Background:
Micro-coring technology (MCT) removes cores of skin without formation of scars, thereby tightening skin and reducing skin wrinkling. The purpose of this study was to evaluate the safety and efficacy of MCT with the dermal micro-coring device for the treatment of facial wrinkles.
Methods:
This prospective, multicenter clinical trial included fifty-one subjects who underwent MCT treatments of the mid to lower face. The primary study endpoint was change in the Lemperle Wrinkle Severity Scale. Secondary study endpoints were change in Global Aesthetic Improvement Scale (GAIS), participant satisfaction, and evaluation of treatment outcome by an independent review panel. All study endpoints were evaluated at 1, 7, 30, 60, and 150 or 180 days after treatment. Procedure bleeding, pain, and early healing profile were also captured.
Results:
The mean Lemperle Wrinkle Severity Scale change was 1.3 grades. Improvement in the GAIS was reported for 89.7% (87/97) of treated sites, and average improvement of GAIS was 1.5. Participants reported satisfaction with 85.6% of treatment sites. The independent review panel correctly identified 84.2% of the post-treatment photographs as post-treatment. Procedure bleeding and pain was mild with good healing responses and patient-reported average down time of 3 days.
Conclusions:
The results of this study demonstrate the safety and efficacy of MCT with the dermal micro-coring device for the treatment of moderate to severe facial wrinkles. MCT led to significant improvement of facial wrinkles with high patient satisfaction and fast recovery time and should be considered in patients who are seeking minimally invasive treatment for wrinkles of the face.
Takeaways
Question: Is MCT safe and effective for treatment of facial wrinkles?
Findings: The mean wrinkle grade change was 1.3 grades. Participants reported satisfaction with 85.6% (83/97) of treatment sites. Procedure bleeding and pain was mild with good healing responses and a patient-reported average down time of 3 days.
Meaning: MCT is safe and effective in treatment of moderate-to-severe facial wrinkles of the mid to lower face, and should be considered in patients who are seeking minimally invasive treatment for wrinkles of the face.
INTRODUCTION
Aging skin is characterized by skin laxity and the appearance of fine lines and wrinkles. Numerous invasive and noninvasive techniques for skin surface area reduction (skin tightening) have been described in the literature. Minimally invasive, nonsurgical treatments such as micro-needling, ablative and nonablative lasers, radiofrequency, and micro-focused ultrasound have been successfully used to treat mild skin redundancy.1–4 However, moderate-to-severe skin redundancy and wrinkling is difficult to improve with minimally invasive techniques. On the other end of the spectrum, facelift surgery provides the most pronounced cosmetic outcomes in reduction of wrinkles and skin laxity.5 However, a facelift does not fully address all areas of facial skin laxity (periorbital and perioral region, nasolabial fold, marionette lines), and is associated with prolonged recovery and the presence of scarring.
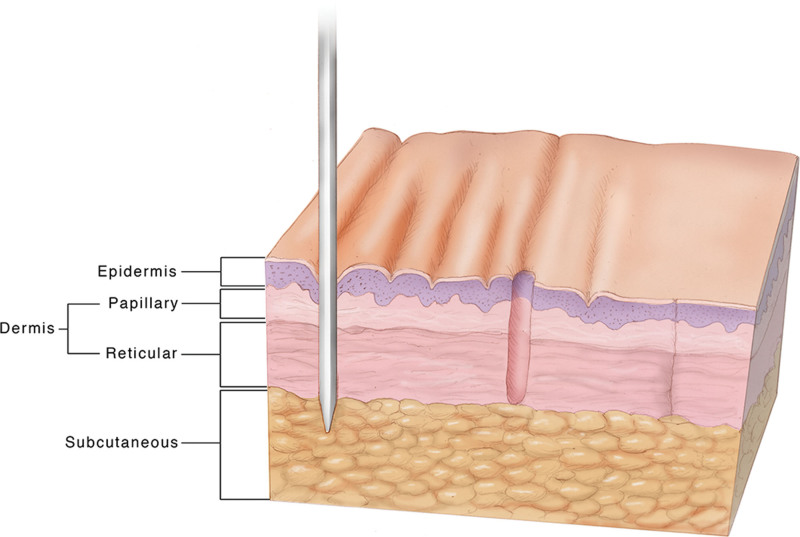
Micro-coring technology (MCT) is an innovative technology that combines the benefits of minimally invasive treatment (fast recovery) with the advantage of scarless skin removal, thereby enabling treatment of moderate to severe skin laxity and wrinkles. The dermal micro-coring device (DMCD) uses hollow coring needles that, when inserted in the skin, excise cores in the size of the needle's inner diameter. Compared with micro-needling that only punctures the skin without removing any tissue, the DMCD needle removes full-thickness cores of skin with diameters in the range of 400 microns. Removal of human skin cores occurs without the formation of a scar (see Figure 1).6
Fig. 1.
