SUMMARY:
We describe a technique termed “resisted inspiration” that could be used during myelography to decrease superior vena cava venous pressure and increase lumbar CSF pressure, potentially aiding in the detection of CSF-venous fistulas.
Spontaneous intracranial hypotension (SIH) can be a debilitating disease that often manifests as orthostatic headaches. One cause of SIH is a CSF-venous fistula (CVF).1 Unfortunately, traditional cross-sectional imaging with CT and MR imaging is unable to detect a CVF.2,3 Therefore, the diagnosis of a CVF is made by myelography under CT (CT myelography), MR imaging (MR myelography), or fluoroscopy (digital subtraction myelography).4-6 All 3 of these techniques are contingent on visualizing contrast that is placed into the subarachnoid space via lumbar puncture (LP) and subsequent passage of contrast from the CSF to a radicular vein. The passage of contrast is driven by the CSF-to-venous pressure gradient. The purpose of our article was to demonstrate a new technique of resisted inspiration that decreases superior vena cava (SVC) pressure while increasing lumbar CSF pressure (thereby maximizing the pressure gradient), a potential adjunct to myelography to improve diagnostic evaluation for a CVF.
MATERIALS AND METHODS
Consecutive patients presenting for venous manometry or LP between April and May of 2022 were included under institutional review board approval. Venous and CSF manometry were performed during a series of breathing maneuvers at a single tertiary medical center (University of California, San Francisco). Pertinent patient demographic variables were also collected for all patients.
Breathing Technique
Changes in SVC venous and lumbar CSF pressures were measured at rest to serve as a baseline. Measurements were also obtained during normal (nonresisted) inspiration and a Valsalva maneuver. Resisted inspiration was performed by inspiring through a 5-mL slip tip syringe (BD Medical). Pressure was measured at mid-resisted inspiration (measured as the half-way point between start and end inspiration) and end-resisted inspiration.
Venous Manometry
Venous pressures were prospectively recorded in 4 patients undergoing a catheter cerebral venography and manometry procedure for a separate clinical indication. All venous manometry was performed in the SVC, which is the drainage pathway of the azygos system, the common venous outflow system of a CVF.7 The manometry zero point was set 1.5 inches anterior to the external auditory canal in accordance with our institution’s standard for cerebral venous manometry. We collected details of the procedure, including access site, catheter type, and venous manometry with the 5 breathing techniques.
CSF Manometry
Lumbar CSF pressures were prospectively recorded in 4 separate patients undergoing a clinically indicated CT-guided LP. Pressure was measured with the patient in the lateral decubitus position with a Compass for Lumbar Puncture disposable pressure transducer (Centurion). Details of the procedure including the patient position, access level, and needle size, length, and type were recorded. CSF manometry was recorded with the same breathing technique used for venous manometry.
RESULTS
Venous Manometry Patients
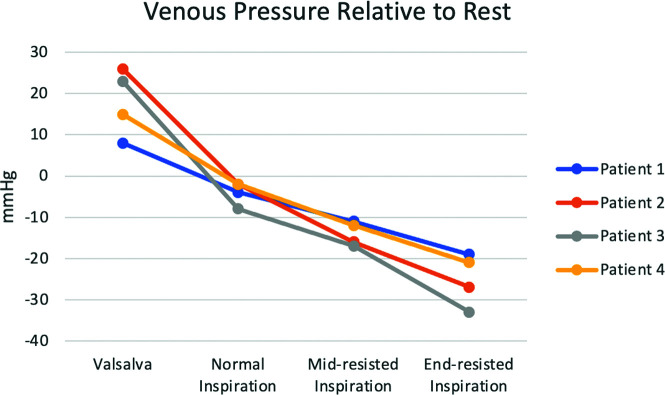
All 4 patients underwent a clinically indicated catheter venogram with manometry. Patient demographics and details of each procedure are listed in Table 1. Venous access was obtained in all 4 patients through the right common femoral vein. Venous pressure was measured with a 6F Benchmark 0.071-inch (Penumbra) guide catheter in patients 1–3 and a 5F UCSF-2/Berenstein III catheter (Cordis) in patient 4. The indication for the venograms included the following: idiopathic intracranial hypertension (IIH) work-up in a patient found to have a transverse sinus stenosis that was subsequently stented (patient 1), 2 cases of pulsatile tinnitus work-up without causative findings on venograms (patients 2 and 3), and 1 follow-up diagnostic case of a prior transverse venous stent for IIH (patient 4). On average and relative to resting pressure, the Valsalva maneuver increased (+18 mm Hg) venous pressure, while inspiration (−4 mm Hg), mid-resisted inspiration (−14 mm Hg), and end-resisted inspiration (−25 mm Hg) decreased venous pressure as depicted in Fig 1.
