Abstract
Objectives:
To understand the global landscape of prevention and control efforts targeting serogroup B meningococcal (MenB) disease and to identify the key challenges and gaps yet to be addressed.
Methods:
We conducted a comprehensive review of policies and practices for the use of protein-based MenB vaccines (Bexsero® [GlaxoSmithKline] and Trumenba® [Pfizer]) in all countries (n = 58) where either or both vaccine is authorized for use. We searched the literature (PubMed) and websites of health ministries and other relevant agencies to identify policy documents and plans and collect information about implementation timelines, target groups, vaccines being used, recommended schedules, and coverage data. Experts in the field were contacted for additional details andclarifications, as needed.
Results:
We found evidence of a national MenB vaccination policy in 24 out of 58 countries where one or both protein-based MenB vaccines are authorized. Of these, 15 countries have included MenB vaccination in their immunization plans for at least one age-based risk group (mostly infants), 21 have issued recommendations for various risk groups based on underlying medical conditions (e.g. asplenia), and 13 have done so for select groups at increased risk of exposure (e.g. laboratory staff). Recommended vaccination schedules and number of doses, where available, varied widely. Vaccination coverage data for age-based risk groups were not obtained for most countries.
Conclusions:
Our findings highlighted the significant heterogeneity in recommendations for MenB vaccination across countries. Greater transparency in reporting MenB vaccination recommendations and more robust data on implementation and the impact of vaccination would better facilitate optimizing MenB prevention strategies.
1. Introduction
Bacterial meningitis, often accompanied by sepsis, is an acute and life-threatening condition, responsible for an estimated 5 million cases and 300,000 deaths annually at global level. Neisseria meningitidis is one of its most common causative agents, affecting all age groups but with highest incidence among children and young adults. Of the 12 meningococcal serogroups that have been identified, six (A, B, C, W, X, and Y) possess epidemic potential and are thus particularly concerning from a public health perspective [1].
The distribution of serogroups is geographically heterogeneous and varies over time, with important implications for prevention and control. Various factors contribute to changes in the meningococcal epidemiology over time, such as mass population movements and gatherings or the introduction of serogroup-specific vaccination campaigns that reduce the prevalence of certain serogroups [2]. Serogroup B contributes to a large proportion of meningococcal disease cases in Europe, North and Latin America, Australia, and North Africa, and MenB disease incidence is highest in these regions [3]. In the absence of prompt and adequate treatment, approximately half of all individuals affected by meningococcal disease die and around 10% develop permanent disabilities.
While effective polysaccharide-based vaccines have been developed to prevent disease caused by other meningococcal serogroups, this approach cannot be used to prevent serogroup B disease owing to the pathogen’s structural characteristics [4]. Hence, the first meningococcal B (MenB) vaccines introduced in the 1980s were based on select proteins of the outer membrane vesicles (OMV) released by bacteria [5]. Although OMV vaccines have been employed for many years to control local outbreaks caused by specific strains in various countries such as Cuba, Norway, Brazil and New Zealand, their protective effect is highly variable and generally limited to selected and context-specific epidemic strains, with immunity rapidly waning over time [6–8].
Over the past decade, with the use of reverse vaccinology and proteomics, two protein-based MenB vaccines aiming to induce broader immunity against a range of serogroup B strains have been developed and approved for use: Bexsero® (4CMenB; GlaxoSmithKline (GSK)) includes four recombinant proteins of serogroup B meningococcus and was first authorized for use by the European Medicines Agency (EMA) in 2013, and Trumenba® (MenB-FHbp; Pfizer) contains two recombinant lipoproteins from serogroup B meningococcus and was first approved by the US Food and Drug Administration (FDA) in 2014 [9]. The introduction of these new vaccines has revolutionized the approach to reducing and minimizing the risk of MenB disease, paving the way for large-scale preventative efforts that were previously not feasible.
In September 2021, the World Health Organization (WHO) launched a global road map to defeat meningitis [10], establishing 5 pillars and 19 strategic goals to prevent and control the primary causes of bacterial meningitis (N. meningitidis (Nm), Streptococcus pneumoniae (Spn), Haemophilus influenzae (Hi) and group B Streptococcus (GBS)), and these goals are very well aligned with the broader agenda for sustainable development [11]. Prevention is essential to alleviating the health, social and economic burden of meningococcal meningitis caused by all serogroups [12]. One of the key targets of the WHO global road map is to “Reduce cases of vaccine-preventable bacterial meningitis by 50% and deaths by 70%”. In areas where MenB is predominant, effective use of MenB vaccines along with other vaccines that can prevent bacterial meningitis caused by different pathogens will be instrumental to achieving this goal. The ambitious goal of developing a global policy for the use of vaccines against pathogens responsible for bacterial meningitis will constitute a step forward in supporting country-level identification of key priorities based on local epidemiology and available resources [10,13].
At present, countries have adopted and implemented a wide range of policies and vaccination programs that take advantage of protein-based MenB vaccines, which became available relatively recently. In order to understand the current global landscape of prevention and control efforts targeting meningococcal disease caused by N. meningitidis serogroup B and to identify the key challenges and gaps yet to be addressed, we conducted a comprehensive review of country-specific MenB vaccination policies and practices to summarize recommendations for the use of MenB vaccines globally.
2. Methods
In order to obtain a list of countries to include in our assessment, we first contacted the developers (GlaxoSmithKline and Pfizer) of the two MenB vaccines of interest (i.e. Bexsero® and Trumenba®) to request the list of countries where their respective MenB vaccines have been approved or authorized for use, along with the year of approval in each country. After confirming these lists through additional online searches, we used a combination of scientific literature searches, review of policy documents, direct contact with experts in the field, and our expertise to identify information about MenB vaccination policies and recommendations in the 58 countries where Bexsero® and/or Trumenba® vaccines have been approved for use (Table S1). OMV vaccines were not considered in our analysis owing to their restricted use during epidemic outbreaks in selected settings.
We systematically searched PubMed using key terms for MenB vaccines (e.g., “Bexsero”, “Trumenba”, “Meningococcal vaccine”, “MenB”, “4CMenB vaccine”, “Meningitis B”, “Meningococcal Vaccines/administration and dosage”[Mesh], “Meningococcal Vaccines/organization and administration”[Mesh], “Meningococcal Vaccines/supply and distribution”[Mesh]) and restricting to articles published from 2013 (i.e. the year of approval of the first protein-based MenB vaccine by a regulatory authority) through October 13, 2021.
