Abstract
Peripheral blood mononuclear cells (PBMC) of human immunodeficiency virus (HIV)-infected children, age-matched HIV-seronegative controls, and HIV-infected asymptomatic and symptomatic adults were compared for their ability to mediate antibody-dependent cellular cytotoxicity (ADCC) and natural killer (NK) cell-mediated cytotoxicity against target cells expressing HIV or herpes simplex virus (HSV) antigens. Target cells consisted of CD4 lymphocytes purified from PBMC of HIV-seronegative adults and incubated with the IIIB strain of HIV, HUT78 cells chronically infected with IIIB, and HSV-infected human fibroblasts. PBMC of asymptomatic HIV-infected adults were generally able to lyse CD4 cells expressing HIV antigens. Direct correlation was found between the magnitude of lysis and absolute CD4 cell counts in these individuals. In contrast to these results, PBMC from HIV-infected children were generally unable to lyse IIIB-expressing CD4 cells, regardless of the children’s clinical status, age, or absolute CD4 cell counts. Cells from HIV-seronegative adults and children did not directly lyse these target cells either but, in contrast to cells of HIV-seropositive children, were able to mediate cell lysis when serum from an HIV-seropositive adult was added. However, effector cells from these HIV-infected children were able to mediate both ADCC against HSV-infected fibroblasts and NK cell-mediated cytotoxicity against IIIB-infected HUT78 cells. Reduced ability of PBMC from vertically HIV-infected children to mediate ADCC against HIV antigen-expressing CD4 cells may contribute to rapid progression to AIDS.
CD4 lymphocytes are known to be targets for human immunodeficiency virus (HIV) infection in vivo. Therefore, the use of HIV antigen-expressing CD4 lymphocytes as target cells in cytotoxicity assays might yield data closely reflecting in vivo events. Previous reports indicated that peripheral blood mononuclear cells (PBMC) of HIV-infected adults are able to lyse CD4 lymphocytes expressing gp120, the major envelope glycoprotein of HIV type 1 (HIV-1) (55, 57, 62). The effector cells responsible for this lysis were shown to be CD16+ natural killer (NK) cells, armed in vivo with cytophilic HIV-specific antibodies. Therefore, this mechanism of cytotoxicity can be classified as antibody-dependent cellular cytotoxicity (ADCC).
To explore possible mechanisms for accelerated disease progression in some perinatally HIV-infected children compared to that in adults (2, 4, 49), we studied ADCC against HIV-1-expressing CD4 lymphocytes in children at various stages of HIV infection. Responses were compared to those of HIV-infected adults and HIV-seronegative age-matched controls.
MATERIALS AND METHODS
Subjects.
Subjects consisted of HIV-infected adults, monitored at the Hospital of the University of Pennsylvania; children with perinatal HIV infection, monitored in the Special Immunology Clinic at The Children’s Hospital of Philadelphia; and age-matched HIV-seronegative healthy volunteers. HIV infection was diagnosed on the basis of at least two positive PCRs and PBMC cultures for HIV. According to Centers for Disease Control and Prevention (CDC) criteria for children and adults (8, 9), HIV-infected children were classified as asymptomatic with normal (P1-A) or abnormal (P1-B) immune function or as symptomatic with nonspecific findings (P2-A) or HIV-related conditions (P2-B-F), whereas adults’ stages were classified as asymptomatic (CDC stage A), symptomatic conditions (stage B), or AIDS-defining conditions (stage C). Patients receiving intravenous immunoglobulin were excluded from the study, since repeated administration of intravenous immunoglobulin may lead to reduced NK cell-mediated cytotoxicity (11) and might affect the ability of PBMC to mediate ADCC. This study was approved by the Institutional Review Boards of the University of Pennsylvania and The Children’s Hospital of Philadelphia.
Effector cells.
PBMC were separated from heparinized venous blood by Ficoll-Hypaque (Pharmacia, Piscataway, N.J.) gradient centrifugation. Monocytes were removed by adherence on plastic surfaces coated with fetal bovine serum (FBS; HyClone, Logan, Utah) as previously described (23). PBMC were used in cytotoxicity assays within 4 h after the blood drawing.
Experiments in which NK cells were depleted from PBMC by incubation with monoclonal antibody anti-Leu 11B (Becton Dickinson, Mountain View, Calif.), which reacts with the FcγIII receptor (CD16) on NK cells, as previously described (3, 39) followed by incubation with baby rabbit complement (Cedarlane Laboratories, Hornby, Ontario, Canada) to destroy antibody-bound cells were performed. The surviving PBMC were used as effector cells in cytotoxicity assays. Arming of effector cells was accomplished by incubating PBMC for 12 h at 37°C with undiluted heat-inactivated heterologous sera obtained from HIV-infected patients and seronegative controls (58). The cells were washed five times before use as effector cells in cytotoxicity assays. To elute putative cytophilic antibodies, freshly isolated PBMC were incubated at 37°C for 12 h and then washed three times (57).
Target cells.
HUT78 cells, derived from a CD4+ lymphoblastoid T-cell line, uninfected and chronically infected with the HIV-1 strain IIIB (16), were kindly provided by J. A. Hoxie, Hospital of the University of Pennsylvania, Philadelphia. K562 cells, derived from an erythroleukemia cell line and known to be sensitive to NK cell-mediated cytotoxicity, were used as target cells in NK cell assays. FS4 cells, human embryonic foreskin fibroblasts (National Institute of Allergy and Infectious Diseases, Bethesda, Md.), were inoculated with the NS strain of herpes simplex virus type 1 (HSV-1) (kindly provided by H. M. Friedman, Hospital of the University of Pennsylvania) at a multiplicity of infection of 5.0, as previously described (37). After 6 h of incubation at 37°C in 5% CO2, the cells were trypsinized, washed, and then stored in the vapor phase of liquid nitrogen. Uninfected FS4 cells were prepared simultaneously.
PBMC of healthy seronegative adults were stimulated with phytohemagglutinin (Sigma, St. Louis, Mo.) and then expanded in the presence of human interleukin 2 (IL-2; Schiapparelli, Fairfield, N.J.) as described previously (56). Thereafter, CD4 cells were selected from these phytohemagglutinin–IL-2-stimulated PBMC by panning (63) with the monoclonal antibody OKT4. Purified CD4+ cells were incubated for 48 h in medium containing 32 U of IL-2 per ml and 20% FBS. The cells were then washed and subjected to low-speed centrifugation. Cell-free supernatant of strain IIIB-infected HUT78 cells was added to pelleted CD4+ cells at a final dilution of 1:10,000. After 1 h of incubation at 37°C, the cell surface expression of viral antigens was confirmed by flow cytometric analysis after immunofluorescent staining with HIV-seropositive human serum and fluorescein-conjugated goat F(ab)2 anti-human immunoglobulin G (IgG) (TAGO, Burlingame, Calif.). After virus inactivation with 4% paraformaldehyde in phosphate-buffered saline, viral antigen expression was quantified by flow cytometry. Unexposed and HIV-coated target cells were electronically gated to exclude aggregates and nonviable cells from evaluation. Fluorescence intensity thresholds of less than 2% positive cells were established by using uninfected target cells incubated with HIV-1 antibody-positive human serum and HIV-1-infected target cells incubated with HIV-1 antibody-negative human serum. HIV antigens were detected on >90% of target cells after 1 h of exposure to IIIB-containing supernatant.
