Keywords: cognitive impairment, diabetes, endothelial cells, microglia, pericytes
Abstract
Vascular contributions to cognitive impairment/dementia (VCID) are a leading cause of dementia, a known neurodegenerative disorder characterized by progressive cognitive decline. Although diabetes increases the risks of stroke and the development of cerebrovascular disease, the cellular and vascular mechanisms that lead to VCID in diabetes are yet to be determined. A growing body of research has identified that cerebrovascular cells within the neurovascular complex display an array of cellular responses that impact their survival and reparative properties, which plays a significant role in VCID development. Specifically, endothelial cells and pericytes are the primary cell types that have gained much attention in dementia-related studies due to their molecular and phenotypic heterogeneity. In this review, we will discuss the various morphological subclasses of endothelial cells and pericytes as well as their relative distribution throughout the cerebrovasculature. Furthermore, the use of diabetic and stroke animal models in preclinical studies has provided more insight into the impact of sex differences on cerebral vascularization in progressive VCID. Understanding how cellular responses and sex differences contribute to endothelial cell and pericyte survival and function will set the stage for the development of potential preventive therapies for dementia-related disorders in diabetes.
INTRODUCTION
Diabetes is an endocrine disease that ravages blood vessels. As such, diabetic nephropathy, retinopathy, and neuropathy have long been categorized as microvascular, whereas heart disease, stroke, and peripheral arterial disease are considered macrovascular complications of this chronic disease (1). Cognitive impairment/dementia has been recently added to this devastating list of diabetes-associated complications. Given that brain is a highly unique organ with no energy reserves and depends upon over 400 mi of intricate cerebrovascular networks as well as a healthy cardiovascular system for constant blood delivery (2, 3), it is finally recognized that dysregulation of vascular function and integrity is a major player in the increased risk of central nervous complications of diabetes (4–7). Diabetes affects the cerebrovasculature at multiple levels. In addition to dysfunction and remodeling of large extracranial and intracranial arteries, diabetes disrupts myogenic, metabolic, chemical, and endocrine processes that are critical for autoregulation of cerebral blood flow (CBF) by parenchymal small vessels and capillaries (3). Diabetes also impairs functional hyperemia, which is achieved by neurovascular coupling as a result of direct and dynamic communication within the neurovascular unit (NVU) (8). Furthermore, blood brain barrier (BBB) integrity is also compromised in diabetes (3). As mentioned earlier, the brain cannot be studied in isolation from all other organ systems, and neurons cannot be studied in isolation from all other cells within the brain. However, for the purposes of this review on the impact of diabetes on cerebrovascular pathobiology, we will briefly introduce the NVU concept (Fig. 1) and then focus on the microvasculature, specifically on endothelial cells and pericytes, for several reasons. First, endothelial cells are the first targets that are hit by metabolic perturbations, i.e., hyperglycemia, dyslipidemia, and hyperinsulinemia, as well as the inflammatory cell and platelet activation triggered by diabetes. Second, capillaries that are lined by endothelial cells and embraced by pericytes constitute the majority of the brain’s vasculature and loss of pericytes affects endothelial cell biology (3, 11, 12). Lastly, very exciting emerging evidence from studies looking at single-cell endothelial transcriptome or translatome via translating ribosome affinity purification coupled with single-cell RNA sequencing demonstrated the vast heterogeneity of endothelial cells along the cerebrovascular tree (9, 10, 12–14). With these in mind, we will first review the literature with respect to what is known about the phenotypic heterogeneity of these two NVU cells. We then briefly summarize cellular responses such as ferroptosis, endothelial mesenchymal transition (EndMT), senescence, and associated secretory phenotype (SASP) that impact survival, trophic, and reparative properties of these cells. In the context of these cellular responses and heterogeneity, we next examine cerebral vascularization and pathological angiogenic response in diabetes and after a stroke event in diabetes with potential sex differences. We conclude with a discussion of the relevance of these microvascular changes to cognitive impairment in diabetes.
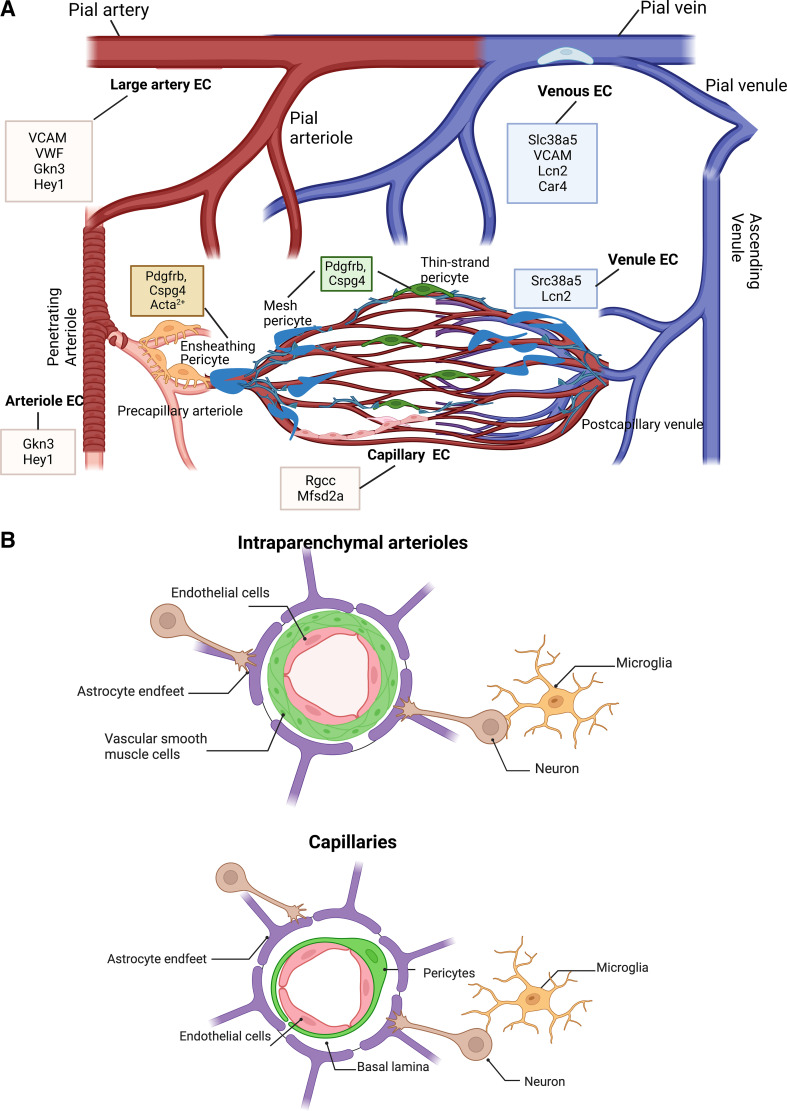
Figure 1.
A: depiction of the molecular markers of endothelial cells and pericytes within the brain as identified by single-cell transcriptomics coupled with in silico analyses (9, 10). Cerebral vasculature runs from surface pial arteries to arterioles to capillaries to venules to large veins. Each vascular segment expresses a unique set of markers that can identify endothelial cells of that particular segment that are shown in the boxes. As the arterioles join capillary bed, smooth muscle cells are replaced by ensheathing pericytes in the precapillary arteriolar zone. These pericytes express the Acta2+ gene encoding α-smooth muscle actin and exhibit contractile properties. Within the capillary bed, mesh pericytes and thin-strand pericytes that do not express the Acta2+ gene form tight connections with capillary endothelial cells. Projections from mesh pericytes continue to extend near the postcapillary venule to interact with venous endothelial cells, as the ascending venule continues along the venous side of the cerebrovascular network. Venous capillary endothelial cells form the inner lining of the pial vein, where there is fewer smooth muscle cells (SMCs). B: a representative schematic of the intricate components of the neurovascular unit (NVU) within the microvasculature, which includes intraparenchymal arterioles and capillaries. The interactive cell types that comprise the NVU include endothelial cells, mural cells [pericytes and vascular smooth muscle cells (VSMCs)], glial cells (astrocytes, oligodendrocytes, and microglia), neurons, and basal lamina. At the intraparenchymal arteriolar level of the cerebrovasculature, VSMCs wrap around the endothelium, but these cells are later replaced by pericytes in the capillaries. Collectively, these cells function to regulate blood brain barrier (BBB) permeability, cerebral blood flow (CBF), and clearance of waste for proper brain health. Created with BioRender.com.
HETEROGENEITY OF BRAIN ENDOTHELIAL CELLS AND PERICYTES
NVU
Despite extensive research, neuroprotection interventions have repeatedly failed to yield any therapeutic targets for acute ischemic stroke. This failure highlighted the need to develop a working model for cellular interactions within the brain and led to the development of the NVU concept at the Stroke Progress Review Group meeting of the National Institute of Neurological Disorders and Stroke in 2001 (6). NVU includes neurons, astrocytes, endothelial cells, mural cells including vascular smooth muscle cells (VSMCs) and pericytes, and basal lamina (Fig. 1B). In recent years, this concept has evolved to include other glial and perivascular cells, and most recently defined as neurovascular complex (8). From a functional perspective, dynamic interactions within the NVU contribute to the regulation of CBF, BBB integrity, clearance of waste material, and trophic functions, all of which are critical for brain health (5). Endothelial cells are at the epicenter of NVU and will be discussed in detail.
Endothelial Cells
Endothelial cells form the lining of all blood vessels throughout the body providing the first barrier at the blood tissue parenchyma interface. In the human brain, microvessels are estimated to be 400 mi long and lined up with approximately five billion endothelial cells (2, 15, 16). The arterial blood supply of the brain is delivered by four major arteries as reviewed (3). In this supply chain, there are three tiers of vascular networks (11). Surface pial arteries and veins form the top-level providing circulation of the surface of the brain. Penetrating arteries stemming from this network are the second level and give rise to penetrating arterioles that dive deep into the brain parenchyma connecting to subsurface capillary circulation. The third level is the three-dimensional capillary network. Most of our current knowledge of the angioarchitecture of the brain has been gained through the assessment of the cortical layers by multiphoton imaging modalities coupled with electrophysiology studies (17). Organization and functional regulation of deeper layers of the vascular networks including arterial and venous circulation especially under flow conditions have been difficult to study due to technical limitations. Although studies over the years have suggested that each tier has unique features that are regulated by the microenvironment and anatomical localization (18, 19), functional and structural similarities and differences of endothelial cells across different vascular segments (arteries-arterioles-capillaries-venules-veins) have also been hard to demonstrate due to technical difficulties in isolating pure endothelial cells from different vascular segments embedded in tissues and mostly acquired from different studies conducted under different conditions in different brain regions (18, 19). With the advancement of single-cell transcriptomic- and translatome-profiling technologies, in recent years, multiple groups have addressed this problem and reported comprehensive analyses of endothelial cell heterogeneity across vascular segments in not only the brain but also other tissues and in the context of other cell types (9, 10, 13, 14, 20, 21). In the following paragraphs, we highlight unique features of brain endothelial cell heterogeneity based on these reports. We refer the readers to these studies for detailed information and a recent review paper (8) that discusses one such transcriptomic study in the context of evolving neurovascular complex concept.
