Abstract
The association between age and language recovery in stroke remains unclear. Here, we used neuroimaging data to estimate brain age, a measure of structural integrity, and examined the extent to which brain age at stroke onset is associated with (i) cross-sectional language performance, and (ii) longitudinal recovery of language function, beyond chronological age alone. A total of 49 participants (age: 65.2 ± 12.2 years, 25 female) underwent routine clinical neuroimaging (T1) and a bedside evaluation of language performance (Bedside Evaluation Screening Test-2) at onset of left hemisphere stroke. Brain age was estimated from enantiomorphically reconstructed brain scans using a machine learning algorithm trained on a large sample of healthy adults. A subsample of 30 participants returned for follow-up language assessments at least 2 years after stroke onset. To account for variability in age at stroke, we calculated proportional brain age difference, i.e. the proportional difference between brain age and chronological age. Multiple regression models were constructed to test the effects of proportional brain age difference on language outcomes. Lesion volume and chronological age were included as covariates in all models. Accelerated brain age compared with age was associated with worse overall aphasia severity (F(1, 48) = 5.65, P = 0.022), naming (F(1, 48) = 5.13, P = 0.028), and speech repetition (F(1, 48) = 8.49, P = 0.006) at stroke onset. Follow-up assessments were carried out ≥2 years after onset; decelerated brain age relative to age was significantly associated with reduced overall aphasia severity (F(1, 26) = 5.45, P = 0.028) and marginally failed to reach statistical significance for auditory comprehension (F(1, 26) = 2.87, P = 0.103). Proportional brain age difference was not found to be associated with changes in naming (F(1, 26) = 0.23, P = 0.880) and speech repetition (F(1, 26) = 0.00, P = 0.978). Chronological age was only associated with naming performance at stroke onset (F(1, 48) = 4.18, P = 0.047). These results indicate that brain age as estimated based on routine clinical brain scans may be a strong biomarker for language function and recovery after stroke.
Keywords: aphasia, neuroimaging, ageing, age, stroke
Kristinsson et al. examined the association between estimated brain age, a measure of cortical atrophy derived from clinical-grade brain scans, and language recovery after acute left hemisphere stroke. Primary findings revealed a strong association between brain age and language function at stroke onset as well as long-term recovery of language.
Graphical Abstract
Graphical abstract.
Introduction
Aphasia is a language impairment that is generally recognized as one of the most disabling consequences of a stroke affecting the language-dominant brain hemisphere.1 Most individuals with aphasia recover some language functions in the days and months following the stroke,2 but the factors associated with recovery remain poorly understood.3 Prior studies indicate that the initial severity of aphasia,4,5 the size of the cortical infarct,6,7 and the lesion site6,8 account for substantial variability in long-term outcomes.
The relationship between recovery and other variables such as age is less clear.9 Neuroplastic properties of the brain decrease with age,10,11 suggesting that age might be an important factor in aphasia recovery. However, older individuals are more likely to present with severe aphasia,1,2 which may indicate that the effect of age on recovery is confounded by aphasia severity, at least in some age groups. Recent research suggests that brain age, which is based on an estimate of cortical tissue integrity, is a more useful indicator of neuroplastic properties of the brain.12,13 In the current study, we report the first acute-to-chronic examination of the impact of estimated brain age for longitudinal language recovery in aphasia.
Healthy ageing is accompanied by reliable changes to structural integrity of the brain; in particular, atrophy of grey matter, reduced volume of white matter connections, and distorted functional connectivity have been observed with magnetic resonance imaging.14–22 The recently coined concept of brain age broadly represents these changes.
Brain age is generally predicted using machine learning algorithms that leverage neuroimaging-derived measures of structural atrophy to estimate how old the brain looks compared to a large sample of healthy control subjects.12 The extent to which brain age deviates from chronological age has been found to be associated with onset of psychiatric and neurologic diseases,13,23 physical functioning,24,25 and cognitive abilities.24,26–28 This suggests that estimated brain age may potentially be implemented as a surrogate measure for cognitive reserve.
The presence of a brain lesion presents a challenge for the estimation of brain age since current approaches depend on the quality of normalization of the neuroimages, i.e. warping individual brains into standard space. Necrotic brain tissue can markedly distort the normalization, which is generally designed to process healthy brain images.29 This issue can be bypassed by applying an enantiomorphic algorithm to native T1 images to effectively ‘heal’ the damaged hemisphere.30 The enantiomorphic ‘healing’ takes advantage of the left-right symmetry across hemispheres to replace tissue in the damaged hemisphere with a mirror image of healthy tissue from the contralateral hemisphere. This approach has been successfully applied in our prior work,31–34 as well as by other groups.35–37
The rate of brain atrophy is increasingly implemented as a clinical biomarker in various neurological disorders characterized by a marked deviation between brain age and chronological age.38–40 In the context of stroke, recent studies have emphasized the detrimental impact of stroke as manifested in accelerated brain age.41,42 Others have observed an association between brain age and stroke risk,43 potentially indicating that biological brain age may both be a biomarker for stroke risk and exacerbated as a consequence of brain damage. Critically, while the association between neurodegeneration and cognitive function has been observed in many neurological disorders,23,26,44 the relationship between brain age and cognitive outcomes in post-stroke functional recovery remains to be studied in detail.45–48
To this end, we examined the association between brain age and language outcomes after stroke. Specifically, we tested the hypothesis that brain age at stroke onset is associated with: (i) cross-sectional language function and (ii) long-term recovery of language function, beyond chronological age. We expected accelerated brain age relative to chronological age to be associated with poorer language function and worse recovery. This study leveraged retrospective clinical neuroimaging data and language assessments collected at stroke onset and at follow-up least 2 years after stroke onset.
Methods
Participants
A total of 49 individuals with acute left hemisphere injury were included in the study. Participants were recruited through the neurology ward at the National University Hospital of Iceland, Reykjavk. Participants were eligible for inclusion in the study if they (i) had incurred a single, unilateral left hemisphere stroke, (ii) were in the acute phase of recovery, (iii) had their stroke confirmed by a CT/MRI scan, (iv) had no history of prior stroke, major psychiatric illness or other neurologic impairment affecting the brain, (v) were native speakers of Icelandic, and (vi) gave informed consent for study participation. All study procedures were approved by the Institutional Review Board of the University of Iceland. For a detailed description of participants and procedures, see Magnusdottir et al.49 and Kristinsson et al.50
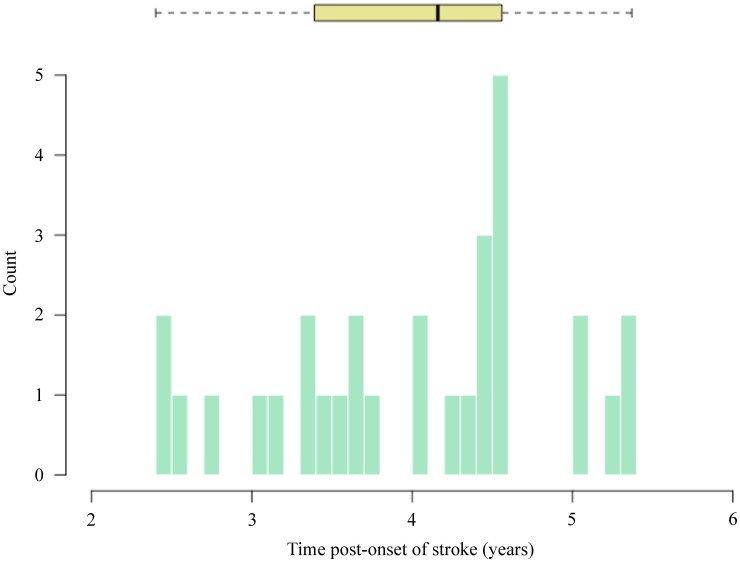
Participants underwent MRI and language assessments within three days of hospital admission. At stroke onset, the average age of the sample was 65.2 years (SD = 12.2 years, range: 34–85 years) and 25 participants were female. A subsample of 30 participants returned for a follow-up language assessment at least 24 months post-onset. At the time of retesting, the average age of the sample was 67.5 years (SD = 10.2 years, range: 43–82 years). The range of time post-stroke across participants was 2.4–5.4 years (mean = 4.0 years, SD = 0.9 years) at retesting. Figure 1 shows time post-onset (TPO) across participants.
