Abstract
Background: Since the 1950s, researchers have studied temperature changes in deep tissues caused by cooling stimuli. However, these changes have not been investigated non-invasively. Moreover, opinions are divided as to whether the temperature in the joints rises or falls. The present study investigated the effects of cooling on various tissues in the body, including muscles.
Methods: Seven healthy subjects (four males and three females) were enrolled (age, 21.5 ± 0.8 years; height, 165.2 ± 10.7 cm; weight, 63.1 ± 12.17 kg). The research was conducted at the Department of Radiology, Okubo Hospital (Akashi, Hyogo, Japan) between March 2015 and December 2020. Magnetic resonance imaging (MRI) was performed on both lower legs in a noncooled resting state. The right lower leg was cooled for 15 minutes using an ice bath, then both legs were examined by MRI (Experiment 1). After two weeks, the left lower leg was cooled, and MRI was performed on both legs (Experiment 2). After the subsequent two weeks, MRI was performed on both legs without cooling (Experiment 3). The target areas were subcutaneous and adipose tissues, muscle, bone, and cartilage. T1 signal intensity changes after cooling were examined for each tissue. Normality was confirmed by the Shapiro-Wilk test in advance, and the effect size (Cohen's d) was calculated as a post-test when a significant difference was found.
Results: In Experiments 1 and 2, T1 signal intensities in subcutaneous tissue, lateral inframalleolar fat pad, the extensor digitorum longus, and abductor hallucis muscles were significantly higher in the cooled than in the noncooled leg (P < 0.05). No significant differences were observed in tissues on the noncooled side.
Conclusions: A 15-minute cold stimulation, such as that used for ankle sprains, reduced temperatures in subcutaneous adipose tissue, muscles, and the lateral inframalleolar fat pad. As the lateral inframalleolar fat pad was effectively cooled, the joint capsule and ligaments immediately below may have also been cooled. It is important to consider the tissue intended for cooling when performing cryotherapy. An ice bath below the lower leg is effective for promoting the recovery of damaged tissue.
Keywords: lower leg, magnetic resonance imaging, t1 signal, deep tissue, intra-articular temperature, cryotherapy
Introduction
Cryotherapy involves conduction cooling (ice and an ice bag), convection cooling (ice water), or vaporization cooling (fluoromethane gas). These methods cool the targeted body area surface, providing pain relief and reducing both swelling and spasticity, which promotes early recovery. Preliminary evidence suggests that intermittent cryotherapy use effectively reduces tissue temperatures to optimal therapeutic levels. Previous studies on humans, dogs, and cats demonstrated that cold stimulation of joints decreased their internal temperature [1-8].
However, Horvath reported that upon cold pack application to humans, though skin surface temperature decreased, there was an increase in intra-articular temperature [9]. These contradictory findings of both increases and decreases in intra-articular temperature require further exploration. As limited information is currently available on the effects of cooling on intra-articular temperatures in the human body, we examined the effects of cooling on various tissues, including muscles.
Magnetic resonance imaging (MRI) provides excellent soft tissue visibility, allowing the measurement of temperature changes in deep tissues based on signal intensities. Herein, we examined temperature change differences between an ice bath cooled lower leg and a noncooled leg by MRI to elucidate the effects of cooling on temperature changes in deep tissues surrounding the ankle joint.
Materials and methods
Subjects
Seven healthy subjects (four males and three females) were enrolled (age, 21.5 ± 0.8 years; height, 165.2 ± 10.7 cm; weight, 63.1 ± 12.17 kg). The study participants were research volunteers who were given oral explanations in advance. They gave consent regarding the purpose, method, safety considerations, and dangers of the experiment. This study was approved by the Ethics Committee of Okubo Hospital (approval no. 2804).
Target tissues in the present study were as follows: subcutaneous tissue 5.0-7.5 cm proximal to the medial malleolus, adipose tissue below the lateral malleolus, bone tissue on the line connecting the medial malleolus and lateral malleolus, articular cartilage on the underside of the tibia, abductor hallucis and extensor digitorum longus muscles. To identify the cooled target by MRI, Adalat tablets were fixed to the skin as markers of the boundary between cooled and noncooled areas. Subjects rested barefoot for 20 minutes before each experiment.
In the group sessions, the measurement method was explained verbally and with illustrations for about 10 minutes to facilitate understanding. Specifically, after arriving at the facility, the subjects rested barefoot for 20 minutes. Then, both lower legs underwent MRI measurement, and one leg was cooled for 15 minutes. Immediately after that, both lower legs underwent MRI measurement. Since the MRI machine installed in the hospital for medical treatment was used for measurement, only one person could be measured at a time, with a measurement period of 4-6 weeks or longer required for each subject.