NK cell-mediated cytotoxicity.
Target cells, consisting of HUT78 cells chronically infected with the IIIB strain of HIV-1 (HUT78/IIIB), uninfected HUT78 cells, and K562 cells were labeled with Na251CrO4 (Amersham, Arlington Heights, Ill.), resuspended in RPMI containing 20% FBS, and aliquoted into round-bottomed 96-well microtiter plate wells. Effector cells, prepared as described above, were added to give effector/target (E:T) cell ratios of 100:1 (5 × 105 PBMC/5,000 targets) in a 200-μl total volume per well. In preliminary experiments with PBMC from HIV-infected adults, maximal levels of NK cell-mediated lysis were detected at this E:T ratio. Owing to small volumes of blood obtainable from children, multiple E:T ratios could not be tested. After 18 h of incubation, 100 μl of supernatant per well was harvested without disturbing the cell pellet. Supernatants were autoclaved to inactivate HIV before being counted in a gamma scintillation counter. All tests were done in triplicate. Total release (100%) was determined by addition of 100 μl of 2% Triton X-100 to target cells in the absence of effector cells. Spontaneous release, determined by adding 100 μl of medium instead of effector cells, was always less than 30%. Percent 51Cr release was calculated by the standard formula.
ADCC against HIV-expressing CD4 lymphocytes.
HIV-expressing and control CD4 lymphocytes, obtained as described above, were labeled with 51Cr, resuspended in RPMI 1640 containing 20% FBS and 32 U of IL-2 per ml, and added to PBMC to give final E:T ratios of 100:1. Microtiter plates were centrifuged (100 × g for 3 min) and incubated for 4 h at 37°C in 5% CO2. 51Cr release in supernatants was determined as described above.
ADCC against HSV-infected FS4 cells.
HSV-infected and uninfected FS4 cells were labeled with 51Cr, resuspended in RPMI containing 20% FBS, and added to effector cells to give E:T ratios of 100:1. The effector cells were added to wells in medium that contained sera from HSV-seropositive or -seronegative individuals at final dilutions of 1:100, previously found to be the optimal concentration for maximal ADCC-mediated killing of HSV-infected cells. After incubation for 4 h at 37°C, 51Cr release in supernatants was determined as described above.
Quantitation of NK cells.
Immunophenotyping was performed by standard procedures. Heparinized venous blood was incubated with the monoclonal antibody B73.1 (kindly provided by G. Trinchieri, Wistar Institute, Philadelphia, Pa.), which reacts with the FcRIII (CD16) receptor present on NK cells and polymorphonuclear leukocytes (39). HIV was inactivated by incubation in 4% paraformaldehyde in phosphate-buffered saline for 16 h at 4°C prior to flow cytometric analysis. Mononuclear lymphocytes were electronically gated to exclude other leukocytes from evaluation. After accumulation of 5,000 events, a fluorescence intensity threshold of less than 2% positive cells was established by using cells incubated with control mouse IgG.
Statistical methods.
The data shown in the figures represent means ± standard errors. The two-sample t test was used to compare mean ADCC antibody titers for HIV-1-infected and uninfected infants. n refers to the total number of determinations for a particular condition.
RESULTS
Cell-mediated cytotoxicity against HIV-expressing CD4 cells.
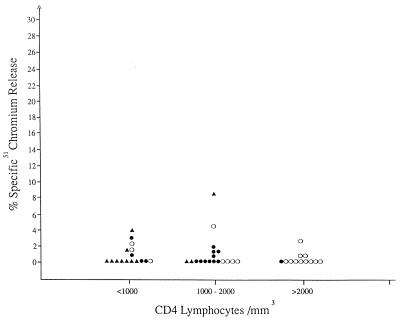
Forty-six children, 3 months to 10 1/2 years of age, with perinatally acquired HIV infection (19 patients at CDC classification stage P1, 15 at stage P2-A, and 12 at stage P2-B-D), were tested for cytotoxic activity against HIV-expressing CD4 cells. As shown in Fig. 1, PBMC from 31 of 46 children failed to lyse HIV-1-expressing CD4 cells, and PBMC of only four children gave >3% lysis. Even for these four, corresponding levels of lysis of control CD4 cells were not significantly different (data not shown). The lack of cytotoxic activity against HIV-expressing CD4 cells did not correlate with the patients’ stage of infection, age, or absolute CD4 lymphocyte counts.
FIG. 1.
Cytotoxicity against IIIB-coated CD4 lymphocytes mediated by PBMC of HIV-infected pediatric patients at different stages of disease: P1 (○), P2-A (●), and P2-B-D (▴), according to the CDC classification for pediatric HIV infection.
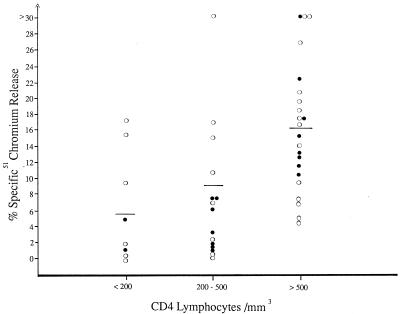
Concurrently with pediatric samples, we tested the ability of PBMC isolated from 45 HIV-seropositive adults to lyse HIV-expressing CD4 cells (Fig. 2). Most of these patients were male homosexuals; 28 were asymptomatic (CDC classification stage A), and 17 were symptomatic (CDC stage B or C). In contrast to the results obtained for HIV-seropositive children, cytotoxicity was observed for the majority of adults tested. The magnitude of target cell lysis correlated positively with absolute CD4 cell counts: the highest magnitude of cytotoxicity was observed when the CD4 cell count exceeded 500/mm3 (mean, 16.3%; range, 5.1 to 36.9%). PBMC of patients with CD4 counts between 200 and 500 cells/mm3 and those with less than 200 cells/mm3 exhibited lower cytotoxic activity (mean cytotoxicity of 9.1 and 5.6%, respectively). Mean cytotoxicity mediated by PBMC of patients with absolute CD4 cell counts of >500 cells/mm3 was significantly higher than that of patients whose CD4 cell counts were <200 cells/mm3 (P < 0.05). Cytotoxicity was not detected against uninfected CD4 cells (data not shown). PBMC of HIV-seronegative adults (n = 30) and children (n = 21) failed to lyse HIV-expressing CD4 lymphocytes (data not shown).
FIG. 2.
Cytotoxicity against IIIB-coated CD4 lymphocytes, mediated by PBMC of HIV-infected adults at different stages of disease: asymptomatic (○) and symptomatic (●).
Characterization of effector cells and requirement for HIV-specific antibodies.