Illustration of micro-coring technology. From left to right, the needle is inserted into the skin, removing a micro-core of tissue that heals without scarring. Reprinted with permission from Plast Reconstr Surg Glob Open. 2021;9:e3905.7 This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-No Derivatives License 4.0 (CCBY-NC-ND), where it is permissible to download and share the work provided it is properly cited. The work cannot be changed in any way or used commercially without permission from the journal.
Based on several preclinical and clinical trials, the DMCD offers a safe and effective treatment for facial wrinkles in the intended patient population. Early safety studies in porcine skin demonstrated favorable wound healing in all treatment areas after 1 week with no evidence of infection or scarring over a three-month period.6 Further, at 1 month, a significant increase in epidermal and papillary dermal thickness, as well as increased collagen content was seen.6
In addition, three prospective clinical safety trials analyzed MCT treatment on abdominal and facial skin (short and long-term).7 All trials showed that MCT treatment was well tolerated by participants‚ with only mild pain and transient, self-limited bleeding during and after the procedure. MCT-treated skin further demonstrated a favorable healing profile with no signs of clinical or histologic scarring. Although the primary focus of all three clinical trials was evaluation of safety, notable signs of skin rejuvenation were observed. Preliminary findings included a significant increase in skin thickness in MCT-treated areas when compared with control in both abdominal and facial skin. Further, the average reduction of the facial skin surface area was 9.4% ± 4.3 at 10% treatment density, which was statistically significant when compared with baseline and control (P < 0.01).
Based on the encouraging findings seen in animal and human studies, the aim of this prospective, multicenter clinical trial was to demonstrate the safety and efficacy of the DMCD for the treatment of moderate-to-severe facial wrinkles in the mid to lower face.
METHODS
This prospective, multicenter clinical trial was approved by the New England Institutional Review Board. All subjects signed informed consent adhering to the guidelines outlined by ISO 14155, 21 US Code of Federal Regulations Parts 50, 54, 56, and 812 (as applicable); US Health Insurance Portability and Accountability Act of 1997 (HIPAA); and International Conference on Harmonization E6 Good Clinical Practice.
Investigational Device Description
The DMCD is composed of a reusable nonsterile hand piece that can be wrapped in a sterile drape. Sterile consumable components consist of a single or triple needle array with tubing and a patient-contact vacuum spacer flange. The hand piece is connected to the system console, which provides power distribution, vacuum control, and the user interface. The device is controlled from the touchscreen graphical user interface. The user interface allows the operator to select a single or three-needle array, the percentage of desired tissue removal (up to 8.5%), and the depth of penetration into the skin (up to 5mm). Only the triple-needle cartridge was used during this study.
The operator moves the device over the treatment area and actuates the system using a foot pedal to engage the vacuum spacer flange and begin treatment actuation. Once the hand piece positioning drive system moves the needles into position, the needles penetrate the skin. The needles are then retracted from the skin, removing a core of tissue from the skin. The tissue is then aspirated by the vacuum pump via the needle-hub tubing into a vacuum-tube system and is collected in an external (disposable) filter. The positioning system and needle continue to move through a predefined pattern to treat an area within a 1 × 1 cm2 square. The system is then moved by the user to the next treatment area. The cycle is repeated until the intended treatment is delivered. The suction system removes the cores from the back end of the needles. The cores build up along the suction path (ie, in the cartridge, tubing, filter, etc). Although there is no feedback from the device, cores are visible in the tubing path. The minimum micro-core count per treatment was 6000 in this study. The maximum number of cores that can be removed by the triple-needle cartridge is 24,000. Therefore, the coring device is still sharp after 6000 uses. The frequency of the puncture rate is up to 12Hz. The 8.5% skin removal pattern takes approximately 2 seconds using the triple-needle cartridge. The maximum needle velocity is 2 m per second. Treatments typically took between 15 and 30 minutes for the area studied.
