Abstract
Patients with heart failure with preserved ejection fraction (HFpEF) have few pharmacologic therapies, and it is not known if supplementing with ubiquinol and/or d-ribose could improve outcomes. The overall objective of this study was to determine if ubiquinol and/ or d-ribose would reduce the symptoms and improve cardiac performance in patients with HFpEF. This was a phase 2 randomized, double-blind, placebo-controlled trial of 216 patients with HFpEF who were ≥ 50 years old with a left ventricular ejection fraction (EF) ≥ 50%. A total of 4 study groups received various supplements over 12 weeks: Group 1 received placebo ubiquinol capsules and d-ribose powder, Group 2 received ubiquinol capsules (600 mg/d) and placebo d-ribose powder, Group 3 received placebo ubiquinol capsules with d-ribose powder (15 g/d), and Group 4 received ubiquinol capsules and d-ribose powder. There were 7 outcome measures for this study: Kansas City Cardiomyopathy Questionnaire (KCCQ) clinical summary score, level of vigor using a subscale from the Profile of Mood States, EF, the ratio of mitral peak velocity of early filling to early diastolic mitral annular velocity (septal E/e’ ratio), B-type natriuretic peptides, lactate/adenosine triphosphate ratio, and the 6-minute walk test. Treatment with ubiquinol and/or d-ribose significantly improved the KCCQ clinical summary score (17.30 to 25.82 points), vigor score (7.65 to 8.15 points), and EF (7.08% to 8.03%) and reduced B-type natriuretic peptides (−72.02 to −47.51) and lactate/adenosine triphosphate ratio (−4.32 to −3.35 × 10 −4). There were no significant increases in the septal E/e’ or the 6-minute walk test. In conclusion, ubiquinol and d-ribose reduced the symptoms of HFpEF and increased the EF. These findings support the use of these supplements in addition to standard therapeutic treatments for patients with HFpEF.
Introduction
Heart failure with preserved ejection fraction (HFpEF), also called diastolic HF, is a debilitating cardiovascular disease. It is characterized by left ventricular diastolic dysfunction defined by the heart’s inability to sufficiently fill with blood during the diastolic period between contractions.1 Treatment options for HFpEF patients currently focus on the management of symptoms, co-morbidities, and risk factors with lifestyle changes and pharmaceutical drugs. Currently, there are no effective therapies targeted for the underlying mechanisms of HFpEF.2 Researchers are investigating mitochondrial dysfunction and impaired cardiac bioenergetics as a possible pathophysiologic mechanism behind the pathogenesis of HFpEF. Treatments that target the mitochondrial pathway to increase the synthesis of adenosine triphosphate (ATP) may be beneficial for patients with HFpEF. Ubiquinol and d-ribose have been studied for their roles in increasing ATP production within the mitochondria, and it is hypothesized that supplementation of these 2 may improve the quality of life of patients with HFpEF.3 The overall objective was to determine if administering ubiquinol and/or d-ribose to patients with HFpEF for 12 weeks would decrease the severity of their complex symptoms and improve their cardiac function.
Methods
For this study, there were 6 hypotheses that are listed in Table 1. We used a randomized, double-blind, placebo-controlled study design. The overall objective was to determine if administering ubiquinol and/or d-ribose to patients with HFpEF for 12 weeks would decrease the severity of their complex symptoms and improve their cardiac function. All patients included in this study were 50 years old or older, with a left ventricular EF ≥ 50%; the other key inclusion and exclusion criteria are listed in Table 2. Detailed study methods and design were published before data collection.3 Once informed consent was obtained, subjects were provided a 1-week supply of placebo ubiquinol capsules (instructed to take 2 capsules per day) and placebo d-ribose powder (instructed to take 3 scoops per day) as a prerandomization run-in period to evaluate adherence to the supplements. Subjects were scheduled for an appointment at the Clinical and Translational Science Unit (CTSU) 1 week after the run-in period. Each subject who demonstrated an 80% adherence rate of ubiquinol intake (as evidenced by pill count) and an 80% adherence rate of d-ribose powder intake (as evidenced by weight of the containers) was eligible to be randomized into a group assignment. The enrolled patients were randomized to 1 of the 4 groups using 2 assignment lists created by a computer-based number generator, 1 for Black/African-Americans and the other for other races to ensure balanced groups with respect to race. The 4 groups were:
Placebo (Group 1): Subjects received no ubiquinol and no d-ribose; they received 2 placebo capsules and 3 scoops of placebo powder per day for 12 weeks.
Ubiquinol group (Group 2): Subjects received 600 mg (300-mg capsules × 2) of ubiquinol and 3 scoops of placebo powder per day for 12 weeks.
d-ribose group (Group 3): Subjects received 2 placebo capsules and 15 g of d-ribose per day (3 scoops) for 12 weeks.
Ubiquinol + d-ribose group (Group 4): Subjects received 600 mg (300-mg capsules × 2) of ubiquinol and 15 g of d-ribose (3 scoops) per day for 12 weeks.
Table 1.
List of hypotheses for the study
| Hypothesis 1: Ubiquinol (600 mg daily), D-ribose (15 g daily), or a combination of the two will enhance the health status of patients with HFpEF as measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ). |
| Hypothesis 2: Ubiquinol (600 mg daily), D-ribose (15 g daily), or a combination of the two will increase the level of vigor in patients with HFpEF as measured by the Vigor subscale of the Profile of Mood States. |
| Hypothesis 3: Ubiquinol (600 mg daily), D-ribose (15 g daily), or a combination of the two will improve ejection fraction and septal E/e’ ratio measured by echocardiographic imaging in patients with HFpEF. |
| Hypothesis 4: Ubiquinol (600 mg daily), D-ribose (15 g daily), or a combination of the two will increase the distance that patients with HFpEF can walk in 6 minutes. |
| Hypothesis 5: Ubiquinol (600 mg daily), D-ribose (15 g daily), or a combination of the two will decrease venous blood B-type natriuretic peptide (BNP) levels in patients with HFpEF. |
| Hypothesis 6: Ubiquinol (600 mg daily), D-ribose (15 g daily), or a combination of the two will decrease the lactate/ATP ratio in patients. |
ATP = Adenosine Triphosphate; BNP = B-type natriuretic peptide; E/e’ = E to early diastolic mitral annular tissue velocity; KCCQ = Kansas City Cardiomyopathy Questionnaire.
Table 2.
Study inclusion and exclusion criteria
| Inclusion Criteria: |
| 1. Have heart failure with preserved ejection fraction (HFpEF), New York HF classification II-III |
| 2. Be ≥ 50 years old |
| 3. Have a left ventricular ejection fraction ≥ 50% documented by an echocardiogram |
| 4. Have had HFpEF within a 6-month period |
| 5. Speak and read English |
| 6. Have a telephone or reliable phone contact |
| 7. Have their own way of transportation to the CTSU |
| Exclusion Criteria |
| 1. Have acute coronary syndrome in the past 12 weeks |
| 2. Have significant valvular heart disease |
| 3. Severe cardiac fibrosis (galectin-3 level > 26 ng/mL) |
| 4. Have constrictive pericardium |
| 5. Have had pulmonary fibrosis |
| 6. Have had congenital heart disease |
| 7. Have had hypertrophic or infiltrative cardiomyopathy |
| 8. Have had a heart transplant |
| 9. Have had a left ventricular assist device |
| 10. Recent percutaneous coronary intervention |
| 10. Have had HF associated hospital admission or ER visit within past 30 days |
| 11. Have had recent percutaneous coronary intervention |
| 12. Have had significant renal and/or hepatic dysfunction (ICD-10 code) |
| 13. Have had severe cognitive impairment |
| 14. Have had consumption of any CoQ10 (ubiquinol) or D-ribose supplements |
CTSU = Clinical and Translational Science Unit; HF = Heart Failure; HFpEF = Heart Failure with Preserved Ejection Fraction; ICD = International Classification of Disease; ng = nanograms.