A series of experiments was carried out to investigate the mechanism of cytotoxicity against HIV-expressing CD4 lymphocytes in adults. CD16+ NK cell-depleted effector cells were tested for their ability to lyse a variety of target cells in 51Cr release assays (Table 1). After depletion of CD16+ cells, specific 51Cr release from all target cells, except from uninfected CD4 lymphocytes, was significantly decreased (P < 0.05). When the effector cells were treated with complement alone prior to being added to cytotoxicity assays, only a slight loss of lytic activity was observed. These experiments confirm that lysis of HIV-expressing CD4 cells was mediated primarily by NK cells.
TABLE 1.
Effect of NK cell depletion on cytotoxic activity
| Treatment of effector cellsa | % Lysis of different
target cellsb
|
|||
|---|---|---|---|---|
| HUT | HUT-IIIB | CD4 | CD4-IIIB | |
| None | 25.6 ± 9.2 | 58.0 ± 5.6 | 0.4 ± 2.6 | 16.0 ± 7.5 |
| Complement | 18.9 ± 8.4 | 41.9 ± 4.8 | 1.4 ± 1.9 | 12.7 ± 6.2 |
| Leu 11B plus complement | 9.0 ± 3.2 | 15.7 ± 1.2 | 0 | 0 |
PBMC of three HIV-infected adults were treated with Leu 11B and complement or complement alone, as described in Materials and Methods, and then used as effector cells in 51Cr release assays.
Means ± standard errors.
After incubation for 12 h at 37°C, PBMC of HIV-seropositive adults lost their ability to lyse HIV antigen-expressing CD4 cells (Table 2). The ability of such PBMC to lyse HIV-expressing CD4 cells was restored when autologous HIV antibody-containing serum was added to assays at a final concentration of 1:100. These findings indicate that decreased cytotoxicity against HIV-expressing CD4 cells after incubation of PBMC is due to elution of cytophilic antibodies from NK cells rather than to defective effector cells. Thus, our data confirm a previous report that lysis of HIV-expressing CD4 lymphocytes occurs via an antibody-dependent mechanism, mediated by NK cells armed in vivo with cytophilic HIV antibodies (62).
TABLE 2.
Effect of incubation on the ability of PBMC to mediate lysis in the absence and presence of autologous serum
| PBMC incubationa | % Lysis
of different target cellsb
|
|||
|---|---|---|---|---|
| CD4 | CD4/IIIB | HUT78 | HUT78/IIIB | |
| 2 h | −1.1 ± 0.6 | 23.5 ± 4.9 | 26.6 ± 5.1 | 49.6 ± 1.8 |
| 12 h | −1.0 ± 1.1 | 6.2 ± 1.1 | 23.2 ± 3.9 | 36.2 ± 1.1 |
| 12 h and autologous serum added to assayc | −1.0 ± 1.7 | 26.4 ± 0.6 | 19.1 ± 4.2 | 37.5 ± 0.7 |
PBMC were obtained from HIV-infected adults with mild or no symptoms. Incubation was in RPMI 1640 containing 20% FBS.
Means ± standard errors.
Autologous serum was added at a final dilution of 1:100.
Mechanism and specificity of the deficient cytotoxicity against HIV-expressing CD4 cells in HIV-infected children.
To determine whether the inability of PBMC from perinatally HIV-infected children to lyse HIV-expressing CD4 cells was due to a reduced number of effector cells, we used flow cytofluorography to determine the percentage of NK cells in PBMC populations. Seventeen HIV-infected pediatric patients, 14 weeks to 8 years of age (stage P1 to P2-D1) had NK cell percentages within the normal range for age-matched seronegative children (range, 11.5 to 22.8%; mean, 17.6%).
To determine whether decreased cytotoxic activity of PBMC from perinatally HIV-infected children was specific for HIV-expressing CD4 cells, we tested PBMC of HIV-infected and uninfected children and adults for their ability to lyse FS4 fibroblasts, infected with HSV-1 strain NS, as previously described (37). Cytotoxicity against HIV-expressing CD4 lymphocytes was studied in parallel. Similar to the results reported above, effector cells from HIV-infected children were unable to lyse HIV-expressing CD4 lymphocytes (Table 3). Addition of HIV-seropositive serum from an adult to the effector cells of HIV-infected children did not significantly increase lysis of HIV-expressing CD4 cells. Effector cells of age-matched HIV-seronegative children were able to kill these targets to a significantly greater extent in the presence of serum from the same HIV-seropositive adult than in the presence of serum from a seronegative adult (P < 0.05).
TABLE 3.
Cytotoxicity against HIV-coated CD4 cells and HIV-infected FS4 cells
| Source of effector cells (n)a | Value for target
cells in the presence of serumb
|
|||||
|---|---|---|---|---|---|---|
| IIIB-coated CD4 cells
|
HSV-infected FS4
cells
|
|||||
| %
Lysisc
|
P valued | %
Lysisc
|
P valued | |||
| HIV seronegative | HIV seropositive | HSV seronegative | HSV seropositive | |||
| HIV-infected children (12) | 0 | 4.4 ± 2.3 | 0.1643 | 11.4 ± 1.6 | 36.4 ± 2.5 | <0.05 |
| Uninfected children (10) | 0 | 8.4 ± 1.3 | <0.05 | 9.0 ± 0.7 | 35.5 ± 3.3 | <0.05 |
| HIV-infected adults (6) | 13.4 ± 5.6 | 16.5 ± 4.0 | 0.3494 | 16.0 ± 1.6 | 41.6 ± 3.7 | <0.05 |
| Uninfected adults (6) | 0.8 ± 1.3 | 15.6 ± 3.4 | <0.05 | 16.6 ± 1.0 | 42.5 ± 3.9 | <0.05 |
PBMC were prepared as described in Materials and Methods.
HIV- and HSV-seropositive and -seronegative sera obtained from adults.
Means ± standard errors.
Data were analyzed by Student’s t test.
In contrast to results with HIV-expressing CD4 lymphocytes, effector cells from HIV-infected children lysed HSV-infected FS4 target cells to a significantly greater extent in the presence of an HSV-seropositive serum sample than in the presence of an HSV-seronegative serum sample (P < 0.05). In addition, effector cells from HIV-infected children were able to lyse HSV-infected targets to the same extent as did effector cells from HIV-seronegative children. These results suggest that NK cells from HIV-infected children are capable of mediating ADCC against target cells infected with a virus other than HIV.
PBMC from HIV-infected adults lysed HIV-expressing lymphocytes equally in the presence and in the absence of HIV-seropositive serum (P > 0.05). Specific cytotoxicity was mediated by PBMC of HIV-seronegative controls when an HIV-seropositive serum sample was added to assay mixtures (P < 0.05). These results were consistent with previous reports that in vitro lysis of HIV-expressing CD4 lymphocytes is mediated by HIV patients’ effector cells armed in vivo with cytophilic antibodies (57, 62). Similar to the observations with children, PBMC from HIV-infected adults and those from HIV-seronegative adults were equally effective in lysing HSV-infected targets in the presence of anti-HSV antibody-containing sera (P > 0.05).