The commercial device is indicated for use by medical professionals for the treatment of moderate and severe wrinkles in the mid and lower face in adults aged 22 years and older with Fitzpatrick skin types I–IV.
Study Design
Subjects who presented to four investigational sites were enrolled in this study (Laser and Skin Surgery Center of New York, Miami Dermatology & Laser Institute, Practice of Brian S. Biesman, M.D., and Laser & Skin Surgery Center of Northern California). The first subject was enrolled on September 10, 2019 and the last subject was enrolled on October 25, 2019. The last follow-up visit was completed on June 26, 2020.
Subject Enrollment
Fifty-nine subjects were screened and enrolled in the study. A total of 59 subjects underwent at least one treatment. A total of 53 subjects underwent two treatments‚ and a total of 49 patients underwent three treatments. A total of five participants (8.5%) discontinued the study or were lost to follow-up before the 90-day follow-up period after the final treatment. Despite the difficulties associated with the COVID-19 pandemic, the overall follow-up visit compliance was excellent at 96.1% (571 actual/594 expected visits). Final follow-up visits after the final treatment were completed for 54 of the 59 treated participants (91.5%). Only subjects who completed at least two treatments were included in the final analysis (n = 51).
Inclusion Criteria
Inclusion criteria included men and women between 40 and 70 years of age at baseline with mid to lower face wrinkles with a grade of 3 (moderately deep wrinkles) and/or 4 (deep wrinkles, well- defined edges) on at least one side using the Lemperle Wrinkle Severity Scale (LWSS) and Fitzpatrick Skin Type I to IV, who were able and willing to provide written informed consent and comply with all study-related procedures and follow-up visits.
Exclusion Criteria
Exclusion criteria included lesions suspicious for any malignancy or the presence of actinic keratosis, melasma, vitiligo, cutaneous papules/nodules, or active inflammatory lesions in the areas to be treated; history of keloid formation or hypertrophic scarring; history of trauma or surgery in the treatment areas in the past 6 months; scar present in the areas to be treated; silicone injections in the areas to be treated, injections of dermal or epidermal fillers in the areas to be treated; fat or botulinum toxin in the areas to be treated, or any other facial procedure within the study treatment areas, within the past 6 months (ie, dermabrasion, laser, radiofrequency, chemical and mechanical peels); active smoking status or having quit within 3 months before treatment; active, chronic, or recurrent infection; history of compromised immune system or currently being treated with immunosuppressive agents; history of sensitivity or allergy to any topical, injectable, or other preparation used during the study, such as Aquaphor, topical or injected anesthetics (ie, lidocaine, benzocaine, procaine, chlorhexidine, povidone-iodine, or epinephrine); excessive sun exposure, use of tanning beds, or tanning creams within 30 days before treatment and for the duration of the study; treatment with aspirin or other blood thinning agents within 14 days before treatment; history or presence of any clinically significant bleeding disorder; treatment with an investigational device or agent within 30 days before treatment or during the study period; currently pregnant or breastfeeding; or planning to become pregnant during the study period.
Study Visits
Participants underwent study-required visits at baseline, treatment (up to three treatments), and at 1, 7, 30, 60, and 90 days after every treatment. Treatments took place 1 month apart. Due to the COVID-19 pandemic, some visits could not be completed at the expected time. Amendments were incorporated to allow follow-up visits at 120, 150, or 180 days as the final follow-up.
Study Endpoints
The primary endpoint of the study was the Lemperle Wrinkle Severity Scale (LWSS) responder rate.8 A responder was defined as a participant with a reduction of one grade or more on the LWSS at the final follow-up visit as determined by the investigator. Further, an independent expert panel consisting of three reviewers (two plastic surgeons and one dermatologist) evaluated baseline and post-treatment photographs and determined LWSS grade (0 = no wrinkles, 1 = just perceptible wrinkles, 2 = shallow wrinkles, 3 = moderately deep wrinkles, 4 = deep wrinkles, well-defined edges, 5 = very deep wrinkles, redundant fold).