Policy documents and vaccination plans were systematically searched for by accessing the websites of ministries of health, national public health agencies, and drug regulatory authorities for each of the countries where at least one of the vaccines had been approved. The manuscripts identified in the literature search were also used to obtain contact information for experts in the field. The authors of relevant publications were then contacted via email to request additional information to augment or clarify the publicly available information we had obtained about individual country policies.
Using these search strategies, for each of the countries where at least one of the two protein-based MenB vaccines had been approved, we identified evidence from reports and official documents for a national policy with regard for the use of MenB vaccine. Whenever we found evidence of a national policy for MenB vaccination, we collected information about implementation timelines, targeted population groups, type of vaccines to be used, and recommended vaccination schedules. For those countries where we found that MenB vaccination is routinely recommended for all infants, we also searched for any vaccination coverage statistics for this age group released by relevant national health authorities, such as the proportion of eligible individuals who had received at least one vaccine dose or had completed the series by calendar year. Coverage levels were investigated conditional on data availability.
3. Results
As of April 2021, Bexsero® and/or Trumenba® have been approved in 58 countries (one in the African Region, 12 in the American Region, 3 in the Eastern Mediterranean Region, 39 in the European Region and 4 in the Western Pacific Region). Trumenba® vaccine was first approved in the United States in 2014, and subsequently approved for use in 50 additional countries, with most countries granting authorization in 2017 or later. Applications for approval of Trumenba® have also been submitted to the national regulatory agency of two additional countries (Saudi Arabia and Turkey) and are stillunder review as we write. Bexsero® vaccine was first authorized by the EMA in 2013, followed by approvals by national regulatory agencies across the European Union and elsewhere, for a total of 47 countries granting authorization in 2013 or later.
Bexsero® is the only option for infant immunization at present given that Trumenba® is currently not authorized for use under the age of ten years. For infants, the manufacturer recommends two to three doses of Bexsero® given at least one or two months apart depending on the age at first dose, starting at age two months (Table S2) [14]. Boosters are also recommended by the manufacturer, usually during the second year of life for those who were first immunized during infancy (i.e. within 12 months of age), with variable intervals relative to the previous dose based on the timing of the primary cycle. Children aged ten and older, adolescents and adults can be immunized with either Bexsero® or Trumenba® depending upon which vaccine is authorized and available in a given country. However, these MenB vaccines are not interchangeable, so that – once started – the immunization cycle must be completed with the same vaccine. According to the manufacturer [15], the recommended schedule for Trumenba® is two doses at least six months apart; however, individuals at increased risk of invasive meningococcal disease should receive the first two doses with a one-month interval, followed by a third dose at least four months after the previous one.
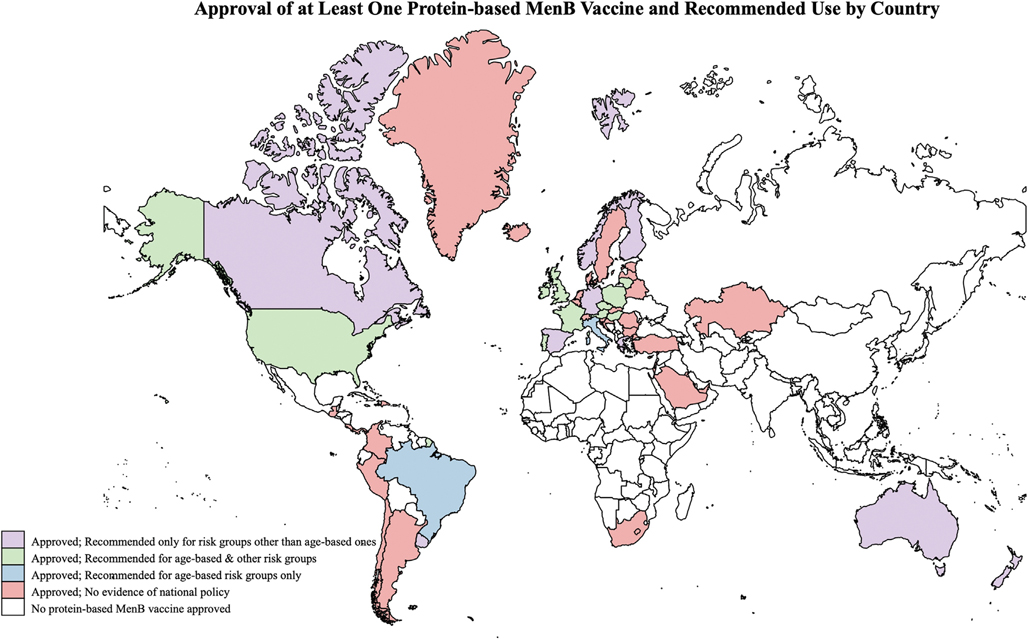
Among the 58 countries where at least one of the protein-based MenB vaccines is currently authorized for use, 24 (41.4%) have included MenB vaccines in their immunization plans and have thus provided recommendations for which groups should receive the vaccine (i.e., age-based risk groups, individuals at risk due to medical conditions or because of increased risk of exposure) (Fig. 1). A detailed description of country-specific policies is provided in Table S3. For the remaining 34 countries where one or both vaccines have been authorized for use, we did not find evidence that a national policy has been made publicly available.
Fig. 1.
Countries where at least one protein-based meningococcal B (MenB) vaccine (Bexsero® and/or Trumenba®) has been approved/authorized for use and where evidence of a national MenB vaccination policy is available are shaded in purple, green, or blue to define the groups recommended for vaccination. Countries where at least one meningococcal B (MenB) vaccine (Bexsero® and Trumenba®) has been approved/authorized for use but with no evidence that a national policy is in place are shaded in red. Risk groups other than age-based ones include individuals with select medical risk conditions and/or those at increased risk of exposure. Australia and Spain are shaded in purple (vaccination recommended for risk groups other than age-based ones) because the recommendation to vaccinate infants in these countries only applies to certain regions and/or ethnic groups. (For further information about the vaccination policies referenced in this figure, see Table S3. For interpretation of the colours (purple, green, blue, red, and white) referenced in this figure legend, see the web version of this article.)
