Abstract
Nirmatrelvir is an antiviral agent active against SARS-CoV-2, the virus causing the pandemic disease COVID-19. It is administrated in combination with the protease inhibitor ritonavir, which acts in case of COVID-19 mainly as enzyme blocking agent preventing the premature metabolic elimination of nirmatrelvir. The combination of the two drugs in separate tablets is marketed under the brand name Paxlovid® and shows good effectivity in preventing the progression of COVID-19 to severe disease state. In this work, we described a LC-MS/MS method for the simultaneous quantification of nirmatrelvir and ritonavir in human plasma of patients treated for COVID-19 with Paxlovid®. After addition of D6-ritonavir as internal standard, plasma proteins were precipitated by the addition of methanol. The analytes were separated by gradient elution on a C18-column and were detected by tandem mass spectrometry. Calibration functions were linear in the ranges of 10 – 10000 ng/mL for nirmatrelvir and 2 – 2000 ng/mL for ritonavir. Inter-day and intra-day precision and accuracy was better than 15 % in the quality control samples and better than 20 % at the LLOQ. The method was successfully applied on samples of hospitalized patients treated for COVID-19 and proved to be capable in supporting therapeutic drug monitoring (TDM).
Keywords: Nirmatrelvir, Ritonavir, LC-MS/MS, Therapeutic drug monitoring
1. Introduction
In the face of the ongoing pandemic of COVID-19, caused by infections with the new coronavirus SARS-CoV-2, there is an urgency for effective anti-viral drug treatments minimizing the risks associated with this disease. The Federal Food and Drug Administration (FDA) of the United States of America and the European Medicines Agency (EMA) of the European Union have recently issued conditional marketing authorizations for Paxlovid® as anti-viral treatment against COVID-19 [1], [2]. Paxlovid® consists of two active substances, nirmatrelvir and ritonavir, in separate tablets. Nirmatrelvir is a SARS-CoV-2 main protease inhibitor, which blocks the processing of virus polyproteins and thus stopping replication of the virus. Ritonavir, a well-known HIV-1 protease inhibitor, acts in this case mainly as an inhibitor of the enzyme cytochrome P450 3A4 and thus prevents premature metabolic deactivation of nirmatrelvir while co-administrated [3].
Paxlovid® shows good effectivity in preventing the progression of COVID-19 to severe disease in high-risk patients [4], [5]. As an oral drug, it can easily be administered to outpatients. However, due to its potential drug-drug interactions by enzyme inhibition, combination with other prescribed drugs is limited [1]. Furthermore, in patients with moderate renal impairment (eGFR between 30 and 60 mL/min), dose adjustment of nirmatrelvir is necessary. In patients with severe renal impairment (eGFR < 30 mL/min) or patients receiving renal replacement therapy, Paxlovid® should not be used [1]. These recommendations exclude major high-risk groups from therapy with Paxlovid®. Facing this dilemma, special treatment courses for patients on dialysis and with severe renal impairment have been developed [6]. A further increase in safety and effectiveness of Paxlovid® in the treatment of these special patient groups may be achieved by therapeutic drug monitoring (TDM) of nirmatrelvir and ritonavir. For ritonavir, as an established HIV anti-viral drug, several quantification methods in human plasma have been reported [7], [8]. However, to the best of our knowledge, no quantification method capable of supporting TDM for nirmatrelvir has been reported. Thus, the goal of the here presented study is the development and validation of a LC-MS/MS method for the simultaneous quantification of nirmatrelvir and ritonavir in plasma of patients treated with Paxlovid®, with special attention to quick and easy processing of the samples to provide timely results.
2. Materials and methods
2.1. Chemicals
Nirmatrelvir (chemical purity > 95 %), ritonavir (chemical purity > 98 %) and the internal standard D6-ritonavir (chemical purity > 98 %, isotopic purity > 99 %) were all obtained from Cayman Chemical Company, (Ann Arbor, Mi, USA). All other chemicals were of analytical grade or better.
