Abstract
Allergen immunotherapy is a form of therapeutic vaccination for established IgE-mediated hypersensitivity to common allergen sources such as pollens, house dust mites and the venom of stinging insects. The classical protocol, introduced in 1911, involves repeated subcutaneous injection of increasing amounts of allergen extract, followed by maintenance injections over a period of 3 years, achieving a form of allergen-specific tolerance that provides clinical benefit for years after its discontinuation. More recently, administration through the sublingual route has emerged as an effective, safe alternative. Oral immunotherapy for peanut allergy induces effective ‘desensitization’ but not long-term tolerance. Research and clinical trials over the past few decades have elucidated the mechanisms underlying immunotherapy-induced tolerance, involving a reduction of allergen-specific T helper 2 (TH2) cells, an induction of regulatory T and B cells, and production of IgG and IgA ‘blocking’ antibodies. To better harness these mechanisms, novel strategies are being explored to achieve safer, effective, more convenient regimens and more durable long-term tolerance; these include alternative routes for current immunotherapy approaches, novel adjuvants, use of recombinant allergens (including hypoallergenic variants) and combination of allergens with immune modifiers or monoclonal antibodies targeting the TH2 cell pathway.
Subject terms: Applied immunology, Translational research
Durham and Shamji review the history and future of allergen immunotherapy for established IgE-mediated hypersensitivity to common allergens. They describe the mechanisms of immunotherapy-induced tolerance and the new strategies being explored to achieve safer, more effective, long-term tolerance.
Introduction
Allergy is a growing problem that affects up to one in three of the population of westernized countries1. For example, the prevalence of physician-diagnosed allergic rhinitis has been estimated at 13% in children2 and 14% in adults3 in the USA and 23% in adults4 in Europe. Allergic rhinitis often has a major impact on sleep quality, work or school performance and leisure activities and is frequently associated with comorbid asthma5. General allergic reactions following Hymenoptera stings have been estimated to occur in 3.4% of children and 7.5% of adults in Europe6. Although diagnostic criteria vary, a convincing history of food allergy with or without a physician diagnosis was found in 8% of children in the USA, 2% of whom were allergic to peanut7. The associated risks of anaphylaxis and occasional fatalities have a major impact on well-being and quality of life for patients and their families. Allergen avoidance, although representing optimal management for allergic diseases, is not often feasible and symptomatic treatment with anti-allergic medication may only be partially effective.
Allergen immunotherapy involves the repeated administration of allergen extracts or products over several years8–10, and may offer a more permanent solution to drugs that only treat symptoms and not the underlying cause. Immunotherapy is offered to individuals who are atopic with IgE-mediated allergic rhinitis and/or allergic asthma due to inhaled allergens, such as pollens and house dust mites (HDMs), who have shown an inadequate response to anti-allergy drugs or who have experienced unacceptable drug side effects. Subcutaneous immunotherapy can be offered to patients who are at risk of anaphylaxis from insect stings6. Oral immunotherapy for allergy to foods has been an experimental approach11. However, for peanut allergy, there is now an approved oral peanut product for use in clinical practice12,13.
In this Perspective, we review the insights gained from past experiences in allergen immunotherapy into the mechanisms of allergic inflammation and immunotherapy-induced tolerance. We describe how current practice has evolved to include both subcutaneous and sublingual routes, and to establish safer and more convenient approaches and to improve patient adherence to immunotherapy. We describe the ways in which current approaches may be further improved in the future owing to advances in molecular allergology, alternative routes of allergen administration and the potential for effective combination of allergen immunotherapy with immune modifiers or monoclonal antibodies that target the allergy-associated T helper 2 (TH2) cell pathway.
Brief history of allergen immunotherapy
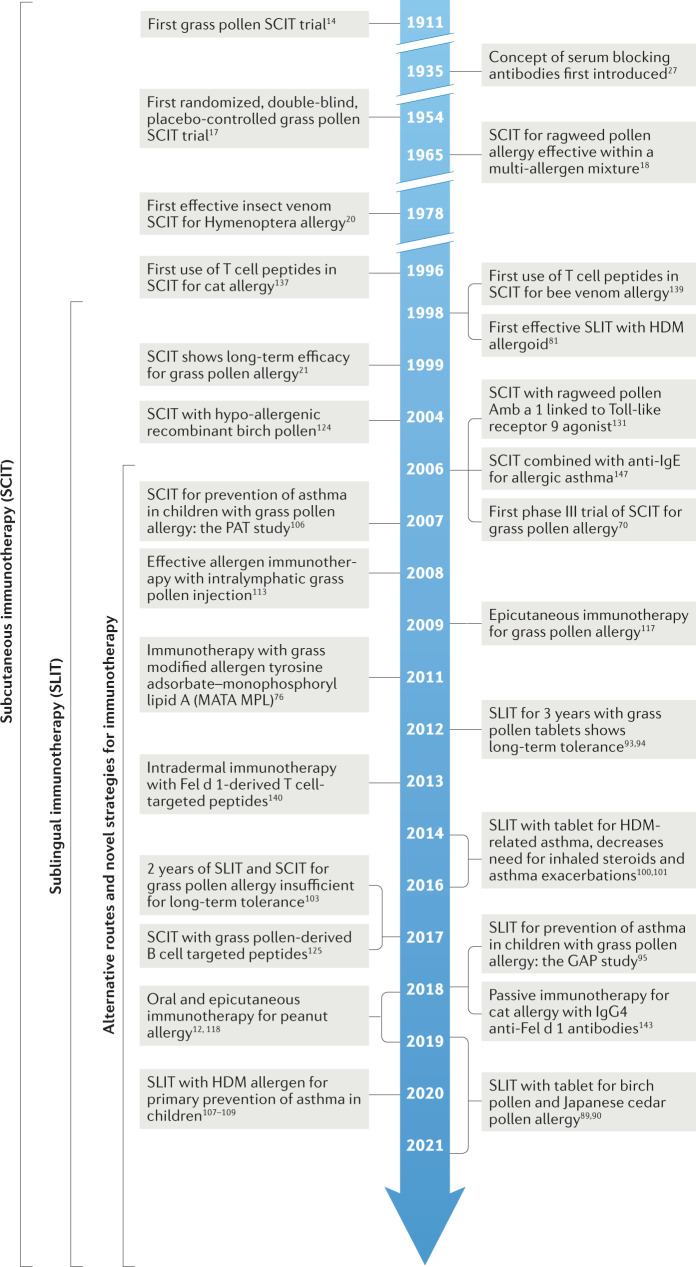
A timeline of key milestones in allergen immunotherapy is shown in Fig.1. In 1911, Leonard Noon was the first to show that repeated injections of crude grass pollen extract into individuals with hay fever reduced immediate conjunctival sensitivity to grass pollen14. Freeman reported that during the following pollen season, they had reduced symptoms of rhinitis and asthma15. Noon’s rationale is not clear, although grass pollen had been recognized as the causal agent in hay fever16 and the concept may have evolved from the parallel development of prophylactic vaccination using killed or modified causal organisms for the prevention of infectious diseases (see https://historyofvaccines.org/history/vaccine-timeline/timeline).
Fig. 1. Key milestones in allergen immunotherapy.
HDM, house dust mite.
In the first double-blind trial, Frankland in 1954 confirmed the efficacy of subcutaneous grass pollen injection therapy17 for seasonal asthma, and showed that the activity responsible for the effect was contained within the high molecular weight protein-containing component of the allergen extract rather than the eluted low molecular weight fractions. Lowell and Franklin18 subsequently demonstrated the efficacy of subcutaneous ragweed pollen extract contained within a multi-allergen mixture. In 1978, Norman and Lichtenstein were the first to demonstrate that allergen immunotherapy was allergen-specific, in that a ragweed pollen extract was effective at relieving symptoms in dual-IgE-sensitized individuals during the ragweed season, but not during the ensuing grass pollen season19. In the same year, Hunt et al. demonstrated the efficacy of subcutaneous purified Hymenoptera venom immunotherapy, in contrast to a whole insect body extract, when compared with placebo in patients with severe insect venom hypersensitivity20.
Remarkably, subcutaneous immunotherapy has changed little over the past 100 years, still involving weekly injections followed by monthly maintenance injections over several years. In 1998, a position paper by the World Health Organization (WHO) summarized the evidence for efficacy and identified the risks, particularly in individuals with uncontrolled asthma8. The report acknowledged progress in the use of more standardized allergen extracts, according to their purification and content of major allergens, and referred to emerging evidence for sublingual immunotherapy as a safer alternative route. This was later supported in a World Allergy Organization position paper on sublingual immunotherapy9. Venom immunotherapy has been established as a highly effective treatment in preventing anaphylaxis following insect stings6.
In 1999, it was reported that 3 years of continuous subcutaneous immunotherapy with grass pollen extract resulted in long-term benefits for 3 years after its discontinuation21. Reproduced on several occasions since22,23, this key observation confirmed the allergen specificity and long-term disease-modifying effects of allergen immunotherapy. Allergen immunotherapy has provided a unique human model to study immunological events underlying long-term antigen-specific tolerance. Randomized controlled trials that have confirmed long-term clinical efficacy after discontinuation are summarized in Supplementary Table S1.
Understanding immune tolerance by allergen immunotherapy
Mechanisms of immunotherapy for allergy to inhalant allergens
In individuals with atopic allergy, natural exposure to low concentrations of environmental allergens results in allergic inflammation involving IgE-mediated activation of mast cells and tissue eosinophilia, events under the regulation of TH2-type cytokines (Fig. 2). In 1921, Prausnitz and Kutsner24 were the first to demonstrate the passive transfer by a serum factor (referred to as ‘reagin’ and, subsequently in 1966, characterized as IgE25,26) of immediate cutaneous IgE sensitivity. In 1935 Cooke and colleagues showed that ‘protective immunity’ after allergen immunotherapy could also be transferred passively27. They showed that serum obtained after immunotherapy from individuals with ragweed pollen hay fever, when injected intradermally into sensitized untreated controls, could block the immediate cutaneous response to ragweed pollen. These two observations illustrated for the first time that hypersensitivity (allergy) and protective immunity (immunotherapy) were dependent on passively transferable serum factors that were subsequently identified as allergen-specific IgE and allergen-specific IgG/IgA-associated IgE-blocking activity, respectively.
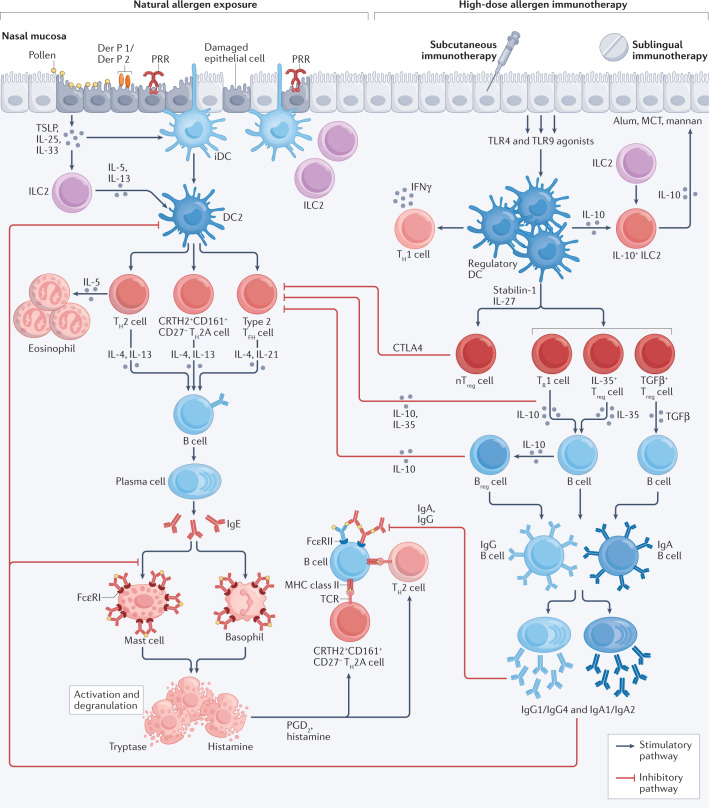
Fig. 2. Mechanisms of allergic inflammation and immunotherapy.