The secondary endpoints of the study were patient satisfaction (0 = extremely dissatisfied, 1 = somewhat dissatisfied, 2 = slightly dissatisfied, 3 = neither satisfied nor dissatisfied, 4 = slightly satisfied, 5 = somewhat satisfied, 6 = extremely satisfied) and global aesthetic improvement as assessed by the investigator comparing baseline and post-treatment photographs on the Global Aesthetic Improvement Scale (GAIS) (3= very much improved; optimal cosmetic result, 2 = much improved; marked improvement in appearance from the initial condition, but not completely optimal, 1 = improved; obvious improvement in appearance from the initial condition, 0 = no change; the appearance is essentially the same as baseline, –1 = worse; the appearance is worse than the original condition, –2 = much worse; marked worsening in appearance from the initial condition, –3= very much worse; obvious worsening in appearance from the initial condition).
Treatment endpoints were bleeding severity during treatment as assessed by the investigator (mild, moderate, severe), patient-reported pain score (0–10), and healing response (absent, trace, mild, moderate, severe for the following categories: delayed bleeding, hematoma, redness, burning, hyperpigmentation, scarring, crusting, hypopigmentation, skin necrosis, dryness/roughness, infection, skin peeling, ecchymosis, inflammation, tenderness, edema, itching, tightness/pulling, erythema, pain/discomfort, tingling).
Micro-coring Treatment
Micro-coring treatments were performed with the DMCD using 22-gauge needles and densities of 6.5%, 6.7%, 7.9%, and/or 8.5% (percent of skin removed per 1 cm2). (See Video [online], which displays the micro-coring treatment. The DMCD uses hollow coring needles that, when inserted in the skin, excise cores in the size of the needle inner diameter.) Coring depths were between 3 and 5 mm. The minimum core count was 6,000 micro-cores per treatment. Treatment location was the mid to lower face (see Fig. 2). Upon completion of the treatment, the area was rinsed with sterile saline, and Aquaphor was applied. Aquaphor was applied daily for a minimum of 7 days with no other dressing. No antibiotics were administered. Injectable local anesthetic was administered before the procedure based on standard office procedures and at the discretion of the physician. Typically, patients were injected with approximately 20 to 40 cc of a lidocaine and epinephrine solution. Occasionally, other forms of analgesia were used in addition to the injected local anesthesia, such as nitrous oxide (Pronox), topical anesthesia (administered for 30 minutes before the local anesthesia injections), or Tylenol.
Fig. 2.
Treatment area. The treatment location for this study was the mid to lower face. © 2022 Mica Duran. Used with permission.
Video 1. displays the micro-coring™ treatment.
Statistical Analysis
Subjects who completed at least two treatments were included in the final analysis (n = 51). SAS Institute Inc. version 9.4 was used for analysis. The change in the LWSS was analyzed using repeated measures analysis of variance modeling methods. The model contained a random effect for subjects and an effect for side (left/right). The mean change from baseline for the LWSS was 1.3 grade [95% CI: 1.22, 1.42]. The lower limit of the 95% confidence interval for the mean change was greater than 1.0 grade, indicating that these data support the primary endpoint conclusion of 1 grade or greater mean improvement, assuming independent observations. The primary hypothesis assumption was that the mean difference from baseline would be 0.78 with a 95% CI of 0.15; this value was exceeded with an observed mean difference of 1.3 [95% CI: 1.22, 1.42] and the endpoint was met. A repeated measures analysis of variance (RMANOVA) was also performed with a repeated factor in the model for side (left/right) and reviewer.1–3 (See table, Supplemental Digital Content 1, which displays the results of the RMANOVA. http://links.lww.com/PRSGO/C165.)
RESULTS
Demographics
The study population consisted of predominantly white, non-Hispanic women over 60 years old with type II or III Fitzpatrick skin types and LWSS scores of 3 or 4 at baseline (Table 1).
Table 1.