Figure 1 illustrates the number of patients eligible for the study and the randomization of subjects into the 4 study groups. There was stratification for African-American race and the number of subjects who failed the run-in period or discontinued participation in the study. The reason for discontinuing in the study was then classified by consent withdrawn, loss of the subject to follow-up calls, nonadherence, or death. Only 1 subject died in the ubiquinol + d-ribose study group during the study period from unrelated causes.
Figure 1.
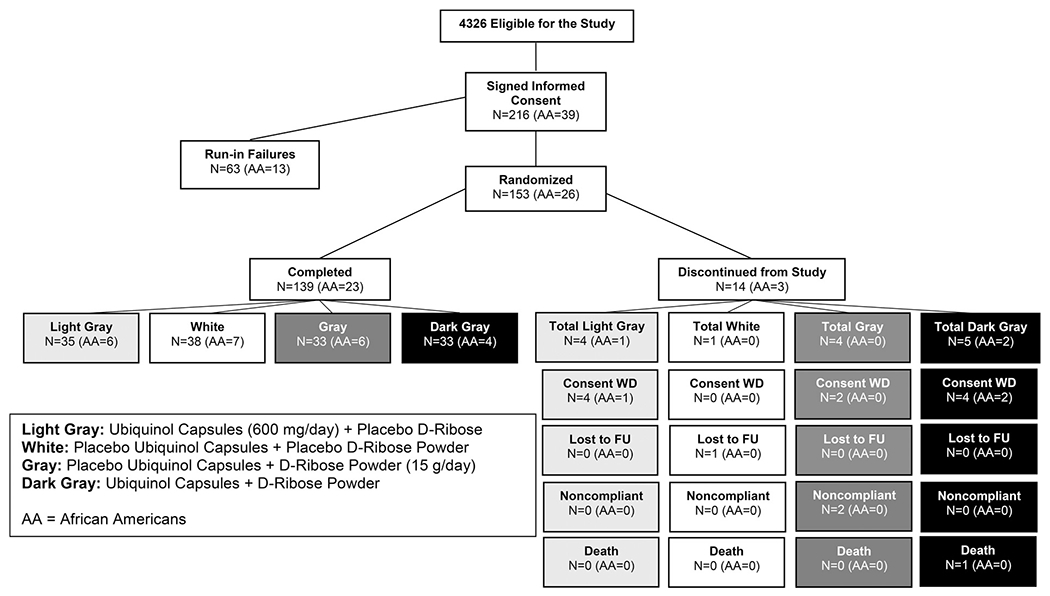
Patients eligible for the study and the randomization of subjects into the 4 study groups.
There were 7 major outcome measures for this clinical trial including: (1) Kansas City Cardiomyopathy Questionnaire (KCCQ), (2) Vigor scale from the Profile of Mood States (POMS), (3) ejection fraction (EF), (4) the ratio of mitral peak velocity of early filling (E) to early diastolic mitral annular velocity (e’) (E/e’ ratio), (5) B-type natriuretic peptide (BNP), (6) lactate/ATP ratio, and (7) the 6-minute walk test (6MWT). At the CTSU, each subject first completed the demographic form at baseline. Next, both the KCCQ and Vigor scale were completed at baseline and at 12 weeks. Participants’ perceptions of their HF symptoms were assessed using the KCCQ, a 23-item questionnaire to which subjects respond on a scale, indicating their limitations due to HF. Completion required in about 5 to 10 minutes. A total of 5 domains of their health status that were relevant to HFpEF were measured: (1) physical limitations, (2) symptoms, (3) self-efficacy, (4) quality of life, and (5) social interference. For example, to measure fatigue, the subject was asked how many times on average over the past 2 weeks has fatigue limited their ability to do what they want? The vigor subscale from the POMS questionnaire allowed patients to rate themselves on 8 adjectives (lively, active, energetic, cheerful, alert, full of pep, carefree, and vigorous) on a 5-point scale (0 = not at all, 1= a little, 2=moderately, 3 = quite a bit, and 4 = extremely). The total time for subjects to complete both questionnaires was approximately 15 minutes. We used the KCCQ and Vigor scale to compare the changes in health status and perception of vigor of subjects across the 4 study groups.
After completing the questionnaires, the subject underwent an echocardiogram that required 30 to 45 minutes to complete. We used a 2-dimensional Doppler echocardiography to measure EF and E/e’ ratio. After the echocardiogram, various blood samples were obtained. Concentrations of BNP and lactate were measured using a portable i-STAT handheld analyzer. Using a finger-stick method, we obtained a 100 μL blood sample at baseline and at 12 weeks to measure BNP concentration and lactate. The BNP measurement was collected before the 6MWT, and the lactate obtained after the 6MWT. For the ATP measurement, a finger-stick blood sample of 5 μL was collected after subjects had completed the questionnaires and echocardiograms. ATP production was measured in an indirect method using a serum lactate/ATP ratio. We also examined the effects of ubiquinol and/or d-ribose on the 6MWT at baseline and at 12 weeks.
The trial was designed to have a sample size of 276 subjects who were to be randomized to 1 of the 4 study groups (69 subjects per group). This sample size had a 98% power to detect an effect size of at least 0.81 in the KCCQ clinical summary score in patients with HFpEF, accounting for possible attrition and 4 comparisons with a Bonferroni correction at a nominal 0.05 significance level.3 Statistical analyses were conducted with the software SAS 9.4.4 The change in the KCCQ clinical summary score from baseline to the end of the 12 weeks of treatment was computed by subtracting the KCCQ score at baseline from the KCCQ score at 12 weeks. A 2-tailed independent sample t test was conducted to examine differences in mean change between subjects on ubiquinol and those on placebo. Similar tests were used to compare d-ribose with placebo, ubiquinol + d-ribose with placebo, and ubiquinol versus ubiquinol + d-ribose. To account for these 4 comparisons, 98.75% confidence intervals (CIs) for the differences in mean change were calculated, which achieved a Bonferroni-corrected nominal 95% level.
For each of the 7 outcome measures, a linear regression model of the change from baseline to Week 12 was used to examine whether ubiquinol and d-ribose interacted synergistically. As independent variables, the model included indicators for ubiquinol and d-ribose treatments as well as the product of the indicators (the interaction term), in addition to the demographics sex and race/ethnicity. A structural equation model was applied to investigate the mechanistic hypothesis that a decrease in lactate/ATP ratio mediated the association between ubiquinol treatment and increase in EF. We controlled for potential confounders such as demographics, baseline lactate/ATP ratio, and baseline EF.
Results
Due to a disruption in the planned study timeline caused by the COVID-19 pandemic and the consequential depletion of study funds, this double-blind study had to be terminated before reaching the target of 276 randomized subjects. A total of 216 participants were enrolled and 153 were randomized (placebo group, n = 39; ubiquinol group, n = 39; d-ribose group, n = 37; and ubiquinol and d-ribose group, n = 38). Overall, the completion rates were similar across the 4 groups (97.4% [38/39] in the placebo group, 89.7% [35/39] in the ubiquinol group, 89.2% [33/37] in the d-ribose group; and 86.8% [33/38] in the ubiquinol and d-ribose group). The most common reason for study discontinuation after randomization was withdrawal of consent that occurred during the COVID-19 pandemic (Figure 1).
