Abstract
Small nonfunctional pancreatic neuroendocrine tumors ≤2 cm have different biological features, and there is no gold standard treatment for them. This study aimed to assess the risk of malignancy of small non-functional pancreatic neuroendocrine tumors and their outcomes after radical resection. The optimal management of small, incidentally detected pancreatic neuroendocrine tumors is controversial, with the aim of identifying factors predicting survival in patients with clinical stage T1N0M0 (cT1N0M0) pancreatic neuroendocrine tumors and whether surgical treatment improves survival. Using the Surveillance, Epidemiology, and End Results database, we identified 637 patients with cT1N0M0 pancreatic neuroendocrine tumors from 2010 to 2015, including clinicopathological characteristics, treatment modalities, and outcome data. From the surveillance, epidemiology, and end results database of 637 patients with cT1N0M0 PNENs, 564 were treated surgically. Age (P = .000), sex (P < .001), and surgery (P < .001) were independent risk factors affecting survival. Patients who have undergone surgery, women and young adults have a higher overall survival rate. The following independent prognostic predictors for cT1N0M0 pNENs were identified: age, sex, and surgery. At last, we concluded that Surgery can increase the overall survival of pancreatic neuroendocrine tumors in T1N0M0.
Keywords: elderly people, multivariate analysis, neuroendocrine tumors, pancreatic neoplasms, prognosis, surgical treatment
1. Introduction
Pancreatic neuroendocrine tumors (PNETs) are the second most common epithelial malignancies of the pancreas. The prevalence of PNETs is low; however, its incidence has significantly increased over the past few decades. According to the American Cancer Society’s estimate, approximately 4032 (>7% of all pancreatic malignancies) individuals will be diagnosed with PNETs by 2020.[1]
PNETs are generally classified into functional and nonfunctional types. Most PNETs are the latter, which comprise about 65% to 90% of all PNETs, and are more aggressive than functional tumors.[2] The best way to achieve a good long-term prognosis for patients with PNET is through a combination of surgical treatments. The surgical strategy should consider the patient’s systemic status and the functional and biological characteristics of the tumor, and carefully assess the risks and benefits of surgery. The incidence of small (≤2 cm), Non-functional pancreatic neuroendocrine tumors (NF-pNETs) has increased in the last decade, and the need for surgery for G1 and G2 asymptomatic pNET with a maximum diameter of <2 cm, without evidence of regional lymph node metastasis or local invasion, is controversial. Incidentally diagnosed sporadic small NF-pNETs may exhibit aggressive behavior and poor prognosis, such as extrapancreatic extension, lymph nodal metastasis, distant metastasis, and recurrence, even causing disease-related death. However, several studies have reported that patients with NF-pNETs ≤ 2 cm had a lower rate of malignant behavior compared with larger ones (>2 cm), and the surgical approach may lead to surgery-related pancreatic complications. However, there is still a lack of level I evidence to convince surgeons to abandon all cases with sporadic small NF-pNETs.[3] Because of the complications associated with surgery, the decision to operate on NF-pNETs ≤ 2 cm in the elderly requires more rigorous and careful evaluation to achieve better outcomes.
Several studies have demonstrated the safety of a watch-and-wait strategy instead of surgery for asymptomatic NF-PNETs < 2 cm.[4] Nevertheless, the shortness of follow-up and the absence of prospective studies suggest a cautious attitude towards this approach.[1] Small NF-PNETs are not immune to potential malignancies. Surgical resection may be considered for small tumors and can provide favorable postoperative and long-term outcomes.[5] Our study focused on patients with only a clinical stage of cT1N0M0 PNET. We aimed to gather sufficient evidence to make strong, well-supported recommendations to support or refute the results of a single published study that demonstrated a significant overall survival (OS) benefit for patients with cT1N0M0 PNET. In addition, we sought to identify the clinical characteristics and prognostic factors associated with cT1N0M0 PNET that could influence OS in this patient cohort. To address these questions, a retrospective survey was conducted using the study population from the internationally renowned surveillance, epidemiology, and end results (SEER) database.