3.1. Countries recommending MenB vaccination for age-based risk groups
In 15 (62.5%) of the 24 countries where we obtained a publicly available national immunization plan, MenB vaccination is recommended routinely for specific age-based risk groups nationwide. In 2015, the United Kingdom was the first country to include MenB vaccination in the routine immunization schedule for all infants (i.e. during the first year of life), regardless of their health status [16]. In the same year, the Brazilian Pediatric Society also introduced MenB vaccine in the national vaccination schedule for all infants, toddlers, children and adolescents [17]. Other countries, almost all in Europe, included MenB in the routine immunization schedule for infants subsequently: Andorra and Ireland in 2016 [18,19]; Italy and San Marino in 2017 [20,21]; Hungary and Lithuania in 2018 [22,23]; Austria, Czech Republic, Malta, Poland and Portugal in 2020 [24–28]; France in 2021 [29]. In Austria and in Hungary, the national plan includes catch-up vaccinations until age 25 [22,24]. Furthermore, in 2020, a recommendation to vaccinate all individuals aged 16 to 23 years based on shared clinical decision-making was made by the Advisory Committee on Immunization Practices in the United States [30].
Two additional countries (Australia and Spain) have issued recommendations for routine MenB vaccination of infants residing in certain regions or belonging to specific ethnic groups. In Australia, a selective MenB infant immunization program was established in 2020, targeting Aboriginal and Torres Strait Islander infants starting at six weeks of age, with a catch-up program for indigenous children aged less than two years up to June 30, 2023 [31]. Beginning in 2018, the state of South Australia is the only Australian state to offer routine MenB vaccination for all infants, at no cost [32]; since 2019, a Bexsero®-based school vaccination program targeting adolescents aged 15–16 years is also in place in this state and publicly funded [33]. With regards to Spain, two autonomous communities (Castilla y León and the Canary Islands) have included Bexsero® in their infant immunization schedules since 2019 [34,35].
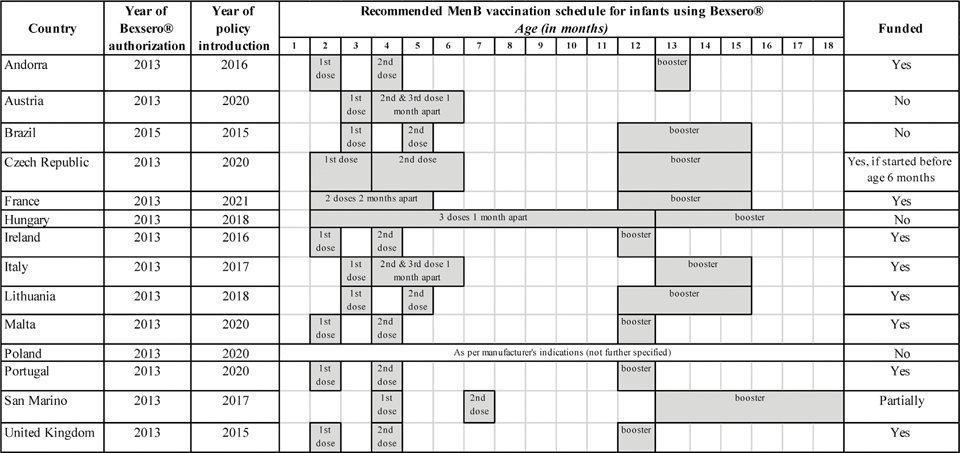
In nine (64.3%) of the 14 countries where MenB vaccination is nationally recommended for infants (Andorra, Czech Republic, France, Ireland, Italy, Lithuania, Malta, Portugal, United Kingdom), this vaccine is offered free of charge to everyone in that age group [16,18–20,23,25,26,28,29]; however, in Czech Republic vaccination is free of charge provided that the vaccination series starts before 6 months of age [25]. A fee is required in the other five countries where MenB vaccination is recommended for all infants (Austria, Brazil, Hungary, Poland, and San Marino) [17,21,22,24,27] (Fig. 2).
Fig. 2.
Countries where meningococcal B (MenB) vaccination in infants is routinely recommended nationwide (n = 14). For each country, the year of Bexsero® approval and the year of policy introduction are summarized, along with the recommended schedule and information on whether this vaccine is funded (i.e. provided free of charge) or provided for a fee. All data shown in this figure were obtained from publicly available country policy documents and/or from experts in the field.
We found that, in countries where Bexsero® is recommended for all infants, the first dose is usually recommended at age two or three months and schedules vary widely within the range indicated by the manufacturer (Fig. 2). Some countries allow for more flexibility with respect to the exact age of first dose among infants, provided that the correct intervals between doses are ensured. Though boosters are recommended up to a year after primary series completion, additional booster doses more than a year after primary series completion are not currently recommended in any country for this age group.
Either vaccine can be utilized to immunize individuals aged 16–23 years in the United States as both are approved for use in this age group [30]. In Brazil, the national policy recommendations state that two doses of Bexsero® are recommended for children and adolescents who had not been vaccinated previously [17]. Trumenba® was approved in Brazil in January 2019, as well, but the last revision of the vaccination policy (2020) available publicly does not include specific recommendations for the use of this vaccine.
3.2. Countries recommending MenB vaccination for other risk groups
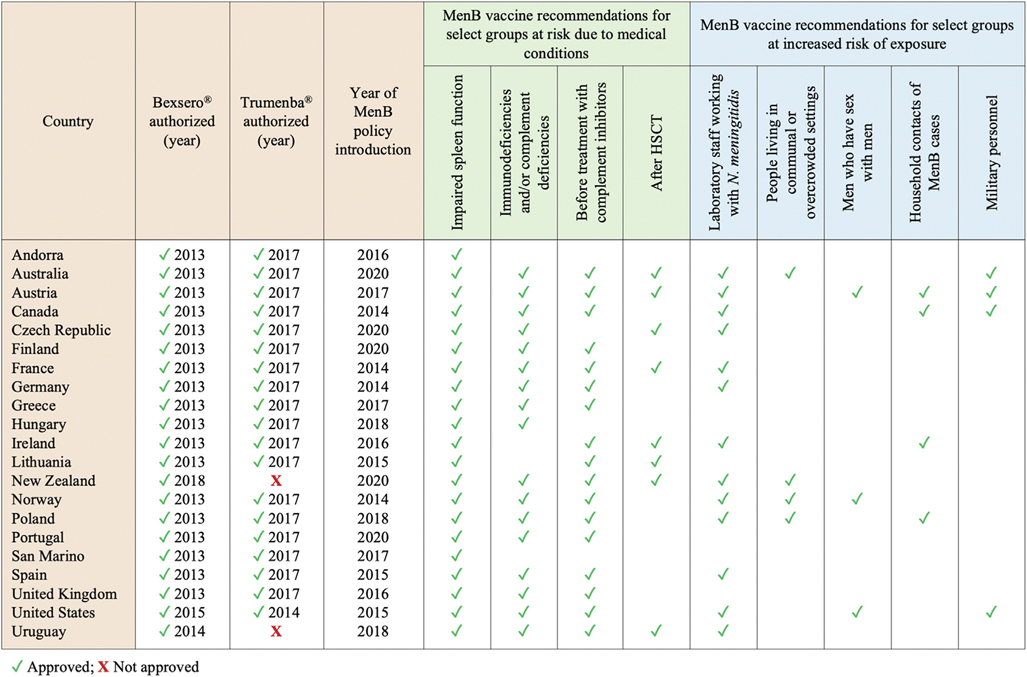
In 21/24 (87.5%) countries with evidence of a publicly available national policy outlining the use of MenB vaccine(s), MenB vaccination is recommended for one or more selected groups based on the presence of conditions that increase the risk of invasive meningococcal disease and/or due to increased risk of exposure related to occupational factors, living conditions or high-risk behaviors (Fig. 3). Among individuals with underlying medical conditions that are associated with an increased risk of invasive disease caused by N. meningitidis, those with impaired spleen function due to anatomic absence of the spleen or functional deficits are recommended to receive MenB vaccination in all 21 countries [16,18,21–25,27–31,36–45]. Vaccination is also recommended before starting treatment with complement inhibitors (e.g., eculizumab) in 18/21 (85.7%) countries, in case of primary or acquired immunodeficiency (particularly complement deficiencies) in 17/21 (81.0%), and after hematopoietic stem cell transplantation (HSCT) in 11/21 (52.4%). Six (28.6%) countries (Australia, Canada, New Zealand, Poland, United States and Uruguay) additionally recommend MenB vaccination for people living with HIV/AIDS (PLWHA) regardless of immune status [27,30,31,36–38]. Individuals with other health conditions that could increase their risk of meningococcal disease are also listed among those recommended for vaccination in some countries, such as individuals receiving cancer chemotherapy or solid organ transplant (SOT) recipients (Table S3).
