Abstract
To determine the mechanism of the purified protein derivative (PPD)-specific hyporesponsiveness in Mycobacterium bovis BCG-vaccinated human T-cell leukemia virus type 1 (HTLV-1)-infected individuals, we examined cytokine production in response to PPD in the following four groups of individuals: (i) HTLV-negative, PPD nonresponders (n = 11; NN); (ii) HTLV-negative, PPD responders (n = 18; NP); (iii) HTLV-positive, PPD nonresponders (n = 15; PN); and (iv) HTLV-positive, PPD responders (n = 15; PP). In vitro stimulation with PPD resulted in both proliferative responses and gamma interferon (IFN-γ) production in NP and PP (P < 0.02), with minimal proliferation and IFN-γ production in the NN and PN groups. Further, PPD-specific interleukin 10 (IL-10) production was significantly reduced in the PN group (P < 0.01), while the other groups had comparable levels. Cytokine reconstitution experiments demonstrated that while addition of recombinant IL-12 (rIL-12) plus anti-IL-4 restored PPD-specific responses in the NN group, it had no effect in the PN group. However, addition of rIL-12 resulted in the increased production of IFN-γ in both nonresponder groups (NN and PN), suggesting that the lack of IFN-γ production was not responsible for the PPD anergy. We conclude that PPD-specific anergy in HTLV-1-infected individuals appears to be due in part to their inability to respond to rIL-12.
Clinical outcomes of infection with human T-cell leukemia or lymphoma virus type 1 (HTLV-1) range from individuals that remain asymptomatic to others who exhibit HTLV-1-associated myelopathy (HAM) and adult T-cell leukemia (ATL) (8). The manifestation of these different clinical outcomes may reflect an individual’s immune response to HTLV-1 infection. Indeed, in vitro analysis and clinical evidence suggest perturbations of the immune function in individuals with asymptomatic HTLV-1 infection, as well as those with ATL or HAM (5, 8). For instance, while individuals with HAM manifest hyperimmune responsiveness to HTLV-1-encoded proteins, those with ATL frequently have immune suppression (13).
We have previously shown decreased reactivity to the purified protein derivative (PPD) of Mycobacterium tuberculosis in HTLV-1-infected persons (11). Such PPD-specific anergy in HTLV-1-infected asymptomatic carriers is thought to be due to immune hyporesponsiveness by the host (9, 11, 15); however, the mechanism underlying the apparent immunological hyporeactivity in HTLV-1 carriers is not known. We hypothesize that distinct Th-cell polarization, manifested as altered cytokine production or responsiveness, may result in hyporesponsiveness to recall antigens. In the present study, we conducted a detailed analysis of PPD-specific responses to better understand the basis for anergy to recall responses in HTLV-1-infected carriers. We provide evidence that PPD-specific anergy in HTLV-1-infected carriers is due to a lack of responsiveness to IL-12 rather than to a reduced Th1 response.
MATERIALS AND METHODS
Subjects.
All participants included (n = 59) in this study are a subset of those from an ongoing study in the Miyazaki cohort (8). All individuals enrolled had a history of M. tuberculosis BCG vaccination and were tested for reactivity to PPD recall antigen in vivo according to criteria used in Japan (11). The BCG vaccination was repeated till PPD reactivity became positive. The PPD reactivity was determined by the presence of induration after an intradermal challenge with 0.05 μg of antigen (Nippon BCG, Tokyo, Japan) at the volar aspect of the forearm (11). After 48 h, the injection site was examined and the positivity was determined on the basis of the diameter of erythema. All participants were tested for PPD reactivity prior to enrollment in the study (9).
All specimens were tested for antibodies to HTLV-1, and positive specimens were further confirmed by PCR. All participants were matched for age and categorized in the following four groups based on HTLV and PPD status: (i) HTLV-negative, PPD nonresponders (n = 11; NN); (ii) HTLV-negative, PPD responders (n = 18; NP); (iii) HTLV-positive, PPD nonresponders (n = 15; PN); and (iv) HTLV-positive, PPD responders (n = 15; PP). All participants were asymptomatic and negative for antibodies to human immunodeficiency virus type 1. None of the patients was on antiretroviral therapy.
Lymphocyte proliferation assays and cytokine production.