To further characterize the functional defect in HIV-infected children, we tested the ability of their PBMC to lyse HUT78 cells chronically infected with the IIIB strain of HIV and uninfected HUT78 cells. PBMC from HIV-infected children and those from uninfected children gave similar results with respect to the ability to lyse uninfected or infected HUT78 cell targets (Table 4). These observations, along with the results for ADCC against HSV-infected targets, indicate that antibody-dependent and -independent NK cell-mediated cytotoxicity is generally intact in perinatally HIV-infected children.
TABLE 4.
NK cell-mediated cytotoxicity against HUT78 cells chronically infected with the HIV-1 strain IIIB
| Source of effector cells (n)a | %
Lysis of target cellsb
|
|
|---|---|---|
| Uninfected HUT78 | IIIB-infected HUT78 | |
| HIV-infected children (34) | 14.8 ± 1.8 | 27.6 ± 2.2 |
| Uninfected children (16) | 12.6 ± 1.6 | 26.2 ± 2.3 |
| HIV-infected adults (28) | 13.8 ± 1.8 | 24.2 ± 3.4 |
| Uninfected adults (19) | 20.5 ± 2.8 | 44.0 ± 3.1 |
PBMC were obtained as described in Materials and Methods.
Means ± standard errors.
To determine whether deficient cytotoxicity against HIV-expressing CD4 cells in HIV-infected children was due to deficient production or to effector cell binding of ADCC-mediating antibodies, assays were carried out by addition of serum from an HIV-seropositive adult throughout the 4-h incubation of the ADCC assay. The presence of seropositive serum was considered to increase the magnitude of cytotoxicity if the mean lysis was higher than the mean cytotoxicity plus 2 standard deviations in the presence of HIV-seronegative serum. For 7 of the 15 HIV-infected children tested, addition of HIV-seropositive serum from an adult had a positive effect on the magnitude of cytotoxicity (Table 5). The presence of adult HIV-seropositive serum also resulted in increased lysis mediated by effector cells of uninfected children.
TABLE 5.
Lysis of IIIB-coated CD4 lymphocytes mediated by PBMC of HIV-infected children in the presence of adult serum
| HIV status of children | Characteristic of
childrenc
|
% Lysis of CD4-IIIB in
the presence of adult serum
|
|||
|---|---|---|---|---|---|
| Age | Stage (CDC classification) | Absolute CD4 cell count/mm3 | HIV seronegativea | HIV seropositiveb | |
| Seropositive | 3 mo | P1-A | 3,000 | 0.6 | 0 |
| 6 mo | P2-C | 950 | 0 | 0 | |
| 7 mo | P1-B | 950 | 1.8 | 1.9 | |
| 1 yr | P1-A | 3,000 | 0 | 0 | |
| 1 yr | P1-A | 5,000 | 4.0 | 12.6 | |
| 1 yr | P2-A | 1,000 | 0 | 0 | |
| 1 yr | P2-A | 1,550 | 1.0 | 8.9 | |
| 1 yr | P2-D1 | 280 | 0 | 6.8 | |
| 1 yr | P2-D1 | 790 | 0 | 20.3 | |
| 2 yr | P2-A | 500 | 0.9 | 0.3 | |
| 2 yr | P2-D1 | 1,200 | 0 | 0 | |
| 2 yr | P2-D1 | 1,000 | 0 | 0 | |
| 4 yr | P2-C | 200 | 0 | 15.5 | |
| 10 yr | P1-B | 185 | 0 | 10.1 | |
| 10 yr | P2-A | 100 | 0 | 13.1 | |
| Seronegative | 15 mo | NA | ND | 0 | 1.3 |
| 2 yr | NA | ND | 0 | 6.9 | |
| 3 yr | NA | ND | 0 | 16.6 | |
| 5 yr | NA | ND | 0 | 10.8 | |
| 5 yr | NA | ND | 0 | 6.5 | |
| 5 yr | NA | ND | 0 | 8.9 | |
| 5 yr | NA | ND | 0 | 7.4 | |
| 8 yr | NA | ND | 0 | 3.7 | |
| 11 yr | NA | ND | 0 | 10.2 | |
Sera obtained from HIV-seronegative adults.
Sera obtained from HIV-infected asymptomatic adults.
NA, not applicable; ND, not determined.
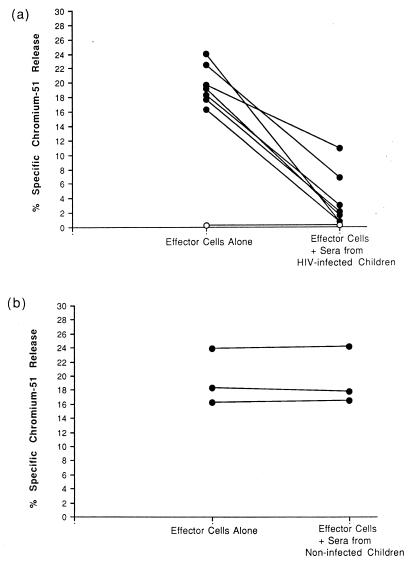
In further studies, we added sera from children with perinatal HIV infection to ADCC assays in which PBMC from HIV-seropositive adults were tested for their ability to lyse HIV-expressing CD4 lymphocytes. As shown in Fig. 3a, the addition of individual sera from seven pediatric HIV patients in seven experiments each time inhibited cytotoxicity mediated by adult PBMC (P < 0.05). In contrast, the presence of sera from uninfected children had no effect on the magnitude of ADCC mediated by PBMC from HIV-infected adults (Fig. 3b).
FIG. 3.
Effects of sera from HIV-seropositive (a) and HIV-seronegative (b) children on cytotoxicity against IIIB-coated CD4 lymphocytes mediated by PBMC of HIV-infected adults (●) at early stages of disease and by PBMC of an HIV-seronegative adult (○).
DISCUSSION
In HIV-infected individuals, humoral and cellular immune mechanisms may act to reduce the quantity of virus in cells and tissues. Studies on the role of cell-mediated cytotoxicity in HIV-infected adults have yielded contradictory results (5, 13, 14, 20, 21, 28, 38, 42, 46, 53, 58). Several recent publications have addressed the correlation between clinical stages of infection and various types of cytotoxic activity detected in vitro, including cytotoxic T-lymphocyte-mediated cytotoxicity, NK cell-mediated cytotoxicity, and ADCC (1, 20, 26–28, 38, 58, 61). In some studies, reduced ADCC (1, 45, 58) and NK cell-mediated cytotoxicity correlated with disease progression (20, 52, 58, 61), whereas in another study increased ADCC activity was detected in patients with AIDS compared to those at earlier stages of infection (38). Some investigators did not find any correlation between cytotoxic activity and clinical stage of HIV infection (26–28). Others described a disparity between NK cell-mediated cytotoxicity, which was observed to decline around the time of first symptoms, and ADCC activity, which remained constant throughout the course of disease (19). In all these ADCC studies with PBMC from adults, HIV immune globulin was added to the assay and HIV-infected T-cell lines were used as targets. Different results among these studies may be explained by the different virus strains, target cell lines, or test conditions employed in the cytotoxicity assays. Even subclones of the same cell line may vary significantly in their susceptibility to ADCC (48). For this reason, we decided to use effector cells, armed in vivo with ADCC-mediating antibodies, to measure cytotoxicity against target cells which appear to be one of the major reservoirs for HIV in infected humans, namely, CD4 lymphocytes.