Table 3 shows the demographic data of subjects who participated in the study with the mean age being 69 years. Table 4 is a comparison of baseline characteristics of study participants in the 4 study groups. Compared with placebo, ubiquinol, d-ribose, and the combination of the 2 significantly enhanced the health status of the subjects according to the KCCQ clinical summary scores (Table 5). Specifically, ubiquinol alone significantly increased the KCCQ scores from baseline to 12 weeks by 22.41 points [Bonferroni-corrected 95% CI, (9.37, 35.45); t = 4.40; df = 70; 2-sided p = 0.0001], d-ribose alone significantly increased the changes in KCCQ scores from baseline by 25.82 points [11.64, 40.00; t = 4.67; df = 68; p <0.0001], and the combination of ubiquinol and d-ribose significantly increased KCCQ score changes by 17.30 points [(2.93, 31.66); t = 3.09; df = 68; p = 0.0116]. The addition of d-ribose to ubiquinol treatment, although did not significantly contribute intention-to-treat analyses that assumed zero changes in the KCCQ scores for the subjects who did not complete the study, yielded similar results. A linear regression model of the changes in the KCCQ clinical summary score that included ubiquinol and d-ribose treatments and their interaction as independent variables suggested a significant antagonistic interaction between the 2 treatments (Figure 2). Group comparisons of symptoms from the KCCQ such as symptom burden and symptom frequencies are listed in Table 6.
Table 3.
Comparison of subjects who completed the 12 week visit vs noncompleters
| Total Randomized | N | Completers 139 |
Noncompleters 14 |
All 153 |
|---|---|---|---|---|
| Sex | ||||
| Female | N (% of column total) | 78 (56.1%) | 8 (57.1%) | 86 (56.2%) |
| Male | N (% of column total) | 61 (43.9%) | 6 (42.9%) | 67 (43.8%) |
| Ethnicity | ||||
| Hispanic or Latino | N (% of column total) | 9 (6.5%) | 0 (0.0%) | 9 (5.9%) |
| Non-Hispanic | N (% of column total) | 129 (92.8%) | 14 (100%) | 143 (93.5%) |
| Unknown | N (% of column total) | 1 (0.7%) | 0 (0.0%) | 1 (0.7%) |
| Race | ||||
| Black | N (% of column total) | 23 (16.5%) | 3 (21.4%) | 26 (17.0%) |
| American Indian or Alaska Native | N (% of column total) | 3 (2.2%) | 0 (0.0%) | 3 (2.0%) |
| Asian | N (% of column total) | 1 (0.7%) | 0 (0.0%) | 1 (0.7%) |
| Not Reported | N (% of column total) | 1 (0.7%) | 0 (0.0%) | 1 (0.7%) |
| Other | N (% of column total) | 4 (2.9%) | 0 (0.0%) | 4 (2.6%) |
| White | N (% of column total) | 107 (77.0%) | 11 (78.6%) | 118 (77.1%) |
| Is the participant a smoker? | ||||
| No | N (% of column total) | 130 (93.5%) | 14 (100%) | 144 (94.1%) |
| Yes | N (% of column total) | 9 (6.5%) | 0 (0.0%) | 9 (5.9%) |
| Does the participant have a history of or on medication for diabetes mellitus? | ||||
| No | N (% of column total) | 72 (51.8%) | 9 (64.3%) | 81 (52.9%) |
| Yes | N (% of column total) | 67 (48.2%) | 5 (35.7%) | 72 (47.1%) |
| Does the participant have a fasting total serum cholesterol level ≥ 4.9 mmol/L or take medication for hypercholesterolemia? | ||||
| No | N (% of column total) | 47 (33.8%) | 8 (57.1%) | 55 (35.9%) |
| Yes | N (% of column total) | 92 (66.2%) | 6 (42.9%) | 98 (64.1%) |
| Does the participant have either systolic or diastolic blood pressure ≥ 140/90 mmHg or take medication for hypertension? | ||||
| No | N (% of column total) | 28 (20.1%) | 4 (28.6%) | 32 (20.9%) |
| Yes | N (% of column total) | 111 (79.9%) | 10 (71.4%) | 121 (79.1%) |
| Does the participant have family history of cardiovascular disease in first-degree relatives before the age of 50 years? | ||||
| No | N (% of column total) | 103 (74.1%) | 14 (100%) | 117 (76.5%) |
| Yes | N (% of column total) | 36 (25.9%) | 0 (0.0%) | 36 (23.5%) |
| Age at Informed Consent | Mean (SD) | 68.7 (10.1) | 74.1 (9.0) | 69.2 (10.1) |
| Baseline KCCQ Clinical Summary Score | Mean (SD) | 47.6 (22.2) | 47.9 (17.2) | 47.6 (21.7) |
| Baseline Vigor Score | Mean (SD) | 13.3 (6.0) | 12.0 (6.3) | 13.1 (6.0) |
| Baseline Septal E/e’ | Mean (SD) | 9.9 (5.1) | 9.3 (6.1) | 9.9 (5.1) |
| Baseline 6MWT Total Distance Walked (m) | Mean (SD) | 268.1 (138.8) | 208.7 (150.1) | 262.7 (140.4) |
| Baseline BNP (pg/mL) | Mean (SD) | 183.1 (250.9) | 373.1 (372.6) | 200.6 (268.6) |
| Baseline Lactate (mmol/L) | Mean (SD) | 2.5 (1.2) | 2.7 (1.4) | 2.5 (1.2) |
| Baseline ATP (RLU) | Mean (SD) | 3312 (931.0) | 3124 (604.1) | 3295 (906.2) |
ATP = Adenosine Triphosphate; BNP = B-type natriuretic peptide; E/e’= E to early diastolic mitral annular tissue velocity; KCCQ = Kansas City Cardiomyopathy Questionnaire; RLU = Relative Light Units; SD = Standard Deviation = 6MWT= 6 Minute Walk Test.
Table 4.
Comparison of baseline characteristics of study participants in the four study groups
| Ubiquinol + D-Ribose |
D-Ribose |
Ubiquinol |
Placebo |
|||||
|---|---|---|---|---|---|---|---|---|
| Completers | Noncompleters | Completers | Noncompleters | Completers | Noncompleters | Completers | Noncompleters | |
| Total per group, N | 33 | 5 | 33 | 4 | 35 | 4 | 38 | 1 |
| Baseline KCCQ Clinical Summary Score, mean (SD) | 45.63 (21.60) | 39.27 (13.50) | 44.05 (19.11) | 62.24 (12.94) | 51.88 (23.17) | 43.23 (20.84) | 48.52 (24.23) | 52.08 (0) |
| Baseline Vigor Score, mean (SD) | 12.21 (5.57) | 12.80 (7.85) | 13.94 (7.11) | 11.25 (6.95) | 13.00 (5.49) | 11.00 (6.06) | 13.79 (5.70 | 15.00 (0) |
| Baseline Ejection Fraction [%], mean (SD) | 51.28 (4.76) | 52.76 (3.55) | 50.79 (4.38) | 49.25 (5.32) | 51.89 (5.68) | 48.00 (2.00) | 51.04 (7.43) | 55.00 (0) |
| Baseline Septal E/e’ Ratio, mean (SD) | 9.35 (4.24) | 8.32 (6.93) | 11.20 (5.97) | 13.76 (7.46) | 9.80 (4.27) | 10.26 (3.13) | 9.41 (5.54) | 0.93 (0) |
| Baseline Total Distance Walked: 6 min Walk Test [m], mean (SD) | 237.85 (131.05) | 166.78 (14.67) | 265.58 (135.96) | 248.20 (211.14) | 322.69 (124.18) | 164.35 (165.96) | 247.87 (150.96) | 438.30 (0) |
| Baseline BNP [pg/ml], mean (SD) | 157.09 (155.27) | 375.80 (462.43) | 152.48 (141.52) | 208.75 (59.53) | 182.51 (211.23) | 613.75 (407.42) | 232.00 (388.28) | 55.00 (0) |
| Baseline lactate [mmol/L], mean (SD) | 2.72 (1.59) | 3.57 (1.29) | 2.34 (1.12) | 2.64 (1.81) | 2.58 (1.04) | 1.95 (0.92) | 2.45 (1.13) | 1.93 (0) |
| Baseline ATP [RLU], mean (SD) | 3,383.24 (954.03) | 3,319.00 (938.38) | 3,312.18 (915.98) | 3,027.00 (478.81) | 3,222.63 (1,040.44) | 3,031.00 (272.74) | 3,332.87 (844.33) | 2,903.00 (0) |
| Baseline lactate/ATP [mmol/L(*RLU)], mean (SD) | 8.58×10−4 (5.53×10−4) | 1.06×10−3 (9.03×10−5) | 7.29×10−4 (3.36×10−4) | 8.72×10−4 (5.44×10−4) | 8.76×10−4 (4.05×10−4) | 6.55×10−4 (3.52×10−4) | 7.93×10−4 (4.03×10−4) | 6.65×10−4 (0) |
Table 5.
