Abstract
Background
Few randomized controlled trials evaluate the long-term efficacy and safety of pharmacotherapy for overactive bladder (OAB). This network meta- analysis compares the long-term (52-week) efficacy and safety of vibegron, mirabegron and anticholinergics for the treatment of OAB.
Methods
A systematic literature review and network meta-analysis were conducted following PRISMA guidelines using MEDLINE, Embase and Cochrane Central Register of Controlled Trials and terms related to OAB. Efficacy outcomes included change from baseline to week 48–52 in mean daily total urinary incontinence (UI) episodes, mean daily number of micturitions and volume voided/micturition. Efficacy outcomes were analysed using Bayesian models. Commonly reported adverse events (AEs) are described.
Results
Of 2098 hits retrieved, 5 publications and 1 study report describing 5 unique randomized controlled trials were included in the analyses. Mean (95% credible interval) change from baseline in total UI episodes for vibegron 75 mg (−2.2; −2.9 to −1.5) showed a significantly greater reduction than mirabegron 50 mg (−1.3; −1.9 to −0.8) and tolterodine 4 mg extended release (−1.6; −2.1 to −1.1). No significant differences were observed between vibegron and comparators for daily micturitions or volume voided/micturition. Within the manuscripts, the 4 most common AEs (range) for anticholinergics included dry mouth (5.2–90.0%), constipation (7.7–65.0%), blurred vision (3.8–35.0%) and hypertension (8.6–9.6%); the 4 most commonly reported AEs for β3-adrenergic agonists included hypertension (8.8–9.2%), urinary tract infection (5.9–6.6%), headache (5.5%) and nasopharyngitis (4.8–5.2%).
Conclusion
Vibegron was associated with significantly greater improvement in daily total UI episodes at 52 weeks than mirabegron and tolterodine. When reported, the most common AE for anticholinergics was dry mouth and for β3-adrenergic agonists was hypertension. Hypertension incidence was similar between drug classes.
Keywords: adrenergic beta-3 receptor agonists, antimuscarinic, medication persistence, micturition, urinary bladder, urinary incontinence
Introduction
Bothersome symptoms of overactive bladder (OAB), which is defined as urinary urgency with frequency and nocturia with or without urinary incontinence (UI),1 affect more than 30 million people in the United States aged ≥40 years.2 First-line treatment of OAB consists of behavioural therapy (for example, bladder training, bladder control strategies), which may be combined with pharmacotherapy.1 Oral anticholinergics and β3-adrenergic receptor agonists are recommended as second-line treatment for OAB.1 Sustained efficacy and a favourable safety and tolerability profile are important considerations for treatment selection given the chronic nature of OAB and the need for continuous treatment.
Anticholinergics have been the mainstays of treatment for OAB. However, side effects, such as dry mouth and constipation, can limit long-term persistence.3–6 Treatment with β3-adrenergic receptor agonists provides efficacy whilst minimizing the risk of anticholinergic-related side effects.7–10 Although long-term persistence with the β3-adrenergic receptor agonist mirabegron is improved compared with anticholinergics,11–13 unmet expectations of treatment are a commonly cited reason for discontinuation.14 Limited information is available regarding the long-term efficacy and safety of oral treatments for OAB. In particular, few randomized controlled trials (RCTs) have evaluated the long-term outcomes. In the absence of long-term RCTs, network meta-analyses enable indirect comparison of drugs that have not been directly compared whilst adjusting for population and trial design differences, and such analyses are often used by health technology assessment bodies to evaluate comparative efficacy.
Vibegron is a novel, selective, oral β3-adrenergic receptor agonist15 approved by the FDA for the treatment of OAB in adults.16 The recommended dosage for vibegron is 75 mg once daily (swallowed whole or crushed and mixed with applesauce), with no requirement for dose titration or dose adjustments. Vibegron is metabolized by CYP3A4 and does not inhibit or induce major human cytochrome P450 enzymes, minimizing interactions with drugs prescribed for this population.15,16 In the phase III, randomized, double-blind, placebo-controlled 12-week EMPOWUR trial and its 40-week double-blind extension, vibegron showed favourable efficacy and safety in patients with OAB.10,17
The long-term real-world efficacy of vibegron relative to other OAB treatments is not yet known; however, the 40-week EMPOWUR extension study,17 which preserved randomization and double-blind treatment for patients receiving active treatment, provides an opportunity to compare long-term efficacy and safety of vibegron to other OAB treatments using data from extended-duration RCTs. Prior network meta-analyses have compared the efficacy of approved pharmacological treatments for OAB,7,18–21 but few19,20 have included trials with long-term (48- to 52-week) outcomes; to our knowledge, none have focused exclusively on the long-term outcomes or included vibegron 75 mg. This network meta-analysis compared the long-term (52-week) efficacy and safety of vibegron 75 mg with mirabegron (both β3–adrenergic agonists) and anticholinergic medications for OAB treatment.
Methods
Search and selection criteria
A systematic literature review was performed in accordance with Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) reporting guidelines. The literature review was guided by the inclusion and exclusion criteria listed in Supplementary Table 1 (available at: https://www.drugsincontext.com/wp-content/uploads/2022/08/dic.2022-4-2-Suppl.pdf) and was conducted in the MEDLINE, Embase and Cochrane Central Register of Controlled Trials databases. The search terms were related to OAB, including “urinary bladder”, “overactive bladder”, “overactive urinary bladder” and “OAB” as well as individual terms for each drug approved for the treatment of OAB. Searches were performed on 16 September 2020 for 48- to 52-week RCTs in adult patients with non-neurogenic OAB. Studies were limited to those in the English language but were not limited by time. Search hits were reviewed for inclusion and exclusion criteria by two teams that included either two or three people, and discrepancies were resolved by the heads of the teams. Reference lists of systematic literature reviews and meta-analyses were compared against search results for the identification of RCTs possibly missed by the searches.
Because this was a systematic literature review and meta-analysis, written informed consent and institutional review board approval were not needed as the data were derived from previously published studies in which informed consent and ethics approval were obtained. Owing to the limited scope, this study was not prospectively registered with PROSPERO.
Data extraction
One reviewer extracted data on study design, baseline values, and selected efficacy and safety outcomes using a standardized extraction form; a second reviewer verified the extracted information. Discrepancies were resolved by consensus.