Peripheral blood mononuclear cells (PBMCs) were cultured at 105 cells per well in 200 μl of medium with or without PPD (0.1 μg/ml; Fuji Rebio) and phytohemagglutinin (PHA; 5 μg/ml) in 96-well round-bottom tissue culture plates for 7 days. Culture supernatants were harvested prior to pulsing for subsequent analysis of cytokine production. Lymphocyte proliferation and cytokine production were performed as described previously (3). Lymphocyte counts above the counts in normal donors plus 3 standard deviations (SD) were considered spontaneous proliferation. Enzyme-linked immunosorbent assay kits (BioSource, Carmillo, Calif.) were used to measure the soluble concentrations of interleukin 10 (IL-10) (sensitivity, <5 pg/ml), tumor necrosis factor alpha (TNF-α; <1 pg/ml), and gamma interferon (IFN-γ; a <4 pg/ml). In some experiments, cultures were treated with recombinant cytokines (recombinant IL-2 [rIL-2] at 20 IU/ml [Cellular Products, Buffalo, N.Y.], rIL-12 at 100 IU/ml, and anti-IL-4 antibody at 10 μg/ml [R&D Systems, Minneapolis, Minn.]).
Statistical analysis.
Student’s t test and analysis of variance were used to determine statistical differences between the groups.
RESULTS
PPD-specific in vitro proliferation mimics in vivo response.
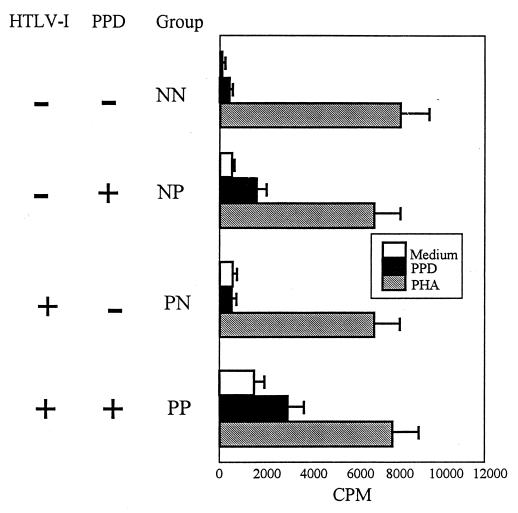
PBMCs from subjects in all four groups were cultured in the presence or absence of PHA and PPD. As expected, most of the PPD nonresponders from the HTLV-1-negative (NN) or -positive (PN) groups demonstrated no PPD-specific lymphocyte proliferation (with the exception of one patient in the NN group), whereas most PPD responders from the HTLV-1-negative (NP) or -positive (PP) groups had PPD-specific responses with a stimulation index ranging from 2 to 31 (Fig. 1). As expected, a high proportion of cells from the PP group demonstrated spontaneous proliferative responses in the absence of antigens (Fig. 2A) (6, 10). The mean value of [3H]thymidine incorporation for PPD responders (NP and PP) were significantly higher than those for PPD nonresponders (P < 0.02). The lack of PPD response in the PPD nonresponder groups was not due to the inability of the cells to proliferate, since PBMCs from each group responded similarly to PHA (Fig. 1). In addition, there was a direct correlation between in vivo and in vitro PPD responses (P < 0.02), suggesting that in vitro PPD responses were mimicking similar responses observed in vivo (data not shown).
FIG. 1.
PPD responder groups proliferate in response to PPD, while all groups proliferate in response to PHA. PBMCs from HTLV-1-positive and -negative groups were cultured in the presence of PPD (0.1 mg/ml) or PHA (0.005%) for 6 days. Eighteen hours prior to harvest, the cells were pulsed with 0.5 μCi of [3H]thymidine. Data are expressed as means ± standard errors of the means.
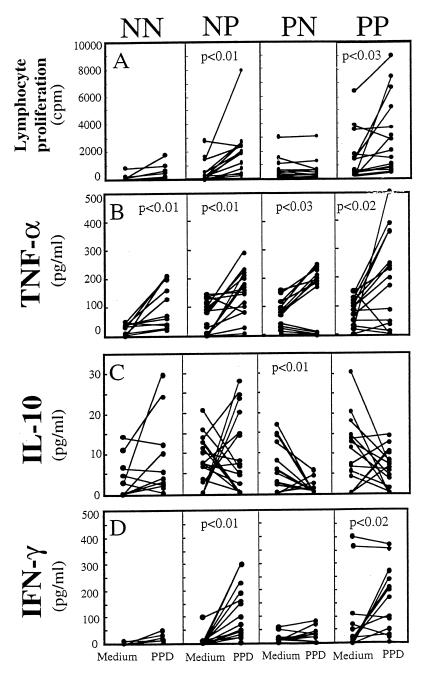
FIG. 2.