Group comparisons of study outcomes using subjects who completed the study
| Ubiquinol vs placebo* | d-ribose vs placebo† | Ubiquinol + d-ribose vs placebo‡ | Ubiquinol + d-ribose vs ubiquinol§ | |
|---|---|---|---|---|
| Change in KCCQ Clinical Summary Score (Hypothesis 1) | ||||
| Difference | 22.41 | 25.82 | 17.30 | −5.11 |
| Degrees of freedom | 70 | 68 | 68 | 66 |
| Uncorrected p value¶ | <0.0001 | <0.0001 | 0.0029 | 0.3113 |
| Corrected p value** | 0.0001 | <0.0001 | 0.0116 | 0.9999 |
| 98.75% confidence interval†† | (9.37, 35.45) | (11.64, 40.00) | (2.93, 31.66) | (−17.98, 7.76) |
| t value | 4.40 | 4.67 | 3.09 | −1.02 |
| Change in Vigor Score (Hypothesis 2) | ||||
| Difference | 7.65 | 7.84 | 8.15 | 0.49 |
| Degrees of freedom | 70 | 68 | 68 | 66 |
| Uncorrected p value¶ | <0.0001 | <0.0001 | <0.0001 | 0.7139 |
| Corrected p value** | <0.0001 | <0.0001 | <0.0001 | 0.9999 |
| 98.75% confidence interval†† | (3.82, 11.49) | (3.49, 12.19) | (4.04, 12.26) | (−2.94, 3.92) |
| t value | 5.12 | 4.63 | 5.08 | 0.37 |
| Change in ejection fraction (%) (Hypothesis 3) | ||||
| Difference | 7.08 | 8.03 | 7.46 | 0.38 |
| Degrees of freedom | 67 | 64 | 64 | 61 |
| Uncorrected p value¶ | 0.0002 | <0.0001 | <0.0001 | 0.8421 |
| Corrected p value** | 0.0006 | <0.0001 | 0.0002 | 0.9999 |
| 98.75% confidence interval†† | (2.55, 11.62) | (3.69, 12.37) | (2.99, 11.93) | (−4.46, 5.21) |
| t value | 4.01 | 4.76 | 4.29 | 0.20 |
| Change in septal E/e’ ratio (Hypothesis 3) | ||||
| Difference | −2.66 | −2.04 | −1.86 | 0.80 |
| Degrees of freedom | 54 | 53 | 50 | 44 |
| Uncorrected p value¶ | 0.1282 | 0.3038 | 0.3249 | 0.5297 |
| Corrected p value** | 0.5128 | 0.9999 | 0.9999 | 0.9999 |
| 98.75% confidence interval†† | (−7.10, 1.79) | (−7.11, 3.04) | (−6.70, 3.00) | (−2.46, 4.10) |
| t value | −1.54 | −1.04 | −0.99 | 0.63 |
| Change in Total Distance Walked: 6 min Walk Test (m) (Hypothesis 4) | ||||
| Difference | 1.59 | 9.80 | 16.59 | 15.00 |
| Degrees of freedom | 69 | 67 | 65 | 60 |
| Uncorrected p value¶ | 0.9132 | 0.5466 | 0.2961 | 0.1401 |
| Corrected p value** | 0.9999 | 0.9999 | 0.9999 | 0.5605 |
| 98.75% confidence interval†† | (−35.75, 38.94) | (−31.72, 51.32) | (−23.88, 57.06) | (−10.84, 40.83) |
| t value | 0.11 | 0.61 | 1.05 | 1.50 |
| Change in BNP (pg/ml) (Hypothesis 5) | ||||
| Difference | −72.02 | −58.64 | −47.51 | 24.51 |
| Degrees of freedom | 69 | 68 | 66 | 61 |
| Uncorrected p value¶ | <0.0001 | 0.0006 | 0.0104 | 0.2080 |
| Corrected p value** | 0.0002 | 0.0024 | 0.0415 | 0.8318 |
| 98.75% confidence interval†† | (−114.8, −29.21) | (−100.4, −16.91) | (−93.76, −1.27) | (−25.05, 74.07) |
| t value | −4.31 | −3.61 | −2.64 | 1.27 |
| Change in Lactate (Mmol/L) | ||||
| Difference | −0.33 | −0.68 | −0.57 | −0.24 |
| Degrees Of Freedom | 69 | 68 | 65 | 60 |
| Uncorrected p Value¶ | 0.1966 | 0.0083 | 0.0419 | 0.2694 |
| Corrected p Value** | 0.7865 | 0.0330 | 0.1675 | 0.9999 |
| 98.75% Confidence Interval†† | (−0.98, 0.32) | (−1.32, −0.04) | (−1.27, 0.14) | (−0.79, 0.31) |
| t value | −1.30 | −2.72 | −2.08 | −1.11 |
| Change in ATP (RLU) | ||||
| Difference | 1,322.6 | 1,096.5 | 875.3 | −447.3 |
| Degrees of freedom | 70 | 68 | 67 | 63 |
| Uncorrected p value¶ | <0.0001 | <0.0001 | 0.0002 | 0.0787 |
| Corrected p value** | <0.0001 | <0.0001 | 0.0008 | 0.3147 |
| 98.75% confidence interval†† | (770.4, 1,874.7) | (533.8, 1,659.2) | (304.7, 1,445.8) | (−1,090.8, 196.2) |
| t value | 6.14 | 5.00 | 3.94 | −1.79 |
| Change in Lactate/ATP Ratio [Mmol/L(*RLU)] (Hypothesis 6) | ||||
| Note: These Tests Are 1 Sided, with the Prespecified Assumption That the Change Would Be Numerically Smaller or More Negative for the Group Listed First | ||||
| Difference | −4.05 × 10−4 | −4.32 × 10−4 | −3.35 × 10−4 | 7.06 × 10−5 |
| Degrees of freedom | 69 | 68 | 65 | 60 |
| Uncorrected p value¶ | <0.0001 | <0.0001 | 0.0002 | 0.8031 |
| Corrected p value** | <0.0001 | <0.0001 | 0.0006 | 0.9999 |
| 98.75% confidence bound†† | (−∞, −2.01 × 10−4) | (−∞, −2.39 × 10−4) | (−∞, −1.33 × 10−4) | (−∞, 2.59 × 10−4) |
| t value | −4.54 | −5.13 | −3.80 | 0.86 |
The difference in changes was calculated as ubiquinol group minus placebo group.
The difference in changes was calculated as d-ribose group minus placebo group.
The difference in changes was calculated as ubiquinol and d-ribose group minus placebo group.
