Keywords: adhesion, integrin, intracellular signaling, lymphocyte, migration
Abstract
Intestinal tissue-resident lymphocytes are critical for maintenance of the mucosal barrier and to prevent enteric infections. The activation of these lymphocytes must be tightly regulated to prevent aberrant inflammation and epithelial damage observed in autoimmune diseases, yet also ensure that antimicrobial host defense remains uncompromised. Tissue-resident lymphocytes express CD103, or αE integrin, which dimerizes with the β7 subunit to bind to E-cadherin expressed on epithelial cells. Although the role of CD103 in homing and retention of lymphocytes to and within peripheral tissues has been well characterized, the molecular signals activated following CD103 engagement remain understudied. Here, we highlight recent studies that elucidate the functional contribution of CD103 in various lymphocyte subpopulations, either as an independent signaling molecule or in the context of TCR co-stimulation. Finally, we will discuss the gaps in our understanding of CD103 biology and the therapeutic potential of targeting CD103 on tissue-resident lymphocytes.
INTRODUCTION
Tissue-resident lymphocytes are essential to provide immunosurveillance and contribute to the maintenance of the intestinal mucosal barrier. In the gut, a single layer of epithelial cell lines the intestinal tract to provide a physical barrier between microbes, dietary antigens, and the underlying mucosa. Within the epithelium resides a subset of lymphocytes referred to as intraepithelial lymphocytes (IELs), which contribute to the first line of defense against invasive microorganisms. Induced IELs are recruited from the periphery after antigen exposure; these IELs express T cell receptor (TCR) αβ and either CD8αβ or CD4 (1). CD8αβ TCRαβ IELs are largely tissue-resident memory (Trm) lymphocytes. In contrast, natural IELs including those that express CD8αα TCRαβ and TCRγδ are considered to be major histocompatibility complex (MHC)-independent and exhibit innate-like functions. Finally, a small fraction of CD4 CD8αα IELs are regulatory T cells (Tregs) that upregulate CD8αα expression following downregulation of ThPOK (2). Regardless of the subset, activation of these tissue-resident IELs must be tightly regulated to prevent aberrant inflammation and/or damage to the epithelium, as observed in inflammatory bowel disease (IBD) and celiac disease (3–5). However, IELs also function to limit microbial infection and lyse infected enterocytes as a key component of mucosal host defense (6).
αE (ITGAE) integrin, or CD103, dimerizes exclusively with β7 (ITGB7) and is expressed on the majority of tissue-resident lymphocytes. To date, the only known ligand for CD103 is E-cadherin (E-cad) (7, 8), an adherens junction protein expressed by epithelial cells. CD103 is largely implicated for its role in the homing and retention of lymphocytes in peripheral tissues (9–13) with less focus on the biological contribution of CD103 ligation beyond enhancing antigen-mediated killing by CD8 T cells. In this review, we will highlight recent findings regarding the molecular mechanisms by which engagement of CD103 leads to independent or co-stimulatory cellular responses, the known signaling pathways activated downstream of CD103/E-cad binding, and how these signals impact lymphocyte function in the context of gastrointestinal disease. Finally, we will define remaining gaps in knowledge regarding CD103 biology that may inform the development of therapeutics targeting CD103/E-cad interactions to improve clinical outcomes in the context of gastrointestinal infection, inflammation, and cancer.
Regulation of CD103 Expression
As mentioned in the INTRODUCTION, CD103 is widely used as a marker to identify tissue-resident immune cells in various organs such as the skin, lungs, and gut (14). CD103 expression is induced on peripheral lymphocytes as a result of local transforming growth factor (TGF)-β production within the tissue microenvironment (15, 16). The role of TGF-β in promoting CD103 expression has been demonstrated in multiple studies: 1) overexpression of TGF-β by tumor cells results in higher CD103 expression in tumor-infiltrating lymphocytes (TILs), 2) T cells with impaired TGF-β type II receptor signaling fail to upregulate CD103 upon migration into the tissue (9, 13, 15, 17), 3) TGF-β is sufficient to induce CD103 in cultured human leukocytes in vitro, and 4) T cell activation in conjunction with TGF-β results in CD103 expression by cytotoxic T lymphocytes (CTL) (9, 15, 18–20).
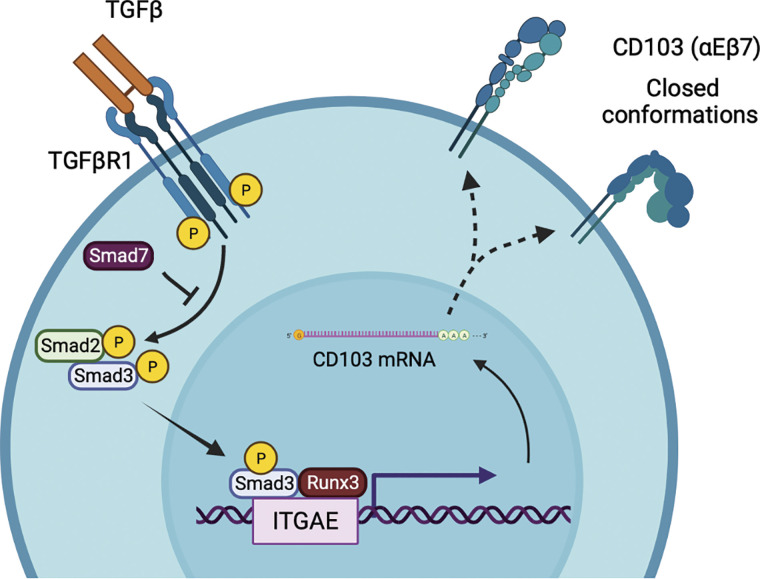
TGF-β ligation to its receptor results in the phosphorylation of Smad2/3, after which phosphorylated Smad3 binds to the promoter region of CD103 in CD8 T cells (21). Since Smad7 interferes with the recruitment and phosphorylation of Smad2/3, transgenic mice overexpressing Smad7 exhibit defective TGF-β signaling and reduced expression of CD103 on IELs (22). In addition to TGF-β, the transcription factor Runx3 was shown to regulate CD103 expression on CD8 single-positive thymocytes and dendritic epidermal T cells (23, 24). Runx3 also plays an important role in the differentiation of CD103-expressing Trm populations (25) by allowing chromatin accessibility within Itgae and other TGF-β regulated genes (26). In addition, T-bet and Tcf1 can directly bind Itgae regulatory regions and inhibit TGF-β-mediated CD103 expression (27, 28). Together, these studies demonstrate that TGF-β-Smad3 signaling and the expression of Runx3 are critical for the induction of CD103 expression on lymphocytes (Fig. 1).