Baseline Demographic Information
| Demographic Variables | Mean ± SD or % (n/N) |
|---|---|
| Gender (women) | 98.0% (50/51) |
| Age (y) | 62.9 ± 5.92 |
| Height (inch.) | 64.1 ± 2.54 |
| Weight (pounds) | 145.8 ± 27.56 |
| Ethnicity (non-Hispanic) | 96.1% (49/51) |
| Race (White) | (51/51) |
| Fitzpatrick skin type (FPST) I | 9.8% (5/51) |
| FPST II | 68.6% (35/51) |
| FPST III | 15.7% (8/51) |
| FPST IV | 5.9% (3/51) |
| Lemperle score (right side)* | |
| 1 | 2.0% (1/51) |
| 2 | 5.9% (3/51) |
| 3 | 58.8% (30/51) |
| 4 | 33.3% (17/51) |
| Lemperle score (left side)* | |
| 1 | 0.0% (0/51) |
| 2 | 2.0% (1/51) |
| 3 | 60.8% (31/51) |
| 4 | 37.3% (19/51) |
Results are presented as mean ± SD or % (n/N).
*Participants were required to have a Lemperle score of 3 or higher on at least one side.
Wrinkle Reduction
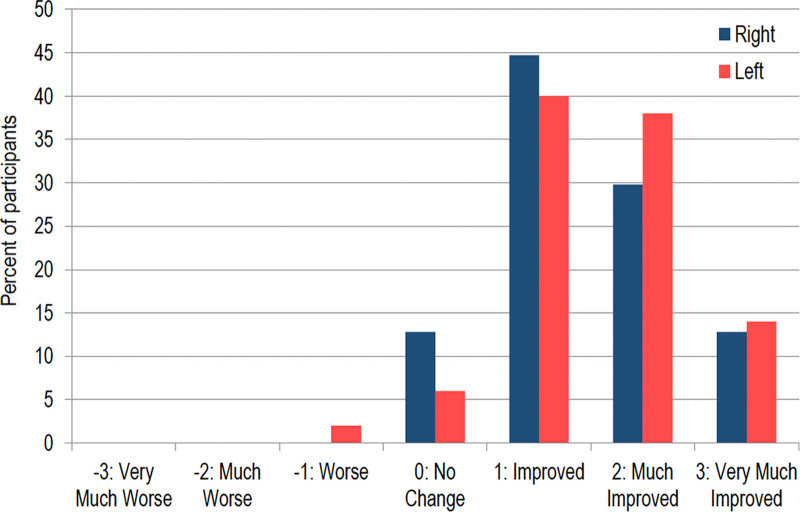
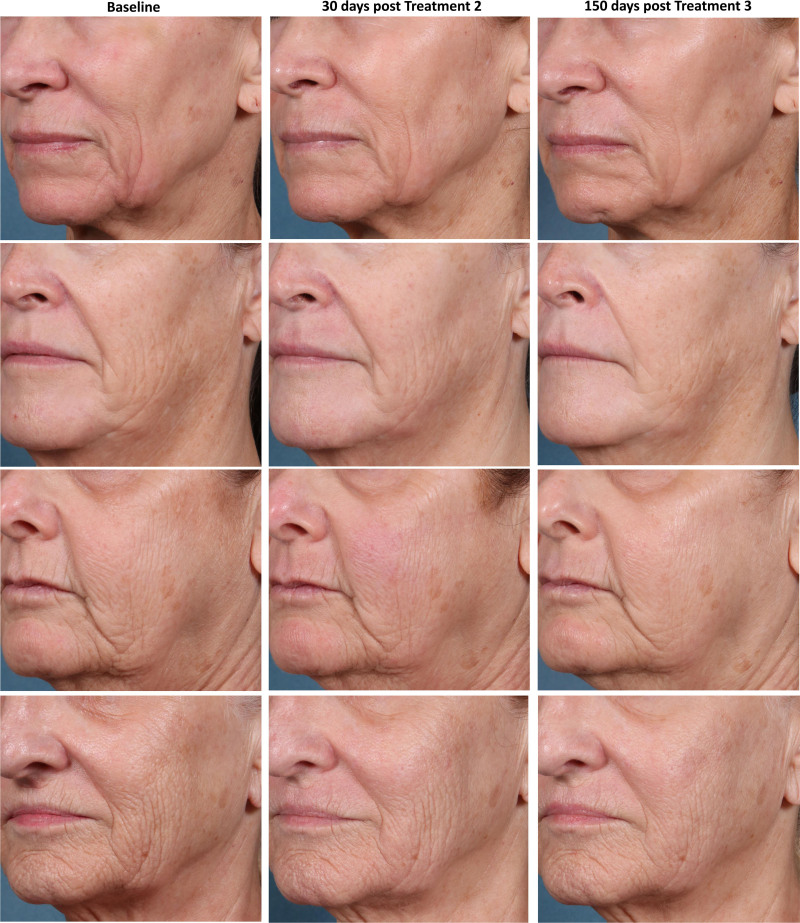
The mean change from baseline for the LWSS was 1.3 grade [95% CI: 1.22, 1.42]. (See figure, Supplemental Digital Content 2, which displays representative results. http://links.lww.com/PRSGO/C166.) The independent reviewer panel correctly identified 84.2% (245/291) of the 90-day post-treatment photographs as post-treatment. Improvement in the GAIS was reported for 89.7% (87/97) of treated sides. The mean change in GAIS demonstrated an average improved score of 1.5 at the last follow-up compared with baseline (Fig. 3).