Outcomes assessed
Efficacy outcomes included in the network meta-analysis were change from baseline to week 48–52 in mean daily total UI episodes, mean daily micturitions and volume voided per micturition. Daily total UI episodes and micturitions were recorded in a 3-day or a 7-day diary for tolterodine and mirabegron and a 7-day diary for vibegron and solifenacin.17,22,23 Volume voided per micturition was recorded prior to each visit over 3 days for mirabegron and solifenacin, over 1 day for vibegron and over 1 or 3 days for tolterodine. Safety outcomes included adverse event (AE) reporting.
Statistical analysis
Micturitions and volume voided per micturition were analysed in the full analysis sets (FAS), defined as all randomized patients with baseline and one or more postbaseline assessment, including those who discontinued the trial for any reason. Total UI episodes were analysed in the FAS for incontinence (FAS-I; all patients in the FAS with one or more incontinence episodes at baseline). Missing efficacy data in Staskin et al.17 were not imputed as the mixed model for repeated measures accounted for missing data, whereas missing efficacy data in Chapple et al.22 and Gratzke et al.23 were imputed using the last observation carried forward approach.17,22,23 Safety outcomes were analysed in the safety analysis sets, which included all patients who received one or more doses of study medication.
Efficacy data were analysed using Bayesian models with normal likelihood and identity link functions. Models were performed in WinBUGS 1.4.3 (ref.24) and R 3.6.1 (ref.25) using the R2WinBUGS package26 and code based on National Institute for Health and Care Excellence technical support documents.27–29 The overall mean (95% credible interval; CrI) change from baseline was calculated for each efficacy outcome in each treatment group. Mean (95% CrI) differences from vibegron 75 mg were also calculated for each outcome in each of the other treatment groups. Changes from baseline with 95% CrIs not overlapping 0 were considered statistically significant. Because placebo arms were not present in the trials, all models used the most commonly reported treatment, tolterodine 4 mg extended release (ER), as the reference. Heterogeneity was assessed by examining the contribution to the total residual deviance of each trial arm. Safety results are presented descriptively.
Results
Studies
A total of 2098 search hits were identified (Figure 1). After the removal of duplicates and review of titles and abstracts, 69 studies remained; most studies were excluded because they did not meet the time frame (48–52 weeks in duration) or were not RCTs. After a review of the full-text articles, 5 publications and 1 study report describing 5 unique RCTs with evaluable data were included in the analyses (Table 1). All trials used parallel group designs and intent-to-treat (ITT) or modified ITT analysis. The 5 trials included comparisons between mirabegron 50 mg and tolterodine 4 mg ER or solifenacin 5 mg (n=1 trial each), between vibegron 75 mg and tolterodine 4 mg ER (n=1 trial) and between imidafenacin 0.1 mg twice daily and solifenacin 5 mg (n=2 trials).
Figure 1.
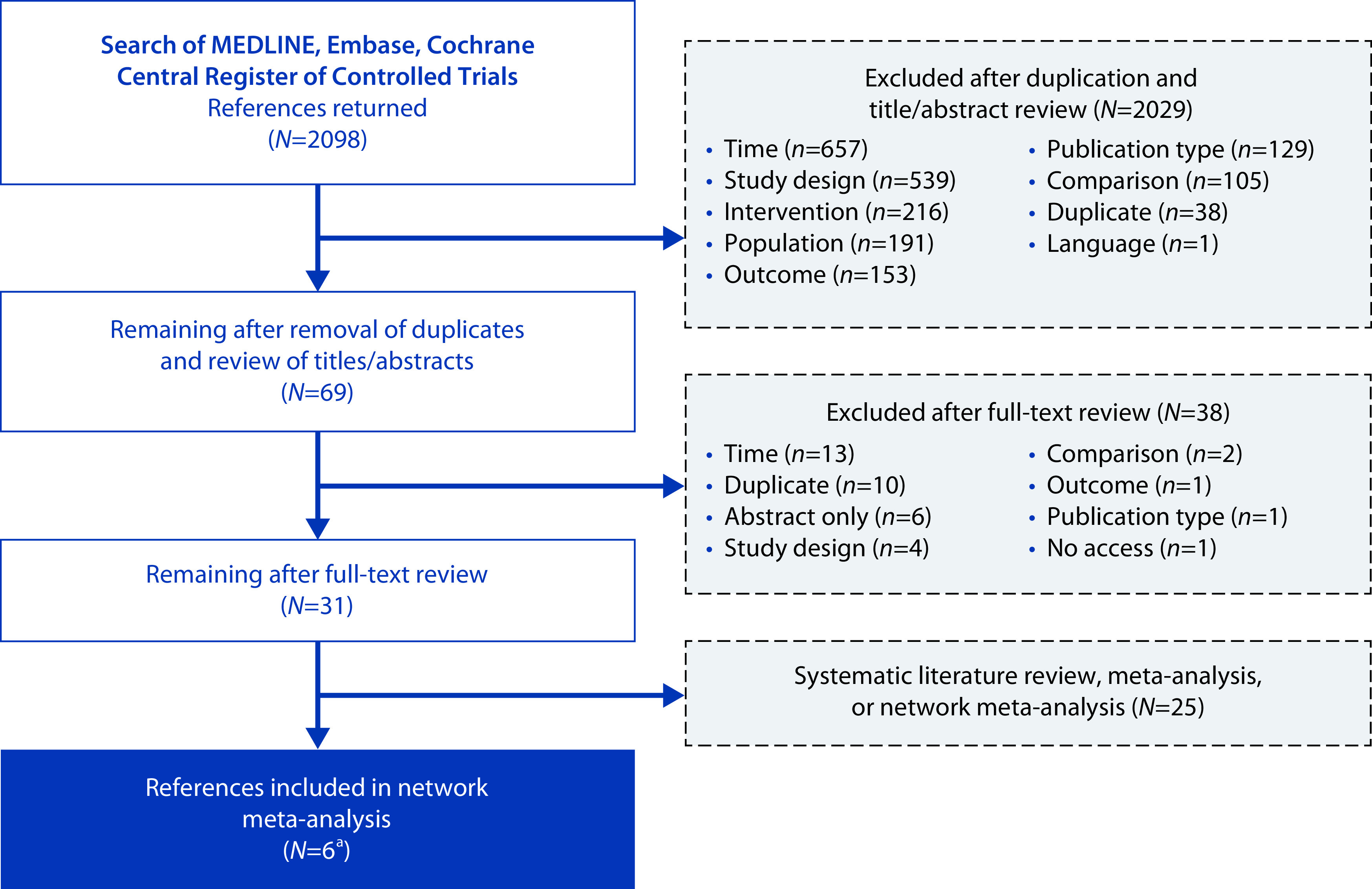
PRISMA flow diagram.
aFive publications and one study report describing five unique randomized controlled trials.
