Abstract
A prospective study was conducted with Bangladeshi children with rotavirus (RV) diarrhea to assess whether nutritional and clinical parameters, RV serotypes, levels of interleukin-10 (IL-10), tumor necrosis factor alpha (TNF-α), and gamma interferon (IFN-γ), and RV-specific antibody titers in plasma and stool were associated with the development of persistent diarrhea. Children with watery diarrhea for 6 to 8 days, selected from the Dhaka Hospital of the International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B), were enrolled in the study and monitored until diarrhea improved. Children were classified as having acute diarrhea (AD) if diarrhea resolved within 14 days of onset and as having persistent diarrhea (PD) if diarrhea persisted for more than 14 days after onset. Uninfected, control children (n = 13) from the Nutrition Follow-Up Unit of ICDDR,B were also enrolled. Of the 149 children with diarrhea enrolled, 29 had diarrhea with RV alone, of which 19 had AD and 10 developed PD. Samples of stool and blood were collected from all children on enrollment. Stool samples were collected again from children when they developed PD. Of the 10 children who had an initial RV infection and then developed PD, only one had persistent RV infection. Plasma levels of IL-10 and TNF-α were higher in children with diarrhea compared to uninfected controls but were similar in children with AD and PD. Plasma IFN-γ levels were higher in children who developed PD than in those with AD (P = 0.008) or uninfected controls (P = 0.001). In stools, the levels of TNF-α, the only cytokine detected, were similar in the three groups of children. RV-specific immunoglobulin G (IgG) titers in plasma were higher in uninfected children than in those with AD (P < 0.001) or PD (P = 0.024) but titers were similar in children with AD and PD. RV-specific IgA titers in plasma and stool were similar in the three groups of children. From all observed parameters, only elevated plasma IFN-γ levels were associated with subsequent development of PD. However, a larger sample size is necessary to substantiate this observation.
Rotavirus (RV) infection is the most common cause of hospitalization due to diarrhea in Bangladeshi children below 5 years of age (31) and is associated with considerable morbidity and mortality. Persistent diarrhea (PD), which is defined as diarrhea lasting for 14 days or more, is responsible for 30 to 50% of deaths due to diarrheal illness in developing countries (13). Although RV infection is not considered a significant risk factor for PD (7, 23), it does cause PD (19) especially in immunodeficient children (28). The cause(s) of PD is not well understood; however, one of the risk factors for PD, identified in children prior to the development of diarrhea, is decreased cell-mediated immunity (4, 9, 20). It is, therefore, possible that children who are already immunodeficient will have an altered immune response to an initial RV infection that may then lead to PD.
Immunity in RV infection involves both cellular and humoral immune responses including cytokines. Several cytokines have been detected in the plasma and stools of children with acute RV infection (15, 21). Also stimulation by RV induces release of interleukin-8 (IL-8), growth-related peptide α, and RANTES from epithelial cells (11) and IL-2 and gamma interferon (IFN-γ) from lymphocytes (35). It has further been shown that IFN-γ and IL-1 inhibit RV entry into human intestinal cell lines (5). Thus, cytokines play a key role in RV infection so that alterations in these cytokines may affect recovery from RV infection. The role of RV-specific immunoglobulins (Ig) in protection from RV infection is controversial. Various studies of natural RV infection or of volunteers challenged with RV have shown that antibodies may be associated with protection but antibodies do not consistently confer protection from infection or illness (6, 8, 12, 14, 18, 24, 33).
This study was aimed at assessing whether children with RV infection have altered levels of IFN-γ, IL-10, tumor necrosis factor alpha (TNF-α), and RV-specific antibodies in plasma and stool, before the onset of PD. These cytokines were selected on the basis of their possible antiviral activities (27) and effects on immunoglobulin A (IgA) secretion (25). Cytokine levels and RV-specific antibody titers were compared in three groups of children as follows: (i) children with RV infection for 6 to 8 days who recovered within 14 days of onset of diarrhea, (ii) children with RV infection for 6 to 8 days in whom diarrhea persisted for more than 14 days after onset, and (iii) uninfected, control children. Also, since malnutrition and cell-mediated immunity have been shown to be independent risk factors for the development of PD (4), the nutritional status and the general immune responses of the three groups of children were compared.
MATERIALS AND METHODS
Patient population.
Children, 7 to 24 months of age, attending the Clinical Research and Service Centre of the International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B), Dhaka, Bangladesh, with complaints of watery diarrhea for 6 to 8 days were initially enrolled. These children were hospitalized until diarrhea improved. If diarrhea improved within 14 days of onset, children were classified as having acute diarrhea (AD), but if diarrhea persisted beyond 14 days, children were classified as having PD. Improvement in diarrhea was defined as a decrease in stool frequency to ≤5 in 24 h and/or change of stool consistency from liquid or loose stools to soft stools. Uninfected, control children were enrolled from the Nutrition Follow-Up Unit of ICDDR,B. This unit is an outpatient unit responsible for nutritional rehabilitation and growth monitoring of children. The children who attend this unit are those who initially attend the Clinical Research and Service Centre of ICDDR,B with severe malnutrition and diarrhea. Children were included as uninfected controls only if they were 7 to 24 months of age and without any apparent infection for at least 1 month.
