Abstract
Introduction
We examined the relationship between current tobacco use and functionally important respiratory symptoms.
Methods
Longitudinal cohort study of 16 295 US adults without COPD in Waves 2–3 (W2–3, 2014–2016) of the Population Assessment of Tobacco and Health Study. Exposure—Ten mutually exclusive categories of tobacco use including single product, multiple product, former, and never use (reference). Outcome—Seven questions assessing wheezing/cough were summed to create a respiratory symptom index; cutoffs of ≥2 and ≥3 were associated with functional limitations and poorer health. Multivariable regressions examined both cutoffs cross-sectionally and change over approximately 12 months, adjusting for confounders.
Results
All tobacco use categories featuring cigarettes (>2/3’s of users) were associated with higher risk (vs. never users) for functionally important respiratory symptoms at W2, for example, at symptom severity ≥ 3, risk ratio for exclusive cigarette use was 2.34 [95% CI, 1.92, 2.85] and for worsening symptoms at W3 was 2.80 [2.08, 3.76]. There was largely no increased symptom risk for exclusive use of cigars, smokeless tobacco, hookah, or e-cigarettes (adjustment for pack-years and marijuana attenuated the cross-sectional e-cigarette association from 1.53(95% CI 0.98, 2.40) to 1.05 (0.67, 1.63); RRs for these products were also significantly lower compared to exclusive use of cigarettes. The longitudinal e-cigarette–respiratory symptom association was sensitive to the respiratory index cutoff level; exclusive e-cigarette use was associated with worsening symptoms at an index cutoff ≥ 2 (RR = 1.63 [1.02, 2.59]) and with symptom improvement at an index cutoff of ≥ 3 (RR = 1.64 [1.04, 2.58]).
Conclusions
Past and current cigarette smoking drove functionally important respiratory symptoms, while exclusive use of other tobacco products was largely not associated. However, the relationship between e-cigarette use and symptoms was sensitive to adjustment for pack-years and symptom severity.
Implications
How noncigarette tobacco products affect respiratory symptoms is not clear; some studies implicate e-cigarettes. We examined functionally important respiratory symptoms (wheezing/nighttime cough) among US adults without COPD. The majority of adult tobacco users smoke cigarettes and have higher risk of respiratory symptoms and worsening of symptoms, regardless of other products used with them. Exclusive use of other tobacco products (e-cigarettes, cigars, smokeless, hookah) was largely not associated with functionally important respiratory symptoms and risks associated with their use was significantly lower than for cigarettes. The association for e-cigarettes was greatly attenuated by adjustment for cigarette pack-years and sensitive to how symptoms were defined.
Introduction
Cigarette smoking causes and exacerbates chronic obstructive pulmonary disease (COPD) and asthma1 and is associated with wheezing and cough in populations without a respiratory diagnosis.2 Quitting cigarettes improves respiratory symptoms3 and limits lung function deterioration.4 While the relationship between cigarette smoking and respiratory symptoms is well-established, the relationship between use of other tobacco products besides cigarettes and respiratory symptoms in adults is less clear.
Changes in the tobacco market, in part, reflect efforts to market products that may cause less harm than cigarettes. Electronic nicotine delivery devices (hereafter referred to as e-cigarettes) may represent such a product. With respect to respiratory symptoms, findings have been mixed, however. Numerous animal and in vitro studies raise theoretical concerns about e-cigarette use and lung disease.5–12 Short-term human experimental studies have linked adult e-cigarette use with wheezing and acute alterations in lung function13 and lower forced expiratory flow.14 One longer term 12-week prospective study of cigarette smokers switching to e-cigarettes found no effects on lung function,15 and two 1-year randomized controlled clinical trials found reduced cough and improved lung function in persons who used e-cigarettes to reduce or quit cigarettes.16,17 Cross-sectional observational studies using Waves (W) 2 and 3 data from the Population Assessment of Tobacco and Health (PATH) Study18,19 have found an association between e-cigarette use and respiratory symptoms. One longitudinal W3–W4 PATH Study analysis found no relation between exclusive e-cigarette use and incident respiratory symptoms but suggested that dual users of cigarettes and e-cigarettes had significantly higher risk for symptom onset compared to exclusive cigarette users.20 Finally, one prospective study of young adults found an association between cannabis vaping (but not nicotine vaping) and respiratory symptoms.21
There are many design issues that make these studies hard to compare. The clinical importance of the respiratory outcome is not clear in most cases because the multiple wheezing questions are analyzed in isolation from each other, or an endorsement of only one item is considered symptomatic. Many of the studies included adults with COPD, which is a diagnosis strongly linked to a history of cigarette smoking, and many people with COPD have chronic severe wheezing and dyspnea. Another concern is residual confounding: Most of the studies showing an association between e-cigarette use and respiratory symptoms failed to adjust for cigarette smoking history and concurrent marijuana use, both associated with respiratory problems and concurrent e-cigarette use. Finally, few studies addressed alternative tobacco product categories besides e-cigarettes.
To better understand these divergent findings on how tobacco product use relates to respiratory health, we analyzed W2 and W3 data from the PATH Study.22 We developed a dependent variable that incorporated all available questions on wheezing and nighttime cough and determined cutoff values associated with functional outcomes. We focused on both cross-sectional and longitudinal associations between functionally important respiratory symptoms and 10 mutually exclusive tobacco product use categories, adjusting for past cigarette smoking history and concurrent marijuana use. We also examined results for two different cutoff values for a respiratory symptom index to test for sensitivity to symptom severity.
Methods
Study Design, Setting, and Participants
Recruitment for W1 (2013–2014) of the PATH Study employed stratified address-based, area-probability sampling with oversampling of adult tobacco users, young adults (18–24 years), and African-Americans. An in-person screener selected youths and adults from households at W1, and audio computer-assisted self-interviews collected data on tobacco use and health outcomes. Respiratory symptoms were assessed in W2 (2014–2015) and W3 (2015–2016), including 28 362 and 28 148 adult participants, respectively (weighted response rates of 83.2% and 78.4%, respectively). Mean time between W2 and W3 adult interviews was 53.8 weeks.
Our analyses utilized the adult W2 and W3 Restricted Use Files (https://doi.org/10.3886/ICPSR36231.v21). We selected all W2 adults without COPD or other nonasthma respiratory diseases (N = 24 798, see flow diagram, Supplementary Figure 1). We report a complete case analysis excluding participants lost to follow up at W3 (N = 2837, 11%) and those with missing data on any variables (N = 5666, 23%) with final analytic sample of 16 295.
PATH Study design and methods,22 interviewing procedures, questionnaires, sampling, weighting, and response rates are in the PATH Study Restricted Use Files User Guide.23 All respondents provided informed consent; Westat’s IRB approved the study.
Outcomes
Functionally Important Respiratory Symptoms
The PATH Study utilized the seven wheezing/cough questions from the International Study of Allergies and Asthma in Childhood (ISAAC) core wheezing module.24 Responses to the ISAAC questions were used to create a respiratory symptom index (range 0 [none] to 9 [worst]). This index was validated in the PATH Study adult sample based on its internal consistency, test-retest reliability, and its strong association with self-reported physician diagnosis of asthma. Respondents with cutoff values of ≥2 and ≥3 had significantly higher risk for physical limitations, fatigue, and poorer perception of health assessed by items from the Patient Reported Outcomes Measurement Information System (physical question bank).25 The full validation of this measure is published elsewhere.26 Because the validation supported cutoff values of of ≥2 and ≥3, we examined both as a test of the sensitivity of the findings to respiratory symptom severity.
Exposures
Tobacco Product Use
Adults reported their lifetime and past 30-day (P30D) use of cigarettes, cigars (traditional cigars, cigarillos, and filtered cigars), pipe tobacco, hookah, snus pouches, other smokeless tobacco (including loose snus, moist snuff, dip, spit, or chewing tobacco), and e-cigarettes, with pictures and descriptions displayed for each product to ensure accuracy. Twelve dummy variables defined all past and current tobacco use possibilities (never use, former use, exclusive use, and polytobacco use; see Supplementary Table 1). The former established tobacco user category includes all established users (e.g., lifetime use of more than 100 cigarettes) who did not use any tobacco product in the past 30 days.