PRISMA, Preferred Reporting Items for Systematic Review and Meta-Analysis.
Table 1.
Trials identified by the systematic literature review.
| Trial | Treatments | SAF, n | FAS, n | FAS-I, n | Mean age, ya | Women, %a |
|---|---|---|---|---|---|---|
|
| ||||||
| Chapple et al., 201322,b TAURUS | Mirabegron 50 mg | 812 | 789 | 479 | 59.4 | 74.0 |
| Mirabegron 100 mgc | 820 | 802 | 483 | |||
| Tolterodine 4 mg ER | 812 | 791 | 488 | |||
|
| ||||||
| Gratzke et al., 201823,b SYNERGY II | Mirabegron 50 mg | 305 | 302 | 301 | 60.5d | 80.0 |
| Solifenacin 5 mg | 303 | 299 | 297 | |||
| Solifenacin 5 mg + mirabegron 50 mgc | 1206 | 1193 | 1184 | |||
|
| ||||||
| Staskin et al., 202117,b,e EMPOWUR Extension | Tolterodine 4 mg ER | 232/141f | 136 | 106 | 61.4 | 78.2 |
| Vibegron 75 mg | 273/181f | 176 | 143 | |||
|
| ||||||
| Yokoyama et al., 201331,e | Imidafenacin 0.1 mg BID | 55 | NR | NR | 71.2 | 62.4 |
| Solifenacin 5 mg | 54 | |||||
|
| ||||||
| Zaitsu et al., 201130 LIST | Imidafenacin 0.1 mg BID | 21 | 11 | NR | 70.7 | 32.0 |
| Solifenacin 5 mg | 20 | 14 | ||||
Weighted mean for treatment groups of interest; reported in the SAF, with the exception of Gratzke et al. (2018) and Yokoyama et al. (2013), in which demographic data were reported in the FAS.
Urinary incontinence analysed in the FAS-I; micturitions and volume voided per micturition analysed in the FAS.
Not a treatment or dose of interest.
Reported as median age.
Efficacy reported for 40-week and 52-week subgroups; only 52-week data were used in these efficacy analyses. Safety reported only for the combination of 40 and 52 weeks.
n for 40-week + 52-week subgroups/n for 52-week subgroup only.
BID, twice a day; ER, extended release; FAS, full analysis set; FAS-I, FAS for incontinence; NR, not reported; SAF, safety analysis set.
Three of the 5 trials contributed to the efficacy analysis and had similar enrollment criteria,17,22,23 which included patients ≥18 years old with symptoms of OAB for ≥3 months with or without UI (only patients with UI in Gratzke et al.23), ≥8 micturitions per day and ≥1 (ref.23) or ≥3 (refs.10,17,22) urgency episodes per day. Of the 2 trials that contributed solely to the safety analyses, 1 trial enrolled patients who were 50–80 years old with OAB symptoms for ≥4 weeks and no previous treatment,30 and the final trial enrolled patients ≥20 years old with OAB and ≥1 urgency episode per day.31 Of the 5 trials, 4 were 52 weeks in length,22,23,30,31 and 1 was a 40-week extension of a 12-week trial.17 During the 40-week extension trial, patients who were randomly assigned to and completed 12 weeks of double-blind treatment with vibegron or tolterodine continued their assigned treatment, whilst patients who completed 12 weeks of placebo were randomized to treatment with double-blind vibegron or tolterodine. Results from subgroup analyses of patients who received vibegron and tolterodine for the full 52 weeks were used for the efficacy analyses. The mean age across the included studies ranged from 59.4 to 70.7 years. With the exception of Zaitsu et al.,30 most patients were women (Table 1).
Efficacy
Two publications, Yokoyama et al.31 and Zaitsu et al.30, assessed efficacy using patient-reported outcomes; Yokoyama et al.31 reported International Prostate Symptom Score, quality of life index and Overactive Bladder Symptom Score (OABSS), whereas Zaitsu et al.30 reported OABSS, OABSS component scores and Kings Health Questionnaire scores. Neither publication reported any of the prespecified efficacy outcomes of interest. Thus, only data from Chapple et al.,22 Gratzke et al.23 and Staskin et al.17 were analysed to compare changes from baseline in efficacy measures over 52 weeks. Mean (95% CrI) changes from baseline to week 52 in efficacy outcomes are described in Table 2.
Table 2.
Change from baseline to week 52 in selected efficacy endpoints (full analysis seta).
| Endpoint, mean (95% CrI) | Vibegron 75 mg n=176 |
Mirabegron 50 mg n=1090 |
Solifenacin 5 mg n=299 |
Tolterodine 4 mg ER n=927 |
|---|---|---|---|---|
| Daily total UI episodesb | −2.2 (−2.9 to −1.5) | −1.3 (−1.9 to −0.8) | −1.6 (−2.3 to −1.0) | −1.6 (−2.1 to −1.1) |
| Daily micturitions | −2.1 (−2.9 to −1.2) | −1.5 (−2.1 to −1.0) | −1.6 (−2.2 to −1.0) | −1.7 (−2.2 to −1.2) |
| Total volume voided per micturition, mLc | 34.2 (17.2 to 51.2) | 17.2 (11.3 to 23.0) | 20.3 (9.9 to 30.6) | 17.6 (14.4 to 20.8) |
Defined as all patients receiving ≥1 dose of double-blind study drug who had a baseline and ≥1 postbaseline assessment.
Analysed in the full analysis set for incontinence, defined as all patients receiving ≥1 dose of double-blind study drug who had a baseline and ≥1 postbaseline assessment and ≥1 UI episode at baseline. Vibegron, n=143; mirabegron, n=780; solifenacin, n=297; tolterodine, n=594.