2.2. Analytical equipment
The HPLC equipment consisted of an Agilent 1260 Infinity system comprising a binary pump, a thermostatted autosampler and a thermostatted column compartment (Agilent Technologies, Waldbronn, Germany). The analytical column was a Zorbax XDB-C18 2.1 × 50 mm, partical size 3.5 µm (Agilent Technologies, Waldbronn, Germany), protected by a SecurityGuard system (Phenomenex, Aschaffenburg, Germany) equipped with a 4 mm × 2 mm C-18 filter insert. The HPLC flow was connected without splitting to a QTRAP 4500MD mass spectrometer (AB SCIEX, Darmstadt, Germany) equipped with a Turbo-Ion-Spray (TSP) source. Instrument control and data handling was carried out by the Analyst MD software package, version 1.6.2.
2.3. Calibration- and quality control samples
Stock solutions of the analytes were prepared in methanol at concentrations of 3 mg/mL nirmatrelvir and 1 mg/mL ritonavir. By spiking 947 µL of drug free human plasma with 33.3 µL of the nirmatrelvir stock solution and 20 µL of the ritonavir stock solution, a calibration stock solution in the concentration of 100 µg/mL nirmatrelvir and 20 µg/mL ritonavir in human plasma was obtained. By stepwise dilution of this stock solution with drug free human plasma, 7 calibration levels at concentrations of 10, 30, 100, 300, 1000, 3000 and 10000 ng/mL nirmatrelvir and 2, 6, 20, 60, 200, 600 and 2000 ng/mL ritonavir were prepared.
Quality control (QC) samples were prepared in a similar way at concentrations of 20 and 4 ng/mL (QC-low), 500 and 100 ng/mL (QC-med) and 6000 and 1200 ng/mL (QC-high) for nirmatrelvir and ritonavir, respectively. QC-samples were stored at −80 °C until analysis.
2.4. Sample preparation
To a 50-µL volume of calibration-, QC- or unknown plasma sample 20 µL of the internal standard solution (1 µg/mL D6-ritonavir in methanol:water 50:50) were added. Proteins were precipitated by the addition of 100 µL of methanol. After centrifugation at 13000 × g for 5 min, 50 µL of the clear supernatant was added to 100 µL aqueous buffer solution (1 g ammoniumformate and 1 mL formic acid in 1 L water, pH 3.5) and the mixture was transferred into autosampler vials with microliter inserts for the HPLC-system.
2.5. Chromatography and detection
After injection of 20 µL of the prepared sample solution, the chromatographic separation of the analytes was achieved by gradient elution at a constant flow rate of 300 µL/min. The gradient started at a composition of 90 % aqueous buffer (1 g ammoniumformate and 1 mL formic acid in 1 L water, pH 3.5) and 10 % acetonitrile. After 1 min at constant ratio, the gradient evolved linearly until 6 min runtime to 30 % buffer and 70 % acetonitrile. This ratio was held constant until 9 min runtime, after which the composition was set back to the starting values. The total cycling time including re-equilibration was 13 min. The column temperature was held constant at 55 °C. Under these conditions, the retention time of nirmatrelvir was 8.2 min and for ritonavir and the IS D6-ritonavir 9.2 min.
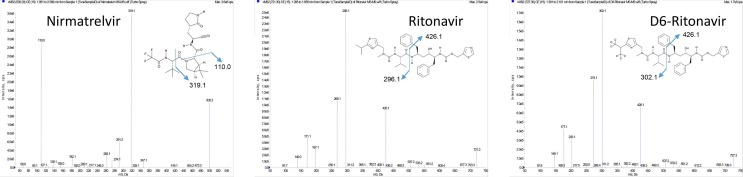
In the time window of 7.5 min to 10.5 min the mobile phase was directed without splitting to the turbo-ion-spray source of the mass spectrometer. The source parameters were curtain gas 40 units, iononization voltage + 4500 V, temperature 500 °C, gas stream 1 50 units, gas stream 2 30 units and declustering potential 100 V. Applying these parameters, the analytes were ionized to the [M + H]+ quasimolecular ions. These parent ions were fragmented at medium collision gas pressure and a fragmentation potential of 35 V to the fragment ions with the mass-to-charge ratios of m/z 500.2 → 319.1 and 110.0 for nirmatrelvir, m/z 721.3 → 426.1 and 296.1 for ritonavir and m/z 727.3 → 426.1 and 302.1 for the IS D6-ritonavir. The first fragment ions for all analytes were recorded for quantification and the second fragment ions for qualification.