Fig. 3.
Countries where meningococcal B (MenB) vaccination is recommended for select health- and exposure-based risk groups. For each country, the following details are summarized: 1) whether Bexsero® and Trumenba® have been authorized; 2) year of vaccine approval, where applicable; 3) year of first introduction of a national policy for the use of MenB vaccines among risk groups other than aged-based ones; 4) evidence of a national recommendation for routine MenB vaccination of select risk groups other than aged-based ones (i.e. select groups of individuals at risk for medical reasons or due to an increased risk of exposure). All data reported in this graph were obtained from publicly available policy documents and/or experts in the field; when updates were issued after the first introduction of the national MenB vaccination plan, the most recent recommendations were reported. In most countries where both MenB vaccines are authorized and available on the market, either can be used to immunize at-risk individuals if age-appropriate; see Table S3 for more details.
A total of 13/24 (54.2%) countries have recommendations for MenB vaccination among select groups at increased risk of exposure, primarily laboratory workers handling meningococcal isolates [18,24,25,27,30,31,36–38,40,42,44,45].
Depending on the country, which vaccine(s) are approved, and the age group, individuals recommended for vaccination as part of these select groups could be eligible to receive either Bexsero® or Trumenba® (Table S3). In most instances where both vaccines are authorized (19 countries), only Bexsero® is explicitly mentioned in publicly available national policies and their related documents, partly reflecting the fact that some policies only focused on infant immunization for which Bexsero is the only option. In addition, some national MenB vaccination policies were introduced after Bexsero® was authorized but before the authorization of Trumenba® in the country, and we found no updated policies issued during the period of time when both vaccines were authorized and available. A considerable between-country heterogeneity in terms of recommended number of doses and intervals between them can be observed across countries, with all schedules falling within manufacturers’ suggested ranges (Table S3).
3.3. MenB vaccination policy implementation
According to our findings, very few policy documents explicitly outline target coverage levels to be reached within a specified period of time, and coverage statistics are not routinely reported in many countries. We observed that vaccination coverage levels were more often reported in countries where MenB vaccination has been included in the routine immunization schedule for age-based risk groups (i.e. an eligibility group whose denominator is more feasible to estimate) and where this policy has been in place for at least a few years. Hence, our assessment of vaccination coverage solely focused on infants.
We found that, in the United Kingdom, coverage statistics were first released in 2017/2018, two years after the inclusion of MenB vaccine in the routine infant schedule, with a vaccine uptake of 92.0 to 92.5% per year until 2020/2021 [46]. In Italy, where MenB vaccine became part of the routine vaccination schedule in 2017, the target coverage for infants was set at ≥ 60% for the first year, ≥75% for 2018, and ≥ 95% for 2019 [20]. Current levels of uptake among those aged up to 24 months appear below target: 66.3% in 2020, with a 2.7% decline compared to the previous year that likely reflects the impact of the coronavirus disease 2019 (COVID-19) pandemic on health services [47]. In Andorra, MenB vaccination coverage among those born from January 2016 onwards exceeds 95% according to what estimated by experts in the field. We were unable to obtain information about MenB immunization coverage levels in other countries in our search.
4. Discussion
Since 2013, 58 countries have approved one or both recently developed protein-based MenB vaccines. Based on our global assessment of country-level MenB vaccination policies, we observed that less than half of the countries that have authorized at least one MenB vaccine have a publicly available national policy for the use of MenB vaccines. Detailed, national vaccination plans are important tools for developing and implementing effective and equitable plans to prevent meningococcal B disease and monitoring their impact. Among the 24 countries with a publicly available national MenB vaccination plan, we found significant variability in recommendations for the use of MenB vaccines, including variability in the age-based, medical risk-based, and exposure-based groups prioritized, number of doses, age/timing of doses, and interval between doses. Given that protein-based MenB vaccines designed to induce broad immunity against a diversity of meningococcal B strains have been developed relatively recently, understanding how countries have incorporated these vaccines into their national vaccination programs can provide important insight into current prevention efforts and help identify opportunities for improvement.