Mirabegron, n=1082; solifenacin, n=293.
CrI, credible interval; ER, extended release; UI, urinary incontinence.
Total urinary incontinence episodes
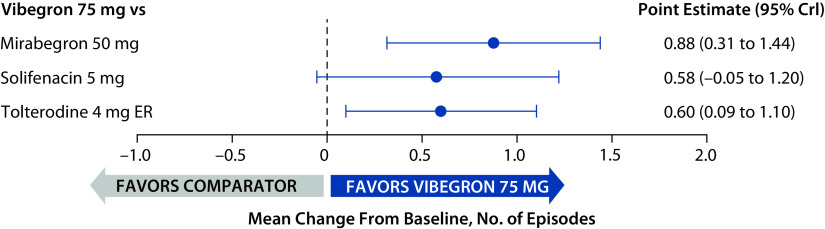
At baseline, the mean (range) daily number of total UI episodes across the 3 trials was 3.11 (2.54–3.65). The mean (95% CrI) change from baseline at week 52 in total UI episodes was statistically significantly greater for vibegron (−2.2; −2.9 to −1.5) than for mirabegron (−1.3; −1.9 to −0.8) and tolterodine (−1.6; −2.1 to −1.1); the 95% CrIs overlapped 0 for the comparison between vibegron and solifenacin, indicating no statistically significant difference (Figure 2).
Figure 2.
Change from baseline to week 52 in mean daily total UI episodes relative to vibegron (full analysis sets for incontinence).
CrI, credible interval; ER, extended release; UI, urinary incontinence.
Daily micturitions
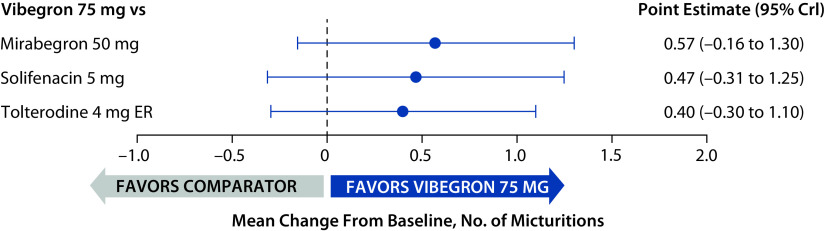
At baseline, the mean (range) daily number of micturitions across the three trials was 11.0 (10.6–11.3). The mean change from baseline at week 52 in daily micturitions was not statistically significantly different for vibegron compared with mirabegron, solifenacin or tolterodine (95% CrIs for all treatment comparisons overlapped 0; Figure 3).
Figure 3.
Change from baseline to week 52 in mean daily micturitions relative to vibegron (full analysis sets).
CrI, credible interval; ER, extended release.
Volume voided per micturition
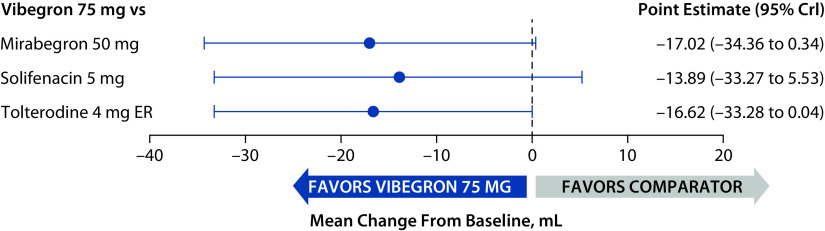
At baseline, the mean (range) volume voided per micturition across all trials was 158.2 (154.0–160.5) mL. The 95% CrIs of point estimates overlapped 0 for all treatment comparisons (Figure 4), indicating that the mean change from baseline at week 52 in volume voided per micturition was not statistically significantly different for vibegron compared with mirabegron, solifenacin or tolterodine.
Figure 4.
Change from baseline to week 52 in mean total volume voided per micturition relative to vibegron (full analysis sets).
CrI, credible interval; ER, extended release.
Safety
Safety was assessed descriptively via AEs reported in each publication. All five publications contributed to the analysis of AEs but publications differed in the way AEs were reported (Table 3). Chapple et al.,22 Gratzke et al.23 and Staskin et al.17 included AEs with an incidence of at least ≥2%. In contrast, Yokoyama et al.31 and Zaitsu et al.30 reported select anticholinergic-related AEs of interest (dry mouth, constipation and blurred vision), and it was therefore not possible to determine if other AEs were reported at an incidence of ≥2%. All five publications included an anticholinergic treatment arm; AEs included in the publication and reported at ≥5% incidence in any anticholinergic treatment arm were dry mouth, constipation, blurred vision, hypertension, nasopharyngitis and urinary tract infection. Three trials included β3–adrenergic agonists; AEs reported at ≥5% incidence in any β3–adrenergic agonist treatment arm were hypertension, urinary tract infection, headache and nasopharyngitis. Across all trials, fewer patients receiving vibegron discontinued due to AEs compared with anticholinergics and with mirabegron (Table 3).
Table 3.
Summary of AEs occurring in ≥5% of patients and reported in each trial (safety analysis set).
| AE, % | Chapple et al., 201322 | Gratzke et al., 201823 | Staskin et al., 202117 | Yokoyama et al., 201331 | Zaitsu et al., 201130 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mirabegron 50 mg n=812 |
Tolterodine 4 mg ER n=812 |
Mirabegron 50 mg n=305 |
Solifenacin 5 mg n=303 |
Vibegron 75 mg n=273 |
Tolterodine 4 mg ER n=232 |
Imidafenacin 0.1 mg BID n=52 |
Solifenacin 5 mg n=52 |
Imidafenacin 0.1 mg BID n=21 |
Solifenacin 5 mg n=20 |
|
| Discontinuation due to AEs | 6.4 | 6.0 | 2.3 | 1.7 | 1.5 | 3.4 | 5.5a | 13.0a | 0 | 15.0 |
| Hypertension | 9.2 | 9.6 | b | b | 8.8 | 8.6 | c | c | c | c |
| UTI | 5.9 | 6.4 | b | b | 6.6 | 7.3 | c | c | c | c |
| Headache | b | b | b | b | 5.5 | 3.9 | c | c | c | c |
| Nasopharyngitis | b | b | 5.2 | 5.0 | 4.8 | 5.2 | c | c | c | c |
| Dry mouth | 2.8 | 8.6 | 3.9 | 5.9 | 1.8 | 5.2 | 38.5 | 40.4 | 71.4 | 90.0 |
| Constipation | b | b | b | b | b | b | 7.7 | 19.2 | 14.3 | 65.0 |
| Blurred vision | b | b | b | b | b | b | 3.8 | 9.6 | 9.5 | 35.0 |
Based on randomization: imidafenacin, n=55; solifenacin, n=54.
