Abstract
Background
Rehabilitation is important in the first months after a stroke for recovery of functional ability, but it is also challenging, since distinct recovery trajectories are seen. Therefore, studying the early changes in muscle characteristics over time (e.g. muscle strength, muscle mass and muscle volume), which are known to be associated with functional abilities, may deepen our understanding of underlying recovery mechanisms of stroke survivors.
Objective
This systematic review aims to describe the longitudinal changes in skeletal muscles, including muscle strength, muscle mass and muscle volume, during the first 3 months post-stroke.
Methods
Electronic searches were conducted in Medline, Scopus and CENTRAL. Longitudinal cohort studies or controlled interventional trials that report data about patients in the first 3 months after stroke were identified. Skeletal muscle characteristics should be measured at least twice within 3 months post-stroke by objective, quantitative assessment methods (e.g. dynamometry, ultrasound, computed tomography). Effect sizes were calculated as Hedges’ g using standardized mean differences.
Results
A total of 38 studies (1,097 subjects) were found eligible. Results revealed an mean increase on the paretic side for upper and lower limb muscle strength (small to moderate effect sizes), whereas muscle thickness decreased (moderate to large effect sizes). Similar, but smaller, effects were found on the non-paretic side. There were insufficient data available to draw conclusions about lean muscle mass and muscle cross-sectional area. No studies aimed at investigating distinct trajectories of the muscle changes.
Conclusion
Muscle strength and thickness changes during the first 3 months after stroke in both the paretic and non-paretic side. Future studies should aim to understand “how” the stroke-induced muscle strength changes are achieved. Exploring existing data from longitudinal studies, by using cluster analyses, such as pattern recognition, could add to the current knowledge-base.
LAY ABSTRACT
After a stroke, it is important to restore functional ability as much as possible. Studying changes in the muscles (e.g. muscle strength, muscle thickness, muscle volume) during the first months post-stroke can help to elucidate individual variations and the underlying repair mechanisms. A systematic search of the literature was performed for studies of objective quantifiable measurements of muscles at least 2 times during the first 3 months after stroke. A total of 38 studies (with in total 1,097 patients) met these criteria. Overall, an improvement in strength was measured on the paretic side for both the upper and lower limbs, but muscle thickness decreased. Similar, but smaller, effects were found on the non-paretic side. For other muscle parameters insufficient data were found to draw conclusions. Because understanding the underlying mechanism of muscle changes post-stroke can improve rehabilitation programmes, further studies should focus on why these muscle strength changes occur.
Key words: stroke, rehabilitation, skeletal muscle, stroke recovery
Stroke is one of the leading causes of acquired physical disability in the adult population (1). Rehabilitation is the most effective way to limit the amount of disability and improve patients’ functionality (2). However, despite continuously improving and innovating interventions, distinct trajectories of recovery are seen. For example, two-third of patients after stroke have residual hemiparesis, resulting in chronic, long-term disability (3). This emphasizes the complex stroke recovery process and highlights the possibility of distinct underlying recovery mechanisms that support the functional ability of stroke survivors. Gaining insight into the mechanisms that contribute to the heterogeneous treatment response in stroke survivors could help us to better understand the complex recovery process and to optimize functional rehabilitation (4).
Skeletal muscle is suggested to be the main effector organ accountable for physical disability in the stroke population (5). This disability traditionally contributed to the neurological insult itself, primarily causing motor symptoms, with muscle weakness, altered muscle tone and decreased muscle control being the most common motor manifestations post-stroke (6). More recently, the functional aspects, structural adaptations and metabolic integrity of peripheral muscle tissue, which remained unrecognized until the past decades, have become an important focus in the evaluation and treatment of the stroke population (7, 8). The relevance of this changing approach is noticeable in the literature, with several reviews synthesizing the available evidence about post-stroke skeletal muscle changes (9–11).
Previous studies have shown that chronic stroke survivors (> 6 months post-stroke) experience a loss in muscle mass and a decrease in muscle strength in both the paretic and non-paretic limbs (9, 10). According to research by Miller and colleagues (12), a decreased central activation can only partly explain bilateral muscle weakness, which might implicate that skeletal muscle changes after stroke can be considered as a multifactorial syndrome depending on various underlying mechanisms besides the brain lesion itself (e.g. neurodegeneration, loss of motor neurones, local muscle metabolic alterations) (8). This assumption is supported by recent research exploring changes after stroke directly at the level of the skeletal muscle (13). Muscle tissue of chronic stroke patients shows an increased intramuscular fat deposition (7) and a major shift from slow-twitch towards fast-twitch fibres in the hemiparetic muscles (14). In addition, the muscle’s architecture, defined as the geometric arrangements of muscle fibres (15), appears to be altered in chronic stroke survivors with a shorter fascicle length on the paretic side. Reports about pennation angle measurements (i.e. fibre orientation) have been less consistent (16).
From a clinical point of view, these (mal-)adaptive skeletal muscle changes have an impact on the recovery process. Muscular atrophy is moderately correlated with decreased gait speed and reduced fitness levels in individuals following stroke in the chronic stage (7). Also, increased severity of gait impairments have been observed as a result of hemiparetic muscle phenotype changes (14), a decline in muscle mass and muscle strength (17). Furthermore, the muscle’s architecture has a profound influence on muscle function, and therefore, structural changes might negatively affect the force-generating process and impact functional recovery (13).
Despite the growing body of evidence about the contribution of underlying skeletal muscle changes to physical disability after stroke, none of the clinical stroke guidelines address these peripheral muscle changes (18, 19). Most of the conventional physiotherapy in stroke rehabilitation includes intensive task-specific exercises aimed at improving motor performance (20). To maximize functional recovery, additional therapy strategies that address alterations of the skeletal muscle are warranted. Since most significant improvements in stroke recovery occur in the first 3 months after stroke onset (21), restorative interventions should be commenced within this critical time window, as suggested by the Stroke Roundtable Consortium (4). Unfortunately, most of the existing evidence about skeletal muscle changes have explored long-term alterations post-stroke (> 6 months), leaving a paucity of knowledge regarding acute and early subacute muscle changes as an important deficiency in literature that needs to be addressed before optimal gains in neurorehabilitation can be achieved.
One way to improve our understanding of how stroke recovery is achieved in the first 3 months following stroke onset is to evaluate changes in the skeletal muscle over time. Although clinical assessment scales are an essential tool in the measurement and evaluation of motor impairments (22), they often have limitations, such as ceiling effects and a lack of sensitivity to detect small, but relevant, changes (23). Therefore, to accurately evaluate peripheral muscle changes, measurements are preferably performed by objective, quantitative assessment methods. Medical imaging techniques, such as ultrasound, medical resonance imaging (MRI) and computed tomography (CT), but also hand-held dynamometers are just a number of the widely used objective tools in research and clinical practice to address changes in function, structure and consistency of the peripheral muscles (23, 24). However, an overview of these measurements representing skeletal muscle changes early after stroke is still lacking.
Therefore, the purpose of this systematic review is to synthesize available evidence describing the time-course of peripheral muscle changes in skeletal muscles (e.g. muscle strength, muscle mass, muscle volume) measured by objective, quantitative assessment methods (e.g. dynamometry, ultrasound, CT) in the first 3 months post-stroke.
METHODS
The PRISMA guidelines were used as a general framework for transparent reporting of this systematic review. Protocol details were registered prospectively on PROSPERO (registration number: CRD42020157647).
Studies were included if they met following inclusion criteria.
Type of participants
Studies involved adult (≥18 years) stroke patients, as defined by the World Health Organization (WHO) (25), in the acute (0–7 days) and/or early subacute (> 7 days to 3 months) stages. The framework of these critical time points post-stroke was defined by the Stroke Roundtable Consortium (4).
Type of outcome
Studies evaluated changes in upper and/or lower extremity skeletal muscle characteristics (i.e. function, structure and composition) (e.g. changes in muscle strength, muscle mass, muscle fibres) measured by objective, quantitative assessment methods (e.g. dynamometer, CT, ultrasound) were included. The muscle characteristics of the subjects needed to be measured at least twice within 3 months post-stroke. Changes in muscle characteristics assessed by clinical measurements (e.g. Medical Research Council Scale, Fugl-Meyer assessment, Modified Ashworth Scale) were excluded.
Type of study design
Longitudinal cohort studies or controlled interventional trials were included. Concerning the latter, in these studies the data of the groups receiving usual care or conventional physiotherapy were considered as longitudinal data, and were included at baseline, post-intervention and follow-up. Data from the experimental groups were excluded. Clinical trial protocols, systematic reviews, narrative reviews, meta-analysis or case reports were excluded.
Type of publication
Peer-reviewed journal articles were included, conference abstracts and proceedings were excluded.
Language
Studies had to be published in English, French or Dutch.
Data sources and searches
A systematic search was undertaken in 3 electronic databases (MEDLINE, Scopus and CENTRAL) from inception until July 2019 and updated in May 2020. The search strategy was structured according to the Population, Intervention, Comparison, Outcome (PICO) method, and combined key words related to stroke and skeletal muscle characteristics. Full electronic search strategies are shown in Appendix 1. Reference lists of related systematic reviews (9, 10) and the citations of included studies were screened for missing studies.
Study selection
To identify eligible studies, titles and abstracts were screened by 2 independent reviewers (LC, NL) with the use of the web application Rayyan (26). If studies were considered relevant, full-text copies were obtained and checked for eligibility by the same independent reviewers. Disagreements between authors were resolved by consensus. If no agreement was reached, additional authors (DB, ES) were consulted.
Data extraction
A data extraction sheet was developed to collect data outcomes of interest. The data extraction sheet was pilot-tested on 3 randomly selected study trials and refined accordingly. One author (LC) collected data from the included studies.
Quality assessment
To critically evaluate the quality of the individual studies, a customized risk of bias tool was created. Key domains and judgement criteria, that had the most potential to introduce bias, were compiled from existing tools (i.e. Cochrane Collaboration’s tool for randomized trials (27), the Methods Guide for Comparative Effectiveness Reviews (28) and the Newcastle-Ottawa Quality Assessment Scale for cohort studies (29)) and adapted into a specified checklist based on the research question of this systematic review. The applicable domains included sampling, confounding, performance, detection, attrition and reporting bias and were rated as “high”, “low” or “unclear”. An overview of the pre-specified judgement criteria is shown in Table SIV in Appendix 2. Two reviewers (LC, WVH) independently evaluated risk of bias for each study. Disagreements were discussed and settled by consensus. If no agreement was reached, an additional author (ES) was consulted.
Data synthesis and analysis
Means, standard deviations (SDs) and significance values of the paretic and non-paretic side were primarily extracted for each evaluation time-point of every outcome measure of interest.
The effect sizes were calculated as Hedges’ g (gH), a bias-adjusted estimate of standardized mean difference between 2 evaluation time-points with an additional correction for small sample sizes (30). Effect sizes were interpreted as small (≤0.2), moderate (0.5), or large (≥0.8), representing an increase (positive values) or decrease (negative values) over time. If the required data was absent or insufficiently reported, the authors were contacted individually by e-mail. When possible, researchers calculated means and SDs themselves or extracted these data from available figures in the included studies. In cases where the desired data was unobtainable, despite the aforementioned methods, a quantitative analysis via effect size calculation was not possible. Nevertheless, these studies were included in the systematic review to avoid reporting bias. For those studies, other available measures of central tendency and dispersion were extracted and reported.