Covariates
Covariates (Supplementary Table 1) were derived from W1 and W2, and included variables associated with both tobacco exposure and functionally important respiratory symptoms. Low socioeconomic status is associated with tobacco use and poorer lung function.27 Sociodemographic variables included age, sex, race/ethnicity, education, income, and urbanicity. Medical conditions that could result from tobacco use and also cause respiratory symptoms included asthma, congestive heart failure, heart attack, diabetes, cancer, being overweight, and use of antihypertensives known to cause coughing or wheezing (beta blockers, angiotensin receptor blockers, and ace inhibitors). Smoke-related exposures included pack-years of cigarette smoking, second-hand smoke exposure, and marijuana use.
Calculating Pack Years of Smoking
We were particularly concerned with adjusting results carefully for each individual’s cigarette smoking history, an important predictor of respiratory outcomes. We derived lifetime pack years to account for cigarette smoking history in this analysis. Lifetime pack years is a clinical metric calculated by multiplying the number of packs of cigarettes per day someone smokes by the number of years they have smoked cigarettes. The following text annotates the algorithm to calculate Wave 1 lifetime pack years. Data from Wave 1 lifetime pack years was used in conjunction with variables describing subsequent cigarette use to determine lifetime pack years at W2 and beyond. Never smokers were assigned a pack years value of zero. All questions used in the algorithm and response categories are listed in Supplementary Table 3.
Because of routing instructions in the PATH Study interview, only those respondents who said that they have smoked cigarettes “fairly regularly” (question R01_AC9002) were asked about how long they have smoked (if a current cigarette smoker) or did smoke (if they had quit smoking cigarettes by Wave 1). For any respondent at Wave 1 who currently smokes regularly or formerly smoked fairly regularly, lifetime pack years was calculated by multiplying the number of cigarette packs smoked per day (assuming 20 cigarettes in a pack) by the number of years they have smoked fairly regularly. Two different formulas were used for this calculation, depending on answers to the questions for variable R01_AC9004 (cigarettes or packs per day) and R01_AC9009 (smoked that same amount since started smoking failry regularly).
1) If a respondent smoked the same frequency per day since they began smoking cigarettes, packs of cigarettes smoked per day was calculated and then multiplied by the number of years a respondent has been smoking regularly.
2)If respondent has not smoked the same amount per day since they started, average packs of cigarettes smoked per day was calculated by taking an average of the respondent’s current smoking frequency and their past smoking frequency. Average packs of cigarettes smoked per day is then multiplied by the number of years a respondent has been smoking regularly.
If a respondent has not smoked fairly regularly, there were additional questions used to estimate lifetime pack years, starting with the question for R01_AC1005 (lifetime cigarettes smoked).
1) If respondent has smoked 100 or more cigarettes in their entire life (R01_AC1005 = 6), the cigarettes per day value, if available, is multiplied by estimated number of years smoking cigarettes.
2) Respondents who have not smoked fairly regularly and have also not smoked more than 100 cigarettes in their entire life (R01_AC1005 response = 1–5) fall into two subcategories:
2a) If a respondent has smoked one or more puffs but never a whole cigarette (response = 1), a pack years value of 0 was assigned.
2b) If a respondent selected responses 2–5, each of these categories was assigned the following pack years value based on the median of the category and estimated years of cigarette use: 1–10 cigarettes = 0.0008; 11–20 cigarettes = 0.002; 21–50 cigarettes = 0.005; 51–99 cigarettes = 0.01.
Statistical Analysis
Missing Data
Some 34% (see Supplementary Figure 1) of the sample had missing data on one or more variable or were lost to follow up. Compared to the analytic sample, the nonanalytic sample was older (see Supplementary Table 2), included more minorities, had lower education and income levels, contained fewer exclusive cigarette smokers (11.4 vs. 16.0%) and exhibited more respiratory symptoms (7.2% vs 13.3%). The longitudinal weights were designed to address nonresponse bias. As a further check, we repeated all main analyses for cutoff of 3 using multiple imputation to address missing data for all predictors and covariates. Because the multiple imputation analyses found only small differences, primarily in the confidence intervals (CI), we report only the weighted complete case analysis results.
Analytical Approach
All main analyses were weighted using the W3 longitudinal (all-waves) full-sample and replicate weights to adjust for the complex sample design and loss to follow up. Variances were estimated using the BRR method28 with Fay’s adjustment set to 0.3 to increase estimate stability.29 Pack-years of cigarette smoking, tobacco product P30D frequency variables (Figures 1 and 2), and second-hand smoke exposure were Winsorized at the 95th, 95th, and 99th percentiles, respectively, to address outliers.30
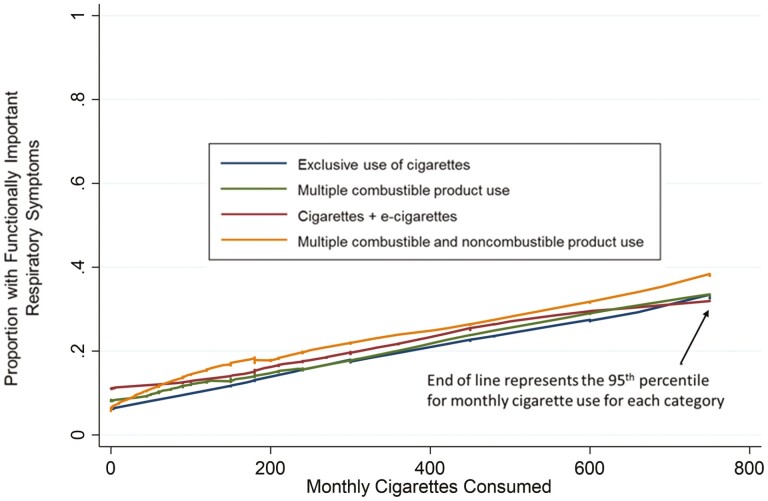
Figure 1.
Unweighted lowess smoothed curvesa illustrating the relation between the proportion with functionally important respiratory symptomsb and intensity of cigarette smoking at Wave 2 of the Population Assessment of Tobacco and Health (PATH) Study for four groups of tobacco users among whom cigarette smoking played a prominent role in their tobacco use.c,d.
aUnweighted lowess smoothed curves show the proportion of the four tobacco use groups that contain cigarette users with functionally important respiratory symptoms with the quantity of monthly cigarettes consumed. Cigarettes per month was calculated by multiplying how many cigarettes a respondent used each day by the number of days they used the products in the past 30 days. This variable was modified by taking outliers above the 95th percentile and recoding them to the 95th percentile value based on descriptive analysis of data for cases in the analysis sample who used more than 0 cigarettes per month. In the PATH Study, only ever cigarette users were asked about their cigarette consumption, so respondents who were never cigarette smokers were then recoded as having consumed 0 cigarettes per month. bCut-off level for what is considered functionally important is a respiratory index value of ≥3. cWeighted proportion (SE) with P30D cigarette use in each polycombustible group: Multiple combustible product use = 90.0% (1.0). Multiple combustible and noncombustible product use = 80.4% (1.5). dWeighted mean (SE) monthly cigarettes consumed for each group. Exclusive use of cigarettes = 313 (5.5). Multiple combustible product use = 224 (9.1). Cigarettes + e-cigarettes = 296 (9.6). Multiple combustible and noncombustible product use = 216 (9.2).
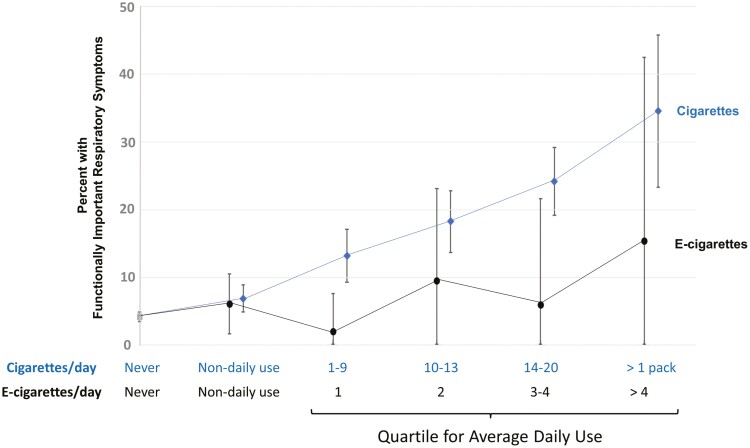
Figure 2.