We showed that PBMC of vertically HIV-infected children were impaired in their ability to lyse HIV-expressing CD4 lymphocytes. In contrast, PBMC of HIV-infected adults lysed such target cells, and the magnitude of lysis correlated with absolute numbers of circulating CD4 lymphocytes. This cytotoxicity was shown to be mediated by NK cells armed in vivo with cytophilic HIV antibodies, confirming previous reports (57, 62). We found normal percentages of CD16+ NK cells in HIV-infected children, confirming previously published data (34).
The inability of PBMC from children with vertical HIV infection to mediate such ADCC against CD4 lymphocytes expressing HIV antigens was a specific and functional, rather than a quantitative, deficiency: effector cells from the same children lysed HSV-infected fibroblasts in ADCC assays and killed HIV-infected HUT78 cells and K562 cells in NK cell-mediated cytotoxicity assays to the same extent as did PBMC from HIV-seronegative children.
Attempts to induce ADCC against HIV-expressing CD4 lymphocytes, by using effector cells of HIV-infected children and serum of an HIV-seropositive adult, gave variable results. PBMC of about one-half of the children showed ADCC-mediated lysis when antibody-containing serum of an HIV-infected adult was added directly to the assays. Furthermore, sera from HIV-infected children interfered with the ability of PBMC from infected adults to mediate ADCC against CD4 lymphocytes. Thus, factors capable of blocking ADCC against HIV-expressing CD4 cells may be present in sera of perinatally HIV-infected children.
NK cells are present in human fetal liver mononuclear cells by the 8th week of gestation (40), mediate cytotoxicity as early as the 9th gestational week (59), and are functionally mature by the 32nd week (47). However, decreased NK cell activity against HIV-expressing targets, as reported for premature (<35 weeks of gestational age) neonates (35), may be due to antenatal glucosteroids, fetal stress, or other critical care issues. Other investigators of pediatric cell-mediated immune responses reported normal NK cell activity and decreased ADCC activity against HIV-expressing target cells in HIV-seropositive neonates (17, 41). Interestingly, HIV-1 gag–cytotoxic T-lymphocyte responses were reported to be also deficient in vertically HIV-infected children (30). Our data indicate that vertical HIV infection does not interfere with maturation of functional NK cells. PBMC of HIV-infected children mediated cytotoxicity against IIIB-infected HUT78 cells in NK cell assays as well as against HSV-infected fibroblasts in ADCC assays. The levels of cytotoxicity observed were comparable to those obtained with PBMC from age-matched healthy peers.
It has been previously shown that decreased NK cell-mediated lysis in AIDS patients could be restored in vitro by addition of cytokines to cytotoxicity assays (1, 24, 25, 44). Bonavida et al. (6) reported that IL-2 triggered release of NK cell cytotoxic factors from PBMC of HIV-infected adults. Ahmad et al. (1) showed in vitro that the addition of IL-2 or gamma interferon enhanced ADCC activity significantly in PBMC from AIDS patients (CD4 counts of <200), which had without cytokine addition significantly lower target lysing ability than those from patients with CD counts of >400. Positive in vitro effects of other cytokines, such as IL-12 and IL-15, on ADCC-mediated lysis in pediatric HIV patients have been reported elsewhere (25). Other cytokines are still under investigation. However, addition of IL-2 to effector cells of HIV-infected children did not enhance their ability to mediate lysis of HIV-expressing CD4 cells (data not shown).
ADCC against HIV-expressing CD4 lymphocytes is mediated by NK cells of HIV-infected adults, linked in vivo to antibodies of the subclass IgG1 (27). Such antibodies are directed against the viral envelope glycoproteins gp120 and gp41 (12, 15, 22, 31, 33, 51, 55, 56, 60, 62, 64) and are distinct from virus-neutralizing antibodies (7, 32, 50). Both ADCC-mediating antibodies and neutralizing antibodies were shown to be present in sera of infants born to HIV-infected mothers (19, 29). The presence of such antibodies, most likely of maternal origin, correlated with a better clinical outcome in one study (32) but had no clinical significance in another investigation (18). However, both reports confirm that the presence of ADCC antibodies failed to prevent vertical transmission of HIV infection. Hypergammaglobulinemia with high levels of IgG1 and IgG3 has been detected in perinatally infected children, but such children frequently have functional hypoglobulinemia and reduced defenses against bacterial opportunistic infections (43). A similar functional inability of antibodies to mediate ADCC against HIV-expressing CD4 lymphocytes might explain the results observed in the present study.
Blocking factors in sera of HIV-infected children might inhibit the arming of effector cells or the contact between effector and target cells. Immune complexes, known to be present more frequently and in higher concentrations in HIV-infected children (10, 27) than in adults (36), might act as such blocking factors. Differences in non-HIV-specific immunoglobulin, HIV-specific noncytophilic antibody, or cytokine profiles between sera of pediatric patients and sera of adult patients may also be accountable for these inhibitive effects.
In summary, our results are similar to several reports about deficient ADCC activity against HIV-expressing target cells in pediatric HIV-infected populations (17, 35, 54) and noncompromised NK cell activity (17, 35). In addition, we demonstrate that the deficit in ADCC activity in HIV-infected children is not due to defective effector cells, appears to be specific to HIV-infected ADCC target cells, and may be caused by undefined serum blocking factors. This deficiency may be a contributing factor to the rapid disease progression often observed with this patient population.
ACKNOWLEDGMENTS
We thank Susan Plaeger and Kenneth Ugen for their extremely valuable help and advice; Barbara Frank for graphic design; and Tracy Gamble, Evan Crawford, and Sihuor Peak for the preparation of the manuscript. Most importantly, we appreciate the patients’ willingness to participate in our study and, above all, our pediatric patients’ valuable blood donations, which made this study possible.
This project was supported by a grant of the Deutsche Forschungsgemeinschaft, the Pediatric AIDS Foundation, and the University of Pennsylvania Research Foundation. U. Ziegner was a scholar of the Pediatric AIDS Foundation.