Allergic inflammation involves IgE-dependent activation of mast cells and local tissue eosinophilia159 under the regulation of T helper 2 (TH2)-type cytokines (IL-4, IL-5, IL-9 and IL-13). The innate immune system, including specialized subsets of dendritic cells28 and innate lymphoid cells (ILCs)47, has a role in both induction and regulation of allergen-induced TH2-type responses. These innate cells are under the influence of epithelial cell-derived cytokines including thymic stromal lymphopoietin (TSLP)160, IL-25 and IL-33. In individuals with atopic allergy, type 2 dendritic cells28 (DC2s) preferentially induce the differentiation of TH2 cells and a population of allergen-stimulated TH2 cells defined by high expression of CRTH2 and CD161 and low expression of CD27 (CRTH2+CD161+CD27–; referred to as TH2A cells). A further subset of cells expressing TH2-type cytokines are type 2 T follicular helper (TFH) cells161, which derive from the marginal zones of lymph nodes. Allergen–IgE cross-linking of adjacent high-affinity IgE receptor (FcεRI) receptors on mast cells releases granule-derived mediators, such as tryptase and histamine, and membrane-associated lipid mediators that include prostaglandin D2 (PGD2) and sulfidopeptide leukotrienes162. Allergen–IgE complexes also bind to surface low-affinity receptor for IgE (FcεRII) on B cells that results in IgE-facilitated TH2 cell development41,163. During subcutaneous immunotherapy and sublingual immunotherapy, high-dose allergen exposure restores regulatory dendritic cells28,29 that produce IL-10 (refs.30,31) and IL-12 (refs.32,46), inhibits TH2 cell responses104,164 and promotes regulatory T (Treg) cell30,34 and regulatory B (Breg) cell50,51 responses and immune deviation in favour of a TH1 cell response32,45. There is preferential B cell isotype switching towards IgG and IgA104,105, resulting in IgE-blocking activity41,163,165, which inhibits both IgE-mediated activation of mast cells and basophils and IgE-facilitated antigen presentation and TH2 cell responses44,163. Red arrows indicate suppressive activities of Treg cells and Breg cells, and IgG/IgA-associated IgE blocking activity. CTLA4, cytotoxic T lymphocyte antigen 4; iDC, immature dendritic cell; MCT, microcrystalline tyrosine; nTreg cell; thymus-derived natural regulatory T cell; PRR, pattern recognition receptor; TCR, T cell receptor; TGFβ, transforming growth factor-β; TLR, Toll-like receptor; TR1 cell, type 1 regulatory T cell (characterized by the co-expression of CD49b and LAG3) 166.
Administration of high doses of allergen either by injection or sublingually during immunotherapy results in the induction of dendritic cells with a pro-regulatory phenotype (tolerogenic dendritic cells)28,29. Within weeks, there is preferential induction of peripherally derived regulatory T (Treg) cells that express IL-10 (refs.30–32), transforming growth factor-β (TGFβ)33 and IL-35 (ref.34), and thymus-derived FOXP3+ Treg cells32. These cells are detectable in the circulation and nasal mucosa35,36 following pollen immunotherapy. Treg cells inhibit the differentiation of TH2 cells via regulatory cytokines as well as by cell–cell interactions37. IL-10 induces B cells to undergo isotype class switching in favour of allergen-specific IgG subclasses including IgG1 (ref.38) and IgG4 (ref.35), whereas TGFβ induces preferential switching of B cells to produce IgA39. These B cells differentiate into plasma cells that synthesize and release allergen-specific IgG1, IgG4, IgA1 or IgA2, which is detectable in the peripheral blood and nasal fluid following immunotherapy40. Allergen-specific IgG and IgA compete with IgE for allergen and directly inhibit the formation of allergen–IgE complexes41, although the blocking activity of IgA relative to IgG has recently been questioned42. These ‘blocking antibodies’ inhibit IgE-dependent activation of mast cells and basophils43. They also inhibit the IgE-facilitated presentation of allergen to low-affinity receptor for IgE (FcεRII)-expressing B cells and to high-affinity IgE receptor (FcεRI)-expressing dendritic cells, with a resulting decrease in TH2 cell development44 (Fig. 2).
Treg cells are induced by allergen-primed regulatory dendritic cells within weeks of starting allergen immunotherapy31,32. By contrast, high-dose allergen exposure, possibly via presentation by non-professional antigen presenting cells, initiates a more delayed immune deviation in favour of allergen-driven TH1 cell responses32,45 that has been detected at 12 months in the blood and target organs. By in situ hybridization of skin biopsies, local increases in IL-12-producing macrophages and interferon-γ-producing T cells following intradermal allergen challenge were accompanied by decreases in skin IL-4-producing T cells and improvement in clinical symptoms46.
Under the regulation of epithelial cytokines, group 2 innate lymphoid cells (ILC2s), although unable to respond directly to antigen, represent an alternative source of TH2-type cytokines to amplify and augment local allergic inflammation47. Grass pollen allergen immunotherapy has been shown to inhibit seasonal increases in ILC2s (ref.48) and to induce a phenotypically distinct subset of ILC2s that express surface KLRG1 and secrete IL-1049. Distinct subsets of regulatory B cells (Breg cells)50,51 and regulatory Treg cells52 also increase and provide further sources of IL-10 after immunotherapy.
Mechanisms of oral immunotherapy for peanut allergy
Compared with immunotherapy for inhalant allergy, immunotherapy for food allergy53 is less well studied, with the exception of peanut allergy for which there is now an approved oral product comprising 300 mg encapsulated whole peanut (AR101) administered daily (the PALISADE study)12. As for grass pollen immunotherapy, successful desensitization to peanut allergen was accompanied by a marked reduction in allergen-stimulated effector memory T cells with a TH2 cell phenotype (characterized as CRTH2+CD161+CD27– and referred to as TH2A cells)54,55. The mechanism of this decrease in peripheral TH2A cells is unknown. Whereas earlier studies of peanut oral immunotherapy showed transient increases in peripheral blood mononuclear cell IL-10 secretion and increases in CD25+FOXP3+ T cells56, this could represent memory effector T cell activation, and subsequent detailed flow cytometry analysis in the PALISADE study failed to show that marked decreases in allergen-stimulated TH2A cells were accompanied by increases in peripheral Treg cells55, although this does not exclude the possibility of local Treg cell induction in the gut. For example, in a mouse model of food allergy, the mucosal induction of FOXP3+ Treg cells depends on local CD103+ dendritic cell activation and involves retinoic acid pathways and secretion of TGFβ rather than IL-10 (ref.57). Individuals with peanut allergy have a higher level of allergen-stimulated basophil activation than IgE-sensitized but non-allergic controls55,58. A striking finding during peanut oral immunotherapy was an early decrease in basophil activation (as indicated by decreased surface CD203c levels) that was accompanied by transient increases in peanut-specific IgE and marked increases in the IgG4 to IgE ratio55 and a decrease in functional IgE-binding assays56.
In contrast to inhalant allergen immunotherapy, oral peanut immunotherapy has not shown convincing evidence of long-term efficacy13,53. Prolonged administration over 2 years in children and young adults (the POISED study59) and over 2.5 years in young children (aged 1–4 years, the IMPACT study60), using high maintenance doses (4,000 mg oral peanut daily), was highly effective in inducing desensitization, but sustained unresponsiveness was only observed at 3 years (12 and 6 months after discontinuation) in 13% and 21% of participants, respectively. However, those who did develop sustained unresponsiveness had lower baseline levels of peanut-specific IgE and of basophil activation, had higher specific IgG4 to IgE ratios and were younger (particularly when initiated in children younger than 1 year old) compared with those who did not. Possible explanations for these findings are the potency of peanut allergen in inducing multivalent and high-affinity IgE responses to conformational rather than linear epitopes61, accompanied by exuberant TH2A cell responses that recur rapidly after immunotherapy is discontinued, and/or absence of effective modulation by sustained antigen-specific Treg cell responses55.
Mechanisms of immunotherapy for allergy to venoms
The availability of purified venom allergens in the 1990s enabled the study of immunological mechanisms of venom allergy in the absence of lipopolysaccharide contamination of crude allergen extracts. Following successful subcutaneous immunotherapy with purified bee venom allergen, stimulation of peripheral blood mononuclear cells with phospholipase A2 (PLA2; a major bee venom allergen) demonstrated reduced allergen-specific T cell proliferation and TH2-type and TH1-type cytokine production62 and induction of IL-10-producing Treg cells30,63. With venom immunotherapy, a rapid desensitization has been shown to be effective and safe using accelerated subcutaneous immunotherapy protocols (‘rush’ and ‘ultra-rush’ protocols)64. A striking early event, within hours or days of immunotherapy administration, was the desensitization of basophils by immune silencing of FcεRI-activated basophils, mediated by histamine 2 receptor (HR2)65, resulting in inhibition of basophil mediator release including histamine, sulfidopeptide leukotrienes and cytokines. The differential regulation of histamine receptor subtypes on T cells was also shown to be important in the development of IL-10-producing Treg cells, with an increased HR2 to HR1 ratio favouring the development of IL-10-producing Treg cells66,67. Subsequent studies of venom immunotherapy highlighted a critical role for the induction of a distinct subset of PLA2-specific regulatory B (Breg) cells50 that produced both IL-10 and allergen-specific IgG4. Allergen-specific IgG4 and IgE-blocking activity was demonstrable in beekeepers exposed to repeated natural stings as well as during successful venom immunotherapy68,69. However, in contrast to immunotherapy with inhalant allergens, following withdrawal of venom immunotherapy there was persistent suppression of allergen-specific IgE responses in the absence of persisting IgG-associated IgE-blocking activity69, implying distinct mechanisms of long-term tolerance. Overall, these studies highlight the importance in venom immunotherapy of rapid early desensitization of basophils, differential expression of histamine receptors on effector cells and T cells and the role of Breg cells as an alternative source of IL-10 and IgG4. There is persistent IgE suppression, rather than a prolonged IgG-associated IgE-blocking activity, following discontinuation of venom immunotherapy68.
Insights from the past 20 years
Although regarded as the gold standard8,9,70, allergen immunotherapy given by subcutaneous injection requires specialist supervision owing to the risk of allergic side effects including anaphylaxis. In the USA, allergen extracts are prepared in 50% glycerin, which acts as a preservative and stabilizer, whereas in Europe extracts are alum-precipitated, which slows the release of allergen on injection and reduces immediate allergic side effects71. In recent years, there have been attempts to improve the safety and convenience for patients whilst retaining efficacy of allergen immunotherapy. These include the use of modified allergens (known as allergoids) and alternative routes of immunotherapy, as discussed below. Figure 3 summarizes the current and novel approaches for allergen immunotherapy.