AE present in <5% of patients in both treatment groups.
AE was not reported in the published manuscript and it was not possible to determine whether it occurred in ≥5% of patients.
AE, adverse event; BID, twice daily; ER, extended release; UTI, urinary tract infection.
Discussion
In this network meta-analysis – which focused exclusively on long-term (48- to 52-week) outcomes and compared vibegron, a novel, selective, oral β3-adrenergic receptor agonist,15 with mirabegron and anticholinergics – vibegron was associated with a statistically significantly greater improvement from baseline to week 52 in the mean number of daily total UI episodes than mirabegron and tolterodine, whereas the comparison between vibegron and solifenacin indicated no statistically significant difference. Generally, a similar efficacy for micturitions was seen for vibegron compared with mirabegron, tolterodine and solifenacin. Results for volume voided per micturition approached significance for the comparisons between vibegron and mirabegron and between vibegron and tolterodine. Our analysis provides long-term data for vibegron that has not been reported in previous network meta-analyses, which have included predominantly short-term trials of anticholinergics,7,18,20,21 mirabegron7,18,19 and onabotulinumtoxin A.19 In general, these network meta-analyses of OAB treatments have shown similar short-term efficacy.7,18,20,21 However, solifenacin has been associated with significant improvements in urge UI relative to tolterodine21 and mirabegron.7 Additionally, onabotulinumtoxin A has been associated with reductions in daily micturitions and incontinence episodes relative to mirabegron.19
In our analysis, the top four reported AEs for the class of anticholinergics were dry mouth, constipation, blurred vision and hypertension, and for the class of β3-adrenergic agonists AEs were hypertension, urinary tract infection, headache and nasopharyngitis. The incidence of hypertension, when reported, was similar amongst all treatment groups (vibegron, 8.8%; mirabegron, 9.2%; tolterodine, 8.6–9.6%). Of the three trials that reported hypertension, Chapple et al.22 and Staskin et al.17 had similar definitions for hypertension, whereas Gratzke et al.23 did not report the definition for hypertension within the publication. The US prescribing information for mirabegron includes a warning stating that it can increase blood pressure and that patients should be periodically monitored.32 A recently published ambulatory blood pressure monitoring study found that treatment with vibegron 75 mg was not associated with significant effects on blood pressure or heart rate overall and in patients with or without pre- existing (controlled) hypertension.33
In addition to the three RCTs that provided efficacy data for this network meta-analysis, several open- label, uncontrolled trials have demonstrated long-term efficacy of anticholinergics and β3-adrenergic receptor agonists in patients with OAB with regards to sustained improvements in micturitions, UI and volume voided.34–38 Mean changes from baseline to week 52 in these outcomes observed with solifenacin, tolterodine and imidafenacin (micturitions/day: −2.3 to −3.0; UI episodes/day: −1.7 to −2.0; volume voided/micturition: 22.8 to 43.2 mL) were generally comparable to those observed in the RCTs. Similarly, in an open-label, uncontrolled trial of mirabegron 50 mg (with a possible dose increase to 100 mg), patients receiving the 50-mg dose experienced statistically significant reductions from baseline to week 52 in mean daily micturitions (−2.2) and incontinence episodes (−1.3).39 In the two RCTs that were excluded from the efficacy analyses because they did not report efficacy outcomes of interest, subjective symptoms of OAB assessed with the OABSS improved significantly from baseline to week 52 amongst patients receiving solifenacin or imidafenacin, with no significant differences between treatment groups.30,31 These findings provide additional support for the long-term efficacy of anticholinergics and β3-adrenergic receptor agonists in OAB.
As expected, anticholinergic-related AEs, such as constipation and dry mouth, were less frequent with vibegron and mirabegron than with tolterodine, solifenacin or imidafenacin. Rates of AEs with vibegron and mirabegron were generally comparable. These findings were consistent with those reported in uncontrolled long-term trials of anticholinergics and mirabegron.34–36,38 Similarly, a systematic review and meta-analysis of RCTs of anticholinergics for OAB showed risk of dry mouth, constipation and blurred vision with anticholinergics.21 Additional meta-analyses have shown that mirabegron was associated with reduced risk of AEs relative to anticholinergics and onabotulinumtoxin A.7,18,19
Long-term use of anticholinergics is associated with an elevated risk of dementia and falls or fractures.40,41 In a meta-analysis of data from six studies, two of which involved treatment for OAB, anticholinergic use for ≥3 months increased the risk of incident dementia by 46% compared with non-use (rate ratio 1.46; 95% CI 1.17–1.81).40 A retrospective matched-cohort study using data from Canadian health databases identified an increased risk of dementia amongst patients with OAB using anticholinergics compared with those prescribed mirabegron (hazard ratio 1.23; 95% CI 1.12–1.35).42 However, whether this difference represents a class effect is unclear. Notably, an online survey of 222 primarily (97%) US urology/gynaecology providers indicated that studies associating anticholinergics with the development of dementia have motivated a shift away from prescribing anticholinergics towards β3-adrenergic receptor agonists for patients with OAB.43
Although both vibegron and mirabegron are selective β3-adrenergic receptor agonists, an in vitro study of β-adrenergic receptor selectivity showed that vibegron has no measurable activity at β1–adrenergic receptors and lower β2-adrenergic receptor activity compared with mirabegron.44 Additionally, vibegron was more potent than mirabegron at activating β3-adrenergic receptors and demonstrated a higher maximum β3-adrenergic receptor response relative to the full agonist isoproterenol (99.2% versus 80.4% with mirabegron). An indirect comparison of vibegron and mirabegron (nine studies) that employed a different statistical methodology than the current analysis found that vibegron was associated with significantly reduced mean daily number of total incontinence episodes at weeks 4 (versus mirabegron 25 and 50 mg) and 52 (versus mirabegron 50 mg) and increased volume voided at weeks 12 (versus mirabegron 25 and 50 mg) and 52 (versus mirabegron 50 mg).45
Treatment persistence with anticholinergics for OAB is generally poor, with 1-year persistence rates in routine clinical practice ranging between 5% and 47%.13 Common factors affecting persistence with OAB treatment include medications not working as expected and the presence of side effects, especially dry mouth.5,11,46–48 Mirabegron has demonstrated significantly longer persistence than anticholinergics in a real-world setting.11 Additional research is needed to explore whether the favourable long-term efficacy and tolerability of vibegron will result in improved treatment persistence.