Lymphocyte proliferation and cytokine production in response to PPD stimulation. (A) PBMCs from HTLV-1-positive and -negative groups were cultured in the presence or absence of PPD (0.1 μg/ml) for 6 days, pulsed, and harvested. Data are expressed as means ± standard errors of the means. (B to D) Production of TNF-α (B), IL-10 (C), and IFN-γ (D) in culture supernatants after PPD stimulation for 6 days is also shown. The data are expressed as means ± standard errors of the means. The statistics shown were calculated by use of the unpaired Student’s t test.
Lack of IFN-γ production in PPD nonresponder groups.
We next examined the levels of soluble cytokines in response to PPD in all groups. Analysis of IFN-γ production in response to PPD demonstrated a significant increase by the PPD responder groups regardless of HTLV-1 status (NP, 7.9 to 93.7 pg/ml; PP, 83.3 to 156.0 pg/ml); in contrast, neither of the PPD nonresponder groups (NN and PN) induced IFN-γ production (Fig. 2D). However, TNF-α production from each group was significantly increased (P < 0.05) in response to PPD when compared with that from the medium controls (Fig. 2B). Analysis of cytokine production by individual donors revealed that neither of the PPD nonresponder groups (NN and PN) induced production of IFN-γ, whereas most donors induced TNF-α production (Fig. 2B). Additionally, induction of TNF-α in response to PPD was comparable in all four groups, suggesting that PPD-specific hyporesponsiveness in the NN and PN groups was specific for IFN-γ production. We were unable to detect IL-2 and IL-4 production, both of which were below the sensitivity level of detection (data not shown).
We next analyzed the production of IL-10 in response to PPD in all groups. Both of the HTLV-1-negative groups produced higher amounts of IL-10 in response to PPD than the HTLV-1-positive groups did (Fig. 2C). In contrast, production of IL-10 by the PN group was significantly reduced (P < 0.01) in response to PPD (6.0 to 0.9 pg/ml; Fig. 2C). PPD-specific IL-10 production was also reduced in the PP group (10.7 to 6.1 pg/ml; Fig. 2C), but the reduction was not statistically different. These data suggested that PPD-specific anergy by the PPD nonresponder groups might be due to the lack of Th1 cytokine production by PBMCs.
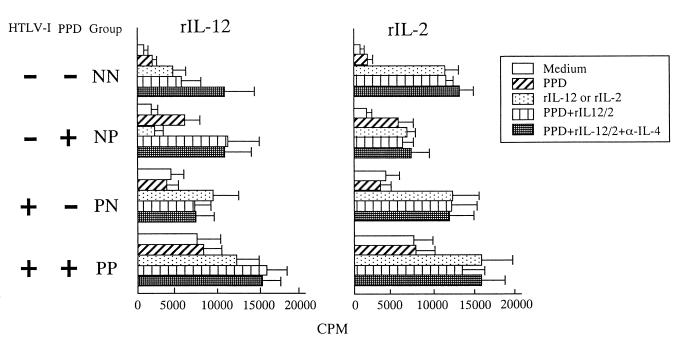
Effects of rIL-2 and rIL-12 on PBMCs in response to PPD.
We next performed reconstitution experiments to determine whether the PPD-specific hyporesponsiveness was due to a low Th1 response. We analyzed the effects of exogenous rIL-2 (20 IU/ml) or rIL-12 (100 IU/ml) on PPD-specific responses, which enhances Th1 responses (13). In general, rIL-2 induced marked PBMC proliferation but did not enhance the PPD-specific responses in any group (Fig. 3). Likewise, rIL-12 also induced PBMC proliferation in all groups except for the NP group, which had minimal proliferation in response to rIL-12. Addition of rIL-12 also enhanced PPD-specific responses in both the HTLV-1-negative (NP) and -positive (PP) PPD responder groups (Fig. 3).
FIG. 3.
Reconstitution of proliferation response and IFN-γ production by rIL-12 or rIL-2 and anti-IL-4. PBMCs were treated with PPD in the absence or presence of rIL-12 (100 U/ml) (left panel) or rIL-2 (20 U/ml) (right panel) in the absence or presence of anti-IL-4 (10 μg/ml). Proliferation was measured by [3H]thymidine incorporation.