Relation between the intensitya of tobacco product use and weighted percentage with functionally important respiratory symptoms for exclusive users of cigarettes or e-cigarettes, at Wave 2 of the Population Assessment of Tobacco and Health (PATH) Study,b after adjusting for pack years of cigarette smoking.
aCigarettes and e-cigarettes per day are calculated by multiplying how many product units a respondent used each day by the number of days they used the products in the past 30 days and then dividing by 30. These variables were modified by taking outliers above the 95th percentile and recoding them to the 95th percentile value based on descriptive analysis of data for cases in the analysis sample who used more than 0 cigarettes or e-cigarettes per month. For e-cigarettes, participants were asked about daily use depending on the type of e-cigarette they regularly used. The five applicable types were disposable e-cigarette, nonrefillable cartridge e-cigarette, refillable cartridge e-cigarette, refillable tank system e-cigarette, or unknown e-cigarette. Depending on their type, respondents were asked on average, how many (1) e-cigarettes; (2) e-cigarette cartridges; or (3) milliliters of e-liquid they now use each day. bN = 9402 adult respondents without chronic obstructive pulmonary disease or other nonasthma respiratory disease and with PATH Study longitudinal (all-waves) weights and complete data on all study variables, plus Wave 2 cigarette and e-cigarette frequency/intensity variables. Unweighted Ns for each mutually exclusive use group: Never tobacco = 5888, Non-daily exclusive cigarette use = 978, Daily exclusive cigarette use Q1 (1–9 per day) = 597, Daily exclusive cigarette use Q2 (10–13 per day) = 543, Daily exclusive cigarette use Q3 (14–20 per day) = 936, Daily exclusive cigarette use Q4 (Greater than 1 pack per day) = 175, Non-daily exclusive e-cigarette use = 174, Daily exclusive e-cigarette use Q1 (1 per day) = 35, Daily exclusive e-cigarette use Q2 (2 per day) = 34, Daily exclusive e-cigarette use Q3 (3-4 per day) = 17, Daily exclusive e-cigarette use Q4 (Greater than 4 e-cigarettes per day) = 25. Quartile values were determined based on descriptive analysis of data for cases in the analysis sample who were exclusive, everyday users of either cigarettes or e-cigarettes. Error bars represent 95% confidence interval.
We examined unadjusted associations between tobacco product use at W2 and the presence of functionally important respiratory symptoms (at a cutoff of 3) then used multivariable weighted Poisson regression to obtain adjusted risk ratios (RR) and 95% CIs for each dichotomous outcome.31 Next, we evaluated longitudinal associations between W2 tobacco product use and changes in respiratory symptoms from W2 to W3. Symptoms “worsened” if the symptom score was <3 at W2 but ≥3 at W3. Symptoms “improved” if the symptom score was ≥3 at W2 but <3 at W3. Finally, we tested the sensitivity of the findings to symptom severity level by rerunning all analyses at a cutoff level of ≥2. For each multivariable analysis, post hoc two-group comparisons (via the method of linear contrasts) were completed to determine if the adjusted risk for each tobacco product use category was significantly different from exclusive cigarette users. All analyses used Stata survey data procedures, version 15.1; standard errors for Tables 3 and 4 and estimates for all covariates for Tables 2–4 are included in Supplementary Tables 4–6.
Table 3.
Effect of Cut-off Level for Respiratory Index on the Longitudinal Association Between Wave 2 Tobacco Use and Worsening Functionally Important Respiratory Symptoms at Wave 3, Population Assessment of Tobacco and Health (PATH) Studya
| Risk factor at baseline | Worsening of respiratory symptoms over time | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1: Respiratory index cutoff ≥3b | Model 2: Respiratory index cutoff ≥2b | |||||||
| Asymptomatic Wave 2 → Symptomatic Wave 3 | Asymptomatic Wave 2 → Symptomatic Wave 3 | |||||||
| (Unweighted N = 14 713) → (5% became symptomatic) | (Unweighted N = 13 956) → (8% became symptomatic) | |||||||
| Unweighted N | Worsen % | Adjusted RRc | 95% CI | Unweighted N | Worsen % | Adjusted RRc | 95% CI | |
| Wave 2 past month tobacco used | ||||||||
| Never | 5638 | 3% | Ref | Ref | 5499 | 5% | Ref | Ref |
| Former | 2131 | 4% | 1.21e | [0.82, 1.80] | 2036 | 8% | 1.32e | [0.97, 1.79] |
| Exclusive use categories | ||||||||
| Cigarette | 2671 | 12% | 2.80 | [2.08, 3.76] | 2444 | 16% | 2.25 | [1.81, 2.81] |
| E-cigarette | 304 | 7% | 1.58e | [0.84, 2.96] | 284 | 12% | 1.63 | [1.02, 2.59] |
| Cigars | 523 | 3% | 0.81e | [0.44, 1.50] | 501 | 4% | 0.70e | [0.42, 1.18] |
| Smokeless tobacco | 421 | 4% | 1.03e | [0.55, 1.90] | 410 | 10% | 1.48e | [0.98, 2.25] |
| Hookah | 307 | 3%† | 0.90 | [0.23, 3.52] | 305 | 5%† | 1.03e | [0.48, 2.21] |
| Multiple use categories | ||||||||
| Cigarette + e-cigarette | 640 | 13% | 2.64 | [1.88, 3.70] | 567 | 17% | 2.20 | [1.67, 2.89] |
| Combustible only | 1086 | 9% | 1.85e | [1.31, 2.61] | 999 | 14% | 1.93 | [1.50, 2.50] |
| Otherf combustible + noncombustible | 933 | 9% | 2.03e | [1.35, 3.05] | 854 | 12% | 1.64e | [1.19, 2.27] |
| Other smoke-related exposures | ||||||||
| Cigarette pack years (per each additional 5 pack years) | 14, 713 | N/A | 1.07 | [1.02, 1.13] | 13 956 | N/A | 1.06 | [1.03, 1.11] |
| Second-hand smoke exposure (per each additional 5 h/week) | 14 713 | N/A | 1.04 | [1.02, 1.05] | 13 956 | N/A | 1.03 | [1.01, 1.04] |
| Past-month marijuana useg | ||||||||
| No | 12 498 | 4% | Ref | Ref | 11 944 | 7% | Ref | Ref |
| Yes | 2215 | 10% | 1.53 | [1.23, 1.90] | 2012 | 14% | 1.38 | [1.15, 1.67] |
N = 16 295 adult respondents without chronic obstructive pulmonary disease or other nonasthma respiratory disease and with PATH Study longitudinal (all-waves) weights and complete data on all variables.
In Model 1, symptom worsening is defined by moving from a respiratory symptom score of <3 to ≥3. In Model 2, symptom worsening is defined by moving from a respiratory symptom score of <2 to ≥2.
All risk ratios (RR) adjust for the variables in the table, age, sex, race/ethnicity, education, income, asthma status, BMI, CHF, heart attack, diabetes, cancer, regular use of beta blockers, angiotensin receptor blockers, or ace inhibitors, and living in an urban area.
Data are not presented for exclusive pipe (N = 44), and dual e-cigarette + smokeless (N = 20) users due to small sample size. Never tobacco user category includes former experimental (e.g., lifetime use of < 100 cigarettes) users; former established user category includes all established users (e.g., lifetime use of more than 100 cigarettes) who did not use a tobacco product in the past 30 days.
These exclusive product risk ratios are significantly different from exclusive cigarette use.
Other than exclusive dual cigarette and e-cigarette users.
Marijuana use variable does not distinguish between combustible and noncombustible use.
Estimate should be interpreted with caution because it has low statistical precision. It is based on a denominator sample size of less than 50, or the coefficient of variation of the estimate or its complement is larger than 30%.
Supplementary Table 5 reports standard errors for all the weighted estimates presented in this table and adjusted RRs for all the variables in the model.
Table 4.