The difference in changes was calculated as ubiquinol and d-ribose group minus ubiquinol group.
p values should be compared to the corrected significance level of 0.05/4 = 0.0125 because of a Bonferroni correction for 4 comparisons.
p values corrected for 4 comparisons.
Confidence intervals are based on a nominal level of 95% confidence and 4 comparisons. Therefore, a Bonferroni corrected 98.75% level was used.
ATP = Adenosine Triphosphate; BNP = B-type natriuretic peptide; E/e’= E to early diastolic mitral annular tissue velocity; KCCQ = Kansas City Cardiomyopathy Questionnaire; RLU = Relative Light Units.
Note: Per protocol, all statistical tests are two-sided, except those for the lactate/ATP ratio which are one-sided.
Figure 2.
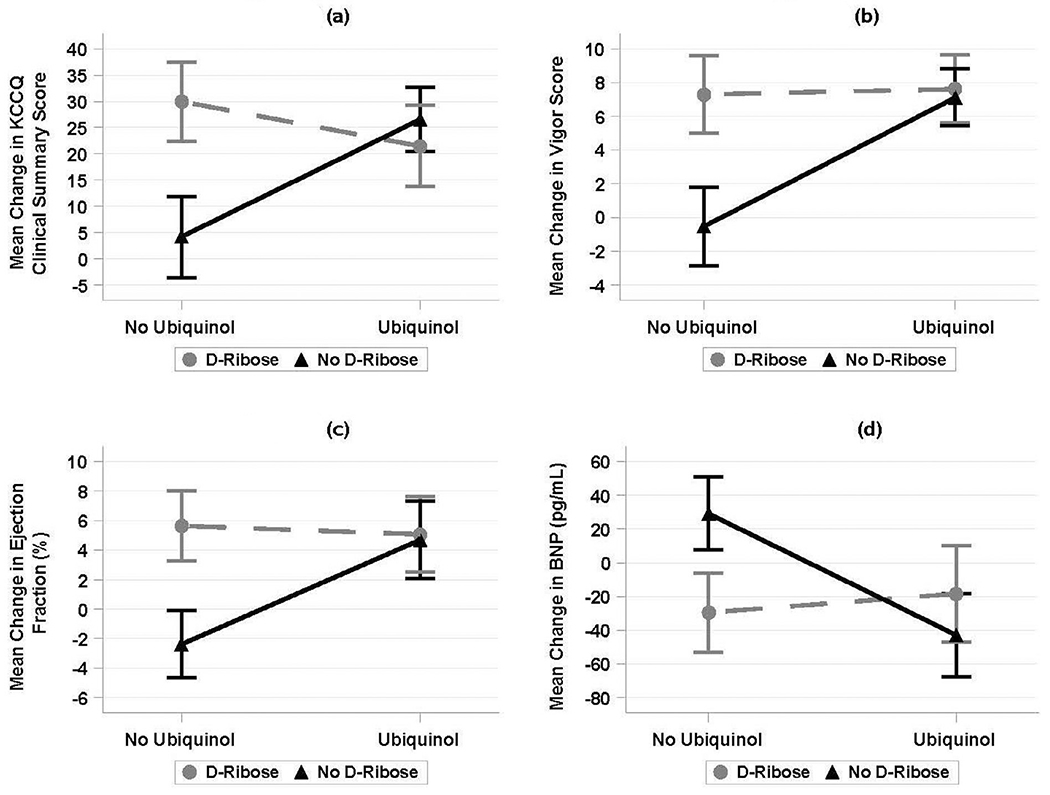
Significant antagonistic interaction between the ubiqinol and d-ribose treatments for KCCQ Clinical Summary Scores, Vigor Scores, Ejection Fraction, and B-type natriuretic peptide.
BNP = B-type natriuretic peptides; KCCQ = Kansas City Cardiomyopathy Questionnaire.
Table 6.
Group comparisons of symptoms from the Kansas City Cardiomyopathy Questionnaire (KCCQ)
| Ubiquinol vs Placebo* | d-ribose vs Placebo† | Ubiquinol and d-ribose vs Placebo‡ | Ubiquinol and d-ribose vs Ubiquinol§ | |
|---|---|---|---|---|
| Change in KCCQ: physical limitation | ||||
| Difference | 24.85 | 29.54 | 21.33 | −3.52 |
| Degrees of freedom | 70 | 68 | 68 | 66 |
| Uncorrected p value¶ | <0.0001 | <0.0001 | 0.0010 | 0.5609 |
| Corrected p value** | 0.0004 | <0.0001 | 0.0040 | 0.9999 |
| 98.75% confidence interval†† | (9.50, 40.20) | (14.31, 44.77) | (5.43, 37.23) | (−18.96, 11.93) |
| t value | 4.15 | 4.98 | 3.44 | −0.58 |
| Change in KCCQ: symptom stability | ||||
| Difference | 17.42 | 20.20 | 8.83 | −8.59 |
| Degrees of freedom | 71 | 69 | 69 | 66 |
| Uncorrected p value¶ | 0.0083 | 0.0027 | 0.1843 | 0.2375 |
| Corrected p value** | 0.0333 | 0.0109 | 0.7374 | 0.9501 |
| 98.75% confidence interval†† | (0.97, 33.88) | (3.54, 36.85) | (−8.06, 25.72) | (−27.11, 9.92) |
| t value | 2.71 | 3.11 | 1.34 | −1.19 |
| Change in KCCQ: symptom frequency | ||||
| Difference | 20.78 | 22.30 | 11.44 | −9.34 |
| Degrees of freedom | 71 | 69 | 69 | 66 |
| Uncorrected p value¶ | 0.0007 | 0.0007 | 0.0952 | 0.1385 |
| Corrected p value** | 0.0026 | 0.0030 | 0.3810 | 0.5541 |
| 98.75% confidence interval†† | (5.85, 35.71) | (6.10, 38.51) | (−5.91, 28.79) | (−25.34, 6.66) |
| t value | 3.57 | 3.53 | 1.69 | −1.50 |
| Change in KCCQ: symptom burden | ||||
| Difference | 18.36 | 21.10 | 14.28 | −4.08 |
| Degrees of freedom | 71 | 69 | 69 | 66 |
| Uncorrected p value¶ | 0.0025 | 0.0033 | 0.0248 | 0.4454 |
| Corrected p value** | 0.0098 | 0.0131 | 0.0993 | 0.9999 |
| 98.75% confidence interval†† | (3.38, 33.35) | (3.33, 38.86) | (−1.68, 30.25) | (−17.74, 9.58) |
| t value | 3.14 | 3.05 | 2.29 | −0.77 |
| Change in KCCQ: Total Symptom Score | ||||
| Difference | 19.57 | 21.70 | 12.86 | −6.71 |
| Degrees of freedom | 71 | 69 | 69 | 66 |
| Uncorrected p value¶ | 0.0006 | 0.0009 | 0.0349 | 0.2078 |
| Corrected p value** | 0.0023 | 0.0034 | 0.1397 | 0.8314 |
| 98.75% confidence interval†† | (5.65, 33.50) | (5.74, 37.66) | (−2.47, 28.19) | (−20.26, 6.84) |
| t value | 3.60 | 3.49 | 2.15 | −1.27 |
| Change in KCCQ: self efficacy | ||||
| Difference | 8.36 | 12.37 | 15.78 | 7.42 |
| Degrees of freedom | 71 | 69 | 69 | 66 |
| Uncorrected p value¶ | 0.1374 | 0.0264 | 0.0203 | 0.2787 |
| Corrected p value** | 0.5494 | 0.1056 | 0.0813 | 0.9999 |
| 98.75% confidence interval†† | (−5.90, 22.61) | (−1.61, 26.35) | (−1.26, 32.82) | (−10.03, 24.88) |
| t value | 1.50 | 2.27 | 2.38 | 1.09 |
| Change in KCCQ: quality of life | ||||
| Difference | 15.46 | 21.94 | 14.61 | −0.84 |
| Degrees of freedom | 71 | 69 | 69 | 66 |
| Uncorrected p value¶ | 0.0088 | 0.0005 | 0.0129 | 0.8856 |
| Corrected p value** | 0.0354 | 0.0018 | 0.0517 | 0.9999 |
| 98.75% confidence interval†† | (0.74, 30.17) | (6.66, 37.22) | (−0.08, 29.30) | (−15.85, 14.17) |
| t value | 2.69 | 3.68 | 2.55 | −0.14 |
| Change in KCCQ: social limitation | ||||
| Difference | 23.41 | 26.50 | 15.83 | −7.58 |
| Degrees of freedom | 66 | 65 | 63 | 63 |
| Uncorrected p value¶ | 0.0018 | 0.0004 | 0.0303 | 0.2794 |
| Corrected p value** | 0.0072 | 0.0016 | 0.1213 | 0.9999 |
| 98.75% confidence interval†† | (4.93, 41.88) | (8.28, 44.73) | (−2.54, 34.19) | (−25.45, 10.29) |
| t value | 3.25 | 3.74 | 2.22 | −1.09 |
| Change in KCCQ: overall summary | ||||
| Difference | 22.50 | 26.17 | 17.63 | −4.86 |
| Degrees of freedom | 65 | 64 | 62 | 63 |
| Uncorrected p value¶ | <0.0001 | <0.0001 | 0.0021 | 0.3354 |
| Corrected p value** | 0.0002 | <0.0001 | 0.0083 | 0.9999 |
| 98.75% confidence interval†† | (9.26, 35.74) | (12.60, 39.73) | (3.52, 31.74) | (−17.74, 8.02) |
| t value | 4.37 | 4.96 | 3.22 | −0.97 |
The difference in changes was calculated as ubiquinol group minus Placebo group.