RESULTS
Study selection
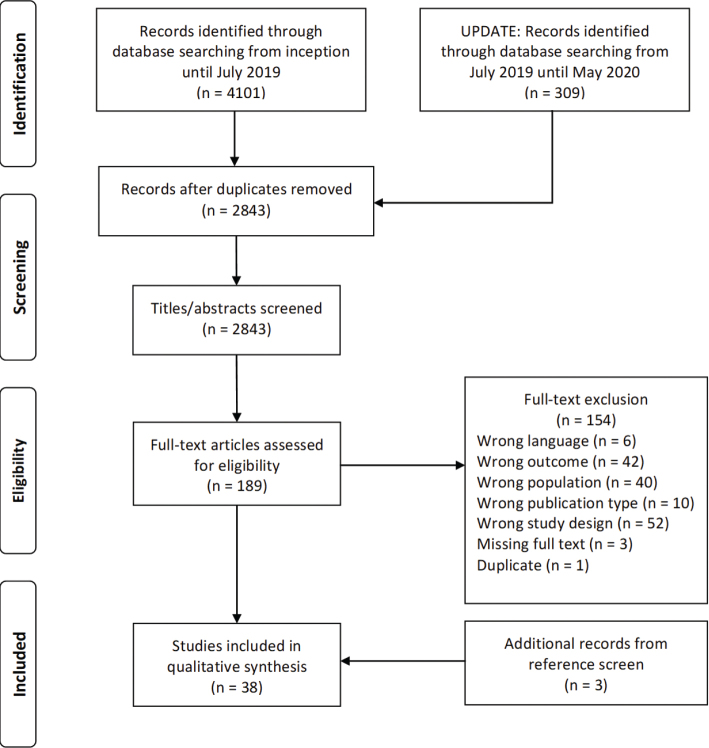
The database search yielded 2,843 unique records, of which a final total of 38 studies was included in this systematic review (31–68). Fig. 1 illustrates a flow diagram of the entire selection process.
Fig. 1.
Flow chart.
Characteristics of included studies
Tables I and II list detailed information about the study characteristics.
Table I.
Muscle strength
| First author (year) | Study design | n | Subject features | Measuring device (unit) | Assessed muscle group | Evaluation time | PSa | NPSa | Change in time | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time point | ES PS (p-value) | ES NPS (p-value) | Drop-out ratio | |||||||||
| LOWER LIMB | ||||||||||||
| Andrews 2003 (31) | Long. | 50 | 26M, 24F Age 61.6 ± 12.7 y PS: 28L, 22R Type: NR Early subacute stage |
Chatillon Hand-held dynamometer (N) | Hip flexors | T0: 12d | 47.0 (44.1) | 94.1 (43.3) | T0 vs T1 | 0.41 (< 0.001*) | 0.25 (< 0.001*) | 0% |
| T1: 29d | 65.1 (44.1) | 105.4 (46.4) | ||||||||||
| Knee extensors | T0: 12d | 129.0 (89.9) | 219.8 (101.5) | T0 vs T1 | 0.68 (< 0.001*) | 0.28 (< 0.001*) | 0% | |||||
| T1: 29d | 191.5 (91.3) | 247.4 (93.1) | ||||||||||
| Ankle dorsiflexors | T0: 12d | 75.6 (72.3) | 164.7 (68.1) | T0 vs T1 | 0.34 (< 0.001*) | 0.31 (< 0.001*) | 0% | |||||
| T1: 29d | 102.4 (86.3) | 185.5 (65.6) | ||||||||||
| Bohannon 2013 (34) | Long. | 55 | 29M, 26F Age 62.8 ± 13.1 y PS: 32L, 23R Type: 49I, 6H Early subacute stage |
Chatillon Hand-held dynamometer (N) |
Hip flexors | T0: 12d | 43.2 (38.1) | 93.7 (42.2) | T0 vs T1 | 0.43 (≤.002*) | 0.24 (≤.002*) | 0% |
| T1: 17d | 59.8 (38.1) | 104.4 (47.4) | ||||||||||
| Knee extensors | T0: 12d | 121.1 (84.4) | 223.4 (100.0) | T0 vs T1 | 0.40 (≤.002*) | 0.19 (≤.002*) | 0% | |||||
| T1: 17d | 155.9 (89.3) | 242.1 (94.1) | ||||||||||
| Ankle dorsiflexors | T0: 12d | 72.5 (68.0) | 163.7 (70.4) | T0 vs T1 | 0.35 (≤.002*) | 0.26 (≤.002*) | 0% | |||||
| T1: 17d | 99.6 (83.7) | 181.9 (67.0) | ||||||||||
| Median (IQR) | Median (IQR) | |||||||||||
| Carin-Levy 2006 (36) | Long. | 18 | 11M, 7F Age 66.0 ± 11.5 y PS: NR Type: NR Acute and early subacute stage |
Isometric strain gauge (N) | Knee extensors | T0: 7d | 258.4 (156.0–360.9) | 270.3 (135.9–404.7)† | T0 vs T1 | NA (NS) | NA (NS) | 22% |
| T1: 14d | 284.9 (178.0–393.8) | 321.4 (178.5–406.3)† | T1 vs T2 | NA (NS) | NA (NS) | 6% | ||||||
| T2: 21d | 183.9 (174.4–344.3) | 222.5 (155.0–370.4)† | T2 vs T3 | NA (NS) | NA (NS) | 0% | ||||||
| T3: 28d | 213.4 (178.0–448.7) | 263.3 (222.1–439.2) † | T0 vs T2 | NA (NS) | NA (NS) | 28% | ||||||
| T0 vs T3 | NA (NS) | NA (NS) | 28% | |||||||||
| Lee 2018 (49) | RCT | 18 | 10M, 8F Age 63.7 ± 11.4 y PS: 10L, 9R Type: 9I, 9H Early subacute stage |
Humac Norm Isokinetic dynamometer (Nm) | Knee extensors | T0: 29d | 39.2 (22.9) | 71.8 (37.0) | T0 vs T1 | 0.31 (0.07) | 0.16 (0.22) | 22% |
| T1: 57d | 46.8 (25.4) | 77.8 (36.4) | ||||||||||
| Knee flexors | T0: 29d | 30.7 (19.7) | 45.7 (25.6) | T0 vs T1 | 0.21 (0.27) | 0.06 (0.56) | 22% | |||||
| T1: 57d | 35.6 (26.4) | 47.2 (24.3) | ||||||||||
| Shapira-Vadler 2015 (62) | Long. | 23 | 12M, 11F Age 58.0 ± 10.5 y PS: 14L, 9R Type: 19I, 2H Early subacute stage |
Hand-held dynamometer (kg/f) | Knee extensors | T0: 20d | 11.6 (4.5) | 13.2 (3.3) ‡ | T0 vs T1 | 0.55 (0.008*) | 0.19 (NR) | 0% |
| T1: 50d | 13.6 (2.6) | 13.8 (2.9) † | ||||||||||
| Solopova 2011 (63) | RCT | 29 | 18M, 11F Age NR PS: 20L, 9R Type: 26I, 0H Early subacute stage |
Strain gauge (Nm) | Knee extensors | T0: 9d | 92.7 (59.8) | 159.9 (51.0) | T0 vs T1 | 0.70 (0.01*) | 0.12 (> 0.05) | 0% |
| T1: 23d | 117.8 (11.1) | 166.0 (50.1) | ||||||||||
| Knee flexors | T0: 9d | 27.4 (11.9) | 64.8 (24.3) | T0 vs T1 | 0.56 (0.01*) | 0.08 (> 0.05) | 0% | |||||
| T1: 23d | 35.9 (18.4) | 67.1 (29.3) | ||||||||||
| UPPER LIMB | ||||||||||||
| Andrews 2003 (31) | Long. | 50 | 26M, 24F Age 61.6 ± 12.7 y PS: 28L, 22R Type: NR Early subacute stage |
Chatillon Hand-held dynamometer (N) | Shoulder abductors | T0: 12d | 52.3 (51.4) | 119.1 (50.8) | T0 vs T1 | 0.28 (< 0.001*) | 0.16 (> 0.001) | 0% |
| T1: 29d | 67.2 (54.5) | 127.1 (49.3) | ||||||||||
| Elbow flexors | T0: 12d | 61.0 (60.0) | 154.2 (57.0) | T0 vs T1 | 0.40 (< 0.001*) | 0.10 (> 0.001) | 0% | |||||
| T1: 29d | 85.8 (64.6) | 159.9 (57.8) | ||||||||||
| Elbow extensors | T0: 12d | 52.7 (52.0) | 120.2 (43.6) | T0 vs T1 | 0.27 (< 0.001*) | 0.08 (> 0.001) | 0% | |||||
| T1: 29d | 66.6 (51.7) | 123.9 (44.8) | ||||||||||
| Wrist extensors | T0: 12d | 26.3 (35.7) | 86.5 (37.8) | T0 vs T1 | 0.36 (< 0.001*) | 0.23 (< 0.001*) | 0% | |||||
| T1: 29d | 40.2 (40.6) | 95.1 (37.1) | ||||||||||
| Au-Yeung 2014 (32) | RCT | 23 | 11M, 12F Age 72.0 ± 9.6 y PS: 11L, 12R Type: 23I, 0H Acute and early subacute stage |
Electronic dynamometer, Digital Grip/Pinch Analyser (kg) | Grip strength | T0: 1d | 2.3 (4.6) | NR | T0 vs T1 | 0.18 (> 0.05) | NR | 30% |
| T1: 29d | 3.1 (4.0) | NR | ||||||||||
| Pinch strength | T0: 1d | 0.7 (1.4) | NR | T0 vs T1 | 0.08 (> 0.05) | NR | 10% | |||||
| T1: 29d | 0.8 (1.2) | NR | ||||||||||
| Au-Yeung 2014 (32) | RCT | 21 | 11M, 10F Age 68.4 ± 9.9 y PS: 12L, 9R Type: 21I, 0H Acute and early subacute stage |
Electronic dynamometer, Digital Grip/Pinch Analyser (kg) | Grip strength | T0: 1d | 0.9 (2.7) | NR | T0 vs T1 | 0.97 (> 0.05) | NR | 30% |
| T1: 29d | 5.6 (7.4) | NR | ||||||||||
| Pinch strength | T0: 1d | 0.5 (1.3) | NR | T0 vs T1 | 0.67 (> 0.05) | NR | 10% | |||||
| T1: 29d | 1.7 (2.2) | NR | ||||||||||
| Mean (range) | Mean (range) | |||||||||||
| Bohannon 1995 (33) | Long. | 10 | 4M, 6F Age 66.7 y PS: 2L, 8R Type: NR Acute and early subacute stage |
Ametek Cadet Force gauge dynamometer (N) | Shoulder abductors | T0: 6d | 27.6 (0.0–90.7) | NR | T0 vs T1 | NA (0.180) | NR | 0% |
| T1: 11d | 34.7 (0.0–93.4) | NR | ||||||||||
| Elbow flexors | T0: 6d | 28.0 (0.0–67.6) | NR | T0 vs T1 | NA (0.109) | NR | 0% | |||||
| T1: 11d | 32.9 (0.0–85.4) | NR | ||||||||||
| Jamar Hand–held dynamometer (N) |
Grip strength | T0: 6d | 44.0 (0–164.5) | NR | T0 vs T1 | NA (0.317) | NR | 0% | ||||
| T1: 11d | 52.0 (0–160.1) | NR | ||||||||||
| Canning 2004 (35) | Long. | 22 | 11M, 11F Age 68.4 ± 6.6 y PS: 10L, 12R Type: NR Early subacute stage |
Load cell (Nm) | Elbow flexors and extensors | T0: 21d | 14.1 (9.4) | 26.9 (NR) | T0 vs T1 | 0.22 (NR) | NA | 0% |
| T1: 35d | 16.4 (10.8) | NR | T1 vs T2 | 0.10 (NR) | NR | 0% | ||||||
| T2: 49d | 17.5 (10.8) | NR | T2 vs T3 | 0.12 (NR) | NR | 0% | ||||||