Effect of Cut-off level for respiratory index on the weighted longitudinal association between Wave 2 tobacco use and selected constructs and improvement in functionally important respiratory symptoms at Wave 3, Population Assessment of Tobacco and Health (PATH) Studya
| Risk factor at baseline | Improvement of respiratory symptoms over time | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1: Respiratory index cutoff ≥ 3b | Model 2: Respiratory index cutoff ≥ 2b | |||||||
| Symptomatic Wave 2→Asymptomatic Wave 3 | Symptomatic Wave 2→Asymptomatic Wave 3 | |||||||
| (Unweighted N = 1582) → (29% became asymptomatic) | (Unweighted N = 2339) → (21% became asymptomatic) | |||||||
| Unweighted N | Improve % | Adjusted RRc | 95% CI | Unweighted N | Improve % | Adjusted RRc | 95% CI | |
| Wave 2 past month tobacco used | ||||||||
| Never | 250 | 36% | Ref | Ref | 389 | 25% | Ref | Ref |
| Former | 160 | 28% | 0.89 | [0.61, 1.30] | 255 | 26% | 1.19e | [0.83, 1.71] |
| Exclusive use categories | ||||||||
| Cigarette | 559 | 26% | 0.86 | [0.63, 1.18] | 786 | 13% | 0.57 | [0.40, 0.82] |
| E-cigarette | 23 | 47%† | 1.64e | [1.04, 2.58] | 43 | 32% | 1.59e | [0.89, 2.85] |
| Cigars | 25 | 29%† | 0.87 | [0.35, 2.20] | 47 | 36% | 1.43e | [0.87, 2.37] |
| Smokeless tobacco | 29 | 21%† | 0.76 | [0.36, 1.59] | 40 | 23% | 1.32e | [0.71, 2.47] |
| Hookah | 14 | 43%† | 1.28 | [0.56, 2.95] | 16 | 32% | 1.60 | [0.54, 4.71] |
| Multiple use categories | ||||||||
| Cigarette + e-cigarette | 152 | 28% | 0.95 | [0.64, 1.42] | 225 | 15% | 0.72 | [0.47, 1.11] |
| Combustible only | 180 | 23% | 0.78 | [0.50, 1.21] | 267 | 17% | 0.76 | [0.52, 1.11] |
| Otherf combustible + noncombustible | 185 | 25% | 0.91 | [0.61, 1.34] | 264 | 20% | 0.97e | [0.67, 1.39] |
| Other smoke-related exposures | ||||||||
| Cigarette pack years (per each additional 5 pack years) |
1582 | N/A | 0.89 | [0.84, 0.94] | 2339 | N/A | 0.92 | [0.87, 0.98] |
| Second-hand smoke exposure (per each additional 5 hrs/week) |
1582 | N/A | 0.98 | [0.96, 1.01] | 2339 | N/A | 0.96 | [0.94, 0.99] |
| Past-month marijuana useg | ||||||||
| No | 1128 | 30% | Ref | Ref | 1682 | 22% | Ref | Ref |
| Yes | 454 | 25% | 0.86 | [0.67, 1.11] | 657 | 17% | 0.87 | [0.65, 1.17] |
N = 16 295 adult respondents without chronic obstructive pulmonary disease or other nonasthma respiratory disease and with PATH Study longitudinal (all-waves) weights and complete data on all variables.
In Model 1, symptom improvement is defined as moving from a symptom score of ≥3 to <3. In Model 2, symptom improvement is defined as moving from a symptom score of ≥2 to <2.
All risk ratios (RR) adjust for the variables in the table, age, sex, race/ethnicity, education, income, BMI, CHF, heart attack, diabetes, cancer, regular use of beta blockers, angiotensin receptor blockers, or ace inhibitors, and living in an urban area.
Data are not presented for exclusive pipe (N = 44), and dual e-cigarette + smokeless (N = 20) users due to small sample size. Never tobacco user category includes former experimental (e.g., lifetime use of <100 cigarettes) users; former established user category includes all established users (e.g., lifetime use of more than 100 cigarettes) who did not use a tobacco product in the past 30 days.
These exclusive product risk ratios are significantly different from exclusive cigarette use.
Other than exclusive dual cigarette and e-cigarette users.
Marijuana use variable does not distinguish between combustible and noncombustible use.
Estimate should be interpreted with caution because it has low statistical precision. It is based on a denominator sample size of less than 50, or the coefficient of variation of the estimate or its complement is larger than 30%.
Supplementary Table 6 reports standard errors for all the weighted estimates presented in this table and adjusted RRs for all the variables in the model.
Table 2.
Weighted Cross-sectional Associations Between Current Tobacco Product Use and Functionally Important Respiratory Symptoms at Wave 2 of the Population Assessment of Tobacco and Health (PATH) Study,a and Influence of Key Confounders
| Risk factor at baseline | Key Confoundersb Included in Multivariable Regression | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No key confounders | Cigarette pack years | Second-hand smoke | Current marijuana use | Full multivariable model | ||||||
| RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | |
| Wave 2 past month tobacco usec | ||||||||||
| Never | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Former | 1.28 | [1.03, 1.60] | 0.96 | [0.77, 1.21] | 1.28 | [1.02, 1.59] | 1.23 | [0.99, 1.53] | 0.93d | [0.74, 1.17] |
| Exclusive use categories | ||||||||||
| Cigarette | 3.82 | [3.18, 4.60] | 2.65 | [2.18, 3.21] | 3.55 | [2.96, 4.26] | 3.52 | [2.92, 4.25] | 2.34 | [1.92, 2.85] |
| E-cigarette | 1.53 | [0.98, 2.40] | 1.14 | [0.73, 1.79] | 1.51 | [0.96, 2.39] | 1.39 | [0.90, 2.14] | 1.05d | [0.67, 1.63] |
| Cigars | 1.04 | [0.63, 1.73] | 0.87 | [0.51, 1.48] | 1.04 | [0.63, 1.72] | 0.94 | [0.56, 1.58] | 0.78d | [0.45, 1.36] |
| Smokeless | 0.86 | [0.55, 1.34] | 0.79 | [0.51, 1.22] | 0.86 | [0.55, 1.34] | 0.84 | [0.54, 1.31] | 0.77d | [0.50, 1.20] |
| Hookah | 1.00 | [0.60, 1.67] | 0.85 | [0.51, 1.41] | 1.00 | [0.60, 1.67] | 0.92 | [0.55, 1.53] | 0.78d | [0.47, 1.29] |
| Multiple use categories | ||||||||||
| Cigarette + e-cigarette | 3.69 | [2.85, 4.78] | 2.52 | [1.97, 3.22] | 3.29 | [2.52, 4.29] | 3.33 | [2.56, 4.33] | 2.13 | [1.64, 2.77] |
| Combustible only | 3.45 | [2.78, 4.29] | 2.61 | [2.11, 3.22] | 3.24 | [2.61, 4.03] | 2.85 | [2.24, 3.63] | 2.08 | [1.64, 2.64] |
| Othere combustible + noncombustible | 3.29 | [2.67, 4.05] | 2.48 | [2.00, 3.08] | 3.04 | [2.45, 3.76] | 2.75 | [2.19, 3.44] | 2.00 | [1.58, 2.53] |
| Other smoke-related exposures | ||||||||||
| Cigarette pack years (per each additional 5 pack years) | N/A | N/A | 1.13 | [1.10, 1.17] | N/A | N/A | N/A | N/A | 1.13 | [1.09, 1.16] |
| Second-hand smoke exposure(per each additional 5 hours/week) | N/A | N/A | N/A | N/A | 1.03 | [1.02, 1.04] | N/A | N/A | 1.02 | [1.01, 1.03] |
| Past-month marijuana usef | ||||||||||
| No | N/A | N/A | N/A | N/A | N/A | N/A | Ref | Ref | Ref | Ref |
| Yes | N/A | N/A | N/A | N/A | N/A | N/A | 1.62 | [1.40, 1.88] | 1.60 | [1.37, 1.87] |
N = 16 295 respondents with complete data at Wave 2. In this table, functionally important respiratory symptoms are defined using a respiratory symptom score of ≥3.
All risk ratios (RR) adjust for the variables in the table, age, sex, race/ethnicity, education, income, CHF, heart attack, diabetes, cancer, BMI, asthma status, regular use of beta blockers, angiotensin receptor blockers or ace inhibitors, and living in an urban area.