On enrollment, freshly collected stools from all children were examined microscopically, cultured for enteric bacteria (34), and assessed for the presence of RV by an enzyme-linked immunosorbent assay (ELISA) as described previously (30). Strains of RV were typed following a scheme described previously (32). Briefly, RV strains were first electropherotyped by polyacrylamide gel electrophoresis (PAGE), and PAGE-positive samples were then typed. Typing was done initially by a monoclonal antibody-based ELISA for G types 1 to 4, followed by reverse transcriptase-PCR (RT-PCR) for G types 1 to 4 and 9 and for P types (32). Presence of enterotoxigenic (ETEC), enteropathogenic (EPEC), and enteroaggregative (EAEC) Escherichia coli was determined by using specific DNA probes as described previously (1, 16). From children who developed PD, second samples of stool were collected at 15 to 18 days after the onset of diarrhea and examined for enteropathogens including RV and diarrheagenic E. coli as described for the first sample. The study was approved by the Ethical Review Committee of ICDDR,B.
For the analysis, among 149 diarrheal children enrolled, only those with RV infection alone on enrollment without copathogens were included. Children were clinically evaluated by their medical history, daily physical examination, and laboratory investigations, which included determination of total and differential counts of leukocytes and serum electrolyte levels as indicated. All children were managed with appropriate fluid replacement therapy. Some children received antibiotics in the hospital if they had concurrent infections such as respiratory tract infection. All samples were collected before antibiotic treatment was started.
Collection and storage of samples.
Samples of stool and venous blood (7 ml) were collected from all diarrheal children on enrollment, i.e., at 6 to 8 days after onset of diarrhea. Four milliliters of blood was collected aseptically in sterile heparinized Vacutainer tubes (Becton Dickinson, Rutherford, N.J.), 2 ml of blood was collected in EDTA-containing Vacutainer tubes (Becton Dickinson), and 1 ml of blood was collected in sterile glass vials. Fresh blood collected in heparinized Vacutainer tubes was separated on Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) by centrifugation at 500 × g for 25 min. Peripheral blood mononuclear cells (PBMs), which formed a band at the interface, were collected, washed, and counted. Separation of plasma (for estimation of cytokines) and serum from fresh blood collected in EDTA-containing Vacutainer tubes and glass vials, respectively, was done by centrifugation for 10 min. Plasma and serum were stored in aliquots at −70 and −20°C, respectively, until assayed. Neat stool was stored at −20°C for RV typing. Stool extracts, for the estimation of cytokines, were prepared and stored as described before (2).
Assays to determine general immune response.
The proliferative response of PBMs was assessed on resting cells by measuring spontaneous DNA synthesis as described previously (3). Results were expressed as counts per minute.
Delayed-type hypersensitivity (DTH) responses were measured by skin tests with the Multitest CMI kit (Pasteur MÉRIEUX, Lyon, France) whereby seven antigens and a glycerin control solution were injected into the skin of the paravertebral area of the back of each child and the induration measured at the end of 48 h. An induration of 2 mm or more was considered to be a positive response. The antigens present in the kit include tetanus (550,000 Mérieux units/ml), diphtheria (1,100,000 Mérieux units/ml), Streptococcus sp. (group C) (2,000 Mérieux units/ml), tuberculin (300,000 IU/ml), Candida albicans (2,000 Mérieux units/ml), Trichophyton mentagrophytes (150 Mérieux units/ml), and Proteus mirabilis (150 Mérieux units/ml). The test was done only on those children who had been immunized against diphtheria, tetanus, and tuberculosis.
Laboratory assays to determine nutritional status.
Transferrin was measured in plasma by turbidimetry in a discrete analyzer (COBAS-BIO, Roche) using a transferrin kit (Boehringer Mannheim, Mannheim, Germany). Results were expressed as milligrams per deciliter and calculated by interpolation from a standard curve by using human serum standards ranging from 59 to 426 mg/dl (Boehringer Mannheim). Controls used included a serum protein, which was provided in the kit, and in addition, samples were arbitrarily spiked with commercially available serum of known transferrin concentration. The coefficient of variation was 4 to 8%.
Albumin was measured in serum with a photometric calorimetric test kit (ALBUMIN liquicolor; Human Diagnostics, Wiesbaden, Germany) and results expressed as grams per liter.
Determination of RV-specific IgA and IgG.