2.6. Validation
Precision and accuracy of the method was tested by repeated measurements of the QC-samples and at the LLOQ (n = 10 intra-day, n = 6 inter-day). The extraction yield was tested by comparing peak areas of the analytes from extracted plasma samples with peak areas of samples spiked after extraction. The effect of the plasma matrix on the ionization efficiency of the mass spectrometer was tested with extracted plasma samples spiked after extraction with the analytes. For comparison, extracts of pure water samples spiked after extraction were measured. Thereby, the undisturbed matrix effect on peak areas could be observed without the influence of any extraction losses. Furthermore, quantification results in plasma samples from six different individual human plasma sources were tested for homogeneity. Stability of the analytes in prepared samples was tested by comparing freshly prepared samples with reinjections after standing 24 h in the autosampler tray. Stability of the analytes in plasma was tested for 4 days at 23 °C and – 20 °C, over 3 month at −80 °C and after 3 freeze–thaw cycles using the QC-samples.
3. Results and discussion
3.1. Sample preparation
Owing to the high selectivity of the mass spectrometric detection of the analytes a very simple and fast sample preparation could be employed. Only the addition of IS and protein precipitation was necessary to obtain sufficiently clean extracts. The ratio of 1:2 between plasma and the precipitation reagent methanol was sufficient to remove the plasma proteins in such a way that no degradation of the chromatographic performance or obstruction of capillaries was observed after several hundreds of sample injections during the validation process and early application of the method. The dilution with the aqueous buffer lowered the solvent strength of the prepared samples adequately to refocus the analytes at the front of the HPLC-column in such a way that sharp and symmetric peaks were obtained. The transfer of the analytes in this sample preparation procedure was quantitative with calculated extraction yields of 98.2 ± 6.3 % for nirmatrelvir, 104.4 ± 5.1 % for ritonavir and 104.9 ± 5.4 % for D6-ritonavir. None of these calculated extraction yields was significantly different from 100 % theoretical yield with p-values of 0.684 for nirmatrelvir, 0.205 for ritonavir and 0.184 for D6-ritonavir (n = 6).
3.2. Chromatography and detection
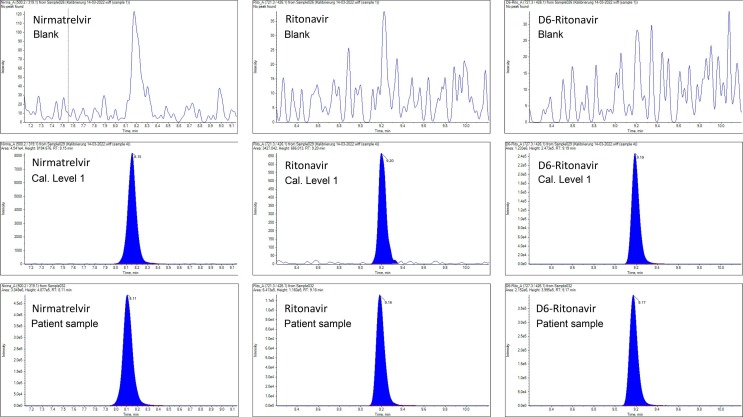
Nirmatrelvir and ritonavir are molecules of moderate to low polarity and are thus suitable for separation on a reversed phase HPLC-column. The mobile phase gradient provided adequate separation of polar endogenous substances at the front of the chromatogram. As it can be seen in Fig. 1 , sharp and symmetric peaks for all analytes were obtained and no interferences from endogenous substances were observed. Surprisingly, the relatively high column temperature of 55 °C was necessary to achieve the depicted resolution. At lower temperatures of 45 °C, 35 °C or 25 °C, increasingly distorted peak shapes of nirmatrelvir were observed. Possibly, second order interactions, which alleviated at increased temperatures, were responsible for this phenomenon.
Fig. 1.
Representative chromatograms of nirmatrelvir and ritonavir in human plasma. Top row: drug free human plasma without internal standard. Middle row: calibration level 1 (LLOQ) containing 10 ng/mL nirmatrelvir and 2 ng/mL ritonavir. Bottom row: patient sample containing 899 ng/mL nirmatrelvir and 131 ng/mL ritonavir.