The difference in changes was calculated as d-ribose group minus Placebo group.
The difference in changes was calculated as ubiquinol and d-ribose group minus Placebo group.
The difference in changes was calculated as ubiquinol and d-ribose group minus ubiquinol group.
p values should be compared to the corrected significance level of 0.05/4=0.0125 because of a Bonferroni correction for 4 comparisons.
p values corrected for 4 comparisons.
Confidence intervals are based on a nominal level of 95% confidence and 4 comparisons. Therefore, a Bonferroni corrected 98.75% level was used.
ATP = Adenosine Triphosphate; BNP = B–type natriuretic peptide; E/e′ = E to early diastolic mitral annular. tissue velocity; KCCQ = Kansas City Cardiomyopathy Questionnaire; RLU = Relative Light Units; SD = standard deviation; 6MWT = 6 min Walk Test.
Values are mean ± standard deviation and mean.
Compared with placebo, ubiquinol, d-ribose, and the combination of the 2 significantly increased the level of vigor (Table 5). Specifically, ubiquinol alone significantly increased the change in vigor scores from baseline to 12 weeks by 7.65 points [Bonferroni-corrected 95% CI, (3.82, 11.49); t = 5.12; df = 70; 2-sided p <0.0001]; d-ribose alone significantly increased the change from baseline by 7.84 points [(3.49, 12.19; t = 4.63); df = 68, p <0.0001]; and the combination of ubiquinol and d-ribose significantly increased the change by 8.15 points [(4.04, 12.26); t = 5.08; df = 68; p <0.0001]. The addition of d-ribose to ubiquinol treatment, however, did not significantly contribute additional increases in vigor scores (Bonferroni-corrected p = 0.9999). A linear regression model of the changes in the vigor score that included ubiquinol and d-ribose treatments and their interaction as independent variables suggested a significant antagonistic interaction between the 2 treatments (Figure 2).
Compared with placebo, ubiquinol or d-ribose alone significantly increased the change in EF from baseline to 12 weeks by 7.08% and 8.03%, respectively (Table 5). However, according to a linear regression model of the changes in EF that included ubiquinol and d-ribose treatments and their interaction as independent variables, there was a significant antagonistic interaction between the 2 treatments. Specifically, the combination of the 2 caused a separate significant reduction of 7.66% (95% CI, −2.65, −2.67; p = 0.0029). As a result, the net effect of adding d-ribose to ubiquinol treatment did not contribute additional improvement in EF; the nonsignificant contribution was 0.38% [95% CI, (−4.46, 5.21); p = 0.2]. Additional models that controlled for sex and race and/or that implemented the intention-to-treat principle yielded similar results. A linear regression model of the changes in the EF that included ubiquinol and d-ribose treatments and their interaction as independent variables suggested a significant antagonistic interaction between the 2 treatments (Figure 2).
We did not find evidence that ubiquinol, d-ribose, or the combination of the 2 significantly improved left ventricular diastolic function measured by septal E/e’ ratio (Table 5). The Bonferroni-corrected p values comparing changes in E/e’ ratio from baseline to 12 weeks for the ubiquinol, d-ribose, or the combination therapy groups with changes for the placebo group were ≥ 0.51. The addition of d-ribose to ubiquinol treatment did not significantly improve the E/e’ ratio either (p = 0.9999). Intention-to-treat analyses that assumed zero changes in the E/e’ ratio for the subjects who did not complete the study provided similar results.
Compared with placebo, ubiquinol, d-ribose, and the combination of the 2 significantly decreased blood BNP levels (Table 5). Ubiquinol alone significantly decreased BNP levels from baseline to 12 weeks by 72.02 pg/ml [Bonferroni-corrected 95% CI, (−114.8, −29.21); t = −4.31; df = 69; 2-sided p = 0.0002], d-ribose alone significantly decreased BNP levels by 58.64 pg/ml [(−100.4, −16.91); t = −3.61; df = 68; p = 0.0024], and the combination of ubiquinol and d-ribose significantly decreased BNP levels by 47.51 pg/ml [(−93.76, −1.27); t = −2.64; df = 66; p = 0.0415]. The addition of d-ribose to ubiquinol treatment, however, did not significantly decrease BNP levels further (Bonferroni-corrected p = 0.8318). Intention-to-treat analyses that assumed zero changes in BNP levels for the subjects who did not complete the study yielded similar results. A linear regression model of the changes in the BNP that included ubiquinol and d-ribose treatments and their interaction as independent variables suggested a significant antagonistic interaction between the 2 treatments (Figure 2).
Compared with placebo, ubiquinol, d-ribose, and the combination of the 2 significantly increased ATP. The ATP increases from baseline to 3 months under ubiquinol were 1,322.6 Relative Light Units (RLU)higher than those under placebo, 1,096.5 RLU higher under d-ribose, and 875.3 RLU higher than those under ubiquinol plus d-ribose (Table 5). Moreover, compared with placebo, the reductions from baseline in lactate levels were significant only for the ubiquinol group and the ubiquinol plus d-ribose group (0.68 and 0.57 greater reductions, respectively, Table 5). The 3 experimental groups, however, exhibited significantly greater reductions in the lactate/ATP ratio compared with placebo. Specifically, ubiquinol alone significantly decreased the change in the ratio from baseline to 12 weeks by 4.05 × 10−4 mmol/(L × RLU) [Bonferroni-corrected 95% bound, (−∞, −2.01 × 10−4); t = −4.54; df = 69; 1-sided p <0.0001] (Table 5); d-ribose alone significantly decreased the change by 4.32 × 10−4 mmol/(L × RLU) [(−∞, −2.39 × 10−4); t = −5.130; df = 68; p <0.0001]; and the combination of ubiquinol and d-ribose significantly decreased the change by 3.35 × 10−4 mmol/ (L × RLU) [(−∞, −1.33 × 10−4) t = −3.80, df = 65, p = 0.0006]. The addition of d-ribose to ubiquinol treatment, however, did not significantly decrease the change in lactate/ATP ratio further (Bonferroni-corrected p = 0.9999).