Flat-bottomed 96-well microtiter plates (MAXISORP, Nunc, Roskilde, Denmark) were coated with rabbit anti-RV serum diluted 1:5,000 in carbonate buffer (pH 9.6) for 3 h at 37°C. After washing, simian RV (SA11), as a standardized dilution (1:1) of the culture supernatant from MA104 cells, in 1% skimmed milk powder (SMP) in phosphate-buffered saline (PBS, pH 7.2)-Tween (PBST) was added, and the mixture was incubated overnight at 4°C. After washing and blocking with 1% SMP-PBST for 1 h at 37°C, samples of stool or plasma, diluted 1:100 in 1% SMP-PBST, were added in threefold dilutions and incubated for 1 h at 37°C. After washing, 100 μl of goat anti-human IgA or IgG conjugated to horseradish peroxidase (Jackson ImmunoResearch, West Grove, Pa.) diluted 1:500 or 1:4,000, respectively, in 1% SMP-PBST was added to each well for 1 h at 37°C. After washing, 100 μl/well of the substrate 3,3′,5,5′,-teramethyl benzidine (TMB; Sigma Chemical Co., St. Louis, Mo.) was added for 10 min, after which point the reaction was stopped with 1 M sulfuric acid. The optical density was read at 450 nm in a spectrophotometer (Titertek Multiskan Plus). Background wells included those where MA104 cells were added instead of SA11 and those where no samples were added. A positive control sample of pooled sera of known titer of RV-specific IgA or IgG was always included. Endpoint titers were calculated as a reciprocal of the interpolated dilution of the sample giving an optical density 0.1 above the background. Calculations were carried out by using a computer-based program (Multi; Data Tree Inc., Wathams, Mass.). Results were expressed as specific titer of IgA or IgG.
Determination of cytokine levels.
IL-10 was measured in plasma and fecal extracts with an ELISA kit (Endogen Inc., Boston, Mass.) which was capable of detecting <3 pg of human IL-10 per ml of sample. Assays were done in duplicate. Concentrations were calculated by interpolation from a standard curve and were expressed as picograms per milliliter of plasma or picograms per gram of stool.
TNF-α was measured by using the DuoSet ELISA Development System (Genzyme Diagnostics, Cambridge, Mass.), and the lowest detection limit was 5.8 pg of TNF-α per ml of sample. Concentrations were calculated by interpolation from a standard curve and were expressed as picograms per milliliter of plasma or picograms per gram of stool.
IFN-γ was measured with an ELISA as described earlier (26), and the detection limit for IFN-γ was 110 pg/ml. Briefly, flat-bottomed 96-well microtiter plates (MAXISORP) were coated overnight at 4°C with a monoclonal antibody (MAb) to human IFN-γ (Chromogenix AB, Molndal, Sweden) at a concentration of 2 μg/ml in PBS. After washing and blocking, undiluted samples and standard recombinant IFN-γ (R&D, Abingdon, United Kingdom) were added at doubling dilutions from 6,000 to 187.5 pg/ml in PBST containing 0.1% bovine serum albumin (BSA) (Sigma). Standards and samples were added in duplicate, and the mixtures were incubated overnight at 4°C. After washing, biotinylated MAb to human IFN-γ (Chromogenix) was added at 1:200 in PBST containing 0.1% BSA for 3 h at room temperature. ExtrAvidin horseradish peroxidase (Sigma) was then added to each well, and the mixtures were incubated at room temperature for 1 h. The substrate, ortho-phenylenediamine dihydrochloride (OPD) (Sigma) at a concentration of 1 mg/ml in 0.1 M citrate buffer (pH 4.5)-0.01% hydrogen peroxide (Sigma), was added for 20 min. The optical density was then measured at 450 nm in a spectrophotometer (Titertek Multiskan Plus). Positive and negative control samples were included in all assays and consisted of pooled supernatants from PBMs collected from healthy individuals and stimulated with phytohemagglutinin (Murex Diagnostics Ltd., Dartford, United Kingdom) (for positive control) or from unstimulated PBMs (for negative control) of known IFN-γ concentration. Concentrations were calculated by interpolation from a standard curve and were expressed as picograms per milliliter of plasma or picograms per gram of stool.
Statistical analysis.
Comparisons among the three groups of children were done with the Kruskal-Wallis test (for nonparametric data) or one-way analysis of variance (for parametric data). Comparisons between two groups were done by using the Mann-Whitney U test (for nonparametric data) or the t test (for parametric data). For comparisons between proportions, the chi-square statistic was used. Multiple regression analysis was carried out to determine the effects of age, nutritional status, sex, and concomitant infection on plasma levels of IFN-γ. Differences were considered to be significant when P was ≤0.05. Data analyses were carried out by using the Statistical Package for Social Sciences (version 7.5 for Windows; SPSS Inc., Chicago, Ill.).
RESULTS
Patient characteristics.