In the ion source of the mass spectrometer, positive charged [M + H]+ quasimolecular ions were produced from the analytes, which were subsequently fragmented producing the corresponding MS/MS spectra depicted in Fig. 2 . Proposed fragmentation pathways leading to these fragment ions were indicated in the molecular formulas of the analytes. From these MS/MS spectra, two fragment ions for each analyte were chosen for quantification and qualification, respectively. In the case of ritonavir and the internal standard, the fragment ions chosen for quantification showed a lower absolute response than the fragment ions used for qualification. However, despite the lower response, they showed nearly identical signal-to-noise ratios and they were advantageous in terms of a slightly better selectivity. In the top-row of Fig. 1, a chromatogram obtained from blank plasma without the addition of internal standard is shown. No peaks from endogenous substances were detected and, thus, the selectivity of the observed ion traces for each analyte was confirmed. In the middle row of Fig. 1, a chromatogram of the lowest calibration sample (i.e. the LLOQ) showed that also the sensitivity is sufficient for the intended calibration range. The matrix effect on the ionization efficiency of plasma extracts in comparison to water extracts was 113.9 ± 8.9 % (n = 6, p < 0.001) for nirmatrelvir. For ritonavir and the internal standard D6-ritonavir, no significant matrix effects were observed (104.7 ± 9.3 %, p = 0.531 and 105.6 ± 8.8 %, p = 0.306). Comparing the quantification results from 6 different plasma matrices, coefficients of variation of 5.56 % for nirmatrelvir and 3.38 % for ritonavir were observed. These results confirmed that matrix effects had only minor influence on the quantification results so that the requirements of the European Medicines Agency for bioanalytical method validation were satisfied [9]. A dedicated isotope labelled internal standard for nirmatrelvir would likely further improve the precision and accuracy of the quantification and would alleviate the matrix effect on this compound.
Fig. 2.
MS/MS spectra of pure standard solutions of nirmatrelvir, ritonavir and the internal standard D6-ritonavir (30 ng/min constant flow infusion). Probable fragmentation pathways leading to these mass spectra were indicated in the molecular formulas of the analytes.
3.3. Calibration
Presently, no empirical concentration values of the active substances of Paxlovid® achieved in clinical practice were published. In the product information of Paxlovid®, Cmax values of 2210 ng/mL for nirmatrelvir and 360 ng/mL for ritonavir were observed after a single standard dose in healthy volunteers [1]. Extrapolating these concentrations with the reported half-lives of 6.1 h for each substance and a standard dosing interval of 12 h, Cmin values of about 550 ng/mL for nirmatrelvir and about 90 ng/mL for ritonavir can be expected. In order to cover these ranges, including margins for enhanced or retarded elimination in special patient groups, calibration ranges of 10 – 10000 ng/mL for nirmatrelvir and 2 – 2000 ng/mL for ritonavir were chosen. In consideration of the hydrophobicity of the analytes, especially in the case of ritonavir, drug-free human plasma instead of pure water was chosen as medium for the dilution series to prepare the calibration samples. Applying this approach, consistent and stable calibration samples were obtained. After application of 1/x weighing, linear calibration functions were calculated with correlation coefficients of 0.9995 for nirmatrelvir and 0.9996 for ritonavir, with corresponding slopes of 0.002534 ± 0.000035 for nirmatrelvir and 0.003495 ± 0.000044 for ritonavir. No significant intercepts of the calibration functions were observed (p = 0.891 for nirmatrelvir and p = 0.053 for ritonavir). Consequently, the intercepts were set to zero. The LLOQ’s were set to the lowest calibration concentration (10 ng/mL for nirmatrelvir and 2 ng/mL for ritonavir). The LLOD’s were found to be 0.5 ng/mL nirmatrelvir and 0.3 ng/mL ritonavir at signal-to-noise ratios of > 3. While real-world clinical data on concentrations of nirmatrelvir and ritonavir in patients receiving Paxlovid®-therapy are currently very scarce, the calibration ranges should be regarded as experimental and may need adaption in future implementations of the method.
3.4. Precision and accuracy
Precision and accuracy data were evaluated both intra-day (n = 10) and inter-day (n = 6) at the LLOQ and the three quality control levels. The results were summarized in Table 1, Table 2 . All values satisfied the requirements for method validation issued by the European Medicines Agency [9]. The concentrations of the QC-samples were chosen to represent concentrations which could be regarded as clearly lower than the therapeutic aim-point (QC-low) [6], at concentrations expected in normal therapy (QC-med) [1], and at concentrations clearly above the therapeutic aim-point (QC-high). In this way, all decision points in TDM of nirmatrelvir and ritonavir were covered by the QC-samples.