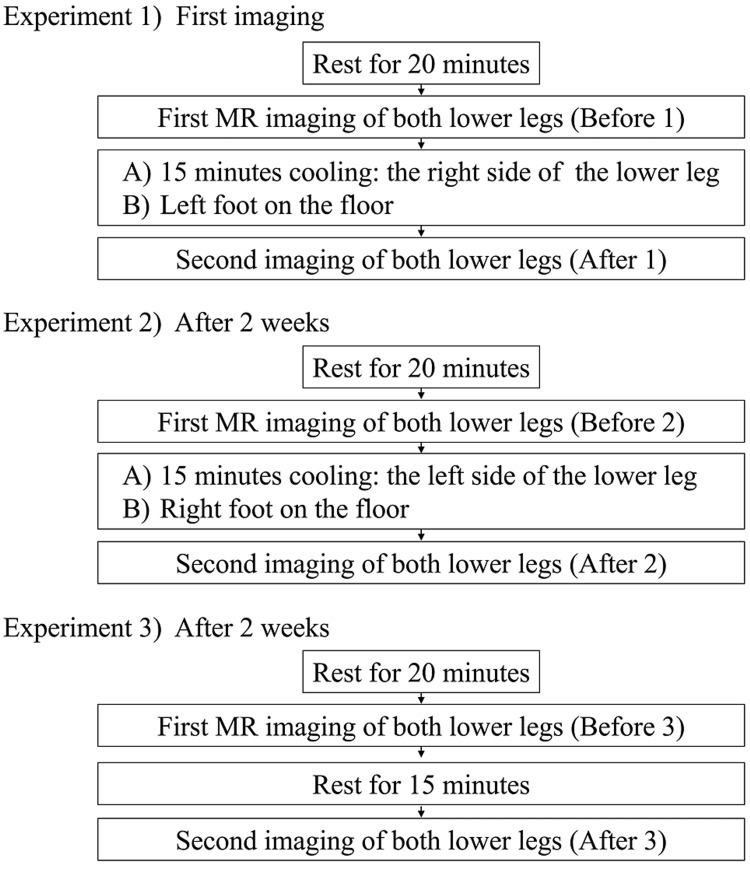
T1 signal intensities of the target tissues on T1-weighted images for both lower legs and feet were then measured. As controls, MRI was performed on both lower limbs before cooling and on the noncooled side (Figure 1).
Figure 1. Cooling and MRI protocols.
In Experiment 1, the lower right leg was submerged in an ice bath containing approximately 3.0 kg of ice and 5 liters of water for 15 minutes. The circumference of the lower leg was about 33.0-38.5 cm. T1 signal intensities in both legs were then measured. The ice bath temperature was 1.8-4.2°C. T1 signal intensities were defined as precooling/post-cooling. After two weeks, the left lower leg was cooled, and the same measurements were performed as in Experiment 1 (Experiment 2). After two weeks, measurements with 15 minutes resting without cooling were performed (Experiment 3). Surface body temperatures on the central part of the anterior lower leg (approximately 5.0 cm proximal to the lateral malleolus level), anterior ankle joint, and dorsal foot were simultaneously measured with a noncontact infrared thermometer before and after cooling. Changes in pain sensitivity after the cooling stimulus were measured on the Numerical Rating Scale of pain (NRS) from 1 to 10 using a pain evaluation roulette device.
MRI measurements
MRI temperature parameters include the T1 relaxation time, T2 relaxation time, water molecule diffusion coefficient, and proton chemical shift. Therefore, T1- and T2-weighted images, diffusion-weighted images, phase images, MR spectroscopy, and proton reference frequency are suitable sequences for temperature measurements.
MRI was performed in the present study using a TOSHIBA Vantage Titan with a magnetic field strength of 1.5 T. The sequence used for imaging was the fast spin echo method, the imaging method was T1-weighted images, with a repetition time of 250 msec, an echo time of 20 msec, and a matrix size of 256 × 256. Five consecutive coronal sections with a 5-mm thickness were imaged, and the mean T1 signal intensity was calculated from three dorsal side images.
The region of interest was a circle with an area of 0.35 cm2 and the field of view was 32 × 45 cm. The temperature of the room in the MRI facility was approximately 25°C and the humidity was 28%-55%.
Evaluation of temperature changes based on the T1 relaxation time
The temperature dependence of the T1 relaxation time increases with temperature and is expressed by the following equation.
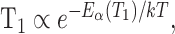
Where Eα(T1) is the activation energy of the relaxation process, k is the Boltzmann constant, and T is the absolute temperature. Within a small temperature, the range T1 is linearly dependent on the temperature [10].