Figure 1.
TGF-β signaling and Runx3 regulate CD103 expression. Activation of TGF-βR1 promotes the recruitment and phosphorylation of Smad2/3. Phosphorylated Smad3 and Runx3 bind to the CD103 promoter to induce transcription. Smad7 negatively regulates Smad2/3 signaling and can inhibit CD103 expression. TGF-β, transforming growth factor β. Image created with BioRender and published with permission.
Bidirectional CD103 Signaling
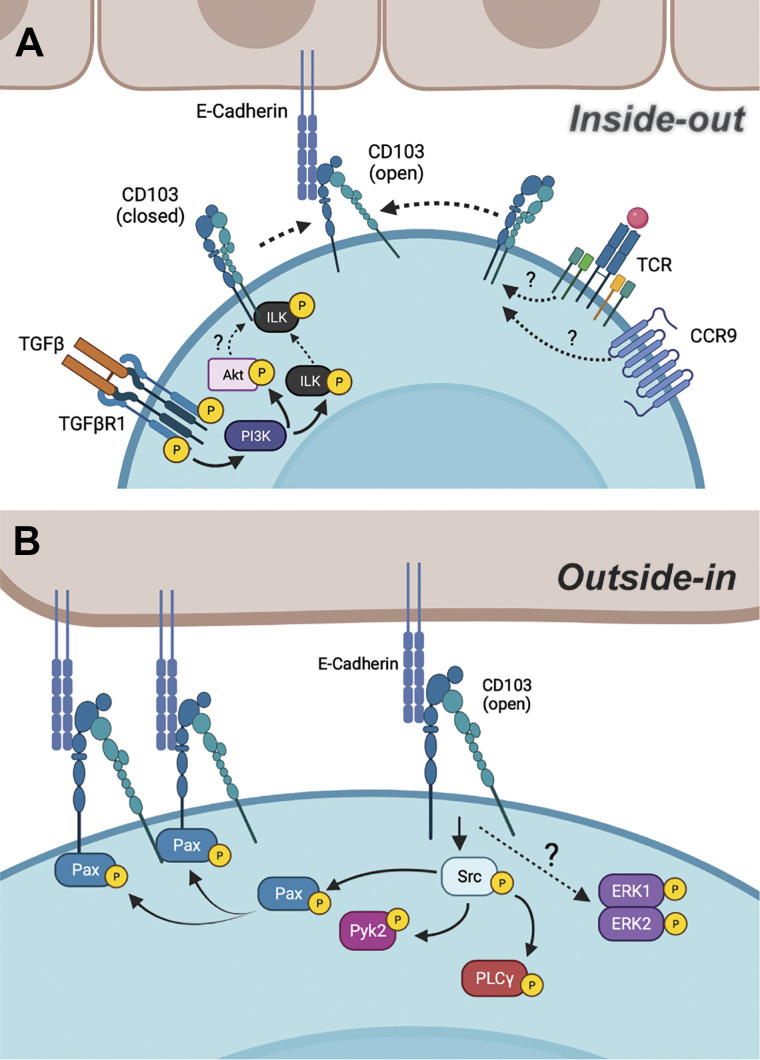
Activation of integrins occurs through two distinct signaling mechanisms: 1) “inside-out” in which intracellular signals downstream of GPCR or TCR signaling promote talin binding to the cytoplasmic tail of the β subunit of integrin, which results in an conformational change to expose the ligand binding region of the extracellular domain, or 2) “outside-in” signaling that is initiated following a high-affinity interaction between the integrin extracellular domain and its binding partner or cations (29). Current evidence suggests that CD103 activation can occur through both mechanisms. Activation of the TCR, CC motif chemokine receptor 9 (CCR9), and TGF-βR1 induces “inside-out” signaling and increases the avidity of the binding between CD103 and E-cad (30–32). Although the molecular mechanisms associated with TCR- or CCR9-mediated CD103 activation remain to be elucidated, signaling through TGF-βR1 promotes PI3K-mediated phosphorylation of integrin-linked kinase (ILK), which subsequently binds to the intracellular tail of CD103 to promote “inside-out” activation (32) (Fig. 2A). Phosphorylation of Akt downstream of TGF-βR1/ILK signaling also contributes to CD103 binding to E-cad; however, the molecular mechanisms by which Akt promotes the confirmational change of CD103 remains unclear. In this study, the authors posit that TGF-β signaling through ILK and Akt strengthened the interaction between CD103/E-cad, which in turn induces “outside-in” signaling to promote CTL adhesion, migration, and cytolytic capacity.
Figure 2.
Bidirectional signaling leading to CD103 activation. A: “inside-out” signaling of CD103 and its known molecular mechanisms, leading to the confirmational change of CD103. B: “outside-in” signaling of CD103 and its known molecular mechanisms, leading to cellular functions. Image created with BioRender and published with permission.
“Outside-in” signaling occurs following high-affinity binding of an integrin to its ligand and mediates cellular responses such as spreading, proliferation, and effector functions through phosphorylation of molecules and/or binding to extracellular matrix. CD103 binding to immobilized recombinant E-cad induced “outside-in” signaling to promote the phosphorylation of the proline-rich tyrosine kinase 2 (Pyk2) and the focal adhesion-associated adaptor protein, paxillin in a Src kinase-dependent manner (33). As a result, phosphorylated paxillin binds to the intracellular tail of clustered CD103, thus enhancing the migratory and effector functions of CD8 T cells. Although TCR coengagement results in CTL degranulation for targeted cell lysis, which may be suggestive of “inside-out” signaling, a later study demonstrated that E-cad engagement alone was sufficient to promote extracellular signaling regulated kinase (ERK) and phospholipase C γ 1 (PLCγ) phosphorylation, resulting in the polarization of cytolytic granules (34). These studies demonstrate that CD103 can be activated via bidirectional signaling, and thus may induce differential downstream signaling pathways in lymphocytes depending on whether CD103 is activated in conjunction with, or independently of, TCR activation (Fig. 2B).