One study provided a molecular atlas of cell types and zonation in the mouse brain vasculature using single-cell transcriptomics (10). This study showed phenotypic changes that is referred as zonation along the arteriovenous tree. For example, well-established markers of endothelial cells such as Von Willebrand factor (Vwf) and vascular cell adhesion molecule (VCAM)-1 were mainly localized to large arteries and veins. A BBB-specific lipid transporter, major facilitator superfamily domain-containing protein 2 (Mfsd2a), was localized to capillaries consistent with its function. Transferrin receptor was found mainly on veins and capillaries but not on arteries (10). Another transcriptomic profiling study with single-cell RNA sequencing compared endothelial cell transcriptome in 11 mouse tissues (9). This comprehensive study identified peptidoglycan recognition protein (Pglyrp)-1 as the only marker expressed in brain endothelial cells and not in endothelial cells in other tissues. Vwf and VCAM-1 were also found to be associated with large vessels. Mfsd2a and regulator of cell cycle (Rgcc) were localized to capillaries. In silico analyses of the identified genes provided a trajectory of markers along vascular segments from large arteries to veins and segments in between and these are depicted in Fig. 1A. This study reported the presence of two unexpected subclusters in the brain termed as “choroid plexus capillary endothelial cells” that were characterized by the expression of plasmalemma vesicle-associated protein (Plvap) gene, and “interferon-activated endothelial cells” characterized by elevated levels of interferon-induced genes. The relative distribution of endothelial cell subclusters in the brain is summarized in Fig. 2. Hierarchical clustering of all subclasses across the tissues suggested that endothelial cell heterogeneity arises from the tissue type and not from the vascular segmentation (9). Given that transcriptomic approach relies on tissue digestion and sorting for cell preparation, other groups took different approaches based on isolation of tissue-specific mRNAs undergoing translation without cell suspension using RiboTag (13, 14). These reports confirmed transcriptomic studies earlier in this paragraph with respect to heterogeneity of endothelial cells in different tissues based on differential gene expression but did not break down the data by vascular segments in a given tissue. However, translatome profiling was reported to be more sensitive and identified very low abundance transcripts that were not picked up by single-cell RNA sequencing (13). Interestingly, Jambusaria and colleagues reported that genes involved in neuronal functions were highly enriched in brain endothelial cells, providing further support to the concept that tissues give rise to endothelial heterogeneity as alluded to above. Moreover, these last two reports looked at endothelial heterogeneity under inflammatory conditions induced by lipopolysaccharide (LPS) injection and identified differential endothelial gene expression patterns in different tissues. For example, P-selectin was markedly upregulated in the brain and heart but not in lungs. These fascinating studies provided seminal information with respect to endothelial cell heterogeneity across vascular beds and segments while opening new avenues of research to better understand the regulation of this complexity in disease states such as diabetes.
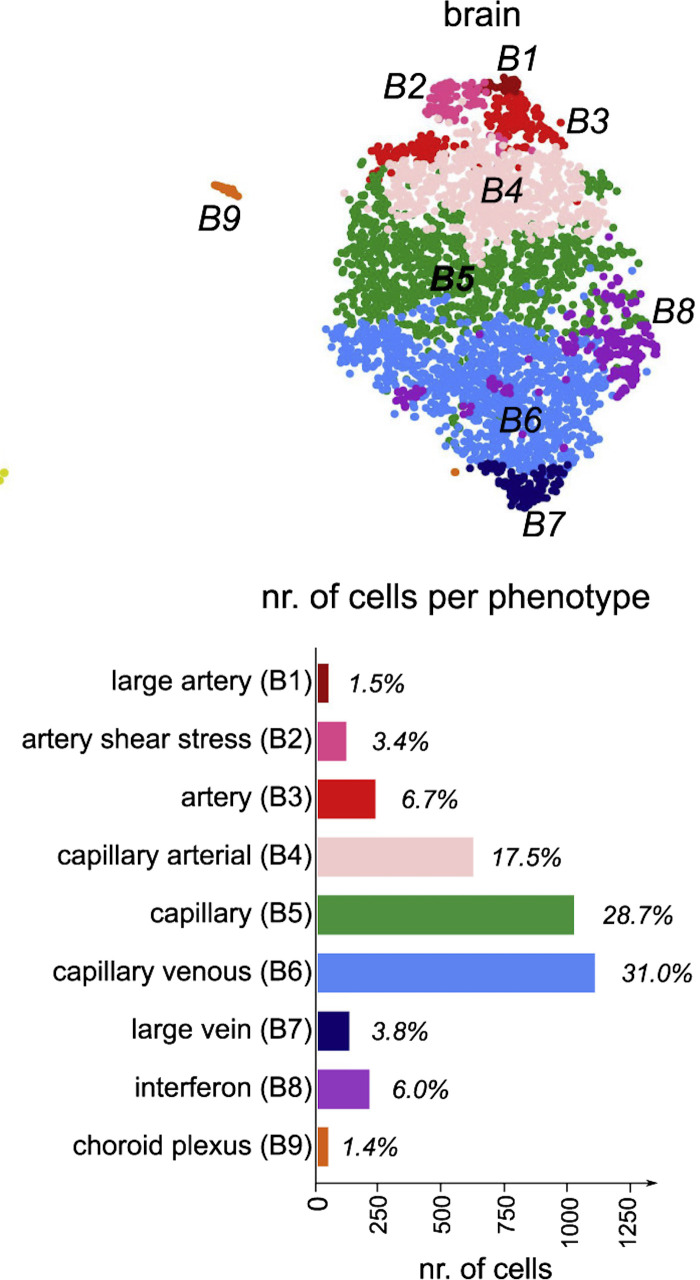
Figure 2.
Endothelial cell subclusters detected by single-cell transcriptomics identify endothelial cells unique to the brain such as choroid plexus capillary endothelial cells and interferon-activated endothelial cells along with heterogeneous endothelial cells distributed along the vascular segments. T-Distributed stochastic neighbor embedding (t-SNE) plot visualization of brain tissue-specific endothelial cell clusters with distinct color codes are shown on top. Bar graph depiction of number of cells and the relative fraction of each subcluster is shown below. Modified from Ref. 9.
Pericytes
Pericytes are multifunctional mural cells that wrap around endothelial cells on the abluminal side of capillaries and arterioles (22–24). Although their presence and contribution to BBB formation and maintenance were known, and their high abundance in cerebral and retinal circulations suggested a possible role in regulation of neurovascular coupling, their function, especially with respect to contractility and regulation of CBF, remained controversial (25). This was in part due to lack of tools and specific markers. With the development of genetic models and multimodality imaging techniques, our understanding of pericyte heterogeneity with respect to function and morphology improved significantly. We refer the readers to excellent reviews for detailed descriptions of the diverse morphology and functions of pericytes within the microvasculature (22–24, 26–28). Below we will briefly summarize pericyte subtypes and topology in the brain.
Alpha-smooth muscle actin (α-SMA), neuron-glial antigen (NG2), and platelet-derived growth factor receptor β (PDGFRβ) are most commonly used markers for pericytes (24). Additional markers that contribute to the growing knowledge on pericyte identification include aminopeptidase A, regulator of G-protein signaling 5 (RGS5), and aldehyde dehydrogenase 1 (Aldh1a1) (21). Evaluating the expression of these cellular markers in pericytes has propelled research further into addressing the vast knowledge gap between pericyte morphology and how it relates to their anatomic location across different vascular beds (12, 27, 29). However, it has to be recognized that the use of these markers still presents several challenges as these markers are also expressed in vascular smooth muscle cells. The current consensus is that there are three morphological subtypes: thin-strand or helical, mesh, and ensheathing pericytes (Fig. 1A). Thin-strand (helical) pericytes are broadly distributed throughout the capillary bed and exhibit distinct features such as long thin processes that form ring structures, which extend along the circumference of microvessels (8, 10, 22, 27, 28). Although thin-strand pericytes have minimal projections, their fine distal branches provide specificity for trajectories toward vascular junctions (30). Regarding α-SMA expression, thin-strand pericytes display little to no α-SMA content, which indicates that this morphological subtype exhibits less powerful contraction (8, 10, 22). Mesh pericytes are primarily located on the proximal side of capillaries and adopt a mesh-like sheath, which surrounds the entire blood vessel. Considering that mesh pericytes have a higher coverage area than thin-strand pericytes, their distinct mesh-like morphology gives them the ability to maintain NVU integrity. Similar to thin-strand pericytes, mesh pericytes also display undetectable α-SMA expression (22, 27). The third morphological subtype, ensheathing pericytes are positioned at the arteriole-capillary transition network and contain densely packed proximal projections that encase the arteriole and cover most of the arteriolar endothelium. Unlike thin-strand and mesh pericytes, ensheathing pericytes express high amounts of α-SMA (8, 22, 27). Acute ablation of cortical pericytes results in neurovascular uncoupling (31). Contractile pericytes determine the direction of CBF at capillary junctions (32). These developments shed light on the controversy regarding the contractile properties of pericytes and their role in regulation of CBF. Current understanding is that ensheathing pericytes that generate powerful contraction due to presence of α-SMA may be involved in rapid responses such as NVU coupling and others are contributing to flow regulation under resting conditions (22).
Recent studies have also provided more in depth understanding of endothelial-pericyte interactions, which is highly relevant to this review. Pericytes and endothelial cells come into close contact through microstructures including gap junctions and peg-socket interactions, where basal lamina, which typically provides a physical separation between these two cell types, is absent (33, 34). Peg-and-socket connections are characterized as protrusions from pericytes or endothelial cells forming indentations on the neighboring cells. This close contact has been proposed to anchor the cells to each other and possibly form a line of communication (34). An earlier study reported that gap junctions between pericytes and endothelial cells are critical for the transfer of biochemical signals required for mural cell differentiation (35). A recent study looked at these special structures using three-dimensional electron microscopy and has revealed that pegs extending from pericytes exhibit a diverse array of morphologies as compared with pegs originating from endothelial cells, which are simpler in structure (34). It was also noted that these pegs from either cell type are enriched around pericyte soma providing further support for the notion that there is more than physical interaction through these sites and warrants future research. The importance of pericytes in regulation of the functional state, i.e., heterogeneity, of endothelial cells was highlighted by a recent transcriptomic study using the pericyte-deficient Pdgfret/ret mice, a model that lacks the heparan sulfate proteoglycan-binding motif in PDGFRβ (12). When pericytes were low, endothelial cells devoid of this coverage showed disruption of arteriovenous zonation and expressed more growth factors and proinflammatory mediators suggesting endothelial transformation.