Figure 1.
Time post-onset. TPO of left hemisphere stroke in years at retesting (min = 2.4y, max = 5.4y; N = 30). The boxplot shown at the top of the figure represents median TPO, quartiles and whiskers denote the range of values.
Language assessments
Speech and language impairment was assessed with the Bedside Evaluation Screening Test-Second Edition (BEST-2).51 The BEST-2 is designed to assess language function at bedside in acute patients who may not be able to complete a full language assessment battery. In addition to providing an assessment of overall language impairment (henceforth, overall score), the BEST-2 assesses several language domains, including naming, speech repetition, and auditory comprehension. As these domains correspond to the main subtests on the Western Aphasia Battery-Revised,52 which is the most widely used aphasia test,53 we included all four scores in the data analyses. Importantly, despite being a short evaluation, our prior work has shown that the BEST-2 is sensitive to language impairment and longitudinal changes in language function.49,50,54
MRI
MRI data were collected as part of routine clinical care in acute stroke on a 1.5 T Siemens scanner. We obtained T1-weighted images, diffusion-weighted imaging (DWI), and fluid-attenuated inversion recovery (FLAIR) scans. The details for these sequences are as follows: T1-weighted image [3D GR\IR sequence, repetition time (TR) = 1160 ms, inversion time (TI) = 600 ms, echo time (TE) = 4.24 ms, flip angle = 15°, the 256 × 256 matrix was reconstructed at 512 × 512, yielding a 0.45 × 0.45 mm2 in axial-plane resolution, with 192 0.9 mm slices), diffusion-weighted images (three scans with B0 = 0, 500, and 1000; TR = 3808 ms, TE = 89 ms, flip angle = 90o, Nx = 4, 192 × 192 matrix, 1.2 × 1.2 mm2 in axial plane, 24 slices, each 5 mm thick with 1.5 mm gap), and T2-weighted FLAIR image (TR = 9000 ms, TI = 2500 ms, TE = 112 ms, flip angle = 15°, 280 × 320 matrix with 0.72 × 0.72 mm2 in axial-plane resolution, 24 slices, each 5 mm thick with 1.5 mm gap). Images were converted from DICOM to NIfTI format using dcm2niix,55 which preserves spatial coordinates (yielding a good starting estimate for the subsequent co-registration of the T1 image to the T2 scan).
An expert neurologist or trained study staff member with extensive experience/training in lesion drawing manually demarcated the brain lesions on FLAIR images using MRIcroGL12.56 DWI images were used to guide lesion drawing as needed to ensure lesion boundaries were precisely demarcated.
Calculating brain age
Each individual’s brain scan was ‘healed’ to exclude the effects of the stroke lesion on automated brain age estimates. First, each participant’s FLAIR/lesion maps were co-registered to align to their own T1 scan. Next, each participant’s T1 and spatially aligned lesion map were used to create an enantiomorphically healed version of their T1.30 The enantiomorphic healing process exploits the symmetrical nature of the brain (i.e. the right and left sides of the brain are roughly symmetrical), as well as the fact that the lesions in our sample were unilateral (and thus all lesions had corresponding contralateral intact brain tissue with which we could repair them). In the current study, enantiomorphic healing involved replacement of damaged tissue in the ipsilesional hemisphere with healthy tissue from homologous areas of the contralateral, non-lesioned hemisphere. This step was completed using the clinical toolbox.57 The enantiomorphically ‘healed’ brain image represents the best estimation of the structural integrity of the brain prior to stroke. All images were subject to visual inspection by study staff blinded to participants’ age. Because the BrainAgeR analysis pipeline expects images in native space as input, we did not normalize the enantiomorphically healed brains.
The BrainAgeR analysis pipeline (github.com/james-cole/brainageR)24 was applied to estimate biological brain age using default settings. First, the DARTEL toolbox58 in SPM12 was used to segment and normalize the T1 images. For quality control, probabilistic tissue maps were visually inspected by an expert neurologist to ensure proper segmentation. Second, cerebrospinal fluid was parcellated out, and grey and white matter probabilistic tissues were vectorized, concatenated, and fed into a principal component analysis (PCA) to reduce dimensionality. The PCA-derived components accounting for the top 80% of variance were retained for estimation of brain age. A pretrained Gaussian regression model in the R package Kernlab was implemented to predict brain age for each individual. The pretrained model was created based on input images from healthy individuals (N = 3377) and validated in a separate sample of healthy individuals (N = 611) between 18 and 90 years old,24 thus serving as inherent control data in the current study.
To adjust for variability in chronological age, we determined the proportional deviation of predicted brain age from chronological age as follows:
Each participant’s proportional brain age difference (PBAD) score indicates whether predicted age is accelerated or decelerated relative to her/his own chronological age. More specifically, positive values indicate premature brain ageing, whereas negative values suggest greater tissue integrity than the same age group in a normative sample.
Statistical analyses
Multiple linear regression models were constructed to test the hypothesis that brain age at stroke onset is independently associated with language function (onset models). Each model included three terms: lesion volume, chronological age, and PBAD. Separate models were run for four outcome variables: overall score, naming, speech repetition, and auditory comprehension subscores. To test our second hypothesis, that brain age is associated with longitudinal recovery of language function, we applied the same paradigm for change in each subscore from stroke onset to follow-up assessment (recovery models). In addition to lesion volume, chronological age, and PBAD, the recovery models were adjusted for baseline performance on each language task (i.e. naming recovery model was adjusted for BEST-2 naming score at baseline). Associations between other variables were explored using Pearson’s or Spearman’s correlation coefficients as appropriate. All analyses were conducted in SPSS version 28.59
Data availability
Data presented in this study is not publicly available at present. However, de-identifiable participant data is available from the primary author upon reasonable request.