We did not find evidence that ubiquinol, d-ribose, or the combination of the 2 increased the distance that subjects can walk in 6 minutes (Table 5). The Bonferroni-corrected p values comparing changes in the total distance walked from baseline to 12 weeks for the ubiquinol, d-ribose, or the combination therapy groups with changes for the placebo group were all close to 1. The addition of d-ribose to ubiquinol treatment did not significantly improve the total distance walked either (p = 0.5605).
Discussion
Among patients with HFpEF who consumed ubiquinol and/or d-ribose for 12 weeks, there were significant improvements in the patients’ perceptions of their health status and level of energy (vigor). Physiologically, there were significant increases in the EF and significantly decreased lactate/ATP ratio and BNP. There were no significant changes in the septal E/e’ ratio or the 6MWT with any of the supplements. However, our data suggest there is no synergistic effect with the addition of d-ribose to ubiquinol.
For heart function, the myocardium needs a steady supply of CoQ10; CoQ10 is essential to the respiratory transport chain for myocardial ATP production.5 Patients with HF also have a depletion of CoQ10 that is associated with the severity of symptoms.6 The Q-SMBIO trial on the effect of CoQ10 on morbidity and mortality in chronic HF found that CoQ10 treatment of patients was safe and reduced symptoms and other adverse events.7,8 We found similar results with ubiquinol in decreasing HF symptoms with few side effects. The benefits of d-ribose have also been studied in different cardiovascular conditions.9–11 Because ribose is a naturally occurring pentose carbohydrate, it can replenish deficient ATP levels by bypassing a key metabolic enzyme needed to produce ATP.12 We found that d-ribose increased blood ATP levels and the myocardial energy, improving EF in patients with HFpEF.
We used the KCCQ and Vigor subscale to evaluate patient-reported outcome measures to incorporate subjects’ experiences into the assessment of ubiquinol and/or d-ribose for the treatment of HFpEF. Many investigators have conducted serial monitoring of health status in patients with HF using the KCCQ and found that health status was prognostic in patients with HFpEF.13–15 On the basis of our findings, using either ubiquinol or d-ribose as a treatment for HFpEF can assist in improving the health status of patients with HFpEF. Investigators have also used the vigor subscale of the POMS to measure the energy level in patients who are fatigued.16–18 Because one of the major symptoms experienced by patients with HFpEF is fatigue, we used this scale to quickly assess changes in fatigue before and after using ubiquinol and/or d-ribose.
Investigators have demonstrated that CoQ10 increased EF, with significant improvement in peripheral endothelial function19 and a reduction in proinflammatory cytokines.20 21 In this study, we also found that either ubiquinol or d-ribose alone increased EF, whereas patients in the placebo group had no change in EF. This is most likely due to ubiquinol’s bioenergetic effect that was demonstrated by the increase in blood ATP values in patients receiving the supplements. Bayram et al9 found that d-ribose (5 g per day) administered for 6 weeks improved EF. However, there were no changes in the septal and lateral E/e’ values. Samuel et al22 found that in elderly patients with HFpEF, treatment with ubiquinol (100 mg/3 times per day) did not significantly affect echocardiographic indexes of diastolic function. We had similar findings for EF and septal E/e’. These data suggest a strong rationale for administering 1 of these 2 supplements in patients with HFpEF as a potential therapeutic agent for maintaining and improving myocardial function.
Onur et al5 found increased NT-proBNP values, lower CoQ10 levels, decreased levels of ubiquinol, and an increase in CoQ10 redox state in patients after a myocardial infarction. In our study, we did find a significant decrease in BNP with ubiquinol supplementation. In the Q-SYMBIO trial where ubiquinone versus ubiquinol was examined, NT-proBNP levels did not decrease after 16 weeks.7,8 This could be a result of using the oxidized form of CoQ10 (ubiquinone) in older patients who have decreased ability to produce ubiquinol used for ATP production. In addition, the dosage of CoQ10 was 100 mg to 200 mg per day, and we used 600 mg per day in our study. We found that the administration of d-ribose also decreased BNP. Vijay et al23 found that d-ribose administration with low-level exercise in patients with HF could improve ventilation. As a naturally occurring pentose carbohydrate, d-ribose can assist with replenishing high-energy phosphates and thereby improve diastolic dysfunction.
In patients with HFpEF, cardiomyocyte mitochondria have structural and energetic abnormalities and causes a mismatch between ATP production and demand.24,25 Chida et al26,27 found that blood lactate/ATP ratio was a sensitive prognostic biomarker. In our study, we evaluated blood lactate/ATP ratio using a rapid ATP measurement. In patients with HFpEF, lactate slightly decreased with ubiquinol and d-ribose, but ATP significantly increased. This illustrates the increase in mitochondrial ATP production and the slight improvement in anaerobic metabolism.
During rest and exercise, patients with HFpEF have increased filling pressures with decreased functional capacity.28 We found no differences in the 6MWT among the 4 groups. There were many other confounding variables, such as orthopedic problems, iron deficiency, morbid obesity, and fear of falling. Other supplements, such as vitamin D, have been used with patients with HF with no change in the 6MWT.29
In conclusion, among patients with HFpEF, a 12-week treatment with ubiquinol (600 mg per day) or d-ribose (15 g per day) reduced HF symptoms, levels of BNP, and lactate and increased EF and ATP production.30 The addition of d-ribose to ubiquinol treatment did not further improve the outcomes, suggesting that either supplement alone is sufficient for improving symptoms and physiologic variables but requires the study dosage. Phase 3 clinical trials are needed to include a larger number of patients conducted in multiple clinics across the country.
Acknowledgments
We would like to thank Dr. Richard Clancy and Mrs. Sally Barhydt.
This work was supported the Department of Health and Human Services, National Institutes of Health, National Institute on Aging (Grant Number: 1R01AG054486-01A1). Trial registration: ClinicalTrials.gov Identifier: NCT03133793, FDA IND number: 134633.
Footnotes
Disclosures
The authors have no conflicts of interest to declare.
References
- 1.Obokata M, Reddy YNV, Borlaug BA. Diastolic dysfunction and heart failure with preserved ejection fraction: understanding mechanisms by using noninvasive methods. JACC Cardiovasc Imaging 2020;13(1 Pt 2):245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oren O, Goldberg S. Heart failure with preserved ejection fraction: diagnosis and management. Am J Med 2017;130(5):510–516. [DOI] [PubMed] [Google Scholar]
- 3.Pierce JD, Mahoney DE, Hiebert JB, Thimmesch AR, Diaz FJ, Smith C, Shen Q, Mudaranthakam DP, Clancy RL. Study protocol, randomized controlled trial: reducing symptom burden in patients with heart failure with preserved ejection fraction using ubiquinol and/or d-ribose. BMC Cardiovasc Disord 2018;18(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Statistical analysis system (SAS); 2021. Available at: https://www.sas.com/en_us/curiosity.html?utm_source=other&utm_medium=cpm&utm_campaign=non-cbo-us&dclid=&gclid=EAIaIQobChMIg9L6yt3w9gIVA2xvBB39FAQ7EAAYASAAEgKRMfD_BwE. Accessed February 8, 2022.