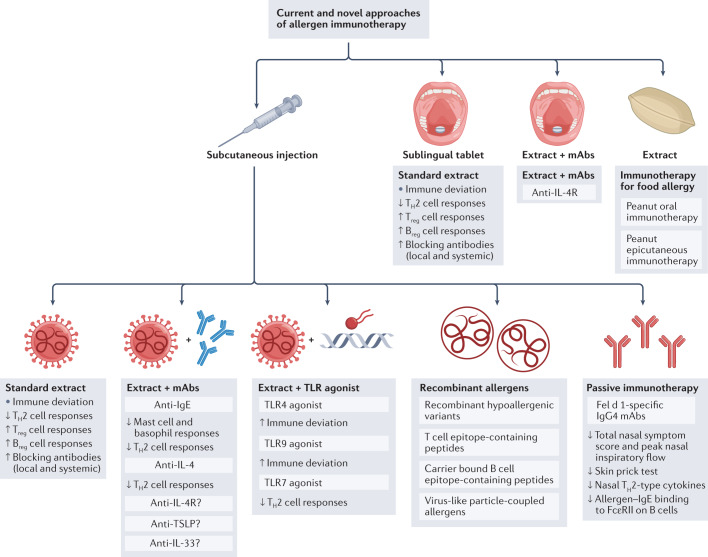
Fig. 3. Current and novel approaches of allergen immunotherapy.
At present, long-term tolerance for inhalant allergies may only be achieved by allergen immunotherapy with standardized whole allergen extracts via either subcutaneous or sublingual routes. To better harness the known underlying mechanisms, novel strategies are being explored to achieve safer, effective, more convenient regimens and more durable long-term tolerance. These include the combination of allergen extract with monoclonal antibodies (mAbs) directed against the T helper 2 (TH2) cell pathway, or with immune modifiers such as Toll-like receptor (TLR) agonists. Molecular allergology has enabled more accurate allergy diagnosis and the development of recombinant whole allergens and hypoallergenic variants that, in the future, may result in a more individualized ‘tailor-made’ allergen immunotherapy. Allergen-derived peptides have been developed to target specifically underlying T or B cell-dependent pathways. These are likely to be safer, although so far they have not shown greater efficacy over whole allergen approaches that target both pathways. On the basis of the known ability of allergen immunotherapy to induce IgE-blocking antibodies, passive immunotherapy by injection of cocktails of IgG4 monoclonal antibodies directed against IgE epitopes of major allergens has proved successful in inhibiting human nasal allergen challenge responses. Oral immunotherapy in children with peanut allergy has been highly effective in inducing ‘desensitization’, whereas long-term tolerance remains elusive, and the treatment is accompanied by occasional serious systemic side effects. Earlier intervention in infancy and early childhood and/or the use of allergen combination strategies may overcome these problems. The epicutaneous approach using peanut allergen patches may be less effective in desensitization but is able to reduce the risk of anaphylaxis on exposure to traces of peanut and this may be a more feasible approach, with lower risk of treatment-related systemic side effects. Breg cell, regulatory B cell; FcεRII, low-affinity receptor for IgE; IL-4R, interleukin-4 receptor; Treg cell, regulatory T cell; TSLP, thymic stromal lymphopoietin.
Modified allergens
Chemical modification of allergens by use of glutaraldehyde or formaldehyde to produce allergoids72,73, with altered tertiary structure and reduced allergenicity, has shown modest efficacy but no obvious benefits over standard extracts in terms of reduced allergic side effects. This unaltered risk of side effects from allergoids could be due to retained IgE epitopes, the exposure of previously latent IgE epitopes or the acquisition of novel IgE epitopes during processing. Similarly, the production of ‘medium length peptides’ from allergens, either by controlled hydrolysis74 or synthetic75 peptides based on the known sequences of major allergens, retains the capacity to generate protective IgG responses and has reduced ability to induce human basophil activation in vitro. These approaches have used shorter treatment regimens, for example with three to six pre-seasonal injections for pollen allergy76. It is therefore not surprising that they have shown only modest efficacy at best and no advantages in terms of safety compared with more prolonged courses over several years using conventional standardized allergen extracts. There have been no head to head comparisons with these approaches and conventional allergen extracts. (Recombinant allergen mixtures and hypoallergenic variants are discussed in ‘Novel immunotherapy strategies’.)
Sublingual immunotherapy
Following the failure of oral immunotherapy for inhalant allergens77, possibly due to gastric digestion of allergens, sublingual immunotherapy was considered as a way of directly accessing local lymphoid tissue and the regional draining lymph nodes shared by the upper respiratory tract. The oral mucosa was known to be an immunologically privileged site as reflected by tolerance to daily exposure to high levels of food proteins without developing hypersensitivity in the vast majority of individuals78. Furthermore, there are high levels of dendritic cells and fewer mast cells in the oral mucosa compared with other mucosal surfaces79. Moreover, the sublingual route was shown to be effective in inducing allergen-specific tolerance in mouse models80.
Studies of allergic rhinitis in humans have shown sublingual immunotherapy to be an effective and safe alternative to subcutaneous immunotherapy9,81. The treatment involves placing allergen extracts daily, in liquid82–84 or tablet85,86 form, under the tongue and can be self-administered following medical supervision of the first dose9. Systematic reviews and meta-analyses have confirmed efficacy of pre-seasonal or co-seasonal immunotherapy for seasonal rhinitis and continuous treatment for perennial rhinitis due to HDM allergens87. Recent adequately powered randomized controlled trials of sublingual tablets have shown consistent efficacy in seasonal allergic rhinitis due to grass85,86, ragweed88, birch89 and Japanese cedar90 pollen allergy. The effect size of the treatment on symptoms and/or use of rescue medication is consistently 30–40% better than placebo treatment, similar to that observed in a phase 3 trial of subcutaneous immunotherapy with grass pollen extract, and compares favourably with the World Allergy Organization’s proposed minimal important difference for clinical efficacy of 20%91. Indirect meta-analysis of sublingual tablet treatment compared with pharmacotherapy revealed efficacy greater than antihistamine tablets and comparable with that observed with intranasal corticosteroids92.
There are now three studies in adults90,93,94 and one in children aged 5–12 years95 that confirm that daily sublingual allergy tablet treatment for 3 years induces long-term clinical benefits for at least 2 years after stopping the treatment (see Supplementary Table 1). Sublingual tablet therapy given over 12 months for HDM-induced perennial rhinitis was shown to be effective in four separate studies96–99. Effect sizes were less than those observed for pollen sublingual immunotherapy, around 17–20% compared with placebo, possibly related to difficulty in selecting those HDM-IgE-sensitized individuals in whom HDM was causal, rather than other perennial non-allergic causes of rhinitis96–99. In HDM-induced allergic asthma, sublingual tablet immunotherapy reduced the requirement for inhaled corticosteroids100 and decreased the rate of acute exacerbations of asthma101. HDM tablet immunotherapy has been approved for these indications in Europe and as an add-on treatment option for patients with HDM-triggered allergic asthma on low-to-high dose inhaled corticosteroids (https://ginasthma.org/reports/).
Subcutaneous versus sublingual allergen immunotherapy
The European Academy of Allergy and Clinical Immunology recently published evidence-based treatment guidelines that confirm the efficacy, safety and long-term benefits of subcutaneous and sublingual allergen immunotherapy for seasonal and perennial allergic rhinoconjunctivitis102. A recent clinical trial compared subcutaneous grass pollen immunotherapy and sublingual grass pollen tablet immunotherapy103. Both routes were effective in terms of the clinical response, but in contrast to 3 years of either treatment, 2 years of treatment was insufficient to maintain tolerance 1 year after stopping therapy. Flow cytometry confirmed that both routes suppressed allergen-specific TH2 cell responses at 2 years that reversed at 3 years, along with the loss of clinical efficacy. Similarly, suppression of TH2-type cytokines (IL-4, IL-5, IL-9 and IL-13) in the nasal mucosa was reversed at 3 years104. By contrast, serum IgE-blocking activity, as reflected by inhibition of IgE-facilitated allergen–IgE binding to B cells and allergen-stimulated activation of basophils, was suppressed and this persisted throughout the 3 years. Blocking antibodies were present in both serum and nasal fluid. For subcutaneous immunotherapy, the IgE-inhibitory activity was predominantly mediated by IgG4, whereas for sublingual immunotherapy local nasal and systemic blocking antibodies were largely IgA1 and/or IgA2 (ref.105). Although speculative, these data support the idea that whereas suppression of TH2-type responses is necessary for the induction of allergen-specific tolerance, prolonged alterations in the B cell compartment may be necessary for persistence of tolerance104.
A secondary preventive role of immunotherapy in asthma was suggested by studies of both subcutaneous106 and sublingual95 immunotherapy in children aged 5–12 years with seasonal rhinitis, in whom 3 years of treatment reduced both symptoms of asthma and requirements for asthma medication at 5 years.
Primary prevention of inhalant allergies in infants has been tested in pilot studies107,108. A placebo-controlled trial of HDM sublingual drops was performed in infants, aged 6–18 months, at high risk of developing allergy. There was a reduction in multiple allergen sensitizations108, as determined by skin testing, and there appeared to be a reduction in asthma prevalence at 6 years109. Although preliminary, these data provide incentive for a larger prospective controlled trial of HDM immunotherapy for primary prevention of asthma in high-risk infants. Whereas whole allergen or recombinant wild type-like allergens boost IgE responses and have potential to induce IgE sensitization, modified allergen derivatives or hypoallergenic peptides are less likely to induce IgE110,111 and may therefore hold more promise in the future for primary prevention.
Alternative routes of immunotherapy
Intralymphatic immunotherapy involves injecting allergen extracts into lymph nodes, generally in the groin, under ultrasound guidance112. The rationale for this approach is that targeting the immune system directly could more efficiently augment allergen presentation to T cells whilst avoiding direct mast cell activation. Small placebo-controlled trials have shown modest benefit using extracts derived from grass pollen113 and tree pollen114. In a small study of participants with cat allergy115, a recombinant cat Fel d 1 allergen was fused with a translocation sequence and a fragment of the human invariant chain to enhance immunogenicity. Three intralymphatic injections of the Fel d 1 fusion protein at 4-weekly intervals protected against nasal challenge with whole cat allergen extract compared with participants treated with placebo. Several studies have shown efficacy of a short course of injections via the intralymphatic route for grass pollen allergy113,114,116, although this has not been confirmed in all studies112.
Oral immunotherapy for inhalant allergens has not been effective77, possibly because allergen extracts are degraded by gastric acid. By contrast, oral peanut immunotherapy is of proven efficacy in children, although it has a high prevalence of side effects. For example, in a phase 3 trial in children 4–17 years old12, oral peanut administered as capsules achieved a predetermined threshold of response to peanut in 67.2% of participants treated with peanut compared with 4.0% of participants treated with placebo, but gastrointestinal side effects were common. Systemic allergic side effects occurred in 14.2% compared with 3.2% in patients treated with placebo, including one case of anaphylaxis. Despite effective desensitization induced by oral peanut immunotherapy59, long-term tolerance, even at high doses with attendant side effects, has been elusive13.