CD103: Implication of Recruitment and Retention
Based on observations that deletion of CD103 reduces the number of tissue-resident lymphocytes in vivo (9–12), the interaction between CD103 and E-cad is implicated in the selective recruitment of lymphocytes to epithelial barriers and anchoring of lymphocytes to the epithelium. However, the requirement for CD103 in tissue homing was challenged when Austrup and colleagues (35) showed that in vitro cultured CD103+ T cells failed to migrate to the gut and other peripheral organs. This suggested that the observed reduction in the number of tissue-resident cells is likely due to impaired retention, and this is supported by infection studies that allow tracking of antigen-specific T cell dynamics. CD103 is not expressed on circulating effector lymphocyte populations or required for T cell trafficking into the tissue, but CD103-deficient Trm populations in the skin, brain, and intestinal epithelium are gradually lost after infection is resolved, indicating a role for CD103 in retention/maintenance of tissue-resident lymphocytes (9, 13, 36). However, this is not absolute, as the intestinal lamina propria contains CD103+ Trm cells that do not require CD103 for their retention within the tissue (9, 37). In addition, many tissues like the liver, salivary gland, and lamina propria contain bona fide Trm cells that are CD103− (38, 39). Together, these studies demonstrate that the expression of CD103 is not essential for the trafficking of lymphocytes to the gut and other tissues, and is likely not a definitive marker of all tissue residents as CD103− Trms are found in various tissues. This highlights the need to determine the functional significance suggesting that CD103, as these data suggest it could be an essential molecule for other cellular functions and not necessarily crucial for immune cells to exist in the tissue environment.
CD103 Functions Both in a Co-Stimulatory Manner and Independently to Promote Lymphocyte Function
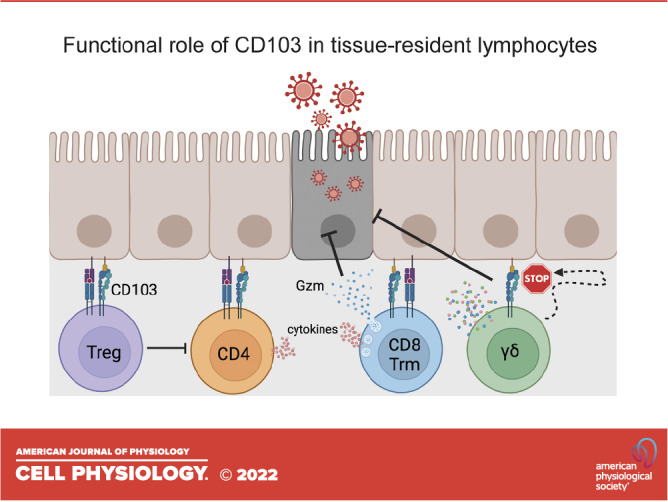
The molecular mechanisms by which CD103/E-cad engagement promotes the cross talk between epithelial cells and lymphocytes remain largely understudied. This is particularly relevant in the context of antigen-specific responses as well as those that are MHC-independent. Regarding antigen-dependent activation, the involvement of CD103 in enhancing CD8 T cell cytolytic capacity is well documented (15, 34, 40). Ligation of CD103 by E-cad leads to the polarization of lytic granules and lowers the activation threshold for the local polarized release of granzymes, resulting in more efficient killing of E-cad-bearing target cells (Table 1) (34, 52). However, CD103 is also involved in enhancing the function of other lymphocyte populations. For example, CD103+ Tregs exhibit more potent suppressive capacity compared with those not expressing CD103, and are critical in limiting allergic contact hypersensitivity (43), colitis (44), and antigen-induced arthritis in animal models (45). Furthermore, coculture of CD103+ CD4+ T cells isolated from the gastric mucosa with H. pylori-primed dendritic cells promotes lymphocyte proliferation and proinflammatory cytokine production that is abrogated by antibody-mediated blockade of CD103 (46). The engagement of CD103+ CD8+ thymocytes with E-cad-expressed primary thymic epithelial cells leads to enhanced proliferation; however, E-cad binding to CD103 alone is insufficient to induce proliferation indicating the necessity for a second signal (47). Taken together, it is clear that CD103 acts as a co-stimulatory molecule in several lymphocyte subsets to enhance proliferation and effector function.
Table 1.
Functional contribution of CD103+ tissue-resident lymphocytes. Engagement of CD103 with E-cad on CD103+ lymphocytes, with or without TCR co-stimulation
| Lymphocyte | Cellular Function | Tissue | Requirement for TCR Co-Stimulation | References |
|---|---|---|---|---|
| CD8 | Enhanced cytotoxicity | Tumors and grafts | Yes | (15, 41, 42) |
| Regulatory T cell (Treg) | Enhanced suppression | Skin, colon, and tumor | Unknown | (18, 43–45) |
| CD4 | Enhanced proliferation and Th1/17 cytokine production | Gastric mucosa | Yes | (46) |
| Thymocytes | Enhanced proliferation | Thymus | Unknown | (47) |
| γδ T cells | Facilitating apoptotic enterocyte shedding via degranulation-independent granzyme release | Villous epithelium | No | (48) |
| Regulating duration of IEL/epithelial contact | Villous epithelium and small intestinal tumors | No | (49–51) |
E-cad, E-cadherin; TCR, T cell receptor.
In contrast, natural IELs expressing the γδ TCR display antigen-independent functionality and uniformly express CD103. We have shown that γδ IELs migrate dynamically along the basement membrane to provide continuous surveillance of the villous epithelium (49). Although CD103 engagement influences lymphocyte shape and motility by promoting actin remodeling (53), we found that CD103-deficient γδ IELs exhibit enhanced migratory speed based on reduced retention within the lateral intercellular space (LIS) between adjacent epithelial cells (49). This suggests that CD103/E-cadherin binding is important for regulating the duration of IEL/epithelial contact (Table 1). As a result of the enhanced γδ IEL surveillance behavior in CD103-deficient mice, the frequency of acute Salmonella typhimurium translocation across the epithelium is reduced (50). Furthermore, administration of anti-γδ TCR antibody has no effect on γδ IEL migratory behavior in response to enteric infection (54) and suggests that CD103 signaling in this capacity does not require TCR activation (Table 1). More recently, we demonstrated that CD103 is required for prolonged interactions between γδ IELs and shedding apoptotic enterocytes both at steady state and in response to TNF exposure (48). Whereas TCR activation is required for γδ IEL degranulation, CD103 engagement by E-cad alone is sufficient to induce the extracellular secretion of granzyme (Gzm) A and GzmB. We found that γδ IEL CD103-mediated Gzm release is required for these lymphocytes to facilitate cell shedding. These observations highlight that CD103 can function independently of TCR activation, which is in line with the more “innate-like” function of these sentinel lymphocytes. It remains to be determined whether CD103 ligation also acts as a co-stimulatory molecule to amplify adaptive γδ IEL responses similar to conventional CD8 T cells. However, many of the downstream molecular pathways that regulate these lymphocyte responses following CD103 engagement, whether TCR-dependent or independent, remain to be elucidated.
