Abstract
Background and Objectives
Increased anxious-depressive symptomatology is observed in the preclinical stage of Alzheimer disease (AD), which may accelerate disease progression. We investigated whether β-amyloid, cortical thickness in medial temporal lobe structures, neuroinflammation, and sociodemographic factors were associated with greater anxious-depressive symptoms during the COVID-19 confinement.
Methods
This retrospective observational study included cognitively unimpaired older adults from the Alzheimer's and Families cohort, the majority with a family history of sporadic AD. Participants performed the Hospital Anxiety and Depression Scale (HADS) during the COVID-19 confinement. A subset had available retrospective (on average: 2.4 years before) HADS assessment, amyloid [18F] flutemetamol PET and structural MRI scans, and CSF markers of neuroinflammation (interleukin-6 [IL-6], triggering receptor expressed on myeloid cells 2, and glial fibrillary acidic protein levels). We performed multivariable linear regression models to investigate the associations of prepandemic AD-related biomarkers and sociodemographic factors with HADS scores during the confinement. We further performed an analysis of covariance to adjust by participants' prepandemic anxiety-depression levels. Finally, we explored the role of stress and lifestyle changes (sleep patterns, eating, drinking, smoking habits, and medication use) on the tested associations and performed sex-stratified analyses.
Results
We included 921 (254 with AD biomarkers) participants. β-amyloid positivity (B = 3.73; 95% CI = 1.1 to 6.36; p = 0.006), caregiving (B = 1.37; 95% CI 0.24–2.5; p = 0.018), sex (women: B = 1.95; 95% CI 1.1–2.79; p < 0.001), younger age (B = −0.12; 95% CI −0.18 to −0.052; p < 0.001), and lower education (B = −0.16; 95% CI −0.28 to −0.042; p = 0.008) were associated with greater anxious-depressive symptoms during the confinement. Considering prepandemic anxiety-depression levels, we further observed an association between lower levels of CSF IL-6 (B = −5.11; 95% CI −10.1 to −0.13; p = 0.044) and greater HADS scores. The results were independent of stress-related variables and lifestyle changes. Stratified analysis revealed that the associations were mainly driven by women.
Discussion
Our results link AD-related pathophysiology and neuroinflammation with greater anxious-depressive symptomatology during the COVID-19-related confinement, notably in women. AD pathophysiology may increase neuropsychiatric symptomatology in response to stressors. This association may imply a worse clinical prognosis in people at risk for AD after the pandemic and thus deserves to be considered by clinicians.
Trial Registration Information
ClinicalTrials.gov Identifier NCT02485730.
There has been a global increase in anxious-depressive symptomatology with the COVID-19 pandemic and home confinement.1,2 This will bring long-term implications for mental health and cognitive decline in vulnerable populations.3-5
In this context, both anxiety3 and depression4-6 are associated with an increased risk for developing cognitive impairment7-9 and Alzheimer disease (AD). The prevalence of AD is higher in women,10 and both women and caregivers reported higher anxiety and depression,11,12 particularly during the COVID-19 pandemic.13
Recent studies suggested an early link between β-amyloid (Aβ) and worsening anxious-depressive symptoms in cognitively unimpaired (CU) adults.14,15 Moreover, AD pathology may alter brain structures that regulate the brain's response to stress and increase the proneness to develop anxious-depressive symptoms.16 Another mechanism linking AD with anxiety-depression might be neuroinflammation, which has an early involvement in the pathogenesis of the disease.17 Notably, the CSF interleukin-6 (IL-6) has been consistently reported to be elevated in both patients with depression and AD.16,18,19 Altogether, it becomes relevant to investigate the COVID-19 confinement-related anxious-depressive symptomatology in adults at risk for cognitive decline and AD, addressing sex/gender differences and caregivers' mental health.
Therefore, here we focused on CU older adults, the majority with a family history (FH) of clinically diagnosed sporadic AD. Older adults with FH of sporadic AD are at a higher risk for cognitive impairment and dementia20 and start showing AD-related pathologic changes early during midlife.21,22 We investigated the associations of Aβ burden, neuroinflammation, and brain structure data acquired approximately 2.4 years before the pandemic with anxious-depressive symptomatology during the COVID-19 confinement. We hypothesized that (1) adults with Aβ burden, higher CSF IL-6 values, and/or lower structural integrity in AD-related regions (medial temporal lobe structures) will show greater anxiety-depression during the confinement and (2) these associations will be independent of the preconfinement anxiety-depression levels. We also hypothesized that women and caregivers will present higher anxious-depressive symptoms during the confinement.