Table 1.
Intra-day precision and accuracy (n = 10).
| Sample |
Nirmatrelvir |
Ritonavir |
||||||
|---|---|---|---|---|---|---|---|---|
| Spike |
Amount detected |
Accuracy* |
CV** |
Spike |
Amount detected |
Accuracy* |
CV** |
|
| (ng/mL) | (ng/mL) | (%) | (%) | (ng/mL) | (ng/mL) | (%) | (%) | |
| LLOQ | 10 | 8.23 | −17.7 | 9.77 | 2 | 1.75 | −12.4 | 9.30 |
| QC-low | 20 | 18.4 | −8.10 | 13.6 | 4 | 3.96 | −1.03 | 5.49 |
| QC-med | 500 | 533 | 6.56 | 2.90 | 100 | 97.9 | −2.12 | 3.80 |
| QC-high | 6000 | 6612 | 10.21 | 3.14 | 1200 | 1239 | 3.25 | 2.25 |
Accuracy: relative difference from expected value.
CV: coefficient of variation.
Table 2.
Inter-day precision and accuracy (n = 6).
| Sample |
Nirmatrelvir |
Ritonavir |
||||||
|---|---|---|---|---|---|---|---|---|
| Spike |
Amount detected |
Accuracy* |
CV** |
Spike |
Amount detected |
Accuracy* |
CV** |
|
| (ng/mL) | (ng/mL) | (%) | (%) | (ng/mL) | (ng/mL) | (%) | (%) | |
| LLOQ | 10 | 8.95 | −10.5 | 7.31 | 2 | 1.68 | −16.2 | 6.40 |
| QC-low | 20 | 21.8 | 9.19 | 9.46 | 4 | 3.63 | −9.28 | 4.31 |
| QC-med | 500 | 536 | 7.30 | 5.56 | 100 | 102.1 | 2.14 | 3.48 |
| QC-high | 6000 | 6222 | 3.70 | 4.48 | 1200 | 1270 | 5.87 | 2.84 |
Accuracy: relative difference from expected value.
CV: coefficient of variation.
3.5. Stability
The stability of the prepared samples was evaluated by re-injecting samples after storage in the autosampler of the HPLC-system for 24 h at room temperature. About 10 % higher peak areas were recorded for all analytes including the internal standard in the re-injected samples, probably due to evaporation of solvent from the prepared samples. However, the quantification of the re-injected samples showed no significant differences form the freshly injected ones because the ratios of nirmatrelvir and ritonavir to the internal standard remained stable. Thus, prepared samples can be stored without special precaution for 24 h at room temperature. Stability over longer delays was not tested because a standard application course of Paxlovid® is completed after 5 days and results need to be timely to have any therapeutic consequences.
Short-term stability of nirmatrelvir and ritonavir in human plasma was assessed using the QC-samples. Freshly prepared human plasma samples were analyzed directly, or otherwise stored for 24 h and 96 h at 23 °C (room temperature) or frozen at −20 °C. Additionally, the stability was tested after three freeze–thaw cycles of the plasma samples. Each sample set was tested in triplicate. The comparison of the results from freshly prepared samples with those undergoing the different storage conditions showed no significant differences in the quantification results (ANOVA and post-hoc comparison after Bonferroni-correction for multiple tests). While typical conditions for sample transport between clinic and laboratory were simulated by these tests, nirmatrelvir and ritonavir could be considered stable in patient samples measured on short-notice for TDM-purposes.
Longer-term storage at −80 °C over three month showed also no significant trends in concentrations of the QC-samples. Thus, the stability at −80 °C of the analytes at the three concentration levels in the QC-samples was verified for at least three months.
3.6. Clinical application
The applicability of the method to support TDM in clinical practice was tested with samples from four hospitalized patients treated for COVID-19 infections with Paxlovid®. An exemplary chromatogram of one of these patient samples is depicted in Fig. 1, bottom row. No peaks from co-administered substances or metabolism products interfering with the analyte peaks were observed. All measured patient samples showed concentrations inside the calibration range of the method with trough levels ranging from 330 to 4730 ng/mL for nirmatrelvir and 5.03 – 638 ng/mL for ritonavir. All trough levels were above the threshold level for efficacy of 292 ng/mL for nirmatrelvir [6]. However, at this time, no further clinical conclusions regarding dose adjustment can be drawn from these values.