This network meta-analysis relied on data from RCTs, which may result in higher treatment persistence than will be seen in the real world and was limited by the small number of studies (n=5) meeting inclusion criteria, which resulted in a smaller dataset. Notably, commonly prescribed anticholinergic agents, such as oxybutynin and fesoterodine, lacked eligible 48- to 52-week trials. Additional studies comparing the long-term efficacy and safety of OAB treatments are needed, particularly in light of recent studies suggesting an association between anticholinergic use and elevated risk of dementia.40,42 Although inclusion of a greater number of trials or the existence of long-term trials with an arm for every comparator of interest may have been able to provide greater insight into comparative efficacy, our network meta-analysis provides relevant data to help inform clinical decision-making. No treatment was represented by more than three trials, and meta-regression was not possible due to the small dataset. No placebo- controlled 48- to 52-week trials were identified in the literature search, likely due to ethical considerations precluding the use of an inactive comparator in a long-term study. Although not all studies were double- blind, the three studies included in the efficacy analyses17,22,23 were conducted in a double-blind manner. These three studies had similar inclusion criteria and mean age and sex distributions but had differences in sample sizes, and our analysis adjusted for such differences in trial population and design. Differences in handling of missing data (for example, imputation via last observation carried forward in Chapple et al.22 and Gratzke et al.23 versus mixed model for repeated measures in Staskin et al.17) may have affected efficacy outcomes, though the low and similar discontinuation rates between treatment groups minimize the potential impact of these differences.
Conclusions
Results from this network meta-analysis suggest a statistically significant improved efficacy for vibegron compared with mirabegron and tolterodine for reduction in total UI episodes. The favourable tolerability and safety profile of β3-adrenergic receptor agonists, in particular the decreased incidence of anticholinergic-related AEs and a potentially lower risk of dementia with long-term use relative to anticholinergics, are important considerations when selecting a medication for OAB. Further research is warranted to evaluate the long-term effectiveness and safety of vibegron in the real-world setting.
Acknowledgements
Medical writing and editorial support was provided by Adrienne Drinkwater, PhD, for The Curry Rockefeller Group, LLC (Tarrytown, NY, USA) and was funded by Urovant Sciences (Irvine, CA, USA). The systematic literature review was performed with the support of Christopher Bly, Cristiano Piron, MPH, Daniel R Murphy, MS, and Julie Myers, MPH, from Medical Decision Modeling, Inc., a consultancy firm working with support from Urovant Sciences; the Bayesian analyses were performed with the support of Jing Voon Chen, PhD, and John Dever, MS, from Medical Decision Modeling, Inc. Portions of this analysis were presented at the 2021 meeting of the Academy of Managed Care Pharmacy and published as an abstract in the Journal of Managed Care and Specialty Pharmacy.49
Footnotes
Contributions: All authors contributed to the study conception and design, acquisition and/or analysis of data, interpretation of results, and preparation and/or revisions of the manuscript and provided final approval for publication. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: MK is a consultant for Allergan, Astellas, Boston Scientific, Coloplast, Laborie, and Urovant Sciences and has received grant or research study funding from Allergan, Amphora, Axonics, Boston Scientific, Coloplast, Cook Myosite, Dignify Therapeutics, EBT Medical, FemPulse, Ipsen, Taris and Uro-1. RW is an employee of Medical Decision Modeling, a consultancy working for Urovant Sciences. ET is an employee of Urovant Sciences. DS and PNM were employees of Urovant Sciences at the time the work was conducted. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2022/08/dic.2022-4-2-COI.pdf
Funding declaration: This work was supported by Urovant Sciences (Irvine, CA, USA).
Correct attribution: Copyright © 2022 Kennelly M, Wielage R, Shortino D, Thomas E, Mudd PN Jr. https://doi.org/10.7573/dic.2022-4-2. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: Submitted; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: 6 Green Lane Business Park, 238 Green Lane, New Eltham, London, SE9 3TL, UK.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Gormley EA, Lightner DJ, Burgio KL, et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline. American Urological Association; 2019. https://www.auanet.org/documents/Guidelines/PDF/OAB-JU.pdf . [Google Scholar]
- 2.Coyne KS, Sexton CC, Vats V, et al. National community prevalence of overactive bladder in the United States stratified by sex and age. Urology. 2011;77:1081–1087. doi: 10.1016/j.urology.2010.08.039. [DOI] [PubMed] [Google Scholar]
- 3.Akino H, Namiki M, Suzuki K, et al. Factors influencing patient satisfaction with antimuscarinic treatment of overactive bladder syndrome: results of a real-life clinical study. Int J Urol. 2014;21:389–394. doi: 10.1111/iju.12298. [DOI] [PubMed] [Google Scholar]
- 4.Athanasopoulos A, Giannitsas K. An overview of the clinical use of antimuscarinics in the treatment of overactive bladder. Adv Urol. 2011;2011:820816. doi: 10.1155/2011/820816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benner JS, Nichol MB, Rovner ES, et al. Patient-reported reasons for discontinuing overactive bladder medication. BJU Int. 2010;105:1276–1282. doi: 10.1111/j.1464-410X.2009.09036.x. [DOI] [PubMed] [Google Scholar]