Data are not presented for exclusive pipe (N = 44), and dual ENDS + Smokeless (N = 20) users due to small sample size. Never tobacco user category includes former experimental (e.g., lifetime use of <100 cigarettes) users; former established user category user includes all established users (e.g., lifetime use of more than 100 cigarettes) who did not use a tobacco product in the past 30 days.
These risk ratios are significantly (p < .05) lower than for exclusive cigarette use.
Other than exclusive dual cigarette and e-cigarette users.
Marijuana use variable does not distinguish between combustible and noncombustible use.
Supplementary Table 4 reports adjusted RRs for all the variables in the model.
Results
Sample Description
The sample included adults 18 and older, and after weights were applied, was representative of the US population in terms of race/ethnicity and socioeconomic status at the time of W1 (demographic and other covariate characteristics are shown in Supplementary Table 7). Cigarettes were the most commonly used product, with 72.2% (SE = 0.7) of all tobacco users smoking cigarettes in the past 30 days. Cigarettes were also the primary product used for individuals in the three multiple use categories. Asthma occurred in 9.2%, diabetes in 14.5%, and medications with respiratory symptom side-effects were used by 3 to 6% of the population.
Cross-sectional Associations
Importance of Cigarette Smoking as a Risk Factor
At W2, the prevalence of functionally important respiratory symptoms (at index score cutoff of ≥ 3) was 7.2% (SE = 0.3). Table 1 shows that respiratory symptoms were more common in the four categories of tobacco use that included cigarettes (exclusive cigarette use, dual cigarette and e-cigarette use, polycombustible use, and polycombustible and noncombustible use), compared to never tobacco use, and among those who used marijuana. Functionally important respiratory symptoms were much more common among those with asthma, and also more common among those with comorbid conditions, obesity, and those using medications known to cause coughing or wheezing (Supplementary Table 7).
Table 1.
Tobacco and Marijuana Use for Participants in Wave 2 of the Population Assessment of Tobacco and Health (PATH) Studya
| Risk factor at baseline | Unweighted N | US population % (SE) | Functionally important symptoms(cutoff ≥ 3)% (SE) |
|---|---|---|---|
| Wave 2 past month tobacco useb | |||
| Never | 5888 | 53.0 (0.7) | 4.2 (0.3) |
| Former | 2291 | 19.2 (0.5) | 5.9 (0.6) |
| Exclusive use categories | |||
| Cigarette | 3230 | 11.4 (0.3) | 16.8 (0.8) |
| E-cigarette | 327 | 1.2 (0.1) | 7.4 (1.6) |
| Cigars | 548 | 2.3 (0.1) | 4.3 (1.1) |
| Smokeless | 450 | 1.9 (0.1) | 6.0 (1.4) |
| Hookah | 321 | 1.0 (0.1) | 3.7† (1.1) |
| Multiple use categories | |||
| Cigarette + e-cigarette | 792 | 2.6 (0.1) | 18.1 (1.5) |
| Combustible only | 1266 | 3.8 (0.1) | 14.8 (1.0) |
| Otherc combustible + noncombustible | 1118 | 3.3 (0.1) | 16.2 (1.3) |
| Other smoke-related exposures | |||
| Cigarette pack years—mean (SE) | 16 295 | 5.4 (0.1) | 10.7 (0.4) |
| Second-hand smoke exposure—mean (SE) | 16 295 | 4.3 (0.1) | 11.0 (0.6) |
| Past-month marijuana used | |||
| No | 13 626 | 91.1 (0.3) | 6.3 (0.3) |
| Yes | 2669 | 8.9 (0.3) | 16.4 (0.8) |
N = 16 295 adult respondents without chronic obstructive pulmonary disease or other nonasthma respiratory disease and with PATH Study longitudinal (all-waves) weights and complete data on all variables.
Never tobacco user category includes former experimental (e.g., lifetime use of <100 cigarettes) users; former established tobacco user category includes all established users (e.g., lifetime use of more than 100 cigarettes) who did not use a tobacco product in the past 30 days. Data are not presented for exclusive pipe (N = 44), and dual ENDS + Smokeless (N = 20) users due to small sample size.
Other than exclusive dual cigarette and e-cigarette users.
Marijuana use variable does not distinguish between combustible and noncombustible use.
Estimate should be interpreted with caution because it has low statistical precision. It is based on a denominator sample size of less than 50, or the coefficient of variation of the estimate or its complement is larger than 30%.
Figure 1 illustrates the unadjusted linear relationship between frequency of cigarette use and proportion of persons with functionally important respiratory symptoms for the four use categories featuring cigarettes. The shape of the dose–response lowess lines were almost identical and the 95th percentile for cigarette use intensity was essentially the same for all four groups, regardless of what other tobacco products were added to cigarettes, emphasizing the importance of cigarettes in these four most prevalent categories of tobacco use.
In the full, adjusted, multivariable cross-sectional model (right-hand column, Table 2), all four tobacco use categories that featured cigarette smoking were associated with a doubling of the risk of functionally important respiratory symptoms vs. never tobacco users (e.g., exclusive cigarettes RR = 2.34 [95% CI 1.92, 2.85]), and risk for the multiple use categories were not significantly different from exclusive cigarette use (e.g., cigarettes + e-cigarettes RR = 2.13 [95% CI 1.64, 2.77]).
Exclusive Use of Noncigarette Products Not Associated With Added Risk
Compared to never users, the risk of functionally important respiratory symptoms were not significantly different for exclusive users of e-cigarette, cigar, hookah and smokeless tobacco; moreover post hoc testing indicated that risk ratios for each of these categories were significantly lower compared to exclusive cigarette use (as indicated by superscript d after the risk ratios for these products, Table 2, right-hand column). None of these cross-sectional results changed when the analysis was repeated at a respiratory index cutoff level of ≥2.
Testing Sensitivity to Key Confounders of the E-cigarette—Respiratory Symptom Association
Cigarette smoking pack-years, second-hand smoke exposure, and marijuana use were also associated with functionally important respiratory symptoms (RRs 1.13 [95% CI, 1.09, 1.16] for each additional 5 pack-years, 1.02 [95% CI, 1.01, 1.03] per each additional 5 hours of weekly second-hand smoke exposure and 1.60 [95% CI, 1.37, 1.87] for past-month marijuana use). Table 2 highlights the importance of cigarette smoking pack-years and past-month marijuana use as confounders of the association between tobacco product use and respiratory symptoms. Cigarette pack-years was a particularly strong confounder; adding this variable alone to the cross-sectional multivariable model attenuated association estimates for cigarettes and cigarettes + e-cigarettes by 30% and for exclusive e-cigarettes by 25%. That was partly because all three groups had a similarly long cigarette smoking history—weighted mean 13.4 (SE = 0.3) cigarette pack-years for exclusive cigarette smokers, 12.9 (SE = 0.4) for the dual users, and 10.8 (SE = 0.9) for exclusive e-cigarette users. Similarly, 19.2% (SE = 3.0) of exclusive e-cigarette users also currently used marijuana; adding P30D marijuana use to the multivariable model attenuated association for e-cigarettes by 9%. Adding all three confounders together attenuated the e-cigarette-respiratory symptom association RR from 1.53(95% CI 0.98, 2.40) to 1.05 (0.67, 1.63).
Exploring the Relationship Between Frequency of Use and Respiratory Symptoms
The categorical analysis did not address whether functionally important respiratory symptoms increased with increasing frequency of use. Figure 2 explored this for cigarettes and e-cigarettes, adjusting for cigarette smoking history. For cigarettes, there was a significant linear increase in the percent with functionally important respiratory symptoms (at a cutoff of ≥3) with higher intensity of use; prevalence of respiratory symptoms was less than 5% for never users and over 30% for those smoking a pack a day or more. There was also an increase in respiratory symptoms with higher intensity of e-cigarette use, but the trend did not reach statistical significance (p = .12).