A total of 149 children with watery diarrhea were enrolled, of whom 108 had AD and 41 developed PD. Of these children, 29 were included who had diarrhea due to RV alone, without other enteropathogens. Of the 29 children with RV infection, 19 had AD and 10 developed PD. The clinical characteristics of the children are shown in Table 1. Children who developed PD were younger than uninfected children (P = 0.008) but were similar in age to children with AD. The frequency of passage of stool and vomiting on enrollment (Table 1) and the extent of dehydration (data not shown) were similar in children with AD and in those who developed PD. From children who developed PD, stool samples were collected again approximately 1 week later, i.e., when they developed PD, and tested for the presence of enteropathogens. Out of these 10 children with PD, RV was detected in 1, EPEC alone was detected in 1, EPEC with Aeromonas sp. was detected in 1, ETEC was detected in 2, EAEC was detected in 2 and Salmonella sp. was detected in 1. No pathogens were detected in two children. The clinical course of the 10 children who developed PD was varied, and after enrollment, they were hospitalized for a median of 13.5 days (range, 8 to 29 days).
TABLE 1.
Clinical features and nutritional status of children on enrollment
| Study group (n) | No. male (%) | Age (mo)a | Frequency of stool/24 ha | Frequency of vomiting/24 ha | No. with lower respiratory tract infection (%) | Weight for age as % of NCHSb median (mean ± SD) | Mean (±SD) serum albumin concn (g/liter) | Plasma transferrin concna (mg/dl) |
|---|---|---|---|---|---|---|---|---|
| Uninfected children (13) | 7 (53.8) | 12.0 (10.5–16.5) | NAc | NA | NA | 72.7 ± 11.4 | 46.2 ± 3.2 | 280.3 (221.6–341.2) |
| Children with AD (19) | 11 (57.9) | 10.0 (8.0–12.0) | 15.0 (9.0–20.0) | 2 (0–5) | 4 (21) | 73.6 ± 10.8 | 43.6 ± 3.8 | 282.7 (211.1–430.7) |
| Children who developed PD (10) | 5 (50.0) | 9.0 (7.0–10.3) | 13.0 (12.0–22.5) | 3 (0–4) | 3 (30) | 77.1 ± 7.9 | 43.7 ± 5.1 | 254.5 (180.0–329.3) |
| Pd | NSe | 0.024f | NS | NS | NS | NS | NS | NS |
Values are medians (25th to 75th quartiles).
National Centre for Health Statistics.
Not applicable.
Comparisons among the three groups were done by using one-way analysis of variance (continuous, parametric data) and the Kruskal-Wallis test (continuous, nonparametric data). Comparisons between two groups were done by using the Mann-Whitney U test; categorical data were compared by using the chi-square statistic.
Not significant.
Children who developed PD were significantly younger than uninfected children (P = 0.008).
The optical density readings of the stool samples used for the detection of RV in ELISA were used as an indirect measure of the viral load. On enrollment, there was no statistically significant difference between the optical densities for children with AD (median optical density, 0.609; 25th to 75th quartiles, 0.257 to 1.020) and those who developed PD (median optical density, 0.337; 25th to 75th quartiles, 0.167 to 0.722).
Nutritional status.
The nutritional statuses (Table 1), as determined by weight-for-age as a percentage of the National Center for Health Statistics median and serum albumin and plasma transferrin concentrations, were similar in all three groups of children.
General immune response.
Spontaneous proliferation was significantly different among the three groups of children (P = 0.013). PBMs from children with AD showed significantly higher spontaneous proliferation (median, 3,785 cpm; 25th to 75th quartiles, 1,627 to 5,767 cpm) than those from uninfected children (median, 984 cpm; 25th to 75th quartiles, 517 to 2,256 cpm; P = 0.005). PBMs from children who developed PD had a proliferative response (median, 2,364 cpm; 25th to 75th quartiles, 1,062 to 3,298 cpm) that was similar to those with AD and those of uninfected children. DTH responses were compared on the basis of the number of children who had a positive response to at least one antigen. The three groups of children were similar with at least one antigen positive in 11 of 12 uninfected children, 13 of 17 children with AD, and in 5 of 7 children who developed PD.
RV-specific antibodies in plasma and stool.
Titers of RV-specific IgA in the plasma and stool were similar in the three groups of children (Table 2). RV-specific IgG titers (Table 2) were significantly different among the three groups of children (P = 0.002) with titers being higher in uninfected children than in those with AD (P < 0.001) or those who developed PD (P = 0.024); titers were similar in children with AD and those who developed PD.
TABLE 2.
RV-specific antibodies in stool and plasma of children on enrollmenta
| Study group | Titer of RV-specific IgA in:
|
Titer of RV-specific IgG in plasma | |
|---|---|---|---|
| Stool | Plasma | ||
| Uninfected children | 406 (329–845) (n = 12) | 1,048 (229–1,546) (n = 11) | 25,954 (5,041–41,199) (n = 9) |
| Children with AD | 690 (171–1,442) (n = 19) | 461 (222–670) (n = 19) | 265 (143–683) (n = 18) |
| Children who developed PD | 130 (59–401) (n = 10) | 213 (77–520) (n = 9) | 840 (153–5,249) (n = 9) |
Values are medians (25th to 75th quartiles). There were no significant differences in RV-specific IgA titers among the three groups. Significant differences in RV-specific IgG titers in plasma among the three groups were as follows. For the control versus the AD and PD groups, P = 0.002. For the control versus the AD group, P < 0.001. For the control versus the PD group, P = 0.024. The Kruskal-Wallis test was used for comparisons among all three groups; the Mann-Whitney U test was used for comparisons between two groups.