REFERENCES
- 1.Ahmad A, Morisset R, Thomas R, Menezes J. Evidence for a defect of antibody-dependent cellular cytotoxic (ADCC) effector function and anti-HIV gp120/41-specific ADCC-mediating antibody titres in HIV-infected individuals. J Acquir Immune Defic Syndr. 1994;7:428–437. [PubMed] [Google Scholar]
- 2.Auger I, Thomas P, DeGruttola V, Morse D, Moore D, Williams R, Truman B, Lawrence C E. Incubation periods for pediatric AIDS patients. Nature. 1988;336:575–577. doi: 10.1038/336575a0. [DOI] [PubMed] [Google Scholar]
- 3.Bandyopadhyay S, Ziegner U, Campbell D E, Miller D S, Hoxie J A, Starr S E. Natural killer cell-mediated lysis of T cell lines chronically infected with HIV-1. Clin Exp Immunol. 1990;79:430–435. doi: 10.1111/j.1365-2249.1990.tb08107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanche S, Tardieu M, Duliege A M, Rouzioux C, Le Deist F, Fukunaga K, Caniglia M, Jacomet C, Messiah A, Griscelli C. Longitudinal study of 94 infants with perinatally acquired immunodeficiency virus infection. Am J Dis Child. 1990;144:1210–1215. doi: 10.1001/archpedi.1990.02150350042021. [DOI] [PubMed] [Google Scholar]
- 5.Blumberg R S, Paradis T, Hartshorn K L, Vogt M, Ho D D, Hirsch M S, Leban J, Sato V L, Schooley R T. Antibody-dependent cell-mediated cytotoxicity against cells infected with the human immunodeficiency virus. J Infect Dis. 1987;156:878–884. doi: 10.1093/infdis/156.6.878. [DOI] [PubMed] [Google Scholar]
- 6.Bonavida B, Katz J D, Gottlieb M. Mechanism of defective NK cell activity in patients with acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. I. Defective trigger on NK cells for NKCF production by target cells and a partial restoration by IL-2. J Immunol. 1986;137:1157–1163. [PubMed] [Google Scholar]
- 7.Böttiger B, Ljunggren K, Karlsson A. Neutralizing antibodies in relation to antibody-dependent cellular cytotoxicity-inducing antibodies against human immunodeficiency virus type 1. Clin Exp Immunol. 1988;73:339–342. [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control. 1993 revised classification system for HIV infection and expanded case definition for AIDS among adolescents and adults. Morbid Mortal Weekly Rep. 1992;41(RR17):1–19. [PubMed] [Google Scholar]
- 9.Centers for Disease Control. Surveillance definition for AIDS—revision of the CDC surveillance case definition for acquired immunodeficiency syndrome. Morbid Mortal Weekly Rep. 1987;36:3S–15S. [Google Scholar]
- 10.Ellaurie M, Carelli T A, Rubinstein A. Human immunodeficiency virus (HIV) circulating immune complexes in infected children. AIDS Res Hum Retroviruses. 1990;6:1437–1441. doi: 10.1089/aid.1990.6.1437. [DOI] [PubMed] [Google Scholar]
- 11.Engelhard D, Waner J L, Kapoor N, Good R A. Effect of intravenous immune globulin on natural killer cell activity: possible association with autoimmune neutropenia and idiopathic thrombocytopenia. J Pediatr. 1986;108:77–81. doi: 10.1016/s0022-3476(86)80772-7. [DOI] [PubMed] [Google Scholar]
- 12.Evans L A, Thomson-Honnebier G, Steimer K, Paoletti E, Perkus M E, Hollander H, Levy J A. Antibody-dependent cellular cytotoxicity is directed against both the gp120 and gp41 envelope proteins of HIV. AIDS. 1989;3:273–276. doi: 10.1097/00002030-198905000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Feremans W W, Huygen K, Menu R. Fifty cases of human immunodeficiency virus (HIV) infection: immuno-ultrastructural study of circulating lymphocytes. J Clin Pathol. 1988;41:62–71. doi: 10.1136/jcp.41.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fontana L, Sirianni M C, DeSanctis G, Carbonari M, Ensoli B, Aiuti F. Deficiency of natural killer activity, but not of natural killer binding, in patients with lymphadenopathy syndrome positive for antibodies to HTLV-III. Immunobiology. 1986;171:425–435. doi: 10.1016/S0171-2985(86)80074-2. [DOI] [PubMed] [Google Scholar]
- 15.Ho D D, Nishanian P, Pomerantz R, Ojo-Amaize E, Reed D, Petteway S, Giorgi J. Fourth International Conference on AIDS. 1988. HIV-1 envelope domains important in binding, antibody neutralization and antibody-dependent cellular cytotoxicity (ADCC) p. 154. Stockholm, Sweden. [Google Scholar]
- 16.Hoxie J A, Haggarty B S, Rackowski J L, Pillsbury N, Levy J A. Persistent noncytopathic infection of normal human T lymphocytes with AIDS-associated retrovirus. Science. 1985;229:1400–1402. doi: 10.1126/science.2994222. [DOI] [PubMed] [Google Scholar]
- 17.Jenkins M, Mills J, Kohl S. Natural killer cytotoxicity and antibody-dependent cellular cytotoxicity of human immunodeficiency virus-infected cells by leukocytes from human neonates and adults. Pediatr Res. 1993;33:469–474. doi: 10.1203/00006450-199305000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Jenkins M, Landers D, Williams-Herman D, Wara D, Viscarello R R, Hammill H A, Kline M W, Shearer W T, Charlebois E D, Kohl S. Association between anti-human immunodeficiency virus type 1 (HIV-1) antibody-dependent cellular cytotoxicity antibody titers at birth and vertical transmission of HIV-1. J Infect Dis. 1994;170:308–312. doi: 10.1093/infdis/170.2.308. [DOI] [PubMed] [Google Scholar]
- 19.Jenson H B. Retrovirus infections and the acquired immunodeficiency syndrome. In: Aronoff S C, Hughes W T, Kohl S, Speck W T, et al., editors. Pediatric infectious diseases. Chicago, Ill: Yearbook Medical Publishers, Inc.; 1990. pp. 93–155. [PubMed] [Google Scholar]
- 20.Katz J D, Mitsuyasu R, Gottlieb M S, Lebow L T, Bonavida B. Mechanism of defective NK cell activity in patients with acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. II. Normal antibody-dependent cellular cytotoxicity (ADCC) mediated by effector cells defective in natural killer (NK) cytotoxicity. J Immunol. 1987;139:55–60. [PubMed] [Google Scholar]
- 21.Katz J D, Nishanian P, Mitsuyasu R, Bonavida B. Antibody-dependent cellular cytotoxicity (ADCC)-mediated destruction of human immunodeficiency virus (HIV)-expressing CD4+ T lymphocytes by acquired immunodeficiency syndrome (AIDS) effector cells. J Clin Immunol. 1988;8:453–458. doi: 10.1007/BF00916950. [DOI] [PubMed] [Google Scholar]