| T3: 63d | 18.8 (10.8) | NR | T3 vs T4 | 0.03 (NR) | NR | 0% | ||||||
| T4: 77d | 19.1 (10.3) | NR | T0 vs T2 | 0.33 (NR) | NR | 0% | ||||||
| T0 vs T3 | 0.46 (NR) | NR | 0% | |||||||||
| T0 vs T4 | 0.49 (NR) | NR | 0% | |||||||||
| Median (IQR) | Median (IQR) | |||||||||||
| Carin-Levy 2006 (36) | Long. | 18 | 11M, 7F Age 66.0 ± 11.5 y PS: NR Type: NR Acute and early subacute stage |
LafayetteHand-held dynamometer (N) | Grip strength | T0: 7d | 78.5 (0–188.7) | 171.6 (103.0–318.7) ‡ | T0 vs T1 | NA (NS) | NA (NS) | 22% |
| T1: 14d | 93.2 (51.5–186.3) | 264.8 (109.1–345.6)‡ | T1 vs T2 | NA (NS) | NA (NS) | 6% | ||||||
| T2: 21d | 88.3 (0–245.2) | 122.6 (107.9–294.2) ‡ | T2 vs T3 | NA (NS) | NA (NS) | 0% | ||||||
| T3: 28d | 103 (83.4–227.9) | 245.2 (117.7–348.1) ‡ | T0 vs T2 | NA (NS) | NA (NS) | 28% | ||||||
| T0 vs T3 | NA (NS) | NA (NS) | 28% | |||||||||
| Chen 2005 (37) | RCT | 14 | 10M, 4F Age 59.6 ± 12.0 y PS: 8L, 6R Type: 8I, 6H Early subacute stage |
Jamar Hand-held dynamometer (kg) |
Grip strength | T0: 12d | 0.0 (0.0) | NR | T0 vs T1 | 0.00 (≥.05) | NR | 0% |
| T1: 19d | 0.0 (0.0) | NR | T1 vs T2 | 0.65 (NR) | NR | 0% | ||||||
| T2: 26d | 0.29 (0.86) | NR | T2 vs T3 | 0.20 (NR) | NR | 0% | ||||||
| T3: 33d | 0.50 (1.23) | NR | T3 vs T4 | 0.11 (NR) | NR | 0% | ||||||
| T4: 40d | 0.65 (1.35) | NR | T4 vs T5 | 0.21 (NR) | NR | 0% | ||||||
| T5: 47d | 0.97 (1.68) | NR | T0 vs T2 | 0.65 (≥.05) | NR | 0% | ||||||
| T0 vs T3 | 0.79 (≥.05) | NR | 0% | |||||||||
| T0 vs T4 | 0.93 (< 0.05*) | NR | 0% | |||||||||
| T0 vs T5 | 1.12 (< 0.05*) | NR | 0% | |||||||||
| Choi 2014 (38) | RCT | 10 | 5M, 5F Age 65.7 ± 11.3 y PS: 6L, 4R Type: 6I, 4H Early subacute stage |
Hand-held dynamometer (kg) | Grip strength | T0: 23d | 6.6 (3.7) | NR | T0 vs T1 | 1.16 (0.005*) | NR | 0% |
| T1: 51d | 12.1 (5.4) | NR | ||||||||||
| Z-Score (SE) | Z-Score (SE) | |||||||||||
| Cortes 2017 (39) | Long. | 18 | 9M, 9F Age 55.0 ± 12.9 y PS: NR Type: 18I, 0H Early subacute stage |
MicroFET2 Hand-held dynamometer (N) | Elbow flexors | T0: 11d | –3.7 (0.4) | NR | T0 vs T1 | NA (0.008*) | NR | 6% |
| T1: 37d | –3.1 (0.2) | NR | ||||||||||
| Ghaziani 2018 (40) | RCT | 49 | 25M, 24F Age 71.0 y PS: NR Type: 37I, 12H Acute and early subacute stage |
Device NR (kg) | Grip strength | T0: 7d | 12.0 (13.0) | NR | T0 vs T1 | 0.35 (< 0.0001*) | NR | 8% |
| T1: 28d | 16.5 (12.8) | NR | ||||||||||
| % Median CL hand | % Median CL hand | |||||||||||
| Groisser 2014 (41) | Long. | 10 | 5M, 5F Age 52.6 ± 5.6 y PS: 8L, 2R Type: NR Acute and early subacute |
Digital dynamometer (unit NR) | Grip strength | T0: 7d | 0.0 | NR | T0 vs T1 | NA (< 0.001*) | NR | 0% |
| T1: 60d | 8.89 | NR | ||||||||||
| Heller 1987 (42) | Long. | 56 | 24M, 32F Age 68.1 ± 11.4 y PS: 25L, 28R Type: NR Acute and early subacute stage |
Hand-held dynamometer (mm Hg) | Grip strength | T0: 4d | NR | NR | T0 vs T1 | NR | NR | NR |
| T1: not specified | NR | NR | ||||||||||
| Hosomi 2016 (43) | RCT | 21 | 13M, 8F Age 63.2 ± 12.5 y PS: 8L, 13R Type: 12I, 9H Early subacute stage |
Device NR (kg) | Grip strength | T0: 45d | 7.1 (8.6) | 26.3 (9.6) | T0 vs T1 | 0.08 (NR) | 0.01 (NR) | 0% |
| T1: 50d | 7.8 (8.4) | 26.4 (9.7) | T1 vs T2 | 0.05 (NR) | –0.07 (NR) | 0% | ||||||
| T2 : 57d | 8.2 (8.9) | 25.7 (9.7) | T2 vs T3 | 0.01 (NR) | 0.11 (NR) | 0% | ||||||
| T3 : 74d | 8.3 (8.6) | 26.8 (9.8) | T0 vs T2 | 0.12 (NR) | –0.06 (NR) | 0% | ||||||
| T0 vs T3 | 0.14 (0.077) | 0.05 (0.388) | 0% | |||||||||
| Kattenstroth 2018 (45) | RCT | 23 | 16M, 7F Age 59.0 y PS: 13L, 10R Type: 23I, 0H Early subacute stage |
Jamar Hand-held dynamometer, (kg) |
Grip strength | T0 : 28d | 20.44 (13.56) | 35.21 (11.43) | T0 vs T1 | 0.07 (0.601) | –0.05 (0.494) | 36% |
| T1 : 40d | 21.28 (10.90) | 34.60 (10.58) | ||||||||||
| Langhammer 2009 (47) | RCT | 40 | 22M, 18F Age 72.0 ± 13.6 y PS: 19L, 21R Type: NR Acute and early subacute stage |
Martin Vigorimeter Hand-held dynamometer (Bar) | Grip strength | T0 : acute stage, not specified | 0.41 (0.26) | NR | T0 vs T1 | 0.29 (NR) | NR | 13% |
| T1 : T0 + 16d | 0.48 (0.29) | NR | ||||||||||
| Larsen 2017 (48) | Long. | 15 | 13M, 2F Age 59.0 ± 15.0 y PS: 7L, 8R Type: 15I, 0H Acute and early subacute stage |
Load cell (N) |
Pinch strength | T0: 3d | 34.0 (14.0) | NR | T0 vs T1 | 0.42 (0.04*) | NR | 13% |
| T1: 38d | 40.0 (14.0) | NR | ||||||||||
| Liuzzi 2014 (50) | Long. | 11 | 7M, 4F Age 63.2 ± 3.0 y PS: 6L, 5R Type: NR Acute and early subacute stage |
Whole hand dynamometer (kg) | Grip strength | T0: 1d | 22.5 (15.3) | 35.1 (9.7) | T0 vs T1 | 0.09 (NR) | –0.15 (NR) | 0% |
| T1: 3d | 23.9 (15.1) | 33.3 (13.3) | T1 vs T2 | 0.10 (NR) | 0.08 (NR) | 0% | ||||||
| T2 : 7d | 25.5 (15.4) | 34.4 (12.8) | T2 vs T3 | 0.23 (NR) | 0.01 (NR) | 0% | ||||||
| T3 : 49d | 29.3 (16.4) | 34.5 (13.1) | T0 vs T2 | 0.19 (NR) | –0.06 (NR) | 0% | ||||||
| T0 vs T3 | 0.41 (< 0.05*) | –0.05 (NR) | 0% | |||||||||
| Matsuura 2015 (51) | RCT | 10 | 5M, 5F Age 74.4 ± 12.7 y PS: 5L, 5R Type: 10I, 0H Early subacute stage |
Hand-held dynamometer (kg) | Grip strength | T0: 9d | 15.0 (2.1) | 22.4 (NR) | T0 vs T1 | 0.39 (NR) | NA (NR) | 0% |
| T1: 14d | 15.9 (2.5) | 23.1 (0.6) | ||||||||||
| Z-Score | Z-Score | |||||||||||
| Noskin (2007)(53) | Long. | 30 | 12M, 18F Age 62.0 ± 11.0 y PS: 18L, 12R Type: NR Acute and early subacute stage |
Hand-held dynamometer (kg) | Grip strength | T0: 2d | –1.1 | –3.1 | T0 vs T1 | NA (NR) | NA (NR) | 27% |
| T1: 7d | –1.0 | –2.6 | ||||||||||
| Median (IQR) | Median (IQR) | |||||||||||
| Powell 1999 (59) | RCT | 30 | 14M, 16F Age 66.4 ± 12.5 y PS: 20L, 10R Type: NR Early subacute stage |
Strain gauge (Nm) | Wrist extensors | T0: 22d | 0.7 (0.0–2.0) | NR (NR) | T0 vs T1 | NA (NR) | NR | 7% |
| T1: 50d | 0.9 (NR) | NR (NR) | T1 vs T2 | NA (NR) | NR | 0% | ||||||
| T2: 78d | 0.9 (NR) | NR (NR) | T0 vs T2 | NA (NR) | NR | 7% | ||||||
| Jamar Hand-held dynamometer (kg) |
Grip strength | T0: 22d | 0.0 (0.0–8.5) | NR (NR) | T0 vs T1 | NA (NR) | NR | 7% | ||||
| T1: 50d | NR (NR) | NR (NR) | T1 vs T2 | NR | NR | 0% | ||||||
| T2: 78d | NR (NR) | NR (NR) | T0 vs T2 | NR | NR | 7% | ||||||
| Prigatano 1997 (60) | Long. | 51 | 29M, 22F Age 69.5 ± 13.0 y PS: 24L, 27R Type: 24I, 20H Early subacute stage |
Stoetting hand dynamometer test | Grip strength | T0: 17d | NR | NR | T0 vs T1 | NR | NR | 0% |
| T1: 28d | NR | NR | ||||||||||
| (Tx–T0)/T0 (SD) | (Tx–T0)/T0 (SD) | |||||||||||
| Renner 2009 (61) | Long. | 16 | 7M, 9F Age 65.0 ± 10.4 y PS: 12L, 4R Type: 16I, 0H Early subacute stage |