Table 1:
Individual patient demographics and SVC manometry readings in 4 patients who underwent a catheter venogram
| Venous Pressure (mm Hg) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Patient | Age (yr)/Sex | Indication | BMI | Rest Pressure | Valsalva | Normal Inspiration | Mid-Resisted Inspiration | End-Resisted Inspiration |
| 1 | 57/F | IIH | 34.5 | 3 | 11 | –1 | –8 | –16 |
| 2 | 63/M | Pulsatile tinnitus | 24.8 | 1 | 27 | –1 | –15 | –26 |
| 3 | 69/F | Pulsatile tinnitus | 31.6 | 2 | 25 | –6 | –15 | –31 |
| 4 | 52/F | IIH | 42.6 | 10 | 25 | 8 | –2 | –11 |
Note:—F indicates female; M, male; BMI, body mass index.
FIG 1.
SVC venous pressure changes with 4 different techniques relative to the SVC venous pressure while breathing at rest. End-resisted inspiration leads to the greatest drop in pressure, which is far greater than with normal inspiration.
CSF Pressure Patients
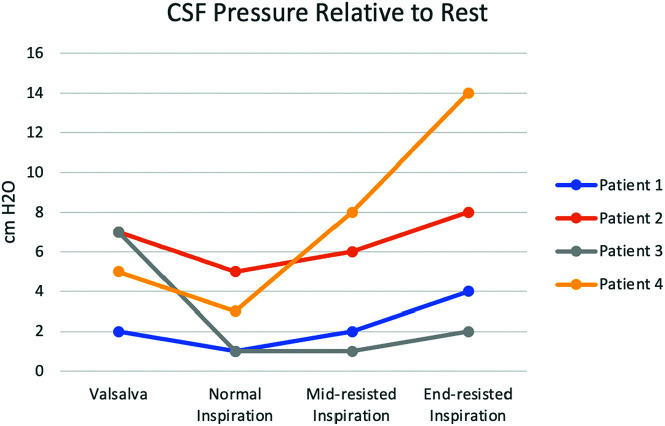
Four patients underwent a CT-guided LP with CSF manometry. CT guidance was chosen per our institutional preference. Patient demographics and procedural details are listed in Table 2. All patients who underwent an LP were in the lateral decubitus position (3 in right lateral, 1 in left lateral). A 9-cm 22-ga Quincke tip needle (BD Medical) was used to access the thecal sac at L5–S1 in each patient. On average and relative to rest breathing, the CSF pressure increased with each maneuver including Valsalva (+5.25 cm H2O), normal inspiration (+2.5 cm H2O), mid-resisted inspiration (+4.25 cm H2O), and end-resisted inspiration (+7 cm H2O) as depicted in Fig 2.
Table 2:
Individual patient demographics and lumbar CSF manometry readings in 4 patients who underwent a CT-guided LP
| CSF Pressure (cm H20) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Patient | Age (yr)/Sex | Indication | BMI | Rest Pressure | Valsalva | Normal Inspiration | Mid-Resisted Inspiration | End-Resisted Inspiration |
| 1 | 69/F | Prior idiopathic leptomeningeal enhancement | 19.6 | 13 | 15 | 14 | 15 | 17 |
| 2 | 45/F | New-onset psychosis | 27.9 | 33 | 40 | 38 | 39 | 41 |
| 3 | 75/M | Intrathecal chemotherapy | 24.2 | 0 | 7 | 1 | 1 | 2 |
| 4 | 59/M | Metastatic lung cancer | 27.3 | 2 | 7 | 5 | 10 | 16 |
Note:—F indicates female; M, male; BMI, body mass index.