The epicutaneous route was developed to take advantage of the high numbers of resident dendritic cells in the skin to facilitate allergen processing of very low allergen concentrations117. Delivery of peanut extract via a skin patch to children (aged 4–11 years) with peanut allergy resulted in desensitization to oral peanut challenge in 35.3% of the children compared with 13.6% of participants treated with placebo and fewer side effects, which were largely confined to local application site reactions118. At present, peanut immunotherapy is not recommended for routine care outside specialist centres. However, early introduction of foods containing peanut for prevention of peanut allergy in infants has been remarkably effective119. Introduction of peanut at 4–6 months of age is now routinely recommended for infants with severe eczema or egg allergy who are at high risk of developing peanut allergy120. Similarly, oral peanut immunotherapy in children aged 1–4 years who are peanut sensitized and symptomatic has been shown to be effective and safer when introduced very early in the course of their disease, when there are very low levels of allergen-specific IgE antibodies60.
Novel immunotherapy strategies
Molecular allergology
Most allergens contained within common inhalants and foods have now been cloned. This has enabled precise molecular diagnosis of IgE sensitivities to major allergens (recognized by more than 50% of individuals) and minor allergens and the identification of cross-reactive epitopes of less clinical relevance121. An accurate molecular diagnosis may assist an individually tailored selection of allergen extracts for immunotherapy that may translate into improved outcomes. There is also the opportunity for more precise monitoring of relevant IgE and IgG responses during treatment. DNA technology has enabled the production of recombinant allergens122, recombinant mixtures123 and hypoallergenic variants for immunotherapy124,125 that precisely match the individual’s sensitivities without the risk of IgE sensitization to irrelevant allergens126. However, although these recombinant vaccines and recombinant hypoallergenic variants have been effective in phase 2 trials122,123, they have not so far shown added benefits in terms of efficacy or safety over currently available standardized allergen extracts122. A recombinant hypoallergenic variant targeting B cells to selectively produce IgG responses is discussed below125.
DNA-based vaccines
DNA-based vaccines127 have been tested in mouse models of allergy127,128 and shown to induce preferential TH1 cell and Treg cell responses and downregulate TH2 cell responses129. There are, however, reservations concerning their repeated use in humans based on theoretical risks of incorporation of plasmid DNA into the human genome inducing carcinogenesis, development of anti-DNA antibodies and the unknown effects of long-term allergen persistence with potential for widespread IgE triggering and risk of anaphylaxis128. One approach tested in patients with allergy to Japanese cedar pollen involved the incorporation of lysosomal-associated membrane protein 1 (LAMP1) into a plasmid vector encoding Cry j 2, a major allergen of Japanese cedar pollen. LAMP1 targets the plasmid to the lysosomal compartment to reduce the risk of release of free allergen from the cell and, thereby, decrease the risk of anaphylaxis130. After four intramuscular injections at 2-weekly intervals, there was inhibition of the immediate skin test response to Cry j 2 at 4 months in 10 of 12 participants, but there was no information on other clinical outcomes.
Another DNA-based approach has been to combine allergen with bacterial DNA sequences that contain CpG motifs to selectively target Toll-like receptor 9 (TLR9) expressed on human B cells and dendritic cells. The major ragweed pollen allergen Amb a 1 covalently linked to a B-type CpG-containing oligodeoxynucleotide (ODN)131 was successful in a phase 2 trial in ragweed pollen-induced hay fever, although this was not confirmed in a phase3 trial and the approach was discontinued. An alternative approach involved mixing HDM extract with an A-type CpG ODN (G10) encapsulated within a virus-like particle derived from the surface protein coat of bacteriophage Qb (QbG10)132. The aim of this approach was to protect against allergen IgE triggering, facilitate allergen uptake and co-stimulate TLR9 to enhance HDM-stimulated Treg cell formation and/or TH1-type immune deviation. In a phase 2 trial, six subcutaneous injections at intervals of 1-2 weeks of allergen-CpG ODN-loaded virus-like particles, when given to patients with allergic rhinoconjunctivitis, were effective in suppressing the immediate conjunctival response to HDM challenge. Paradoxically, QbG10 alone was as effective as the QbG10–HDM combination, suggesting that a direct effect of CpG ODN virus-like particles on the innate immune response may have been sufficient to block the allergen-specific response132. However, subsequent placebo-controlled trials of QbG10 alone in severe allergic asthma have yielded inconsistent results133,134.
Finally, vaccines based on mRNA constructs have been successful in inducing type 1 immune deviation and suppressing allergic inflammation in mouse models of allergy135. Their success against SARS-CoV-2 in the COVID-19 pandemic will inevitably stimulate interest in whether mRNA vaccines may have a future role in treating allergic diseases.
Targeted approaches
An important question for allergen immunotherapy is whether novel strategies should target predominantly the T cell response with minimal or no risk of anaphylaxis136 or, alternatively, target the predominant B cell response that favours generation of IgG and IgA responses with IgE-blocking potential104.
Antigen-specific approaches targeting T cells have involved the use of short-chain T cell peptide combinations that have been developed based on in vitro human T cell epitope mapping of individual major allergens with a broad HLA-restriction repertoire to enable peptide recognition by most of the targeted allergic population. The aim has been to selectively drive ‘protective’ T cell responses in the absence of significant IgG antibody responses136–138. Although minimizing the risk of IgE-mediated anaphylaxis, there is the possibility of inducing T cell-dependent late-phase asthmatic responses. In a pilot study, patients with a history of bee venom allergy received increasing doses of a mixture of three T cell epitope peptides of PLA2 subcutaneously over 2 months. Whereas all five treated participants were able to tolerate an intracutaneous challenge with PLA, two of the five developed systemic reactions after a live bee sting challenge, implying incomplete protection139. In patients with cat allergy, a mixture of 7 Fel d 1 peptides (comprising 13–17 amino acids) administered intradermally at intervals of 2–4 weeks over 12 weeks reduced rhinoconjunctivitis symptom scores in an environmental challenge chamber during controlled exposure to whole cat allergen. At 1 year, there was a marked reduction in symptoms that suggested induction of long-term clinical tolerance140. However, a phase 3 field trial of Fel d 1 peptide immunotherapy (ClinicalTrials.gov, NCT01620762)141 was unsuccessful. This may have been due to an observed high placebo response in the control population or selection of cat owners (as opposed to cat avoiders) who may already have exhibited a degree of tolerance due to natural allergen exposure. Following a further unsuccessful phase 3 trial of immunotherapy with HDM-derived T cell peptide (NCT02150343)142 the programme was halted.
Selective targeting of B cell responses during allergen immunotherapy is supported by the detection of blocking antibodies during conventional allergen immunotherapy27,40 and by recent studies identifying increases in IL-10-producing Breg cells during immunotherapy for bee venom50 and HDM allergies51. This concept receives further support from a trial of passive immunization in individuals with cat allergy. A single subcutaneous injection of a mixture of two recombinant anti-Fel d 1 antibodies conferred protection against nasal challenge with whole cat allergen extract that persisted for almost 3 months143. There was a reduction in nasal fluid TH2-type cytokines and accompanying increases in serum and nasal IgE-blocking activity144. This approach has been replicated in seasonal birch pollen allergy where a cocktail of three monoclonal antibodies directed against the major birch allergen Bet v 1 was effective in inhibiting the clinical response to birch pollen nasal challenge for at least 2 months145.
A complementary approach involving selective induction of allergen-specific blocking antibodies with minimal T cell responses involves active immunization with recombinant allergen-derived B cell peptides. A mixture of recombinant non-IgE reactive linear peptides (BM32) derived from the grass pollen allergens Phleum p 1, 2, 5 and 6 were fused to a carrier protein (Pre-S protein, derived from hepatitis C virus)125. A placebo-controlled field study in 181 participants demonstrated increases in allergen-specific IgG1 and IgG4 and minimal changes in IgE responses after BM32 treatment. The primary analysis of seasonal combined symptom medication scores was encouraging but did not achieve significance, although asthma symptom scores and quality of life scores improved125. Results of phase 3 trials are awaited.
Allergen combination strategies
Combination strategies of allergen either plus TLR agonists or in combination with the recently available monoclonal antibodies targeting the TH2 cell pathway provide new opportunities for improving efficacy and long-term tolerance. Targeting TLRs146 such as TLR4 with selective agonists in combination with allergen, with the aim of favouring TH1 cell responses over TH2 cell responses, has been tested in a phase 3 trial. Four injections of the TLR4 agonist monophosphoryl lipid A combined with a grass pollen allergoid showed modest efficacy, with a 13% reduction in combined symptom–medication scores76.
The combination of allergen with anti-IgE monoclonal antibody (omalizumab) as an adjunct or pretreatment with inhalant allergen immunotherapy reduced symptom scores and systemic IgE-mediated side effects but had no impact on long-term tolerance147,148.
Grass pollen subcutaneous immunotherapy combined with an anti-IL-4 antibody downregulated circulating IL-4-expressing TH2 cells in blood but had no effect on the magnitude or duration of suppression of allergen-induced late skin responses compared with allergen alone149. The combination of grass pollen allergen with an anti-IL-4 receptor (anti-IL-4R) antibody would target both IL-4-dependent and IL-13-dependent pathways, and is currently being tested with both inhalant immunotherapy (NCT04502966)150,151 and oral peanut immunotherapy (NCT03682770)152.
The importance of innate immune responses involving the respiratory epithelium and distinct subsets of dendritic cells and ILCs that preferentially drive TH2 cell responses is increasingly recognized47. Anti-OX40 in combination with allergen represents an alternative strategy to divert TH2-type responses153. Combinations of allergen with antibodies against pro-allergic epithelial cytokines such as thymic stromal lymphopoietin (TSLP) (NCT02237196)154 and IL-33 (ref.155) are currently under evaluation.
Perspectives
Currently, our best approach for achieving long-term tolerance for inhalant allergies is allergen immunotherapy with whole allergen extracts for 3 years102. This is based on proven long-term clinical efficacy paralleled by downregulation of allergen-specific TH2 cell responses and durable increases in ‘protective’ antibody responses that persist long after discontinuation of treatment21,23,93. For the subcutaneous route, the IgE-inhibitory activity is predominantly IgG, whereas for sublingual immunotherapy IgA is the dominant isotype105. The fact that sublingual and subcutaneous immunotherapy involve distinct mechanisms implies that it may be possible to combine both treatments in resistant cases. Immunotherapy for asthma remains a substantial unmet need for which the sublingual route may be preferable on grounds of safety and proven effects of HDM sublingual tablets on reducing steroid burden100 and preventing asthma exacerbations101.
Advances in molecular allergology have provided recombinant allergens that facilitate more precision for allergy diagnosis and for the selection of patients for allergen immunotherapy126. At present, recombinant allergen immunotherapy has not added value in terms of efficacy or safety over currently available whole allergen extracts122,123 but recombinant hypoallergenic variants may have such potential. In the future, immunotherapy could involve tailor-made vaccines containing major allergens or hypoallergenic variants, based on personalized profiles of IgE sensitivity121. Allergen modifications to preferentially target T cell responses and reduce IgE-dependent side effects have so far met with limited success140, suggesting that targeting both T cell and B cell arms is important. However, passive immunotherapy with recombinant IgG4 antibodies or targeting B cells using active immunization with recombinant allergen peptides that favour preferential IgG antibody responses are proving to be safe and effective125, with phase 3 trials now in progress. Given the key requirement for IgE-blocking antibodies for long-term clinical efficacy, future studies are needed to determine whether this requires ongoing affinity maturation of B cell IgG responses (that is, with more effective binding of individual epitopes) on prolonged exposure or increased avidity of blocking antibodies due to increased polyclonality (that is, recognition of more epitopes with more effective blockade of IgE cross-linking156).
