S. Galis, Ph.D., Lorie Harper, M.D., Namasivayam Ambalavanan, M.D., Nancy L. Geller, Ph.D., Suzanne Oparil, M.D., Gary R. Cutter, Ph.D., and William W. Andrews, M.D., Ph.D.
The authors’ affiliations are as follows: the Department of Obstetrics and Gynecology (A.T.T., W.W.A.), the Center for Women’s Reproductive Health (A.T.T., J.M.S., N.A., S.O., G.R.C., W.W.A.), the Department of Biostatistics (J.M.S., G.R.C.), the Division of Neonatology, Department of Pediatrics (N.A.), and the Division of Cardiovascular Disease, Department of Medicine (S.O.), University of Alabama, Birmingham; the Department of Obstetrics and Gynecology, University of North Carolina at Chapel Hill (K.B.), and the Department of Obstetrics and Gynecology, Duke University, Durham (B.L.H.) — both in North Carolina; the Department of Obstetrics and Gynecology, University of Pennsylvania (L.D.), and the Department of Obstetrics and Gynecology, Drexel University College of Medicine (L.P.), Philadelphia, St. Luke’s University Health Network, Fountain Hill (J.B.), and the Department of Obstetrics and Gynecology, Magee Women’s Hospital, University of Pittsburgh, Pittsburgh (H.N.S.) — all in Pennsylvania; the Department of Obstetrics and Gynecology, University of Texas (B.S.), and the Department of Obstetrics and Gynecology, Baylor College of Medicine and Texas Children’s Hospital, Houston (K.A.), the Department of Obstetrics and Gynecology, University of Texas Southwestern Medical Center, Dallas (B.C.), the Department of Obstetrics and Gynecology, University of Texas Medical Branch, Galveston (G.R.S.), and the Department of Women’s Health, University of Texas, Austin (L.H.); the Department of Obstetrics and Gynecology, Columbia University (K.L.), Weill Cornell University (P.A.), and the Department of Obstetrics and Gynecology, New York Presbyterian Queens Hospital (D.S.), New York, and the Department of Obstetrics and Gynecology, Winthrop University Hospital, Mineola (W.K.) — all in New York; the Department of Obstetrics and Gynecology, University of Oklahoma Health Sciences, Oklahoma City (R.K.E.); MetroHealth System, Cleveland (K.G.); the Department of Obstetrics and Gynecology, Indiana University, Indianapolis (D.M.H.); the Department of Obstetrics and Gynecology, University of Utah (T.M.), and Intermountain Healthcare (S.E.), Salt Lake City; Ochsner Baptist Medical Center, New Orleans (S.L.); Christiana Care Health Services, Newark, DE (M.H.); the Department of Obstetrics and Gynecology, UnityPoint Health–Meriter Hospital/Marshfield Clinic, Madison (K.K.H.), and the Department of Obstetrics and Gynecology, Medical College of Wisconsin, Milwaukee (A.P.); St. Peters University Hospital (J.F.) and the Department of Obstetrics and Gynecology, Robert Wood Johnson Medical School, Rutgers University (T.R.), New Brunswick, NJ; the Department of Obstetrics and Gynecology, Washington University, St. Louis (M.T.); the Department of Obstetrics and Gynecology, University of Mississippi Medical Center, Jackson (M.Y.O.); the Department of Obstetrics and Gynecology, Ohio State University, Columbus (H.F.); the Department of Obstetrics and Gynecology, University of South Alabama, Mobile (S.B.); the Department of Obstetrics and Gynecology, Yale University, New Haven, CT (U.M.R.); the Department of Obstetrics and Gynecology, University of Colorado, Boulder (E.S.), and the Department of Obstetrics and Gynecology, Denver Health, Denver (N.N.); the Department of Obstetrics and Gynecology, Emory University, Atlanta (I.K.); the Department of Obstetrics and Gynecology, University of California, San Francisco, and Zuckerberg San Francisco General Hospital (M.E.N.), San Francisco, the Department of Obstetrics and Gynecology, Stanford University, Stanford (Y.Y.E.-S.), and the Department of Obstetrics and Gynecology, Arrowhead Regional Medical Center, Colton (D.O.); Beaumont Hospital, Southfield, MI (D.O.); and the Division of Cardiovascular Sciences (Z.S.G.) and the Office of Biostatistics Research (N.L.G.), National Heart, Lung, and Blood Institute, Bethesda, MD.
Footnotes
The authors’ full names, academic degrees, and affiliations are listed in the Appendix. Dr. Tita can be contacted at atita@uab.edu or at the Department of Obstetrics and Gynecology, Center for Women’s Reproductive Health, Marnix E. Heersink School of Medicine, University of Alabama at Birmingham, 619 19th St. S., Birmingham, AL 35249.