FIG 2.
CSF pressure changes with 4 different techniques relative to the CSF pressure while breathing at rest. CSF pressure increased the most with end-resisted inspiration, which was even greater compared with the Valsalva maneuver.
DISCUSSION
In the current study, we found that resisted inspiration can dramatically decrease SVC venous pressure and concurrently increase CSF pressure, which has the effect of increasing the CSF-to-venous pressure gradient. Increasing this gradient will likely improve detection of a CVF using myelography, a notoriously challenging diagnosis. This is the first description of resisted inspiration as an adjunctive maneuver during myelography to potentially help increase the conspicuity of a CVF.
MR myelography, CT myelography, and digital subtraction myelography have been described as different diagnostic techniques to identify a CVF.4,5 Each of these techniques requires the passage of contrast from the CSF to the venous system, which is driven by the CSF-to-venous pressure gradient. One prior article has described the effects of respiration on CVF detection. Amrhein et al8 have shown the benefit of end inspiration with the visualization of CVF on a CT myelogram and digital subtraction myelography. We have built on this work by showing that resisted inspiration dramatically decreases the venous pressure and increases the CSF pressure to an even greater extent compared with normal (nonresisted) inspiration.
Deep inspiration leads to negative intrathoracic pressure, increased cardiac return, and subsequent decreased venous pressure.9,10 If normal inspiration can generate decreased SVC venous pressure, then resisted inspiration that requires greater effort from the muscles of inspiration should further decrease venous pressure. Gutzeit et al11 showed that resisted inspiration increases the SVC/inferior vena cava blood flow for opacification of the pulmonary arteries and thereby aids in the detection of pulmonary emboli on CT angiography. While this effect was seen with resisted inspiration, they only noted minimal effect with normal inspiration.
Most interesting and somewhat unexpected, inspiration (both resisted and normal) leads to an increase in CSF pressure. Downward contraction of the diaphragm increases intrabdominal pressure and leads to an influx of blood into the epidural veins and, thus, increases pressure on the thecal sac.12
The CSF-to-venous pressure gradient should dictate the flow of CSF and, therefore, intrathecal contrast across a fistula. A 1-way valve between the SVC and azygos vein resists reflux of high pressure from the SVC into the azygos system but would facilitate low pressure in the SVC into the azygos system.13 This outcome is important as new and effective treatments become available for CVF, which include transvenous endovascular embolization14 in addition to surgical ligation and percutaneous fibrin glue injection.15
There are several limitations to our study. First, we studied a small number of separate patients for venous and CSF manometry. Ideally, we would study the pressure differences in a larger number of patients who would undergo both venous and CSF manometry. However, the venous manometry changes with resisted inspiration were large and consistent across all 4 patients and markedly different from both breathing at rest and normal inspiration. These results have already caused a change in the myelography technique at our institution. Finally, we used SVC pressure as a surrogate for pressure within the azygos system. While this is the most common venous drainage pathway of CVF, there are other venous drainage patterns7 that may not be affected by pressure changes in the SVC to the same degree.
CONCLUSIONS
We describe a technique termed “resisted inspiration” that could be used during myelography to decrease SVC venous pressure and increase lumbar CSF pressure, which may potentially aid in the detection of CVFs.