Results
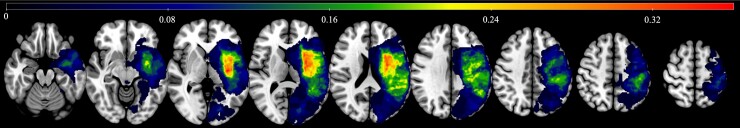
Fig. 2 presents a lesion overlay map for the study sample. Most participants presented with relatively small lesions (average lesion volume = 5.5 ± 6.0 cm3). Across the group, lesions primarily covered the middle cerebral artery peri-Sylvian region, with greatest overlap observed in the insula extending into inferior frontal territory.
Figure 2.
Lesion overlay map. Lesion overlap across participants. The colour bar represents proportional overlap (max = 37% overlap).
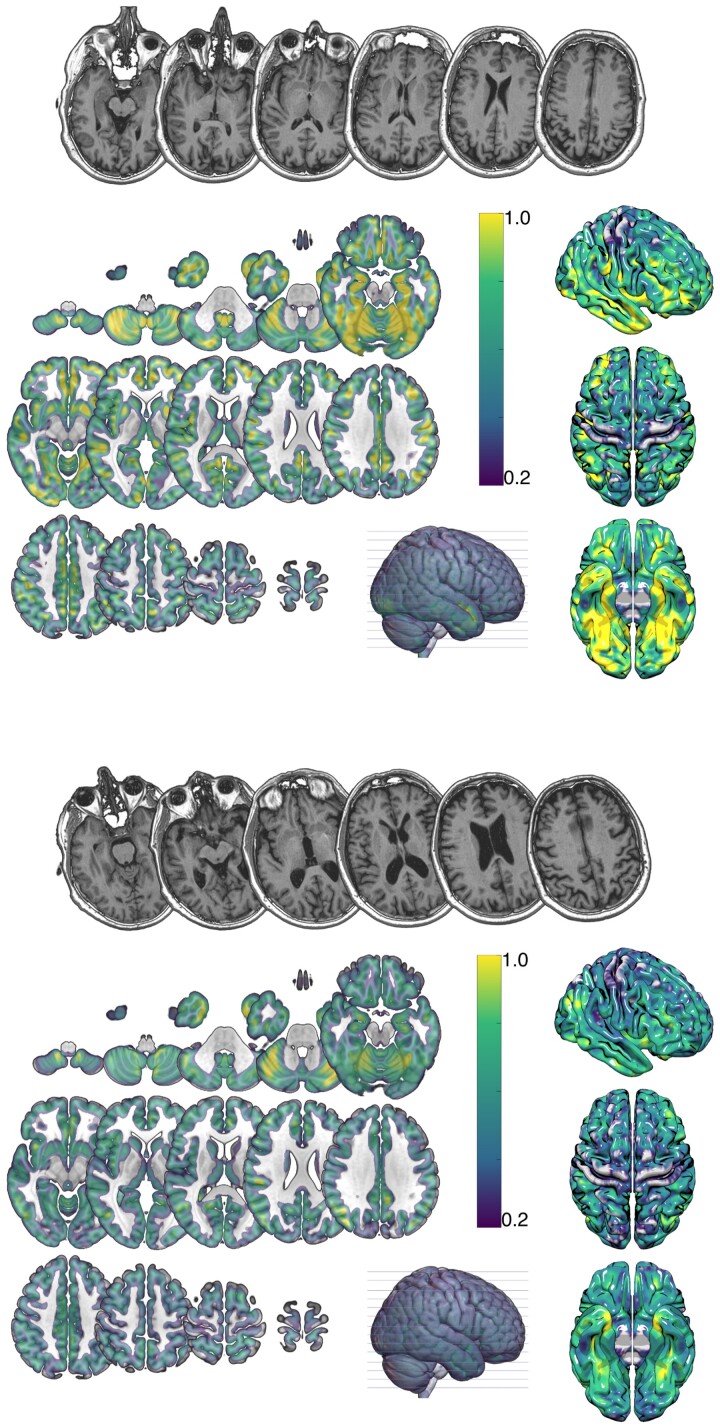
Brain age at stroke onset was estimated for all 49 participants. Estimated brain age was on average decelerated by 3.7 ± 7.5 years (range: −24.1 to 10.1 years) relative to chronological age. The corresponding PBAD values were −0.06 ± 0.11 (range: −0.40 to 0.16). Fig. 3 demonstrates an example of two participants of similar chronological age and with comparable lesion profiles but vastly different brain age.
Figure 3.
Example grey matter volume maps. Probabilistic grey matter estimates from two representative participants. Top panel: male, chronological age = 60.2 years, brain age = 36.9 years, PBAG = −0.39); bottom panel: male, chronological age = 62.4 years, brain age = 71.7, PBAG = 0.15). The colour bar represents the probabilistic measure of grey matter volume (darker colours suggesting less grey matter).
Estimated brain age correlated significantly with chronological age (ρ = 0.80, P < 0.001) and with lesion volume (ρ = −0.29, P = 0.042). Chronological age was similarly correlated with lesion volume (ρ = −0.32, P = 0.026). Critically, PBAD was neither correlated with chronological age (ρ = −0.06, P = 0.704) nor with lesion volume (ρ = −0.07, P = 0.631).
Brain age is associated with language function at stroke onset
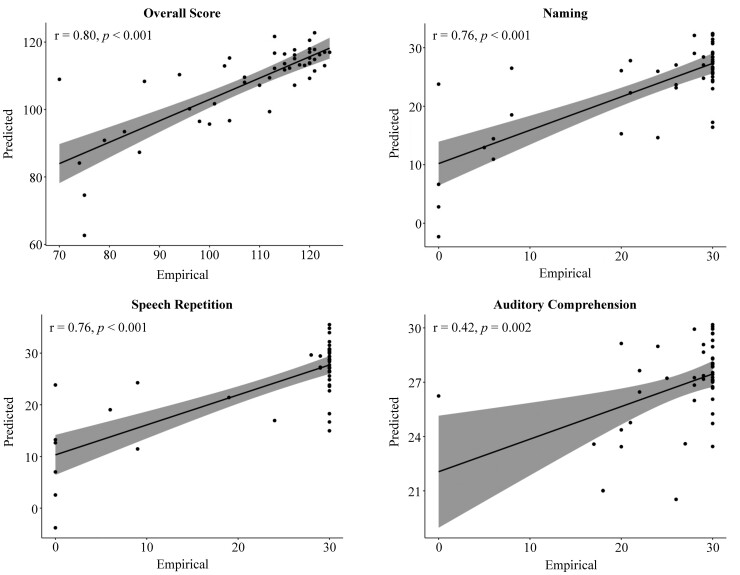
Our first aim sought to test the hypothesis that estimated brain age is associated with language function at stroke onset independently of chronological age. To this end, multiple regression models were used to predict language outcomes based on lesion volume, chronological age, and PBAD. We found that PBAD was a significant predictor of overall score (F(1, 48) = 5.65, P = 0.022), naming (F(1, 48) = 5.13, P = 0.028), and speech repetition (F(1, 48) = 8.49, P = 0.006), but not auditory comprehension (F(1, 48) = 2.06, P = 0.158). In each case, the standardized beta (ß) value for PBAD was negative (−0.29 to −0.22; Table 1), suggesting a negative association between accelerated brain age relative to chronological age and language performance. Lesion volume was a significant predictor of all language outcomes (P = <0.001 to.005). Chronological age emerged as a significant predictor of naming (F(1, 48) = 4.18, P = 0.047), but the effect of chronological age was not significant in other models (all P > 0.20). Model parameters are shown in Table 1. Figure 4 demonstrates actual and predicted language scores based on onset models.