Longitudinal Associations—Worsening Respiratory Symptoms 12 Months Later
Consistent Associations With Cigarette Smoking
Table 3 gives results for the two longitudinal models (respiratory index cutoff levels of ≥2 and ≥3) for worsening respiratory symptoms (asymptomatic at W2, symptomatic at W3). Symptoms worsened for 5% and 8%, respectively, for cutoff levels of ≥3 and ≥2. Symptom worsening was most common in the four categories featuring cigarette use, with risk ratios for worsening symptoms for the four categories ranging from 1.64 to 2.80, and always significantly higher than for never users, regardless of threshold. Also regardless of threshold, post hoc testing indicated that risk ratios for dual use of cigarettes + e-cigarettes were never different compared with exclusive cigarette use, whereas combustible plus noncombustible use was always associated with lower risk. Cigarette pack-years, second-hand smoke exposure, and marijuana use at W2 were also associated with symptom worsening at W3, at both cutoff levels.
Exclusive Use of Noncigarette Products—Testing Sensitivity to Respiratory Symptom Severity
There were no statistically significant associations between exclusive use of cigars, smokeless tobacco or hookah (vs. never use) and worsening of respiratory symptoms compared to never users. Post hoc testing indicated that risk ratios were significantly smaller than for exclusive use of cigarettes, regardless of cutoff level for the respiratory symptom outcome (except hookah use was not significantly lower at a threshold of ≥3). In contrast, findings for exclusive e-cigarette use were sensitive to symptom severity, showing a significant association with worsening symptoms at a threshold of ≥2 (RR 1.63 [1.02, 2.59] compared to never users (RR not significantly lower than for exclusive cigarette use), but not at a symptom threshold of ≥3 (RR 1.58 [0.84, 2.96], (here RR was significantly lower than for exclusive cigarette use).
Longitudinal Analyses—Improving Respiratory Symptoms 12 Months Later
Table 4 gives results for the two longitudinal models (respiratory index cutoff levels of ≥2 and ≥3) for improving respiratory symptoms (symptomatic at W2, asymptomatic at W3). Symptoms improved for 21% and 29%, respectively, for cutoff levels of ≥2 and ≥3. In contrast to symptom worsening models, tobacco use was less apt to be associated with improvement and more sensitive to cutoff threshold. Categories of use featuring cigarettes were not reliably less likely to be associated with symptom improvement compared to never users; only exclusive use of cigarettes at a threshold of ≥2 was associated with lower risk ratio for symptom improvement (RR 0.57 [0.40, 0.82]). At this threshold, former smokers, e-cigarette, cigar and smokeless tobacco users were all significantly more likely to show symptom improvement compared to exclusive cigarette users (but were not significantly different from never users). This was also true for e-cigarette users at a threshold of ≥3, where e-cigarette users were also more likely show symptom improvement compared to never users (RR 1.64 [1.04, 2.58]).
Discussion
This study underscores the adverse consequences of continued cigarette smoking among people without COPD or other nonasthma respiratory disease on functionally important respiratory symptoms. Consistent with other studies,32 a longer history of cigarette smoking (pack-years) predicted worsening respiratory symptoms and decreased chances of improvement, independent of P30D cigarette smoking, underlining the importance of cigarette smoke exposure in the development or worsening of respiratory symptoms.
The consequences of cigarette use were the same regardless of which additional tobacco products were used. As shown previously, dual users of cigarettes and e-cigarettes smoked cigarettes as frequently as exclusive cigarette smokers,33 their respiratory response to cigarette smoking intensity was essentially the same as exclusive cigarette users, and they had indistinguishable risk for symptom worsening.19 We found no evidence to support the idea that dual use of cigarettes and e-cigarettes carries higher risk for respiratory symptom worsening compared to exclusive cigarettes for the symptom outcomes we examined. This contrasts with increased risk of dual use in the analyses of PATH Study data reported by Reddy et al.,20 an analysis that involved a different period (W3–W4), and adjusted only for demographics; we doubt the finding reported by Reddy would have remained statistically significant after adjustment for the multiple confounders included in the present analysis.
In contrast, respiratory symptom risk for exclusive users of other tobacco products was significantly lower than for cigarettes, and was largely not significantly different from never or former tobacco users. The finding for e-cigarettes contradicts two cross-sectional studies of tobacco use and respiratory symptoms, one using PATH Study W2 data18 and one using W3 data,19 both concluding that there was an association between e-cigarette use and wheezing. These studies examined the association with each item on the respiratory index and neither adjusted for cigarette smoking history or marijuana use.34 Based on the present study findings—lack of a crude dose-response for e-cigarette frequency illustrated in Figure 2 and the confounding analysis in Table 2—we conclude that the reported associations in these papers were likely spurious, primarily because of the failure to adjust for cigarette smoking history. Our Supplementary Materials include a method for determining cigarette pack-years from PATH Study data to support the inclusion of this important confounder by other users of these data.
The longitudinal results seem contradictory if the reference of focus is never users—e-cigarette users are significantly more likely to have symptoms worsen at one cutoff level and significantly more likely to have symptoms improve at another—an example of how results for e-cigarette users may be sensitive to how health outcomes are determined. But another viewpoint is that potentially reduced harm tobacco products are judged also by how health risks of the product compare to the health risks for cigarette smokers. With cigarette users as the referent category, the analysis suggests that exclusive e-cigarette users are less likely to have their respiratory symptoms worsen, along with consistent findings (at both thresholds), that they are more likely to have their symptoms improve. In sum, with respect to short-term changes in functionally important respiratory symptoms, the results suggest risk for exclusive e-cigarette users are intermediate—increased harm compared to never tobacco users, but reduced harm compared to cigarette users.
Cigar smokers had consistently lower risk for functionally important respiratory symptoms compared to exclusive cigarette smokers, as was previously reported for some of the single respiratory symptom items in another PATH Study report.19 Cigar smoking has been associated with higher (compared to never tobacco users) mortality from respiratory disease and lung cancer,35 increased risk for diagnosis of lung cancer and COPD,36,37 decreased lung function and airflow obstruction,38,39 and respiratory symptoms.38–40 In all studies including cigarette smokers, risks associated with cigars were lower than for cigarettes; former cigarette smokers switching to cigars had higher risk vs. those who had smoked only cigars.41,42 Respiratory symptom risk among hookah smokers has not been studied extensively but was intermediate between never smokers and cigarette smokers in one study.43
Lower symptom risk with exclusive cigar use may be explained by reduced smoke inhalation.42 In contrast to cigarettes, cigar tobacco is fermented, and many cigars are smoked with lower frequency. Cigar smokers also tend to inhale less deeply because of smoke alkalinity which also enhances oral nicotine absorption. Only 15% of exclusive cigar smokers report actively inhaling the smoke, compared to two-thirds for users of both cigars and cigarettes (which posed high risk for respiratory symptoms in this study).42
Marijuana was associated with functionally important respiratory symptoms, consistent with 8 of 10 previous studies.44,45 The findings are backed by research involving dual users of marijuana and cigarettes showing higher puff volumes, deeper inhalation, and greater tar retention from marijuana vs. cigarettes,46 animal research documenting pulmonary cell changes with chronic marijuana smoking, and prospective research showing changes in lung function among marijuana smokers.47 Marijuana use was also a confounder of the e-cigarette—respiratory symptoms association. One study showing an association between e-cigarette use and cough among young never cigarette smokers, failed to adjust for marijuana use in the multivariable model (even though the data for marijuana use were presented in an earlier table). Another study of adult PATH Study W4 data found vaping with marijuana to be associated with wheezing (but not vaping without marijuana), consistent with our findings.48 Two other studies of youth, one using PATH Study data, have shown that the e-cigarette—respiratory outcome is confounded by marijuana use49 and marijuana vaping.21 Clinicians need to be aware of the association between marijuana use and respiratory symptoms as use increases.50
The study strengths include a nationally representative sample, a validated respiratory outcome related to functional impairment, and adjustment for multiple confounding influences. Limitations include small numbers in some product groups, increasing the probability of a chance finding. Because switching from cigarette smoking to exclusive e-cigarette use is an uncommon event, randomized e-cigarette switching trials may be required to better assess how e-cigarette substitution affects wheezing symptoms among adult cigarette smokers. Risk of marijuana smoking on respiratory symptoms may be underestimated because marijuana use may have included noncombustible products.51,52 Relying on self-report of COPD may have resulted in some who were unaware of their diagnosis being retained in the study. The findings relate only to short-term changes in wheezing and nighttime cough, not other bothersome symptoms (cough with phlegm production, or dyspnea), longer-term symptom effects, relation to respiratory disease onset, or vaping-related acute lung injury—medical issues that underline concern about any inhaled product use. The analysis included many comparisons and nevertheless employed a p-value of 0.05; the associations reported should be confirmed in other samples. Finally, future analyses with the latest available data from the PATH Study may provide a more refined look at the questions addressed in the present study.