ABBREVIATIONS:
- CVF
CSF-venous fistula
- IIH
idiopathic intracranial hypertension
- LP
lumbar puncture
- SIH
spontaneous intracranial hypotension
- SVC
superior vena cava
Footnotes
Each of the authors (I.T.M., V.N.S., M.R.A., K.H.N., M.T.C., S.T., W.P.D.) contributed to all categories established by the International Committee of Medical Journal Editors, including conception and design, or acquisition of data, or analysis and interpretation of data, drafting the article or revising it critically for important intellectual content, final approval of the version to be published, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.Schievink WI, Moser FG, Maya MM. CSF-venous fistula in spontaneous intracranial hypotension. Neurology 2014;83:472–73 10.1212/WNL.0000000000000639 [DOI] [PubMed] [Google Scholar]
- 2.Schievink WI, Maya MM, Jean-Pierre S, et al. A classification system of spontaneous spinal CSF leaks. Neurology 2016;87:673–79 10.1212/WNL.0000000000002986 [DOI] [PubMed] [Google Scholar]
- 3.Amrhein TJ, Kranz PG. Spontaneous intracranial hypotension: imaging in diagnosis and treatment. Radiol Clin North Am 2019;57:439–51 10.1016/j.rcl.2018.10.004 [DOI] [PubMed] [Google Scholar]
- 4.Schievink WI, Moser FG, Maya MM, et al. Digital subtraction myelography for the identification of spontaneous spinal CSF-venous fistulas. J Neurosurg Spine 2016;24:960–64 10.3171/2015.10.SPINE15855 [DOI] [PubMed] [Google Scholar]
- 5.Chazen JL, Robbins MS, Strauss SB, et al. MR myelography for the detection of CSF-venous fistulas. AJNR Am J Neuroradiol 2020;41:938–40 10.3174/ajnr.A6521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim DK, Brinjikji W, Morris PP, et al. Lateral decubitus digital subtraction myelography: tips, tricks, and pitfalls. AJNR Am J Neuroradiol 2020;41:21–28 10.3174/ajnr.A6368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kranz PG, Amrhein TJ, Gray L. CSF venous fistulas in spontaneous intracranial hypotension: imaging characteristics on dynamic and CT myelography. AJR Am J Roentgenol 2017;209:1360–66 10.2214/AJR.17.18351 [DOI] [PubMed] [Google Scholar]
- 8.Amrhein TJ, Gray L, Malinzak MD, et al. Respiratory phase affects the conspicuity of CSF-venous fistulas in spontaneous intracranial hypotension. AJNR Am J Neuroradiol 2020;41:1754–56 10.3174/ajnr.A6663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinsky MR. Cardiopulmonary interactions: physiologic basis and clinical applications. Ann Am Thorac Soc 2018;15:S45–48 10.1513/AnnalsATS.201704-339FR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magder S. Volume and its relationship to cardiac output and venous return. Crit Care 2016;20:271 10.1186/s13054-016-1438-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutzeit A, Roos JE, Hergan K, et al. Suction against resistance: a new breathing technique to significantly improve the blood flow ratio of the superior and inferior vena cava. Eur Radiol 2014;24:3034–41 10.1007/s00330-014-3328-1 [DOI] [PubMed] [Google Scholar]
- 12.Lloyd RA, Butler JE, Gandevia SC, et al. Respiratory cerebrospinal fluid flow is driven by the thoracic and lumbar spinal pressures. J Physiol 2020;598:5789–805 10.1113/JP279458 [DOI] [PubMed] [Google Scholar]
- 13.Yeh BM, Coakley FV, Sanchez HC, et al. Azygos arch valves: prevalence and appearance at contrast-enhanced CT. Radiology 2004;230:111–15 10.1148/radiol.2301021216 [DOI] [PubMed] [Google Scholar]
- 14.Brinjikji W, Savastano LE, Atkinson JL, et al. A novel endovascular therapy for CSF hypotension secondary to CSF-venous fistulas. AJNR Am J Neuroradiol 2021;42:882–87 10.3174/ajnr.A7014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mamlouk MD, Shen PY, Sedrak MF, et al. CT-guided fibrin glue occlusion of cerebrospinal fluid-venous fistulas. Radiology 2021;299:409–18 10.1148/radiol.2021204231 [DOI] [PubMed] [Google Scholar]