Evolving understanding of the diverse roles of pericytes in acute and chronic regulation of CBF, BBB integrity, and endothelial heterogeneity has drawn attention to the involvement of these mural cells in brain injury/repair and neurodegenerative diseases ranging from acute ischemic stroke to different forms of dementias (23, 36–38). However, our knowledge of pericyte phenotypic and functional changes in diabetes is limited. Thus, further research in pericyte biology is needed to advance our fundamental knowledge of these complex cells in health and disease to identify targets for intervention.
CELLULAR PROCESSES THAT IMPACT THE SURVIVAL AND REPARATIVE PROPERTIES OF THE MICROVASCULAR ENDOTHELIAL CELLS AND PERICYTES
Ferroptosis
Based on emerging evidence, novel cell death mechanisms that contribute to the pathophysiology of specific neurodegenerative diseases and brain injury have gained significant attention (39–44). One such mechanism is ferroptosis, a recently discovered iron-dependent form of regulated cell death accompanied by the accumulation of reactive oxygen species (ROS), leading to lipid peroxidation and membrane permeabilization (45–47). Morphologically, it is characterized by small mitochondria with reduction in cristae and lack of condensation in the nucleus. Inhibition of plasma membrane cystine/glutamate antiporter known as system XC− leads to decreased cysteine levels, which is needed for glutathione biosynthesis. When glutathione levels drop, glutathione peroxidase 4 (GPX4) can no longer function to clear lipid peroxides leading to cell death (42, 47). This is further enhanced by activation of iron-responsive element-binding proteins (IREBs). Ferroptosis is particularly important for the brain as glutamate, which causes excitotoxic cell death, also inhibits system XC−. Hallmarks of ferroptosis include decreased glutathione GPX4 and ferritin levels as well as increased Acyl-CoA synthetase long-chain family member 4 (ACSL4) (46). Although it is recognized that it may differ in certain experimental conditions, current recommendations for ferroptosis studies are that there should be accumulation of lipid peroxides, and ferroptosis should be suppressed by iron chelators such as deferoxamine (DFX) (46). We refer the readers to a recent review article for detailed description of molecular, biochemical, and morphological markers of ferroptosis (47).
It is well established that accumulation of iron triggers lasting neurotoxic effects and ferroptosis contributes to neuronal death in intracerebral hemorrhage (ICH) models (40, 41, 48–51). It was reported that accumulation of free iron in endothelial cells and pericytes promotes secondary brain injury in ICH but ferroptosis markers were not investigated (52). The role(s) of iron and ferroptosis in brain endothelial cell viability is relatively less understood. This is particularly relevant to diabetes because it is well established that ischemic stroke events are associated with greater occurrence of hemorrhagic transformation (HT), a secondary form of bleeding into the brain (53–55). If bleeding is petechial and not space-occupying, it is not treated (56, 57). However, the role and mechanisms by which iron accumulation resulting from bleeding affect repair and recovery after stroke in diabetes are not well known. Evidence from ICH literature shows robust activation of the innate immunity, especially Toll-like receptor 4 (TLR4) (58, 59). A possible role of TLR4 and the impact of iron chelation by DFX on neurovascular repair, in particular on endothelial cell ferroptosis, will be further discussed in CEREBRAL VASCULARIZATION IN EXPERIMENTAL MODELS OF DIABETES AND STROKE.
EndMT
The endothelial to mesenchymal transition (EndMT) is a relatively new cellular transdifferentiation process that was originally described as a physiological process associated with cardiac development in which endothelial cells lining the atrioventricular canal and the outflow canal transform to form the septa and valves (60–62). EndMT is a physiological program necessary for development, but it can become maladaptive and reactivated in pathological conditions such as pulmonary hypertension, cancer, and cardiac fibrosis (60, 63–65). We refer the readers to excellent recent reviews on EndMT in cardiovascular diseases (66, 67). Although different stimuli may trigger EndMT process in different organs, downstream molecular signals converge on TGF-β superfamily signaling including TGF-β isoforms, bone morphogenetic protein-6 (BMP6), and their cognate receptors (65–68). Cells that escape stress-induced cell death progress into transdifferentiation and lose their endothelial features such as VE-cadherin, CD31, eNOS, and claudin-5 while gaining fibroblast-like features including α-smooth muscle actin (SMA) and fibronectin (60, 63, 69). This transformation can range from a complete transdifferentiation to an incomplete transformation retaining some endothelial and some mesenchymal cell characteristics that made studying EndMT very difficult.
In the brain, EndMT has been shown to contribute to pathological vascular lesions observed in cerebral cavernous malformations (CCMs) in experimental models (70, 71). It was later reported that EndMT also occurs in human brain arteriovenous malformations (AVMs) (72). A 2017 study showed that endothelial TLR4 drives CCMs (73). Although CCM and AVMs are completely different disease entities, these intriguing studies suggested the possibility of TLR4-mediated EndMT in other cerebrovascular pathologies. Indeed, two recent studies reported that EndMT is involved in regulation of BBB integrity after stroke injury (74, 75). Using lineage-tracking mice, Chen et al. (75) showed the development of EndMT after ischemic stroke, which was prevented by endothelial-specific overexpression of microRNA let-7i. As discussed earlier, TLR4 activation by iron or other blood products can also lead to ferroptosis, suggesting a cross talk between processes that regulate endothelial cell survival and phenotype. Thus, we will discuss a possible role of EndMT in cerebrovascular plasticity in diabetes and after stroke in the context of our findings in cerebral vascularization patterns in male and female rats in cerebral vascularization in experimental models of diabetes and stroke.
Cellular Senescence
Senescence, like EndMT, can be both a typical physiological process and in other instances deleterious to the homeostasis of the surrounding cellular environment (76–78). It is now recognized that senescent phenotype is heterogeneous and can be categorized as replicative senescence (RC), stress-induced premature senescence (SIPS), and oncogene-induced senescence (OIS). We refer the readers to excellent recent review articles that describe the molecular processes, mechanisms, and the characterization of senescent phenotypes (79–82) and provide a brief summary relevant to the purpose of this review.
The primary physiological trigger of senescence is DNA damage that is most often linked to the DNA damage response (DDR) but it can also be observed in the normal processes of cellular aging (81, 82). Cells continue to replicate until the telomeres are entirely depleted and the DNA would no longer be copied in its entirety. This phenomenon prevents mutations that would result from incomplete DNA replication and is termed replicative senescence (83). It is thought to be a normal process of aging due to the strong correlation between age and increased senescence markers (81, 82). Senescent inflammatory phenotype can also be triggered by metabolic disturbances as seen in diabetes and obesity, major comorbidities for individuals suffering from Alzheimer’s disease and related dementias (ADRD), and led to the concept of premature metabolic aging (84, 85). This is also known as SIPS and is independent of telomere length, but reliant on constitutive DDR signaling (81). Senescent cells can undergo changes such as flattening, stochastic gene expression thought to derive from chromatin remodeling, altered paracrine activity, and increased production of reactive oxygen species (78, 79, 82, 86). These changes in cell morphology as well as gene expression prevent the cell from carrying out its intended processes that could potentially lead to tissue dysfunction that come with chronological aging as well as age-related disorders such as ADRD including vascular contributions to cognitive impairment (VCID) (80). The most studied hallmark of senescent cells is their secretory phenotype known as the SASPs that is associated with high levels of inflammatory cytokines, immune modulators, growth factors, and protease (76, 79, 81, 82, 84). As such, senescence that starts in the smallest working unit of the brain can trigger neuroinflammation, an important component of VCID.
There is evidence that metabolic disturbances like hyperglycemia and advanced glycation end products (AGEs) induce endothelial senescence but most, if not all, of this evidence comes from other vascular beds (87–90). Evidence for senescence in brain endothelial cells, especially in diabetic conditions, is limited. A recent study showed greater expression of genes associated with senescence in the microvessels isolated from postmortem brains from patients with AD (91). Another study reported cellular senescence as one of the major signaling pathways that is significantly affected in the neurons of brains of patients with type 2 diabetes in which endothelial cells showed robust formation of AGEs (92). Kiss and colleagues (15) identified senescent microvascular endothelial cells in the aged mouse brain using complex analyses of single-cell RNA sequencing data. An intriguing study reported that the loss of CCM1 and CCM2 genes in endothelial cells, genes involved in brain vascular malformations as mentioned above in EndMT, promote SASP phenotype (93), drawing attention to the potential similarities in EndMT and endothelial senescence. Although there are no direct studies on pericyte senescence in diabetes, senescent pericytes were reported to be involved in impaired barrier function (94). Moreover, Liu et al. (95) have shown that increased ROS and AGEs production concomitant with pericyte dysfunction in cerebral blood vessels from old diabetic rats and cultured pericytes grown in high glucose conditions. We have shown reduced pericyte coverage in the brains of diabetic rats that exhibit pathological vascularization and cognitive deficits (96, 97), which we will review.
CEREBRAL VASCULARIZATION IN EXPERIMENTAL MODELS OF DIABETES AND STROKE
There is clinical evidence that early onset of microvascular dysfunction in diabetes leads to structural changes in the cerebral microvasculature over time compromising NVU, as elegantly reviewed in Ref. 98. It is also established that vascular rarefaction and enlarged perivascular space are part of the neurovascular pathologies observed in ADRD (26, 98–100). Experimental models of diabetes and metabolic disease exhibit similar alterations providing a platform to investigate underlying mechanisms (3). As discussed earlier, the revised definition of NVU as neurovascular complex includes relatively larger vessels like pial arteries feeding into the microvasculature, which is typically defined as vessels between first-order arterioles and first-order venules (8). Thus, we will include leptomeningeal arteries in our review.