Table 1.
Multiple regression models (df = 48) predicting language performance at stroke onset
| F | t | ß | η2 | P | |
|---|---|---|---|---|---|
| Overall score | |||||
| Model | 25.97 | 0.63 (R2 = 0.61) | <0.001** | ||
| Lesion volume | 72.72 | −8.53 | −0.82 | 0.62 | <0.001** |
| Chronological age | 0.41 | −0.64 | −0.06 | 0.01 | 0.525 |
| PBAD | 5.65 | 2.38 | −0.22 | 0.11 | 0.022* |
| Naming | |||||
| Model | 19.96 | 0.57 (R2 = 0.54) | <0.001** | ||
| Lesion volume | 58.99 | −7.68 | −0.80 | 0.57 | <0.001** |
| Chronological age | 4.18 | −2.05 | −0.21 | 0.09 | 0.047* |
| PBAD | 5.13 | 2.26 | −0.23 | 0.10 | 0.028* |
| Speech repetition | |||||
| Model | 20.70 | 0.58 (R2 = 0.55) | <0.001** | ||
| Lesion volume | 57.91 | −7.61 | −0.79 | 0.56 | <0.001** |
| Chronological age | 1.18 | −1.08 | −0.11 | 0.03 | 0.284 |
| PBAD | 8.49 | 2.91 | −0.29 | 0.16 | 0.006** |
| Auditory comprehension | |||||
| Model | 3.29 | 0.18 (R2 = 0.13) | 0.029 | ||
| Lesion volume | 8.93 | −2.99 | −0.43 | 0.17 | 0.005** |
| Chronological age | 1.68 | −1.30 | −0.18 | 0.04 | 0.202 |
| PBAD | 2.06 | 1.44 | −0.20 | 0.04 | 0.158 |
P < 0.05.
P < 0.01.
Figure 4.
Actual and predicted language scores. Empirical versus predicted language scores at stroke onset. Multiple regression models included lesion volume, chronological age and brain age as independent terms.
Brain age is associated with longitudinal language recovery
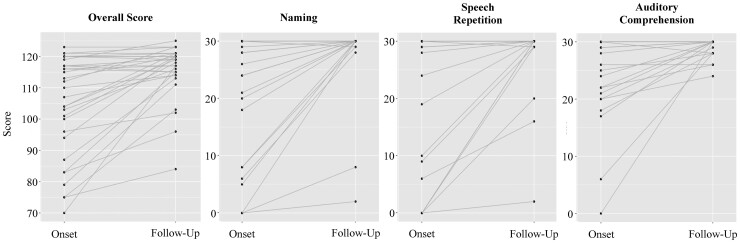
On average, the subsample of participants who returned for a second language assessment showed a significant improvement on all language outcomes from stroke onset to follow-up (paired t(29) range: 2.8–4.8, all P < 0.01; Fig. 5). In order to examine the effects of brain age on longitudinal recovery of language function, we applied the same paradigm to model change in language performance from stroke onset to follow-up assessments (recovery models). Given the strong correlation between baseline and follow-up language assessments (r = 0.45–0.67, all P < 0.05), each recovery model was additionally adjusted for baseline BEST scores.
Figure 5.
Longitudinal recovery of language. Longitudinal recovery across language domains. A paired-samples t-test revealed a significant average improvement on all language outcomes from stroke onset to follow-up (df = 29, toverall = 4.8, tnaming = 3.4, trepetition = 3.0, tcomprehension = 2.8, all P < 0.01).
PBAD emerged as a significant predictor of change in overall language function from stroke onset to follow-up (F(1, 26) = 5.45, P = 0.028). The standardized beta coefficient was negative (ß = −0.22), suggesting that decelerated brain age relative to chronological age (negative PBAD value) was associated with better language recovery, whereas relatively accelerated brain age (positive PBAD value) was associated with poorer recovery. No statistically significant main effects of PBAD were observed for change in naming (F(1, 26) = 0.23, P = 0.880) or speech repetition (F(1, 26) = 0.11, P = 0.978), and PBAD marginally failed to reach statistical significance for change in auditory comprehension (F(1, 26) = 2.87, P = 0.103).
As expected, baseline performance was the strongest predictor in each model (all P < 0.001), independently accounting for > 70% of variability in language recovery. Lesion volume emerged as a significant predictor of change in overall score (P = 0.003), naming (P < 0.001), and auditory comprehension (P = 0.005) but marginally failed to reach statistical significance for speech repetition (P = 0.064). Chronological age was not associated with longitudinal change in any recovery model (all P > 0.20).
Given the variability in TPO at the follow-up assessment, we performed a post hoc analysis to investigate the effects of TPO on language recovery. TPO did not emerge as a significant factor in any model (all P > 0.30) and did not impact other results. Full models are shown in Table 2.
Table 2.
General linear models predicting raw change in language performance from stroke onset to follow-up adjusted for baseline severity (BEST)
| F | t | ß | η2 | P | |
|---|---|---|---|---|---|
| Overall score | |||||
| Model | 30.06 | 0.83 (R2 = 0.80) | <0.001 | ||
| Baseline score | 90.66 | −9.52 | −1.19 | 0.78 | <0.001 |
| Lesion volume | 10.52 | −3.24 | −0.44 | 0.30 | 0.003 |
| Chronological age | 1.66 | 1.29 | 0.12 | 0.06 | 0.210 |
| PBAD | 5.45 | −2.34 | −0.22 | 0.18 | 0.028* |
| Naming | |||||
| Model | 36.63 | 0.85 (R2 = 0.83) | <0.001 | ||
| Baseline score | 114.15 | −10.68 | −1.14 | 0.82 | <0.001 |
| Lesion volume | 18.91 | −4.35 | −0.51 | 0.43 | <0.001 |
| Chronological age | 0.64 | 0.80 | 0.07 | 0.03 | 0.433 |
| PBAD | 0.23 | 0.15 | 0.01 | 0.00 | 0.880 |
| Speech repetition | |||||
| Model | 28.77 | 0.82 (R2 = 0.79) | <0.001 | ||
| Baseline score | 69.13 | −8.31 | −1.05 | 0.73 | <0.001 |
| Lesion volume | 3.77 | −1.94 | −0.26 | 0.13 | 0.064 |
| Chronological age | 0.69 | 0.83 | 0.08 | 0.03 | 0.413 |
| PBAD | 0.00 | 0.03 | 0.00 | 0.00 | 0.978 |
| Auditory comprehension | |||||
| Model | 159.39 | 0.96 (R2 = 0.96) | <0.001 | ||
| Baseline score | 547.88 | −23.41 | −1.03 | 0.96 | <0.001 |
| Lesion volume | 9.56 | −3.09 | −0.15 | 0.28 | 0.005 |
| Chronological age | 0.58 | −0.76 | −0.03 | 0.02 | 0.453 |
| PBAD | 2.87 | −1.69 | −0.07 | 0.10 | 0.103 |
P < 0.05.