Statistical analysis
R (ver.4.1.2) was used for statistical analysis, to compare the cooled and noncooled T1 signal values by the paired t-test. The significance was set at 5%. Normality was confirmed by the Shapiro-Wilk test in advance, and the effect size (Cohen's d) was calculated as a post-test when a significant difference was found. In cases where normality was not rejected, the t-test was used, and in cases where normality was rejected, the Mann-Whitney U test evaluated the similarity between left and right-side distributions.
Before cooling at rest in all experiments, normality was significantly rejected in the right subcutaneous tissue, right and left body fat, right and left tibias, right and left flexor muscles, and abductor right and left muscles. As a result, no significant difference was found in any experiments.
After cooling in Experiment 1, normality was significantly rejected in the left fat pad, left tibia, right and left cartilage, right and left flexor muscles, and left abductor muscle. As a result, significant differences were observed among the subcutaneous tissue, fat pad, flexor muscle, and abductor muscle.
After cooling in Experiment 2, normality was significantly rejected on the left tibia, left cartilage, and right abductor muscle. As a result, significant differences were observed among the subcutaneous tissue, fat pad, flexor muscle, and abductor muscle. Cohen’s d showed significantly large size effects in these tissues. Before cooling, no significant differences were observed between the left and right sides in any experiment.
Results
The mean axillary temperature was 36.48 ± 0.039. The mean skin surface temperature at six sites on the left and right legs at rest before cooling was approximately 32.0°C. The surface temperature of the cooled leg in Experiments 1 and 2 decreased by approximately 20°C, whereas the non-cooled side decreased by about 1°C (Table 1).
Table 1. Skin surface temperatures.
Values are mean ± standard deviation
| Center of the lower leg | Center of the ankle | Center of the dorsal foot | ||||
| Experiment 1 | Cooled side (right) | Non-cooled side (left) | Cooled side (right) | Non-cooled side (left) | Cooled side (right) | Non-cooled side (left) |
| Pre-cooling 1 | 32.30 ± 1.30 | 31.62 ± 1.77 | 32.25 ± 1.36 | 32.32 ± 1.25 | 32.29 ± 1.30 | 32.26 ± 1.31 |
| Post-cooling 1 | 13.90 ± 1.01 | 33.71 ± 0.75 | 13.59 ± 0.91 | 33.0 ± 1.43 | 13.16 ± 1.08 | 33.32 ± 1.31 |
| Experiment 2 | Cooled side (right) | Non-cooled side (left) | Cooled side (right) | Non-cooled side (left) | Cooled side (right) | Non-cooled side (left) |
| Pre-cooling 2 | 32.37 ± 1.66 | 32.16 ± 1.73 | 32.36 ± 1.62 | 32.39 ± 1.60 | 32.47 ± 1.66 | 32.44 ± 1.63 |
| Post-cooling 2 | 13.07 ± 0.82 | 33.58 ± 0.73 | 12.77 ± 0.94 | 33.27 ± 1.25 | 12.67 ± 0.94 | 33.24 ± 1.55 |
No significant differences were observed in the T1 signal intensities in tissues in both legs before cooling in Experiment 1 or 2 (pre-cooling 1 and pre-cooling 2, respectively, in Tables 2, 3). T1 signal intensities in subcutaneous tissue, the lateral inframalleolar fat pad, and extensor digitorum longus and abductor hallucis muscles were significantly elevated after cooling compared with before cooling in both Experiments 1 and 2 (Tables 2, 3).
Table 2. Experiment 1: T1 signal strength before and after cooling.