Piezoelectric force sensor (Nm) | Elbow flexors | T0: 29d | T0 vs T1 | NA (NR) | NR | 0% | ||
| T1: 50d | 0.48 (0.84) | NR | T1 vs T2 | NA (NR) | NR | 0% | ||||||
| T2: 71d | 0.78 (0.99) | NR | T0 vs T2 | NA (NR) | NR | 0% | ||||||
| Elbow extensors | T0: 29d | T0 vs T1 | NA (NR) | NR | 0% | |||||||
| T1: 50d | 0.48 (0.83) | NR | T1 vs T2 | NA (NR) | NR | 0% | ||||||
| T2: 71d | 0.90 (1.67) | NR | T0 vs T2 | NA (NR) | NR | 0% | ||||||
| Wrist extensors | T0: 29d | T0 vs T1 | NA (NR) | NR | 0% | |||||||
| T1: 50d | 0.48 (0.83) | NR | T1 vs T2 | NA (NR) | NR | 0% | ||||||
| T2: 71d | 0.90 (1.67) | NR | T0 vs T2 | NA (NR) | NR | 0% | ||||||
| Digital multi- myometer (N) | Grip strength | T0: 29d | T0 vs T1 | NA (NR) | NR | 0% | ||||||
| T1: 50d | 0.62 (0.90) | NR | T1 vs T2 | NA (NR) | NR | 0% | ||||||
| T2: 71d | 0.73 (1.22) | NR | T0 vs T2 | NA (NR) | NR | 0% | ||||||
| Suzuki 2011 (64) | Long. | 21 | 10M, 11F Age 73.5 ± 11.8 y PS: 4L, 17R Type: 17I, 4H Early subacute stage |
µTAS MT-1 Hand-held dynamometer (Nm) | Elbow flexors | T0: 7d | 12.1 (13.7) | 23.5 (14.1) | T0 vs T1 | 0.17 (> 0.05) | 0.38 (0.001*) | 0% |
| T1: 14d | 14.5 (14.4) | 29.9 (18.7) | T1 vs T2 | 0.17 (NR) | 0.07 (NR) | 0% | ||||||
| T2: 21d | 17.1 (16.3) | 28.7 (16.4) | T2 vs T3 | –0.05 (NR) | 0.10 (NR) | 0% | ||||||
| T3: 28d | 16.3 (15.5) | 30.4 (17.4) | T0 vs T2 | 0.33 (< 0.0001*) | 0.33 (0.009*) | 0% | ||||||
| T0 vs T3 | 0.28 (0.001*) | 0.43 (< 0.0001*) | 0% | |||||||||
| Elbow extensors | T0: 7d | 9.6 (10.1) | 18.1 (9.7) | T0 vs T1 | 0.29 (0.006*) | 0.29 (0.038*) | 0% | |||||
| T1: 14d | 13.2 (14.1) | 21.3 (11.8) | T1 vs T2 | 0.01 (NR) | 0.01 (NR) | 0% | ||||||
| T2: 21d | 13.1 (13.4) | 21.4 (10.9) | T2 vs T3 | –0.06 (NR) | 0.08 (NR) | 0% | ||||||
| T3: 28d | 12.3 (11.7) | 22.4 (13.3) | T0 vs T2 | 0.29 (0.008*) | 0.31 (0.027*) | 0% | ||||||
| T0 vs T3 | 0.24 (0.046*) | 0.37 (0.003*) | 0% | |||||||||
| Jamar Hand-held dynamometer (kg) |
Grip strength | T0: 7d | 7.6 (9.2) | 16.3 (8.7) | T0 vs T1 | 0.13 (> 0.05) | 0.30 (0.001*) | 0% | ||||
| T1: 14d | 8.8 (9.4) | 19.1 (9.5) | T1 vs T2 | 0.10 (NR) | 0.03 (NR) | 0% | ||||||
| T2: 21d | 9.8 (10.4) | 19.4 (9.0) | T2 vs T3 | 0.02 (NR) | 0.09 (NR) | 0% | ||||||
| T3: 28d | 10.0 (10.6) | 20.2 (9.1) | T0 vs T2 | 0.22 (0.012*) | 0.34 (< 0.0001*) | 0% | ||||||
| T0 vs T3 | 0.24 (0.005*) | 0.43 (< 0.0001*) | 0% | |||||||||
| Turner 2012 (65) | Long. | 10 | 5M, 5F Age 68.0 ± 4.0 y PS: 5L, 5R Type 7I, 3H Early subacute stage |
Hand joystick robotic device, IMT2 shoulder/arm robot (N) | Shoulder flexors | T0: 20d | 25.0 (9.5) | NR | T0 vs T1 | –0.13 (> 0.05) | NR | 0% |
| T1: 27d | 23.0 (19.0) | NR | T1 vs T2 | 0.17 (> 0.05) | NR | 0% | ||||||
| T2: 34d | 26.0 (15.8) | NR | T2 vs T3 | –0.12 (> 0.05) | NR | 0% | ||||||
| T3: 41d | 24.0 (15.8) | NR | T3 vs T4 | –0.06 (> 0.05) | NR | 0% | ||||||
| T4: 48d | 23.0 (15.8) | NR | T0 vs T2 | 0.08 (> 0.05) | NR | 0% | ||||||
| T0 vs T3 | 0.08 (> 0.05) | NR | 0% | |||||||||
| T0 vs T4 | –0.15 (> 0.05) | NR | 0% | |||||||||
| Shoulder Extensors | T0: 20d | 19.0 (12.6) | NR | T0 vs T1 | –0.35 (> 0.05) | NR | 0% | |||||
| T1: 27d | 15.0 (9.5) | NR | T1 vs T2 | 0.76 (> 0.05) | NR | 0% | ||||||
| T2: 34d | 25.0 (15.8) | NR | T2 vs T3 | –0.18 (> 0.05) | NR | 0% | ||||||
| T3: 41d | 22.0 (15.8) | NR | T3 vs T4 | 0.30 (> 0.05) | NR | 0% | ||||||
| T4: 48d | 27.0 (15.8) | NR | T0 vs T2 | 0.40 (> 0.05) | NR | 0% | ||||||
| T0 vs T3 | 0.20 (> 0.05) | NR | 0% | |||||||||
| T0 vs T4 | 0.54 (> 0.05) | NR | 0% | |||||||||
| Turner 2012 (65) | Long. | 10 | 5M, 5F Age 68.0 ± 4.0 y PS: 5L, 5R Type 7I, 3H Early subacute stage |
Hand joystick robotic device, IMT2 shoulder/arm robot (N) | Shoulder Abductors | T0: 20d | 22.0 (9.5) | NR | T0 vs T1 | 0.08 (> 0.05) | NR | 0% |
| T1: 27d | 23.0 (15.8) | NR | T1 vs T2 | 0.13 (> 0.05) | NR | 0% | ||||||
| T2: 34d | 25.0 (12.6) | NR | T2 vs T3 | –0.38 (> 0.05) | NR | 0% | ||||||
| T3: 41d | 20.0 (12.6) | NR | T3 vs T4 | 0.00 (> 0.05) | NR | 0% | ||||||
| T4: 48d | 20.0 (12.6) | NR | T0 vs T2 | 0.26 (> 0.05) | NR | 0% | ||||||
| T0 vs T3 | –0.17 (> 0.05) | NR | 0% | |||||||||
| T0 vs T4 | –0.17 (> 0.05) | NR | 0% | |||||||||
| Shoulder Adductors | T0: 20d | 10.0 (12.6) | NR | T0 vs T1 | 0.26 (> 0.05) | NR | 0% | |||||
| T1: 27d | 13.0 (9.5) | NR | T1 vs T2 | 0.30 (> 0.05) | NR | 0% | ||||||
| T2: 34d | 16.0 (9.5) | NR | T2 vs T3 | 0.17 (> 0.05) | NR | 0% | ||||||
| T3: 41d | 18.0 (12.6) | NR | T3 vs T4 | –0.43 (> 0.05) | NR | 0% | ||||||
| T4: 48d | 13.0 (9.5) | NR | T0 vs T2 | 0.52 (> 0.05) | NR | 0% | ||||||
| T0 vs T3 | 0.61 (> 0.05) | NR | 0% | |||||||||
| T0 vs T4 | 0.26 (> 0.05) | NR | 0% | |||||||||
| Umeki 2019 (66) | RCT | 25 | 14M, 11F Age 65.3 ± 14.1 y PS: 11L, 14R Type: 14I, 11H Acute and early subacute stage |
Takei Hand dynamometer (kg) | Grip strength | T0: 6d | 17.7 (13.9) | NR | T0 vs T1 | 0.12 (> 0.05) | NR | 0% |
| T1: 16d | 19.4 (14.6) | NR | ||||||||||
| Vanbellingen 2017 (67) | Long. | 82 | 47M, 35F Age 67.9 ± 13.8 y PS: 0L, 82R Type: 61I, 21H Early subacute stage |
Jamar Hand-held dynamometer (kg) |
Grip strength | T0: 11d | 19.7 (11.1) | NR | T0 vs T1 | 0.25 (< 0.0001*) | NR | 0% |
| T1: 44d | 22.5 (10.3) | NR | ||||||||||
| Vanoglio 2016 (68) | RCT | 15 | 7M, 8F Age 73.0 ± 14.0 y PS: 10L, 5R Type: 9I, 6H Early subacute stage |
Jamar Plus+ Hand-held dynamometer (kg/BMI) |
Grip strength | T0: 17d | 0.19 (0.43) | NR | T0 vs T1 | 0.06 (0.085) | NR | 13% |
| T1: 59d | 0.22 (0.48) | |||||||||||
| Vanoglio 2016 (68) | RCT | 15 | 7M, 8F Age 73.0 ± 14.0 y PS: 10L, 5R Type: 9I, 6H Early subacute stage |
Jamar Plus+ Hand-held dynamometer (kg/BMI) |
Pinch strength | T0: 17d | 0.04 (0.07) | NR | T0 vs T1 | 0.12 (0.087) | NR | 13% |
| T1: 59d | 0.05 (0.09) | NR | ||||||||||
n: number of participants; PS: paretic side; NPS: non-paretic side; ES: effect size; Long.: longitudinal; RCT: randomized controlled trial; M: male; F: female; L: left; R: right; I: ischaemic; H: haemorrhagic; T: time-point of evaluation; d: mean days after stroke; NR: not reported; NS: not significant; NA: not analysed; IQR: interquartile range; SD: standard deviation; N: Newton; kg: kilogram; BMI: body mass index; Nm: Newton meter; mmHg: millimetres of mercury; kg/f: kilogram/force.
Values of the paretic and non-paretic side are reported as means and standard deviations unless otherwise mentioned.
Statistically significant.
Studies reporting differences between the paretic and non-paretic side are marked with
(no difference between both sides (p≥0.05)) or
(significant difference between both sides (p < 0.05)).
Table II.