Cytokine levels in plasma and stool.
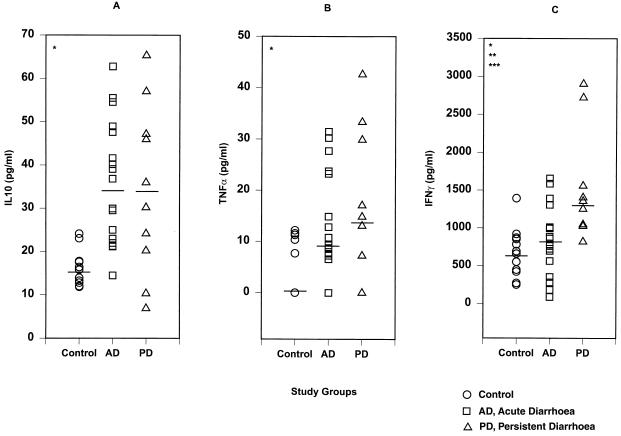
On enrollment, plasma levels of IL-10 (Fig. 1A), TNF-α (Fig. 1B), and IFN-γ (Fig. 1C) were significantly different among the three groups of children (P = 0.001, 0.021, and 0.001, respectively). Compared to uninfected children, children with RV infection, whether with AD or PD, had higher plasma levels of IL-10 (P < 0.001 and 0.026, respectively) and TNF-α (P = 0.022 and 0.013, respectively). IL-10 and TNF-α levels in the plasma were similar in children with AD and those who developed PD. IFN-γ levels in the plasma (Fig. 1C) were higher in children who developed PD than in uninfected children (P = 0.001) or those with AD (P = 0.008); there was no difference in levels between children with AD and uninfected controls. In order to assess whether differences in plasma IFN-γ levels were significant due to higher values in two children who developed PD (Fig. 1C), comparisons were repeated without these two outliers. Such comparisons showed that significant differences in IFN-γ levels in the plasma remained when the three groups were compared (P = 0.008) and when children with AD and those who developed PD were compared (median, 1,151 pg/ml; 25th to 75th quartiles, 1,032 to 1393 pg/ml; P = 0.038).
FIG. 1.
Levels of IL-10 (A), TNF-α (B), and IFN-γ (C) in the plasma of uninfected control children, children with acute rotavirus diarrhea (AD) and children who developed persistent diarrhea from rotavirus infection (PD). For diarrheal children (AD or PD) samples were collected at 6 to 8 days after the onset of diarrhea. Each symbol represents a single child, and the horizontal bars are median values. P values are given only where differences are statistically significant. ∗, Comparison of controls, children with AD, and those who developed PD. For IL-10 P = 0.001, for TNF-α P = 0.021, and for IFN-γ, P = 0.001. ∗∗, Comparison of controls and children who developed PD. P = 0.001. ∗∗∗, Comparison of children with AD and those who developed PD. P = 0.008.
In the stools, IL-10 and IFN-γ were not detectable in the three groups of children. Although TNF-α was detectable in the stools, levels were similar in the three groups of children (data not shown).
Effects of other factors on IFN-γ levels in the plasma.
As other factors such as age, nutritional status, sex, and concomitant infections could have an influence on IFN-γ levels, multiple regression analysis was carried out to assess their effects on plasma IFN-γ levels. None of the variables tested had a significant effect.
G and P types of RV strains.
Stool samples from 19 children with diarrhea (AD, n = 13; PD, n = 6) were available for typing RV strains. No PAGE pattern was obtained from nine samples, suggestive of a low concentration of virus particles in stool, and these samples were, therefore, not typed. RV strains from seven children with AD and three children who developed PD were typed, and the results are shown in Table 3. The overall frequency of the strain types observed is similar to that found in the community (32), and there were too few strains to ascertain differences between children with AD and those who developed PD.
TABLE 3.
Rotavirus G and P types in children with diarrhea
| Patient status | G type | P type |
|---|---|---|
| AD | 2 | 0a |
| AD | 4 | 0a |
| AD | 4 | 8 + 4 |
| AD | 1 | 4 |
| AD | 1 | 4 |
| AD | 4 | 8 |
| AD | 0a | 8 |
| PD | 4 | 0a |
| PD | 2 + 4 | 4 |
| PD | 0a | 4 |
Not typeable.
DISCUSSION
The immune response in PD due to a defined etiology has not been investigated before. For this purpose, children with a 6- to 8-day history of diarrhea from RV infection were studied. The high percentage of PD from an initial RV infection observed here (10 of 29) could be because children who already had prolonged RV infection were selected. Children with a diarrhea duration of 6 to 8 days were chosen on the basis that a reasonable number would develop PD. Enrollment of children at a more acute stage of diarrhea would result in too large a sample size for the study to be manageable.