| Experiment 1 | ||||||||||||
| Subcutaneous tissue | Lateral inframalleolar fat pad | Tibia (bone) | Cartilage on the underside of the tibia | Extensor digitorum longus | Abductor hallucis | |||||||
| Resting before cooling (Before 1) | ||||||||||||
| Right | Left | Right | Left | Right | Left | Right | Left | Right | Left | Right | Left | |
| Mean ± SD | 1026 ± 110.16 | 1033 ± 113.26 | 763 ± 135.21 | 758.54 ± 118.13 | 964.16 ± 169.13 | 982.32 ± 179.6 | 86.95 ± 25.74 | 88.29 ± 23.62 | 192 ± 49.81 | 196.15 ± 52.48 | 204.83 ± 54.32 | 203.45 ± 50.06 |
| Mean difference | −7.30 | 4.57 | −18.17 | −1.34 | −3.31 | 1.38 | ||||||
| Shapiro–Wilk test (p-value) | 0.0371 | 0.0581 | 0.0012 | 0.0145 | 0.0011 | 0.0015 | 0.4186 | 0.3984 | 0.0000 | 0.0000 | 0.0001 | 0.0002 |
| T test (p-value) | 0.3134 | 0.6761 | 0.2746 | 0.5926 | 0.1145 | 0.5628 | ||||||
| Cohen's d | 0.0638 | 0.0351 | 0.1016 | 0.0528 | 0.0632 | 0.0258 | ||||||
| Effect size | Negligible | Negligible | Negligible | Negligible | Negligible | Negligible | ||||||
| Mann– Whitney U test (p-value) | 0.8014 | 0.9010 | 0.7462 | 0.8617 | 0.5667 | 1.0000 | ||||||
| After cooling the right foot (After 1) | ||||||||||||
| Cooling (right) | No cooling (left) | Cooling (right) | No cooling (left) | Cooling (right) | No cooling (left) | Cooling (right) | No cooling (left) | Cooling (right) | No cooling (left) | Cooling (right) | No cooling (left) | |
| Mean ± SD | 1080.68 ± 142.06 | 979.81 ± 110.78 | 794.53 ± 148.66 | 719.34 ± 117.68 | 965.54 ± 172.33 | 969 ± 178.44 | 92.90 ± 23.48 | 88.52 ± 24.51 | 211.81 ± 46.48 | 184.71 ± 36.63 | 236.20 ± 51.88 | 199.56 ± 53.04 |
| Mean difference | 100.87 | 75.19 | -3.95 | 4.38 | 27.10 | 36.64 | ||||||
| Shapiro-Wilk test (p-value) | 0.3484 | 0.3951 | 0.2370 | 0.0102 | 0.2697 | 0.0281 | 0.0138 | 0.0221 | 0.0159 | 0.0627 | 0.2248 | 0.0065 |
| T test (p-value) | 0.0000 | 0.0000 | 0.7409 | 0.1498 | 0.0000 | 0.0000 | ||||||
| Cohen's d | 0.7728 | 0.5473 | 0.0220 | 0.1779 | 0.6321 | 0.6816 | ||||||
| Effect size | Medium | Medium | Negligible | Negligible | Medium | Medium | ||||||
| Mann - Whitney U test (p-value) | 0.0336 | 0.0062 | 0.9010 | 0.3923 | 0.0295 | 0.0073 | ||||||
Table 3. Experiment 2: T1 signal strength before and after cooling.
| Subcutaneous tissue | Lateral inframalleolar fat pad | Tibia (bone) | Cartilage on the underside of the tibia | Extensor digitorum longus | Abductor hallucis | |||||||
| Resting before cooling (Before 2) | ||||||||||||
| Right | Left | Right | Left | Right | Left | Right | Left | Right | Left | Right | Left | |
| Mean ± SD | 1027.34 ± 96 | 1037.99 ± 116.93 | 739.15 ± 97.91 | 744.56 ± 127.53 | 957.55 ± 163.02 | 968.30 ± 158.42 | 72.47 ± 32.89 | 74.10 ± 38.28 | 186.77 ± 22.35 | 188.79 ± 16.59 | 200.06 ± 30.14 | 198.94 ± 30.28 |
| Mean difference | −10.65 | −5.41 | −10.75 | −1.62 | −2.02 | 1.13 | ||||||
| Shapiro–Wilk test (p-value) | 0.1792 | 0.1835 | 0.3760 | 0.3498 | 0.0037 | 0.002135 | 0.1508 | 0.0323 | 0.0882 | 0.1914 | 0.2068 | 0.1209 |
| T test (p-value) | 0.4165 | 0.6575 | 0.4434 | 0.6514 | 0.4290 | 0.7328 | ||||||
| Cohen's d | 0.0972 | 0.0464 | 0.0653 | 0.0444 | 0.0999 | 0.0364 | ||||||
| Effect size | Negligible | Negligible | Negligible | Negligible | Negligible | Negligible | ||||||
| Mann - Whitney U test (p-value) | 0.7275 | 0.8617 | 0.6904 | 0.9405 | 0.7842 | 0.8422 | ||||||
| After cooling the right foot (After 2) | ||||||||||||
| Cooling (right) | No cooling (left) | Cooling (right) | No cooling (left) | Cooling (right) | No cooling (left) | Cooling (right) | No cooling (left) | Cooling (right) | No cooling (left) | Cooling (right) | No cooling (left) | |
| Mean ± SD | 1227.18 ± 110.34 | 969.34 ± 45.65 | 837.82 ± 111.99 | 712.18 ± 98.89 | 989.73 ± 155.74 | 992.62 ± 159.72 | 78.10 ± 31.69 | 79.70 ± 41.71 | 182.92 ± 27.77 | 227.03 ± 26.31 | 208.01 ± 46.27 | 235.53 ± 21.30 |
| Mean difference | 257.84 | 116.63 | −2.89 | −1.60 | −44.11 | −27.52 | ||||||
| Shapiro–Wilk test (p-value) | 0.1079 | 0.4230 | 0.0964 | 0.1886 | 0.7057 | 0.0120 | 0.1653 | 0.0076 | 0.4539 | 0.5006 | 0.0007 | 0.8673 |
| T test (p-value) | 0.0000 | 0.0000 | 0.8342 | 0.7620 | 0.0000 | 0.0093 | ||||||
| Cohen's d | 2.9800 | 1.0774 | 0.0179 | 0.0421 | 1.5915 | 0.7456 | ||||||
| Effect size | Large | Large | Negligible | Negligible | Large | Medium | ||||||
| Mann– Whitney U test (p-value) | 0.0000 | 0.0012 | 0.9010 | 0.4539 | 0.0000 | 0.0010 | ||||||
No significant differences were observed in the T1 signal intensities of tissues in both legs before cooling in Experiment 1 or 2 (pre-cooling 1 and pre-cooling 2, respectively, in Tables 2, 3). T1 signal intensities in subcutaneous tissue, the lateral inframalleolar fat pad, and extensor digitorum longus and abductor hallucis muscles were significantly elevated after cooling compared with before cooling in both Experiments 1 and 2 (Tables 2, 3).