2. Patients and Methods
2.1. Patients
The study population was extracted from the SEER database using an exclusive software (SEER*Stat 8.4.0). The SEER program was established as one of the first steps in the War on Cancer declared by President Nixon’s Administration (National Cancer Act of 1971). Prior to that time, basic cancer statistics were gathered at the state level, but there was no national system for cancer surveillance. The SEER registry began collecting information on January 1, 1973 in the states of Connecticut, Iowa, New Mexico, Utah, Hawaii, and the metropolitan areas of Detroit and San Francisco/Oakland. Other areas were added to the SEER database over the years. These included the rest of California, the metropolitan area of Atlanta and rural Georgia, the 13-county Seattle-Puget Sound area, New Jersey, Louisiana, Kentucky, Arizona, and the Alaska native tumor registry. After the year 2000, SEER captured approximately 25% of all cancer cases diagnosed in the United States each year, and the registries from which it received its data covered roughly 25% of the US population. The SEER database is very useful for population-based cancer research because it provides detailed information on a large number of individual cancer cases with firm assurance of confidentiality and extensive quality control.[6] Patients were identified using ICD-O-3, as described elsewhere. Patients with PNET were identified using the primary site code for the pancreas (C25.0–C25.9) and the histology codes for carcinoid tumors (8240), neuroendocrine carcinoids (8246), islet-cell adenocarcinoma (8150), malignant beta-cell tumor (8151), malignant alpha-cell tumor (8152), G-cell tumor (8153), VIPoma (8155), malignant somatostatinoma (8156), malignant enteroglucagonoma (8157), argentaffin carcinoid tumor (8241), enterochromaffin cell tumor (8242), mucocarcinoid tumor (8243), and atypical carcinoid tumor (8249). For the purpose of this study, the functionality of tumors was defined by histology codes as functional (8151, 8152, 8153, and 8155) or nonfunctional (8150, 8240, 8241, and 8246) because the SEER database offers no information on symptoms at presentation. We included carcinoid and enterochromaffin tumors of the pancreas (histology codes 8240–8242) and neuroendocrine carcinoma of the pancreas (8246) in order to capture all neuroendocrine pancreatic tumors.[7,8] We selected only patients with primary American Joint Committee on Cancer stage I PNET. As our study used established data and did not involve interactions with human patients, institutional review board approval was not required.
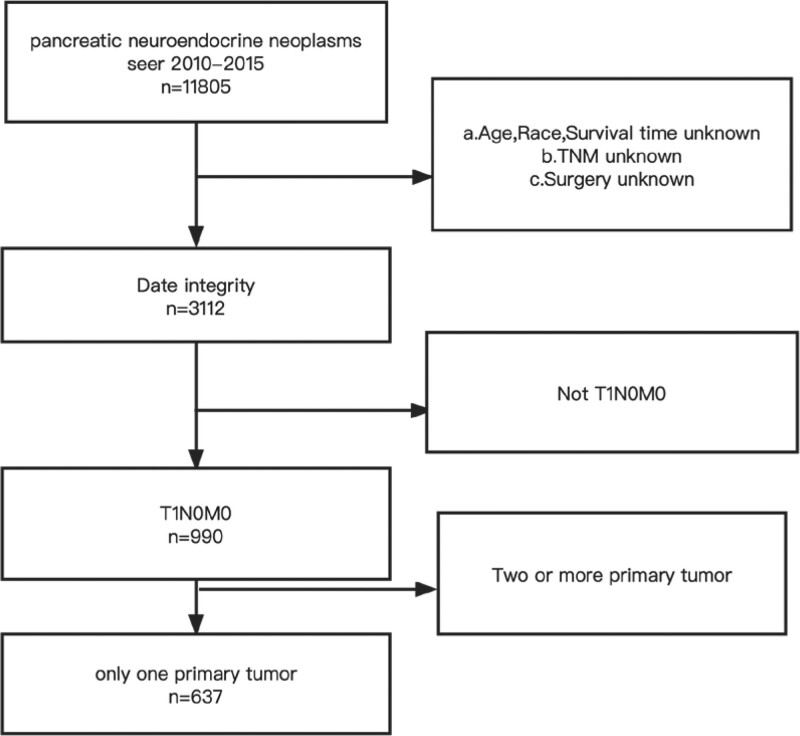
Preliminary selection criteria for study cases included: diagnosis of PNET, clinical stage T1N0M0 (cT1N0M0) PNET, and diagnosis between 2010 and 2015. Only a single primary tumor. Exclusion criteria were: not the first primary malignancy; unknown SEER summary stage; unknown survival time; unknown treatment. As shown in Figure 1.