Our findings highlight some critical areas for improvement in meningococcal disease prevention in general and MenB in particular. First, robust surveillance is essential for guiding policy decision-making and adapting immunization strategies to local needs as it allows to better understand where and to what extent MenB is a problem while also monitoring changes in epidemiology. Meningococcal disease is generally subject to mandatory notification, and substantial progress has been made over the past two decades, but laboratory capacity and surveillance mechanisms – where existent – vary widely, thus hindering an accurate assessment of local epidemiology and proper comparisons across countries. In the absence of routine serogroup-specific meningococcal disease surveillance, passive surveillance of bacterial isolates has revealed that serogroup B meningococcus has been identified at least once in dozens of countries over the past two decades [48]. However, the burden of meningococcal disease and the proportion of cases due to serogroup B differ across settings and are likely underestimated in several contexts, especially those without routine surveillance [49]. Improved efforts to understand the role of serogroup B meningococcus in the epidemiology of meningococcal disease is needed to determine whether evidence exists to support the need for protein-based MenB vaccines in those countries where they are not currently authorized. Of note, neither Bexsero® or Trumenba® are currently authorized for use in any of the North African countries, where N. meningitidis is an important cause of bacterial meningitis and serogroup B is responsible for the vast majority of cases of bacterial meningitis (up to 80–95% according to some reports) [50,51]. As of now, none of the MenB vaccines is included in the WHO’s recommendations for routine immunization, which may play a role in shaping countries’ decisions about which vaccines should be prioritized. In addition to implementing enhanced surveillance, it may be necessary to conduct thorough analyses, such as cost-effectiveness and feasibility assessments using surveillance data, to ensure evidence-based policy recommendations for the most efficient use of the vaccine in areas where there may be resource limitations or implementation challenges. With more detailed surveillance especially among high-risk populations in certain regions, national health authorities would have better and more complete data to determine the most effective MenB vaccination strategy given the local context. These data are needed to assess whether MenB vaccines should be routinely recommended for all age groups or certain age groups, recommended for those in specific geographic regions where meningococcal disease cases are more frequently detected or outbreak risks are high, and/or whether this vaccination should only be utilized for select risk groups that are considered at higher risk due to underlying medical conditions or other factors. Some countries with well-established surveillance systems have designed their own policies on the basis of cost-effectiveness models that have taken into account the local epidemiology, the estimated vaccine coverage of prominent strains, the characteristics of the general population and specific risk groups [52].
The WHO’s recent commitment to support the development, licensure, and introduction of new effective, affordable and safe MenB vaccines may change the way MenB vaccination is perceived and implemented where serogroup B meningococcus is more prevalent [10]. Most notably, under strategic goal 2 of pillar 1 (prevention and epidemic control) of the global road map, WHO encourages the adoption of multivalent conjugate vaccines and/or serogroup B vaccines, as relevant, in at least ten countries by 2026. In the European Region, where serogroup B accounts for a large proportion of meningococcal disease cases [3], this target seems within reach if not already met, with at least nine countries that, between 2015 and 2020, have included Bexsero® among recommended routine immunizations for all infants, at no cost for recipients. Although we focused only on protein-based MenB vaccines in this policy review, it is important to note that the Finlay Institute’s OMV-based MenB/C vaccine VA-MENGOC-BC® has played a major role in successfully controlling serogroup B and C meningococcal disease in Cuba and other countries throughout Latin America since 1989 [8].
Second, greater transparency regarding how countries are utilizing MenB vaccines could be a critical global asset. In fact, the widespread lack of detailed and easily accessible documents has been the biggest barrier we have faced in our attempt to summarize the global landscape with respect to MenB vaccination policies. Some countries already provide clear schematics concerning their strategy, typically if MenB vaccination is included in their routine immunization program for age-based risk groups. Establishing a publicly accessible central repository may be a good way to improve transparency in reporting on policy developments and an opportunity for countries to learn from one another, especially those with similar epidemiologic situations. Existing tools such as the Meningitis Progress Tracker [53], developed by the Meningitis Research Foundation (MRF) and endorsed by WHO, could be leveraged for this purpose. The WHO’s global call for meningococcal meningitis control presents a timely opportunity to encourage a more transparent, clear, and standardized way of reporting, summarizing, and monitoring the impact of national strategies. In this regard, the development of a global policy for use of MenB vaccines to be achieved by 2022 as per strategic goal 3 of the WHO road map’s pillar 1 could provide some form of high-level guidance [10]. An ideal national policy document should clearly outline the rationale and evidence base for the recommendations proposed, identify which specific population group(s) are to be prioritized, describe which vaccine(s) can be utilized for immunizing target groups along with the recommended schedule(s), indicate whether the cost of vaccination for each category is partially or fully covered by public funding or other forms of financial support, and be publicly accessible through web-based platforms. Furthermore, a clearly outlined plan describing efforts to monitor implementation, including coverage goals for specific risk groups where appropriate, would be an asset.
If identifying and summarizing existing national MenB vaccination policies has been a challenging task, understanding whether and to what extent these policies are implemented in the field presented an even greater challenge and was beyond the scope of this work. More data about vaccination uptake are thus required to assess the degree to which national recommendations are being implemented and to identify key barriers to scaling up vaccination programs.
Our work provides the first comprehensive overview of the global landscape of protein-based MenB vaccination policies. We undertook this work given the urgent need to understand the current policy landscape in light of the recently launched global goals via the WHO road map. Our findings make important contributions to understanding how countries are approaching serogroup B meningococcal disease prevention, with implications for both clinical and public health practice. Among the biggest strengths of this policy review is that we have conducted a very thorough systematic search for relevant documents in any language. We have also made every effort to reach out to experts in the field in order to fill key gaps, whenever possible. However, we note that our review is only as complete as the publicly available sources we identified. It is possible that we may not have had access to relevant non-electronic resources or non-publicly accessible strategic documents and thus additional countries may have implemented MenB vaccination programs beyond those reported here, or we may have missed important details in countries with existing policies. For instance, while all dosing schedules listed in this review reflect most up to date vaccination policy reports obtained by our research team, it is possible that the current dosing recommendations may have changed or may not have been publicly available. This limitation is inherent to any ad-hoc policy review and another reason that a centralized policy database would lead to greater transparency and improved reporting.
Numerous studies have demonstrated that protein-based MenB vaccines are safe and effective in preventing MenB diseases, though these vaccines do not have an impact on meningococcal carriage [54,55]. The development of MenB vaccines presents an opportunity to contribute to the goals of the WHO’s global road map and significantly advance efforts to prevent meningococcal disease worldwide [10]. Furthermore, OMV and protein-based MenB vaccines could have a beneficial impact on other conditions such as gonorrhea, by conferring some degree of cross-protection against gonococcal strains and representing an interesting starting point for the development of specific gonococcal vaccines [56]. Our review has demonstrated the significant heterogeneity in national MenB vaccination policies and the opportunity for countries to learn from one another in designing, implementing, and monitoring MenB vaccination programs. We have also highlighted the need for enhanced epidemiologic surveillance to support evidence-based policy, greater transparency with respect to policy recommendations, and better data on implementation and uptake. The future introduction of pentavalent vaccines targeting all major meningococcal serogroups (MenABCWY) will further help curb the burden of meningococcal disease globally; these combined products, that are currently under investigation [57,58], may be a more efficient way to improve immunization coverages particularly where surveillance of circulating serogroups is still suboptimal.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge Dr. Jamie Findlow for providing the list of countries where Trumenba® is currently authorized along with the year of approval in each country.