In summary, this study of a nationally representative sample of US adults without severe respiratory disease found an association between cigarette smoking and functionally important respiratory symptoms—and substantially less evidence of associations between respiratory symptoms and exclusive noncigarette tobacco product use.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr.
Acknowledgments
Tragically, co-author Lisa Schwartz, MD, MS, our extraordinary friend and colleague, died before publication of this article.
We gratefully acknowledge the assistance of Jennifer Hebb, BSN who was essential in coordinating the group effort and the electronic submission of the manuscript. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the U.S. Department of Health and Human Services or any of its affiliated institutions or agencies.
Contributor Information
James D Sargent, Geisel School of Medicine at Dartmouth, The C. Everett Koop Institute at Dartmouth, Lebanon, NH, USA.
Michael J Halenar, Westat, Rockville, MD, USA.
Kathryn C Edwards, Westat, Rockville, MD, USA.
Steven Woloshin, Dartmouth Institute for Health Policy and Clinical Practice, The C. Everett Koop Institute at Dartmouth, The Lisa Schwartz Foundation, Lebanon, NH, USA.
Lisa Schwartz, Dartmouth Institute for Health Policy and Clinical Practice, The C. Everett Koop Institute at Dartmouth, The Lisa Schwartz Foundation, Lebanon, NH, USA.
Jennifer Emond, Geisel School of Medicine at Dartmouth, The C. Everett Koop Institute at Dartmouth, Lebanon, NH, USA.
Susanne Tanski, Geisel School of Medicine at Dartmouth, The C. Everett Koop Institute at Dartmouth, Lebanon, NH, USA.
Kristie A Taylor, Westat, Rockville, MD, USA.
John P Pierce, Moore’s Cancer Center, University of California at San Diego, San Diego, CA, USA.
Jason Liu, Westat, Rockville, MD, USA.
Maciej L Goniewicz, Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA.
Raymond Niaura, New York University, New York, NY, USA.
Gabriella Anic, U.S. Food and Drug Administration, Center for Tobacco Products, Bethesda, MD, USA.
Yanling Chen, U.S. Food and Drug Administration, Center for Tobacco Products, Bethesda, MD, USA.
Priscilla Callahan-Lyon, U.S. Food and Drug Administration, Center for Tobacco Products, Bethesda, MD, USA.
Lisa D Gardner, U.S. Food and Drug Administration, Center for Tobacco Products, Bethesda, MD, USA.
Theresa Thekkudan, U.S. Food and Drug Administration, Center for Tobacco Products, Bethesda, MD, USA.
Nicolette Borek, U.S. Food and Drug Administration, Center for Tobacco Products, Bethesda, MD, USA.
Heather L Kimmel, National Institute on Drug Abuse, National Institutes of Health, Bethesda, MD, USA.
K Michael Cummings, Medical University of South Carolina, Charleston, SC, USA.
Andrew Hyland, Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA.
Mary Brunette, Geisel School of Medicine at Dartmouth, The C. Everett Koop Institute at Dartmouth, Lebanon, NH, USA.
Funding
This manuscript is supported with Federal funds from the National Institute on Drug Abuse, National Institutes of Health, and the Center for Tobacco Products, Food and Drug Administration, Department of Health and Human Services, under contract to Westat (Contract No. HHSN271201100027C).
Declaration of Interests
K. Michael Cummings provides expert testimony on the health effects of smoking and tobacco industry tactics in lawsuits filed against the tobacco industry. He has also received payment as a consultant to Pfizer, Inc., for services on an external advisory panel to assess ways to improve smoking cessation delivery in health care settings. Maciej Goniewicz has received a research grant from Pfizer and served as a member of scientific advisory board to Johnson & Johnson, pharmaceutical companies that manufacture smoking cessation medications. Raymond Niaura receives funding from the Food and Drug Administration Center for Tobacco Products via contractual mechanisms with Westat and the National Institutes of Health. Within the past 3 years, he has served as a paid consultant to the Government of Canada via a contract with Industrial Economics Inc. and has received an honorarium for a virtual meeting from Pfizer Inc. Dr. Niaura was an unpaid grant reviewer for the Foundation for a Smoke Free World.
Data Availability
Data from the PATH Study Wave 1 to Wave 3 are available for download as Restricted Use Files (https://www.icpsr.umich.edu/icpsrweb/NAHDAP/studies/36231). Request guidelines and conditions of use are available at the website above.
References
- 1. US Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 2. Woodruff PG, Barr RG, Bleecker E, et al. Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med. 2016;374(19):1811–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tashkin DP, Clark VA, Coulson AH, et al. The UCLA population studies of chronic obstructive respiratory disease. VIII. Effects of smoking cessation on lung function: a prospective study of a free-living population. Am Rev Respir Dis. 1984;130(5):707–715. [DOI] [PubMed] [Google Scholar]
- 4. Fletcher C, Peto R.. The natural history of chronic airflow obstruction. Br Med J. 1977;1(6077):1645–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vasanthi Bathrinarayanan P, Brown JEP, Marshall LJ, Leslie LJ.. An investigation into E-cigarette cytotoxicity in-vitro using a novel 3D differentiated co-culture model of human airways. Toxicol In Vitro. 2018;52:255–264. [DOI] [PubMed] [Google Scholar]
- 6. Szyfter K, Napierala M, Florek E, et al. Molecular and health effects in the upper respiratory tract associated with tobacco smoking other than cigarettes. Int J Cancer. 2019;144(11):2635–2643. [DOI] [PubMed] [Google Scholar]
- 7. Sosnowski TR, Jablczynska K, Odziomek M, Schlage WK, Kuczaj AK.. Physicochemical studies of direct interactions between lung surfactant and components of electronic cigarettes liquid mixtures. Inhal Toxicol. 2018;30(4–5):159–168. [DOI] [PubMed] [Google Scholar]
- 8. Scott A, Lugg ST, Aldridge K, et al. Pro-inflammatory effects of e-cigarette vapour condensate on human alveolar macrophages. Thorax. 2018;73(12):1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miyashita L, Suri R, Dearing E, et al. E-cigarette vapour enhances pneumococcal adherence to airway epithelial cells. Eur Respir J. 2018;51(2):1701592. doi: 10.1183/13993003.01592-2017. PMID: 29437942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee HW, Park SH, Weng MW, et al. E-cigarette smoke damages DNA and reduces repair activity in mouse lung, heart, and bladder as well as in human lung and bladder cells. Proc Natl Acad Sci USA. 2018;115(7):E1560–E1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hiemstra PS, Bals R.. Effects of E-Cigarette use on human lung tissue. On harm reduction and causing harm. Am J Respir Crit Care Med. 2018;198(1):6–7. [DOI] [PubMed] [Google Scholar]
- 12. Crotty Alexander LE, Drummond CA, Hepokoski M, et al. Chronic inhalation of e-cigarette vapor containing nicotine disrupts airway barrier function and induces systemic inflammation and multiorgan fibrosis in mice. Am J Physiol Regul Integr Comp Physiol. 2018;314(6):R834–R847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vardavas CI, Anagnostopoulos N, Kougias M, et al. Short-term pulmonary effects of using an electronic cigarette: impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest. 2012;141(6):1400–1406. [DOI] [PubMed] [Google Scholar]
- 14. Meo SA, Ansary MA, Barayan FR, et al. Electronic cigarettes: Impact on lung function and fractional exhaled nitric oxide among healthy adults. Am J Mens Health. 2019;13(1):1557988318806073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cravo AS, Bush J, Sharma G, et al. A randomised, parallel group study to evaluate the safety profile of an electronic vapour product over 12 weeks. Regul Toxicol Pharmacol. 2016;81(Suppl 1):S1–S14. [DOI] [PubMed] [Google Scholar]
- 16. Cibella F, Campagna D, Caponnetto P, et al. Lung function and respiratory symptoms in a randomized smoking cessation trial of electronic cigarettes. Clin Sci (Lond). 2016;130(21):1929–1937. [DOI] [PubMed] [Google Scholar]
- 17. Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of E-Cigarettes versus nicotine-replacement therapy. N Eng J Med. 2019;380(7):629–637. [DOI] [PubMed] [Google Scholar]
- 18. Li D, Sundar IK, McIntosh S, et al. Association of smoking and electronic cigarette use with wheezing and related respiratory symptoms in adults: cross-sectional results from the Population Assessment of Tobacco and Health (PATH) study, wave 2. Tob Control. 2020;29(2):140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schneller LM, Quiñones Tavárez Z, Goniewicz ML, et al. Cross-sectional association between exclusive and concurrent use of cigarettes, ENDS, and Cigars, the three most popular tobacco products, and wheezing symptoms among U.S. adults. Nicotine Tob Res. 2020;22(Suppl 1):S76–s84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reddy KP, Schwamm E, Kalkhoran S, et al. Respiratory symptom incidence among people using electronic cigarettes, combustible tobacco, or both. Am J Respir Crit Care Med. 2021;204(2):231–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Braymiller JL, Barrington-Trimis JL, Leventhal AM, et al. Assessment of nicotine and cannabis vaping and respiratory symptoms in young adults. JAMA Netw Open. 2020;3(12):e2030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hyland A, Ambrose BK, Conway KP, et al. Design and methods of the Population Assessment of Tobacco and Health (PATH) study. Tob Control. 2017;26(4):371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. National Addiction & HIV Data Archive Program. Population Assessment of Tobacco and Health (PATH) Study Series. 2018; https://www.icpsr.umich.edu/icpsrweb/NAHDAP/series/606. Accessed November 18, 2019.