- 22.Koup R A, Sullivan J L, Levine P H, Brewster F, Mahr A, Mazzara G, McKenzie S, Panicali D. Antigenic specificity of antibody-dependent cell-mediated cytotoxicity directed against human immunodeficiency virus in antibody-positive sera. J Virol. 1989;63:584–590. doi: 10.1128/jvi.63.2.584-590.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumagai K, Itoh K, Hinuma S, Tada M. Pretreatment of plastic petri dishes with fetal calf serum: a simple method for macrophage isolation. Immunol Methods. 1979;29:17–25. doi: 10.1016/0022-1759(79)90121-2. [DOI] [PubMed] [Google Scholar]
- 24.Lew F, Tsang P, Soloman S, Selikoff I J, Bekesi J G. Natural killer cell function and modulation and α-IFN and IL-2 in AIDS patients and prodromal subjects. J Clin Lab Immunol. 1984;14:115–121. [PubMed] [Google Scholar]
- 25.Lin S J, Roberts R L, Ank B J, Nguyen Q H, Thomas E K, Stiehm E R. Effect of interleukin (IL)-12 and IL-15 on activated natural killer (ANK) and antibody-dependent cellular cytotoxicity (ADCC) in HIV infection. J Clin Immunol. 1998;18:335–345. doi: 10.1023/a:1023290932154. [DOI] [PubMed] [Google Scholar]
- 26.Ljunggren K, Böttiger B, Biberfeld G, Karlson A, Fenyo E M, Jondal M. Antibody-dependent cellular cytotoxicity-inducing antibodies against human immunodeficiency virus: presence at different clinical stages. J Immunol. 1987;139:2263–2267. [PubMed] [Google Scholar]
- 27.Ljunggren K, Broliden P A, Morfeldt-Manson L. IgG subclass response to HIV in relation to antibody-dependent cellular cytotoxicity at different clinical stages. Clin Exp Immunol. 1988;73:343–347. [PMC free article] [PubMed] [Google Scholar]
- 28.Ljunggren K, Karlson A, Fenyö E M, Jondal M. Natural and antibody-dependent cytotoxicity in different clinical stages of human immunodeficiency virus type 1 infection. Clin Exp Immunol. 1989;75:184–189. [PMC free article] [PubMed] [Google Scholar]
- 29.Ljunggren K, Moschese V, Broliden P A, Giaquinto C, Quinti I, Fenyo E M, Wahren B, Rossi P, Jondal M. Antibodies mediating cellular cytotoxicity and neutralization correlate with a better clinical stage in children born to human immunodeficiency virus-infected mothers. J Infect Dis. 1989;161:198–202. doi: 10.1093/infdis/161.2.198. [DOI] [PubMed] [Google Scholar]
- 30.Luzuriaga K, Koup R A, Pikora C A, Brettler D B, Sullivan J L. Deficient human immunodeficiency vertically infected children. J Pediatr. 1989;119:230–236. doi: 10.1016/s0022-3476(05)80732-2. [DOI] [PubMed] [Google Scholar]
- 31.Lyerly H K, Matthews T J, Langlois A J, Bolognesi D P, Weinhold K J. Human T-cell lymphotrophic virus IIIB glycoprotein (gp120) bound to CD4 determinants on normal lymphocytes and expressed by infected cells serves as target for immune attack. Proc Natl Acad Sci USA. 1987;84:4601–4605. doi: 10.1073/pnas.84.13.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyerly H K, Pratz J E, Tyler D S, Matthews T J, Langlois A L, Bolognesi D P, Weinhold K J. Discordant virus neutralization and anti-HIV ADCC activity in HIV seropositive patient sera. In: Ginsberg H, Brown F, Lernier R, et al., editors. Vaccines 88: new chemical and genetic approaches to vaccination. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1988. pp. 323–326. [Google Scholar]
- 33.Lyerly H K, Reed D L, Matthews T J, Langlois A J, Ahearne P A, Petteway S R, Jr, Weinhold K J. Anti-gp120 antibodies from HIV positive individuals mediate broadly reactive anti-HIV ADCC. AIDS Res Hum Retroviruses. 1987;3:409–422. doi: 10.1089/aid.1987.3.409. [DOI] [PubMed] [Google Scholar]
- 34.Maccario R, Arico M, Ugazio A, Plebani A, Montagna D, Avanzini A, Marseglia G, Caselli D, Burgio G. Acquired immune deficiency syndrome in childhood: impaired production of interleukin-2 by HIV (LAV/HTLVIII) infected patients. Infection. 1987;15:99–104. doi: 10.1007/BF01650205. [DOI] [PubMed] [Google Scholar]
- 35.Merrill J D, Sigaroudinia M, Kohl S. Characterization of natural killer and antibody-dependent cellular cytotoxicity of preterm infants against human immunodeficiency virus-infected cells. Pediatr Res. 1996;40:498–503. doi: 10.1203/00006450-199609000-00021. [DOI] [PubMed] [Google Scholar]
- 36.Morrow W J W, Wharton M, Stricker R B, Levy J A. Circulating immune complexes in patients with acquired immune deficiency syndrome contain the AIDS-associated retrovirus. Clin Immunol Immunopathol. 1986;40:515–524. doi: 10.1016/0090-1229(86)90196-0. [DOI] [PubMed] [Google Scholar]
- 37.Oh S H, Bandyopadhyay S, Miller D S, Starr S E. Cooperation between CD16 (Leu-11B)+ NK cells and HLA-DR+ cells in natural killing of herpes virus-infected fibroblasts. J Immunol. 1987;139:2799–2802. [PubMed] [Google Scholar]
- 38.Ojo-Amaize E, Nishanian P G, Heitjan D F, Rezai A, Esmail I, Korns E, Detels R, Fahey J, Giorgi J V. Serum and effector-cell antibody-dependent cellular cytotoxicity (ADCC) activity remains high during human immunodeficiency virus (HIV) disease progression. J Clin Immunol. 1989;9:454–461. doi: 10.1007/BF00918014. [DOI] [PubMed] [Google Scholar]
- 39.Perussia B, Starr S, Abraham S, Fanning V, Trinchieri G. Human natural killer cells analyzed by B73.1, a monoclonal antibody blocking Fc-receptor functions: characterization of the lymphocyte subset reactive with B73.1. J Immunol. 1983;130:2133–2141. [PubMed] [Google Scholar]
- 40.Phan D T, Mihalik R, Benczur M, Domotori J, Kiss C, Petranyi G G, Hollan S R. Expression of NK-cell associated antigen on human fetal liver cells. Thymus. 1988;11:253–256. [PubMed] [Google Scholar]
- 41.Pugatch D, Sullivan J L, Pikora C A, Luzuriaga K. Delayed generation of antibodies mediating human immunodeficiency virus type 1-specific antibody-dependent cellular cytotoxicity in vertically infected infants. J Infect Dis. 1997;176:643–648. doi: 10.1086/514085. [DOI] [PubMed] [Google Scholar]
- 42.Robinson W E, Mitchell W M, Chambers W H, Schuffman S S, Montefiori D C, Oeltmann T N. Natural killer cell infection and inactivation in vitro by the human immunodeficiency virus. Hum Pathol. 1988;19:535–540. doi: 10.1016/s0046-8177(88)80200-4. [DOI] [PubMed] [Google Scholar]