However, addition of rIL-12 alone did not reverse PPD-specific hyporesponsiveness in either of the PPD nonresponder groups (NN and PN) (Fig. 3). Addition of neutralizing anti-IL-4 in conjunction with rIL-12 enhanced PPD-specific responses in the HTLV-1-negative groups but had no effect on the PPD-specific responses in the HTLV-1-positive groups (Fig. 3), suggesting that PPD-specific hyporesponsiveness in HTLV-1-infected individuals might be due to the lack of IL-12 signaling.
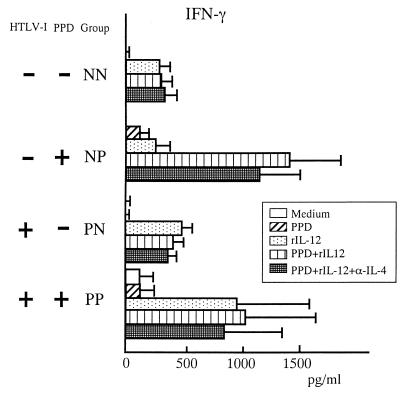
Addition of rIL-12 results in IFN-γ production.
It has been previously shown that IFN-γ production is dependent on IL-12 signaling for optimal induction (4, 14). To examine if the PPD-specific hyporesponsiveness by the HTLV-1-positive donors was due to defective IL-12 signaling, we tested for the production of IFN-γ following IL-12 treatment. All groups, including HTLV-1-positive PPD nonresponders, had enhanced production of IFN-γ (Fig. 4). These data suggest that the lack of PPD responsiveness in the HTLV-1-positive group (PN) was not due to defective IL-12 signaling. These data also imply that signals that induce IFN-γ production do not necessarily reverse the PPD-specific hyporesponsiveness in HTLV-1-infected carriers.
FIG. 4.
Effects of rIL-12 on IFN-γ production in response to PPD. PBMCs were cultured with or without rIL-12 (100 U/ml) and anti-IL-4 (10 μg/ml) monoclonal antibody. On day 6, IFN-γ levels were measured by an enzyme-linked immunosorbent assay. The data are expressed as means ± standard errors of the means.
DISCUSSION
Several studies have established that infection with HTLV-1 results in subclinical immune suppression, manifesting as secondary infections with bacterial and parasitic pathogens (2, 5). The direct evidence of immune suppression comes from studies in Japan, where a great majority of HTLV-1 carriers failed to respond to PPD (9, 11, 15). The cellular immune response involves activation of T cells with production of cytokines, such as IL-2 and IFN-γ; the altered magnitude of the response can have an impact on the clinical outcome in patients infected with HTLV-1. In the present study, we show that the PPD-specific anergy among HTLV-1 carriers is due to a selective defect in IFN-γ production, without affecting TNF-α production. The PPD-specific anergy could not be restored in the PN group by the addition of rIL-2, rIL-12, or anti-IL-4 antibody, although rIL-12 did restore IFN-γ production.
The hyporesponsiveness to PPD in both HTLV-1-positive and -negative groups appeared to be specific for PPD, since PHA responses were comparable in all groups as previously observed in vivo (9). Analysis of cytokine production in response to PPD revealed that the IFN-γ level was markedly increased in the PPD responder groups relative to that of the PPD nonresponder groups. Interestingly, both HTLV-1-positive and -negative PPD nonresponder groups were incapable of inducing IFN-γ production. Several possible mechanisms may explain the low production of IFN-γ in PPD nonresponders. First, the frequency of antigen-specific cells capable of producing IFN-γ may be low in the PPD nonresponder groups. However, we were able to demonstrate that both HTLV-1-positive and -negative hyporesponder groups were capable of inducing similar levels of TNF-α. Second, the presence of regulating cytokines, such as IL-10, might be involved in the PPD-specific anergy. IL-10 has been shown to inhibit Th1-driven proliferation by suppressing the production of both IL-2 and IL-12 (12). However, in accordance with our recent observation of down-regulation of IL-10 in HTLV-infected donors (3), we observed reduced production of IL-10 in the HTLV-1-positive group. Thus, the presence of IL-10 could not have accounted for the PPD-specific anergy in the HTLV-1-positive group.