Muscle size
| Author (year) | Study design | n | Subject features | Measuring device (unit) | Assessed muscle group | Evaluation time | PSa | NPSa | Change in time | ||||||
| Time point | ES PS (p-value) | ES NPS (p-value) | Drop-out ratio | ||||||||||||
| CROSS-SECTIONAL AREA (UPPER LIMB) | |||||||||||||||
| Median (IQR) | Median (IQR) | ||||||||||||||
| Carin-Levy 2006 (36) | Long. | 18 | 11M, 7F Age 66.0 ± 11.5 y PS: NR Type: NR Acute and early subacute stag |
Skin fold measurements (cm2) | Upper arm muscles | T0: 7d | 60.5 (58.1–62.8) | 62.9 (61.1–64.8) | T0 vs T1 | NA (NS) | NA (NS) | 22% | |||
| T1: 14d | 65.3 (58.3–2.6) | 66.0 (62.2–68.7)† | T1 vs T2 | NA (NS) | NA (NS) | 6% | |||||||||
| T2: 21d | 60.2 (44.0–76.8) | 60.1 (44.9–75.8)† | T2 vs T3 | NA (NS) | NA (NS) | 0% | |||||||||
| T3: 28d | 64.3 (58.3–75.6) | 65.5 (59.4–72.0)† | T0 vs T2 | NA (NS) | NA (NS) | 28% | |||||||||
| T0 vs T3 | NA (NS) | NA (NS) | 28% | ||||||||||||
| Median (IQR) | Median (IQR) | ||||||||||||||
| Forearm muscles | T0: 7d | 35.9 (33.3–38.5) | 45.3 (37.4–47.7)† | T0 vs T1 | NA (NS) | NA (NS) | 22% | ||||||||
| T1: 14d | 47.2 (41.4–48.9) | 45.9 (43.3–48.2)† | T1 vs T2 | NA (NS) | NA (NS) | 6% | |||||||||
| T2: 21d | 43 (34.2–50.1) | 42.8 (34.9–51.4)† | T2 vs T3 | NA (NS) | NA (NS) | 0% | |||||||||
| T3: 28d | 47.2 (42.0–50.5) | 45.9 (41.8–49.7)† | T0 vs T2 | NA (NS) | NA (NS) | 28% | |||||||||
| T0 vs T3 | NA (NS) | NA (NS) | 28% | ||||||||||||
| CROSS-SECTIONAL AREA (LOWER LIMB) | |||||||||||||||
| Median (IQR) | Median (IQR) | ||||||||||||||
| Carin-Levy 2006 (36) | Long. | 18 | 11M, 7F Age 66.0 ± 11.5 y PS: NR Type: NR Acute and early subacute stag |
Skin fold measurements (cm2) | Thigh muscles | T0: 7d | 143.6 (118.2–169.0) | 155.4 (123.6–187.1)† | T0 vs T1 | NA (NS) | NA (NS) | 22% | |||
| T1: 14d | 162.7 (133.4–171.7) | 163.2 (136.3–170.7)† | T1 vs T2 | NA (NS) | NA (NS) | 6% | |||||||||
| T2: 21d | 155.7 (113.4–157.1) | 133.5 (107.2–170.7)† | T2 vs T3 | NA (NS) | NA (NS) | 0% | |||||||||
| T3: 28d | 148.2 (115.5–168.2) | 152.8 (120.6–159.7)† | T0 vs T2 | NA (NS) | NA (NS) | 28% | |||||||||
| T0 vs T3 | NA (NS) | NA (NS) | 28% | ||||||||||||
| Carin-Levy 2006 (36) | Long. | 18 | 11M, 7F Age 66.0 ± 11.5 y PS: NR Type: NR Acute and early subacute stag |
Skin fold measurements (cm2) | Calf muscles | T0: 7d | 65.6 (63.0–106.7) | 69.5 (64.9–105.5)† | T0 vs T1 | NA (NS) | NA (NS) | 22% | |||
| T1: 14d | 94.2 (83.6–101.8) | 96.7 (78.0–97.7)† | T1 vs T2 | NA (NS) | NA (NS) | 6% | |||||||||
| T2: 21d | 80.2 (61.1–100) | 82.0 (60.1–101.5)† | T2 vs T3 | NA (NS) | NA (NS) | 0% | |||||||||
| T3: 28d | 94.7 (76.7–101.5) | 95.6 (71.4–99.5)† | T0 vs T2 | NA (NS) | NA (NS) | 28% | |||||||||
| T0 vs T3 | NA (NS) | NA (NS) | 28% | ||||||||||||
| MUSCLE THICKNESS (UPPER LIMB) | |||||||||||||||
| Moukas 2002 (52) | Long. | 16 | 8M, 8F Age 61.0 ± 11.0 y PS: NR Type: NR Acute and early subacute stage |
Ultrasound imaging, M-mode, 10 MHz transducer (cm) | Elbow extensors | T0: 1d | 2.21 (0.43) | NR | T0 vs T1 | –1.01 (< 0.001*) | NR | 0% | |||
| T1: 11d | 1.78 (0.40) | NR | |||||||||||||
| MUSCLE THICKNESS (LOWER LIMB) | |||||||||||||||
| Kokura 2020 (46) | Long. | 57 39 |
23M, 34F Age 82.1 ± 7.6 y PS: 29L, 28R Type: 45I, 12H Acute and early subacute stage |
Ultrasound imaging, B-mode, 8 MHz transducer (mm) | Knee extensors | T0: 1d | 25.6 (7.2) | 25.0 (7.7) | T0 vs T1 | –0.27 (< 0.001*) | –0.08 (> 0.05) | 0% | |||
| T1: 7d | 23.6 (7.7) | 24.4 (7.2) | T1 vs T2 | –0.13 (NR) | –0.11 (NR) | 0% | |||||||||
| T2: 14d | 22.6 (7.6) | 23.6 (7.6) | T2 vs T3 | –0.07 (NR) | –0.01 (NR) | 0% | |||||||||
| T3: 21d | 22.1 (7.3) | 23.5 (6.9) | T3 vs T4 | –0.11 (NR) | –0.03 (NR) | 0% | |||||||||
| T4: 28d | 21.3 (7.1) | 23.3 (6.8) | T0 vs T2 | –0.40 (< 0.001*) | –0.18 (< 0.05*) | 0% | |||||||||
| T0 vs T3 | –0.48 (< 0.001*) | –0.20 (< 0.05*) | 0% | ||||||||||||
| T0 vs T4 | –0.60 (< 0.001*) | –0.23 (< 0.05*) | 0% | ||||||||||||
| 19M, 20F Age 80.8 ± 9.8 y PS: 22L, 17R Type: 28I, 11H Acute and early subacute stage |
Ultrasound imaging, B-mode, 8 MHz transducer (mm) | Knee extensors | T0: 1d | 26.7 (6.7) | 26.4 (7.5) | T0 vs T1 | –0.34 (< 0.05*) | –0.22 (< 0.05*) | 0% | ||||||
| T1: 7d | 24.4 (6.8) | 24.8 (6.8) | T1 vs T2 | –0.15 (NR) | –0.09 (NR) | 0% | |||||||||
| T2: 14d | 23.4 (6.7) | 24.2 (7.0) | T2 vs T3 | –0.27 (NR) | –0.09 (NR) | 0% | |||||||||
| T3: 21d | 21.7 (5.8) | 23.6 (6.9) | T3 vs T4 | –0.05 (NR) | –0.15 (NR) | 0% | |||||||||
| T4: 28d | 21.4 (6.2) | 22.6 (6.4) | T0 vs T2 | –0.49 (< 0.001*) | –0.30 (< 0.001*) | 0% | |||||||||
| T0 vs T3 | –0.79 (< 0.001*) | –0.39 (< 0.001*) | 0% | ||||||||||||
| T0 vs T4 | –0.81 (< 0.001*) | –0.54 (< 0.001*) | 0% | ||||||||||||
| Nozoe 2016 (54) | Long. | 31 | 22M, 9F Age 70.3 ± 12.8 y PS: 17L, 14R Type: 18I, 13H Acute and early subacute stage |
Ultrasound imaging, B-mode, 8 MHz transducer (mm) | Knee extensors | T0: 2d | 28.0 (7.1) | 28.6 (7.3) | T0 vs T1 | –0.84 (< 0.001*) | –0.59 (< 0.001*) | 0% | |||
| T1: 16d | 22.1 (6.8) | 24.1 (7.7)‡ | |||||||||||||
| Nozoe 2017 (56) | CCT | 10 | 7M, 3F Age 63.1 ± 11.4 y PS: 6L, 4R Type: NR Acute and early subacute stage |
Ultrasound imaging, B-mode, 8 MHz transducer (cm) | Knee extensors | T0: 2d | 3.07 (0.60) | 3.29 (0.59) | T0 vs T1 | –1.30 (.005*) | –0.85 (.005*) | 0% | |||
| T1: 16d | 2.20 (0.68) | 2.62 (0.92) | |||||||||||||
| Nozoe 2018 (55) | Long. | 17 | 4M, 13F Age 66.0 ± 11.0 y PS: NR Type: NR Acute and early subacute |
Ultrasound imaging, B-mode, 8 MHz transducer (cm) | Knee extensors | T0: 3d | 2.98 (0.72) | NR | T0 vs T1 | –0.41 (.001*) | NR | 0% | |||
| T1: 17d | 2.67 (0.74) | NR | |||||||||||||
| MD T1–T0 (SD) | MD T1–T0 (SD) | ||||||||||||||
| Nozoe 2020a (57) | Long. | 55 | 41M, 14F Age 65.0 ± 10.0 y PS: 31L, 24R Type: 27I, 28H Acute and early subacute |
Ultrasound imaging, B-mode, 8 MHz transducer (%) | Knee extensors | T0: 7d | –16.9 (14.7) | –11.3 (12.7) | T0 vs T1 | NA (NR) | NA (NR) | 0% | |||
| T1: 21d | |||||||||||||||
| Median (IQR) | Median (IQR) | ||||||||||||||
| Nozoe 2020b (58) | Long. | 18 | 14M, 4F Age 66.3 ± 10.3 y PS: 7L, 11R Type: 10I, 8H Acute and early subacute |
Ultrasound imaging, B-mode, 8 MHz transducer (cm) | Knee extensors | T0: 2d | 3.1 (1.7) | 3.5 (1.4)† | T0 vs T1 | NA (.014*) | NA (.003*) | 0% | |||
| T1: 16d | 2.6 (1.4) | 2.9 (1.4)‡ | |||||||||||||
| LEAN MUSCLE MASS (LOWER LIMB) | |||||||||||||||
| Jorgensen 2011 (44) | Long. | 12 | Not able to walk by 2 months Acute and early subacute stage |
DEXA scanner (gr) | Lower limb | T0: 7d | 6796.42 (1529.94) | 6832.83 (1315.65) | T0 vs T1 | –0.36 (< 0.05*) | –0.23 (< 0.05*) | 11% | |||
| T1: 56d | 6294.92 (1154.28) | 6518.58 (1282.65) | |||||||||||||
| 13 | Able to walk by 2 months Acute and early subacute stage |
DEXA scanner (gr) | Lower limb | T0: 7d | 7074.31 (1482.91) | 6945.69 (1420.88) | T0 vs T1 | –0.10 (> 0.05) | –0.05 (> 0.05) | 11% | |||||
| T1: 56d | 6931.23 (1389.81) | 6871.38 (1363.01) | |||||||||||||
n: number of participants; PS: paretic side; NPS: non-paretic side; ES: effect size; Long.: Longitudinal; CCT: clinical controlled trial; M: Male; F: female; L: left; R: right; I: ischaemic; H: haemorrhagic; T: time-point of evaluation; d: mean days after stroke; NR: not reported; NS: not significant; NA: not analysed; gr: gram; Cm: centimetres; mm: millimetres; IQR: interquartile range; MD: mean difference; SD: standard deviation.
Values of the paretic and non-paretic side are reported as means and standard deviations unless otherwise mentioned
Statistically significant Studies reporting differences between the paretic and non-paretic side are marked with
(no difference between both sides (p≥0.05)) or
(significant difference between both sides (p < 0.05)).
Study design
Of the 38 included studies, 24 were longitudinal cohort studies in which muscle characteristics were measured at least twice within 3 months post-stroke (31, 33–36, 39, 41, 42, 44, 46, 48, 50, 52–55, 57, 58, 60–62, 64, 65, 67). The remaining eligible studies represent 13 randomized controlled trials (RCTs) (32, 37, 38, 40, 43, 45, 47, 49, 51, 59, 63, 66, 68) and 1 clinical controlled trial (CCT) (56). Participants in the control groups of these interventional trials were considered as longitudinal data as they received conventional physiotherapy (32, 37, 38, 40, 47, 49, 56, 59, 63, 66, 68) or a placebo intervention combined with usual care (32, 43, 45, 51).
Patient characteristics
In total, 1,097 stroke survivors (598 males, 499 females; mean age 67 years) participated in the included studies. Muscle characteristics of the subjects were assessed in the acute and early subacute stage post-stroke (32, 33, 36, 40–42, 44, 46–48, 50, 52–54, 56–58, 66) or exclusively measured in the acute (55) or early subacute stage (Tables I and II) (31, 34, 35, 37–39, 43, 45, 49, 51, 59–65, 67, 68).