Diabetes
A comprehensive study compared pial collateral number as well as diameter in response to an ischemic insult in both sexes in different mouse models of hyperlipidemia, obesity, and diabetes (101). In obese mice (Lepob/ob), pial collateral numbers were equivalent to wild type but collateral diameter was reduced. However, fewer native pial collaterals were observed in mice with hyperlipidemia and moderate obesity (Ldlr−/− fed a Western diet), hyperlipidemic mice (Apoe−/− fed a normal diet), and type 1 diabetic mice (Ins2Akita). Mice with metabolic syndrome (Lepob/ob; Ldlr−/−) that have type 2 diabetes, hypertension, moderate obesity, and hyperlipidemia had pronounced reduction in perfusion as well as number and diameter of pial collaterals (101). The authors did not report any sex differences. In contrast, tortuosity as well as collateral numbers and size were found to be greater in the pial vasculature of male Goto Kakizaki (GK) rats, a nonobese model of diabetes that presents with relatively moderate blood glucose levels (102). Further studies using three-dimensional reconstruction of the fluorescein isothiocyanate (FITC)-filled vessels showed greater cortical and striatal vascularization as evidenced by greater vascular density, volume, surface area, and branching in the GK model as compared with control Wistar rats (96, 97). Furthermore, colocalization of the FITC signal with isolectin B4, an endothelial cell marker, showed that there was relatively more isolectin staining in diabetic sections suggesting the presence of more nonperfused vessels (96). These presumably newly formed/remodeled vessels lacked proper pericyte coverage. These changes were unique to the brain and retinal vasculature. Primary brain microvascular endothelial cells isolated from diabetic GK rats retained their angiogenic potential in culture and exhibited more tube formation and migration properties when compared with primary cells from control Wistar rats in a vascular endothelial growth factor (VEGF)-dependent manner (96). Moreover, this proangiogenic response involved reactive oxygen/nitrogen species signaling and downregulation of the guidance molecule Robo-4 (96, 103). Inhibition of VEGF signaling or upregulation of Robo-4 restored pericyte coverage of the microvasculature and prevented pathological cerebral vascularization (103). Glycemic control with metformin partially prevented aberrant vascularization (Fig. 3) (97, 104). These alterations that occurred early in the disease process did not progress as animals aged (104).
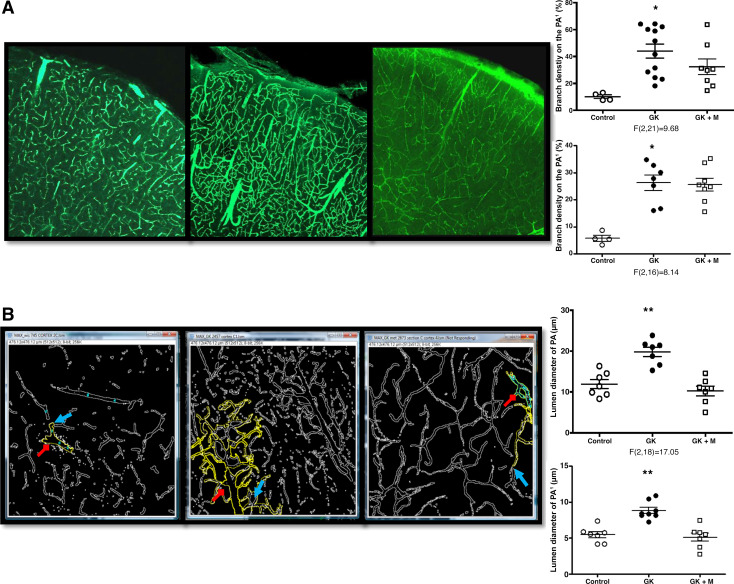
Figure 3.
Diabetes increases branch density, lumen diameter, and tortuosity of penetrating arterioles (PAs) and its branches in the GK model of diabetes. Representative images showing vascular branching on PAs and surface cortical vessels taken under 10× (A) and inner vessel walls outlined using the Fiji software (Red arrows represent the PA and the blue arrows depict the immediate branched from the PA (PA1) (B). Although there was no significant change in branching of PAs with glycemic control with metformin (GK+M), lumen diameter was reduced. *P < 0.01 vs. control, **P < 0.001 vs. control or treatment, ***P < 0.0001 vs. control or treatment. Means ± SE, n = 6–8. Modified from Ref. 97.
A greater branch density and tortuosity with a trend for decreased lumen diameter of the penetrating arterioles was also observed in the Leprdb/db model of diabetes. There was an increase in vascular volume and surface area that was more dominant in the microvasculature (97). Enlarged perivascular space (PVS) is considered a hallmark of small vessel disease of the brain based on MRI imaging in humans (105, 106). Intriguingly, diabetic animals that develop progressive cognitive decline also exhibit this vascular structural pathology (107). A recent elegant study from another group using light-sheet microscopy confirmed and expanded these findings in male db/db mice (108). Authors reported the presence of extensive neovascularization and imbalanced distribution of micro and macrovessels as well as loss of pericytes in diabetic mice. It was noted that as animals aged, there was an increase in branch points but a decrease in endpoint hinting the formation of loop structures, and authors suggested that this may contribute to poor perfusion in diabetic animals. The perfusion volume of macrovessels (arteries larger than 90 µm) was also increased when compared with microvessels, suggesting redistribution of flow in favor of larger vessels further worsening the perfusion. Greater vascularization was also observed in male rats in which diabetes was induced by a high-fat diet/low streptozotocin (HFD/STZ) combination albeit to a lesser extent (109).
It has long been suggested that eye provides a window to the brain. Eye contains blood retina barrier and retinal NVU (110–112). It is well established that diabetic retinopathy is a microvascular complication of the disease that leads to blindness. Vascular pathologies similar to cerebrovascular changes discussed above including aberrant neovascularization, intraretinal hemorrhage, and microaneurysms are hallmarks of diabetic retinopathy (111). Relevant to this review, premature senescence was reported in retinal endothelial cells in diabetes (113, 114). Recent studies also call attention to the dynamic interplay between vascular, neuronal, glial, and immune cells in the retina (110–112).
Sex differences.
In most of the studies discussed above, only male animals were use. In the HFD/STZ model discussed in the diabetes section (109), when the same brain regions were probed using the same FITC space-filling method in female rats, there was no difference in cortical or striatal vascular volume, surface area, or density between control and HFD/STZ diabetic rats, but vascular density appeared to be greater in both control and diabetic female rats than male rats (unpublished results). It is important to note that there may also be regional differences. A direct comparison of the vascularization of the dentate gyrus, an important domain for learning and memory, showed that while diabetes was associated with higher branch density in both sexes, surface area was lower in diabetic males but higher in diabetic females in this diet-induced model. Interestingly, neuronal counts were lower in male diabetic rats as compared to control male rats, but this was not seen in females (115, 116).
Stroke
These differences noted in the integrity and patterns of cerebral vascularization in diabetic animals may predispose them to greater risk of neurological complications such as cognitive impairment and ischemic stroke. Furthermore, neurovascular injury, especially after a second hit such as ischemic stroke, may be greater in diabetic conditions (117–119). It has been shown that male GK and HFD/STZ diabetic rats that exhibit pathological excessive vascularization as described above develop greater HT without significant increases in infarct size (120–122).
A healthy vasculature and adaptive/reparative vascular plasticity is critical for repair process after an injury (1, 118). Although control male rats exhibited greater vascularization in the lesional hemisphere, especially around the periinfarct area, indicative of reparative angiogenesis, GK diabetic male rats that develop greater HT after ischemic stroke presented profound loss of vasculature 14 days after stroke (123). Excessive formation of peroxynitrite after ischemia/perfusion injury was found to switch VEGF-akt1-cell survival pathway to VEGF-MAPK-cell death pathway leading to endothelial apoptosis (124). Treatment with metformin during the 14-day recovery period following stroke prevented vasoregression and this effect was not solely due to glycemic control (124). Studies with the HFD/STZ model of diabetes suggested that the presence and magnitude of HT may also play a role in poststroke vasoregression observed in male diabetic rats (109). Iron chelation by DFX after thromboembolic stroke was found to prevent cerebral vasoregression and this was associated with better functional outcomes 14 days after stroke in male diabetic rats (125).
In type 1 model of diabetes, cerebral vascularization after stroke seems to be different. For example, one study reported increased vascular density in the periinfarct area 14 days after stroke in male animals (126). Another study measured vessel density and sprouting with in vivo time lapse imaging after photothrombotic stroke and showed increased vascular density in both control and diabetic animals (127). Authors postulated that this finding is result of the robust dilation rather than the sprouting of vessels. Interestingly, this study also reported lesser branch points indicative of increased pruning after stroke as seen in GK rats (127). These differences in cerebrovascularization may be due to differences between animal strains, experimental models, duration of insult, and the number of metabolic risk factors. However, at least in the HFD/TZ model, same strain and species of animals were matched for age and duration/severity of diabetes.
Sex differences.
When brain microvascular endothelial cells (BMVECs) isolated from control and diabetic male animals were challenged with iron to mimic HT, there was increased lipid peroxidation and expression of various ferroptosis markers and DFX prevented this ferroptotic response improving BMVEC viability. Interestingly, when female diabetic rats were subjected to the same stroke model and DFX treatment regimen, results were different than those observed in males (unpublished results). There was no obvious vasoregression after stroke despite significant HT in female diabetic rats and DFX treatment caused a decrease in vascularization indices in both control and diabetic rats (unpublished results). In vitro studies using male and female brain microvascular endothelial cell lines suggest that male cells may be more susceptible to ferroptosis, an iron-dependent form of cell death, when challenged with iron or hypoxia (125), whereas, female cells survive and undergo phenotypic changes such as EndMT under similar conditions (unpublished results). In accordance with these findings, a previous study reported that endothelial cells isolated from female animals are more resistant to cell death (128).
In the context of endothelial phenotypic changes, ferroptosis and senescence discussed earlier, a common finding in the diabetic male and female rats that present with these cerebrovascular structural abnormalities before and after an ischemic event is the heightened expression of TLR4, especially in the microvasculature (125, 129). As discussed above, endothelial TLR4 signaling mediates EndMT (73) and contributes to ferroptosis (59, 75, 125). Growing body of evidence indicates a role for TLR4 in cellular senescence as well (130–132). Although diabetes is an endocrine disease in its origin, it is also a vascular and chronic inflammatory disease and overactivation of TLR4, one of the gatekeepers of the innate immunity can be a common mechanism in endothelial alterations (refer to graphical abstract).