- 6.Chancellor MB, Migliaccio-Walle K, Bramley TJ, et al. Long-term patterns of use and treatment failure with anticholinergic agents for overactive bladder. Clin Ther. 2013;35:1744–1751. doi: 10.1016/j.clinthera.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 7.Kelleher C, Hakimi Z, Zur R, et al. Efficacy and tolerability of mirabegron compared with antimuscarinic monotherapy or combination therapies for overactive bladder: a systematic review and network meta-analysis. Eur Urol. 2018;74:324–333. doi: 10.1016/j.eururo.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 8.Mitcheson HD, Samanta S, Muldowney K, et al. Vibegron (RVT-901/MK-4618/KRP-114V) administered once daily as monotherapy or concomitantly with tolterodine in patients with an overactive bladder: a multicenter, phase IIb, randomized, double-blind, controlled trial. Eur Urol. 2019;75:274–282. doi: 10.1016/j.eururo.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Nitti VW, Khullar V, van Kerrebroeck P, et al. Mirabegron for the treatment of overactive bladder: a prespecified pooled efficacy analysis and pooled safety analysis of three randomised, double-blind, placebo-controlled, phase III studies. Int J Clin Pract. 2013;67:619–632. doi: 10.1111/ijcp.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Staskin D, Frankel J, Varano S, et al. International phase III, randomized, double-blind, placebo and active controlled study to evaluate the safety and efficacy of vibegron in patients with symptoms of overactive bladder: EMPOWUR. J Urol. 2020;204:316–324. doi: 10.1097/JU.0000000000000807. [DOI] [PubMed] [Google Scholar]
- 11.Soda T, Tashiro Y, Koike S, Ikeuchi R, Okada T. Overactive bladder medication: persistence, drug switching, and reinitiation. Neurourol Urodyn. 2020;39:2527–2534. doi: 10.1002/nau.24527. [DOI] [PubMed] [Google Scholar]
- 12.Song YS, Lee HY, Park JJ, Kim JH. Persistence and adherence of anticholinergics and beta-3 agonist for the treatment of overactive bladder: systematic review and meta-analysis, and network meta-analysis. J Urol. 2021;205:1595–1604. doi: 10.1097/JU.0000000000001440. [DOI] [PubMed] [Google Scholar]
- 13.Yeowell G, Smith P, Nazir J, et al. Real-world persistence and adherence to oral antimuscarinics and mirabegron in patients with overactive bladder (OAB): a systematic literature review. BMJ Open. 2018;8:e021889. doi: 10.1136/bmjopen-2018-021889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wada N, Watanabe M, Banjo H, et al. Long-term persistence with mirabegron in a real-world clinical setting. Int J Urol. 2018;25:501–506. doi: 10.1111/iju.13558. [DOI] [PubMed] [Google Scholar]
- 15.Edmondson SD, Zhu C, Kar NF, et al. Discovery of vibegron: a potent and selective β3 adrenergic receptor agonist for the treatment of overactive bladder. J Med Chem. 2016;59:609–623. doi: 10.1021/acs.jmedchem.5b01372. [DOI] [PubMed] [Google Scholar]
- 16.Urovant Sciences. GEMTESA® (vibegron) Full Prescribing Information. 2020. [Accessed August 23, 2022]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213006s000lbl.pdf .
- 17.Staskin D, Frankel J, Varano S, et al. Once-daily vibegron 75 mg for overactive bladder: long-term safety and efficacy from a double-blind extension study of the international phase 3 trial (EMPOWUR) J Urol. 2021;205:1421–1429. doi: 10.1097/JU.0000000000001574. [DOI] [PubMed] [Google Scholar]
- 18.Lozano-Ortega G, Walker DR, Johnston K, et al. Comparative safety and efficacy of treatments for overactive bladder among older adults: a network meta-analysis. Drugs Aging. 2020;37:801–816. doi: 10.1007/s40266-020-00792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lozano-Ortega G, Walker D, Rogula B, et al. The relative efficacy and safety of mirabegron and onabotulinumtoxinA in patients with overactive bladder who have previously been managed with an antimuscarinic: a network meta-analysis. Urology. 2019;127:1–8. doi: 10.1016/j.urology.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Herbison P, McKenzie JE. Which anticholinergic is best for people with overactive bladders? A network meta-analysis. Neurourol Urodyn. 2019;38:525–534. doi: 10.1002/nau.23893. [DOI] [PubMed] [Google Scholar]
- 21.Nazir J, Kelleher C, Aballea S, et al. Comparative efficacy and tolerability of solifenacin 5 mg/day versus other oral antimuscarinic agents in overactive bladder: a systematic literature review and network meta-analysis. Neurourol Urodyn. 2018;37:986–996. doi: 10.1002/nau.23413. [DOI] [PubMed] [Google Scholar]
- 22.Chapple CR, Kaplan SA, Mitcheson D, et al. Randomized double-blind, active-controlled phase 3 study to assess 12-month safety and efficacy of mirabegron, a β3-adrenoceptor agonist, in overactive bladder. Eur Urol. 2013;63:296–305. doi: 10.1016/j.eururo.2012.10.048. [DOI] [PubMed] [Google Scholar]
- 23.Gratzke C, van Maanen R, Chapple C, et al. Long-term safety and efficacy of mirabegron and solifenacin in combination compared with monotherapy in patients with overactive bladder: a randomised, multicentre phase 3 study (SYNERGY II) Eur Urol. 2018;74:501–509. doi: 10.1016/j.eururo.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Lunn D, Thomas A, Best N, Spiegelhalter D. WinBUGS – a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput. 2000;10:325–337. [Google Scholar]
- 25.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2019. https://www.r-project.org/ [Google Scholar]
- 26.Sturtz S, Ligges U, Gelman A. R2WinBUGS: a package for running WinBUGS from R. J Stat Softw. 2005;12:1–16. [Google Scholar]
- 27.Dias S, Welton N, Sutton A, Ades A. NICE DSU technical support document 1 introduction to evidence synthesis for decision making. National Institute for Health and Clinical Excellence; 2012. https://pubmed.ncbi.nlm.nih.gov/27905715/ [PubMed] [Google Scholar]
- 28.Dias S, Welton N, Sutton A, Ades A. NICE DSU technical support document 2 a generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials. National Institute for Health and Clinical Excellence Decision Support Unit; 2016. https://www.ncbi.nlm.nih.gov/books/NBK310366/ [PubMed] [Google Scholar]
- 29.Dias S, Welton N, Sutton A, Ades A. NICE DSU technical support document 3 heterogeneity: subgroups, meta-regression, bias and bias-adjustment. National Institute for Health and Clinical Excellence Decision Support Unit; 2016. https://pubmed.ncbi.nlm.nih.gov/27905717/ [PubMed] [Google Scholar]
- 30.Zaitsu M, Mikami K, Ishida N, Takeuchi T. Comparative evaluation of the safety and efficacy of long-term use of imidafenacin and solifenacin in patients with overactive bladder: a prospective, open, randomized, parallel-group trial (the LIST study) Adv Urol. 2011;2011:854697. doi: 10.1155/2011/854697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yokoyama T, Koide T, Hara R, et al. Long-term safety and efficacy of two different antimuscarinics, imidafenacin and solifenacin, for treatment of overactive bladder: a prospective randomized controlled study. Urol Int. 2013;90:161–167. doi: 10.1159/000345055. [DOI] [PubMed] [Google Scholar]
- 32.Astellas Pharma US Inc. MYRBETRIQ® (mirabegron extended-release tablets). Full Prescribing Information. 2018. [Accessed August 23, 2022]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/202611s011lbl.pdf .