Combination strategies provide new opportunities to improve efficacy and safety of conventional immunotherapy and achieve long-term tolerance with shorter more convenient courses. Based on the known mechanisms of immunotherapy, these ‘allergen+’ strategies include combinations with TLR agonists76,131 or monoclonal antibodies targeting IgE148 or the TH2 cell pathway150,151. An attractive approach is the combination of allergen with antibodies directed ‘upstream’ at epithelial cytokines that regulate TH2 cell pathways154,155.
Long-term tolerance after oral immunotherapy for food allergies has proved elusive53. A recent breakthrough has been that very early intervention with peanut oral immunotherapy in young children less than 4 years old, when associated with low specific IgE levels and reduced basophil activation, may result in sustained unresponsiveness to oral peanut challenge for several months (IMPACT study)60. Pending safety studies, combination strategies in this age group that include either anti-TSLP or anti-IL-33 antibodies would seem logical to induce more durable tolerance154,155. Targeting dendritic cells directly with epicutaneous peanut immunotherapy has been shown to be safer, albeit less effective, than the oral route118. However, continued epicutaneous peanut immunotherapy may reduce the risk of anaphylaxis upon accidental exposure to trace amounts, which may be a more realistic goal, rather than attempting to achieve long-term tolerance via oral peanut immunotherapy with attendant risks of anaphylaxis due to the treatment13.
As a result of the remarkable success and safety of early introduction of peanut as a primary preventive strategy119,120, similar studies in infants who are at risk are ongoing for prevention of other food allergies including shrimp, cashew157 and milk158 allergy. It is therefore logical to consider primary preventive strategies against developing inhalant allergies. For example, preliminary data have shown that with sublingual HDM treatment in infants who are at risk, it may be possible to prevent allergic asthma developing later in childhood108,109.
Supplementary information
Acknowledgements
The authors thank M. Penagos for providing Fig. 1 and Supplementary Table 1 and for reviewing the manuscript.
Author contributions
Both authors researched data for the article, contributed substantially to discussion of the content, and reviewed and/or edited the manuscript before submission. S.R.D. wrote the article.
Peer review
Peer review information
Nature Reviews Immunology thanks C. Akdis, R. Valenta and the other, anonymous, reviewer for their contribution to the peer review of this work.
Competing interests
S.R.D. reports research grants from the Immune Tolerance Network, National Institutes of Allergy and Infectious Diseases USA, Medical Research Council UK and GlaxoSmithKline; has received lecture fees from Abbott laboratories, ALK, Allergopharma, Pneumo Update GmbH and Stallergenes Greer; and has received consultancy fees from ALK, ANGANY Inc. and Revolo Biotherapeutics. M.H.S. reports grants from Leti, Regeneron, Merck, ANGANY Inc., Allergy Therapeutics and the Immune Tolerance Network; reports personal fees from Allergopharma; and reports grants and personal fees from ALK and Allergy Therapeutics.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ClinicalTrials.gov: https://clinicaltrials.gov/
Global Initiative for Asthma Guidelines: https://ginasthma.org/reports/
The History of Vaccines: https://historyofvaccines.org/history/vaccine-timeline/timeline
Contributor Information
Stephen R. Durham, Email: s.durham@imperial.ac.uk
Mohamed H. Shamji, Email: m.shamji99@imperial.ac.uk
Supplementary information
The online version contains supplementary material available at 10.1038/s41577-022-00786-1.
References
- 1.Lee TH. Allergy: the unmet need. Clin. Med. 2003;3:303–305. doi: 10.7861/clinmedicine.3-4-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meltzer EO, et al. Burden of allergic rhinitis: results from the pediatric allergies in America survey. J. Allergy Clin. Immunol. 2009;124:S43–S70. doi: 10.1016/j.jaci.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Meltzer EO, et al. Burden of allergic rhinitis: allergies in America, Latin America, and Asia-Pacific adult surveys. Allergy Asthma Proc. 2012;33:S113–S141. doi: 10.2500/aap.2012.33.3603. [DOI] [PubMed] [Google Scholar]
- 4.Bauchau V, Durham SR. Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur. Respir. J. 2004;24:758–764. doi: 10.1183/09031936.04.00013904. [DOI] [PubMed] [Google Scholar]
- 5.Bousquet J, et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) Allergy. 2008;63:8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 6.Sturm GJ, et al. EAACI guidelines on allergen immunotherapy: hymenoptera venom allergy. Allergy. 2018;73:744–764. doi: 10.1111/all.13262. [DOI] [PubMed] [Google Scholar]
- 7.Gupta RS, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128:e9–e17. doi: 10.1542/peds.2011-0204. [DOI] [PubMed] [Google Scholar]
- 8.Bousquet J, et al. Allergen immunotherapy: therapeutic vaccines for allergic diseases. World Health Organization. American Academy of Allergy, Asthma and Immunology. Ann. Allergy Asthma Immunol. 1998;81:401–405. doi: 10.1016/S1081-1206(10)63136-5. [DOI] [PubMed] [Google Scholar]
- 9.Canonica GW, et al. Sublingual immunotherapy: World Allergy Organization position paper 2013 update. World Allergy Organ. J. 2014;7:6. doi: 10.1186/1939-4551-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cox L, et al. Allergen immunotherapy: a practice parameter third update. J. Allergy Clin. Immunol. 2011;127:S1–S55. doi: 10.1016/j.jaci.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 11.Pajno GB, et al. EAACI guidelines on allergen immunotherapy: IgE-mediated food allergy. Allergy. 2018;73:799–815. doi: 10.1111/all.13319. [DOI] [PubMed] [Google Scholar]
- 12.PALISADE Group of Clinical Investigators. AR101 oral immunotherapy for peanut allergy. N. Engl. J. Med. 2018;379:1991–2001. doi: 10.1056/NEJMoa1812856. [DOI] [PubMed] [Google Scholar]
- 13.Chu DK, et al. Oral immunotherapy for peanut allergy (PACE): a systematic review and meta-analysis of efficacy and safety. Lancet. 2019;393:2222–2232. doi: 10.1016/S0140-6736(19)30420-9. [DOI] [PubMed] [Google Scholar]
- 14.Noon L. Prophylactic inoculation against hay fever. Lancet. 1911;1:1572–1573. doi: 10.1016/S0140-6736(00)78276-6. [DOI] [Google Scholar]
- 15.Freeman J. Vaccination against hay fever: report of results during the last three years. Lancet. 1914;183:1178–1180. doi: 10.1016/S0140-6736(01)56900-7. [DOI] [Google Scholar]
- 16.Blackley, C. Experimental Researches on the Nature and Causes of Catarrhus Aestivus (Hay-fever or Hay-asthma) (Oxford Historical Books, 1873).
- 17.Frankland AW, Augustin R. Prophylaxis of summer hay-fever and asthma: a controlled trial comparing crude grass-pollen extracts with the isolated main protein component. Lancet. 1954;266:1055–1057. doi: 10.1016/S0140-6736(54)91620-7. [DOI] [PubMed] [Google Scholar]
- 18.Lowell FC, Franklin W. A double-blind study of the effectiveness and specificity of injecton therapy in ragweed hay fever. N. Engl. J. Med. 1965;273:675–679. doi: 10.1056/NEJM196509232731302. [DOI] [PubMed] [Google Scholar]
- 19.Norman PS, Lichtenstein LM. The clinical and immunologic specificity of immunotherapy. J. Allergy Clin. Immunol. 1978;61:370–377. doi: 10.1016/0091-6749(78)90116-1. [DOI] [PubMed] [Google Scholar]
- 20.Hunt KJ, et al. A controlled trial of immunotherapy in insect hypersensitivity. N. Engl. J. Med. 1978;299:157–161. doi: 10.1056/NEJM197807272990401. [DOI] [PubMed] [Google Scholar]
- 21.Durham SR, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N. Engl. J. Med. 1999;341:468–475. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- 22.Bozek A, Cudak A, Walter Canonica G. Long-term efficacy of injected allergen immunotherapy for treatment of grass pollen allergy in elderly patients with allergic rhinitis. Allergy Asthma Proc. 2020;41:271–277. doi: 10.2500/aap.2020.41.200035. [DOI] [PubMed] [Google Scholar]
- 23.Penagos M, Durham SR. Allergen immunotherapy for long-term tolerance and prevention. J. Allergy Clin. Immunol. 2022;149:802–811. doi: 10.1016/j.jaci.2022.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Prausnitz C, Küstner H. Studien über die Überempfindlichkeit [German] Zentralbl Bakteriol. [Orig. A] 1921;86:160–169. [Google Scholar]
- 25.Johansson SG. Raised levels of a new immunoglobulin class (IgND) in asthma. Lancet. 1967;2:951–953. doi: 10.1016/S0140-6736(67)90792-1. [DOI] [PubMed] [Google Scholar]
- 26.Ishizaka K, Ishizaka T, Hornbrook MM. Physicochemical properties of reaginic antibody. V. Correlation of reaginic activity with γ-E-globulin antibody. J. Immunol. 1966;97:840–853. doi: 10.4049/jimmunol.97.6.840. [DOI] [PubMed] [Google Scholar]
- 27.Cooke RA, Barnard JH, Hebald S, Stull A. Serological evidence of immunity with coexisting sensitization in a type of human allergy (hay fever) J. Exp. Med. 1935;62:733–750. doi: 10.1084/jem.62.6.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gueguen C, et al. Changes in markers associated with dendritic cells driving the differentiation of either TH2 cells or regulatory T cells correlate with clinical benefit during allergen immunotherapy. J. Allergy Clin. Immunol. 2016;137:545–558. doi: 10.1016/j.jaci.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 29.Zimmer A, et al. A regulatory dendritic cell signature correlates with the clinical efficacy of allergen-specific sublingual immunotherapy. J. Allergy Clin. Immunol. 2012;129:1020–1030. doi: 10.1016/j.jaci.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 30.Akdis CA, Blesken T, Akdis M, Wuthrich B, Blaser K. Role of interleukin 10 in specific immunotherapy. J. Clin. Invest. 1998;102:98–106. doi: 10.1172/JCI2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Francis JN, et al. Grass pollen immunotherapy: IL-10 induction and suppression of late responses precedes IgG4 inhibitory antibody activity. J. Allergy Clin. Immunol. 2008;121:1120–1125.e2. doi: 10.1016/j.jaci.2008.01.072. [DOI] [PubMed] [Google Scholar]
- 32.Bohle B, et al. Sublingual immunotherapy induces IL-10-producing T regulatory cells, allergen-specific T-cell tolerance, and immune deviation. J. Allergy Clin. Immunol. 2007;120:707–713. doi: 10.1016/j.jaci.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 33.O’Hehir RE, et al. House dust mite sublingual immunotherapy: the role for transforming growth factor-β and functional regulatory T cells. Am. J. Respir. Crit. Care Med. 2009;180:936–947. doi: 10.1164/rccm.200905-0686OC. [DOI] [PubMed] [Google Scholar]