Figure 1.
Inclusion exclusion criteria.
2.2. Statistical analysis
Clinical and demographic features were compared across subgroups using the chi-square or Fisher exact test, survival curves were generated using the Kaplan–Meier method, and the log-rank test was used to evaluate survival differences between subgroups. Adjusted hazard ratios and 95% confidence intervals were calculated using multivariate Cox proportional hazard regression models. Statistical analysis was performed using SPSS Statistics version 26 (IBM Corp, Armonk, NY). Statistical significance was set at P values < .05 were considered statistically significant, and all statistical tests were 2-tailed.
3. Results
3.1. Summary statistics
In total, 637 patients with cT1N0M0 PNET who fulfilled all the inclusion criteria were identified: 308 (48.4%) were male and 329 (51.6%) were female. The follow-up period for the cohort ranged from 0 to 107 months, with a mean follow-up of 56.65 months. Nearly 63.7% of the patients in this cohort were aged < 65 years. Most patients were white (n = 476; 74.7%), followed by other races (n = 98; 15.4%), and black (n = 63; 9.9%). Most patients were married (n = 416; 65.3%), 188 (29.5%) were single, and 33 (5.2%) were listed as others. The number of patients with histological grade 1, 2, or 3/4 disease was 570 (89.5%), 63 (9.9%), and 4 (0.6%), respectively.
In this cohort, the vast majority of the patients (n = 564, 88.5%) underwent surgery, whereas 73 (11.5%) Only 73 patients were under clinical observation. A total of 394 patients (61.9%) underwent lymph node dissection. Except for sex (P = .001), age (P = .000), and surgery (P = .001), we did not find any differences when considering other characteristics, including tumor site, size, tumor grade, marital status, and lymphadenectomy (P > .05). The basic clinical and demographic characteristics are summarized in Table 1, and notable differences were detected between the subgroups.
Table 1.
Epidemiologic and clinicodemographic characteristics of patients with cT1N0M0 PNET.
| Variable | Alive | Dead | Total | P value |
|---|---|---|---|---|
| Sex | .001 | |||
| Female | 314 | 15 | 329 | |
| Male | 271 | 37 | 308 | |
| Age | .000 | |||
| <65 | 196 | 17 | 231 | |
| ≥65 | 389 | 35 | 406 | |
| Race | .923 | |||
| White | 438 | 38 | 476 | |
| Black | 58 | 5 | 63 | |
| Other | 89 | 9 | 98 | |
| Marital status | .775 | |||
| Married | 380 | 36 | 416 | |
| Single | 175 | 13 | 188 | |
| Other | 30 | 3 | 33 | |
| Primary site | ||||
| Head of pancreas | 137 | 10 | 147 | .759 |
| Body of pancreas | 138 | 15 | 153 | |
| Tail of pancreas | 210 | 17 | 227 | |
| Other | 100 | 10 | 110 | |
| Grade | ||||
| 1 | 525 | 45 | 570 | .423 |
| 2 | 57 | 6 | 63 | |
| 3/4 | 3 | 1 | 4 | |
| Surg | .001 | |||
| No | 60 | 13 | 73 | |
| Yes | 525 | 39 | 564 | |
| LN | .346 | |||
| No | 220 | 23 | 245 | |
| Yes | 365 | 29 | 394 | |
| Tumor size | .998 | |||
| >1 cm | 179 | 16 | 195 | |
| 1.0 to 1.5 cm | 235 | 21 | 256 | |
| 1.5 to 2.0 cm | 171 | 15 | 186 | |
LN = lymph node dissection, PNET = pancreatic neuroendocrine tumor, Surg = surgery.