Finally, we ran each recovery model without adjustment for baseline BEST scores to enable direct comparison to the cross-sectional results reported above. Together, lesion volume, chronological age, and PBAD accounted for 19%, 33%, 14%, and 20% of variability in change scores in naming, speech repetition, auditory comprehension, and overall score, respectively. Without adjustment for baseline performance, PBAD was associated with change in speech repetition (P = 0.017) and marginally failed to reach statistical significance for change in naming (P = 0.072), whereas chronological age was not a significant factor in any model (all P > 0.100; Supplementary Table 1).
Discussion
This study tested the hypothesis that brain age, as estimated based on neuroimaging-derived measures of brain atrophy, is associated with language function and recovery following stroke independent of chronological age. Our results support this hypothesis. Specifically, we found that accelerated brain age relative to chronological age is negatively associated with both language function at stroke onset and long-term language recovery. This effect was independent of overall lesion volume and TPO. Thus, the present study demonstrates for the first time the utility of brain age estimated based on routine clinical-grade brain scans to inform longitudinal recovery of language function following left hemisphere stroke. The significance of these findings is discussed below.
Association between age and language performance in stroke
Neuroplastic properties of the brain deteriorate with age due to progressive atrophy of grey and white matter tissue.60,61 As a consequence, healthy ageing is accompanied by gradual cognitive decline,62,63 including in language function.64 The rate of age-related cognitive decline is associated with increased risk of neurogenic diseases, such as dementia.62 Moreover, the diminished structural integrity of the brain has been shown to be associated with worse functional outcomes in stroke recovery.65,66
Despite ample evidence suggesting a strong causal link between structural integrity of intact brain regions and recovery, prior work has failed to find a consistent relationship between age and language recovery in post-stroke aphasia.9 Several potential reasons for this contradiction have been postulated. For instance, some studies have observed more severe language deficits in older patients at stroke onset.1,2 As aphasia severity is generally considered a strong predictor of language recovery,4,5 this may negate any possible independent effects of age. Alternatively, the large interindividual variability in age-related brain changes22,67 may reduce statistical power to detect effects of interest in a literature that is dominated by single-subject and small group studies.68
Predicted brain age largely bypasses these issues and offers a novel approach to inform the true integrity of the brain.44 Our results revealed a positive correlation between chronological age and brain age (ρ = 0.80, P < 0.001), suggesting that these two measures are strongly related. Notwithstanding, we found that the relative deviance between estimated brain age and age was associated with performance on naming and speech repetition, as well as overall score at stroke onset (see Table 1) and longitudinal recovery of overall language function (see Table 2) when variability explained by chronological age was accounted for. In further post hoc analysis, all significant main effects were replicated after ceiling scores were removed (Supplementary Tables 2 and 3). These findings are consistent with the notion that there is not a direct correspondence between chronological age and cognitive decline69,70 and, instead, indicate that estimated brain age accounts for unique variability unrelated to chronological age.
Recent research has shown that other cerebrovascular risk factors are similarly correlated with brain age, such as blood pressure71 and BMI.13 Cerebrovascular biomarkers are unequivocally associated with overall brain health and structural brain atrophy.72,73 To this end, estimated brain age may capture atrophy explained by other factors than chronological age. In the context of the current study, these additional factors account for a significant amount of variability in language function and recovery. Importantly, our results echo findings in other neurogenic diseases74–76 and corroborate recent findings reported in the stroke recovery literature.42,45,46
Implications
While prior studies in the aphasia literature have not incorporated an estimate of brain age to inform language function, various approaches have been successfully implemented to reveal a strong association between structural integrity of intact brain regions and language performance.77,78 The novelty of the current study lies instead in the approach used. We applied enantiomorphic ‘healing’ to clinical T1-weighted brain scans to avoid complications introduced by lesioned brain tissue and enable accurate computation of brain age. Proportional brain age gap was unrelated to lesion volume, indicating that the healed brain image was unaffected by lesion characteristics. This is important for two main reasons. First, the sheer extent of lesion damage is a critical determinant of the subsequent functional consequences.7 This notion is strongly supported by our findings as lesion volume was a strong predictor in most regression models, typically accounting for one- to two-thirds of variability in the dependent variable. Critically, the effect of brain age was independent of lesion volume.
Second, measures of structural integrity used to investigate language function in post-stroke aphasia are frequently derived from DWI, T2-weighted scans, or other sophisticated imaging modalities that use long acquisition times, multi-echo sequences, and ultra-high field resolution only possible on high field strength (3 T) scanners. These research-grade scans are generally not collected as part of routine clinical care in stroke, where the primary goal is to acquire time-sensitive information about coarse lesion characteristics. In the current study, the scans came from a 1.5 T scanner, which is common for clinical scans. The ability to derive clinically meaningful prognostic information from clinical scans offers the potential to substantially improve prognostication procedures in aphasia.79
Therefore, the current study serves as a proof-of-principle for a novel, effective and simple to use approach to inform post-stroke language recovery. The extent to which brain age, as indicative of total and/or regional brain atrophy, can be implemented as a tool to guide clinical decision making in aphasia remains to be examined. Future studies will need to determine the unique contribution of brain age relative to other lesion, neuropsychological, and biographical factors associated with language outcomes. As a biomarker of cognitive reserve, brain age is less dependent on factors like language, education, and socio-economic status, which frequently influence cognitive testing.80,81 At the same time, brain age is sensitive both to modifiable environmental factors, such as training,28,39 and changes in cognitive abilities.82,83 Thus, brain age may be a particularly promising marker of long-term therapy success.
Limitations
The results reported herein, despite being promising, should be interpreted with caution given the novel approach implemented. Several other important limitations of the study design warrant discussion. First, and perhaps most importantly, we included a relatively small sample size that may not support generalization of the results to another sample. Although the sample size is fairly typical for aphasia research,84 the heterogenous nature of language deficits in aphasia reduces statistical power to detect subtle effects of interest.85 Notwithstanding, it is worth noting that the strength of the association between brain age and language performance in the current study increases our confidence that these findings are not spurious.
Second, estimated brain age was considerably lower on average than chronological age (mean = −3.7 years). This estimate is lower than that reported in most prior studies.86 There are several potential reasons for this; one potential reason is that cerebrovascular health statistics are generally good in Iceland, especially for women.87 Importantly, 25/49 participants in the current study were women. Additionally, the Icelandic population has comparatively good access to high quality health care at a low out-of-pocket cost.88
Third, one potential criticism of this work is that the enantiomorphic healing process could have introduced artefacts into the brain images that were then used by BrainAgeR to estimate age. We argue that this is unlikely due to our finding that lesion size (and thus the extent to which damaged tissue was replaced with healthy tissue) was not significantly related to estimated brain age differences. Last, the BEST-2 is a coarse measure that may not be sensitive to subtle changes in language function. However, given the substantial functional changes expected in the acute recovery phase89 in addition to observed improvements across all language tests, this should not affect our results.