- 5.Onur S, Niklowitz P, Jacobs G, Lieb W, Menke T, Döring F. Association between serum level of ubiquinol and nt-probnp, a marker for chronic heart failure, in healthy elderly subjects. BioFactors 2015;41(1):35–43. [DOI] [PubMed] [Google Scholar]
- 6.Kalén A, Appelkvist EL, Dallner G. Age-related changes in the lipid compositions of rat and human tissues. Lipids 1989;24(7):579–584. [DOI] [PubMed] [Google Scholar]
- 7.Mortensen SA, Rosenfeldt F, Kumar A, Dolliner P, Filipiak KJ, Pella D, Alehagen U, Steurer G, Littarru GP, Q-SYMBIO Study Investigators. The effect of coenzyme q10 on morbidity and mortality in chronic heart failure: results from Q-SYMBIO: a randomized double-blind trial. JACC Heart Fail 2014;2(6):641–649. [DOI] [PubMed] [Google Scholar]
- 8.Mortensen AL, Rosenfeldt F, Filipiak KJ. Effect of coenzyme q10 in Europeans with chronic heart failure: a sub-group analysis of the q-symbio randomized double-blind trial. Cardiol J 2019;26(2):147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bayram M, St Cyr JA, Abraham WT. D-ribose aids heart failure patients with preserved ejection fraction and diastolic dysfunction: a pilot study. Ther Adv Cardiovasc Dis 2015;9(3):56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pliml W, von Arnim T, Stäblein A, Hofmann H, Zimmer HG, Erdmann E. Effects of ribose on exercise-induced ischaemia in stable coronary artery disease. Lancet 1992;340(8818):507–510. [DOI] [PubMed] [Google Scholar]
- 11.Derosa G, Pasqualotto S, Catena G, D’Angelo A, Maggi A, Maffioli P. A randomized, double-blind, placebo-controlled study to evaluate the effectiveness of a food supplement containing creatine and d-ribose combined with a physical exercise program in increasing stress tolerance in patients with ischemic heart disease. Nutrients 2019;11(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ingwall JS, Weiss RG. Is the failing heart energy starved? On using chemical energy to support cardiac function. Circ Res 2004;95(2):135–145. [DOI] [PubMed] [Google Scholar]
- 13.Huang W, Teng TK, Tay WT, Richards AM, Kadam U, Lawson CA, Shimizu W, Loh SY, Anand I, Lam CSP. Patient-reported outcomes in heart failure with preserved vs. reduced ejection fraction: focus on physical independence. ESC Heart Fail 2020;7(5):2051–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pokharel Y, Khariton Y, Tang Y, Nassif ME, Chan PS, Arnold SV, Jones PG, Spertus JA. Association of serial kansas city cardiomyopathy questionnaire assessments with death and hospitalization in patients with heart failure with preserved and reduced ejection fraction: a secondary analysis of 2 randomized clinical trials. JAMA Cardiol 2017;2(12):1315–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deaton C, Forsyth F, Mant J, Edwards D, Hobbs R, Taylor C, Aziz A, Schiff R, Odone J, Zaman J. Characteristics and health status of patients with and without confirmed HFpEF. Eur Heart J 2020;41(Supplement_2):3139. [Google Scholar]
- 16.Fink AM, Eckhardt AL, Fennessy MM, Jones J, Kruse D, VanderZwan KJ, Ryan CJ, Zerwic JJ. Psychometric properties of three instruments to measure fatigue with myocardial infarction. West J Nurs Res 2010;32(7):967–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukuoka Y, Lindgren TG, Rankin SH, Cooper BA, Carroll DL. Cluster analysis: a useful technique to identify elderly cardiac patients at risk for poor quality of life. Qual Life Res 2007;16(10):1655–1663. [DOI] [PubMed] [Google Scholar]
- 18.Fennessy MM, Fink AM, Eckhardt AL, Jones J, Kruse DK, VanderZwan KJ, Ryan CJ, Zerwic JJ. Gender differences in fatigue associated with acute myocardial infarction. J Cardiopulm Rehabil Prev 2010;30(4):224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawashima C, Matsuzawa Y, Konishi M, Akiyama E, Suzuki H, Sato R, Nakahashi H, Kikuchi S, Kimura Y, Maejima N, Iwahashi N, Hibi K, Kosuge M, Ebina T, Tamura K, Kimura K. Ubiquinol improves endothelial function in patients with heart failure with reduced ejection fraction: a single-center, randomized double-blind placebo-controlled crossover pilot study. Am J Cardiovasc Drugs 2020;20(4):363–372. [DOI] [PubMed] [Google Scholar]
- 20.Kumar A, Singh RB, Saxena M, Niaz MA, Josh SR, Chattopadhyay P, Mechirova V, Pella D, Fedacko J. Effect of carni q-gel (ubiquinol and carnitine) on cytokines in patients with heart failure in the tishcon study. Acta Cardiol 2007;62(4):349–354. [DOI] [PubMed] [Google Scholar]
- 21.Sander S, Coleman CI, Patel AA, Kluger J, White CM. The impact of coenzyme q10 on systolic function in patients with chronic heart failure. J Card Fail 2006;12(6):464–472. [DOI] [PubMed] [Google Scholar]
- 22.Samuel TY, Hasin T, Gotsman I, Weitzman T, Ben Ivgi F, Dadon Z, Asher E, Amir O, Glikson M, Alcalai R, Leibowitz D. Coenzyme q10 in the treatment of heart failure with preserved ejection fraction: a prospective, randomized, double-blind, placebo-controlled trial. Drugs RD 2022;22(1):25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vijay N, MacCarter D, Shecterle LM, St Cyr JA. D-ribose benefits heart failure patients. J Med Food 2008;11(1):199–200. [DOI] [PubMed] [Google Scholar]
- 24.Dai DF, Chen T, Szeto H, Nieves-Cintrón M, Kutyavin V, Santana LF, Rabinovitch PS. Mitochondrial targeted antioxidant peptide ameliorates hypertensive cardiomyopathy. J Am Coll Cardiol 2011;58(1):73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phan TT, Abozguia K, Nallur Shivu G, Mahadevan G, Ahmed I, Williams L, Dwivedi G, Patel K, Steendijk P, Ashrafian H, Henning A, Frenneaux M. Heart failure with preserved ejection fraction is characterized by dynamic impairment of active relaxation and contraction of the left ventricle on exercise and associated with myocardial energy deficiency. J Am Coll Cardiol 2009;54(5):402–409. [DOI] [PubMed] [Google Scholar]
- 26.Chida J, Kido H. Extraction and quantification of adenosine triphosphate in mammalian tissues and cells. Methods Mol Biol 2014;1098:21–32. [DOI] [PubMed] [Google Scholar]
- 27.Chida J, Ono R, Yamane K, Hiyoshi M, Nishimura M, Onodera M, Nakataki E, Shichijo K, Matushita M, Kido H. Blood lactate/atp ratio, as an alarm index and real-time biomarker in critical illness. PLOS ONE 2013;8(4):e60561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolsk E, Kaye D, Borlaug BA, Burkhoff D, Kitzman DW, Komtebedde J, Lam CSP, Ponikowski P, Shah SJ, Gustafsson F. Resting and exercise haemodynamics in relation to six-minute walk test in patients with heart failure and preserved ejection fraction. Eur J Heart Fail 2018;20(4):715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Witte KK, Byrom R, Gierula J, Paton MF, Jamil HA, Lowry JE, Gillott RG, Barnes SA, Chumun H, Kearney LC, Greenwood JP, Plein S, Law GR, Pavitt S, Barth JH, Cubbon RM, Kearney MT. Effects of vitamin D on cardiac function in patients with chronic HF: the VINDICATE study. J Am Coll Cardiol 2016;67(22):2593–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roell KR, Reif DM, Motsinger-Reif AA. An introduction to terminology and methodology of chemical synergy-perspectives from across disciplines. Front Pharmacol 2017;8:158. [DOI] [PMC free article] [PubMed] [Google Scholar]