3.2. Survival analysis
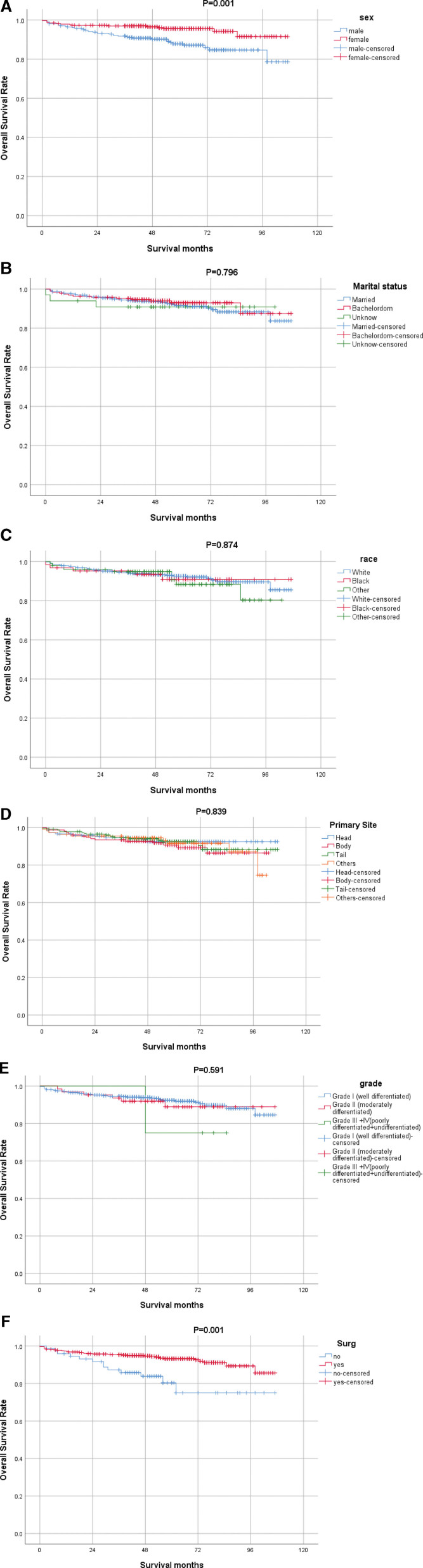
Survival analysis was performed using the Kaplan–Meier estimate for OS. There were significant differences in OS according to age at diagnosis (P < .000), sex (P = .001), and surgery (P = .001) (Fig. 1).
Younger (<65 years) patients had better OS than older (≥65 years) patients (Fig. 2I), and male had poorer OS than female (Fig. 2A). Patients who chose surgery had a higher OS than those who did not (Fig. 2F). However, race (Fig. 2C), primary site (Fig. 2D), tumor grade (Fig. 2E), marital status (Fig. 2B), tumor size (Fig. 2H), and lymph node dissection (Fig. 2G) did not have a significant impact on survival (P > .05).
Figure 2.
OS curves of patients with cT1N0M0 PNET compared according to (A) Sex, (B) Marital status, (C) Race, (D) Primary Site, (E) Grade, (F) Surg, (G) LN, (H) Tumor size, (I) Age. PNET = pancreatic neuroendocrine tumor. OS = overall survival.