Of the 10 children who developed PD, only one was found to have persistent RV infection when second samples of stools were tested for enteropathogens at 15 to 18 days after the onset of diarrhea. This data suggests that PD from an initial RV infection is not due to persistence of RV but to an alteration in some host factor(s) which makes the children more susceptible to other infections or which leads to prolonged malabsorption. Other studies have also shown that PD is often due to sequential infection by different organisms rather than by a single organism (10). Our finding that children with AD and those who developed PD cannot be differentiated clinically during the acute stage of illness corroborates previous findings, which showed that clinical characteristics have a low positive predictive value for the development of PD (22).
In contrast to earlier studies (4, 9, 20) we found that the general immune responses of children were not lower in those who developed PD. Comparisons between the present study and previous studies on the role of the immune response in the development of PD is difficult as our study was conducted with children who already had diarrhea for 6 to 8 days while previous studies were conducted with children prior to the development of diarrhea. In this study, children who developed PD were not significantly malnourished, which is in contrast to an earlier study where malnutrition during AD was found to be a risk factor for the development of PD (23). It is possible that when specific etiologies are considered, the overall risk factors are different. Thus, it has been shown that RV infection occurs more commonly in better-nourished children (31) so that PD from RV infection also may not be related to malnutrition.
The role of RV-specific antibodies in protection against RV infection and illness is controversial (6, 8, 12, 14, 18, 24, 33). RV-specific IgG in serum correlates negatively with illness from RV (14), and this corroborates our finding of higher titers of RV-specific IgG in the plasma of uninfected children than in diarrheal children (both AD and PD). The results from this study suggest that RV-specific IgG or IgA has no role in the development of PD following an initial RV infection. However, a larger sample size is required to confirm this.
The role of IL-10 in RV infection has not been investigated, and the significance of our finding that IL-10 levels in the plasma are increased in children with RV infection (whether AD or PD) is not clear. As IL-10 promotes B-cell proliferation and IgA secretion (25) we hypothesized that in RV infection IL-10 could have a beneficial effect by stimulating the secretion of RV-specific IgA. However, as IL-10 levels were not different between children with AD and those who developed PD, it is unlikely that IL-10 is important in protection against prolonged diarrhea or as a marker of disease severity. TNF-α is an antiviral cytokine (27). The increased levels observed during the first week of RV infection (whether in AD or PD) may reflect antiviral activity. However, as there is no association between the development of PD and plasma levels of TNF-α, the relevance of TNF-α levels in plasma to the resolution of diarrhea from RV remains unclear.
IFN-γ is produced by human PBMs stimulated with RV (35), and IFN-γ can inhibit entry of RV into cultured epithelial cells (5). In contrast, clearance of RV by CD8+ T cells appears not to be dependent on IFN-γ (17). Thus the role of IFN-γ in RV infection is not clear. In the present study, children with AD and uninfected children had similar levels of IFN-γ, while children who developed PD had higher plasma IFN-γ levels than those with AD or uninfected children. The implications of these findings are not clear but they suggest that (i) IFN-γ has no role in protection against prolonged diarrhea following RV infection, (ii) IFN-γ is a correlate of a more serious pathology, and/or (iii) elevated levels of IFN-γ during the acute stage of RV infection are detrimental. IFN-γ can stimulate the immune response such that it leads to lysis of bystander cells, thereby causing more tissue damage (29). Furthermore, products of IFN-γ-induced cell lysis may cause inflammation of tissue, as has been shown for lymphocytic choriomeningitis virus infection (29). As in this study PD following an initial RV infection was not due to persistence of RV, it is possible that another factor, such as enhanced inflammation during the acute stage of the infection, could have led to prolonged diarrhea. However, we have no evidence to support this hypothesis.
In summary, the results of the present study show that during acute RV infection, IL-10 and TNF-α are elevated in the plasma and the development of PD is associated with a higher IFN-γ response. However, a larger sample size is required to confirm these trends.
ACKNOWLEDGMENTS
This research was supported by the ICDDR,B Centre for Health and Population Research and USAID under cooperative agreement #DPE-5986-A-00-1009-00.