No significant changes were observed in the tissues on the noncooled side in Experiments 1 and 2, or in the noncooled lower limbs after resting for 15 minutes in Experiment 3 (Table 4).
Table 4. Experiment 3: T1 signal strength after a 15-minute rest.
| Subcutaneous tissue | Lateral inframalleolar fat pad | Tibia (bone) | Cartilage on the underside of the tibia | Extensor digitorum longus | Abductor hallucis | |||||||
| Before resting (Before 3) | ||||||||||||
| Right | Left | Right | Left | Right | Left | Right | Left | Right | Left | Right | Left | |
| Mean ± SD | 1029.55 ± 114.27 | 1028.97 ± 85.68 | 865.43 ± 105.97 | 870.66 ± 122.22 | 1034.27 ± 116.00 | 10591.73 ± 84.73 | 89.34 ± 21.13 | 85.42 ± 24.44 | 201.24 ± 51.57 | 193.02 ± 37.43 | 227.84 ± 59.06 | 222.43 ± 40.47 |
| Mean difference | 0.58 | −5.23 | −25.46 | 3.91 | 8.22 | 5.42 | ||||||
| Shapiro–Wilk test (p-value) | 0.02292 | 0.4074 | 0.7807 | 0.364 | 0.05109 | 0.3946 | 0.07906 | 0.02666 | 0.0978 | 0.5114 | 0.05082 | 0.2408 |
| T test (p-value) | 0.9815 | 0.7827 | 0.2213 | 0.1705 | 0.1446 | 0.5366 | ||||||
| Cohen's d | 0.005573731 | −0.04463965 | −0.2446001 | 0.1670672 | 0.1780801 | 0.1043971 | ||||||
| Effect size | Negligible | Negligible | Small | Negligible | Negligible | Negligible | ||||||
| Mann–Whitney U test (p-value) | 0.8813 | 0.8813 | 0.2399 | 0.3391 | 0.8228 | 0.8617 | ||||||
| After a 15-minute rest (After 3) | ||||||||||||
| Cooling (right) | No cooling (left) | Cooling (right) | No cooling (left) | Cooling (right | No cooling (left) | Cooling (right) | No cooling (left) | Cooling (right) | No cooling (left) | Cooling (right) | No cooling (left) | |
| Mean ± SD | 1062.80 ± 103.23 | 1080 ± 107.09 | 921.55 ± 125.29 | 910.78 ± 146.84 | 1037.40 ± 118.64 | 1062.38 ± 128.65 | 83.92 ± 17.47 | 89.01 ± 19.63 | 190.58 ± 27.10 | 187.60 ± 32.08 | 213.30 ± 52.75 | 209 ± 48.72 |
| Mean difference | -17.52 | 10.77 | -24.99 | -5.09 | 2.98 | 3.92 | ||||||
| Shapiro- Wilk test (p-value) | 0.682 | 0.3242 | 0.4723 | 0.851 | 0.03357 | 0.05222 | 0.9379 | 0.9778 | 0.239 | 0.305 | 0.002 | 0.003 |
| T test (p-value) | 0.4982 | 0.6483 | 0.3292 | 0.1588 | 0.5423 | 0.4571 | ||||||
| Cohen's d | −0.1625596 | 0.07698071 | −0.1970634 | −0.2673158 | 0.09805097 | 0.07528159 | ||||||
| Effect size | Negligible | Negligible | Negligible | Small | Negligible | Negligible | ||||||
| Mann– Whitney U test (p-value) | 0.6184 | 0.8813 | 0.4104 | 0.4395 | 0.7842 | 0.9207 | ||||||
The NRS for determining pain sensation changes after cooling was close to 0 in both Experiments 1 and 2 (Table 5). NRS on all noncooled sides was 10.