The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the Department of Health and Human Services.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Contributor Information
A.T. Tita, Department of Obstetrics and Gynecology, University of Alabama, Birmingham; Center for Women’s Reproductive Health, University of Alabama, Birmingham
J.M. Szychowski, Department of Obstetrics and Gynecology, University of Alabama, Birmingham; Center for Women’s Reproductive Health, University of Alabama, Birmingham
K. Boggess, Department of Obstetrics and Gynecology, University of North Carolina at Chapel Hill, North Carolina
L. Dugoff, Department of Obstetrics and Gynecology, University of Pennsylvania, Pennsylvania
B. Sibai, Department of Obstetrics and Gynecology, University of Texas, New York
K. Lawrence, Department of Obstetrics and Gynecology, Columbia University, New York
B.L. Hughes, Department of Obstetrics and Gynecology, Duke University, Durham, North Carolina
J. Bell, Philadelphia, St. Luke’s University Health Network, Fountain Hill, Pennsylvania
K. Aagaard, Department of Obstetrics and Gynecology, Baylor College of Medicine and Texas Children’s Hospital, Houston, New York
R.K. Edwards, Department of Obstetrics and Gynecology, University of Oklahoma Health Sciences, Oklahoma City
K. Gibson, MetroHealth System, Cleveland
D.M. Haas, Department of Obstetrics and Gynecology, Indiana University, Indianapolis
L. Plante, Department of Obstetrics and Gynecology, Drexel University College of Medicine, Pennsylvania
T. Metz, Department of Obstetrics and Gynecology, University of Utah, Salt Lake City
B. Casey, Department of Obstetrics and Gynecology, University of Texas Southwestern Medical Center, Dallas, New York
S. Esplin, Intermountain Healthcare, Salt Lake City
S. Longo, Ochsner Baptist Medical Center, New Orleans
M. Hoffman, Christiana Care Health Services, Newark, DE
G.R. Saade, Department of Obstetrics and Gynecology, University of Texas Medical Branch, Galveston, New York
K.K. Hoppe, Department of Obstetrics and Gynecology, UnityPoint Health–Meriter Hospital/Marshfield Clinic, Madison, New Brunswick, NJ
J. Foroutan, St. Peters University Hospital, New Brunswick, NJ
M. Tuuli, Department of Obstetrics and Gynecology, Washington University, St. Louis
M.Y. Owens, Department of Obstetrics and Gynecology, University of Mississippi Medical Center, Jackson
H.N. Simhan, Department of Obstetrics and Gynecology, Magee Women’s Hospital, University of Pittsburgh, Pittsburgh, Pennsylvania
H. Frey, Department of Obstetrics and Gynecology, Ohio State University, Columbus
T. Rosen, Department of Obstetrics and Gynecology, Robert Wood Johnson Medical School, Rutgers University, New Brunswick, NJ
A. Palatnik, Department of Obstetrics and Gynecology, Medical College of Wisconsin, Milwaukee
S. Baker, Department of Obstetrics and Gynecology, University of South Alabama, Mobile
P. August, Department of Obstetrics and Gynecology, Columbia University (K.L.), Weill Cornell University, New York
U.M. Reddy, Department of Obstetrics and Gynecology, Yale University, New Haven, CT
W. Kinzler, Department of Obstetrics and Gynecology, Winthrop University Hospital, Mineola, New York
E. Su, Department of Obstetrics and Gynecology, University of Colorado, Boulder
I. Krishna, Department of Obstetrics and Gynecology, Emory University, Atlanta
N. Nguyen, Department of Obstetrics and Gynecology, Denver Health, Denver
M.E. Norton, Department of Obstetrics and Gynecology, University of California, San Francisco, and Zuckerberg San Francisco General Hospital, San Francisco
D. Skupski, Department of Obstetrics and Gynecology, New York Presbyterian Queens Hospital, New York
Y.Y. El-Sayed, San Francisco, the Department of Obstetrics and Gynecology, Stanford University, Stanford
D. Ogunyemi, Department of Obstetrics and Gynecology, Arrowhead Regional Medical Center, Colton
Z.S. Galis, Division of Cardiovascular Sciences, National Heart, Lung, and Blood Institute, Bethesda, MD
L. Harper, Department of Women’s Health, University of Texas, Austin
N. Ambalavanan, Center for Women’s Reproductive Health, University of Alabama, Birmingham; Division of Neonatology, Department of Pediatrics, University of Alabama, Birmingham
N.L. Geller, Office of Biostatistics Research, National Heart, Lung, and Blood Institute, Bethesda, MD
S. Oparil, Division of Cardiovascular Disease, Department of Medicine, University of Alabama, Birmingham
G.R. Cutter, Center for Women’s Reproductive Health, University of Alabama, Birmingham; Department of Biostatistics, University of Alabama, Birmingham