4. Conclusion
The described method is capable of the simultaneous quantification of nirmatrelvir and ritonavir in patients receiving Paxlovid® for the treatment of COVID-19. The calibration range covered all concentrations measured so far and the selectivity of the LC-MS/MS detection was sufficient to exlude all interferences. Both analytes were stable in human plasma during typical transport times of samples from clinic to laboratory without special precautions. The method may be helpful to support TDM and thus increase safety and efficacy of the treatment with Paxlovid®. Especially patients, which are at high risk to develop severe disease after COVID-19 infections, but are currently recommended not to be treated with Paxlovid®, may benefit from this option. Future improvement options for the here described method are the introduction of a dedicated isotope labelled internal standard for nirmatrelvir and the adjustment of the calibration ranges and concentrations of the QC-samples after more real-world clinical data were collected.
CRediT authorship contribution statement
Jens Martens-Lobenhoffer: Investigation. Corinna R. Böger: Investigation. Jan Kielstein: Conceptualization. Stefanie M. Bode-Böger: Conceptualization.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: [Jens Martens-Lobenhoffer reports financial support was provided by Pfizer Pharma GmbH Germany.].
Acknowledgement
This study was financially supported by Pfizer Pharma GmbH, Germany. The sponsor was not involved in the acquisition and interpretation of the data. The authors declare to have no personal conflicts of interest.
Data availability
Data will be made available on request.
References
- 1.Paxlovid, European Medicines Agency, 2022, https://www.ema.europa.eu/en/medicines/human/EPAR/paxlovid, accessed 4 May 2022.
- 2.US Food and Drug Administration, Emergency use authorization 105, 2022, https://www.fda.gov/media/155049/download, accessed 26 July 2022.
- 3.L.D. Saravolatz, S. Depcinski, M. Sharma, Molnupiravir and Nirmatrelvir-Ritonavir: Oral COVID Antiviral Drugs, Clin. Infect. Dis. (2022) Online ahead of print. https://doi.org/10.1093/cid/ciac180. [DOI] [PMC free article] [PubMed]
- 4.R. Najjar-Debbiny, N. Gronich, G. Weber, J. Khoury, M. Amar, N. Stein, L.H. Goldstein, W. Saliba, Effectiveness of Paxlovid in Reducing Severe COVID-19 and Mortality in High Risk Patients, Clin. Infect. Dis. (2022) Online ahead of print. https://doi.org/10.1093/cid/ciac443. [DOI] [PMC free article] [PubMed]
- 5.Hammond J., Leister-Tebbe H., Gardner A., Abreu P., Bao W., Wisemandle W., Baniecki M., Hendrick V.M., Damle B., Simón-Campos A., Pypstra R., Rusnak J.M. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. N. Engl. J. Med. 2022;386:1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hiremath S., McGuinty M., Argyropoulos C., Brimble K.S., Brown P.A., Chagla Z., Cooper R., Hoar S., Juurlink D., Treleaven D., Walsh M., Yeung A., Blake P. Prescribing Nirmatrelvir/Ritonavir for COVID-19 in Advanced CKD. CJASN. 2022;17(8):1247–1250. doi: 10.2215/CJN.05270522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Habler K., Brügel M., Teupser D., Liebchen U., Scharf C., Schönermarck U., Vogeser M., Paal M. Simultaneous quantification of seven repurposed COVID-19 drugs remdesivir (plus metabolite GS-441524), chloroquine, hydroxychloroquine, lopinavir, ritonavir, favipiravir and azithromycin by a two-dimensional isotope dilution LC-MS/MS method in human serum. J. Pharm. Biomed. Anal. 2021;196 doi: 10.1016/j.jpba.2021.113935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Estrela R.C.E., Ribeiro F.S., Seixas B.V., Suarez-Kurtz G. Determination of lopinavir and ritonavir in blood plasma, seminal plasma, saliva and plasma ultra-filtrate by liquid chromatography/tandem mass spectrometry detection. Rapid Commun. Mass Spectrom. 2008;22:657–664. doi: 10.1002/rcm.3411. [DOI] [PubMed] [Google Scholar]
- 9.Bioanalytical method validation, European Medicines Agency, 2012, https://www.ema.europa.eu/en/bioanalytical-method-validation, accessed 4 November 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.