Abstract
A reverse-sandwich enzyme-linked immunosorbent assay (ELISA), in which an antibody is sandwiched by antigens, was established for the titration of antibodies to verocytotoxins (VT) in human and animal sera. This assay has two advantages over a conventional indirect ELISA: (i) higher specificity and sensitivity and (ii) the ability to comparably titrate antibodies from different species. The VT1 (Shiga-like toxin 1) antibody-positive rates were 5% in 202 normal adult humans and 99% in 93 normal cattle at a dairy farm. This ELISA is most suitable for seroepidemiologic studies of infections with VT-producing Escherichia coli in humans and various animal species.
Verocytotoxin (VT)-producing Escherichia coli (VTEC), or enterohemorrhagic E. coli, infection is a communicable disease whose mode of transmission is both animal to human and person to person. Simple methods for titrating antibodies from different species are needed for seroepidemiologic studies of this infection. A neutralization test (2, 7, 11) is not suitable for processing large numbers of serum specimens because it requires tissue cultures and much expertise. Indirect enzyme-linked immunosorbent assays (indirect ELISA) (1, 3, 6, 12) also are unsuitable because results cannot be compared among different species. To overcome these difficulties, we investigated the applicability of the reverse-sandwich (RS) ELISA (8), in which an antibody is sandwiched by antigens. In 1988, we developed the RS ELISA in order to detect the minute amount of pollen allergen-specific immunoglobulin G (IgG) present in a large amount of the total IgG in serum from pollinosis patients, because with the indirect ELISA it was difficult to detect such low-concentration antibodies due to the high nonspecific IgG background (8). Recently, Horimoto et al. used the RS ELISA to titrate Borna virus antibody in animal sera (4). In some recent commercial kits (e.g., from Roche and Abbott) for the detection of human immunodeficiency virus antibodies, this technique is called the double-antigen sandwich method.
MATERIALS AND METHODS
VTs and reference antisera.
VT1, or Shiga-like toxin 1, was produced in the VT1 gene recombinant strain E. coli 87-27, and VT2 was produced in the VT2 gene recombinant strain E. coli Tp8 (9). Both toxins were purified as described by Ito et al. (5). Biotinylation was done as described by Nerurkar et al. (10), and the toxins were stored in 50% (vol/vol) glycerol at 4°C. Hyperimmune antiserum produced in rabbits by immunization with formaldehyde-inactivated toxins in Freund complete adjuvant (Difco Laboratories, Detroit, Mich.) was the reference serum for the antibody titration.
Serum specimens for antibody titration.
Adult human serum samples were collected from 1994 to 1997 from 202 healthy adults 19 to 41 years old. Samples from VTEC-infected persons were collected from 1996 to 1997 from 12 patients with diarrhea and from 8 healthy carriers from whom VTEC had been isolated (age range, 2 to 59 years old). Cattle serum samples were collected from 1993 to 1997 from 93 healthy cattle that were 1 to 9 years old and that were reared on a dairy farm.
RS ELISA.
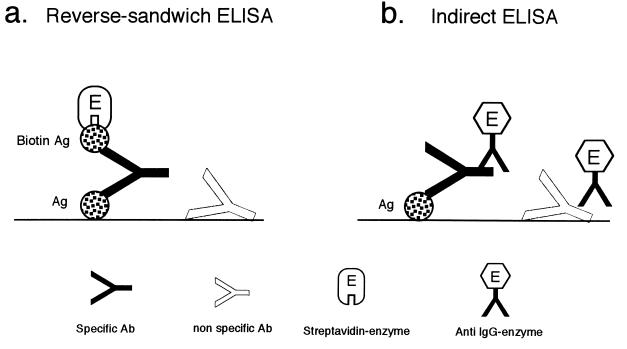
The RS ELISA method of Miyazawa et al. (8) was used. A schematic diagram of the RS ELISA is shown in Fig. 1a. A 0.1-ml quantity of VT1 (0.3 μg/ml) or VT2 (0.9 μg/ml) plus bovine serum albumin (BSA; 25 μg/ml) in 0.5 M NaCl–0.1% NaN3–0.05 M sodium carbonate (pH 9.6) was added to wells of Maxisorp microplates (Nalge Nunc, Copenhagen, Denmark). The plates were incubated overnight at 4°C for antigen immobilization. After the wells were washed test sera diluted 1:4, 1:40, and 1:400 with FBS-PBST (10% [vol/vol] fetal bovine serum [FBS], 0.1% NaN3–phosphate-buffered saline [PBS]–0.05% Tween 20 [PBST]) were added, and the plates were incubated for 60 min at room temperature. Seven threefold serial dilutions of the reference serum were used. After another wash, biotinylated VT1 or VT2 (0.05 μg/ml) in FBS-PBST was added to the wells, and the reaction was allowed to take place for 60 min at room temperature. The wells were washed again, streptavidin-conjugated β-d-galactosidase (GIBCO BRL, Life Technologies Inc., Rockville, Md.; diluted 1:50,000 in PBST containing 1% BSA) was added, and the plates were incubated for 60 min at room temperature. After another wash, 0.2 mM 4-methylumbelliferyl-β-d-galactoside (Sigma Chemical Co., St. Louis, Mo.) in 0.1 M NaCl–1 mM MgCl2–0.1% BSA–0.1% NaN3–0.01 M sodium phosphate (pH 7.0) was added. The wells were sealed with tape, and the plates were immersed in 37°C water for 60 min. Finally, 0.1 ml of 0.1 M glycine-NaOH (pH 10.2) was added to each well to stop the enzyme reaction. The fluorescence units (FU) in each well were measured with a Fluoroskan II apparatus (Flow Laboratories, Rockville, Md.). The antibody concentrations of the test sera were calculated from the titration curve of the reference serum with known antibody units per milliliter.