Table 5. Changes in pain after cooling by NRS.
| Experiment 1 | |||
| Center in front of lower leg (in ice water) | Center in front of ankle | Center of foot back | |
| Cooling (right) | Cooling (right) | Cooling (right) | |
| Maximum | 1 | 1 | 1 |
| Minimum | 0 | 0 | 0 |
| Average | 0.44 | 0.22 | 0.33 |
| Experiment 2 | |||
| Cooling (left) | Cooling (left) | Cooling (left) | |
| Maximum | 2 | 1 | 1 |
| Minimum | 0 | 0 | 0 |
| Average | 0.78 | 0.33 | 0.22 |
Discussion
Accurately monitoring temperature changes in vivo and a detailed understanding of their effects are important for the proper application of 15-minute cryotherapy. In the present study, the surface temperature of the cooled area decreased by approximately 20°C accompanied by significantly increased T1 signal intensities in subcutaneous tissue, the lateral inframalleolar fat pad, and the extensor digitorum longus and abductor hallucis muscles.
This study used the fast spin echo method to image tissues, requiring less than 2 seconds to image each frame, with near real-time data obtained. As ligament water content the around the ankle joint is low, clear visualization is difficult due to the requirements of T1-weighted images.
A linear relationship exists between T1 relaxation times and temperature. The slope of this relationship varies depending on the tissue type and was previously reported to range between approximately 5-12 ms/°C [11-14].
It is important to note that T1 signal intensity is a relative value due to the characteristics of MRI in which images are constructed to improve visualization. Therefore, in the noncooled legs in Experiments 1, 2, and 3, T1 signal intensities may differ even in the absence of a significant change in skin surface temperature. However, T1 signal intensities may be compared when the left and right lower legs are simultaneously imaged on the same screen.
Regarding changes in the T1 signal intensity of each target tissue, it is estimated that the temperature decreased by 13.6°C or more in the subcutaneous tissue, 11.0°C or more in the lateral ankle fat pad, 4.2°C or more in the extensor digitorum longus muscle, and 4.1°C or more in the abductor hallucis muscle. The temperature of the skin surface was 13 to 27°C lower than the original body temperature, and the skin temperature was reported to be 9.5 to 15.0°C, supporting our findings [3,15-18].
According to Van’t Hoff’s law, a 10% decrease in body temperature results in a 50% decrease in the rate of metabolism of body tissues. Therefore, the T1 signal intensities of the subcutaneous adipose tissue and the lateral sub-ankle fat pad decreased by more than 50%, and those of the extensor digitorum longus and abductor hallucis muscle decreased by about 20%, indicating a decrease in metabolism. On the other hand, cooling the lower leg with an ice bath for 15 minutes did not result in an increase in T1 signal intensity for the central tibia or subtibial cartilage. Therefore, no significant changes were observed in intra-articular temperature with cooling.
The thermal conductivity and body tissue-specific heat are as follows: 2.78 (cal/sec °C × 103) and 0.38 (cal/g °C) for bone, 1.53 and 0.895 for muscle, 1.31 and 0.87 for blood, 0.898 and 0.9 for skin, and 0.45 and 0.55 for subcutaneous adipose tissue [19]. Thermal conductivity is higher in the order of bone, muscle, blood, skin, and subcutaneous fat, indicating differences in heat transfer even as a temperature stimulus is applied. Therefore, there were temperature differences (T1 values) among sites cooled at the same temperature (Tables 2, 3).
In the present study, body surface cooling may transfer arterial blood warmth through veins and arteriovenous anastomosis. The specific heat of body tissue is higher in the order of skin, muscle, blood, subcutaneous adipose tissue, and bone. The cold stimulus cooling effects used in this study showed that distal bone and ankle joint cartilage were not significantly affected by the cold stimulus in terms of T1 signal intensity.
When body temperature is decreased by 10°C, the metabolic rate is halved, which suppresses oxygen activity and reduces collagen fiber degeneration [20,21]. This represents an effective approach for reducing secondary damage. On the other hand, a temperature of 42°C has been shown to promote macrophage migration and satellite cell differentiation. Further studies are needed to examine the effects of cold stimulation on skeletal muscle recovery [22-25]. When skin temperature decreases below 20°C, pain impulse conduction decreases, and γ nerve fibers are suppressed, resulting in decreased muscle tone [26,27].