REFERENCES
- 1.Albert M J, Faruque S M, Faruque A S G, Neogi P K B, Ansaruzzaman M, Bhuiyan N A, Alam K, Akbar M S. A controlled study of Escherichia coli diarrheal infections in Bangladeshi children. J Clin Microbiol. 1995;33:973–977. doi: 10.1128/jcm.33.4.973-977.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azim T, Halder R C, Sarker M S, Ahmed S, Hamadani J, Chowdhury A, Qadri F, Salam M A, Sack R B, Albert M J. Cytokines in the stools of children with complicated shigellosis. Clin Diagn Lab Immunol. 1995;2:492–495. doi: 10.1128/cdli.2.4.492-495.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azim T, Sarker M S, Hamadani J, Khanum N, Halder R C, Salam M A, Albert M J. Alterations in lymphocyte phenotype and function in children who develop complications in shigellosis. Clin Diagn Lab Immunol. 1996;3:191–196. doi: 10.1128/cdli.3.2.191-196.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baqui A H, Black R E, Sack R B, Chowdhury H R, Yunus M, Siddique A K. Malnutrition, cell mediated immune deficiency, and diarrhea: a community-based longitudinal study in rural Bangladeshi children. Am J Epidemiol. 1993;137:355–365. doi: 10.1093/oxfordjournals.aje.a116682. [DOI] [PubMed] [Google Scholar]
- 5.Bass D M. Interferon gamma and interleukin 1, but not interferon alfa, inhibit rotavirus entry into human intestinal cell lines. Gastroenterology. 1997;113:81–89. doi: 10.1016/S0016-5085(97)70083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernstein D I, Sander D S, Smith V E, Gilbert M S, Ward R L. Protection from rotavirus reinfection: a 2 year prospective study. J Infect Dis. 1991;164:277–283. doi: 10.1093/infdis/164.2.277. [DOI] [PubMed] [Google Scholar]
- 7.Bhardwaj A, Aggarwal V, Chakravarty A, Mittal S K. Does rotavirus infection cause persistent diarrhoea in childhood? Trop Gastroenterol. 1996;17:18–21. [PubMed] [Google Scholar]
- 8.Black R E, Greenberg H B, Kapikian A Z, Brown K H, Becker S. Acquisition of serum antibody to Norwalk virus and rotavirus and relation to diarrhea in a longitudinal study of young children in rural Bangladesh. J Infect Dis. 1982;145:483–489. doi: 10.1093/infdis/145.4.483. [DOI] [PubMed] [Google Scholar]
- 9.Black R E, Lanata C F, Lazo F. Delayed cutaneous hypersensitivity: epidemiologic factors affecting and usefulness in predicting diarrheal incidence in young Peruvian children. Pediatr Infect Dis J. 1989;8:210–215. [PubMed] [Google Scholar]
- 10.Black R E. Persistent diarrhea in children of developing countries. Pediatr Infect Dis J. 1993;12:751–761. doi: 10.1097/00006454-199309000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Casola A, Estes M K, Crawford S E, Ogra P L, Ernst P B, Garofalo R P, Crowe S E. Rotavirus infection of cultured intestinal epithelial cells induces secretion of CXC and CC chemokines. Gastroenterology. 1998;114:947–955. doi: 10.1016/s0016-5085(98)70314-2. [DOI] [PubMed] [Google Scholar]
- 12.Chiba S, Yokoyama T, Nakata S, Morita Y, Urasawa T, Taniguchi K, Urasawa S, Nakao T. Protective effect of naturally acquired homotypic and heterotypic rotavirus antibodies. Lancet. 1986;ii:417–421. doi: 10.1016/s0140-6736(86)92133-1. [DOI] [PubMed] [Google Scholar]
- 13.Child Health Research Project. Synopsis: persistent diarrhea algorithm, no. 1. Washington, D.C: U.S. AID; 1997. [Google Scholar]
- 14.Clemens J D, Ward R L, Rao M R, Sack D A, Knowlton D R, van Loon F P L, Huda S, McNeal M, Ahmed F, Schiff G. Seroepidemiologic evaluation of antibodies to rotavirus as correlates of the risk of clinically significant rotavirus diarrhea in rural Bangladesh. J Infect Dis. 1992;165:161–165. doi: 10.1093/infdis/165.1.161. [DOI] [PubMed] [Google Scholar]
- 15.De Boissieu D, Lebon P, Badoual J, Bompard Y, Dupont C. Rotavirus induces α-interferon release in children with gastroenteritis. J Pediatr Gastroenterol Nutr. 1992;16:29–32. doi: 10.1097/00005176-199301000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Faruque S M, Haider K, Albert M J, Ahmad Q S, Nahar S, Tzipori S. A comparative study of specific gene probes and standard bioassays to identify diarrhoeagenic Escherichia coli in paediatric patients with diarrhoea in Bangladesh. J Med Microbiol. 1992;36:37–40. doi: 10.1099/00222615-36-1-37. [DOI] [PubMed] [Google Scholar]
- 17.Franco M A, Tin C, Rott L S, VanCott J L, McGhee J R, Greenberg H B. Evidence for CD8+ T-cell immunity to murine rotavirus in the absence of perforin, fas, and gamma interferon. J Virol. 1997;71:479–486. doi: 10.1128/jvi.71.1.479-486.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kapikian A Z, Wyatt R G, Levine M M, Yolken R H, VanKirk D H, Dolin R, Greenberg H B, Chanock R M. Oral administration of human rotavirus to volunteers: induction of illness and correlates of resistance. J Infect Dis. 1983;147:95–106. doi: 10.1093/infdis/147.1.95. [DOI] [PubMed] [Google Scholar]