Fig. 3.
GAIS change from baseline to final follow-up by side. Improvement in the GAIS was reported for 89.7% (87/97) of treated sides. The mean change in GAIS indicates an improved score of 1.5 at the last follow-up compared with the baseline.
Patient Satisfaction
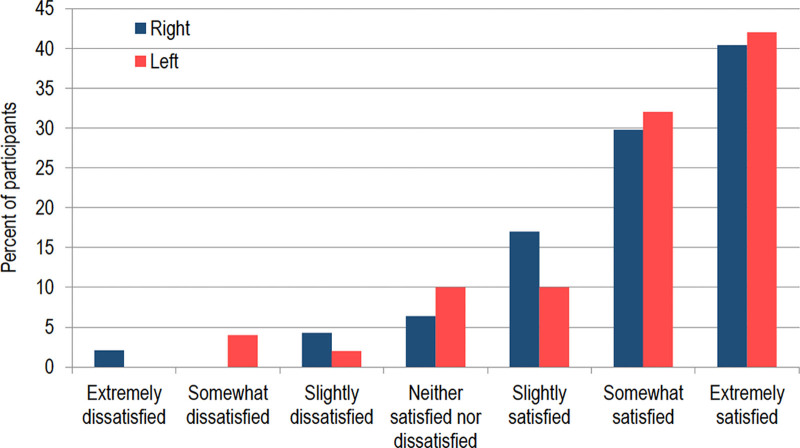
When considering all treatment sides, the overall satisfaction rate (including slightly, somewhat, and extremely satisfied) was 85.6% (83/97) (Figure 4).
Fig. 4.
Participant satisfaction by side. When considering all treatment sides, the overall satisfaction rate (including somewhat, slightly, and extremely satisfied) was 85.6% (83/97).
Treatment Endpoints
Procedure bleeding was mild in most cases (≥78%). There were no reports of severe bleeding. Procedure pain was mild (see Table 2). Most (90%) participants had absent, trace, or mild healing responses by 7 days after treatment (see Figure 5). Moderate ecchymosis, edema, erythema, hyperpigmentation, itching, pain/discomfort, redness, tenderness, and tightness were seen in less than 10% of cases at 7 days. A limited number (<5%) of participants reported moderate dryness, ecchymosis, erythema, hyperpigmentation, and redness at 30 days after treatment. No skin reactions were reported at 90 days after treatment. The mean reported down time was three days.
Table 2.
Procedure Pain
| Side | Pain Score | ||
|---|---|---|---|
| Treatment 1 | Treatment 2 | Treatment 3 | |
| Left | 1.5 ± 1.98 (50) | 2.0 ± 1.77 (50) | 2.8 ± 2.21 (46) |
| Right | 1.3 ± 1.76 (47) | 1.9 ± 1.57 (47) | 2.7 ± 1.99 (43) |
The mean pain experienced during treatment indicates minimal discomfort for all treatments. Results are presented as mean ± SD (number of treated areas).
Fig. 5.
Healing profile. Most (90%) participants had absent, trace, or mild healing responses by 7 days after treatment.
Adverse Events
No serious adverse events were reported. A total of nine adverse events were reported in five participants. Five of the adverse events were not related to the device or treatment. Four of the adverse events in four participants were considered adverse device effects (ADEs). The ADEs were black eye, cheek numbness, redness, and prolonged needle marks on cheek. The ADEs were mild to moderate in severity and did not require any intervention. All adverse events were resolved by the end of the study.