RELEVANCE TO COGNITIVE IMPAIRMENT
The critical role of the structural and functional integrity of the peripheral and cerebral vasculature in brain health is undisputable (5, 7, 26, 99, 106). Cerebrovascular dysfunction is recognized as disregarded partner of cognitive demise, highlighting the importance of the vascular system in the pathogenesis and progression of neurodegenerative diseases. Cerebrovascular disease is a common pathology between diabetes and cognitive impairment (133). Although epidemiological studies have long established the coexistence of cognitive deficits and diabetes (134), only in recent years, it has become clearer that cognitive deficits might be a direct complication of diabetes due to microvascular and macrovascular disease (4, 135, 136). BBB, formed by endothelial cells and pericytes, is the interface between the brain and blood, and endothelial cells are also the first line of barrier subjected to metabolic changes mediated by diabetes. Thus, we suggest that better understanding of the heterogeneity, sex differences, and cellular responses that are critical for endothelial cell and pericyte survival and function would aid in identifying preventive and disease-modifying targets and strategies for ADRD in diabetes.
GRANTS
This study was supported by Veterans Affairs (VA) Merit Review (BX000347), VA Senior Research Career Scientist Award (IK6 BX004471), National Institutes of Health (NIH) RF1 NS083559 (formerly RO1 NS083559), and R01 NS104573 (multi-PI, Susan C. Fagan as co-PI) (to A.E.). M.E-F. was supported by Initiative for Maximizing Student Development (IMSD) (5R25GM072643-16) and RF1 NS083559-S1.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.E-F. and A.E. prepared figures; M.E-F. and A.E. drafted manuscript; M.E-F. and A.E. edited and revised manuscript; A.E. approved final version of manuscript.
ACKNOWLEDGMENTS
Graphical abstract was created with BioRender.com.
REFERENCES
- 1. Ergul A, Kelly-Cobbs A, Abdalla M, Fagan SC. Cerebrovascular complications of diabetes: focus on stroke. Endocr Metab Immune Disord Drug Targets 12: 148–158, 2012. doi: 10.2174/187153012800493477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Begley DJ, Brightman MW. Structural and functional aspects of the blood-brain barrier. Prog Drug Res 61: 39–78, 2003. doi: 10.1007/978-3-0348-8049-7_2. [DOI] [PubMed] [Google Scholar]
- 3. Coucha M, Abdelsaid M, Ward R, Abdul Y, Ergul A. Impact of metabolic diseases on cerebral circulation: structural and functional consequences. Compr Physiol 8: 773–799, 2018. doi: 10.1002/cphy.c170019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Sloten TT, Sedaghat S, Carnethon MR, Launer LJ, Stehouwer CDA. Cerebral microvascular complications of type 2 diabetes: stroke, cognitive dysfunction, and depression. Lancet Diabetes Endocrinol 8: 325–336, 2020. doi: 10.1016/S2213-8587(19)30405-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gorelick PB, Furie KL, Iadecola C, Smith EE, Waddy SP, Lloyd-Jones DM, Bae HJ, Bauman MA, Dichgans M, Duncan PW, Girgus M, Howard VJ, Lazar RM, Seshadri S, Testai FD, van Gaal S, Yaffe K, Wasiak H, Zerna C; American Heart Association/American Stroke Association. Defining optimal brain health in adults: a presidential advisory from the American Heart Association/American Stroke Association. Stroke 48: e284–e303, 2017. doi: 10.1161/STR.0000000000000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron 96: 17–42, 2017. doi: 10.1016/j.neuron.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sweeney MD, Montagne A, Sagare AP, Nation DA, Schneider LS, Chui HC, et al. Vascular dysfunction-The disregarded partner of Alzheimer's disease. Alzheimers Dement 15: 158–167, 2019. doi: 10.1016/j.jalz.2018.07.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schaeffer S, Iadecola C. Revisiting the neurovascular unit. Nat Neurosci 24: 1198–1209, 2021. doi: 10.1038/s41593-021-00904-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kalucka J, de Rooij L, Goveia J, Rohlenova K, Dumas SJ, Meta E, Conchinha NV, Taverna F, Teuwen LA, Veys K, Garcia-Caballero M, Khan S, Geldhof V, Sokol L, Chen R, Treps L, Borri M, de Zeeuw P, Dubois C, Karakach TK, Falkenberg KD, Parys M, Yin X, Vinckier S, Du Y, Fenton RA, Schoonjans L, Dewerchin M, Eelen G, Thienpont B, Lin L, Bolund L, Li X, Luo Y, Carmeliet P. Single-cell transcriptome atlas of murine endothelial cells. Cell 180: 764–779.e20, 2020. doi: 10.1016/j.cell.2020.01.015. [DOI] [PubMed] [Google Scholar]
- 10. Vanlandewijck M, He L, Mäe MA, Andrae J, Ando K, Del Gaudio F, Nahar K, Lebouvier T, Laviña B, Gouveia L, Sun Y, Raschperger E, Rasanen M, Zarb Y, Mochizuki N, Keller A, Lendahl U, Betsholtz C. A molecular atlas of cell types and zonation in the brain vasculature. Nature 554: 475–480, 2018. [Erratum in Nature 560: E3, 2018]. doi: 10.1038/nature25739. [DOI] [PubMed] [Google Scholar]
- 11. Shih AY, Rühlmann C, Blinder P, Devor A, Drew PJ, Friedman B, Knutsen PM, Lyden PD, Mateo C, Mellander L, Nishimura N, Schaffer CB, Tsai PS, Kleinfeld D. Robust and fragile aspects of cortical blood flow in relation to the underlying angioarchitecture. Microcirculation 22: 204–218, 2015. doi: 10.1111/micc.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mäe MA, He L, Nordling S, Vazquez-Liebanas E, Nahar K, Jung B, Li X, Tan BC, Chin Foo J, Cazenave-Gassiot A, Wenk MR, Zarb Y, Lavina B, Quaggin SE, Jeansson M, Gu C, Silver DL, Vanlandewijck M, Butcher EC, Keller A, Betsholtz C. Single-cell analysis of blood-brain barrier response to pericyte loss. Circ Res 128: e46–e62, 2021. doi: 10.1161/CIRCRESAHA.120.317473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cleuren ACA, van der Ent MA, Jiang H, Hunker KL, Yee A, Siemieniak DR, Molema G, Aird WC, Ganesh SK, Ginsburg D. The in vivo endothelial cell translatome is highly heterogeneous across vascular beds. Proc Natl Acad Sci USA 116: 23618–23624, 2019. doi: 10.1073/pnas.1912409116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jambusaria A, Hong Z, Zhang L, Srivastava S, Jana A, Toth PT, Dai Y, Malik AB, Rehman J. Endothelial heterogeneity across distinct vascular beds during homeostasis and inflammation. eLife 9: e51413, 2020. doi: 10.7554/eLife.51413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kiss T, Nyúl-Tóth Á, Balasubramanian P, Tarantini S, Ahire C, DelFavero J, Yabluchanskiy A, Csipo T, Farkas E, Wiley G, Garman L, Csiszar A, Ungvari Z. Single-cell RNA sequencing identifies senescent cerebromicrovascular endothelial cells in the aged mouse brain. Geroscience 42: 429–444, 2020. doi: 10.1007/s11357-020-00177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Quaegebeur A, Lange C, Carmeliet P. The neurovascular link in health and disease: molecular mechanisms and therapeutic implications. Neuron 71: 406–424, 2011. doi: 10.1016/j.neuron.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 17. Driscoll JD, Shih AY, Drew PJ, Cauwenberghs G, Kleinfeld D. Two-photon imaging of blood flow in the rat cortex. Cold Spring Harb Protoc 2013: 759–767, 2013. doi: 10.1101/pdb.prot076513. [DOI] [PubMed] [Google Scholar]
- 18. Simionescu M, Simionescu N, Palade GE. Segmental differentiations of cell junctions in the vascular endothelium. The microvasculature. J Cell Biol 67: 863–885, 1975. doi: 10.1083/jcb.67.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Augustin HG, Koh GY. Organotypic vasculature: from descriptive heterogeneity to functional pathophysiology. Science 357: eaal2379, 2017. doi: 10.1126/science.aal2379. [DOI] [PubMed] [Google Scholar]
- 20. Rohlenova K, Goveia J, García-Caballero M, Subramanian A, Kalucka J, Treps L, et al. Single-cell RNA sequencing maps endothelial metabolic plasticity in pathological angiogenesis. Cell Metab 31: 862–877.e14, 2020. doi: 10.1016/j.cmet.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 21. Saunders A, Macosko EZ, Wysoker A, Goldman M, Krienen FM, de Rivera H, Bien E, Baum M, Bortolin L, Wang S, Goeva A, Nemesh J, Kamitaki N, Brumbaugh S, Kulp D, McCarroll SA. Molecular diversity and specializations among the cells of the adult mouse brain. Cell 174: 1015–1030.e16, 2018. doi: 10.1016/j.cell.2018.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hartmann DA, Coelho-Santos V, Shih AY. Pericyte control of blood flow across microvascular zones in the central nervous system. Annu Rev Physiol, 84: 331–354, 2021. doi: 10.1146/annurev-physiol-061121-040127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sweeney MD, Ayyadurai S, Zlokovic BV. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat. Neurosci 19: 771–783, 2016. doi: 10.1038/nn.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bohannon DG, Long D, Kim WK. Understanding the heterogeneity of human pericyte subsets in blood-brain barrier homeostasis and neurological diseases. Cells 10: 890, 2021. doi: 10.3390/cells10040890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Attwell D, Mishra A, Hall CN, O'Farrell FM, Dalkara T. What is a pericyte? J Cereb Blood Flow Metab 36: 451–455, 2016. doi: 10.1177/0271678X15610340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Blood-brain barrier: from physiology to disease and back. Physiol Rev 99: 21–78, 2019. doi: 10.1152/physrev.00050.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grant RI, Hartmann DA, Underly RG, Berthiaume AA, Bhat NR, Shih AY. Organizational hierarchy and structural diversity of microvascular pericytes in adult mouse cortex. J Cereb Blood Flow Metab 39: 411–425, 2019. doi: 10.1177/0271678X17732229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bernier LP, Brunner C, Cottarelli A, Balbi M. Location matters: navigating regional heterogeneity of the neurovascular unit. Front Cell Neurosci 15: 696540, 2021. doi: 10.3389/fncel.2021.696540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stark K, Pekayvaz K, Massberg S. Role of pericytes in vascular immunosurveillance. Front Biosci (Landmark Ed) 23: 767–781, 2018. doi: 10.2741/4615. [DOI] [PubMed] [Google Scholar]