FIG. 1.
Different principles for antibody assays by the RS ELISA and the indirect ELISA. Ab, antibody; Ag, antigen.
Indirect ELISA.
The indirect ELISA method of Karmali et al. (7) was used. Briefly, 0.1 ml of VT1 (3 μg/ml) in PBS was added to Maxisorp microplate wells for antigen immobilization. After postcoating of the wells with 1% BSA in PBS, human serum diluted 1:100 in 1% BSA-PBST was added. The plates were incubated for 60 min at room temperature. The wells were washed, goat anti-human IgG conjugated to alkaline phosphatase (Biosource International, Camarillo, Calif.; diluted 1:1,000) was added, and the plates were incubated for 60 min. After another wash, color was developed with p-nitrophenyl phosphate.
Inhibition ELISAs for antigen specificity.
Equal volumes of test sera (diluted 1:2 to 1:200) and VT1 or VT2 (1 μg/ml) were mixed. After incubation for 60 min at room temperature, the mixture was added to the antigen-coated wells used in the RS ELISA.
Absorption of IgG.
Thirty-one microliters of test serum and 300 μl of a 25% (vol/vol) suspension of recombinant protein G-agarose (Zymed Laboratories, San Francisco, Calif.) were mixed and shaken for 60 min at room temperature. After centrifugation for 5 min at 2,000 × g, the supernatant (1:10-diluted serum) was subjected to the RS ELISA for antibody activity.
Statistics.
The Mann-Whitney U test was used to examine whether there was a significant difference in the geometric means of the antibody concentrations. The chi-square test was used to examine whether there was a significant difference in the antibody-positive rates.
RESULTS
Titration curves for anti-VT hyperimmune sera.
Figure 2 shows the titration curve for anti-VT1 rabbit serum. When the FU cutoff value was set at twice the blank well value, the end-point dilution was 1:14,000,000, and the antibody activity of this serum was designated 14,000,000 arbitrary antibody units (AbU) per ml. This serum was diluted to an antibody activity of 500 AbU/ml and used as the anti-VT1 reference serum in the RS ELISA. Similarly, anti-VT2 rabbit serum designated as having an antibody activity of 7,000,000 AbU/ml (data not shown) was diluted for use as the anti-VT2 reference serum.
FIG. 2.
Antibody titration curve for anti-VT1 hyperimmune rabbit serum. Fourfold serial dilutions of the serum were added to microplate wells in the RS ELISA. Antibody activity was expressed as FU.
Antibody activity of human sera.
Figure 3a shows the results of antibody titration for 202 normal adult serum samples. Ten persons (5%) had VT1 antibody activity (geometric mean, 107 AbU/ml; range, 5.1 to 9,531 AbU/ml). Only one person, who had no VT1 antibody, had VT2 antibody. Figure 3b shows the results for 20 VTEC-infected persons from whom serum was collected for 10 to 131 days, mostly over 30 days, after VTEC isolation. Eight persons (40%) had VT1 antibody, but the mean value was not significantly lower than that for antibody-positive normal adults (Mann-Whitney U test). Two persons (10%) had VT2 and VT1 antibodies.
FIG. 3.
Distribution of VT1 and VT2 antibody activities (AbU/ml) in human sera. (a) Normal adults. (b) VTEC-infected persons.
Specificity of antibody activity.
An inhibition ELISA was done with the 10 VT1 antibody-positive sera from normal adults to determine the specificity of the antibody activity for the antigen. Activity was inhibited by VT1 antigen in all the sera, indicating that the antibody was specific for the antigen. Findings for four representative sera are shown in Fig. 4.
FIG. 4.