- 43.Roilides E, Black C, Reimer C, Rubin M, Venzon D, Pizzo P A. Serum immunoglobulin G subclasses in children infected with human immunodeficiency virus type 1. Pediatr Infect Dis J. 1991;10:134–139. doi: 10.1097/00006454-199102000-00012. [DOI] [PubMed] [Google Scholar]
- 44.Rook A H, Hooks J J, Quinnan G V, Lane H C, Manischewitz J F, Macher A M, Masur H, Fauci A S, Djeu J Y. Interleukin 2 enhances the natural killer cell activity of acquired immunodeficiency syndrome through a γ-interferon-independent mechanism. J Immunol. 1985;134:1503–1507. [PubMed] [Google Scholar]
- 45.Rook A H, Lane H C, Folks T, McCoy S, Alter H, Fauci A S. Sera from HTLVIII/LAV antibody-positive individuals mediate antibody-dependent cellular cytotoxicity against HTLVIII/LAV-infected T cells. J Immunol. 1987;138:1064–1067. [PubMed] [Google Scholar]
- 46.Ruscetti F W, Mikovits J A, Kalyanaraman V S, Overton R, Stevenson H, Stromberg K, Herberman R B, Farrar W L, Ortaldo J R. Analysis of effector mechanisms against HTLV-1- and HTLV-III/LAV-infected lymphoid cells. J Immunol. 1986;36:3619–3624. [PubMed] [Google Scholar]
- 47.Saito S, Saito M, Moriyama I, Hino K, Ichijo M. Ontogenic development of human natural killer (NK) cell and lymphokine activated killer (LAK) cell. Acta Obstet Gynaecol Jpn. 1987;39:591–598. [PubMed] [Google Scholar]
- 48.Scheppler J A, Mawle A C, McDougal J S. Evaluation of target cells for HIV-1-specific antibody-dependent cellular cytotoxicity assays. J Immunol Methods. 1988;115:247–253. doi: 10.1016/0022-1759(88)90294-3. [DOI] [PubMed] [Google Scholar]
- 49.Scott G B, Hutto C, Makuch R W, Mastrucci M T, O’Connors T, Mitchell C D, Trapido E J, Parks W P. Survival in children with perinatally acquired human immunodeficiency virus type 1 infection. N Engl J Med. 1989;321:1791–1796. doi: 10.1056/NEJM198912283212604. [DOI] [PubMed] [Google Scholar]
- 50.Shepp D H, Hendry R M, Vujcic L, Quinnan G V. Program and abstracts of the Twenty-Seventh Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1987. Neutralizing antibodies (Nt), antibody dependent cellular cytotoxicity (ADCC) and immunoblot pattern in human immunodeficiency virus (HIV) infection, abstr. 10; p. 98. [Google Scholar]
- 51.Shepp D H, Chakrabarti S, Moss S B, Quinnan G V., Jr Antibody-dependent cellular cytotoxicity specific for the envelope antigens of human immunodeficiency virus. J Infect Dis. 1988;157:1260–1264. doi: 10.1093/infdis/157.6.1260. [DOI] [PubMed] [Google Scholar]
- 52.Sirianni M C, De Sanctis G, Macchi B, Soddu S, Ensoli F, Aiuti F, Fontana L. Natural killer activity from normal peripheral blood lymphocytes against a human T-lymphotrophic retrovirus type III (HTLV-III)-infected cell line. Diagn Clin Immunol. 1988;5:297–303. [PubMed] [Google Scholar]
- 53.Sirianni M C, Soddus S, Malorni W, Arancia G, Aiuti F. Mechanism of defective natural killer cell activity in patients with AIDS is associated with defective distribution of tubulin. J Immunol. 1988;140:2565–2568. [PubMed] [Google Scholar]
- 54.Szelc C M, Mitcheltree C, Roberts R L, Stiehm E R. Deficient polymorphonuclear cell and mononuclear cell antibody-dependent cellular cytotoxicity in pediatric and adult human immunodeficiency virus infection. J Infect Dis. 1992;166:486–493. doi: 10.1093/infdis/166.3.486. [DOI] [PubMed] [Google Scholar]
- 55.Tanneau F, McChesney M, Lopez O, Sansonetti P, Montagnier L, Riviere Y. Primary cytotoxicity against envelope glycoprotein of human immunodeficiency virus-1: evidence for antibody-dependent cellular cytotoxicity in vivo. J Infect Dis. 1990;162:837–843. doi: 10.1093/infdis/162.4.837. [DOI] [PubMed] [Google Scholar]
- 56.Tyler D, Zolla-Pazner S, Gorny M, Shadduck P P, Langlois A J, Matthews T J, Bolognesi D P, Palker T J, Weinhold K J. Fifth International Conference on AIDS. 1989. Identification of sites within gp41 which serve as targets for ADCC using human monoclonal antibodies; p. 521. Montreal, Canada. [PubMed] [Google Scholar]
- 57.Tyler D S, Nastala C A, Stanley S D, Matthews T J, Lyerly H K, Bolognesi D P, Weinhold K J. GP120 specific cellular cytotoxicity in HIV-1 seropositive individuals: evidence for circulatory CD16+ effector cells armed in vivo with cytophilic antibody. J Immunol. 1989;142:1177–1182. [PubMed] [Google Scholar]
- 58.Tyler D S, Stanley S D, Nastala C A, Austin A A, Bartlett J A, Stine K C, Lyerly H K, Bolognesi D P, Weinhold K J. Alterations in antibody-dependent cellular cytotoxicity during the course of HIV-1 infection. J Immunol. 1990;144:3375–3384. [PubMed] [Google Scholar]
- 59.Uksila J, Lassila O, Hirvonen T, Toivanen P. Development of natural killer cell function in the human fetus. J Immunol. 1983;130:153–156. [PubMed] [Google Scholar]
- 60.Vogel T, Kurth R, Norley S. The majority of neutralizing Abs in HIV-1-infected patients recognize linear V3 loop sequences. Studies using HIV-1MN multiple antigenic peptides. J Immunol. 1994;153:1895–1904. [PubMed] [Google Scholar]
- 61.Voth R, Rossol S, Gräff E, Laubenstein H P, Schroder H C, Muller W E, Meyer zum Buschenfelde K H, Hess G. Natural killer cell activity as a prognostic parameter in the progression to AIDS. J Infect Dis. 1988;157:851–852. doi: 10.1093/infdis/157.4.851. [DOI] [PubMed] [Google Scholar]
- 62.Weinhold K J, Lyerly H K, Matthews T J, Tyler D S, Ahearne P M, Stine K C, Langlois A J, Durack D T, Bolognesi D P. Cellular anti-GP120 cytolytic reactivities in HIV-1 seropositive individuals. Lancet. 1988;i:902–905. doi: 10.1016/s0140-6736(88)91713-8. [DOI] [PubMed] [Google Scholar]
- 63.Wysocki L T, Sato V L. “Panning” for lymphocytes: a method for cell selection. Proc Natl Acad Sci USA. 1978;75:2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ziegner U, Frank I, Bernatowicz A, Starr S E, Streckert H J. Antibody-dependent cellular cytotoxicity is directed against immunodominant epitopes of the envelope proteins of the human immunodeficiency virus-1 (HIV-1) Viral Immunol. 1992;5:273–281. doi: 10.1089/vim.1992.5.273. [DOI] [PubMed] [Google Scholar]