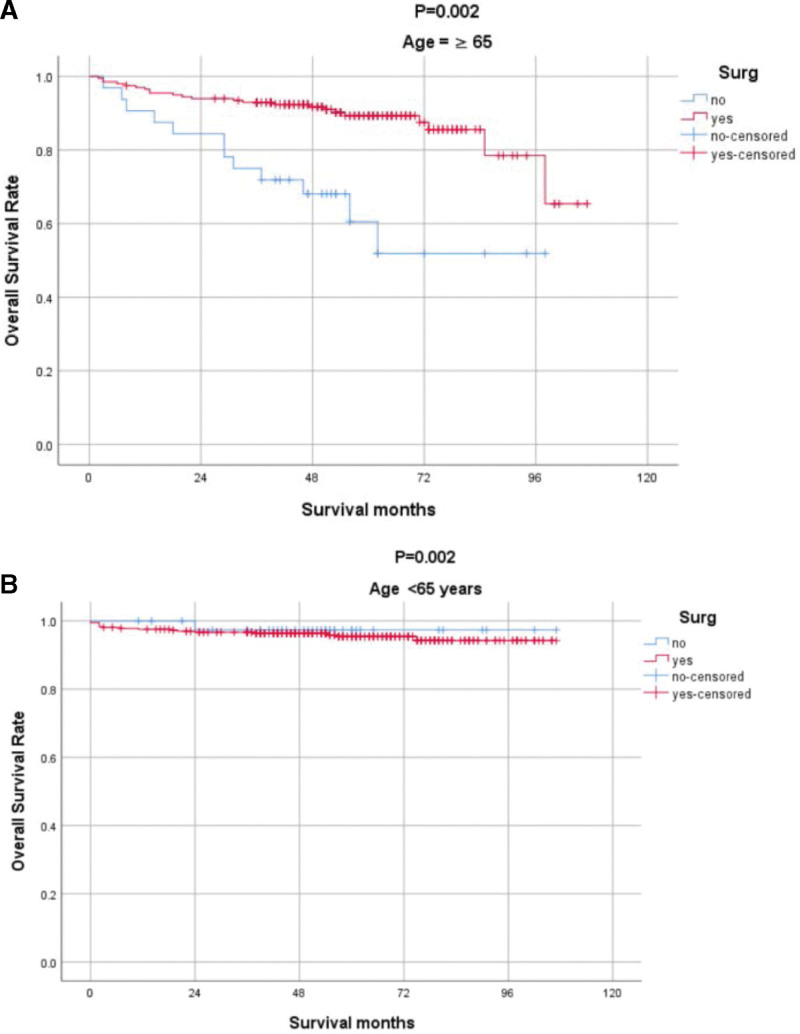
As age and surgery are independent influences on OS, to explore whether for older people surgery is beneficial, we conducted a subgroup analysis stratified by age to determine the impact of surgery on prognosis (Fig. 3).
Figure 3.
Subgroup analysis according to age.
3.3. Multivariate analysis
All factors identified as significant in the survival analysis were entered into multivariate analysis based on the Cox regression model. Multivariate Cox regression analysis showed that sex, age, and surgery were important independent prognostic indicators. As shown in Table 2.
Table 2.
Cox proportional hazards regression model showing the association of variables with OS.
| Variable | HR (95% CI) | Multivariable |
|---|---|---|
| P value | ||
| Sex | ||
| Male | 1 [Reference] | NA |
| Female | 0.426 (0.222–0.817) | .010 |
| Surgical | ||
| No | 1 [Reference] | NA |
| Yes | 0.345 (0.150–0.834) | .018 |
| Age | ||
| <65 | 1 [Reference] | NA |
| ≥65 | 3.368 (1.855–6.117) | .000 |
| M | ||
| Married | 1 [Reference] | NA |
| Single | 1.086 (0.548–2.150) | .813 |
| Other | 1.360 (0.403–4.589) | .621 |
| Primary Site | ||
| Head of pancreas | 1 [Reference] | NA |
| Body of pancreas | 1.269 (0.557–2.891) | .571 |
| Tail of pancreas | 1.016 (0.449–2.300) | .969 |
| other | 1.107 (0.445–2.694) | .823 |
| Grade | ||
| 1 | 1 [Reference] | NA |
| 2 | 1.241 (0.521–2.955) | .625 |
| 3/4 | 1.544 (0.197–12.103) | .679 |
| LN | ||
| No | 1 [Reference] | NA |
| Yes | 1.187 (0.571–2.470) | .646 |
| Tumor size | ||
| >1cm | 1 [Reference] | NA |
| 1.0 to 1.5 | 1.055 (0.542–2.055) | .875 |
| 1.5 to 2.0 | 1.057 (0.510–2.194) | .881 |
| Tace | ||
| White | 1 [Reference] | NA |
| Black | 1.486 (0.556–3.970) | .43 |
| Other | 1.458 (0.694–3.063) | .319 |
95% CI = 95% confidence intervals, HR = Hazard ratio, LN = lymph node dissection, OS= overall survival, Surg = surgery.