CD103 in Gastrointestinal Disease
Intestinal host defense involves the coordinated response of both natural and induced IELs, as the speed and potency of this response are critical to prevent disruption of the epithelial barrier and unchecked pathogen replication and dissemination. γδ IELs are activated early after infection, and alterations in their surveillance behavior are observed within minutes to hours following Salmonella or Toxoplasma encounter, respectively (50, 54). Increased migration into the LIS following the loss of CD103 expression likely allows γδ IELs to elicit a highly localized and conserved antimicrobial response to limit pathogen translocation (50, 55). Trm cells can control pathogen replication in the intestinal tissue (38, 56), but the mechanism and role of CD103 in this process are less well defined. However, Trm cells in other tissues can undergo bystander activation by innate cytokines to support pathogen control (57). Subsequent processing and presentation of pathogen-derived antigens lead to the activation of Trm IELs to potentially eliminate infected enterocytes and produce cytokines that stimulate activation and recruitment of additional immune cells (58, 59).
Obesity can promote epithelial barrier dysfunction and low levels of chronic inflammation (60, 61). Feeding of a high-fat diet to young mice results in the downregulation of CD103 on CD4 and γδ IELs leading to a reduction in the persistence of IELs within the mucosa; however, IEL number and CD103 expression are restored following diet-induced weight loss (62). Although this study focuses on the role of CD103 in the retention or maintenance of these tissue-resident populations, these findings suggest that obesity can indirectly regulate CD103 expression and may have additional implications for tissue-resident lymphocyte function.
IBD, which includes both ulcerative colitis (UC) and Crohn’s disease (CD), is a multifactorial disease associated with relapsing and remitting intestinal inflammation. In the T cell transfer model of colitis, CD103 is not required for homing of lymphocytes to the intestinal lamina propria and the transfer of either WT or CD103-deficient effector T cells results in equally severe disease (63). In contrast, the identification of CD8+ CD103+ Tregs revealed that these cells exhibit the capacity to attenuate ileitis and suppress T-cell-mediated colitis (64, 65). Although γδ T cells are typically considered to be protective in the context of IBD (3), Do and colleagues identified a peripheral population of CD103+ T cells that are proinflammatory and can exacerbate colitis (66). Genetic or antibody-mediated inhibition of β7 integrin ameliorates colitis (67, 68); however, specific targeting of β7 integrin will affect both α4β7 and αEβ7 (CD103) integrins. While vedolizumab, a humanized monoclonal antibody that selectively targets α4β7, is efficacious in the treatment of moderate to severe UC and CD (69), etrolizumab, a humanized monoclonal antibody against β7 integrin, appeared to be promising in phase 2 clinical trial (70); but failed to yield consistent results in a phase 3 trial (71). It was thought that inhibiting both integrins would reduce intestinal inflammation by inhibiting lymphocyte trafficking to the gut, yet it is possible that blocking CD103 may be a double-edged sword and ultimately limit the protective functions of tissue-resident IELs, Trms, and Tregs.
In solid tumors, CD103 expression on CD8 TILs results in a favorable prognosis and increased overall survival (41). CD103 expression is thought to retain CTLs within the tumor epithelium (19), and a reduction of colonic CD103+ γδ IELs or Trm IELs is observed in patients with familial adenomatous polyposis (FAP) (72). Moreover, CD103 expression was also reduced in lamina propria T cells in patients with FAP. Deletion of CD103 on lymphocytes was recently shown to impair tumor immunosurveillance by reducing the frequency of IEL/epithelial interactions leading to an increase in small intestinal tumors in an APCmin model (51); however, this study did not directly address whether loss of CD103 affected cytolytic effector function. Furthermore, enrichment of TGF-β in the tumor microenvironment induces CD103 expression on tumor-infiltrating Tregs (18). Thus, the enhanced suppressive nature of CD103+ Tregs (44) may lead to increased tumor burden and negative prognosis, as observed in patients with ovarian and breast cancer (73, 74). Together, these findings indicate a need to better understand the functional contribution of CD103 within distinct lymphocyte subsets in the context of infection, chronic intestinal inflammation, and cancer.
CONCLUDING REMARKS
In summary, CD103 contributes to several aspects of protective T cell-mediated immunity in the gut including antigen-dependent activation, surveillance behavior, and control of enteric infection. These studies clearly indicate that CD103 expression on various lymphocyte populations has additional functions beyond tissue homing and retention. Despite the multifunctional contribution of CD103 to tissue-resident lymphocyte biology, much remains unknown regarding how positive and/or negative regulation of signaling downstream of CD103 contributes to the modulation of lymphocyte effector function. For example, inside-out activation of integrins can promote the release of calcium from intracellular stores (75), which may contribute to changes in lymphocyte motility, cytolytic function, and effector cytokine production. Moreover, our knowledge regarding how CD103 signaling in gut-resident lymphocytes is affected in response to different diets, celiac disease, or aging, is extremely limited. Studies to date suggest that the functional role of CD103 may differ based on lymphocyte subset; therefore, investigating the molecular pathways activated by CD103 engagement, either in response to co-stimulation or independent of TCR signaling, will significantly advance our understanding of how these tissue-resident cells are regulated and activated under homeostatic and pathological conditions at barrier sites.
GRANTS
This work was supported by the Agency for Science, Technology and Research (A*STAR) postdoctoral fellowship (to W.X.); the National Institutes of Health (NIH) Grants R01 AI153096 and R21 AI148900 (to T.B.); and NIH R01 DK119349, R21 DK123488, and R21 AI171959 (to K.L.E.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.X. and K.L.E. prepared figures; W.X. drafted manuscript; W.X., T.B., and K.L.E. edited and revised manuscript; W.X., T.B., and K.L.E. approved final version of manuscript.
ACKNOWLEDGMENTS
Figures and Graphical abstract created with BioRender and published with permission.