Conclusions
In conclusion, our results show for the first time that neuroimaging-based estimation of brain age—as a measure of overall structural integrity of the brain—is associated with language function and recovery following acute stroke. Critically, brain age explained more variability in language performance than chronological age alone. These results hold substantial promise to enhance understanding of the neural bases of aphasia recovery and to improve prognostication in the clinical management of aphasia.
Supplementary Material
Abbreviations
- BEST-2
Bedside Evaluation Screening Test-Second Edition
- DWI
diffusion-weighted imaging
- FLAIR
fluid-attenuated inversion recovery
- PBAD
proportional brain age difference
- PCA
principal component analysis
- SD
standard deviation
- TE
echo time
- TI
inversion time
- TPO
time post-onset
- TR
repetition time.
Contributor Information
Sigfus Kristinsson, Center for the Study of Aphasia Recovery, University of South Carolina, Columbia, SC 29208, USA.
Natalie Busby, Center for the Study of Aphasia Recovery, University of South Carolina, Columbia, SC 29208, USA.
Christopher Rorden, Center for the Study of Aphasia Recovery, University of South Carolina, Columbia, SC 29208, USA; Department of Psychology, University of South Carolina, Columbia, SC 29208, USA.
Roger Newman-Norlund, Center for the Study of Aphasia Recovery, University of South Carolina, Columbia, SC 29208, USA; Department of Psychology, University of South Carolina, Columbia, SC 29208, USA.
Dirk B den Ouden, Center for the Study of Aphasia Recovery, University of South Carolina, Columbia, SC 29208, USA; Department of Communication Sciences and Disorders, Columbia, SC 29208, USA.
Sigridur Magnusdottir, Department of Medicine, University of Iceland, Reykjavik 00107, Iceland.
Haukur Hjaltason, Department of Medicine, University of Iceland, Reykjavik 00107, Iceland; Department of Neurology, Landspitali University Hospital, Reykjavik 00101, Iceland.
Helga Thors, Department of Medicine, University of Iceland, Reykjavik 00107, Iceland.
Argye E Hillis, Center for the Study of Aphasia Recovery, University of South Carolina, Columbia, SC 29208, USA; Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MA 21218, USA.
Olafur Kjartansson, Department of Neurology, Landspitali University Hospital, Reykjavik 00101, Iceland.
Leonardo Bonilha, Center for the Study of Aphasia Recovery, University of South Carolina, Columbia, SC 29208, USA; Department of Neurology, Medical University of South Carolina, Charleston, SC 29425, USA.
Julius Fridriksson, Center for the Study of Aphasia Recovery, University of South Carolina, Columbia, SC 29208, USA; Department of Communication Sciences and Disorders, Columbia, SC 29208, USA.
Funding
This study was supported by the following grant sponsors: National Institute on Deafness and Other Communication Disorders (P50 DC014664; DC008355); National Institute of Neurological Disorders and Stroke (NS054266).
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain Communications online.
References
- 1. Engelter ST, Gostynski M, Papa S, et al. . Epidemiology of aphasia attributable to first ischemic stroke: Incidence, severity, fluency, etiology, and thrombolysis. Stroke. 2006;37(6):1379–1384. [DOI] [PubMed] [Google Scholar]
- 2. Pedersen PM, Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS. Aphasia in acute stroke: Incidence, determinants, and recovery. Ann Neurol. 1995;38(4):659–666. [DOI] [PubMed] [Google Scholar]
- 3. Watila MM, Balarabe SA. Factors predicting post-stroke aphasia recovery. J Neurol Sci. 2015;352(1–2):12–18. [DOI] [PubMed] [Google Scholar]
- 4. REhabilitation and recovery of peopLE with Aphasia after StrokE (RELEASE) Collaborators . Predictors of poststroke aphasia recovery. Stroke. 2021;52:1778–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lazar RM, Minzer B, Antoniello D, Festa JR, Krakauer JW, Marshall RS. Improvement in aphasia scores after stroke is well predicted by initial severity. Stroke. 2010;41(7):1485–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benghanem S, Rosso C, Arbizu C, et al. . Aphasia outcome: The interactions between initial severity, lesion size and location. J Neurol. 2019;266(6):1303–1309. [DOI] [PubMed] [Google Scholar]
- 7. Forkel SJ, Thiebaut de Schotten M, Dell’Acqua F, et al. . Anatomical predictors of aphasia recovery: A tractography study of bilateral Perisylvian language networks. Brain. 2014; 137(Pt 7):2027–2039. [DOI] [PubMed] [Google Scholar]
- 8. Hillis AE, Beh YY, Sebastian R, et al. . Predicting recovery in acute poststroke aphasia. Ann Neurol. 2018;83(3):612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ellis C, Urban S. Age and aphasia: A review of presence, type, recovery and clinical outcomes. Top Stroke Rehabil. 2016;23(6):430–439. [DOI] [PubMed] [Google Scholar]
- 10. Toth E, Gersner R, Wilf-Yarkoni A, et al. . Age-dependent effects of chronic stress on brain plasticity and depressive behavior. J Neurochem. 2008;107(2):522–532. [DOI] [PubMed] [Google Scholar]
- 11. Vara H, Muñoz-Cuevas J, Colino A. Age-dependent alterations of long-term synaptic plasticity in thyroid-deficient rats. Hippocampus. 2003;13(7):816–825. [DOI] [PubMed] [Google Scholar]
- 12. Cole JH, Marioni RE, Harris SE, Deary IJ. Brain age and other bodily “ages”: Implications for neuropsychiatry. Mol Psychiatry. 2019;24(2):266–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wrigglesworth J, Ward P, Harding IH, et al. . Factors associated with brain ageing - a systematic review. BMC Neurol. 2021;21(1):312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bennett IJ, Madden DJ. Disconnected aging: Cerebral white matter integrity and age-related differences in cognition. Neuroscience. 2014;276:187–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bonifazi P, Erramuzpe A, Diez I, et al. . Structure-function multi-scale connectomics reveals a major role of the fronto-striato-thalamic circuit in brain aging. Hum Brain Mapp. 2018;39(12):4663–4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Damoiseaux JS. Effects of aging on functional and structural brain connectivity. Neuroimage. 2017;160:32–40. [DOI] [PubMed] [Google Scholar]
- 17. Fjell AM, Walhovd KB. Structural brain changes in aging: Courses, causes and cognitive consequences. Rev Neurosci. 2010;21(3):187–221. [DOI] [PubMed] [Google Scholar]
- 18. Fjell AM, Westlye LT, Grydeland H, et al. . Accelerating cortical thinning: Unique to dementia or universal in aging? Cereb Cortex. 2014;24(4):919–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fotenos AF, Snyder AZ, Girton LE, Morris JC, Buckner RL. Normative estimates of cross-sectional and longitudinal brain volume decline in aging and AD. Neurology. 2005;64(6):1032–1039. [DOI] [PubMed] [Google Scholar]
- 20. Grajauskas LA, Siu W, Medvedev G, Guo H, D’Arcy RCN, Song X. MRI-based evaluation of structural degeneration in the ageing brain: Pathophysiology and assessment. Ageing Res Rev. 2019;49:67–82. [DOI] [PubMed] [Google Scholar]