- 30. Hartmann DA, Underly RG, Grant RI, Watson AN, Lindner V, Shih AY. Pericyte structure and distribution in the cerebral cortex revealed by high-resolution imaging of transgenic mice. Neurophotonics 2: 041402, 2015. doi: 10.1117/1.NPh.2.4.041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kisler K, Nikolakopoulou AM, Sweeney MD, Lazic D, Zhao Z, Zlokovic BV. Acute ablation of cortical pericytes leads to rapid neurovascular uncoupling. Front Cell Neurosci 14: 27, 2020. doi: 10.3389/fncel.2020.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gonzales AL, Klug NR, Moshkforoush A, Lee JC, Lee FK, Shui B, Tsoukias NM, Kotlikoff MI, Hill-Eubanks D, Nelson MT. Contractile pericytes determine the direction of blood flow at capillary junctions. Proc Natl Acad Sci USA 117: 27022–27033, 2020. doi: 10.1073/pnas.1922755117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dore-Duffy P, Wang S, Mehedi A, Katyshev V, Cleary K, Tapper A, Reynolds C, Ding Y, Zhan P, Rafols J, Kreipke CW. Pericyte-mediated vasoconstriction underlies TBI-induced hypoperfusion. Neurol Res 33: 176–186, 2011. doi: 10.1179/016164111X12881719352372. [DOI] [PubMed] [Google Scholar]
- 34. Ornelas S, Berthiaume AA, Bonney SK, Coelho-Santos V, Underly RG, Kremer A, Guérin CJ, Lippens S, Shih AY. Three-dimensional ultrastructure of the brain pericyte-endothelial interface. J Cereb Blood Flow Metab 41: 2185–2200, 2021. doi: 10.1177/0271678X211012836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hirschi KK, Burt JM, Hirschi KD, Dai C. Gap junction communication mediates transforming growth factor-beta activation and endothelial-induced mural cell differentiation. Circ Res 93: 429–437, 2003. doi: 10.1161/01.RES.0000091259.84556.D5. [DOI] [PubMed] [Google Scholar]
- 36. Lendahl U, Nilsson P, Betsholtz C. Emerging links between cerebrovascular and neurodegenerative diseases-a special role for pericytes. EMBO Rep 20: e48070, 2019. doi: 10.15252/embr.201948070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Uemura MT, Maki T, Ihara M, Lee VMY, Trojanowski JQ. Brain microvascular pericytes in vascular cognitive impairment and dementia. Front Aging Neurosci 12: 80, 2020. doi: 10.3389/fnagi.2020.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Winkler EA, Sagare AP, Zlokovic BV. The pericyte: a forgotten cell type with important implications for Alzheimer's disease? Brain Pathol 24: 371–386, 2014. doi: 10.1111/bpa.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Magtanong L, Dixon SJ. Ferroptosis and brain injury. Dev Neurosci 40: 382–395, 2018. doi: 10.1159/000496922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weiland A, Wang Y, Wu W, Lan X, Han X, Li Q, Wang J. Ferroptosis and its role in diverse brain diseases. Mol Neurobiol 56: 4880–4893, 2019. doi: 10.1007/s12035-018-1403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fang Y, Gao S, Wang X, Cao Y, Lu J, Chen S, Lenahan C, Zhang JH, Shao A, Zhang J. Programmed cell deaths and potential crosstalk with blood-brain barrier dysfunction after hemorrhagic stroke. Front Cell Neurosci 14: 68, 2020. doi: 10.3389/fncel.2020.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Majernikova N, den Dunnen WFA, Dolga AM. The potential of ferroptosis-targeting therapies for Alzheimer's disease: from mechanism to transcriptomic analysis. Front Aging Neurosci 13: 745046, 2021. [Erratum in Front Aging Neurosci 14: 863205, 2022]. doi: 10.3389/fnagi.2021.745046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. DeGregorio-Rocasolano N, Martí-Sistac O, Gasull T. Deciphering the iron side of stroke: neurodegeneration at the crossroads between iron dyshomeostasis, excitotoxicity, and ferroptosis. Front Neurosci 13: 85, 2019. doi: 10.3389/fnins.2019.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yan N, Zhang JJ. The emerging roles of ferroptosis in vascular cognitive impairment. Front Neurosci 13: 811, 2019. doi: 10.3389/fnins.2019.00811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149: 1060–1072, 2012. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascon S, Hatzios SK, Kagan VE, Noel K, Jiang X, Linkermann A, Murphy ME, Overholtzer M, Oyagi A, Pagnussat GC, Park J, Ran Q, Rosenfeld CS, Salnikow K, Tang D, Torti FM, Torti SV, Toyokuni S, Woerpel KA, Zhang DD. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171: 273–285, 2017. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chen X, Comish PB, Tang D, Kang R. Characteristics and biomarkers of ferroptosis. Front Cell Dev Biol 9: 637162, 2021. doi: 10.3389/fcell.2021.637162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Diao X, Cui Q, Tian N, Zhou Z, Xiang W, Jiang Y, Deng J, Liao H, Lin X, Li Q, Liao R. Hemorrhage-induced sphingosine kinase 1 contributes to ferroptosis-mediated secondary brain injury in intracerebral hemorrhage. Mol Neurobiol 59: 1381–1397, 2022. doi: 10.1007/s12035-021-02605-5. [DOI] [PubMed] [Google Scholar]
- 49. Wan J, Ren H, Wang J. Iron toxicity, lipid peroxidation and ferroptosis after intracerebral haemorrhage. Stroke Vasc Neurol 4: 93–95, 2019. doi: 10.1136/svn-2018-000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zille M, Karuppagounder SS, Chen Y, Gough PJ, Bertin J, Finger J, Milner TA, Jonas EA, Ratan RR. Neuronal death after hemorrhagic stroke in vitro and in vivo shares features of ferroptosis and necroptosis. Stroke 48: 1033–1043, 2017. doi: 10.1161/STROKEAHA.116.015609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zille M, Oses-Prieto JA, Savage SR, Karuppagounder SS, Chen Y, Kumar A, Morris JH, Scheidt KA, Burlingame AL, Ratan RR. Hemin-induced death models hemorrhagic stroke and is a variant of classical neuronal ferroptosis. J Neurosci 42: 2065–2079, 2022. doi: 10.1523/JNEUROSCI.0923-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Imai T, Iwata S, Hirayama T, Nagasawa H, Nakamura S, Shimazawa M, Hara H. Intracellular Fe(2+) accumulation in endothelial cells and pericytes induces blood-brain barrier dysfunction in secondary brain injury after brain hemorrhage. Sci Rep 9: 6228, 2019. doi: 10.1038/s41598-019-42370-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bruno A, Levine SR, Frankel MR, Brott TG, Lin Y, Tilley BC, Lyden PD, Broderick JP, Kwiatkowski TG, Fineberg SE; NINDS rt-PA Stroke Study Group. Admission glucose level and clinical outcomes in the NINDS rt-PA stroke trial. Neurology 59: 669–674, 2002. doi: 10.1212/wnl.59.5.669. [DOI] [PubMed] [Google Scholar]
- 54. Hafez S, Coucha M, Bruno A, Fagan SC, Ergul A. Hyperglycemia, acute ischemic stroke, and thrombolytic therapy. Transl Stroke Res 5: 442–453, 2014. doi: 10.1007/s12975-014-0336-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Thomas SE, Plumber N, Venkatapathappa P, Gorantla V. A review of risk factors and predictors for hemorrhagic transformation in patients with acute ischemic stroke. Int J Vasc Med 2021: 4244267, 2021. doi: 10.1155/2021/4244267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Larrue V, von Kummer RR, Muller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II). Stroke 32: 438–441, 2001. doi: 10.1161/01.str.32.2.438. [DOI] [PubMed] [Google Scholar]
- 57. Yong M, Kaste M. Dynamic of hyperglycemia as a predictor of stroke outcome in the ECASS-II trial. Stroke 39: 2749–2755, 2008. doi: 10.1161/STROKEAHA.108.514307. [DOI] [PubMed] [Google Scholar]
- 58. Chen D, Sui L, Chen C, Liu S, Sun X, Guan J. Atorvastatin suppresses NLRP3 inflammasome activation in intracerebral hemorrhage via TLR4- and MyD88-dependent pathways. Aging (Albany NY) 14: 462–476, 2022. doi: 10.18632/aging.203824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Xiong XY, Liu L, Wang FX, Yang YR, Hao JW, Wang PF, Zhong Q, Zhou K, Xiong A, Zhu WY, Zhao T, Meng ZY, Wang YC, Gong QW, Liao MF, Wang J, Yang QW. Toll-like receptor 4/MyD88-mediated signaling of hepcidin expression causing brain iron accumulation, oxidative injury, and cognitive impairment after intracerebral hemorrhage. Circulation 134: 1025–1038, 2016. doi: 10.1161/CIRCULATIONAHA.116.021881. [DOI] [PubMed] [Google Scholar]
- 60. Piera-Velazquez S, Jimenez SA. Endothelial to mesenchymal transition: role in physiology and in the pathogenesis of human diseases. Physiol Rev 99: 1281–1324, 2019. doi: 10.1152/physrev.00021.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. van Meeteren LA, ten Dijke P. Regulation of endothelial cell plasticity by TGF-β. Cell Tissue Res 347: 177–186, 2012. doi: 10.1007/s00441-011-1222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nakajima Y, Yamagishi T, Hokari S, Nakamura H. Mechanisms involved in valvuloseptal endocardial cushion formation in early cardiogenesis: roles of transforming growth factor (TGF)-beta and bone morphogenetic protein (BMP). Anat Rec 258: 119–127, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 63. Pérez L, Muñoz-Durango N, Riedel CA, Echeverria C, Kalergis AM, Cabello-Verrugio C, Simon F. Endothelial-to-mesenchymal transition: cytokine-mediated pathways that determine endothelial fibrosis under inflammatory conditions. Cytokine Growth Factor Rev 33: 41–54, 2017. doi: 10.1016/j.cytogfr.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 64. Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res 67: 10123–10128, 2007. doi: 10.1158/0008-5472.CAN-07-3127. [DOI] [PubMed] [Google Scholar]
- 65. Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 13: 952–961, 2007. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 66. Alvandi Z, Bischoff J. Endothelial-mesenchymal transition in cardiovascular disease. Arterioscler Thromb Vasc Biol 41: 2357–2369, 2021. doi: 10.1161/ATVBAHA.121.313788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kovacic JC, Dimmeler S, Harvey RP, Finkel T, Aikawa E, Krenning G, Baker AH. Endothelial to mesenchymal transition in cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol 73: 190–209, 2019. doi: 10.1016/j.jacc.2018.09.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Morikawa M, Derynck R, Miyazono K. TGF-β and the TGF-β family: context-dependent roles in cell and tissue physiology. Cold Spring Harb Perspect Biol 8: a021873, 2016. doi: 10.1101/cshperspect.a021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Derada Troletti C, de Goede P, Kamermans A, de Vries HE. Molecular alterations of the blood-brain barrier under inflammatory conditions: the role of endothelial to mesenchymal transition. Biochim Biophys Acta 1862: 452–460, 2016. doi: 10.1016/j.bbadis.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 70. Bravi L, Malinverno M, Pisati F, Rudini N, Cuttano R, Pallini R, Martini M, Larocca LM, Locatelli M, Levi V, Bertani GA, Dejana E, Lampugnani MG. Endothelial cells lining sporadic cerebral cavernous malformation cavernomas undergo endothelial-to-mesenchymal transition. Stroke 47: 886–890, 2016. doi: 10.1161/STROKEAHA.115.011867. [DOI] [PubMed] [Google Scholar]