Inhibition of VT1 antibody activity by VT1 antigen in the RS ELISA. Serum was mixed with 1 μg of VT1 or VT2 per ml before the assay. ELISA activity is shown in FU.
IgG specificity was examined next. Activity was abolished after protein G absorption (data not shown), evidence that the antibody activity was that of IgG.
Comparison of the RS ELISA with the indirect ELISA.
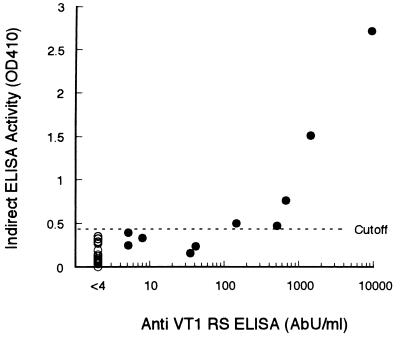
The 10 VT1 antibody-positive sera and 20 randomly chosen negative sera were subjected to the indirect ELISA. Only 5 of the 10 positive sera were positive in the indirect ELISA due to the high background of the negative sera (Fig. 5).
FIG. 5.
Comparison of the RS ELISA with the indirect ELISA for the detection of VT1 antibody in 30 human adult sera. The abscissa shows RS ELISA activity (AbU/ml). The ordinate shows indirect ELISA activity as the absorbance (optical density at 410 nm [OD 410]). The cutoff value (mean + two standard deviations) in the indirect ELISA was determined from the absorbance value of 20 RS ELISA-negative sera (○).
Antibody activity of cattle sera.
VT1 and VT2 antibody activities in 93 normal cattle serum samples are shown in Fig. 6. Ninety-two of the cattle (99%) had VT1 antibody, and 68 (73%) had VT2 antibody. The geometric mean of the VT1 antibody levels was 6,030 AbU/ml, 56-fold that for normal adult humans (Mann-Whitney U test; P <0.001). To determine the antigen specificity of the VT1-positive sera, 20 randomly chosen sera were subjected to an inhibition ELISA. Antibody activity in all the sera was inhibited by VT1 antigen (data not shown).
FIG. 6.
Distribution of VT1 and VT2 antibody activities (AbU/ml) in normal cattle sera. Sera from 93 cattle at a dairy farm were titrated for VT1 and VT2 antibody activities by the RS ELISA.
DISCUSSION
The greatest advantage of the RS ELISA is its ability to detect a small amount of IgG antibody in as low as a 1:4 dilution of serum, compared with the indirect ELISA, in which a serum dilution of 1:100 to 1:1,000 is usual (1, 3, 6, 12). In the RS ELISA, a high-affinity IgG antibody binds to the antigen on the solid phase at one of the two binding sites and captures the biotinylated antigen in the liquid phase at another site (Fig. 1a) when protein antigens with no repeating epitopes are immobilized on the solid phase without forming clusters or aggregates. To ensure this condition, we used a large amount of an unrelated protein, e.g., BSA, and a high ionic strength (0.5 M NaCl) to coat the solid phase (8). In the RS ELISA, IgG molecules that are nonspecifically attached to the solid phase do not capture the labeled antigen (Fig. 1a). In the indirect ELISA, however, nonspecific IgG produces a nonspecific enzyme reaction that gives high background levels (Fig. 1b). Therefore, the RS ELISA can titrate a small quantity of antibodies with higher specificity and sensitivity than the indirect ELISA.
Another advantage of the RS ELISA is that antibodies from any species can be titrated comparably. Hyperimmune serum produced in animals can be used as the reference antiserum for quality control of this ELISA. Further, this ELISA should prove useful for seroepidemiologic surveys of zoonoses, in which antibody prevalence rates and geometric mean concentrations are compared among humans and various animal species.
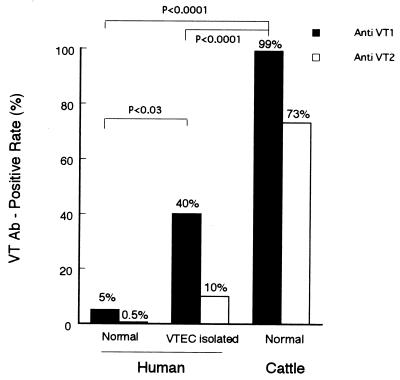
Figure 7 shows the VT1 and VT2 antibody-positive rates in humans and cattle. The VT1 and VT2 antibody-positive rates in VTEC-infected persons are 40 and 10%, respectively, indicating that VT1 and VT2 may be poor immunogens for humans and that even this sensitive ELISA may not be useful for serodiagnosis. The fact that the positivity rate in cattle was 99%, however, is striking. Further seroepidemiologic studies with this ELISA and sera from humans, cattle, and other domestic animals and pets will be needed to clarify the natural history of VTEC infection as a zoonosis.
FIG. 7.