- 21. Gunning-Dixon FM, Brickman AM, Cheng JC, Alexopoulos GS. Aging of cerebral white matter: A review of MRI findings. Int J Geriatr Psychiatry. 2009;24(2):109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Storsve AB, Fjell AM, Tamnes CK, et al. . Differential longitudinal changes in cortical thickness, surface area and volume across the adult life span: Regions of accelerating and decelerating change. J Neurosci. 2014;34(25):8488–8498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jonsson BA, Bjornsdottir G, Thorgeirsson TE, et al. . Brain age prediction using deep learning uncovers associated sequence variants. Nat Commun. 2019;10(1):5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cole JH, Ritchie SJ, Bastin ME, et al. . Brain age predicts mortality. Mol Psychiatry. 2018;23(5):1385–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smith SM, Vidaurre D, Alfaro-Almagro F, Nichols TE, Miller KL. Estimation of brain age delta from brain imaging. Neuroimage. 2019;200:528–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Elliott ML, Belsky DW, Knodt AR, et al. . Brain-age in midlife is associated with accelerated biological aging and cognitive decline in a longitudinal birth cohort. Mol Psychiatry. 2021;26(8):3829–3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cole JH, Leech R, Sharp DJ. Prediction of brain age suggests accelerated atrophy after traumatic brain injury. Ann Neurol. 2015;77(4):571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smith AE, Wade AT, Olds TS, et al. . Characterising activity and diet compositions for dementia prevention: Protocol for the ACTIVate prospective longitudinal cohort study. BMJ Open. 2022;12:e047888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brett M, Leff AP, Rorden C, Ashburner J. Spatial normalization of brain images with focal lesions using cost function masking. Neuroimage. 2001;14(2):486–500. [DOI] [PubMed] [Google Scholar]
- 30. Nachev P, Coulthard E, Jäger HR, Kennard C, Husain M. Enantiomorphic normalization of focally lesioned brains. Neuroimage. 2008;39(3):1215–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kristinsson S, Yourganov G, Xiao F, et al. . Brain-derived neurotrophic factor genotype-specific differences in cortical activation in chronic aphasia. J Speech Lang Hear Res. 2019;62(11):3923–3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kristinsson S, Zhang W, Rorden C, et al. . Machine learning-based multimodal prediction of language outcomes in chronic aphasia. Hum Brain Mapp. 2021;42(6):1682–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marebwa BK, Fridriksson J, Yourganov G, et al. . Chronic post-stroke aphasia severity is determined by fragmentation of residual white matter networks. Sci Rep. 2017;7:8188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yourganov G, Fridriksson J, Rorden C, Gleichgerrcht E, Bonilha L. Multivariate connectome-based symptom mapping in post-stroke patients: Networks supporting language and speech. J Neurosci. 2016;36(25):6668–6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moura LM, Luccas R, de Paiva JPQ, et al. . Diffusion tensor imaging biomarkers to predict motor outcomes in stroke: A narrative review. Front Neurol. 2019;10:445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ptak R, Bourgeois A, Cavelti S, Doganci N, Schnider A, Iannotti GR. Discrete patterns of cross-hemispheric functional connectivity underlie impairments of spatial cognition after stroke. J Neurosci. 2020;40(34):6638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Salvalaggio A, De Filippo De Grazia M, Zorzi M, Thiebaut de Schotten M, Corbetta M. Post-stroke deficit prediction from lesion and indirect structural and functional disconnection. Brain. 2020;143(7):2173–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dubois B, Chupin M, Hampel H, et al. . Donepezil decreases annual rate of hippocampal atrophy in suspected prodromal Alzheimer’s disease. Alzheimers Dement. 2015;11(9):1041–1049. [DOI] [PubMed] [Google Scholar]
- 39. Johnson L, Werden E, Shirbin C, et al. . The post ischaemic stroke cardiovascular exercise study: Protocol for a randomised controlled trial of fitness training for brain health. Eur Stroke J. 2018;3(4):379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Langeskov-Christensen M, Grøndahl Hvid L, Nygaard MKE, et al. . Efficacy of high-intensity aerobic exercise on brain MRI measures in multiple sclerosis. Neurology. 2021;96(2):e203–e213. [DOI] [PubMed] [Google Scholar]
- 41. Egorova N, Liem F, Hachinski V, Brodtmann A. Predicted brain age after stroke. Front Aging Neurosci. 2019;11:348–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Richard G, Kolskår K, Ulrichsen KM, et al. . Brain age prediction in stroke patients: Highly reliable but limited sensitivity to cognitive performance and response to cognitive training. Neuroimage Clin. 2020;25:102159–102159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. de Lange AMG, Anatürk M, Suri S, et al. . Multimodal brain-age prediction and cardiovascular risk: The Whitehall II MRI sub-study. NeuroImage. 2020;222:117292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Franke K, Gaser C. Ten years of BrainAGE as a neuroimaging biomarker of brain aging: What insights have we gained? Front Neurol. 2019;10:789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bretzner M, Bonkhoff A, Schirmer M, et al. . Radiomics derived brain age predicts functional outcome after acute ischemic stroke [preprint] 2021. 10.21203/rs.3.rs-923769/v1 [DOI]
- 46. Brodtmann A, Werden E, Khlif MS, et al. . Neurodegeneration over 3 years following ischaemic stroke: Findings from the cognition and neocortical volume after stroke study. Front Neurol. 2021;12:754204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Veldsman M, Cheng HJ, Ji F, et al. . Degeneration of structural brain networks is associated with cognitive decline after ischaemic stroke. Brain Commun. 2020;2(2):fcaa155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Veldsman M, Werden E, Egorova N, Khlif MS, Brodtmann A. Microstructural degeneration and cerebrovascular risk burden underlying executive dysfunction after stroke. Sci Rep. 2020;10(1):17911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Magnusdottir S, Fillmore P, den Ouden DB, et al. . Damage to left anterior temporal cortex predicts impairment of complex syntactic processing: A lesion-symptom mapping study. Hum Brain Mapp. 2013; 34(10):2715–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kristinsson S, Thors H, Yourganov G, et al. . Brain damage associated with impaired sentence processing in acute aphasia. J Cogn Neurosci. 2020;32(2):256–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fitch-West J, Sands ES, Ross-Swain D. Bedside Evaluation Screening Test–Second Edition (BEST-2).: Pro-Ed; 1998. [Google Scholar]
- 52. Kertesz A. Western Aphasia Battery–Revised (WAB-R). Pearson; 2007. [Google Scholar]
- 53. Wallace SJ, Worrall L, Rose T, et al. . A core outcome set for aphasia treatment research: The ROMA consensus statement. Int J Stroke. 2019;14(2):180–185. [DOI] [PubMed] [Google Scholar]