REFERENCES
- 1. Cheroutre H, Lambolez F, Mucida D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat Rev Immunol 11: 445–456, 2011. doi: 10.1038/nri3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reis BS, Rogoz A, Costa-Pinto FA, Taniuchi I, Mucida D. Mutual expression of the transcription factors Runx3 and ThPOK regulates intestinal CD4+ T cell immunity. Nat Immunol 14: 271–280, 2013. doi: 10.1038/ni.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hu MD, Edelblum KL. Sentinels at the frontline: the role of intraepithelial lymphocytes in inflammatory bowel disease. Curr Pharmacol Rep 3: 321–334, 2017. doi: 10.1007/s40495-017-0105-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Konjar S, Ferreira C, Blankenhaus B, Veldhoen M. Intestinal barrier interactions with specialized CD8 T cells. Front Immunol 8: 1281, 2017. doi: 10.3389/fimmu.2017.01281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abadie V, Discepolo V, Jabri B. Intraepithelial lymphocytes in celiac disease immunopathology. Semin Immunopathol 34: 551–566, 2012. doi: 10.1007/s00281-012-0316-x. [DOI] [PubMed] [Google Scholar]
- 6. Hu MD, Jia L, Edelblum KL. Policing the intestinal epithelial barrier: innate immune functions of intraepithelial lymphocytes. Curr Pathobiol Rep 6: 35–46, 2018. doi: 10.1007/s40139-018-0157-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cepek KL, Shaw SK, Parker CM, Russell GJ, Morrow JS, Rimm DL, Brenner MB. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the αEβ7 integrin. Nature 372: 190–193, 1994. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- 8. Karecla PI, Bowden SJ, Green SJ, Kilshaw PJ. Recognition of E-cadherin on epithelial cells by the mucosal T cell integrin alpha M290 β7 (αEβ7). Eur J Immunol 25: 852–856, 1995. doi: 10.1002/eji.1830250333. [DOI] [PubMed] [Google Scholar]
- 9. Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK, Lucas PJ, Artis D, Wherry EJ, Hogquist K, Vezys V, Masopust D. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J Immunol 188: 4866–4875, 2012. doi: 10.4049/jimmunol.1200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schön MP, Arya A, Murphy EA, Adams CM, Strauch UG, Agace WW, Marsal J, Donohue JP, Her H, Beier DR, Olson S, Lefrancois L, Brenner MB, Grusby MJ, Parker CM. Mucosal T lymphocyte numbers are selectively reduced in integrin alpha E (CD103)-deficient mice. J Immunol 162: 6641–6649, 1999. [PubMed] [Google Scholar]
- 11. Schön MP, Schön M, Parker CM, Williams IR. Dendritic epidermal T cells (DETC) are diminished in integrin alphaE(CD103)-deficient mice. J Invest Dermatol 119: 190–193, 2002. doi: 10.1046/j.1523-1747.2002.17973.x. [DOI] [PubMed] [Google Scholar]
- 12. Chodaczek G, Papanna V, Zal MA, Zal T. Body-barrier surveillance by epidermal γδ TCRs. Nat Immunol 13: 272–282, 2012. doi: 10.1038/ni.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML, Vega-Ramos J, Lauzurica P, Mueller SN, Stefanovic T, Tscharke DC, Heath WR, Inouye M, Carbone FR, Gebhardt T. The developmental pathway for CD103+CD8+ tissue-resident memory T cells of skin. Nat Immunol 14: 1294–1301, 2013. doi: 10.1038/ni.2744. [DOI] [PubMed] [Google Scholar]
- 14. Szabo PA, Miron M, Farber DL. Location, location, location: tissue resident memory T cells in mice and humans. Sci Immunol 4: eaas9673, 2019. doi: 10.1126/sciimmunol.aas9673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. El-Asady R, Yuan R, Liu K, Wang D, Gress RE, Lucas PJ, Drachenberg CB, Hadley GA. TGF-β-dependent CD103 expression by CD8+ T cells promotes selective destruction of the host intestinal epithelium during graft-versus-host disease. J Exp Med 201: 1647–1657, 2005. doi: 10.1084/jem.20041044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qiu Z, Chu TH, Sheridan BS. TGF-β: many paths to CD103+ CD8 T cell residency. Cells 10: 989, 2021. doi: 10.3390/cells10050989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang N, Bevan MJ. Transforming growth factor-β signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity 39: 687–696, 2013. doi: 10.1016/j.immuni.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Anz D, Mueller W, Golic M, Kunz WG, Rapp M, Koelzer VH, Ellermeier J, Ellwart JW, Schnurr M, Bourquin C, Endres S. CD103 is a hallmark of tumor-infiltrating regulatory T cells. Int J Cancer 129: 2417–2426, 2011. doi: 10.1002/ijc.25902. [DOI] [PubMed] [Google Scholar]
- 19. Ling KL, Dulphy N, Bahl P, Salio M, Maskell K, Piris J, Warren BF, George BD, Mortensen NJ, Cerundolo V. Modulation of CD103 expression on human colon carcinoma-specific CTL. J Immunol 178: 2908–2915, 2007. doi: 10.4049/jimmunol.178.5.2908. [DOI] [PubMed] [Google Scholar]
- 20. Kilshaw PJ. Alpha E beta 7. Mol Pathol 52: 203–207, 1999. doi: 10.1136/mp.52.4.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mokrani M, Klibi J, Bluteau D, Bismuth G, Mami-Chouaib F. Smad and NFAT pathways cooperate to induce CD103 expression in human CD8 T lymphocytes. J Immunol 192: 2471–2479, 2014. doi: 10.4049/jimmunol.1302192. [DOI] [PubMed] [Google Scholar]
- 22. Suzuki R, Nakao A, Kanamaru Y, Okumura K, Ogawa H, Ra C. Localization of intestinal intraepithelial T lymphocytes involves regulation of αEβ7 expression by transforming growth factor-beta. Int Immunol 14: 339–345, 2002. doi: 10.1093/intimm/14.4.339. [DOI] [PubMed] [Google Scholar]