Comparison of VT antibody (Ab)-positive rates between cattle and humans who were healthy or VTEC infected.
ACKNOWLEDGMENT
We thank Shoko Sakamoto for technical assistance.
REFERENCES
- 1.Barrett T J, Green J H, Griffin P M, Pavia A T, Ostroff S M, Wachsmuth I K. Enzyme-linked immunosorbent assays for detecting antibodies to Shiga-like toxin I, Shiga-like toxin II and Escherichia coli in human serum. Curr Microbiol. 1991;23:189–195. [Google Scholar]
- 2.Caprioli A, Luzzi I, Rosmini F, Pasquini P, Cirrincione R, Gianviti A, Matteucci M C, Rizzoni G. Hemolytic-uremic syndrome and Vero cytotoxin-producing Escherichia coli infection in Italy. J Infect Dis. 1992;166:154–158. doi: 10.1093/infdis/166.1.154. [DOI] [PubMed] [Google Scholar]
- 3.Greatorex J S, Thorne G M. Humoral immune responses to Shiga-like toxins and Escherichia coli O157 lipopolysaccharide in hemolytic-uremic syndrome patients and healthy subjects. J Clin Microbiol. 1994;32:1172–1178. doi: 10.1128/jcm.32.5.1172-1178.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horimoto T, Takahashi H, Sakaguchi M, Horikoshi K, Iritani S, Kazamatsuri H, Ikeda K, Tashiro M. A reverse-type sandwich enzyme-linked immunosorbent assay for detecting antibodies to Borna disease virus. J Clin Microbiol. 1997;35:1661–1666. doi: 10.1128/jcm.35.7.1661-1666.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito H, Yutsudo T, Hirayama T, Takeda Y. Isolation and some properties of A and B subunits of Vero toxin 2 and in vitro formation of hybrid toxins between subunits of Vero toxin 1 and Vero toxin 2 from Escherichia coli O157:H7. Microb Pathog. 1988;5:189–195. doi: 10.1016/0882-4010(88)90021-6. [DOI] [PubMed] [Google Scholar]
- 6.Karmali M A, Petric M, Winkler M, Bielaszewska M, Brunton J, van de Kar N, Morooka T, Nair G B, Richardson S E, Arbus G S. Enzyme-linked immunosorbent assay for detection of immunoglobulin G antibodies to Escherichia coli Vero cytotoxin 1. J Clin Microbiol. 1994;32:1457–1463. doi: 10.1128/jcm.32.6.1457-1463.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karmali M A, Petric M, Lim C, Fleming P C, Arbus G S, Lior H. The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J Infect Dis. 1985;151:775–782. doi: 10.1093/infdis/151.5.775. [DOI] [PubMed] [Google Scholar]
- 8.Miyazawa H, Inouye S, Sakaguchi M, Koizumi K. A reverse-sandwich ELISA for IgG antibody to a pollen allergen. J Allergy Clin Immunol. 1988;82:407–413. doi: 10.1016/0091-6749(88)90013-9. [DOI] [PubMed] [Google Scholar]
- 9.Naito S, Horino A, Komiya T, Fukuda Y, Takahashi M, Ami Y, Suzaki Y, Satoh S, Gondaira F, Sugiyama J, Nakano Y, Mori M, Awai K, Nishinohara S, Komuro K, Uchida T. Protection against verocytotoxin in mice induced by liposome-coupled verocytotoxin. Int Arch Allergy Immunol. 1997;114:293–297. doi: 10.1159/000237682. [DOI] [PubMed] [Google Scholar]
- 10.Nerurkar L S, Namba M, Brashears G, Jacob A J, Lee Y J, Sever J L. Rapid detection of herpes simplex virus in clinical specimens by use of a capture biotin-streptavidin enzyme-linked immunosorbent assay. J Clin Microbiol. 1984;20:109–114. doi: 10.1128/jcm.20.1.109-114.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reymond D, Johnson R P, Karmali M A, Petric M, Winkler M, Johnson S, Rahn K, Renwick S, Wilson J, Clarke R C, Spika J. Neutralizing antibodies to Escherichia coli Vero cytotoxin 1 and antibodies to O157 lipopolysaccharide in healthy farm family members and urban residents. J Clin Microbiol. 1996;34:2053–2057. doi: 10.1128/jcm.34.9.2053-2057.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamada S, Matsushita S, Kai A, Sasaki M, Tsuji A, Kanemitsu T, Yamashita N, Anzai E, Kudoh Y. Detection of verocytotoxin from stool and serological testing of patients with diarrhea caused by Escherichia coli O157:H7. Microbiol Immunol. 1993;37:111–118. doi: 10.1111/j.1348-0421.1993.tb03187.x. [DOI] [PubMed] [Google Scholar]