Outcome measure characteristics
Data extraction revealed 4 outcome measures of interest: muscle strength (i.e. lower limb (31, 34, 36, 49, 62, 63), upper limb (31, 33, 35, 39, 59, 61, 64, 65), grip (32, 33, 36–38, 40–43, 45, 47, 50, 51, 53, 59–61, 64, 66–68) and pinch strength (32, 48, 68)), muscle thickness (46, 52, 54–58), muscle cross-sectional area (CSA) (36) and lean muscle mass (44). No data on muscle fibre characteristics were reported. The preferred assessment tool to objectively quantify muscle strength was a hand-held dynamometer, which was used in the majority of the included studies (73%) (31–34, 36-39, 41, 42, 45, 47, 49–51, 53, 59, 62, 64, 66–68). Muscle CSA was determined via anthropometric skin fold measurements (36), whereas a Dual Energy Xray absorptiometry (DEXA) scanner and an ultrasound system were used to respectively measure lean muscle mass (44) and muscle thickness (46, 52, 54–58). The assessed muscle groups in the lower limb were hip flexors (31, 34), knee extensors (31, 34, 36, 46, 49, 54–58, 62, 63), knee flexors (49, 63) and ankle dorsiflexors (31, 34). The muscles of the upper extremity being evaluated were the shoulder abductors (31, 33, 65), adductors (65), flexors (65) and extensors (65), elbow flexors (31, 33, 35, 39, 61, 64) and extensors (31, 35, 52, 61, 64), and wrist extensors (31, 59, 61). Studies focused mainly on the evaluation of the muscle groups on the paretic side. Data of the non-paretic side were reported in less than half of the included trials (45%) (31, 34, 36, 43–46, 49–51, 53, 54, 56–58, 62–64).
Quality assessment
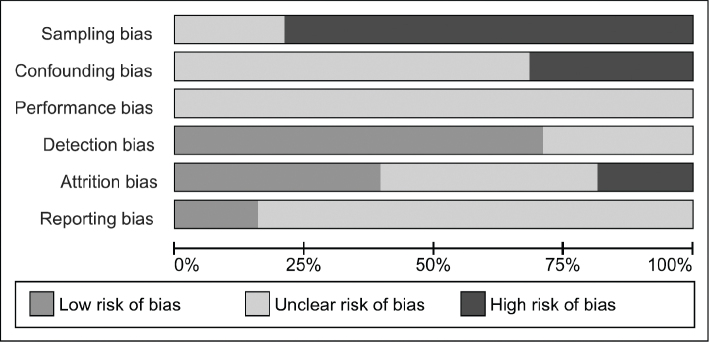
Fig. 2 represents a summary of the authors’ judgement about each risk of bias item. Sampling bias was rated “high” in 30 studies because recruitment of the target population took place in only 1 centre or because a specific target population was recruited for the study (31, 32, 36–46, 48–52, 54–59, 61, 62, 64, 65, 67, 68). The remaining 8 studies were rated “unclear” (33–35, 47, 53, 60, 63, 66). Due to insufficiently reported information about 1 of the pre-specified confounding variables, confounding bias was rated “unclear” in 26 studies (32, 33, 35–40, 43, 44, 46, 47, 49, 50, 52, 54–60, 62, 64, 66, 68), whereas the remaining 12 studies were rated “high” because of the large age differences at study onset (31, 34, 41, 42, 45, 48, 51, 53, 61, 63, 65, 67). Performance bias was unclear in every study because there was insufficient information about the timing of the assessments during the day. Detection bias was low in 27 studies (31–34, 37–39, 41, 42, 44–47, 49–52, 54–58, 61, 64, 66–68) and unclear in the remaining 11 studies because information about the measurement device was insufficiently reported (35, 36, 40, 43, 48, 53, 59, 60, 62, 63, 65). Attrition bias was rated “low” in 15 studies (38, 40, 43, 46–51, 54, 57, 59, 60, 65, 68), “unclear” in 16 (31–35, 41, 42, 45, 52, 55, 61–64, 66, 67) and “high” in the other 7 studies (36, 37, 39, 44, 53, 56, 58). Only 6 of the included studies reported a pre-specified protocol and therefore reporting bias was rated “low” (40, 43, 45, 51, 66, 68), whereas reporting bias of the other 32 studies was rated “unclear” (31–39, 41, 42, 44, 46–50, 52–65, 67).
Fig. 2.
Risk of bias assessment.
Synthesis of the results
The means, SDs, significance values and effect sizes of all included studies are shown in Tables I and II. Twelve studies did not report mean values and SDs and were excluded from quantitative data-analysis and therefore will not be discussed in the following paragraphs (33, 35, 36, 39, 41, 42, 53, 57–61). Detailed information about these studies is reported in Tables I and II.
Muscle strength
The examined muscle groups of the lower limbs (i.e. hip flexors, knee flexors, knee extensors and ankle dorsiflexors), measured within the early subacute stage, showed a significant increase in muscle strength on the paretic side with moderate, positive effect sizes (p < 0.05, gH: 0.34–0.70) (31, 34, 62, 63). A single study did not report significant changes in knee flexors and extensors, but did find a similar trend towards an increase in muscle strength over time with positive, small effect sizes (knee extensors: p = 0.07, gH: 0.31; knee flexors: p = 0.27, gH: 0.21) (49). Concerning the non-paretic side, muscle strength of the lower limbs significantly increased in the hip flexors (p < 0.05, gH: 0.24–0.25) and ankle dorsiflexors (p < 0.05, gH: 0.26–0.31) (31, 34), whilst studies that investigated the knee extensors and flexors reported mixed results (p < 0.001 to p > 0.05, gH: 0.06–0.28) (31, 34, 49, 62, 63).
The muscle groups of the upper limbs were assessed in the early subacute stage after stroke. Studies reported a significant increase over time for elbow flexors, elbow extensors and wrist extensors on the paretic side with small to moderate effect sizes (p < 0.001 to p = 0.046, gH: 0.17–0.40) (31, 64). Changes in muscle strength of the shoulder abductors were inconsistent between studies (p < 0.001 to p > 0.05, gH: –0.15 to 0.28) (31, 65). The muscle groups of the shoulder flexors, extensors and adductors were examined only in a single study which showed non-significant changes over time (p > 0.05, gH: –0.43 to 0.76) (65). Regarding the non-paretic side, studies reported a significant increase for the wrist extensors (p < 0.001, gH: 0.23) (31), a non-significant change over time for the shoulder abductors (p > 0.001, gH: 0.16) (31) and mixed results for the elbow flexors and extensors (p < 0.05 to p > 0.001, gH: –0.05 to 0.43) (31, 64).
Studies that measured grip strength within the early subacute stage demonstrated positive effect sizes representing an increase over time. However, significance values and the size of the effects showed large discrepancies between studies measuring grip strength on the paretic side, with non-significant changes and small effect sizes being reported on the paretic side (p > 0.05, gH: 0.01–0.14) (43, 45, 68), whilst other studies showed a significant increase with small to large effect sizes (p < 0.05, gH: 0.25–1.16) (37, 38, 64, 67). This variability is also presented in studies evaluating grip strength changes between the acute and early subacute stage with effect sizes ranging from small to large (gH: 0.12–0.97) and both significant (40, 50) and non-significant (32, 66) changes are found. Studies investigating grip strength on the non-paretic side showed an overall non-significant change over time, with only 1 study reporting significant changes with moderate, positive effect sizes (p < 0.05, gH: 0.33–0.38) (64).
Pinch strength was examined in 1 study within the early subacute stage, which showed a non-significant change over time on the paretic side with a small effect size (p = 0.087, gH = 0.12) (68). Two other studies reported pinch strength data assessed between the acute and early subacute stage with small to moderate positive effect sizes (gH: 0.08–0.67) representing an increase on the paretic side (p = 0.04 and p > 0.05) (32, 48). No studies examined pinch strength on the non-paretic side.
Muscle thickness
Muscle thickness was measured in the muscle groups of the knee extensors (46, 54–57) and elbow extensors (52). Studies assessing stroke survivors between the acute and early subacute stage found a significant decrease in muscle thickness of the knee extensors on the paretic side with moderate to large effect sizes (p < 0.001 to p < 0.05, gH: –0.40 to –1.30), whereas small to large effects were observed on the non-paretic side (p < 0.001 to p < 0.05; gH: –0.18 to –0.85). One study reported changes in the knee extensors’ muscle thickness within the acute and early subacute stage, showing a significant decrease on both sides (p < 0.05, gH: –0.01 to –0.34) (46). Although the elbow extensors were only addressed in a single study, a large negative effect size was found representing a significant decrease in muscle thickness between the acute and early subacute stage (p < 0.001, gH = –1.01) (52).
Lean muscle mass
Jorgensen et al.’s study was the only one investigating lean muscle mass in the early stage post-stroke (44). They divided the stroke population into 2 subgroups based on the subjects ambulation type. Results showed a significant decrease in lean muscle mass on the paretic and non-paretic side for patients who were not able to walk 2 months post-stroke with small to moderate effect sizes (paretic side: p < 0.05, gH = –0.36; non-paretic side: p < 0.05, gH = –0.23). In contrast, subjects who were ambulant after 2 months showed no significant decrease in lean muscle mass on both sides (paretic side: p > 0.05, gH = –0.10; non-paretic side: p > 0.05, gH = –0.05).
Cross-sectional area
Only 1 study investigated the cross-sectional area in the acute and subacute phase after stroke, using skin-fold measurements (cm2) of the upper arm muscles, forearm muscles, tight muscles and calf muscles. No significant changes were reported between the different time-points for the paretic and the non-paretic side.
DISCUSSION
Summary of the evidence
The aim of this systematic review was to synthesize available evidence describing the time-course of peripheral skeletal muscle changes measured by objective, quantitative assessment methods in the first 3 months post-stroke.
A total of 38 studies investigating time-related changes in muscle characteristics in 4 different outcome measures were identified. In general, the results revealed an overall mean increase in muscle strength on the paretic side, whereas muscle thickness in the upper and lower limb decreased. Lean muscle mass and muscle CSA were only examined in a single study, but results showed a trend towards a decrease in lean muscle mass in the lower limbs of non-ambulant stroke survivors, whereas muscle CSA seemed to remain unchanged in the early subacute stages post-stroke. Similar results were found on the non-paretic side, but these changes were smaller compared with the paretic side.
The general increase in muscle strength in the first 3 months of stroke recovery is probably due to inpatient rehabilitation, where clinicians nowadays are using a broad range of evidence-based interventions to improve motor recovery (2). In addition, spontaneous recovery mechanisms will contribute to this early gain in muscle strength (21). Despite the overall increase in muscle strength, which might indicate early recovery, results should be interpreted with caution.
The benefits of early stroke rehabilitation are beyond doubt, yet the calculated effect sizes for muscle strength were generally small to moderate, indicating that there is still room for improvement. Moreover, an early decrease in muscle thickness was found despite participants receiving conventional therapy. This finding is in line with previous research that reported stroke-induced muscle wasting (7, 8). Moreover, it seems that this phenomenon is not only due to sarcopaenia (i.e. age-related loss of muscle mass and muscle strength) since stroke survivors show higher prevalence rates of muscle wasting compared with healthy people, even after controlling for age, sex and race (69). These results might indicate that more attention for specific therapy regimens, directly aimed at the level of the skeletal muscle, is warranted to prevent loss of function and to optimize recovery (70).
One might question if the observed changes in muscle strength are the result of true changes in the underlying physiology and mechanical properties of the skeletal muscle or due to neural adaptation. Understanding of “how” muscle strength changes are achieved early after stroke is essential to better understand the complex stroke recovery process. Besides the increase in muscle strength, the results of this review revealed a concomitant decrease in muscle thickness, which might seem contradictory at first sight. Yet, previous research has shown that there is no one-to-one relationship between those 2 parameters, suggesting that other factors contribute to the recovery of muscle strength (71). One such factor, besides neuromuscular changes, may be the architectural properties of the skeletal muscle, which play an integer role in the force-generation process. Muscle fibre length, pennation angle and physiological CSA are deemed the most important architectural parameters and therefore, structural changes of these properties might influence force production (15). However, to the best of our knowledge, the impact of such changes on force production has not been investigated in (sub)acute stroke patients, and, therefore, this should be considered speculative and warrants further investigation. Nevertheless, previous research in chronic stroke survivors has already presented evidence that changes in the architecture of the muscle occur (i.e. decreased fibre length) and contribute to strength deficits (16), but no information for (sub)acute stroke patients is available. Only a single study measuring muscle CSA was included in this systematic review, which made it difficult to draw uniform conclusions towards its influence on recovery (36). Therefore, the structure-function relationship in the early recovery process after stroke remains unclear because no other studies investigating the architectural properties were found during the systematic search of this review.