- 33.Weber MA, Haag-Molkenteller C, King J, et al. Effects of vibegron on ambulatory blood pressure in patients with overactive bladder: results from a double-blind, placebo-controlled trial. Blood Press Monit. 2022;27:128–134. doi: 10.1097/MBP.0000000000000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haab F, Cardozo L, Chapple C, Ridder AM for the Solifenacin Study Group. Long-term open-label solifenacin treatment associated with persistence with therapy in patients with overactive bladder syndrome. Eur Urol. 2005;47:376–384. doi: 10.1016/j.eururo.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Homma Y, Yamaguchi O. Long-term safety, tolerability, and efficacy of the novel anti-muscarinic agent imidafenacin in Japanese patients with overactive bladder. Int J Urol. 2008;15:986–991. doi: 10.1111/j.1442-2042.2008.02152.x. [DOI] [PubMed] [Google Scholar]
- 36.Kreder K, Mayne C, Jonas U. Long-term safety, tolerability and efficacy of extended-release tolterodine in the treatment of overactive bladder. Eur Urol. 2002;41:588–595. doi: 10.1016/s0302-2838(02)00177-x. [DOI] [PubMed] [Google Scholar]
- 37.Masumori N. Long-term safety, efficacy, and tolerability of imidafenacin in the treatment of overactive bladder: a review of the Japanese literature. Patient Prefer Adherence. 2013;7:111–120. doi: 10.2147/PPA.S28160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takei M, Homma Y Japanese Tolterodine Study Group. Long-term safety, tolerability and efficacy of extended-release tolterodine in the treatment of overactive bladder in Japanese patients. Int J Urol. 2005;12:456–464. doi: 10.1111/j.1442-2042.2005.01066.x. [DOI] [PubMed] [Google Scholar]
- 39.Yamaguchi O, Ikeda Y, Ohkawa S. Phase III study to assess long-term (52-week) safety and efficacy of mirabegron, a β3-adrenoceptor agonist, in Japanese patients with overactive bladder. Low Urin Tract Symptoms. 2017;9:38–45. doi: 10.1111/luts.12107. [DOI] [PubMed] [Google Scholar]
- 40.Dmochowski RR, Thai S, Iglay K, et al. Increased risk of incident dementia following use of anticholinergic agents: a systematic literature review and meta-analysis. Neurourol Urodyn. 2021;40:28–37. doi: 10.1002/nau.24536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Szabo SM, Gooch K, Schermer C, et al. Association between cumulative anticholinergic burden and falls and fractures in patients with overactive bladder: US-based retrospective cohort study. BMJ Open. 2019;9:e026391. doi: 10.1136/bmjopen-2018-026391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Welk B, McArthur E. Increased risk of dementia among patients with overactive bladder treated with an anticholinergic medication compared to a beta-3 agonist: a population-based cohort study. BJU Int. 2020;126:183–190. doi: 10.1111/bju.15040. [DOI] [PubMed] [Google Scholar]
- 43.Menhaji K, Cardenas-Trowers OO, Chang OH, et al. Anticholinergic prescribing pattern changes of urogynecology providers in response to evidence of potential dementia risk. Int Urogynecol J. 2021;32:2819–2826. doi: 10.1007/s00192-021-04736-8. [DOI] [PubMed] [Google Scholar]
- 44.Brucker BM, King J, Mudd PN, Jr, McHale K. Selectivity and maximum response of vibegron and mirabegron for β3-adrenergic receptors. Curr Ther Res. 2022;96:100674. doi: 10.1016/j.curtheres.2022.100674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kennelly MJ, Rhodes T, Girman CJ, et al. Efficacy of vibegron and mirabegron for overactive bladder: a systematic literature review and indirect treatment comparison. Adv Ther. 2021;38:5452–5464. doi: 10.1007/s12325-021-01902-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ali M, Grogan S, Powell S, et al. Qualitative analysis of factors influencing patient persistence and adherence to prescribed overactive bladder medication in UK primary care. Adv Ther. 2019;36:3110–3122. doi: 10.1007/s12325-019-01098-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diokno AC, Sand PK, Macdiarmid S, Shah R, Armstrong RB. Perceptions and behaviours of women with bladder control problems. Fam Pract. 2006;23:568–577. doi: 10.1093/fampra/cml018. [DOI] [PubMed] [Google Scholar]
- 48.Kinjo M, Sekiguchi Y, Yoshimura Y, Nutahara K. Long-term persistence with mirabegron versus solifenacin in women with overactive bladder: prospective, randomized trial. Low Urin Tract Symptoms. 2018;10:148–152. doi: 10.1111/luts.12151. [DOI] [PubMed] [Google Scholar]
- 49.Kennelly M, Wielage R, Shortino D, Thomas E, Mudd PN., Jr Long-term efficacy and safety of vibegron versus mirabegron and anticholinergics for overactive bladder: a systematic review and network meta-analysis. J Manag Care Spec Pharm. 2021;27:S115–S116. doi: 10.7573/dic.2022-4-2. https://www.jmcp.org/toc/jmcsp/27/4-a+Suppl . [DOI] [PMC free article] [PubMed] [Google Scholar]