- 23. Grueter B, Petter M, Egawa T, Laule-Kilian K, Aldrian CJ, Wuerch A, Ludwig Y, Fukuyama H, Wardemann H, Waldschuetz R, Möröy T, Taniuchi I, Steimle V, Littman DR, Ehlers M. Runx3 regulates integrin αE/CD103 and CD4 expression during development of CD4-/CD8+ T cells. J Immunol 175: 1694–1705, 2005. [Erratum in J Immunol 175: 6238, 2005]. doi: 10.4049/jimmunol.175.3.1694. [DOI] [PubMed] [Google Scholar]
- 24. Woolf E, Brenner O, Goldenberg D, Levanon D, Groner Y. Runx3 regulates dendritic epidermal T cell development. Dev Biol 303: 703–714, 2007. doi: 10.1016/j.ydbio.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 25. Milner JJ, Toma C, Yu B, Zhang K, Omilusik K, Phan AT, Wang D, Getzler AJ, Nguyen T, Crotty S, Wang W, Pipkin ME, Goldrath AW. Runx3 programs CD8+ T cell residency in non-lymphoid tissues and tumours. Nature 552: 253–257, 2017. doi: 10.1038/nature24993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fonseca R, Burn TN, Gandolfo LC, Devi S, Park SL, Obers A, Evrard M, Christo SN, Buquicchio FA, Lareau CA, McDonald KM, Sandford SK, Zamudio NM, Zanluqui NG, Zaid A, Speed TP, Satpathy AT, Mueller SN, Carbone FR, Mackay LK. Runx3 drives a CD8+ T cell tissue residency program that is absent in CD4+ T cells. Nat Immunol 23: 1236–1245, 2022. doi: 10.1038/s41590-022-01273-4. [DOI] [PubMed] [Google Scholar]
- 27. Laidlaw BJ, Zhang N, Marshall HD, Staron MM, Guan T, Hu Y, Cauley LS, Craft J, Kaech SM. CD4+ T cell help guides formation of CD103+ lung-resident memory CD8+ T cells during influenza viral infection. Immunity 41: 633–645, 2014. doi: 10.1016/j.immuni.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu J, Madi A, Mieg A, Hotz-Wagenblatt A, Weisshaar N, Ma S, Mohr K, Schlimbach T, Hering M, Borgers H, Cui G. T cell Factor 1 suppresses CD103+ lung tissue-resident memory T cell development. Cell Rep 31: 107484, 2020. doi: 10.1016/j.celrep.2020.03.048. [DOI] [PubMed] [Google Scholar]
- 29. Takagi J, Petre BM, Walz T, Springer TA. Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell 110: 599–611, 2002. doi: 10.1016/s0092-8674(02)00935-2. [DOI] [PubMed] [Google Scholar]
- 30. Higgins JM, Mandlebrot DA, Shaw SK, Russell GJ, Murphy EA, Chen YT, Nelson WJ, Parker CM, Brenner MB. Direct and regulated interaction of integrin αEβ7 with E-cadherin. J Cell Biol 140: 197–210, 1998. doi: 10.1083/jcb.140.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ericsson A, Svensson M, Arya A, Agace WW. CCL25/CCR9 promotes the induction and function of CD103 on intestinal intraepithelial lymphocytes. Eur J Immunol 34: 2720–2729, 2004. doi: 10.1002/eji.200425125. [DOI] [PubMed] [Google Scholar]
- 32. Boutet M, Gauthier L, Leclerc M, Gros G, de Montpreville V, Théret N, Donnadieu E, Mami-Chouaib F. TGFβ signaling intersects with CD103 integrin signaling to promote T-lymphocyte accumulation and antitumor activity in the lung tumor microenvironment. Cancer Res 76: 1757–1769, 2016. doi: 10.1158/0008-5472.CAN-15-1545. [DOI] [PubMed] [Google Scholar]
- 33. Gauthier L, Corgnac S, Boutet M, Gros G, Validire P, Bismuth G, Mami-Chouaib F. Paxillin binding to the cytoplasmic domain of CD103 promotes cell adhesion and effector functions for CD8+ resident memory T cells in tumors. Cancer Res 77: 7072–7082, 2017. doi: 10.1158/0008-5472.CAN-17-1487. [DOI] [PubMed] [Google Scholar]
- 34. Le Floc'h A, Jalil A, Franciszkiewicz K, Validire P, Vergnon I, Mami-Chouaib F. Minimal engagement of CD103 on cytotoxic T lymphocytes with an E-cadherin-Fc molecule triggers lytic granule polarization via a phospholipase Cγ-dependent pathway. Cancer Res 71: 328–338, 2011. doi: 10.1158/0008-5472.CAN-10-2457. [DOI] [PubMed] [Google Scholar]
- 35. Austrup F, Rebstock S, Kilshaw PJ, Hamann A. Transforming growth factor-β1-induced expression of the mucosa-related integrin αE on lymphocytes is not associated with mucosa-specific homing. Eur J Immunol 25: 1487–1491, 1995. doi: 10.1002/eji.1830250602. [DOI] [PubMed] [Google Scholar]
- 36. Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci USA 107: 17872–17879, 2010. doi: 10.1073/pnas.1010201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sheridan BS, Pham QM, Lee YT, Cauley LS, Puddington L, Lefrançois L. Oral infection drives a distinct population of intestinal resident memory CD8+ T cells with enhanced protective function. Immunity 40: 747–757, 2014. doi: 10.1016/j.immuni.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bergsbaken T, Bevan MJ. Proinflammatory microenvironments within the intestine regulate the differentiation of tissue-resident CD8+ T cells responding to infection. Nat Immunol 16: 406–414, 2015. doi: 10.1038/ni.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Christo SN, Evrard M, Park SL, Gandolfo LC, Burn TN, Fonseca R, Newman DM, Alexandre YO, Collins N, Zamudio NM, Souza-Fonseca-Guimaraes F, Pellicci DG, Chisanga D, Shi W, Bartholin L, Belz GT, Huntington ND, Lucas A, Lucas M, Mueller SN, Heath WR, Ginhoux F, Speed TP, Carbone FR, Kallies A, Mackay LK. Discrete tissue microenvironments instruct diversity in resident memory T cell function and plasticity. Nat Immunol 22: 1140–1151, 2021. doi: 10.1038/s41590-021-01004-1. [DOI] [PubMed] [Google Scholar]
- 40. Franciszkiewicz K, Le Floc'h A, Boutet M, Vergnon I, Schmitt A, Mami-Chouaib F. CD103 or LFA-1 engagement at the immune synapse between cytotoxic T cells and tumor cells promotes maturation and regulates T-cell effector functions. Cancer Res 73: 617–628, 2013. doi: 10.1158/0008-5472.CAN-12-2569. [DOI] [PubMed] [Google Scholar]