After ice bath cooling for 15 minutes in Experiments 1 and 2, the mean NRS of the lower leg, ankle, and instep were 0.14 and 0.71, respectively, showing a marked reduction in pain sensation (Table 5). This suggests that ice bath cooling sufficiently reduces pain impulse conduction. Thus, longer cooling times may increase the effects of lowering the pain sensation, but sufficient pain-relieving effects can be obtained even with cooling for 15 minutes.
This study has some limitations. First, the effects of heat generation by water molecules induced by the RF pulse wave during MRI measurements with a magnetic field strength of 3.0 T represent an increase of approximately 0.5°C over 200 seconds. In this study, however, the inductive heating effect of MRI in 1.5 T over 120 seconds was negligible compared to normal body temperature changes.
Conclusions
As the lateral inframalleolar fat pad was sufficiently cooled, the joint capsule and ligaments immediately below are also likely to be cooled. We conclude that a 15-minute ice bath on the lower leg does not affect the bone marrow or cartilage in the joints, but it can lower the temperature of many other deep body tissues. This method has been found to be effective in the early treatment of many soft tissue injuries. When performing cryotherapy, it is important to carefully consider the tissue intended for cooling. In the future, it will be necessary to improve consistency between direct temperature measurements in living tissues and noninvasive temperature measurements using MRI.
Acknowledgments
We thank Dr. Hidemi Fujino (Professor, Department of Rehabilitation Science, Kobe University Graduate School of Health Sciences), Dr. Hideki Tanaka (Director, Department of Orthopedic Surgery, Meimai Central Hospital), Chief Engineer Yusuke Nito (Chief, Department of Radiology, Okubo Hospital) and volunteer students of the Faculty of Rehabilitation, Kobe International University for their cooperation in this study. We also thank Dr. Yasuhiko Tomita (Professor, International University of Health and Welfare) for their comments and Medical English service for the English language editing.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study. Ethics Committee of Okubo Hospital issued approval 2804
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Effect of arthroscopy and continuous cryotherapy on the intra-articular temperature of the knee. Sánchez-Inchausti G, Vaquero-Martín J, Vidal-Fernández C. Arthroscopy. 2005;21:552–556. doi: 10.1016/j.arthro.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 2.The effect of local heat and cold therapy on the intraarticular and skin surface temperature of the knee. Oosterveld FG, Rasker JJ, Jacobs JW, Overmars HJ. Arthritis Rheum. 1992;35:146–151. doi: 10.1002/art.1780350204. [DOI] [PubMed] [Google Scholar]
- 3.Temperature changes produced by spraying with ethyl chloride. Borken N, Bierman W. https://pubmed.ncbi.nlm.nih.gov/14362745/ Arch Phys Med Rehabil. 1955;36:288–290. [PubMed] [Google Scholar]
- 4.Three years experience in direct intraarticular temperature measurement. Haimovici N. https://pubmed.ncbi.nlm.nih.gov/7167499/ Prog Clin Biol Res. 1982;107:453–461. [PubMed] [Google Scholar]
- 5.The effect of ice on intra-articular temperature in the knee of the dog. Bocobo C, Fast A, Kingery W, Kaplan M. Am J Phys Med Rehabil. 1991;70:181–185. doi: 10.1097/00002060-199108000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Blood flow to the knee joint of the dog; effect of heating, cooling and adrenaline. Cobbold AF, Lewis OJ. J Physiol. 1956;132:379–383. doi: 10.1113/jphysiol.1956.sp005531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Influence of physical agents and of certain drugs on intra-articular temperature. Wakim KG, Porter AN, Krusen FH. https://pubmed.ncbi.nlm.nih.gov/14886159/ Arch Phys Med Rehabil. 1951;32:714–721. [PubMed] [Google Scholar]
- 8.Intra-articular knee joint temperature variations in response to cooling and heating of the skin in the cat. Ekholm J, Skoglund S. Acta Physiol Scand. 1960;50:175–185. doi: 10.1111/j.1748-1716.1960.tb02088.x. [DOI] [PubMed] [Google Scholar]