Since both IL-2 and IL-12 are potent stimulators of IFN-γ production (4, 14), it is possible that a lack of their production in the PPD nonresponder group may have accounted for PPD-specific anergy. Indeed, addition of rIL-12 plus anti-IL-4 was able to partially reverse PPD-specific anergy in the HTLV-negative group. The enhancing effect of IL-12 on the PPD-specific response could be due to the activation of unresponsive T cells or replacement of insufficient IL-12 produced by the individuals PBMCs. Taken together, these data suggest that PPD-specific anergy in the NN group was due to the lack of IL-12 production, as well as to an increased production of down-regulating Th2 cytokines.
In contrast, the addition of neither rIL-12 nor anti-IL-4 reversed PPD-specific anergy in the HTLV-1-positive, PPD nonresponder group. Further, the lack of IL-12 responsiveness was not due to defective IL-12 signaling since treatment with rIL-12 resulted in production of IFN-γ, a cytokine dependent on IL-12 for optimal induction (4, 14). IL-12-induced IFN-γ production did not restore PPD-specific anergy in the HTLV-1-positive, PPD nonresponder group. However, IL-12 was able to enhance the proliferative response to PPD in the HTLV-1-positive PPD responder group, although the stimulation indices never reached levels similar to those of the HTLV-1-negative, PPD responder group. These results are consistent with the ability of IL-12 to enhance the proliferative response to antigens, alloantigens, and recall antigens in healthy individuals. The lack of comparable stimulation indices between HTLV-1-positive and -negative groups may be due to the high background counts in the HTLV-1-positive groups. We and others have shown that lymphocytes from individuals infected with HTLV-1 or -2 exhibit spontaneous proliferation in the absence of exogenous cytokines or mitogens (6, 10). The PPD-specific proliferative response could be detected in most of the PBMCs in the HTLV-1-positive PPD responder group, despite high background counts. Thus, the PN group remained anergic despite reconstitution with IL-2 or IL-12, the two Th1 cytokines involved in antigen-specific responses.
A possibility that HTLV-1 infection could have resulted in a loss of antigen-specific responses exists. Several reports suggest that, under certain conditions, immune T cells lose their antigen-specific reactivity following infection with HTLV-1 (16). For instance, some of the CD8+ cytotoxic T lymphocyte clones lose cytotoxic activity following HTLV-1 infection and continue to proliferate without stimulation with the appropriate antigen (17). Further, immunoreactivity of HTLV-1-specific T-cell clones has been shown to vary greatly: while some clones lose antigen specificity, others retain antigen specificity (16, 17). These in vitro studies suggest that HTLV-1 infection has the potential to result in a loss of T-cell function under some conditions. Such may be the case here, where most HTLV-1-infected individuals retained their PPD-specific responsiveness although a subset was anergic to PPD. While the exact mechanism(s) of such differential PPD responses was not examined, it is possible that the site(s) or the number of proviral integrations in the PPD nonresponder group might have resulted in an altered functional capacity or even a selective depletion of antigen-reactive clones from the periphery. In vitro data support the hypothesis that the functional consequence of HTLV-1 infection in immune T cells might be affected by a proviral integration pattern (7, 16). Further studies are needed to evaluate the exact role(s) of HTLV-1 infection on recall antigen anergy in HTLV-1-infected carriers.
ACKNOWLEDGMENTS
M. Suzuki was supported in part by a fellowship from Harvard University, Boston, Mass.
We gratefully acknowledge R. Mosley for editorial comments.