- 34.Shamji MH, et al. Role of IL-35 in sublingual allergen immunotherapy. J. Allergy Clin. Immunol. 2019;143:1131–1142.e4. doi: 10.1016/j.jaci.2018.06.041. [DOI] [PubMed] [Google Scholar]
- 35.Nouri-Aria KT, et al. Grass pollen immunotherapy induces mucosal and peripheral IL-10 responses and blocking IgG activity. J. Immunol. 2004;172:3252–3259. doi: 10.4049/jimmunol.172.5.3252. [DOI] [PubMed] [Google Scholar]
- 36.Radulovic S, Jacobson MR, Durham SR, Nouri-Aria KT. Grass pollen immunotherapy induces Foxp3-expressing CD4+CD25+ cells in the nasal mucosa. J. Allergy Clin. Immunol. 2008;121:1467–1472.e1. doi: 10.1016/j.jaci.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 37.Ling EM, et al. Relation of CD4+CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet. 2004;363:608–615. doi: 10.1016/S0140-6736(04)15592-X. [DOI] [PubMed] [Google Scholar]
- 38.Ejrnaes AM, Bodtger U, Larsen JN, Svenson M. The blocking activity of birch pollen-specific immunotherapy-induced IgG4 is not qualitatively superior to that of other IgG subclasses. Mol. Immunol. 2004;41:471–478. doi: 10.1016/j.molimm.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 39.Pilette C, et al. Grass pollen immunotherapy induces an allergen-specific IgA2 antibody response associated with mucosal TGF-β expression. J. Immunol. 2007;178:4658–4666. doi: 10.4049/jimmunol.178.7.4658. [DOI] [PubMed] [Google Scholar]
- 40.Shamji MH, et al. Nasal allergen-neutralizing IgG4 antibodies block IgE-mediated responses: novel biomarker of subcutaneous grass pollen immunotherapy. J. Allergy Clin. Immunol. 2019;143:1067–1076. doi: 10.1016/j.jaci.2018.09.039. [DOI] [PubMed] [Google Scholar]
- 41.van der Heijden FL, Joost van Neerven RJ, van Katwijk M, Bos JD, Kapsenberg ML. Serum-IgE-facilitated allergen presentation in atopic disease. J. Immunol. 1993;150:3643–3650. doi: 10.4049/jimmunol.150.8.3643. [DOI] [PubMed] [Google Scholar]
- 42.He Y, et al. The IgE blocking activity induced by Dermatophagoides pteronyssinus subcutaneous immunotherapy does not correlate with specific IgA but with IgG4 in both serum and saliva. Int. Arch. Allergy Immunol. 2021;182:1231–1244. doi: 10.1159/000517152. [DOI] [PubMed] [Google Scholar]
- 43.Shamji MH, et al. Effector cell signature in peripheral blood following nasal allergen challenge in grass pollen allergic individuals. Allergy. 2015;70:171–179. doi: 10.1111/all.12543. [DOI] [PubMed] [Google Scholar]
- 44.Wurtzen PA, et al. A double-blind placebo-controlled birch allergy vaccination study II: correlation between inhibition of IgE binding, histamine release and facilitated allergen presentation. Clin. Exp. Allergy. 2008;38:1290–1301. doi: 10.1111/j.1365-2222.2008.03020.x. [DOI] [PubMed] [Google Scholar]
- 45.Durham SR, et al. Grass pollen immunotherapy inhibits allergen-induced infiltration of CD4+ T lymphocytes and eosinophils in the nasal mucosa and increases the number of cells expressing messenger RNA for interferon-γ. J. Allergy Clin. Immunol. 1996;97:1356–1365. doi: 10.1016/S0091-6749(96)70205-1. [DOI] [PubMed] [Google Scholar]
- 46.Hamid QA, Schotman E, Jacobson MR, Walker SM, Durham SR. Increases in IL-12 messenger RNA+ cells accompany inhibition of allergen-induced late skin responses after successful grass pollen immunotherapy. J. Allergy Clin. Immunol. 1997;99:254–260. doi: 10.1016/S0091-6749(97)70106-4. [DOI] [PubMed] [Google Scholar]
- 47.Licona-Limon P, Kim LK, Palm NW, Flavell RA. TH2, allergy and group 2 innate lymphoid cells. Nat. Immunol. 2013;14:536–542. doi: 10.1038/ni.2617. [DOI] [PubMed] [Google Scholar]
- 48.Lao-Araya M, Steveling E, Scadding GW, Durham SR, Shamji MH. Seasonal increases in peripheral innate lymphoid type 2 cells are inhibited by subcutaneous grass pollen immunotherapy. J. Allergy Clin. Immunol. 2014;134:1193–1195.e4. doi: 10.1016/j.jaci.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 49.Golebski K, et al. Induction of IL-10-producing type 2 innate lymphoid cells by allergen immunotherapy is associated with clinical response. Immunity. 2021;54:291–307.e7. doi: 10.1016/j.immuni.2020.12.013. [DOI] [PubMed] [Google Scholar]
- 50.van de Veen W, et al. IgG4 production is confined to human IL-10-producing regulatory B cells that suppress antigen-specific immune responses. J. Allergy Clin. Immunol. 2013;131:1204–1212. doi: 10.1016/j.jaci.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 51.Boonpiyathad T, et al. Role of Der p 1-specific B cells in immune tolerance during 2 years of house dust mite-specific immunotherapy. J. Allergy Clin. Immunol. 2019;143:1077–1086.e10. doi: 10.1016/j.jaci.2018.10.061. [DOI] [PubMed] [Google Scholar]
- 52.Sharif H, et al. Altered chromatin landscape in circulating T follicular helper and regulatory cells following grass pollen subcutaneous and sublingual immunotherapy. J. Allergy Clin. Immunol. 2021;147:663–676. doi: 10.1016/j.jaci.2020.10.035. [DOI] [PubMed] [Google Scholar]
- 53.Yu W, Freeland DMH, Nadeau KC. Food allergy: immune mechanisms, diagnosis and immunotherapy. Nat. Rev. Immunol. 2016;16:751–765. doi: 10.1038/nri.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wambre E, et al. A phenotypically and functionally distinct human TH2 cell subpopulation is associated with allergic disorders. Sci. Transl. Med. 2017;9:eaam9171. doi: 10.1126/scitranslmed.aam9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bajzik V, et al. Oral desensitization therapy for peanut allergy induces dynamic changes in peanut-specific immune responses. Allergy. 2022;77:2534–2548. doi: 10.1111/all.15276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vickery BP, et al. Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy. J. Allergy Clin. Immunol. 2014;133:468–475. doi: 10.1016/j.jaci.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J. Exp. Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Santos AF, et al. Biomarkers of severity and threshold of allergic reactions during oral peanut challenges. J. Allergy Clin. Immunol. 2020;146:344–355. doi: 10.1016/j.jaci.2020.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chinthrajah RS, et al. Sustained outcomes in oral immunotherapy for peanut allergy (POISED study): a large, randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2019;394:1437–1449. doi: 10.1016/S0140-6736(19)31793-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jones SM, et al. Efficacy and safety of oral immunotherapy in children aged 1–3 years with peanut allergy (the Immune Tolerance Network IMPACT trial): a randomised placebo-controlled study. Lancet. 2022;399:359–371. doi: 10.1016/S0140-6736(21)02390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suarez-Farinas M, et al. Evolution of epitope-specific IgE and IgG4 antibodies in children enrolled in the LEAP trial. J. Allergy Clin. Immunol. 2021;148:835–842. doi: 10.1016/j.jaci.2021.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jutel M, et al. Bee venom immunotherapy results in decrease of IL-4 and IL-5 and increase of IFN-γ secretion in specific allergen-stimulated T cell cultures. J. Immunol. 1995;154:4187–4194. doi: 10.4049/jimmunol.154.8.4187. [DOI] [PubMed] [Google Scholar]
- 63.Akdis CA, et al. Epitope-specific T cell tolerance to phospholipase A2 in bee venom immunotherapy and recovery by IL-2 and IL-15 in vitro. J. Clin. Invest. 1996;98:1676–1683. doi: 10.1172/JCI118963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stock R, et al. Safety and tolerability of venom immunotherapy: evaluation of 581 rush- and ultra-rush induction protocols (safety of rush and ultra-rush venom immunotherapy) World Allergy Organ. J. 2021;14:100496. doi: 10.1016/j.waojou.2020.100496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Novak N, et al. Early suppression of basophil activation during allergen-specific immunotherapy by histamine receptor 2. J. Allergy Clin. Immunol. 2012;130:1153–1158.e2. doi: 10.1016/j.jaci.2012.04.039. [DOI] [PubMed] [Google Scholar]
- 66.Akdis C, Jutel M, Akdis M. Regulatory effects of histamine and histamine receptor expression in human allergic immune responses. Chem. Immunol. Allergy. 2008;94:67–82. doi: 10.1159/000154858. [DOI] [PubMed] [Google Scholar]
- 67.Jutel M, et al. Histamine regulates T-cell and antibody responses by differential expression of H1 and H2 receptors. Nature. 2001;413:420–425. doi: 10.1038/35096564. [DOI] [PubMed] [Google Scholar]
- 68.Varga EM, et al. Time course of serum inhibitory activity for facilitated allergen–IgE binding during bee venom immunotherapy in children. Clin. Exp. Allergy. 2009;39:1353–1357. doi: 10.1111/j.1365-2222.2009.03303.x. [DOI] [PubMed] [Google Scholar]
- 69.Varga EM, et al. Tolerant beekeepers display venom-specific functional IgG4 antibodies in the absence of specific IgE. J. Allergy Clin. Immunol. 2013;131:1419–1421. doi: 10.1016/j.jaci.2012.08.037. [DOI] [PubMed] [Google Scholar]
- 70.Frew AJ, Powell RJ, Corrigan CJ, Durham SR. Efficacy and safety of specific immunotherapy with SQ allergen extract in treatment-resistant seasonal allergic rhinoconjunctivitis. J. Allergy Clin. Immunol. 2006;117:319–325. doi: 10.1016/j.jaci.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 71.Norman PS, Lichtenstein LM. Comparisons of alum-precipitated and unprecipitated aqueous ragweed pollen extracts in the treatment of hay fever. J. Allergy Clin. Immunol. 1978;61:384–389. doi: 10.1016/0091-6749(78)90118-5. [DOI] [PubMed] [Google Scholar]
- 72.Marsh DG, Lichtenstein LM, Campbell DH. Studies on “allergoids” prepared from naturally occurring allergens. I. Assay of allergenicity and antigenicity of formalinized rye group I component. Immunology. 1970;18:705–722. [PMC free article] [PubMed] [Google Scholar]
- 73.Worm M, et al. Efficacy and safety of birch pollen allergoid subcutaneous immunotherapy: a 2-year double-blind, placebo-controlled, randomized trial plus 1-year open-label extension. Clin. Exp. Allergy. 2019;49:516–525. doi: 10.1111/cea.13331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mosges R, et al. A randomized, double-blind, placebo-controlled, dose-finding trial with Lolium perenne peptide immunotherapy. Allergy. 2018;73:896–904. doi: 10.1111/all.13358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kettner A, et al. Benefit of Bet v 1 contiguous overlapping peptide immunotherapy persists during first follow-up season. J. Allergy Clin. Immunol. 2018;142:678–680.e7. doi: 10.1016/j.jaci.2018.01.052. [DOI] [PubMed] [Google Scholar]