- 54. Fridriksson J, Holland AL, Coull BM, Plante E, Trouard TP, Beeson P. Aphasia severity: Association with cerebral perfusion and diffusion. Aphasiology. 2002;16(9):859–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li X, Morgan PS, Ashburner J, Smith J, Rorden C. The first step for neuroimaging data analysis: DICOM to NIfTI conversion. J Neurosci Methods. 2016;264:47–56. [DOI] [PubMed] [Google Scholar]
- 56. Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12(4):191–200. [DOI] [PubMed] [Google Scholar]
- 57. Rorden C, Bonilha L, Fridriksson J, Bender B, Karnath HO. Age-specific CT and MRI templates for spatial normalization. Neuroimage. 2012;61(4):957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26(3):839–851. [DOI] [PubMed] [Google Scholar]
- 59. IBM Corp . IBM SPSS Statistics for Windows, Version 28.0.: IBM Corp. 2021. [Google Scholar]
- 60. Sibille E. Molecular aging of the brain, neuroplasticity, and vulnerability to depression and other brain-related disorders. Dialogues Clin Neurosci. 2013;15(1):53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Del Maschio N, Sulpizio S, Gallo F, Fedeli D, Weekes BS, Abutalebi J. Neuroplasticity across the lifespan and aging effects in bilinguals and monolinguals. Brain Cogn. 2018;125:118–126. [DOI] [PubMed] [Google Scholar]
- 62. Bishop NA, Lu T, Yankner BA. Neural mechanisms of ageing and cognitive decline. Nature. 2010;464(7288):529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Deary IJ, Corley J, Gow AJ, et al. . Age-associated cognitive decline. Br Med Bull. 2009;92:135–152. [DOI] [PubMed] [Google Scholar]
- 64. Shafto MA, Tyler LK. Language in the aging brain: The network dynamics of cognitive decline and preservation. Science. 2014;346(6209):583–587. [DOI] [PubMed] [Google Scholar]
- 65. Etherton MR, Wu O, Rost NS. Recent advances in leukoaraiosis: White matter structural integrity and functional outcomes after acute ischemic stroke. Curr Cardiol Rep. 2016;18(12):123. [DOI] [PubMed] [Google Scholar]
- 66. van Meer MPA, Otte WM, van der Marel K, et al. . Extent of bilateral neuronal network reorganization and functional recovery in relation to stroke severity. J Neurosci. 2012;32(13):4495–4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1 Pt 1):21–36. [DOI] [PubMed] [Google Scholar]
- 68. Fridriksson J, Hillis AE. Current approaches to the treatment of post-stroke aphasia. J Stroke. 2021;23(2):183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kirkwood TBL. Understanding the odd science of aging. Cell. 2005;120(4):437–447. [DOI] [PubMed] [Google Scholar]
- 70. Kirkwood TBL. A systematic look at an old problem. Nature. 2008;451(7179):644–647. [DOI] [PubMed] [Google Scholar]
- 71. Cherbuin N, Walsh EI, Shaw M, et al. . Optimal blood pressure keeps our brains younger. Front Aging Neurosci. 2021;13:694982–694982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cox S, Lyall D, Ritchie S, et al. . Associations between vascular risk factors and brain MRI indices in UK biobank. Eur Heart J. 2019;40:2290–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Launer LJ, Lewis CE, Schreiner PJ, et al. . Vascular factors and multiple measures of early brain health: CARDIA brain MRI study. PLoS One. 2015;10(3):e0122138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gaser C, Franke K, Klöppel S, Koutsouleris N, Sauer H. BrainAGE in mild cognitive impaired patients: Predicting the conversion to Alzheimer’s disease. PLoS One. 2013;8(6):e67346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pardoe HR, Cole JH, Blackmon K, Thesen T, Kuzniecky R. Structural brain changes in medically refractory focal epilepsy resemble premature brain aging. Epilepsy Res. 2017;133:28–32. [DOI] [PubMed] [Google Scholar]
- 76. Schnack HG, van Haren NEM, Nieuwenhuis M, Hulshoff Pol HE, Cahn W, Kahn RS. Accelerated brain aging in schizophrenia: A longitudinal pattern recognition study. Am J Psychiatry. 2016;173(6):607–616. [DOI] [PubMed] [Google Scholar]
- 77. Schlaug G, Marchina S, Norton A. Evidence for plasticity in white-matter tracts of patients with chronic Broca’s aphasia undergoing intense intonation-based speech therapy. Ann N Y Acad Sci. 2009;1169:385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bonilha L, Gleichgerrcht E, Nesland T, Rorden C, Fridriksson J. Success of anomia treatment in aphasia is associated with preserved architecture of global and left temporal lobe structural networks. Neurorehabil Neural Repair. 2016;30(3):266–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cheng BBY, Worrall LE, Copland DA, Wallace SJ. Prognostication in post-stroke aphasia: How do speech pathologists formulate and deliver information about recovery? Int J Lang Commun Disord. 2020;55(4):520–536. [DOI] [PubMed] [Google Scholar]
- 80. Makin SD, Doubal FN, Shuler K, et al. . The impact of early-life intelligence quotient on post stroke cognitive impairment. Eur Stroke J. 2018;3(2):145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Harrison SL, Sajjad A, Bramer WM, Ikram MA, Tiemeier H, Stephan BCM. Exploring strategies to operationalize cognitive reserve: A systematic review of reviews. J Clin Exp Neuropsychol. 2015;37(3):253–264. [DOI] [PubMed] [Google Scholar]
- 82. Jack CRJ, Lowe VJ, Weigand SD, et al. . Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: Implications for sequence of pathological events in Alzheimer’s disease. Brain. 2009;132(Pt 5):1355–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jack CRJ, Knopman DS, Jagust WJ, et al. . Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wilson SM, Schneck SM. Neuroplasticity in post-stroke aphasia: A systematic review and meta-analysis of functional imaging studies of reorganization of language processing. Neurobiol Lang (Camb). 2021;2(1):22–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lorca-Puls DL, Gajardo-Vidal A, White J, et al. . The impact of sample size on the reproducibility of voxel-based lesion-deficit mappings. Neuropsychologia. 2018;115:101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Baecker L, Garcia-Dias R, Vieira S, Scarpazza C, Mechelli A. Machine learning for brain age prediction: Introduction to methods and clinical applications. EBioMedicine. 2021;72:103600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Levi F, Lucchini F, Negri E, La Vecchia C. Trends in mortality from cardiovascular and cerebrovascular diseases in Europe and other areas of the world. Heart. 2002;88(2):119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gustafsdottir SS, Fenger K, Halldorsdottir S, Bjarnason T. Social justice, access and quality of healthcare in an age of austerity: Users’ perspective from rural Iceland. Int J Circumpolar Health. 2017;76(1):1347476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Laska AC, Hellblom A, Murray V, Kahan T, Von Arbin M. Aphasia in acute stroke and relation to outcome. J Intern Med. 2001;249(5):413–422. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data presented in this study is not publicly available at present. However, de-identifiable participant data is available from the primary author upon reasonable request.