- 41. Kim Y, Shin Y, Kang GH. Prognostic significance of CD103+ immune cells in solid tumor: a systemic review and meta-analysis. Sci Rep 9: 3808, 2019. doi: 10.1038/s41598-019-40527-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Smyth LJ, Kirby JA, Cunningham AC. Role of the mucosal integrin αE(CD103)β7 in tissue-restricted cytotoxicity. Clin Exp Immunol 149: 162–170, 2007. doi: 10.1111/j.1365-2249.2007.03385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Braun A, Dewert N, Brunnert F, Schnabel V, Hardenberg J-H, Richter B, Zachmann K, Cording S, Claßen A, Brans R, Hamann A, Huehn J, Schön MP. Integrin αE(CD103) is involved in regulatory T-cell function in allergic contact hypersensitivity. J Invest Dermatol 135: 2982–2991, 2015. doi: 10.1038/jid.2015.287. [DOI] [PubMed] [Google Scholar]
- 44. Lehmann J, Huehn J, de la Rosa M, Maszyna F, Kretschmer U, Krenn V, Brunner M, Scheffold A, Hamann A. Expression of the integrin αEβ7 identifies unique subsets of CD25+ as well as CD25- regulatory T cells. Proc Natl Acad Sci USA 99: 13031–13036, 2002. doi: 10.1073/pnas.192162899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Huehn J, Siegmund K, Lehmann JC, Siewert C, Haubold U, Feuerer M, Debes GF, Lauber J, Frey O, Przybylski GK, Niesner U, de la Rosa M, Schmidt CA, Bräuer R, Buer J, Scheffold A, Hamann A. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J Exp Med 199: 303–313, 2004. doi: 10.1084/jem.20031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen P, Ming S, Lao J, Li C, Wang H, Xiong L, Zhang S, Liang Z, Niu X, Deng S, Geng L, Wu M, Wu Y, Gong S. CD103 promotes the pro-inflammatory response of gastric resident CD4+ T cell in Helicobacter pylori-positive gastritis. Front Cell Infect Microbiol 10: 436, 2020. doi: 10.3389/fcimb.2020.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kutlesa S, Wessels JT, Speiser A, Steiert I, Müller CA, Klein G. E-cadherin-mediated interactions of thymic epithelial cells with CD103+ thymocytes lead to enhanced thymocyte cell proliferation. J Cell Sci 115: 4505–4515, 2002. doi: 10.1242/jcs.00142. [DOI] [PubMed] [Google Scholar]
- 48. Hu MD, Golovchenko NB, Burns GL, Nair PM, Kelly TJ 4th, Agos J, Irani MZ, Soh WS, Zeglinski MR, Lemenze A, Bonder EM, Sandrock I, Prinz I, Granville DJ, Keely S, Watson AJM, Edelblum KL. γδ Intraepithelial lymphocytes facilitate pathological epithelial cell shedding via CD103-mediated granzyme release. Gastroenterology 162: 877–889.e7, 2022. doi: 10.1053/j.gastro.2021.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Edelblum KL, Shen L, Weber CR, Marchiando AM, Clay BS, Wang Y, Prinz I, Malissen B, Sperling AI, Turner JR. Dynamic migration of γδ intraepithelial lymphocytes requires occludin. Proc Natl Acad Sci USA 109: 7097–7102, 2012. doi: 10.1073/pnas.1112519109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Edelblum KL, Singh G, Odenwald MA, Lingaraju A, El Bissati K, McLeod R, Sperling AI, Turner JR. γδ Intraepithelial lymphocyte migration limits transepithelial pathogen invasion and systemic disease in mice. Gastroenterology 148: 1417–1426, 2015. doi: 10.1053/j.gastro.2015.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Morikawa R, Nemoto Y, Yonemoto Y, Tanaka S, Takei Y, Oshima S, Nagaishi T, Tsuchiya K, Nozaki K, Mizutani T, Nakamura T, Watanabe M, Okamoto R. Intraepithelial lymphocytes suppress intestinal tumor growth by cell-to-cell contact via CD103/E-cadherin signal. Cell Mol Gastroenterol Hepatol 11: 1483–1503, 2021. doi: 10.1016/j.jcmgh.2021.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Le Floc'h A, Jalil A, Vergnon I, Le Maux Chansac B, Lazar V, Bismuth G, Chouaib S, Mami-Chouaib F. αEβ7 integrin interaction with E-cadherin promotes antitumor CTL activity by triggering lytic granule polarization and exocytosis. J Exp Med 204: 559–570, 2007. doi: 10.1084/jem.20061524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schlickum S, Sennefelder H, Friedrich M, Harms G, Lohse MJ, Kilshaw P, Schön MP. Integrin αE(CD103)β7 influences cellular shape and motility in a ligand-dependent fashion. Blood 112: 619–625, 2008. doi: 10.1182/blood-2008-01-134833. [DOI] [PubMed] [Google Scholar]
- 54. Hoytema van Konijnenburg DP, Reis BS, Pedicord VA, Farache J, Victora GD, Mucida D. Intestinal epithelial and intraepithelial T cell crosstalk mediates a dynamic response to infection. Cell 171: 783–794.e13, 2017. doi: 10.1016/j.cell.2017.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ismail AS, Severson KM, Vaishnava S, Behrendt CL, Yu X, Benjamin JL, Ruhn KA, Hou B, Defranco AL, Yarovinsky F, Hooper LV. γδ Intraepithelial lymphocytes are essential mediators of host-microbial homeostasis at the intestinal mucosal surface. Proc Natl Acad Sci USA 108: 8743–8748, 2011. doi: 10.1073/pnas.1019574108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tomov VT, Palko O, Lau CW, Pattekar A, Sun Y, Tacheva R, Bengsch B, Manne S, Cosma GL, Eisenlohr LC, Nice TJ, Virgin HW, Wherry EJ. Differentiation and protective capacity of virus-specific CD8(+) t cells suggest murine norovirus persistence in an immune-privileged enteric niche. Immunity 47: 723–738.e5, 2017. doi: 10.1016/j.immuni.2017.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ge C, Monk IR, Pizzolla A, Wang N, Bedford JG, Stinear TP, Westall GP, Wakim LM. Bystander activation of pulmonary Trm cells attenuates the severity of bacterial pneumonia by enhancing neutrophil recruitment. Cell Rep 29: 4236–4244.e3, 2019. doi: 10.1016/j.celrep.2019.11.103. [DOI] [PubMed] [Google Scholar]