More importantly, surgery was an independent protective factor that decreased the risk of death by 65% (hazard ratios = 0.345, 95% confidence Interval 0.150–0.834).
4. Discussion
Previously, marital status is an independent prognostic factor for survival in PNETs patients.[9]
In contrast, in our survival analyses, marital status was not associated with cT1N0M0 PNET Prognosis, However, more research is needed on the association of marital status with increased survival. With regard to sex, our results showed that women at diagnosis had a significant independent protective effect on cT1N0M0 PNENs survival. Watchful waiting is recommended for patients with nonfunctional PNETs < 1 cm. Further evidence is needed to determine whether surgery for nonfunctional PNETs of 1–2 cm would be of benefit or if surgery should be individualized.[10] Our study divided size into three subgroups (>1 cm; 1.0–1.5 cm; 1.5–2.0 cm), and the results showed no significant difference in survival among the three groups.
PNETs are among the slowest and fastest growing human cancers.[11] The incidence of small PNETs (T1, size ≤ 2 cm) has significantly increased in the past few decades owing to improvements in diagnostic techniques and the frequency of imaging.[12] Surgical resection is considered the mainstay of treatment for locoregional PNET and represents the only chance for a cure. However, the optimal management of incidentally discovered small NF-PNETs is controversial. Although consensus guidelines exist, surgical management requires a more in-depth assessment and tailored approach to individual patients and their tumor types. In addition, with the greater detection of smaller incidentally found PNETs, there is a pressing need for the development of appropriate surveillance and surgical treatment algorithms to guide management.
Surgical management of PNETs is indicated for nonfunctional tumors ≥ 2 cm, tumors that are functional, symptomatic, or have evidence of aggressive features (local invasion, lymphatic metastases). With the increasing incidence of PNETs < 2 cm, there is concern for the potential to surgically overtreat, with patients being “Victims of Modern Imaging Technology”.[13] In Germany, the ENETS guidelines for surgery of small PNENs are not yet well-accepted.[14]
A Korean study, which included 76 cases of NF-PNET with tumor size ≤ 2 cm, concluded that intensive follow-up may be an acceptable approach for small (especially < 1.5 cm), asymptomatic NF-PNET.[15] In a study that included 101 patients with ≤ 2 cm PNET, 72% received active monitoring and 28% underwent surgery. Seventy-three patients treated conservatively survived with no signs of distant metastases, and none of them underwent surgery. Only five patients (20%) had tumor growth.[16] In our study, the majority of patients (89%) opted for surgical treatment.
In a series of studies, it was mentioned that although the overall perioperative comorbidity of small NF-PNETs was as high as 64.3%, only a small proportion had serious complications (11.1%); however, there were no deaths.[5]
We found a significantly lower OS in patients who did not undergo surgical resection than in those who did. Contrary to many current recommendations, the majority of patients with PNETs ≤ 2.0 cm in the US underwent surgical resection and had a survival advantage with surgical treatment. In the present study, 63 (9.9%) and 4 (0.6%) patients were classified as G2, G3/4 respectively, and the data demonstrated that 15 patients eventually died of distant metastases, suggesting that T1N0M0 tumors have malignant potential. In summary, nonfunctional PNET of all sizes should be considered potentially malignant, and surgical resection should be considered. This suggests that the current recommendations should be revised. This finding is consistent with that of a previous population-based study.[17]
The risks and benefits of surgical resection should be carefully considered for a given patient. Considering that the disease is usually inert, it seems reasonable to observe elderly patients with small, well-differentiated, and/or severely comorbid cT1N0M0 PNENs. This is because the risks of surgery may outweigh the benefits of resection. Therefore, we performed a subgroup analysis according to age and found that for elderly patients (≥65 years), surgical treatment significantly improved OS.