Furthermore, the results reported by the included studies were highly variable, with effect sizes ranging from small to large and noticeable discrepancies in the significance values. This heterogeneity is likely due to researchers using different measurement tools, units, and procedures to address their outcome measure and variable time-windows to assess muscle changes. In addition, stroke research has shown that recovery profiles vary greatly between individual stroke survivors (72). This might raise the question of whether different processes underlie recovery for subjects recovering better and faster compared with others. Indeed, the results reported by all of the included studies are summarized values (e.g. means) which represent stroke recovery at group level. However, with the different recovery profiles in mind, it might be interesting to closely observe individual muscle changes rather than focusing on the group level. Although none of the trials in this systematic review statistically established these individual muscle trajectories, some of the authors plotted individual data in figures (39, 55) or additional tables (50). Cortes et al., for example, illustrated the time-course of biceps muscle strength for each individual stroke participant (39). This figure shows a clear diversity in the recovery patterns of stroke survivors and confirms that not every patient has the same potential to recover after a stroke incident. Knowledge of the underlying mechanisms contributing to this large recovery variability within the stroke population is fundamental for both research and clinical practice. The possibility to distinguish subgroups within a certain population would make it easier to select a specific group for testing a given treatment or personalize therapeutic stroke interventions (4, 73).
Study limitations
This systematic review has a number of limitations. An important key issue was the large variability between the included studies, which made it difficult to compare results. By dividing the time after stroke into clear cut-off phases, as suggested by the Stroke Consortium (4), we tried to facilitate comparison. However, timing of the measurements regarding stroke onset differed greatly between the included studies. Research has shown that most recovery occurs in the first 3 months post-stroke, but knowledge about the exact time-window during which muscle-related recovery takes place remains unclear.
Another constraint was the sources of bias in the included trials. The overall high risk of sampling bias is important to consider, since most studies recruited participants in only 1 centre or recruiting a specific target population (e.g. subjects with low baseline strength). Although the patient’s baseline level of functioning and stroke severity were not taken into account in this systematic review, it might be noticed that this will probably have clinical implications towards the reported muscle changes. In stroke recovery, a rule of thumb is that subjects with initially more severe deficits are less likely to make a good recovery. However, not every patient seems to fit this proportional recovery rule (74). This once again highlights the importance of reporting individual changes over time and the need to specify clear subgroups in stroke survivors.
Furthermore, the pre-specified confounding variables were insufficiently reported in most studies, especially nutrition, smoking and physical activity status, which could result in a potential bias as these confounding factors might have an influence on muscle changes post-stroke.
Finally, studies that used surface electromyography as a measuring device to assess muscle activity were excluded from this systematic review because data are likely to be influenced by the position of the electrodes, the analysis methods, rapportage of the results, which would complicate comparison between studies (75). However, the results of these measurements could be of important clinical relevance and might therefore be an interesting topic for future research.
Future research
This review gathered all information about skeletal muscle changes in the acute and early subacute stroke population, yet it also uncovered important shortcomings in the existing stroke literature that should be considered in future research about this topic.
First, this review identified a lack of studies investigating architectural properties in the first 3 months after stroke. Future studies addressing these outcome measures are recommended, as they might help to better understand the recovery process.
Secondly, standardized time-intervals to assess post-stroke muscle changes are lacking and causing a large heterogeneity between studies. Although the time after stroke was divided into phases in this review, the evaluation time-points did not entirely fit into these clear-cut sequences. Defining critical time-points in stroke rehabilitation to explore muscle trajectories in a uniform matter and to determine the optimal timing to implement interventions is recommended.
Thirdly, muscle changes are only reported at group level. Since stroke patients show an individual recovery pattern, it seems advisable to report individual muscle trajectories rather than trajectories at group level. Research into those individual trajectories would make it possible to define subgroups within the stroke population for research or clinical purpose and might permit future research to investigate the effects of interventions tailored to these specific patient profiles.
Finally, potential confounding factors, which might have an influence on peripheral muscle changes, were only taken into account to a limited extent. A number of these confounding factors are already proposed in this review within the context of confounding bias and based on experts’ advice (e.g. age, nutrition, physical activity) (Appendix 2). These suggested confounding factors are not exhaustive and should be further explored in future research.
CONCLUSION
Skeletal muscle changes in the first 3 months of stroke recovery show a clear trajectory at the group level on the paretic side, with an increase in strength (muscle strength, grip strength and pinch strength) and a decrease in muscle thickness, whereas lean muscle mass and muscle CSA were insufficiently investigated to draw firm conclusions. More attention on specific therapy regimens, directly aimed at the skeletal muscle, is warranted to prevent loss of function and to optimize recovery post-stroke. Understanding of “how” muscle strength changes are achieved early after stroke could lay important foundations for advances in future stroke rehabilitation interventions.
Funding
This work was supported by the Wetenschappelijk Fonds Willy Gepts of the UZ Brussel. Co-author E.D.K. is a Strategic Basic Research fellow funded by the Research Foundation – Flanders (FWO).
Supplementary Material
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Katan M, Luft A. Global Burden of Stroke. Semin Neurol 2018; 38: 208-211. [DOI] [PubMed] [Google Scholar]
- 2.Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurol 2009; 8: 741–754. [DOI] [PubMed] [Google Scholar]
- 3.Kelly-Hayes M, Beiser A, Kase CS, Scaramucci A, D’Agostino RB, Wolf PA. The influence of gender and age on disability following ischemic stroke: the Framingham study. J Stroke Cerebrovasc Dis 2003; 12: 119–126. [DOI] [PubMed] [Google Scholar]
- 4.Bernhardt J, Hayward KS, Kwakkel G, Ward NS, Wolf SL, Borschmann K, et al. Agreed definitions and a shared vision for new standards in stroke recovery research: The Stroke Recovery and Rehabilitation Roundtable taskforce. Int J Stroke 2017; 12: 444–450. [DOI] [PubMed] [Google Scholar]
- 5.Scherbakov N, Doehner W. Sarcopenia in stroke-facts and numbers on muscle loss accounting for disability after stroke. J Cachex Sarcopen Musc 2011; 2: 5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gracies JM. Pathophysiology of spastic paresis. I: paresis and soft tissue changes. Muscle Nerve 2005; 31: 535–551. [DOI] [PubMed] [Google Scholar]
- 7.Ryan AS, Dobrovolny CL, Smith GV, Silver KH, Macko RF. Hemiparetic muscle atrophy and increased intramuscular fat in stroke patients. Arch Phys Med Rehabil 2002; 83: 1703–1707. [DOI] [PubMed] [Google Scholar]
- 8.Scherbakov N, Sandek A, Doehner W. Stroke-related sarcopenia: specific characteristics. J Am Med Direct Assoc 2015; 16: 272–276. [DOI] [PubMed] [Google Scholar]
- 9.English C, McLennan H, Thoirs K, Coates A, Bernhardt J. Loss of skeletal muscle mass after stroke: a systematic review. Int J Stroke 2010; 5: 395–402. [DOI] [PubMed] [Google Scholar]
- 10.Hunnicutt JL, Gregory CM. Skeletal muscle changes following stroke: a systematic review and comparison to healthy individuals. Top Stroke Rehabil 2017; 24: 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hafer-Macko CE, Ryan AS, Ivey FM, Macko RF. Skeletal muscle changes after hemiparetic stroke and potential beneficial effects of exercise intervention strategies. J Rehabil Res Dev 2008; 45: 261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller M, Flansbjer UB, Lexell J. Voluntary activation of the knee extensors in chronic poststroke subjects. Am J Phys Med Rehabil 2009; 88: 286–291. [DOI] [PubMed] [Google Scholar]
- 13.Schillebeeckx F, De Groef A, De Beukelaer N, Desloovere K, Verheyden G, Peers K. Muscle and tendon properties of the spastic lower leg after stroke defined by ultrasonography: a systematic review. Eur J Phys Rehabil Med 2020. [DOI] [PubMed]
- 14.De Deyne PG, Hafer-Macko CE, Ivey FM, Ryan AS, Macko RF. Muscle molecular phenotype after stroke is associated with gait speed. Muscle Nerve 2004; 30: 209–215. [DOI] [PubMed] [Google Scholar]
- 15.Lieber RL, Fridén J. Functional and clinical significance of skeletal muscle architecture. Muscle Nerve 2000; 23: 1647–1666. [DOI] [PubMed] [Google Scholar]
- 16.Gray V, Rice CL, Garland SJ. Factors that influence muscle weakness following stroke and their clinical implications: a critical review. Physiother Can 2012; 64: 415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patterson SL, Forrester LW, Rodgers MM, Ryan AS, Ivey FM, Sorkin JD, et al. Determinants of walking function after stroke: differences by deficit severity. Arch Phys Med Rehabil 2007; 88: 115–119. [DOI] [PubMed] [Google Scholar]
- 18.White WB, Strand V, Roberts R, Whelton A. Effects of the cyclooxygenase-2 specific inhibitor valdecoxib versus nonsteroidal antiinflammatory agents and placebo on cardiovascular thrombotic events in patients with arthritis. Am J Ther 2004; 11: 244–250. [DOI] [PubMed] [Google Scholar]
- 19.Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2016; 47: e98–e169. [DOI] [PubMed] [Google Scholar]
- 20.Veerbeek JM, van Wegen E, van Peppen R, van der Wees PJ, Hendriks E, Rietberg M, et al. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLoS One 2014; 9: e87987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet 2011; 377: 1693–1702. [DOI] [PubMed] [Google Scholar]
- 22.Lamola G, Bertolucci F, Rossi B, Chisari C. Clinical assessments for predicting functional recovery after stroke. Int J Neurorehabil 2015; 2: 174. [Google Scholar]
- 23.Bohannon RW. Considerations and practical options for measuring muscle strength: a narrative review. Biomed Res Int 2019; 2019: 8194537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engelke K, Museyko O, Wang L, Laredo JD. Quantitative analysis of skeletal muscle by computed tomography imaging – state of the art. J Orthop Translat 2018; 15: 91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Truelsen T, Begg S. The global burden of cerebrovascular disease. Geneva: World Health Organization; 2006. [Google Scholar]
- 26.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan – a web and mobile app for systematic reviews. Syst Rev 2016; 5: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta-analyses. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.1 [updated September 2008]. Higgins JPT, Green S, The Cochrane Collaboration 2008: 9.1–9.43.