- 71. Maddaluno L, Rudini N, Cuttano R, Bravi L, Giampietro C, Corada M, Ferrarini L, Orsenigo F, Papa E, Boulday G, Tournier-Lasserve E, Chapon F, Richichi C, Retta SF, Lampugnani MG, Dejana E. EndMT contributes to the onset and progression of cerebral cavernous malformations. Nature 498: 492–496, 2013. doi: 10.1038/nature12207. [DOI] [PubMed] [Google Scholar]
- 72. Shoemaker LD, McCormick AK, Allen BM, Chang SD. Evidence for endothelial-to-mesenchymal transition in human brain arteriovenous malformations. Clin Transl Med 10: e99, 2020. doi: 10.1002/ctm2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tang AT, Choi JP, Kotzin JJ, Yang Y, Hong CC, Hobson N, Girard R, Zeineddine HA, Lightle R, Moore T, Cao Y, Shenkar R, Chen M, Mericko P, Yang J, Li L, Tanes C, Kobuley D, Võsa U, Whitehead KJ, Li DY, Franke L, Hart B, Schwaninger M, Henao-Mejia J, Morrison L, Kim H, Awad IA, Zheng X, Kahn ML. Endothelial TLR4 and the microbiome drive cerebral cavernous malformations. Nature 545: 305–310, 2017. doi: 10.1038/nature22075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bai Y, Zhang Y, Han B, Yang L, Chen X, Huang R, Wu F, Chao J, Liu P, Hu G, Zhang JH, Yao H. Circular RNA DLGAP4 Ameliorates Ischemic Stroke Outcomes by Targeting miR-143 to regulate endothelial-mesenchymal transition associated with blood-brain barrier integrity. J Neurosci 38: 32–50, 2018. [Erratum in J Neurosci 40: 8601, 2020]. doi: 10.1523/JNEUROSCI.1348-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chen D, Li L, Wang Y, Xu R, Peng S, Zhou L, Deng Z. Ischemia-reperfusion injury of brain induces endothelial-mesenchymal transition and vascular fibrosis via activating let-7i/TGF-βR1 double-negative feedback loop. FASEB J 34: 7178–7191, 2020. doi: 10.1096/fj.202000201R. [DOI] [PubMed] [Google Scholar]
- 76. Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka F, Andrulis M, Pascual G, Morris KJ, Khan S, Jin H, Dharmalingam G, Snijders AP, Carroll T, Capper D, Pritchard C, Inman GJ, Longerich T, Sansom OJ, Benitah SA, Zender L, Gil J. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol 15: 978–990, 2013. doi: 10.1038/ncb2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Childs BG, Durik M, Baker DJ, van Deursen JM. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med 21: 1424–1435, 2015. doi: 10.1038/nm.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. He S, Sharpless NE. Senescence in health and disease. Cell 169: 1000–1011, 2017. doi: 10.1016/j.cell.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Burton DG, Krizhanovsky V. Physiological and pathological consequences of cellular senescence. Cell Mol Life Sci 71: 4373–4386, 2014. doi: 10.1007/s00018-014-1691-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sikora E, Bielak-Zmijewska A, Dudkowska M, Krzystyniak A, Mosieniak G, Wesierska M, Wlodarczyk J. Cellular senescence in brain aging. Front Aging Neurosci 13: 646924, 2021. doi: 10.3389/fnagi.2021.646924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sikora E, Bielak-Zmijewska A, Mosieniak G. A common signature of cellular senescence; does it exist? Ageing Res Rev 71: 101458, 2021. doi: 10.1016/j.arr.2021.101458. [DOI] [PubMed] [Google Scholar]
- 82. Gorgoulis V, Adams PD, Alimonti A, Bennett DC, Bischof O, Bishop C, Campisi J, Collado M, Evangelou K, Ferbeyre G, Gil J, Hara E, Krizhanovsky V, Jurk D, Maier AB, Narita M, Niedernhofer L, Passos JF, Robbins PD, Schmitt CA, Sedivy J, Vougas K, von Zglinicki T, Zhou D, Serrano M, Demaria M. Cellular senescence: defining a path forward. Cell 179: 813–827, 2019. doi: 10.1016/j.cell.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 83. Liu J, Wang L, Wang Z, Liu JP. Roles of telomere biology in cell senescence, replicative and chronological ageing. Cells 8: 54, 2019. doi: 10.3390/cells8010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Burton DGA, Faragher RGA. Obesity and type-2 diabetes as inducers of premature cellular senescence and ageing. Biogerontology 19: 447–459, 2018. doi: 10.1007/s10522-018-9763-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Palmer AK, Tchkonia T, LeBrasseur NK, Chini EN, Xu M, Kirkland JL. Cellular senescence in type 2 diabetes: a therapeutic opportunity. Diabetes 64: 2289–2298, 2015. doi: 10.2337/db14-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Burton DG, Faragher RG. Cellular senescence: from growth arrest to immunogenic conversion. Age (Dordr) 37: 27, 2015. doi: 10.1007/s11357-015-9764-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Haspula D, Vallejos AK, Moore TM, Tomar N, Dash RK, Hoffmann BR. Influence of a hyperglycemic microenvironment on a diabetic versus healthy rat vascular endothelium reveals distinguishable mechanistic and phenotypic responses. Front Physiol 10: 558, 2019. doi: 10.3389/fphys.2019.00558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Prattichizzo F, De Nigris V, Mancuso E, Spiga R, Giuliani A, Matacchione G, Lazzarini R, Marcheselli F, Recchioni R, Testa R, La Sala L, Rippo MR, Procopio AD, Olivieri F, Ceriello A. Short-term sustained hyperglycaemia fosters an archetypal senescence-associated secretory phenotype in endothelial cells and macrophages. Redox Biol 15: 170–181, 2018. doi: 10.1016/j.redox.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Rhee SY, Kim YS. The role of advanced glycation end products in diabetic vascular complications. Diabetes Metab J 42: 188–195, 2018. doi: 10.4093/dmj.2017.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Senthil KKJ, Gokila VM, Wang SY. Activation of Nrf2-mediated anti-oxidant genes by antrodin C prevents hyperglycemia-induced senescence and apoptosis in human endothelial cells. Oncotarget 8: 96568–96587, 2017. doi: 10.18632/oncotarget.19951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bryant AG, Hu M, Carlyle BC, Arnold SE, Frosch MP, Das S, Hyman BT, Bennett RE. Cerebrovascular senescence is associated with tau pathology in Alzheimer's disease. Front Neurol 11: 575953, 2020. doi: 10.3389/fneur.2020.575953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bury JJ, Chambers A, Heath PR, Ince PG, Shaw PJ, Matthews FE, Brayne C, Simpson JE, Wharton SB, Cognitive F, Ageing S; Cognitive Function and Ageing Study. Type 2 diabetes mellitus-associated transcriptome alterations in cortical neurones and associated neurovascular unit cells in the ageing brain. Acta Neuropathol Commun 9: 5, 2021. doi: 10.1186/s40478-020-01109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Vannier DR, Shapeti A, Chuffart F, Planus E, Manet S, Rivier P, Destaing O, Albiges-Rizo C, Van Oosterwyck H, Faurobert E. CCM2-deficient endothelial cells undergo a ROCK-dependent reprogramming into senescence-associated secretory phenotype. Angiogenesis 24: 843–860, 2021. doi: 10.1007/s10456-021-09809-2. [DOI] [PubMed] [Google Scholar]
- 94. Yamazaki Y, Baker DJ, Tachibana M, Liu CC, van Deursen JM, Brott TG, Bu G, Kanekiyo T. Vascular cell senescence contributes to blood-brain barrier breakdown. Stroke 47: 1068–1077, 2016. doi: 10.1161/STROKEAHA.115.010835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Liu Y, Zhang H, Wang S, Guo Y, Fang X, Zheng B, Gao W, Yu H, Chen Z, Roman RJ, Fan F. Reduced pericyte and tight junction coverage in old diabetic rats are associated with hyperglycemia-induced cerebrovascular pericyte dysfunction. Am J Physiol Heart Circ Physiol 320: H549–H562, 2021. doi: 10.1152/ajpheart.00726.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Prakash R, Somanath PR, El-Remessy AB, Kelly-Cobbs A, Stern JE, Dore-Duffy P, Johnson M, Fagan SC, Ergul A. Enhanced cerebral but not peripheral angiogenesis in the Goto-Kakizaki model of type 2 diabetes involves VEGF and peroxynitrite signaling. Diabetes 61: 1533–1542, 2012. doi: 10.2337/db11-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Prakash R, Johnson M, Fagan SC, Ergul A. Cerebral neovascularization and remodeling patterns in two different models of type 2 diabetes. PLoS One 8: e56264, 2013. doi: 10.1371/journal.pone.0056264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Horton WB, Barrett EJ. Microvascular dysfunction in diabetes mellitus and cardiometabolic disease. Endocr Rev 42: 29–55, 2021. doi: 10.1210/endrev/bnaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sweeney MD, Kisler K, Montagne A, Toga AW, Zlokovic BV. The role of brain vasculature in neurodegenerative disorders. Nat Neurosci 21: 1318–1331, 2018. doi: 10.1038/s41593-018-0234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Fouda AY, Fagan SC, Ergul A. Brain vasculature and cognition. Arterioscler Thromb Vasc Biol 39: 593–602, 2019. doi: 10.1161/ATVBAHA.118.311906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Moore SM, Zhang H, Maeda N, Doerschuk CM, Faber JE. Cardiovascular risk factors cause premature rarefaction of the collateral circulation and greater ischemic tissue injury. Angiogenesis 18: 265–281, 2015. doi: 10.1007/s10456-015-9465-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Li W, Prakash R, Kelly-Cobbs AI, Ogbi S, Kozak A, El-Remessy AB, Schreihofer DA, Fagan SC, Ergul A. Adaptive cerebral neovascularization in a model of type 2 diabetes: relevance to focal cerebral ischemia. Diabetes 59: 228–235, 2010. doi: 10.2337/db09-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Abdelsaid M, Coucha M, Hafez S, Yasir A, Johnson MH, Ergul A. Enhanced VEGF signalling mediates cerebral neovascularisation via downregulation of guidance protein ROBO4 in a rat model of diabetes. Diabetologia 60: 740–750, 2017. doi: 10.1007/s00125-017-4214-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Abdelsaid M, Kaczmarek J, Coucha M, Ergul A. Dual endothelin receptor antagonism with bosentan reverses established vascular remodeling and dysfunctional angiogenesis in diabetic rats: relevance to glycemic control. Life Sci 118: 268–273, 2014. doi: 10.1016/j.lfs.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wardlaw JM, Benveniste H, Nedergaard M, Zlokovic BV, Mestre H, Lee H, Doubal FN, Brown R, Ramirez J, MacIntosh BJ, Tannenbaum A, Ballerini L, Rungta RL, Boido D, Sweeney M, Montagne A, Charpak S, Joutel A, Smith KJ, Black SE; colleagues from the Fondation Leducq Transatlantic Network of Excellence on the Role of the Perivascular Space in Cerebral Small Vessel D. Perivascular spaces in the brain: anatomy, physiology and pathology. Nat Rev Neurol 16: 137–153, 2020. doi: 10.1038/s41582-020-0312-z. [DOI] [PubMed] [Google Scholar]