DISCUSSION
MCT is an innovative, minimally invasive treatment method that enables removal of skin without formation of scar, thereby effectively removing redundant skin and skin wrinkles. This study analyzed DMCD for the treatment of moderate to severe wrinkles of the face. We demonstrate that MCT treatment of facial skin is well tolerated with minimal pain and bleeding during treatment, as well as short recovery time and good healing profile. Further, DMCD leads to significant improvement of moderate to severe facial wrinkles with high patient satisfaction.
Moderate to severe skin wrinkles of the lower face in the perioral region including the nasolabial fold and marionette lines are difficult to treat with currently available minimally invasive techniques or facelift surgery. There is a need for novel devices to treat skin redundancy in this area effectively. The current study showed that treatment with DMCD improved mid to lower face wrinkles with a mean change of 1.3 on the LWSS at 90 days after treatment. (See figure, Supplemental Digital Content 2, http://links.lww.com/PRSGO/C166.) In comparison, a single-center, open label study of 48 subjects undergoing four sessions of micro-needling 30 days apart showed a mean change in wrinkle severity of 0.4 for nasolabial folds and 0.3 for marionette lines according to the LWSS at 90 days after treatment.9 Therefore, the DMCD may provide a greater efficacy in the reduction of wrinkles in these key areas. The change in the LWSS for the DCMD was further reflected by the improvements seen in overall aesthetic appearance of the lower face on the GAIS in the vast majority (89.7%) of treated sides. Participants were satisfied with the treatment outcome in most cases (85.6%). Therefore, DMCD appears to be three times as effective as micro-needling in reduction of wrinkles. This change in the LWSS was further reflected by the improvements seen in overall aesthetic appearance of the lower face on the GAIS in the vast majority (89.7%) of treated sides. Participants were satisfied with the treatment outcome in most cases (85.6%).
As demonstrated in prior safety studies, the procedure was very well tolerated with local anesthesia. Bleeding was mild in most cases and the mean procedure pain scores were between 1.2–2.8 (on a scale of 0–10). In comparison, subjects undergoing micro-needling treatment of the face have been shown to experience a mean pain score of 5.3 out of 10 (range 3.8–6.1) during treatment.9 A comparison of pain scores is difficult, given that micro-needling treatment typically does not require local anesthesia when compared with micro-coring treatment.
Comparable to prior clinical trials, the MCT healing profile was favorable with most patients experiencing full recovery after treatment at 7 days (≥78%).6 The most common skin reactions (erythema, edema, ecchymosis, redness, itching, tightness) described by the investigators were comparable to those seen after micro-needling procedures.10 In addition, although the MCT skin reactions were comparable to those seen with fractional CO2 laser treatments, less common but significant complications that have been reported after laser treatment such as bacterial and viral infections and scarring were not observed with the DCMD.11,12 The reported skin reactions were trace to mild, and no moderate or severe skin responses to treatment were observed. Given the mild nature of skin changes seen after MCT treatment, application of makeup starting 48–72h after treatment can mask skin changes. The skin recovery time from MCT appears to be slightly longer than for micro-needling procedures, and significantly shorter than for fractional CO2 laser resurfacing, after which most patients experience side effects for around 14 days.11,13 However, participants after MCT treatment were able to return to their normal activities of living almost immediately with little down time (three days on average).
No long-term skin reactions were observed at the last follow-up visit, including hypopigmentation or hyperpigmentation. This is an advantage over laser-based treatments that are associated with postinflammatory hyperpigmentation, as well as hypopigmentation.12 Although the underlying pathomechanism of pigmentation disorders remains unknown, it is possible that energy-based devices such as lasers increase the risk of pigment disturbances as compared to non-energy-based devices such as DMCD.
Around 15% of patients did not experience the expected results, which also correlates with the 15% of patients; the independent review panel was not able to identify a difference between pre- and post-treatment pictures. Other minimally invasive rejuvenation techniques have reported similar patient satisfaction rates.14 One possible confounding factor that has been reported in the literature are high patient expectations pre-procedure that lead to lower satisfaction rates with other devices such as micro-needling.15 However, for any type of procedure, we would not expect all patients to be satisfied.