- 76.DuBuske LM, et al. Ultrashort-specific immunotherapy successfully treats seasonal allergic rhinoconjunctivitis to grass pollen. Allergy Asthma Proc. 2011;32:239–247. doi: 10.2500/aap.2011.32.3453. [DOI] [PubMed] [Google Scholar]
- 77.Taudorf E, Weeke B. Orally administered grass pollen. Allergy. 1983;38:561–564. doi: 10.1111/j.1398-9995.1983.tb04140.x. [DOI] [PubMed] [Google Scholar]
- 78.Novak N, Haberstok J, Bieber T, Allam JP. The immune privilege of the oral mucosa. Trends Mol. Med. 2008;14:191–198. doi: 10.1016/j.molmed.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 79.Allam JP, et al. Distribution of Langerhans cells and mast cells within the human oral mucosa: new application sites of allergens in sublingual immunotherapy? Allergy. 2008;63:720–727. doi: 10.1111/j.1398-9995.2007.01611.x. [DOI] [PubMed] [Google Scholar]
- 80.Brimnes J, Kildsgaard J, Jacobi H, Lund K. Sublingual immunotherapy reduces allergic symptoms in a mouse model of rhinitis. Clin. Exp. Allergy. 2007;37:488–497. doi: 10.1111/j.1365-2222.2006.02624.x. [DOI] [PubMed] [Google Scholar]
- 81.Passalacqua G, et al. Randomised controlled trial of local allergoid immunotherapy on allergic inflammation in mite-induced rhinoconjunctivitis. Lancet. 1998;351:629–632. doi: 10.1016/S0140-6736(97)07055-4. [DOI] [PubMed] [Google Scholar]
- 82.Creticos PS, et al. Randomized, double-blind, placebo-controlled trial of standardized ragweed sublingual-liquid immunotherapy for allergic rhinoconjunctivitis. J. Allergy Clin. Immunol. 2014;133:751–758. doi: 10.1016/j.jaci.2013.10.041. [DOI] [PubMed] [Google Scholar]
- 83.Okamoto Y, et al. Efficacy and safety of sublingual immunotherapy for two seasons in patients with Japanese cedar pollinosis. Int. Arch. Allergy Immunol. 2015;166:177–188. doi: 10.1159/000381059. [DOI] [PubMed] [Google Scholar]
- 84.Ott H, et al. Efficacy of grass pollen sublingual immunotherapy for three consecutive seasons and after cessation of treatment: the ECRIT study. Allergy. 2009;64:1394–1401. doi: 10.1111/j.1398-9995.2009.02194.x. [DOI] [PubMed] [Google Scholar]
- 85.Dahl R, et al. Efficacy and safety of sublingual immunotherapy with grass allergen tablets for seasonal allergic rhinoconjunctivitis. J. Allergy Clin. Immunol. 2006;118:434–440. doi: 10.1016/j.jaci.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 86.Didier A, et al. Optimal dose, efficacy, and safety of once-daily sublingual immunotherapy with a 5-grass pollen tablet for seasonal allergic rhinitis. J. Allergy Clin. Immunol. 2007;120:1338–1345. doi: 10.1016/j.jaci.2007.07.046. [DOI] [PubMed] [Google Scholar]
- 87.Dhami S, et al. Allergen immunotherapy for allergic rhinoconjunctivitis: a systematic review and meta-analysis. Allergy. 2017;72:1597–1631. doi: 10.1111/all.13201. [DOI] [PubMed] [Google Scholar]
- 88.Creticos PS, et al. Randomized controlled trial of a ragweed allergy immunotherapy tablet in North American and European adults. J. Allergy Clin. Immunol. 2013;131:1342–1349.e6. doi: 10.1016/j.jaci.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 89.Biedermann T, et al. The SQ tree SLIT-tablet is highly effective and well tolerated: results from a randomized, double-blind, placebo-controlled phase III trial. J. Allergy Clin. Immunol. 2019;143:1058–1066.e6. doi: 10.1016/j.jaci.2018.12.1001. [DOI] [PubMed] [Google Scholar]
- 90.Yonekura S, et al. Disease-modifying effect of Japanese cedar pollen sublingual immunotherapy tablets. J. Allergy Clin. Immunol. Pract. 2021;9:4103–4116. doi: 10.1016/j.jaip.2021.06.060. [DOI] [PubMed] [Google Scholar]
- 91.Canonica GW, et al. Recommendations for standardization of clinical trials with allergen specific immunotherapy for respiratory allergy. a statement of a World Allergy Organization (WAO) taskforce. Allergy. 2007;62:317–324. doi: 10.1111/j.1398-9995.2006.01312.x. [DOI] [PubMed] [Google Scholar]
- 92.Durham SR, et al. Treatment effect of sublingual immunotherapy tablets and pharmacotherapies for seasonal and perennial allergic rhinitis: pooled analyses. J. Allergy Clin. Immunol. 2016;138:1081–1088.e4. doi: 10.1016/j.jaci.2016.04.061. [DOI] [PubMed] [Google Scholar]
- 93.Durham SR, et al. SQ-standardized sublingual grass immunotherapy: confirmation of disease modification 2 years after 3 years of treatment in a randomized trial. J. Allergy Clin. Immunol. 2012;129:717–725.e5. doi: 10.1016/j.jaci.2011.12.973. [DOI] [PubMed] [Google Scholar]
- 94.Didier A, Malling HJ, Worm M, Horak F, Sussman GL. Prolonged efficacy of the 300IR 5-grass pollen tablet up to 2 years after treatment cessation, as measured by a recommended daily combined score. Clin. Transl. Allergy. 2015;5:12. doi: 10.1186/s13601-015-0057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Valovirta E, et al. Results from the 5-year SQ grass sublingual immunotherapy tablet asthma prevention (GAP) trial in children with grass pollen allergy. J. Allergy Clin. Immunol. 2018;141:529–538.e13. doi: 10.1016/j.jaci.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 96.Bergmann KC, et al. Efficacy and safety of sublingual tablets of house dust mite allergen extracts in adults with allergic rhinitis. J. Allergy Clin. Immunol. 2014;133:1608–1614.e6. doi: 10.1016/j.jaci.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 97.Demoly P, et al. Effective treatment of house dust mite-induced allergic rhinitis with 2 doses of the SQ HDM SLIT-tablet: results from a randomized, double-blind, placebo-controlled phase III trial. J. Allergy Clin. Immunol. 2016;137:444–451.e8. doi: 10.1016/j.jaci.2015.06.036. [DOI] [PubMed] [Google Scholar]
- 98.Okubo K, et al. Efficacy and safety of the SQ house dust mite sublingual immunotherapy tablet in Japanese adults and adolescents with house dust mite-induced allergic rhinitis. J. Allergy Clin. Immunol. 2017;139:1840–1848.e10. doi: 10.1016/j.jaci.2016.09.043. [DOI] [PubMed] [Google Scholar]
- 99.Demoly P, et al. A 300 IR sublingual tablet is an effective, safe treatment for house dust mite-induced allergic rhinitis: an international, double-blind, placebo-controlled, randomized phase III clinical trial. J. Allergy Clin. Immunol. 2021;147:1020–1030.e10. doi: 10.1016/j.jaci.2020.07.036. [DOI] [PubMed] [Google Scholar]
- 100.Mosbech H, et al. Standardized quality (SQ) house dust mite sublingual immunotherapy tablet (ALK) reduces inhaled corticosteroid use while maintaining asthma control: a randomized, double-blind, placebo-controlled trial. J. Allergy Clin. Immunol. 2014;134:568–575.e7. doi: 10.1016/j.jaci.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 101.Virchow JC, et al. Efficacy of a house dust mite sublingual allergen immunotherapy tablet in adults with allergic asthma: a randomized clinical trial. JAMA. 2016;315:1715–1725. doi: 10.1001/jama.2016.3964. [DOI] [PubMed] [Google Scholar]
- 102.Roberts G, et al. EAACI guidelines on allergen immunotherapy: allergic rhinoconjunctivitis. Allergy. 2018;73:765–798. doi: 10.1111/all.13317. [DOI] [PubMed] [Google Scholar]
- 103.Scadding GW, et al. Effect of 2 years of treatment with sublingual grass pollen immunotherapy on nasal response to allergen challenge at 3 years among patients with moderate to severe seasonal allergic rhinitis: the GRASS randomized clinical trial. JAMA. 2017;317:615–625. doi: 10.1001/jama.2016.21040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Renand A, et al. Synchronous immune alterations mirror clinical response during allergen immunotherapy. J. Allergy Clin. Immunol. 2018;141:1750–1760.e1. doi: 10.1016/j.jaci.2017.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shamji MH, et al. Differential induction of allergen-specific IgA responses following timothy grass subcutaneous and sublingual immunotherapy. J. Allergy Clin. Immunol. 2021;148:1061–1071.e11. doi: 10.1016/j.jaci.2021.03.030. [DOI] [PubMed] [Google Scholar]
- 106.Jacobsen L, et al. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007;62:943–948. doi: 10.1111/j.1398-9995.2007.01451.x. [DOI] [PubMed] [Google Scholar]
- 107.Holt PG, et al. Prophylactic use of sublingual allergen immunotherapy in high-risk children: a pilot study. J. Allergy Clin. Immunol. 2013;132:991–993.e1. doi: 10.1016/j.jaci.2013.04.049. [DOI] [PubMed] [Google Scholar]
- 108.Zolkipli Z, et al. Randomized controlled trial of primary prevention of atopy using house dust mite allergen oral immunotherapy in early childhood. J. Allergy Clin. Immunol. 2015;136:1541–1547.e11. doi: 10.1016/j.jaci.2015.04.045. [DOI] [PubMed] [Google Scholar]
- 109.Alviani C, et al. Primary prevention of asthma in high-risk children using HDM SLIT: assessment at age 6 years. J. Allergy Clin. Immunol. 2020;145:1711–1713. doi: 10.1016/j.jaci.2020.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kundig TM, et al. Der p 1 peptide on virus-like particles is safe and highly immunogenic in healthy adults. J. Allergy Clin. Immunol. 2006;117:1470–1476. doi: 10.1016/j.jaci.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 111.Campana R, et al. Vaccination of nonallergic individuals with recombinant hypoallergenic fragments of birch pollen allergen Bet v 1: safety, effects, and mechanisms. J. Allergy Clin. Immunol. 2019;143:1258–1261. doi: 10.1016/j.jaci.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hoang MP, Seresirikachorn K, Chitsuthipakorn W, Snidvongs K. Intralymphatic immunotherapy for allergic rhinoconjunctivitis: a systematic review and meta-analysis. Rhinology. 2021;59:236–244. doi: 10.4193/Rhin20.572. [DOI] [PubMed] [Google Scholar]
- 113.Senti G, et al. Intralymphatic allergen administration renders specific immunotherapy faster and safer: a randomized controlled trial. Proc. Natl Acad. Sci. USA. 2008;105:17908–17912. doi: 10.1073/pnas.0803725105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Konradsen JR, et al. Intralymphatic immunotherapy in pollen-allergic young adults with rhinoconjunctivitis and mild asthma: a randomized trial. J. Allergy Clin. Immunol. 2020;145:1005–1007.e7. doi: 10.1016/j.jaci.2019.11.017. [DOI] [PubMed] [Google Scholar]
- 115.Zaleska A, et al. Immune regulation by intralymphatic immunotherapy with modular allergen translocation MAT vaccine. Allergy. 2014;69:1162–1170. doi: 10.1111/all.12461. [DOI] [PubMed] [Google Scholar]