- 24. Asher M, Keil U, Anderson H, et al. International Study of Asthma and Allergies in Childhood (ISAAC): Rationale and methods. Eur Respir J. 1995;8(3):483–491. [DOI] [PubMed] [Google Scholar]
- 25. US Health and Human Services. Patient-Reported Outcomes Measurement System (PROMISE). 2019; http://www.healthmeasures.net/explore-measurement-systems/promis. Accessed May 5, 2019.
- 26. Halenar MJ, Sargent JD, Edwards KC, et al. Validation of an index for functionally important respiratory symptoms among adults in the nationally representative population assessment of tobacco and health study, 2014–2016. Int J Environ Res Public Health. 2021;18(18):9688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hegewald MJ, Crapo RO.. Socioeconomic status and lung function. Chest. 2007;132(5):1608–1614. [DOI] [PubMed] [Google Scholar]
- 28. McCarthy PJ. Pseudoreplication: further evaluation and applications of the balanced half-sample technique. Vital Health Stat 2. 1969;(31):1–24. [PubMed] [Google Scholar]
- 29. Judkins DR. Fay’s method for variance estimation. J Off Stat. 1990;6(3):223–239. [Google Scholar]
- 30. Rivest L-P. Statistical properties of Winsorized means for skewed distributions. Biometrika. 1994;81(2):373–383. [Google Scholar]
- 31. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. [DOI] [PubMed] [Google Scholar]
- 32. Eagan TM, Bakke PS, Eide GE, Gulsvik A.. Incidence of asthma and respiratory symptoms by sex, age and smoking in a community study. Eur Respir J. 2002;19(4):599–605. [DOI] [PubMed] [Google Scholar]
- 33. Wang JB, Olgin JE, Nah G, et al. Cigarette and e-cigarette dual use and risk of cardiopulmonary symptoms in the Health eHeart Study. PLoS One. 2018;13(7):e0198681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sargent JD, Tanski S, Brunette MF, Emond JA, Woloshin S.. Up in smoke: the reported association between e-cigarette use and wheezing in this study is probably spurious. Tob Control. 2019. https://tobaccocontrol-bmj-com.dartmouth.idm.oclc.org/content/early/2019/02/13/tobaccocontrol-2018-054694.responses#up-in-smoke-the-reported-association-between-e-cigarette-use-and-wheezing-in-this-study-is-probably-spurious. Accessed April 29, 2019. [Google Scholar]
- 35. Christensen CH, Rostron B, Cosgrove C, et al. Association of cigarette, cigar, and pipe use with mortality risk in the US population. JAMA Intern Med. 2018;178(4):469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Benhamou S, Benhamou E, Flamant R.. Lung cancer risk associated with cigar and pipe smoking. Int J Cancer. 1986;37(6):825–829. [DOI] [PubMed] [Google Scholar]
- 37. Iribarren C, Tekawa IS, Sidney S, Friedman GD.. Effect of cigar smoking on the risk of cardiovascular disease, chronic obstructive pulmonary disease, and cancer in men. N Engl J Med. 1999;340(23):1773–1780. [DOI] [PubMed] [Google Scholar]
- 38. Rodriguez J, Jiang R, Johnson WC, et al. The association of pipe and cigar use with cotinine levels, lung function, and airflow obstruction: a cross-sectional study. Ann Intern Med. 2010;152(4):201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tambi Medabala RB, Glad Mohesh M, Praveen Kumar M.. Effect of cigarette and cigar smoking on peak expiratory flow rate. J Clin Diagn Res. 2013;7(9):1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brown CA, Woodward M, Tunstall-Pedoe H.. Prevalence of chronic cough and phlegm among male cigar and pipe smokers: results of the Scottish Heart Health Study. Thorax. 1993;48(11):1163–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wald NJ, Watt HC.. Prospective study of effect of switching from cigarettes to pipes or cigars on mortality from three smoking related diseases. BMJ. 1997;314(7098):1860–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Baker F, Ainsworth SR, Dye JT, et al. Health risks associated with cigar smoking. JAMA. 2000;284(6):735–740. [DOI] [PubMed] [Google Scholar]
- 43. Raad D, Gaddam S, Schunemann HJ, et al. Effects of water-pipe smoking on lung function: A systematic review and meta-analysis. Chest. 2011;139(4):764–774. [DOI] [PubMed] [Google Scholar]
- 44. Tashkin DP. Marijuana and lung disease. Chest. 2018;154(3):653–663. [DOI] [PubMed] [Google Scholar]
- 45. Ghasemiesfe M, Ravi D, Vali M, et al. Marijuana Use. Respiratory symptoms, and pulmonary function: a systematic review and meta-analysis. Ann Inter Med. 2018;169(2):106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wu TC, Tashkin DP, Djahed B, Rose JE.. Pulmonary hazards of smoking marijuana as compared with tobacco. N Engl J Med. 1988;318(6):347–351. [DOI] [PubMed] [Google Scholar]
- 47. Meier MH, Caspi A, Cerda M, et al. Associations between cannabis use and physical health problems in early midlife: a longitudinal comparison of persistent cannabis vs tobacco users. JAMA Psychiatry. 2016;73(7):731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xie Z, Li D.. Cross-Sectional association between lifetime use of electronic cigarettes with or without marijuana and self-reported past 12-month respiratory symptoms as well as lifetime respiratory diseases in US adults. Nicotine Tob Res. 2020;22(Suppl 1):S70–S75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Boyd CJ, McCabe SE, Evans-Polce RJ, Veliz PT.. Cannabis, vaping, and respiratory symptoms in a probability sample of U.S. youth. J Adolesc Health. 2021;69(1):149–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fligiel SE, Beals TF, Tashkin DP, et al. Marijuana exposure and pulmonary alterations in primates. Pharmacol Biochem Behav. 1991;40(3):637–642. [DOI] [PubMed] [Google Scholar]
- 51. Borodovsky JT, Crosier BS, Lee DC, Sargent JD, Budney AJ.. Smoking, vaping, eating: is legalization impacting the way people use cannabis? Int J Drug Policy. 2016;36:141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schauer GL, King BA, Bunnell RE, Promoff G, McAfee TA.. Toking, vaping, and eating for health or fun: marijuana use patterns in Adults, U.S., 2014. Am J Prev Med. 2016;50(1):1–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the PATH Study Wave 1 to Wave 3 are available for download as Restricted Use Files (https://www.icpsr.umich.edu/icpsrweb/NAHDAP/studies/36231). Request guidelines and conditions of use are available at the website above.