The National Comprehensive Cancer Network guidelines advocate formal resection with lymphadenectomy in tumors > 2 cm, but there is no firm consensus for smaller tumors.[18] Additionally, regional lymphadenectomy may lead to the inclusion of splenectomy, increased blood loss, longer operating time and hospital stay, and increased lymphocele development. Thus, the benefits and risks of lymphadenectomy should be evaluated carefully. Zhang et al confirmed the positive significance of regional lymphadenectomy in grade 2/3 patients, while adequate lymphadenectomy is not recommended for grade 1 patients because Lymph node metastasis shows key prognostic information about survival.[19] A multicenter international study concluded that in resected sporadic NF-PNETs, the risk of lymph node metastases is correlated with tumor size. Considering that sporadic NF-PNETs between 1.1 and 2 cm had a higher risk of lymph node metastases and recurrence compared to tumors ≤ 1 cm, the decision to perform surgery in this subgroup of patients should be individualized in surgically fit patients.[20] However, our study indicated that increased lymph node dissection was not associated with OS, and there was no significant difference in OS between patients who underwent lymph node dissection and those who did not.
Similar to other studies that used SEER as a data source, our study has some limitations. First, as we only included PNETs of T1N0M0, and the incidence was relatively low, the sample size of our study was small. In addition, in this study, there were only 15 functioning pancreatic neuroendocrine cell tumors, representing 2% of the total; therefore, they were not excluded. The second limitation is the lack of important variables, such as surgical margins, Ki-67, and other molecular biomarkers. The Ki-67 index and surgical margin play important roles in the prognosis of pNEN.[21] Third, we classified surgery and lymph node removal as yes or no; we did not categorize them in detail, for example, 1 to 3 regional lymph nodes removed, 4 or more regional lymph nodes removed, biopsy or aspiration of regional lymph node, sentinel lymph node biopsy, sentinel node biopsy, and lym and removed same/unstated time. Finally, because the SEER database is a retrospective database, selection bias cannot be completely avoided.
5. Conclusions
The OS rate of PNETs in T1N0M0 is extremely high, and surgical treatment may provide a survival benefit, especially for older people (≥65 years).
Author contributions
Guo conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, and approved the final draft.
Zhen Liang conceived and designed the experiments, analyzed the data, reviewed the drafts of the paper, and approved the final draft.
Li Jiao Xiong analyzed the data, reviewed the drafts of the paper, and approved the final draft.
Conceptualization: Nian Guo, Zhen Liang, Li Jiao Xiong.
Data curation: Nian Guo, Zhen Liang.
Formal analysis: Nian Guo.
Funding acquisition: Zhen Liang.
Investigation: Nian Guo.
Methodology: Nian Guo.
Resources: Zhen Liang, Li Jiao Xiong.
Validation: Li Jiao Xiong.
Writing – original draft: Nian Guo.
Abbreviations:
- cT1N0M0 =
- clinical stage T1N0M0
- NF-PNETs =
- non-functional pancreatic neuroendocrine tumors
- OS =
- overall survival
- PNETs =
- pancreatic neuroendocrine tumors
- SEER =
- surveillance, epidemiology, and end results.
The datasets generated during and/or analyzed during the current study are publicly available.
This study was supported by grants from the Shenzhen People’s Hospital (the Natural Science Foundation of Shenzhen City, China to ZL [No. KCXFZ20201221173600001]).
How to cite this article: Guo N, Liang Z, Xiong L. Clinicopathological characteristics, survival outcomes and prognostic factors in the cT1N0M0 pancreatic neuroendocrine tumors: A SEER-based study. Medicine 2022;101:41(e31019).
Contributor Information
Nian Guo, Email: qwert123_1011@qq.com.
Li Jiao Xiong, Email: smallstar64@163.com.