- 58. Schenkel JM, Fraser KA, Beura LK, Pauken KE, Vezys V, Masopust D. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science 346: 98–101, 2014. doi: 10.1126/science.1254536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Han SJ, Glatman Zaretsky A, Andrade-Oliveira V, Collins N, Dzutsev A, Shaik J, Morais da Fonseca D, Harrison OJ, Tamoutounour S, Byrd AL, Smelkinson M, Bouladoux N, Bliska JB, Brenchley JM, Brodsky IE, Belkaid Y. White adipose tissue is a reservoir for memory T cells and promotes protective memory responses to infection. Immunity 47: 1154–1168.e6, 2017. doi: 10.1016/j.immuni.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Brun P, Castagliuolo I, Di Leo V, Buda A, Pinzani M, Palù G, Martines D. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol 292: G518–G525, 2007. doi: 10.1152/ajpgi.00024.2006. [DOI] [PubMed] [Google Scholar]
- 61. Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57: 1470–1481, 2008. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 62. Park C, Cheung KP, Limon N, Costanzo A, Barba C, Miranda N, Gargas S, Johnson AMF, Olefsky JM, Jameson JM. Obesity modulates intestinal intraepithelial T cell persistence, CD103 and CCR9 expression, and outcome in dextran sulfate sodium-induced colitis. J Immunol 203: 3427–3435, 2019. doi: 10.4049/jimmunol.1900082. [DOI] [PubMed] [Google Scholar]
- 63. Annacker O, Coombes JL, Malmstrom V, Uhlig HH, Bourne T, Johansson-Lindbom B, Agace WW, Parker CM, Powrie F. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J Exp Med 202: 1051–1061, 2005. doi: 10.1084/jem.20040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ho J, Kurtz CC, Naganuma M, Ernst PB, Cominelli F, Rivera-Nieves J. A CD8+/CD103high T cell subset regulates TNF-mediated chronic murine ileitis. J Immunol 180: 2573–2580, 2008. doi: 10.4049/jimmunol.180.4.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Liu Y, Lan Q, Lu L, Chen M, Xia Z, Ma J, Wang J, Fan H, Shen Y, Ryffel B, Brand D, Quismorio F, Liu Z, Horwitz DA, Xu A, Zheng SG. Phenotypic and functional characteristic of a newly identified CD8+ Foxp3- CD103+ regulatory T cells. J Mol Cell Biol 6: 81–92, 2014. doi: 10.1093/jmcb/mjt026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Do JS, Kim S, Keslar K, Jang E, Huang E, Fairchild RL, Pizarro TT, Min B. γδ T cells coexpressing gut homing α4β7 and αE integrins define a novel subset promoting intestinal inflammation. J Immunol 198: 908–915, 2017. doi: 10.4049/jimmunol.1601060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Picarella D, Hurlbut P, Rottman J, Shi X, Butcher E, Ringler DJ. Monoclonal antibodies specific for β7 integrin and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) reduce inflammation in the colon of scid mice reconstituted with CD45RBhigh CD4+ T cells. J Immunol 158: 2099–2106, 1997. [PubMed] [Google Scholar]
- 68. Sydora BC, Wagner N, Lohler J, Yakoub G, Kronenberg M, Muller W, Aranda R. β7 Integrin expression is not required for the localization of T cells to the intestine and colitis pathogenesis. Clin Exp Immunol 129: 35–42, 2002. doi: 10.1046/j.1365-2249.2002.01892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Scribano ML. Vedolizumab for inflammatory bowel disease: from randomized controlled trials to real-life evidence. World J Gastroenterol 24: 2457–2467, 2018. doi: 10.3748/wjg.v24.i23.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Vermeire S, O'Byrne S, Keir M, Williams M, Lu TT, Mansfield JC, Lamb CA, Feagan BG, Panes J, Salas A, Baumgart DC, Schreiber S, Dotan I, Sandborn WJ, Tew GW, Luca D, Tang MT, Diehl L, Eastham-Anderson J, De Hertogh G, Perrier C, Egen JG, Kirby JA, van Assche G, Rutgeerts P. Etrolizumab as induction therapy for ulcerative colitis: a randomised, controlled, phase 2 trial. Lancet 384: 309–318, 2014. doi: 10.1016/S0140-6736(14)60661-9. [DOI] [PubMed] [Google Scholar]
- 71. Rubin DT, Dotan I, DuVall A, Bouhnik Y, Radford-Smith G, Higgins PDR, Mishkin DS, Arrisi P, Scalori A, Oh YS, Tole S, Chai A, Chamberlain-James K, Lacey S, McBride J, Panés J; HIBISCUS Study Group. Etrolizumab versus adalimumab or placebo as induction therapy for moderately to severely active ulcerative colitis (HIBISCUS): two phase 3 randomised, controlled trials. Lancet Gastroenterol Hepatol 7: 17–27, 2022. [Erratum in Lancet Gastroenterol Hepatol 7: e8, 2022]. doi: 10.1016/S2468-1253(21)00338-1. [DOI] [PubMed] [Google Scholar]
- 72. Noble A, Durant L, Dilke SM, Man R, Martin I, Patel R, Hoyles L, Pring ET, Latchford A, Clark SK, Carding SR, Knight SC. Altered mucosal immune-microbiota interactions in familial adenomatous polyposis. Clin Transl Gastroenterol 13: e00428, 2022. doi: 10.14309/ctg.0000000000000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 10: 942–949, 2004. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 74. Gobert M, Treilleux I, Bendriss-Vermare N, Bachelot T, Goddard-Leon S, Arfi V, Biota C, Doffin AC, Durand I, Olive D, Perez S, Pasqual N, Faure C, Ray-Coquard I, Puisieux A, Caux C, Blay JY, Ménétrier-Caux C. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res 69: 2000–2009, 2009. doi: 10.1158/0008-5472.CAN-08-2360. [DOI] [PubMed] [Google Scholar]
- 75. Longhurst CM, Jennings LK. Integrin-mediated signal transduction. Cell Mol Life Sci 54: 514–526, 1998. doi: 10.1007/s000180050180. [DOI] [PMC free article] [PubMed] [Google Scholar]