There are several limitations to this study. First, the study population consisted predominantly of white, non-Hispanic women over 60 years old with type II or III Fitzpatrick skin type. Further studies will be required to demonstrate safety and efficacy in subjects with Fitzpatrick skin type IV and higher, as well as in different gender, age groups and ethnicities. In addition, there was no control area that was left untreated. However, standardized photography allowed for comparison to baseline.
CONCLUSIONS
The results of this micro-coring technology study demonstrate the safety and efficacy of the DMCD for the reduction of moderate to severe facial wrinkles in the intended patient population. DMCD led to significant improvement of facial wrinkles in the mid to lower face with high patient satisfaction and fast recovery time and should be considered in patients who are seeking minimally invasive treatment for wrinkles of the face.
Supplementary Material
Footnotes
Published online 17 October 2022.
Disclosure: All authors are consultants for Cytrellis Biosystems Inc.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Manstein D, Herron GS, Sink RK, et al. Fractional photothermolysis: a new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg Med. 2004;34:426–438. [DOI] [PubMed] [Google Scholar]
- 2.Beasley KL, Weiss RA. Radiofrequency in cosmetic dermatology. Dermatol Clin. 2014;32:79–90. [DOI] [PubMed] [Google Scholar]
- 3.Alster TS, Graham PM. Microneedling: a review and practical guide. Dermatol Surg. 2018;44:397–404. [DOI] [PubMed] [Google Scholar]
- 4.Angra K, Alhaddad M, Boen M, et al. Prospective clinical trial of the latest generation of noninvasive monopolar radiofrequency for the treatment of facial and upper neck skin laxity. Dermatol Surg. 2021;47:762–766. [DOI] [PubMed] [Google Scholar]
- 5.Stuzin JM. MOC-PSSM CME article: Face lifting. Plast Reconstr Surg. 2008;121(1 Suppl):1–19. [DOI] [PubMed] [Google Scholar]
- 6.Fernandes JR, Samayoa JC, Broelsch GF, et al. Micro-mechanical fractional skin rejuvenation. Plast Reconstr Surg. 2013;131:216–223. [DOI] [PubMed] [Google Scholar]
- 7.Pozner JN, Kilmer SL, Geronemus RG, et al. Cytrellis: A novel microcoring technology for scarless skin removal: summary of three prospective clinical trials. Plast Reconstr Surg Glob Open. 2021;9:e3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lemperle G, Holmes RE, Cohen SR, et al. A classification of facial wrinkles. Plast Reconstr Surg. 2001;108:1735–50; discussion 1751. [DOI] [PubMed] [Google Scholar]
- 9.Ablon G. Safety and effectiveness of an automated microneedling device in improving the signs of aging skin. J Clin Aesthet Dermatol. 2018;11:29–34. [PMC free article] [PubMed] [Google Scholar]
- 10.Gowda A, Healey B, Ezaldein H, et al. A systematic review examining the potential adverse effects of microneedling. J Clin Aesthet Dermatol. 2021;14:45–54. [PMC free article] [PubMed] [Google Scholar]
- 11.Berlin AL, Hussain M, Phelps R, et al. A prospective study of fractional scanned nonsequential carbon dioxide laser resurfacing: a clinical and histopathologic evaluation. Dermatol Surg. 2009;35:222–228. [DOI] [PubMed] [Google Scholar]
- 12.Graber EM, Tanzi EL, Alster TS. Side effects and complications of fractional laser photothermolysis: experience with 961 treatments. Dermatol Surg. 2008;34:301–5; discussion 305. [DOI] [PubMed] [Google Scholar]
- 13.Karsai S, Czarnecka A, Jünger M, et al. Ablative fractional lasers (CO(2) and Er:YAG): a randomized controlled double-blind split-face trial of the treatment of peri-orbital rhytides. Lasers Surg Med. 2010;42:160–167. [DOI] [PubMed] [Google Scholar]
- 14.Dadkhahfar S, Fadakar K, Robati RM. Efficacy and safety of long pulse Nd:YAG laser versus fractional erbium:YAG laser in the treatment of facial skin wrinkles. Lasers Med Sci. 2019;34:457–464. [DOI] [PubMed] [Google Scholar]
- 15.Iriarte C, Awosika O, Rengifo-Pardo M, et al. Review of applications of microneedling in dermatology. Clin Cosmet Investig Dermatol. 2017;10:289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.