W.W. Andrews, Department of Obstetrics and Gynecology, University of Alabama, Birmingham; Center for Women’s Reproductive Health, University of Alabama, Birmingham
REFERENCES
- 1.Bateman BT, Bansil P, Hernandez-Diaz S, Mhyre JM, Callaghan WM, Kuklina EV. Prevalence, trends, and outcomes of chronic hypertension: a nationwide sample of delivery admissions. Am J Obstet Gynecol 2012;206(2):134.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ananth CV, Duzyj CM, Yadava S, Schwebel M, Tita ATN, Joseph KS. Changes in the prevalence of chronic hypertension in pregnancy, United States, 1970 to 2010. Hypertension 2019;74:1089–95. [DOI] [PubMed] [Google Scholar]
- 3.American College of Obstetricians and Gynecologists. Hypertension in pregnancy: report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013;122:1122–31. [DOI] [PubMed] [Google Scholar]
- 4.American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins — Obstetrics. ACOG practice bulletin no. 203: chronic hypertension in pregnancy. Obstet Gynecol 2019;133(1):e26–e50. [DOI] [PubMed] [Google Scholar]
- 5.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311:507–20. [DOI] [PubMed] [Google Scholar]
- 6.Whelton PK, Carey RM, Aronow WS, et al. 2017. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018;71(19):e127–e248. [DOI] [PubMed] [Google Scholar]
- 7.Magee LA, Ornstein MP, von Dadelszen P. Fortnightly review: management of hypertension in pregnancy. BMJ 1999;318:1332–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abalos E, Duley L, Steyn DW, Gialdini C. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev 2018;10:CD002252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.SMFM Publications Committee. SMFM statement: benefit of antihypertensive therapy for mild-to-moderate chronic hypertension during pregnancy remains uncertain. Am J Obstet Gynecol 2015;213:3–4. [DOI] [PubMed] [Google Scholar]
- 10.von Dadelszen P, Ornstein MP, Bull SB, Logan AG, Koren G, Magee LA. Fall in mean arterial pressure and fetal growth restriction in pregnancy hypertension: a meta-analysis. Lancet 2000;355:87–92. [DOI] [PubMed] [Google Scholar]
- 11.Magee LA, Elran E, Bull SB, Logan A, Koren G. Risks and benefits of beta-receptor blockers for pregnancy hypertension: overview of the randomized trials. Eur J Obstet Gynecol Reprod Biol 2000;88:15–26. [DOI] [PubMed] [Google Scholar]
- 12.Sinkey RG, Battarbee AN, Bello NA, Ives CW, Oparil S, Tita ATN. Prevention, diagnosis, and management of hypertensive disorders of pregnancy: a comparison of international guidelines. Curr Hypertens Rep 2020;22:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garovic VD, Dechend R, Easterling T, et al. Hypertension in pregnancy: diagnosis, blood pressure goals, and pharmacotherapy: a scientific statement from the American Heart Association. Hypertension 2022;79(2):e21–e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ankumah NA, Cantu J, Jauk V, et al. Risk of adverse pregnancy outcomes in women with mild chronic hypertension before 20 weeks of gestation. Obstet Gynecol 2014;123:966–72. [DOI] [PubMed] [Google Scholar]
- 15.American College of Obstetricians and Gynecologists. Method for estimating due date: committee opinion no. 611. Obstet Gynecol 2014;124:863–6. [DOI] [PubMed] [Google Scholar]
- 16.Duryea EL, Hawkins JS, McIntire DD, Casey BM, Leveno KJ. A revised birth weight reference for the United States. Obstet Gynecol 2014;124:16–22. [DOI] [PubMed] [Google Scholar]
- 17.Sterne JAC, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sibai BM, Mabie WC, Shamsa F, Villar MA, Anderson GD. A comparison of no medication versus methyldopa or labetalol in chronic hypertension during pregnancy. Am J Obstet Gynecol 1990;162:960–6. [DOI] [PubMed] [Google Scholar]
- 19.Magee LA, von Dadelszen P, Rey E, et al. Less-tight versus tight control of hypertension in pregnancy. N Engl J Med 2015;372:407–17. [DOI] [PubMed] [Google Scholar]
- 20.Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension. 1. Overview, meta-analyses, and meta-regression analyses of randomized trials. J Hypertens 2014;32:2285–95. [DOI] [PubMed] [Google Scholar]
- 21.Hardy ST, Loehr LR, Butler KR, et al. Reducing the blood pressure-related burden of cardiovascular disease: impact of achievable improvements in blood pressure prevention and control. J Am Heart Assoc 2015;4(10):e002276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grover S, Brandt JS, Reddy UM, Ananth CV. Chronic hypertension, perinatal mortality and the impact of preterm delivery: a population-based study. BJOG 2022;129:572–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