- 28.Viswanathan M, Ansari MT, Berkman ND, Chang S, Hartling L, McPheeters M, Santaguida PL, Shamliyan T, Singh K, Tsertsvadze A, Treadwell JR. Assessing the Risk of Bias of Individual Studies in Systematic Reviews of Health Care Interventions. 2012 Mar 8. In: Methods Guide for Effectiveness and Comparative Effectiveness Reviews [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2008. PMID: . [PubMed] [Google Scholar]
- 29.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25: 603–605. [DOI] [PubMed] [Google Scholar]
- 30.Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol 2013; 4: 863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andrews AW, Bohannon RW. Short-term recovery of limb muscle strength after acute stroke. Arch Phys Med Rehabil 2003; 84: 125–130. [DOI] [PubMed] [Google Scholar]
- 32.Au-Yeung SS, Hui-Chan CW. Electrical acupoint stimulation of the affected arm in acute stroke: a placebo-controlled randomized clinical trial. Clin Rehabil 2014; 28: 149–158. [DOI] [PubMed] [Google Scholar]
- 33.Bohannon RW. Consistency of paretic upper extremity motor performance soon after stroke. J Phys Ther Sci 1995; 7: 49–51. [Google Scholar]
- 34.Bohannon RW, Andrews AW, Glenney SS. Responsiveness of measurements of lower-limb muscle strength obtained with a hand-held dynamometer from patients with stroke. Isokinetics Exercise Sci 2013; 21: 129–134. [Google Scholar]
- 35.Canning CG, Ada L, Adams R, O’Dwyer NJ. Loss of strength contributes more to physical disability after stroke than loss of dexterity. Clin Rehabil 2004; 18: 300–308. [DOI] [PubMed] [Google Scholar]
- 36.Carin-Levy G, Greig C, Young A, Lewis S, Hannan J, Mead G. Longitudinal changes in muscle strength and mass after acute stroke. Cerebrovasc Dis 2006; 21: 201–207. [DOI] [PubMed] [Google Scholar]
- 37.Chen JC, Liang CC, Shaw FZ. Facilitation of sensory and motor recovery by thermal intervention for the hemiplegic upper limb in acute stroke patients: a single-blind randomized clinical trial. Stroke 2005; 36: 2665–2669. [DOI] [PubMed] [Google Scholar]
- 38.Choi JH, Han EY, Kim BR, Kim SM, Im SH, Lee SY, et al. Effectiveness of commercial gaming-based virtual reality movement therapy on functional recovery of upper extremity in subacute stroke patients. Ann Rehabil Med 2014; 38: 485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cortes JC, Goldsmith J, Harran MD, Xu J, Kim N, Schambra HM, et al. A Short and distinct time window for recovery of arm motor control early after stroke revealed with a global measure of trajectory kinematics. Neurorehabil Neural Repair 2017; 31: 552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghaziani E, Couppe C, Siersma V, Sondergaard M, Christensen H, Magnusson SP. Electrical somatosensory stimulation in early rehabilitation of arm paresis after stroke: a randomized controlled trial. Neurorehabil Neural Repair 2018; 32: 899–912. [DOI] [PubMed] [Google Scholar]
- 41.Groisser BN, Copen WA, Singhal AB, Hirai KK, Schaechter JD. Corticospinal tract diffusion abnormalities early after stroke predict motor outcome. Neurorehabil Neural Repair 2014; 28: 751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heller A, Wade DT, Wood VA, Sunderland A, Hewer RL, Ward E. Arm function after stroke: measurement and recovery over the first three months. Journal of Neurology, Neurosurgery & Psychiatry 1987; 50: 714–719. doi: 10.1136/jnnp.50.6.714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hosomi K, Morris S, Sakamoto T, Taguchi J, Maruo T, Kageyama Y, et al. Daily repetitive transcranial magnetic stimulation for poststroke upper limb paresis in the subacute period. J Stroke Cerebrovasc Dis 2016; 25: 1655–1664. [DOI] [PubMed] [Google Scholar]
- 44.Jørgensen L, Jacobsen BK. Changes in muscle mass, fat mass, and bone mineral content in the legs after stroke: a 1 year prospective study. Bone. 2001. Jun; 28(6): 655–9. doi: 10.1016/s8756-3282(01)00434-3. PMID: . [DOI] [PubMed] [Google Scholar]
- 45.Kattenstroth JC, Kalisch T, Sczesny-Kaiser M, Greulich W, Tegenthoff M, Dinse HR. Daily repetitive sensory stimulation of the paretic hand for the treatment of sensorimotor deficits in patients with subacute stroke: RESET, a randomized, sham-controlled trial. BMC Neurol 2018; 18: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kokura Y, Kato M, Taniguchi Y, Kimoto K, Okada Y. Energy intake during the acute phase and changes in femoral muscle thickness in older hemiplegic inpatients with stroke. Nutrition (Burbank, Los Angeles County, CA) 2020; 70: 110582. [DOI] [PubMed] [Google Scholar]
- 47.Langhammer B, Stanghelle JK, Lindmark B. An evaluation of two different exercise regimes during the first year following stroke: a randomised controlled trial. Physiother Theory Pract 2009; 25: 55–68. [DOI] [PubMed] [Google Scholar]
- 48.Larsen LH, Zibrandtsen IC, Wienecke T, Kjaer TW, Christensen MS, Nielsen JB, et al. Corticomuscular coherence in the acute and subacute phase after stroke. Clin Neurophysiol 2017; 128: 2217–2226. [DOI] [PubMed] [Google Scholar]
- 49.Lee SY, Im SH, Kim BR, Han EY. The effects of a motorized aquatic treadmill exercise program on muscle strength, cardiorespiratory fitness, and clinical function in subacute stroke patients: a randomized controlled pilot trial. Am J Phys Med Rehabil 2018; 97: 533–540. [DOI] [PubMed] [Google Scholar]
- 50.Liuzzi G, Hörniß V, Lechner P, Hoppe J, Heise K, Zimerman M, Gerloff C, Hummel FC. Development of movement-related intracortical inhibition in acute to chronic subcortical stroke. Neurology Jan 2014; 82(3): 198–205. doi: 10.1212/WNL.0000000000000028 [DOI] [PubMed] [Google Scholar]
- 51.Matsuura A, Onoda K, Oguro H, Yamaguchi S. Magnetic stimulation and movement-related cortical activity for acute stroke with hemiparesis. Eur J Neurol 2015; 22: 1526–1532. [DOI] [PubMed] [Google Scholar]
- 52.Moukas M, Vassiliou MP, Amygdalou A, Mandragos C, Takis F, Behrakis PK. Muscular mass assessed by ultrasonography after administration of low-dose corticosteroids and muscle relaxants in critically ill hemiplegic patients. Clin Nutr 2002; 21: 297–302. [DOI] [PubMed] [Google Scholar]
- 53.Noskin O, Krakauer JW, Lazar RM, Festa JR, Handy C, O’Brien KA, et al. Ipsilateral motor dysfunction from unilateral stroke: implications for the functional neuroanatomy of hemiparesis. J Neurol Neurosurg Psychiatry 2008; 79: 401–406. [DOI] [PubMed] [Google Scholar]
- 54.Nozoe M, Kanai M, Kubo H, Kitamura Y, Yamamoto M, Furuichi A, et al. Changes in quadriceps muscle thickness, disease severity, nutritional status, and c-reactive protein after acute stroke. J Stroke Cerebrovasc Dis 2016; 25: 2470–2474. [DOI] [PubMed] [Google Scholar]
- 55.Nozoe M, Kanai M, Kubo H, Kobayashi M, Yamamoto M, Shimada S, et al. Quadriceps muscle thickness changes in patients with aneurysmal subarachnoid hemorrhage during the acute phase. Top Stroke Rehabil 2018; 25: 209–213. [DOI] [PubMed] [Google Scholar]
- 56.Nozoe M, Kanai M, Kubo H, Takeuchi Y, Kobayashi M, Yamamoto M, et al. Efficacy of neuromuscular electrical stimulation for preventing quadriceps muscle wasting in patients with moderate or severe acute stroke: a pilot study. NeuroRehabilitation 2017; 41: 143–149. [DOI] [PubMed] [Google Scholar]
- 57.Nozoe M, Kanai M, Kubo H, Yamamoto M, Shimada S, Mase K. Non-paretic lower limb muscle wasting during acute phase is associated with dependent ambulation in patients with stroke. J Clin Neurosci 2020; 74: 141–145. [DOI] [PubMed] [Google Scholar]
- 58.Nozoe M, Kubo H, Kanai M, Yamamoto M, Shimada S, Mase K. Peripheral motor nerve conduction abnormality, muscle strength, and muscle wasting in patients with acute stroke: a pilot study. J Clin Neurosci 2020; 75: 80–84. [DOI] [PubMed] [Google Scholar]
- 59.Powell J, Pandyan AD, Granat M, Cameron M, Stott DJ. Electrical Stimulation of Wrist Extensors in Poststroke Hemiplegia. Stroke 1999; 30: 1384–1389. doi: 10.1161/01.str.30.7.1384. [DOI] [PubMed] [Google Scholar]
- 60.Prigatano GP, Wong JL. Speed of finger tapping and goal attainment after unilateral cerebral vascular accident. Arch Phys Med Rehabil. 1997. Aug; 78(8): 847–52. doi: 10.1016/s0003-9993(97)90198-2. PMID: . [DOI] [PubMed] [Google Scholar]
- 61.Renner CI, Bungert-Kahl P, Hummelsheim H. Change of strength and rate of rise of tension relate to functional arm recovery after stroke. Arch Phys Med Rehabil 2009; 90: 1548–1556. [DOI] [PubMed] [Google Scholar]
- 62.Shapira-Vadler O, Treger I, Katz-Leurer M. muscle strength, function and heart autonomic regulation system recovery at the sub-acute stage post stroke. Eur Neurol 2015; 74: 154–157. [DOI] [PubMed] [Google Scholar]
- 63.Solopova IA, Tihonova DY, Grishin AA, Ivanenko YP. Assisted leg displacements and progressive loading by a tilt table combined with FES promote gait recovery in acute stroke. NeuroRehabilitation 2011; 29: 67–77. [DOI] [PubMed] [Google Scholar]
- 64.Suzuki M, Omori Y, Sugimura S, Miyamoto M, Sugimura Y, Kirimoto H, et al. Predicting recovery of bilateral upper extremity muscle strength after stroke. J Rehabil Med 2011; 43: 935–943. [DOI] [PubMed] [Google Scholar]
- 65.Turner DL, Tang X, Winterbotham W, Kmetova M. Recovery of submaximal upper limb force production is correlated with better arm position control and motor impairment early after a stroke. Clin Neurophysiol 2012; 123: 183–192. [DOI] [PubMed] [Google Scholar]
- 66.Umeki N, Murata J, Higashijima M. Effects of training for finger perception on functional recovery of hemiplegic upper limbs in acute stroke patients. Occupat Ther Int 2019; 2019: 6508261–6508261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vanbellingen T, Ottiger B, Maaijwee N, Pflugshaupt T, Bohlhalter S, Muri RM, et al. Spatial neglect predicts upper limb use in the activities of daily living. Cerebrovasc Dis 2017; 44: 122–127. [DOI] [PubMed] [Google Scholar]
- 68.Vanoglio F, Bernocchi P, Mule C, Garofali F, Mora C, Taveggia G, et al. Feasibility and efficacy of a robotic device for hand rehabilitation in hemiplegic stroke patients: a randomized pilot controlled study. Clin Rehabil 2017; 31: 351–360. [DOI] [PubMed] [Google Scholar]
- 69.Ryan AS, Ivey FM, Serra MC, Hartstein J, Hafer-Macko CE. Sarcopenia and physical function in middle-aged and older stroke survivors. Arch Phys Med Rehabil 2017; 98: 495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beckwee D, Lefeber N, Bautmans I, Cuypers L, De Keersmaecker E, De Raedt S, et al. Muscle changes after stroke and their impact on recovery: time for a paradigm shift? Review and commentary. Top Stroke Rehabil 2021; 28: 104–111. [DOI] [PubMed] [Google Scholar]
- 71.McGregor RA, Cameron-Smith D, Poppitt SD. It is not just muscle mass: a review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longevity Healthspan 2014; 3: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kwakkel G, Kollen B, Lindeman E. Understanding the pattern of functional recovery after stroke: facts and theories. Restorative Neurol Neurosci 2004; 22: 281–299. [PubMed] [Google Scholar]
- 73.Boyd LA, Hayward KS, Ward NS, Stinear CM, Rosso C, Fisher RJ, et al. Biomarkers of stroke recovery: consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable. Int J Stroke 2017; 12: 480–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vliet R, Selles RW, Andrinopoulou ER, Nijland R, Ribbers GM, Frens MA, et al. Predicting upper limb motor impairment recovery after stroke: a mixture model. Ann Neurol 2020; 87: 383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Konrad P. The ABC of EMG, A Practical Introduction to Kinesiological Electromyography; Noraxon Inc.: Scottsdale, AZ, USA, 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.