References
- [1].Pavel M, Öberg K, Falconi M, et al. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:844–60. [DOI] [PubMed] [Google Scholar]
- [2].Franko J, Feng W, Yip L, et al. Non-functional neuroendocrine carcinoma of the pancreas: incidence, tumor biology, and outcomes in 2,158 patients. J Gastrointest Surg. 2010;14:541–8. [DOI] [PubMed] [Google Scholar]
- [3].Yang G, Ji M, Chen J, et al. Surgery management for sporadic small (≤2 cm), non-functioning pancreatic neuroendocrine tumors: a consensus statement by the Chinese Study Group for Neuroendocrine Tumors (CSNET). Int J Oncol. 2017;50:567–74. [DOI] [PubMed] [Google Scholar]
- [4].Partelli S, Cirocchi R, Crippa S, et al. Systematic review of active surveillance versus surgical management of asymptomatic small non-functioning pancreatic neuroendocrine neoplasms. Br J Surg. 2016;104:34–41. [DOI] [PubMed] [Google Scholar]
- [5].Liu X, Chin W, Pan C, et al. Risk of malignancy and prognosis of sporadic resected small (≤2 cm) nonfunctional pancreatic neuroendocrine tumors. Gland Surgery. 2021;10:219–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Scosyrev E, Messing J, Noyes K, et al. Surveillance Epidemiology and End Results (SEER) program and population-based research in urologic oncology: an overview. Urol Oncol. 2012;30:126–32. [DOI] [PubMed] [Google Scholar]
- [7].Halfdanarson TR, Rabe KG, Rubin J, et al. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol. 2008;19:1727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhang X-F, Xue F, Dong D-H, et al. New nodal staging for primary pancreatic neuroendocrine tumors: a multi-institutional and national data analysis. Ann Surg. 2021;274:e28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhou H, Zhang Y, Song Y, et al. Marital status is an independent prognostic factor for pancreatic neuroendocrine tumors patients: an analysis of the Surveillance, Epidemiology, and End Results (SEER) database. Clin Res Hepatol Gastroenterol. 2017;41:476–86. [DOI] [PubMed] [Google Scholar]
- [10].Ziogas IA, Schmitz R, Moris D, et al. The role of surgery for pancreatic neuroendocrine tumors. Anticancer Res. 2022;42:629–39. [DOI] [PubMed] [Google Scholar]
- [11].Mpilla GB, Philip PA, El-Rayes B, et al. Pancreatic neuroendocrine tumors: therapeutic challenges and research limitations. World J Gastroenterol. 2020;26:4036–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3:1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Johnston II ME, Carter MM, Wilson GC, et al. Surgical management of primary pancreatic neuroendocrine tumors. J Gastrointest Oncol. 2020;11:578–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mintziras I, Keck T, Werner J, et al. Implementation of current ENETS guidelines for surgery of small (≤2 cm) pancreatic neuroendocrine neoplasms in the German surgical community: an analysis of the prospective DGAV StuDoQ|Pancreas registry. World J Surg. 2019;43:175–82. [DOI] [PubMed] [Google Scholar]
- [15].Jung JG, Lee KT, Woo YS, et al. Behavior of small, asymptomatic, nonfunctioning pancreatic neuroendocrine tumors (NF-PNETs). Medicine. 2015;94:e983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Partelli S, Mazza M, Andreasi V, et al. Management of small asymptomatic nonfunctioning pancreatic neuroendocrine tumors: limitations to apply guidelines into real life. Surgery. 2019;166:157–63. [DOI] [PubMed] [Google Scholar]
- [17].Chivukula SV, Tierney JF, Hertl M, et al. Operative resection in early stage pancreatic neuroendocrine tumors in the United States: are we over- or undertreating patients? Surgery. 2020;167:180–6. [DOI] [PubMed] [Google Scholar]
- [18].Shah MH, Goldner WS, Halfdanarson TR, et al. NCCN guidelines insights: neuroendocrine and adrenal tumors, Version 2.2018. J Natl Compr Canc Netw. 2018;16:693–702. [DOI] [PubMed] [Google Scholar]
- [19].Zhang Z, Wang F, Li Z, et al. Value of lymphadenectomy in patients with surgically resected pancreatic neuroendocrine tumors. BMC Surg. 2022;22:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Perinel J, Nappo G, Zerbi A, et al. Sporadic nonfunctional pancreatic neuroendocrine tumors: Risk of lymph node metastases and aggressiveness according to tumor size: a multicenter international study. Surgery. 2022. 172:975–81. [DOI] [PubMed] [Google Scholar]
- [21].Watzka FM, Meyer F, Staubitz JI, et al. Prognostic assessment of non-functioning neuroendocrine pancreatic neoplasms as a basis for risk-adapted resection strategies. World J Surg. 2020;44:594–603. [DOI] [PubMed] [Google Scholar]