- 9.Intra-articular temperature as a measure of joint reaction. Horvath SM, Hollander JL. J Clin Invest. 1949;28:469–473. doi: 10.1172/JCI102092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magnetic resonance temperature imaging for guidance of thermotherapy. Quesson B, de Zwart JA, Moonen CT. J Magn Reson Imaging. 2000;12:525–533. doi: 10.1002/1522-2586(200010)12:4<525::aid-jmri3>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 11.Interventional MRI: update. Lufkin RB, Gronemeyer DH, Seibel RM. Eur Radiol. 1997;7 Suppl 5:187–200. doi: 10.1007/pl00006891. [DOI] [PubMed] [Google Scholar]
- 12.Tissue temperature monitoring for thermal interventional therapy: comparison of T1-weighted MR sequences. Matsumoto R, Mulkern RV, Hushek SG, Jolesz FA. J Magn Reson Imaging. 1994;4:65–70. doi: 10.1002/jmri.1880040114. [DOI] [PubMed] [Google Scholar]
- 13.Measurement of changes in tissue temperature using MR imaging. Dickinson RJ, Hall AS, Hind AJ, Young IR. https://pubmed.ncbi.nlm.nih.gov/3700752/ J Comput Assist Tomogr. 1986;10:468–472. [PubMed] [Google Scholar]
- 14.Observation by MR imaging of in vivo temperature changes induced by radio frequency hyperthermia. Hall AS, Prior MV, Hand JW, Young IR, Dickinson RJ. J Comput Assist Tomogr. 1990;14:430–436. doi: 10.1097/00004728-199005000-00021. [DOI] [PubMed] [Google Scholar]
- 15.The effect on muscular temperature produced by cooling normal and ultraviolet radiated skin. Bing HI, Carlsten A, Chistiansen SV. Acta Med Scand. 1945;121:577–591. doi: 10.1111/j.0954-6820.1945.tb06900.x. [DOI] [PubMed] [Google Scholar]
- 16.The physiologic effects of ice massage. Waylonis GW. https://pubmed.ncbi.nlm.nih.gov/6016565/ Arch Phys Med Rehabil. 1967;48:37–42. [PubMed] [Google Scholar]
- 17.The magnitude of tissue cooling during cryotherapy with varied types of compression. Tomchuk D, Rubley MD, Holcomb WR, Guadagnoli M, Tarno JM. J Athl Train. 2010;45:230–237. doi: 10.4085/1062-6050-45.3.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Effects of a specific ecutaneous cold stimulus on single motor unit activity of medial gastrocnemium muscle in man. Wolf SL, Letbetter WD, Basmajian JV. https://pubmed.ncbi.nlm.nih.gov/961831/ Am J Phys Med. 1976;55:177–183. [PubMed] [Google Scholar]
- 19.Hecox B, Mehreteab TA, Weisberg J, Mohrotoab TA. Weisberg J, Mohrotoab TA: Physical Agents: A Comprehensive Text for Physical Therapists. Appleton & Lange, Norwalk. Norwalk: Appleton & Lange; 1994. Physical Agents: A Comprehensive Text for Physical Therapists. [Google Scholar]
- 20.Unconventional applications of the Arrhenius law. Laidler KJ. J Chem Educ. 1972;49:343. [Google Scholar]
- 21.The influence of temperature and fibril stability on degradation of cartilage collagen by rheumatoid synovial collagenase. Harris ED Jr, McCroskery PA. N Engl J Med. 1974;290:1–6. doi: 10.1056/NEJM197401032900101. [DOI] [PubMed] [Google Scholar]
- 22.Influence of icing on muscle regeneration after crush injury to skeletal muscles in rats. Takagi R, Fujita N, Arakawa T, Kawada S, Ishii N, Miki A. J Appl Physiol (1985) 2011;110:382–388. doi: 10.1152/japplphysiol.01187.2010. [DOI] [PubMed] [Google Scholar]
- 23.Heat stress promotes skeletal muscle regeneration after crush injury in rats. Takeuchi K, Hatade T, Wakamiya S, Fujita N, Arakawa T, Miki A. Acta Histochem. 2014;116:327–334. doi: 10.1016/j.acthis.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Continuous local cooling for pain relief following total hip arthroplasty. Saito N, Horiuchi H, Kobayashi S, Nawata M, Takaoka K. J Arthroplasty. 2004;19:334–337. doi: 10.1016/j.arth.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Does cryotherapy improve outcome with soft tissue injury? Hubbard TJ, Denegar CR. https://pubmed.ncbi.nlm.nih.gov/15496998/ J Athl Train. 2004;39:278–279. [PMC free article] [PubMed] [Google Scholar]
- 26.The effect of cooling on mammalian muscle spindles. Eldred E, Lindsley DF, Buchwald JS. Exp Neurol. 1960;2:144–157. doi: 10.1016/0014-4886(60)90004-2. [DOI] [PubMed] [Google Scholar]
- 27.The effects of muscle cooling and stretch on muscle spindle secondary endings in the cat. Michalski WJ, Séguin JJ. J Physiol. 1975;253:341–356. doi: 10.1113/jphysiol.1975.sp011193. [DOI] [PMC free article] [PubMed] [Google Scholar]