The authors gratefully acknowledge Dr. Saber Yezli and Dr. Mireia G. Carrasco for sharing their expertise and providing important information regarding the use of meningococcal B vaccines in the Kingdom of Saudi Arabia and the Principality of Andorra, respectively.
Footnotes
Declaration of Competing Interest
R.B. performs contract research on behalf of UK Health Security Agency for GlaxoSmithKline, Pfizer and Sanofi Pasteur. All other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Financial Disclosure Statement
Research reported in this publication was supported by the National Institute Of Allergy And Infectious Diseases of the National Institutes of Health under Award Number R01AI132496 (PI: Dr. Nicole E. Basta). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.The funders had no role in the design and conduct of the study, results interpretation, and decision to publish.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.04.101.
References
- [1].Rosenstein NE, Perkins BA, Stephens DS, Popovic T, Hughes JM. Meningococcal disease. N Engl J Med 2001;344(18):1378–88. [DOI] [PubMed] [Google Scholar]
- [2].Pelton SI. The Global Evolution of Meningococcal Epidemiology Following the Introduction of Meningococcal Vaccines. J Adolesc Health 2016;59(2):S3–S11. [DOI] [PubMed] [Google Scholar]
- [3].Sridhar S, Greenwood B, Head C, Plotkin SA, Sáfadi MA, Saha S, et al. Global incidence of serogroup B invasive meningococcal disease: a systematic review. Lancet Infect Dis 2015;15(11):1334–46. [DOI] [PubMed] [Google Scholar]
- [4].Finne J, Leinonen M, Mäkelä PH. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet 1983;322(8346):355–7. [DOI] [PubMed] [Google Scholar]
- [5].Holst J, Oster P, Arnold R, Tatley M, Næss L, Aaberge I, et al. Vaccines against meningococcal serogroup B disease containing outer membrane vesicles (OMV): lessons from past programs and implications for the future. Hum Vaccin Immunother 2013;9(6):1241–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Panatto D, Amicizia D, Lai PL, Cristina ML, Domnich A, Gasparini R. New versus old meningococcal group B vaccines: how the new ones may benefit infants & toddlers. Indian J Med Res 2013;138:835–46. [PMC free article] [PubMed] [Google Scholar]
- [7].Harder T, Koch J, Wichmann O, Hellenbrand W. Predicted vs observed effectiveness of outer membrane vesicle (OMV) vaccines against meningococcal serogroup B disease: Systematic review. J Infect 2017;75(2):81–94. [DOI] [PubMed] [Google Scholar]
- [8].Sierra-González VG. Cuban Meningococcal Vaccine VA-MENGOC-BC:30 Years of Use and Future Potential. MEDICC Rev 2019;21:19–27. [DOI] [PubMed] [Google Scholar]
- [9].Rivero-Calle I, Raguindin PF, Gómez-Rial J, Rodriguez-Tenreiro C, Martinón-Torres F. Meningococcal Group B Vaccine For The Prevention Of Invasive Meningococcal Disease Caused By Neisseria meningitidis Serogroup B. Infect Drug Resist 2019;12:3169–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Defeating meningitis by 2030: a global road map. Geneva, Switzerland: World Health Organization (WHO); 2021. Available at: https://www.who.int/publications/i/item/9789240026407. [Google Scholar]
- [11].Transforming our world: the 2030 agenda for sustainable development. New York, NY, USA: United Nations (UN); 2015. Available at: https://sdgs.un.org/sites/default/files/publications/21252030%20Agenda%20for%20Sustainable%20Development%20web.pdf. [Google Scholar]
- [12].Borrow R, Alarcón P, Carlos J, Caugant DA, Christensen H, Debbag R, et al. The Global Meningococcal Initiative: global epidemiology, the impact of vaccines on meningococcal disease and the importance of herd protection. Expert Rev Vaccines 2017;16(4):313–28. [DOI] [PubMed] [Google Scholar]
- [13].Greenwood B, Sow S, Preziosi M-P. Defeating meningitis by 2030 – an ambitious target. Trans R Soc Trop Med Hyg 2021;115(10):1099–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Product monograph: Bexsero. Multicomponent Meningococcal B Vaccine (recombinant, absorbed). ATC Code: J07AH09. Mississauga, ON, Canada: GlaxoSmithKline Inc.; 2013. (Last revised: March 31, 2022). Available at: https://ca.gsk.com/media/1212390/bexsero.pdf. [Google Scholar]
- [15].Product monograph including patient medication information: Trumenba. Meningococcal group B vaccine [Bivalent recombinant lipoprotein (rLP2086)]. Kirkland, QC, Canada: Pfizer Canada ULC; 2017. (Last revised: May 24, 2019). Available at: https://www.pfizer.ca/sites/default/files/201907/Trumenba_PM_E_220373_24May2019.pdf. [Google Scholar]
- [16].JCVI position statement on use of Bexsero® meningococcal B vaccine in the UK. London, United Kingdom: Joint Committee on Vaccination and Imminisation, Health Department, Government of the United Kingdom; 2014. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/294245/JCVI_Statement_on_MenB.pdf. [Google Scholar]
- [17].Quadro do Calendário Vacinal da SBP 2015. Sociedade Brasileira de Pediatria; 2015. Available at: https://www.sbp.com.br/fileadmin/user_upload/2015/02/calendario-vacinal2015-2.pdf. [Google Scholar]
- [18].HSE National Immunization Office | Meningococcal B. Dublin, Ireland: Health Service Executive (HSE) National Immunization Office. Available at: https://www.hse.ie/eng/health/immunisation/hcpinfo/othervaccines/meningococcalb/. Last accessed: May 4, 2022. [Google Scholar]
- [19].[Vaccination Plan]. Andorra: Health Department, Government of Andorra. Available at: https://www.salut.ad/departament-de-salut/pla-de-vacunacions. Last accessed: May 4, 2022. [Google Scholar]
- [20].[National Vaccine Prevention Plan 2017–2019]. Rome, Italy: Directorate General for Health Prevention, Ministry of Health; 2017. Available at: https://www.salute.gov.it/imgs/C_17_pubblicazioni_2571_allegato.pdf. [Google Scholar]