- 19.Khoshoo V, Bhan M K, Jayashree S, Kumar R. Rotavirus infection and persistent diarrhoea in young children. Lancet. 1990;336:1314–1315. doi: 10.1016/0140-6736(90)92995-t. [DOI] [PubMed] [Google Scholar]
- 20.Koster F T, Palmer D L, Chakraborty J, Jackson T, Curlin G C. Cellular immune competence and diarrheal morbidity in malnourished Bangladeshi children: a prospective field study. Am J Clin Nutr. 1987;46:115–120. doi: 10.1093/ajcn/46.1.115. [DOI] [PubMed] [Google Scholar]
- 21.Kutukeuler N, Caglayan S. Tumor necrosis factor-α and interleukin-6 in stools of children with bacterial and viral gastroenteritis. J Pediatr Gastroenterol Nutr. 1997;25:556–558. doi: 10.1097/00005176-199711000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Lanata C F, Black R E, Gilman R H, Lazo F, Aguila R D. Epidemiologic, clinical, and laboratory characteristics of acute vs. persistent diarrhea in periurban Lima, Peru. J Pediatr Gastroenterol Nutr. 1991;12:82–88. doi: 10.1097/00005176-199101000-00017. [DOI] [PubMed] [Google Scholar]
- 23.Mahalanabis D, Alam A N, Rahman N, Hasnat A. Prognostic indicators and risk factors for increased duration of acute diarrhoea and for persistent diarrhoea in children. Int J Epidemiol. 1991;20:1064–1072. doi: 10.1093/ije/20.4.1064. [DOI] [PubMed] [Google Scholar]
- 24.Matson D O, O’Ryan M L, Herrera I, Pickering L K, Estes M K. Fecal antibody responses to symptomatic and asymptomatic rotavirus infections. J Infect Dis. 1993;167:577–583. doi: 10.1093/infdis/167.3.577. [DOI] [PubMed] [Google Scholar]
- 25.Moore K W, O’Garra A, Malefyt R de W, Viera P, Mosmann T R. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 26.Quiding M, Nordstrom I, Kilander A, Andersson G, Hanson L A, Holmgren J, Czerkinsky C. Intestinal immune responses in humans. Oral cholera vaccination induces strong intestinal antibody responses and interferon-γ production and evokes local immunological memory. J Clin Investig. 1991;88:143–148. doi: 10.1172/JCI115270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramsay A J, Ruby J, Ramshaw I A. A case for cytokines as effector molecules in the resolution of virus infection. Immunol Today. 1993;14:155–157. doi: 10.1016/0167-5699(93)90277-R. [DOI] [PubMed] [Google Scholar]
- 28.Saulsbury F T, Winkelstein J A, Yolken R H. Chronic rotavirus infection in immunodeficiency. J Pediatr. 1980;97:61–65. doi: 10.1016/s0022-3476(80)80131-4. [DOI] [PubMed] [Google Scholar]
- 29.Smith M J, Trapani J A. The relative role of lymphocyte granule exocytosis versus death receptor-mediated cytotoxicity in viral pathophysiology. J Virol. 1998;72:1–9. doi: 10.1128/jvi.72.1.1-9.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Unicomb L E, Bignan F, Rahim Z, Banu N N, Gomes J G, Podder G, Munshi M H, Tzipori S R. A one-year survey of rotavirus strains from three locations in Bangladesh. Arch Virol. 1993;132:201–208. doi: 10.1007/BF01309854. [DOI] [PubMed] [Google Scholar]
- 31.Unicomb L E, Kilgore P E, Faruque A S G, Hamadani J D, Fuchs G J, Albert M J, Glass R I. Anticipating rotavirus vaccines: hospital-based surveillance for rotavirus diarrhea and estimates of disease burden in Bangladesh. Pediatr Infect Dis J. 1997;16:947–951. doi: 10.1097/00006454-199710000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Unicomb L E, Podder G, Gentsch J R, Hasan K Z, Faruque A S G, Albert M J, Glass R I. Evidence of high-frequency genomic reassortment of group A rotavirus strains in Bangladesh: emergence of type G9 in 1995. J Clin Microbiol. 1999;37:1885–1891. doi: 10.1128/jcm.37.6.1885-1891.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ward R L, Bernstein D I, Shukla R, Young E C, Sherwood J R, McNeal M M, Walker M C, Schiff G M. Effects of antibody to rotavirus on protection of adults challenged with a human rotavirus. J Infect Dis. 1989;159:79–88. doi: 10.1093/infdis/159.1.79. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. Manual for laboratory investigations of acute enteric infections. Geneva, Switzerland: World Health Organization; 1987. pp. 9–20. [Google Scholar]
- 35.Yasukawa M, Nakagomi O, Kobayashi Y. Rotavirus induces proliferative response and augments non-specific cytotoxic activity of lymphocytes in humans. Clin Exp Immunol. 1990;80:49–55. doi: 10.1111/j.1365-2249.1990.tb06440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]