- 106. Brown R, Benveniste H, Black SE, Charpak S, Dichgans M, Joutel A, Nedergaard M, Smith KJ, Zlokovic BV, Wardlaw JM. Understanding the role of the perivascular space in cerebral small vessel disease. Cardiovasc Res 114: 1462–1473, 2018. doi: 10.1093/cvr/cvy113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Chandran R, Li W, Ahmed HA, Dong G, Ward RA, He L, Doueiry C, Ergul A. Diabetic rats are more susceptible to cognitive decline in a model of microemboli-mediated vascular contributions to cognitive impairment and dementia. Brain Res 1749: 147132, 2020. doi: 10.1016/j.brainres.2020.147132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Liu Y, Chen D, Smith A, Ye Q, Gao Y, Zhang W. Three-dimensional remodeling of functional cerebrovascular architecture and gliovascular unit in leptin receptor-deficient mice. J Cereb Blood Flow Metab 41: 1547–1562, 2021. doi: 10.1177/0271678X211006596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Li W, Valenzuela JP, Ward R, Abdelbary M, Dong G, Fagan SC, Ergul A. Post-stroke neovascularization and functional outcomes differ in diabetes depending on severity of injury and sex: potential link to hemorrhagic transformation. Exp Neurol 311: 106–114, 2019. doi: 10.1016/j.expneurol.2018.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Alarcon-Martinez L, Shiga Y, Villafranca-Baughman D, Belforte N, Quintero H, Dotigny F, Cueva Vargas JL, Di Polo A. Pericyte dysfunction and loss of interpericyte tunneling nanotubes promote neurovascular deficits in glaucoma. Proc Natl Acad Sci USA 119: e2110329119, 2022. doi: 10.1073/pnas.2110329119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Duh EJ, Sun JK, Stitt AW. Diabetic retinopathy: current understanding, mechanisms, and treatment strategies. JCI Insight 2: e93751, 2017. doi: 10.1172/jci.insight.93751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hanaguri J, Yokota H, Kushiyama A, Kushiyama S, Watanabe M, Yamagami S, Nagaoka T. The effect of sodium-dependent glucose cotransporter 2 inhibitor tofogliflozin on neurovascular coupling in the retina in type 2 diabetic mice. Int J Mol Sci 23: 1362, 2022. doi: 10.3390/ijms23031362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Rojas M, Lemtalsi T, Toque HA, Xu Z, Fulton D, Caldwell RW, Caldwell RB. NOX2-induced activation of arginase and diabetes-induced retinal endothelial cell senescence. Antioxidants (Basel) 6: 43, 2017. doi: 10.3390/antiox6020043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Shosha E, Xu Z, Narayanan SP, Lemtalsi T, Fouda AY, Rojas M, Xing J, Fulton D, Caldwell RW, Caldwell RB. Mechanisms of diabetes-induced endothelial cell senescence: role of arginase 1. Int J Mol Sci 19: 1215, 2018. doi: 10.3390/ijms19041215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ward R, Valenzuela JP, Li W, Dong G, Fagan SC, Ergul A. Poststroke cognitive impairment and hippocampal neurovascular remodeling: the impact of diabetes and sex. Am J Physiol Heart Circ Physiol 315: H1402–H1413, 2018. doi: 10.1152/ajpheart.00390.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wolf V, Abdul Y, Li W, Ergul A. Impact of diabetes and ischemic stroke on the cerebrovasculature: a female perspective. Neurobiol Dis 167: 105667, 2022. doi: 10.1016/j.nbd.2022.105667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Sweetnam D, Holmes A, Tennant KA, Zamani A, Walle M, Jones P, Wong C, Brown CE. Diabetes impairs cortical plasticity and functional recovery following ischemic stroke. J Neurosci 32: 5132–5143, 2012. doi: 10.1523/JNEUROSCI.5075-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ergul A, Abdelsaid M, Fouda AY, Fagan SC. Cerebral neovascularization in diabetes: implications for stroke recovery and beyond. J Cereb Blood Flow Metab 34: 553–563, 2014. doi: 10.1038/jcbfm.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ergul A, Valenzuela JP, Fouda AY, Fagan SC. Cellular connections, microenvironment and brain angiogenesis in diabetes: lost communication signals in the post-stroke period. Brain Res 1623: 81–96, 2015. doi: 10.1016/j.brainres.2015.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ergul A, Elgebaly MM, Middlemore ML, Li W, Elewa H, Switzer JA, Hall C, Kozak A, Fagan SC. Increased hemorrhagic transformation and altered infarct size and localization after experimental stroke in a rat model type 2 diabetes. BMC Neurol 7: 33, 2007. doi: 10.1186/1471-2377-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Li W, Qu Z, Prakash R, Chung C, Ma H, Hoda MN, Fagan SC, Ergul A. Comparative analysis of the neurovascular injury and functional outcomes in experimental stroke models in diabetic Goto-Kakizaki rats. Brain Res 1541: 106–114, 2013. doi: 10.1016/j.brainres.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Li W, Ward R, Valenzuela JP, Dong G, Fagan SC, Ergul A. Diabetes worsens functional outcomes in young female rats: comparison of stroke models, tissue plasminogen activator effects, and sexes. Transl Stroke Res, 2017. doi: 10.1007/s12975-017-0525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Prakash R, Li W, Qu Z, Johnson MA, Fagan SC, Ergul A. Vascularization pattern after ischemic stroke is different in control versus diabetic rats: relevance to stroke recovery. Stroke 44: 2875–2882, 2013. doi: 10.1161/STROKEAHA.113.001660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Abdelsaid M, Prakash R, Li W, Coucha M, Hafez S, Johnson MH, Fagan SC, Ergul A. Metformin treatment in the period after stroke prevents nitrative stress and restores angiogenic signaling in the brain in diabetes. Diabetes 64: 1804–1817, 2015. doi: 10.2337/db14-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Abdul Y, Li W, Ward R, Abdelsaid M, Hafez S, Dong G, Jamil S, Wolf V, Johnson MH, Fagan SC, Ergul A. Deferoxamine treatment prevents post-stroke vasoregression and neurovascular unit remodeling leading to improved functional outcomes in type 2 male diabetic rats: role of endothelial ferroptosis. Transl Stroke Res 12: 615–630, 2021. doi: 10.1007/s12975-020-00844-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Ye X, Chopp M, Cui X, Zacharek A, Cui Y, Yan T, Shehadah A, Roberts C, Liu X, Lu M, Chen J. Niaspan enhances vascular remodeling after stroke in type 1 diabetic rats. Exp Neurol 232: 299–308, 2011. doi: 10.1016/j.expneurol.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Tennant KA, Brown CE. Diabetes augments in vivo microvascular blood flow dynamics after stroke. J Neurosci 33: 19194–19204, 2013. doi: 10.1523/JNEUROSCI.3513-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Gupta NC, Davis CM, Nelson JW, Young JM, Alkayed NJ. Soluble epoxide hydrolase: sex differences and role in endothelial cell survival. Arterioscler Thromb Vasc Biol 32: 1936–1942, 2012. doi: 10.1161/ATVBAHA.112.251520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Abdul Y, Abdelsaid M, Li W, Webb RC, Sullivan JC, Dong G, Ergul A. Inhibition of toll-like receptor-4 (TLR-4) improves neurobehavioral outcomes after acute ischemic stroke in diabetic rats: possible role of vascular endothelial TLR-4. Mol Neurobiol 56: 1607–1617, 2019. doi: 10.1007/s12035-018-1184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Azam S, Jakaria M, Kim IS, Kim J, Haque ME, Choi DK. Regulation of toll-like receptor (TLR) signaling pathway by polyphenols in the treatment of age-linked neurodegenerative diseases: focus on TLR4 signaling. Front Immunol 10: 1000, 2019. doi: 10.3389/fimmu.2019.01000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Wilhelm I, Nyúl-Tóth Á, Kozma M, Farkas AE, Krizbai IA. Role of pattern recognition receptors of the neurovascular unit in inflamm-aging. Am J Physiol Heart Circ Physiol 313: H1000–H1012, 2017. doi: 10.1152/ajpheart.00106.2017. [DOI] [PubMed] [Google Scholar]
- 132. Zhan X, Stamova B, Sharp FR. Lipopolysaccharide associates with amyloid plaques, neurons and oligodendrocytes in Alzheimer's disease brain: a review. Front Aging Neurosci 10: 42, 2018. doi: 10.3389/fnagi.2018.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Li W, Abdul Y, Ward R, Ergul A. Endothelin and diabetic complications: a brain-centric view. Physiol Res 67: S83–S94, 2018. doi: 10.33549/physiolres.933833. [DOI] [PubMed] [Google Scholar]
- 134. Cheng G, Huang C, Deng H, Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J 42: 484–491, 2012. doi: 10.1111/j.1445-5994.2012.02758.x. [DOI] [PubMed] [Google Scholar]
- 135. Biessels GJ, Despa F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol 14: 591–604, 2018. doi: 10.1038/s41574-018-0048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Srikanth V, Sinclair AJ, Hill-Briggs F, Moran C, Biessels GJ. Type 2 diabetes and cognitive dysfunction-towards effective management of both comorbidities. Lancet Diabetes Endocrinol 8: 535–545, 2020. doi: 10.1016/S2213-8587(20)30118-2. [DOI] [PubMed] [Google Scholar]