- [21].[Recommended Vaccinations]. San Marino Republic: Istituto per la Sicurezza Sociale, San Marino. Available at: https://www.iss.sm/on-line/home/vaccini-e-vaccinazioni/vaccinazioni-raccomandate.html. Last accessed: May 4, 2022. [Google Scholar]
- [22].[Childhood immunizations. VACSATC Hungary]. Budapest, Hungary: VACSATC; 2018. Available at: http://www.vacsatc.hu/?Gyermekkori-v%E9d%F5olt%E1sok&pid=24. Last accessed: May 4, 2022. [Google Scholar]
- [23].[Order No. 22 on approval of the calendar of preventive vaccinations in children]. Vilnius, Lithuania: Ministry of Health of the Republic of Lithuania; 2018. Available at: https://e-seimas.lrs.lt/portal/legalAct/lt/TAD/f4a925d0f50f11e79a1bc86190c2f01a. [Google Scholar]
- [24].Impfplan Öesterreich 2022. Vienna, Austria: Bundesministerium Soziales, Gesundheit, Pflege und Konsumentenschutz; 2022. Available at: https://www.sozialministerium.at/Themen/Gesundheit/Impfen/Impfplan-Österreich.html. Last accessed: May 4, 2022. [Google Scholar]
- [25].Recommendation of the Czech Vaccinological Society of the Czech Medical Association for vaccination against invasive meningococcal diseases. Prague, Czech Republic: Czech Vaccinological Society, Czech Medical Association; 2020. Available at: http://www.szu.cz/uploads/IMO/2020_Recommendation_vaccination_IMD.pdf. [Google Scholar]
- [26].National Immunization Schedule. Malta: Ministry of Health, Government of Malta; 2020. Available at: https://deputyprimeminister.gov.mt/en/phc/pchyhi/Pages/National-Immunisation-Schedule.aspx. Last accessed: May 4, 2022. [Google Scholar]
- [27].[Announcement of the Ministry of Health on the 2019 Preventive Vaccination Program]. Warsaw, Poland: Ministry of Health, Governemnt of Poland; 2018. Available at: https://gis.gov.pl/wp-content/uploads/2018/01/akt.pdf. [Google Scholar]
- [28].Programa Nacional de Vacinação 2020. Lisbon, Portugal: Serviço Nacional de Saude, República Portuguesa; 2020. Available at: https://www.dgs.pt/normas-orientacoes-e-informacoes/normas-e-circulares-normativas/norma-n-0182020-de-27092020-pdf.aspx. [Google Scholar]
- [29].Stratégie de vaccination pour la prévention des infections invasives à méningocoques: Le sérogroupe B et la place de BEXSERO®. Recommandation Vaccinale. Paris, France: Haute Autorité de Santé (HAS); 2021. Available at: https://www.has-sante.fr/upload/docs/application/pdf/2021-06/strategie_de_vaccination_pour_la_prevention_des_infections_invasives_a_meningocoques_le_serogroupe_b_et_la_place_de_bexsero.pdf. [Google Scholar]
- [30].Mbaeyi SA, Bozio CH, Duffy J, Rubin LG, Hariri S, Stephens DS, et al. Meningococcal Vaccination: Recommendations of the Advisory Committee on Immunization Practices, United States, 2020. MMWR Recomm Rep 2020;69(9):1–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].National Immunisation Program Schedule - Department of Health, Australian Government. Canberra, Australia: Department of Health, Australian Government; 2021. Available at: https://www.health.gov.au/health-topics/immunisation/immunisation-throughout-life/national-immunisation-program-schedule. Last accessed: May 4, 2022. [Google Scholar]
- [32].A Meningococcal B Program for South Australia. Public Report. Adelaide, SA, Australia: South Australian Meningococcal B Expert Working Group. Government of South Australia; 2018. Available at: https://www.sahealth.sa.gov.au/wps/wcm/connect/b82a9fb7-061a-48b9-be37-54e88a1907d1/2018-06+Optimal+Men+B+Program+for+SA+Public+Report+%282%29.pdf?MOD=AJPERES&CACHEID=ROOTWORKSPACE-b82a9fb7-061a-48b9-be37-54e88a1907d1-nwKSghQ. [Google Scholar]
- [33].Meningococcal B Immunisation Program. Adelaide, SA, Australia: SA Health, Government of South Australia; 2021. Available at: https://www.sahealth.sa.gov.au/wps/wcm/connect/public+content/sa+health+internet/conditions/immunisation/immunisation+programs/meningococcal+b+immunisation+program?finderTab=tab-2. Last accessed: May 4, 2022. [Google Scholar]
- [34].[Vaccination calendar throughout life in Castilla and Leon]. Servicio de Salud de Castilla y Leon, Junta de Castilla y Leon; 2019. Available at: https://www.saludcastillayleon.es/en/vacunaciones/calendario-vacunal-toda-vida-castilla-leon. Last accessed: May 4, 2022. [Google Scholar]
- [35].[New vaccination calendar for all ages]. Las Palmas, Canary Islands: Government of Canary Islands; 2019. Available at: https://www3.gobiernodecanarias.org/sanidad/scs/contenidoGenerico.jsp?idDocument=3cc62be0-9746-11e0-ba66-75bd8cf93e41&idCarpeta=fd4cc535-588f-11e1-92c3-9195656fdecf. Last accessed: May 4, 2022. [Google Scholar]
- [36].Immunisation Handbook 2020. Wellington, New Zealand: Ministry of Health, New Zealand Government; 2020. Available at: https://www.health.govt.nz/system/files/documents/publications/immunisation-handbook-2020-sep20-v8.pdf. [Google Scholar]
- [37].Vacunas antimeningocócicas en Uruguay. Montevideo, Uruguay: Ministerio de Salud; 2018. Available at: https://www.gub.uy/ministerio-salud-publica/sites/ministerio-salud-publica/files/documentos/noticias/Postura%20sobre%20vacunas%20antimeningoc%C3%B3cicas%20en%20Uruguay%202310.pdf. Last accessed: May 4, 2022. [Google Scholar]
- [38].Advice for the use of the multicomponent meningococcal serogroup B (4CMenB) vaccine. Ottawa, Canada: Advisory Committee Statement (ACS), National Advisory Committee on Immunization (NACI), Public Health Agency of Canada (PHAC); 2014. Available at: http://publications.gc.ca/collections/collection_2014/aspc-phac/HP40-104-2014-eng.pdf. [Google Scholar]
- [39].Infectious Disease and Vaccinations - Meningococcus B vaccine. Helsinki, Finland: Finnish Institute for Health and Welfare; 2021. Available at: https://thl.fi/en/web/infectious-diseases-and-vaccinations/vaccines-a-to-z/meningococcal-vaccines/meningococcus-b-vaccine. Last accessed: May 4, 2022. [Google Scholar]