- 116.Skaarup SH, Schmid JM, Skjold T, Graumann O, Hoffmann HJ. Intralymphatic immunotherapy improves grass pollen allergic rhinoconjunctivitis: a 3-year randomized placebo-controlled trial. J. Allergy Clin. Immunol. 2021;147:1011–1019. doi: 10.1016/j.jaci.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 117.Senti G, et al. Epicutaneous allergen administration as a novel method of allergen-specific immunotherapy. J. Allergy Clin. Immunol. 2009;124:997–1002. doi: 10.1016/j.jaci.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 118.Zylke JW. Epicutaneous immunotherapy vs placebo for peanut protein ingestion among peanut-allergic children. JAMA. 2019;321:956. doi: 10.1001/jama.2019.1133. [DOI] [PubMed] [Google Scholar]
- 119.Du Toit G, et al. Effect of avoidance on peanut allergy after early peanut consumption. N. Engl. J. Med. 2016;374:1435–1443. doi: 10.1056/NEJMoa1514209. [DOI] [PubMed] [Google Scholar]
- 120.Togias A, et al. Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases-sponsored expert panel. J. Allergy Clin. Immunol. 2017;139:29–44. doi: 10.1016/j.jaci.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Incorvaia C, et al. Personalized medicine for allergy treatment: allergen immunotherapy still a unique and unmatched model. Allergy. 2021;76:1041–1052. doi: 10.1111/all.14575. [DOI] [PubMed] [Google Scholar]
- 122.Pauli G, et al. Efficacy of recombinant birch pollen vaccine for the treatment of birch-allergic rhinoconjunctivitis. J. Allergy Clin. Immunol. 2008;122:951–960. doi: 10.1016/j.jaci.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 123.Jutel M, et al. Allergen-specific immunotherapy with recombinant grass pollen allergens. J. Allergy Clin. Immunol. 2005;116:608–613. doi: 10.1016/j.jaci.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 124.Niederberger V, et al. Vaccination with genetically engineered allergens prevents progression of allergic disease. Proc. Natl Acad. Sci. USA. 2004;101:14677–14682. doi: 10.1073/pnas.0404735101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Niederberger V, et al. Safety and efficacy of immunotherapy with the recombinant B-cell epitope-based grass pollen vaccine BM32. J. Allergy Clin. Immunol. 2018;142:497–509.e9. doi: 10.1016/j.jaci.2017.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dorofeeva Y, et al. Past, present, and future of allergen immunotherapy vaccines. Allergy. 2021;76:131–149. doi: 10.1111/all.14300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Scheiblhofer S, Thalhamer J, Weiss R. DNA and mRNA vaccination against allergies. Pediatr. Allergy Immunol. 2018;29:679–688. doi: 10.1111/pai.12964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Slater JE, Paupore E, Zhang YT, Colberg-Poley AM. The latex allergen Hev b 5 transcript is widely distributed after subcutaneous injection in BALB/c mice of its DNA vaccine. J. Allergy Clin. Immunol. 1998;102:469–475. doi: 10.1016/S0091-6749(98)70137-X. [DOI] [PubMed] [Google Scholar]
- 129.Hartl A, Hochreiter R, Stepanoska T, Ferreira F, Thalhamer J. Characterization of the protective and therapeutic efficiency of a DNA vaccine encoding the major birch pollen allergen Bet v 1a. Allergy. 2004;59:65–73. doi: 10.1046/j.1398-9995.2003.00335.x. [DOI] [PubMed] [Google Scholar]
- 130.Su Y, et al. Safety and long-term immunological effects of CryJ2-LAMP plasmid vaccine in Japanese red cedar atopic subjects: a phase I study. Hum. Vaccin. Immunother. 2017;13:2804–2813. doi: 10.1080/21645515.2017.1329070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Creticos PS, et al. Immunotherapy with a ragweed-Toll-like receptor 9 agonist vaccine for allergic rhinitis. N. Engl. J. Med. 2006;355:1445–1455. doi: 10.1056/NEJMoa052916. [DOI] [PubMed] [Google Scholar]
- 132.Senti G, et al. Use of A-type CpG oligodeoxynucleotides as an adjuvant in allergen-specific immunotherapy in humans: a phase I/IIa clinical trial. Clin. Exp. Allergy. 2009;39:562–570. doi: 10.1111/j.1365-2222.2008.03191.x. [DOI] [PubMed] [Google Scholar]
- 133.Beeh KM, et al. The novel TLR-9 agonist QbG10 shows clinical efficacy in persistent allergic asthma. J. Allergy Clin. Immunol. 2013;131:866–874. doi: 10.1016/j.jaci.2012.12.1561. [DOI] [PubMed] [Google Scholar]
- 134.Casale TB, et al. CYT003, a TLR9 agonist, in persistent allergic asthma — a randomized placebo-controlled phase 2b study. Allergy. 2015;70:1160–1168. doi: 10.1111/all.12663. [DOI] [PubMed] [Google Scholar]
- 135.Roesler E, et al. Immunize and disappear-safety-optimized mRNA vaccination with a panel of 29 allergens. J. Allergy Clin. Immunol. 2009;124:1070–1077.e1–e11. doi: 10.1016/j.jaci.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 136.Moldaver D, Larche M. Immunotherapy with peptides. Allergy. 2011;66:784–791. doi: 10.1111/j.1398-9995.2011.02610.x. [DOI] [PubMed] [Google Scholar]
- 137.Norman PS, et al. Treatment of cat allergy with T-cell reactive peptides. Am. J. Respir. Crit. Care Med. 1996;154:1623–1628. doi: 10.1164/ajrccm.154.6.8970345. [DOI] [PubMed] [Google Scholar]
- 138.Oldfield WL, Larche M, Kay AB. Effect of T-cell peptides derived from Fel d 1 on allergic reactions and cytokine production in patients sensitive to cats: a randomised controlled trial. Lancet. 2002;360:47–53. doi: 10.1016/S0140-6736(02)09332-7. [DOI] [PubMed] [Google Scholar]
- 139.Muller U, et al. Successful immunotherapy with T-cell epitope peptides of bee venom phospholipase A2 induces specific T-cell anergy in patients allergic to bee venom. J. Allergy Clin. Immunol. 1998;101:747–754. doi: 10.1016/S0091-6749(98)70402-6. [DOI] [PubMed] [Google Scholar]
- 140.Patel D, et al. Fel d 1-derived peptide antigen desensitization shows a persistent treatment effect 1 year after the start of dosing: a randomized, placebo-controlled study. J. Allergy Clin. Immunol. 2013;131:e101–e107. doi: 10.1016/j.jaci.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 141.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT01620762 (2016).
- 142.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT02150343 (2017).
- 143.Orengo JM, et al. Treating cat allergy with monoclonal IgG antibodies that bind allergen and prevent IgE engagement. Nat. Commun. 2018;9:1421. doi: 10.1038/s41467-018-03636-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shamji MH, et al. Passive prophylactic administration with a single dose of anti-Fel d 1 monoclonal antibodies REGN1908-1909 in cat allergen-induced allergic rhinitis: a randomized, double-blind, placebo-controlled clinical trial. Am. J. Respir. Crit. Care Med. 2021;204:23–33. doi: 10.1164/rccm.202011-4107OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gevaert P, et al. Novel antibody cocktail targeting Bet v 1 rapidly and sustainably treats birch allergy symptoms in a phase 1 study. J. Allergy Clin. Immunol. 2022;149:189–199. doi: 10.1016/j.jaci.2021.05.039. [DOI] [PubMed] [Google Scholar]
- 146.Kirtland ME, Tsitoura DC, Durham SR, Shamji MH. Toll-like receptor agonists as adjuvants for allergen immunotherapy. Front. Immunol. 2020;11:599083. doi: 10.3389/fimmu.2020.599083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Casale TB, et al. Omalizumab pretreatment decreases acute reactions after rush immunotherapy for ragweed-induced seasonal allergic rhinitis. J. Allergy Clin. Immunol. 2006;117:134–140. doi: 10.1016/j.jaci.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 148.Dantzer JA, Wood RA. The use of omalizumab in allergen immunotherapy. Clin. Exp. Allergy. 2018;48:232–240. doi: 10.1111/cea.13084. [DOI] [PubMed] [Google Scholar]
- 149.Chaker AM, et al. Short-term subcutaneous grass pollen immunotherapy under the umbrella of anti-IL-4: a randomized controlled trial. J. Allergy Clin. Immunol. 2016;137:452–461.e9. doi: 10.1016/j.jaci.2015.08.046. [DOI] [PubMed] [Google Scholar]
- 150.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT04502966 (2020).
- 151.Corren J, et al. Short-term subcutaneous allergy immunotherapy and dupilumab are well tolerated in allergic rhinitis: a randomized trial. J. Asthma Allergy. 2021;14:1045–1063. doi: 10.2147/JAA.S318892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT03682770 (2021).
- 153.Burrows KE, et al. OX40 blockade inhibits house dust mite driven allergic lung inflammation in mice and in vitro allergic responses in humans. Eur. J. Immunol. 2015;45:1116–1128. doi: 10.1002/eji.201445163. [DOI] [PubMed] [Google Scholar]
- 154.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT02237196 (2019).
- 155.Chinthrajah S, et al. Phase 2a randomized, placebo-controlled study of anti-IL-33 in peanut allergy. JCI Insight. 2019;4:e131347. doi: 10.1172/jci.insight.131347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Holm J, Willumsen N, Wurtzen PA, Christensen LH, Lund K. Facilitated antigen presentation and its inhibition by blocking IgG antibodies depends on IgE repertoire complexity. J. Allergy Clin. Immunol. 2011;127:1029–1037. doi: 10.1016/j.jaci.2011.01.062. [DOI] [PubMed] [Google Scholar]
- 157.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT03504774 (2022).
- 158.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT03462030 (2021).
- 159.Shamji MH, Durham SR. Mechanisms of allergen immunotherapy for inhaled allergens and predictive biomarkers. J. Allergy Clin. Immunol. 2017;140:1485–1498. doi: 10.1016/j.jaci.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 160.Corren J, Ziegler SF. TSLP: from allergy to cancer. Nat. Immunol. 2019;20:1603–1609. doi: 10.1038/s41590-019-0524-9. [DOI] [PubMed] [Google Scholar]
- 161.Varricchi G, et al. T follicular helper (TFH) cells in normal immune responses and in allergic disorders. Allergy. 2016;71:1086–1094. doi: 10.1111/all.12878. [DOI] [PubMed] [Google Scholar]
- 162.Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J. Allergy Clin. Immunol. 2010;125:S73–S80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wachholz PA, Soni NK, Till SJ, Durham SR. Inhibition of allergen-IgE binding to B cells by IgG antibodies after grass pollen immunotherapy. J. Allergy Clin. Immunol. 2003;112:915–922. doi: 10.1016/S0091-6749(03)02022-0. [DOI] [PubMed] [Google Scholar]
- 164.Wambre E, et al. Differentiation stage determines pathologic and protective allergen-specific CD4+ T-cell outcomes during specific immunotherapy. J. Allergy Clin. Immunol. 2012;129:544–551. doi: 10.1016/j.jaci.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shamji MH, et al. Functional rather than immunoreactive levels of IgG4 correlate closely with clinical response to grass pollen immunotherapy. Allergy. 2012;67:217–226. doi: 10.1111/j.1398-9995.2011.02745.x. [DOI] [PubMed] [Google Scholar]
- 166.Gagliani N, et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat. Med. 2013;19:739–746. doi: 10.1038/nm.3179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.