REFERENCES
- 1.Abbas A K, Murphy K M, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–791. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 2.Bryan J M. Infectious complications of human T cell leukemia/lymphoma virus: type I infection. Clin Infect Dis. 1996;23:138–145. doi: 10.1093/clinids/23.1.138. [DOI] [PubMed] [Google Scholar]
- 3.Dezzutti C S, Sasso D R, Rudolph D L, Lal R B. Down-regulation of interleukin-10 expression and production is associated with spontaneous proliferation by lymphocytes from human T-lymphotropic virus type II-infected persons. J Infect Dis. 1998;177:1489–1496. doi: 10.1086/515311. [DOI] [PubMed] [Google Scholar]
- 4.Germann T, Gately M K, Schoenhaut D S, Lohoff M, Mattner F, Fischer S, Jin S, Schmitt E, Rude E. Interleukin-12/T-cell stimulating factor, a cytokine with multiple effects on T-helper type 1 (Th1) but not Th2 cells. Eur J Immunol. 1994;23:1762–1770. doi: 10.1002/eji.1830230805. [DOI] [PubMed] [Google Scholar]
- 5.Hollesberg P, Hafler D A. Pathogenesis of disease induced by human lymphotropic virus type I infection. N Engl J Med. 1993;328:1173–1182. doi: 10.1056/NEJM199304223281608. [DOI] [PubMed] [Google Scholar]
- 6.Lal R B, Rudolph D L, Dezzutti C S, Linsley P S, Prince H E. Costimulatory effects of T cell proliferation during infection with human T lymphotropic virus types I and II are mediated through CD80 and CD86 ligands. J Immunol. 1996;157:1288–1296. [PubMed] [Google Scholar]
- 7.Mitsuya H, Jarrett R F, Cossman J, Cohen O J, Kao C S, Guo H G, Reitz M S, Broder S. Infection of human T lymphotropic virus-I-specific immune T cell clones by human T lymphotropic virus-I. J Clin Invest. 1986;78:1302–1310. doi: 10.1172/JCI112715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mueller N, Tachibana N, Stuver S O, Okayama A, Ishizaki J, Shishime E, Murai K, Shioiri S, Tsuda K. Epidemiologic perspectives of HTLV-I. In: Blattner W, editor. Human retrovirology: HTLV. New York, N.Y: Raven Press; 1990. pp. 281–293. [Google Scholar]
- 9.Murai K, Tachibana N, Shioiri S, Shishime E, Okayama A, Ishizaki J, Tsuda K, Mueller N. Suppression of delayed-type hypersensitivity to PPD and PHA in elderly HTLV-I carriers. J Acquired Immune Defic Syndr. 1990;3:1006–1009. [PubMed] [Google Scholar]
- 10.Prince H E, York J, Golding J, Owen S M, Lal R B. Spontaneous lymphocyte proliferation in human T-cell lymphotropic virus type I (HTLV-I) and HTLV-II infection: T-cell subset response and their relationships to the presence of provirus and viral antigen production. Clin Diagn Lab Immunol. 1994;1:273–282. doi: 10.1128/cdli.1.3.273-282.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tachibana N, Okayama A, Ishizaki J, Yokota T, Shishime E, Murai K, Shioiri S, Tsuda K, Essex M, Mueller N. Suppression of tuberculin skin reaction in healthy HTLV-I carriers from Japan. Int J Cancer. 1988;42:829–831. doi: 10.1002/ijc.2910420605. [DOI] [PubMed] [Google Scholar]
- 12.Taga K, Mostowski H, Tosato G. Human interleukin 10 can directly inhibit T-cell growth. Blood. 1993;81:2964–2971. [PubMed] [Google Scholar]
- 13.Tendler C L, Greenberg S J, Burton J D, Danielpour D, Kim S J, Blattner W A, Manns A, Waldmann T A. Cytokine induction in HTLV-I associated myelopathy and adult T-cell leukemia: alternate molecular mechanisms underlying retroviral pathogenesis. J Cell Biochem. 1991;46:302–311. doi: 10.1002/jcb.240460405. [DOI] [PubMed] [Google Scholar]
- 14.Trinchieri G, Gerosa F. Immunoregulation by interleukin-12. J Leukoc Biol. 1996;59:505–511. doi: 10.1002/jlb.59.4.505. [DOI] [PubMed] [Google Scholar]
- 15.Welles S L, Tachibana N, Okayama A, Shioiri S, Ishihara S, Murai K, Mueller N E. Decreased reactivity to PPD among HTLV-I carriers in relation to virus and hematologic status. Int J Cancer. 1994;56:337–340. doi: 10.1002/ijc.2910560307. [DOI] [PubMed] [Google Scholar]
- 16.Yarchoan R, Guo H G, Reitz M, Maluish A, Mitsuya H, Broder S. Alterations in cytotoxic and helper T cell function after infection of T cell clones with human T cell leukemia virus type I. J Clin Invest. 1986;77:1466–1470. doi: 10.1172/JCI112459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yssel H, Malefyt R D W, Dodon M D, Blanchard D, Gazzolo L, de Vries J E, Spits H. Human T cell leukemia/lymphoma virus type I infection of a CD4+ proliferative/cytotoxic T cell clone progresses in at least two distinct phases based on changes in function and phenotype of the infected cells. J Immunol. 1989;142:2279–2284. [PubMed] [Google Scholar]