- [40].Calendrier des vaccinations et recommandations vaccinales 2014. Paris, France: Ministère des Affaires Sociales et de la Santé; 2014. Available at: https://solidarites-sante.gouv.fr/IMG/pdf/Calendrier_vaccinal_ministere_sante_2014-2.pdf. [Google Scholar]
- [41].Stratégie de vaccination pour la prévention des infections invasives à méningocoques: Le sérogroupe B et la place de TRUMENBA®. Recommandation Vaccinale. Paris, France: Haute Autorité de Santé (HAS); 2021. Available at: https://www.has-sante.fr/upload/docs/application/pdf/2021-06/strategie_de_vaccination_pour_la_prevention_des_infections_invasives_a_meningocoques_-_le_serogroupe_b_et_la_place_de_trumen.pdf. [Google Scholar]
- [42].Statement of the German Standing Committee on Vaccination at the RKI Recommendations of the Standing Committee on Vaccination (STIKO) at the Robert Koch Institute – 2017/2018. Epidemiologisches Bulletin. Berlin, Germany: Robert Koch Institut; 2017. Available at: https://www.rki.de/EN/Content/infections/Vaccination/recommandations/34_2017_engl.pdf?__blob=publicationFile. [Google Scholar]
- [43].[Vaccination Program for Children & Adolescents 2020]. Athens, Greece: Greek Republic, Ministry of Health, General Directorate of Public Health and Quality of life; 2020. Available at: https://www.moh.gov.gr/articles/health/dieythynsh-dhmosias-ygieinhs/emboliasmoi/ethniko-programma-emboliasmwn-epe-paidiwn-kai-efhbwn/7246-programma-emboliasmwn-paidiwn-efhbwn-2020. Last accessed; May 4, 2022. [Google Scholar]
- [44].FHI | Meningokokkvaksine - veileder for helsepersonell. Oslo, Norway: Norwegian Institute of Public Health; 2020. Available at: https://www.fhi.no/nettpub/vaksinasjonsveilederen-for-helsepersonell/vaksiner-mot-de-enkelte-sykdommene/meningokokkvaksinasjon—veileder-f/. Last accessed: May 4, 2022. [Google Scholar]
- [45].Recomendaciones de utilización de la vacuna frente a enfermedad meningocócica por serogrupo B. Madrid, Spain: Ministerio de Sanidad, Servicios Sociales e Igualidad; 2015. Available at: https://www.mscbs.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/comoTrabajamos/docs/MenB_situacEspeciales.pdf. [Google Scholar]
- [46].Childhood Vaccination Coverage Statistics. National Statistics. London, United Kingdom: National Health System (NHS) and Public Health England (PHE); 2021. Available at: https://digital.nhs.uk/data-and-information/publications/statistical/nhs-immunisation-statistics/england—2020-21. Last accessed: May 4, 2022. [Google Scholar]
- [47].Coperture vaccinali dell’età pediatrica e dell’adolescente. Commento ai dati aggiornati al 31 dicembre 2020. Rome, Italy: Directorate General of Health Prevention, Ministry of Health; 2021. Available at: https://www.salute.gov.it/imgs/C_17_tavole_20_9_7_file.pdf. Last accessed: May 4, 2022. [Google Scholar]
- [48].Jolley KA, Maiden MCJ. BIGSdb: Scalable analysis of bacterial genome variation at the population level. BMC Bioinf 2010;11:595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Acevedo R, Bai X, Borrow R, Caugant DA, Carlos J, Ceyhan M, et al. The Global Meningococcal Initiative meeting on prevention of meningococcal disease worldwide: Epidemiology, surveillance, hypervirulent strains, antibiotic resistance and high-risk populations. Expert Rev Vaccines 2019;18(1):15–30. [DOI] [PubMed] [Google Scholar]
- [50].Borrow R, Caugant DA, Ceyhan M, Christensen H, Dinleyici EC, Findlow J, et al. Meningococcal disease in the Middle East and Africa: Findings and updates from the Global Meningococcal Initiative. J Infect 2017;75(1):1–11. [DOI] [PubMed] [Google Scholar]
- [51].Taha M-K, Presa J, Serra L. A Review of the Epidemiology of Invasive Meningococcal Disease and Vaccination Strategies in North Africa. Int J Infect Dis 2021;104:189–97. [DOI] [PubMed] [Google Scholar]
- [52].Huang L, Mauskopf J, Farkouh R, Masaquel C. Use of Cost-Effectiveness Analyses for Decisions About Vaccination Programs for Meningococcal Disease in the United States, United Kingdom, The Netherlands, and Canada. Expert Review of Vaccines 2021;20(1):59–72. [DOI] [PubMed] [Google Scholar]
- [53].Meningitis Progress Tracker. Bristol, United Kingdom: Meningitis Research Foundation (MRF); 2021. Available at: https://www.meningitis.org/mpt. [Google Scholar]
- [54].Martinón-Torres F, Banzhoff A, Azzari C, De Wals P, Marlow R, Marshall H, et al. Recent advances in meningococcal B disease prevention: real-world evidence from 4CMenB vaccination. J Infect 2021;83(1):17–26. [DOI] [PubMed] [Google Scholar]
- [55].Perez JL, Absalon J, Beeslaar J, Balmer P, Jansen KU, Jones TR, et al. From research to licensure and beyond: clinical development of MenB-FHbp, a broadly protective meningococcal B vaccine. Expert Rev Vaccines 2018;17(6):461–77. [DOI] [PubMed] [Google Scholar]
- [56].WHO preferred product characteristics for gonococcal vaccines. Geneva, Switzerland: World Health Organization (WHO); 2021. Available at: https://www.who.int/publications/i/item/9789240039827. [Google Scholar]
- [57].Vesikari T, Brzostek J, Ahonen A, Paassilta M, Majda-Stanislawska E, Szenborn L, et al. Immunogenicity and safety of different schedules of the meningococcal ABCWY vaccine, with assessment of long-term antibody persistence and booster responses - results from two phase 2b randomized trials in adolescents. Hum Vaccin Immunother 2021;17(11):4689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Sáez-Llorens X, Beltran-Rodriguez J, Novoa Pizarro JM, Mensi I, Keshavan P, Toneatto D. Four-year antibody persistence and response to a booster dose of a pentavalent MenABCWY vaccine administered to healthy adolescents and young adults. Hum Vaccin Immunother 2018;14(5):1161–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.