Methods
Participants
Participants were recruited from the ALzheimer's and FAmilies (ALFA) and ALFA+ cohorts established at the Barcelonaβeta Brain Research Center in Barcelona, Spain, as a research platform to characterize preclinical AD.20 The ALFA cohort includes 2743 CU (Clinical Dementia Rating [CDR] score = 0) older adults aged between 45 and 74 years, enriched for FH of AD (86% had at least 1 parent diagnosed with dementia) and APOE ε4 genotypes. At the baseline visit (2013–2014), sociodemographic, clinical, epidemiologic, genetic, and cognitive data were collected. Participants from the ALFA cohort were invited to participate in the ALFA+ study following a genetic risk enrichment strategy (APOE ε4 carriership and FH of sporadic AD). Four hundred fifty participants from the ALFA cohort were enrolled in the nested ALFA+ study. These participants underwent advanced MRI and PET, lumbar puncture, clinical interviews, cognitive testing, lifestyle, and risk factors evaluations. The inclusion criteria of ALFA+ participants were as follows: (1) participation in the ALFA study; (2) aged 45–75 years at inclusion in the ALFA study; and (3) long-term commitment to follow-up visits, assessments, and study procedures. ALFA+ exclusion criteria were as follows: (1) cognitive impairment (CDR score >0, Mini-Mental State Examination <27, and semantic fluency <12); (2) any unstable medical condition or significant systemic illness that could interfere with protocol compliance; (3) any contraindication to the tests or study procedures; and (4) FH of monogenic AD.20
In the current study, sociodemographic, genetic, clinical, and neuroimaging data collected between 2016 and 2019 from the ALFA and/or ALFA+ participants were used and are referred to as preconfinement measurements. On May 8, 2020, during the de-escalation phases of the COVID-19 confinement, the invitation to participate in the current study was sent via email to 2,582 ALFA participants. On March 14, 2020, during the first wave of the COVID-19 pandemic in Spain, the Spanish government declared a state of emergency and started a national lockdown to control the increasing number of COVID-19 cases in the country. From March 15, all residents were confined to their homes except to make necessary purchases, work, and emergencies.23 On May 2, the government started to implement de-escalation phases to ease the confinement restrictions. Between May 8 and August 31, the period referred to as confinement hereafter, 967 ALFA participants agreed to take part in the current study and completed an online assessment battery that included the Hospital Anxiety and Depression Scale (HADS), the Perceived Stress Scale (PSS), the Brief Resilience Scale (BRS), and an ad hoc evaluation on caregiving and changes in lifestyle patterns (sleep patterns, eating, drinking, smoking habits, and medication use). Of these, 265 were from the ALFA+ study and referred to as biomarker sample in this article. The average time from preconfinement to confinement assessments was 2.4 (±0.8) years.
Standard Protocol Approvals, Registrations, and Patient Consents
The ALFA and ALFA+ study protocols have been approved by the Independent Ethics Committee “Parc de Salut Mar,” Barcelona, and registered at ClinicalTrials.gov (ALFA Identifier: NCT01835717; ALFA+ Identifier: NCT02485730). The COVID-19 protocol (CovidImpact_BBRC2020) has been approved by the Independent Ethics Committee “Parc de la Salud” on March 16, 2020 (Identifier: 2020/9255). All participants signed informed consent that had also been approved by the Independent Ethics Committee “Parc de la Salud”.
Clinical Measurements
Anxiety and Depression
Anxiety and depression were measured with the HADS consisting of 7-item anxiety and 7-item depression subscales. Each subscale has a possible total score ranging from 0 to 21 (≤7 normal, 8–10 borderline, and ≥11 probable anxiety or depression).24
Stress-Related Measurements
We measured self-perceived stress using the 10-item PSS,25 with higher scores indicating greater stress perception. We also assessed the ability to resist or recover from stress with the 6-item BRS.26 Higher scores reflect greater stress resilience.
Caregiver Status and Changes in Lifestyle Patterns
Caregiver status was defined with the following question in the ad hoc evaluation: “Are you a caregiver for a dependent person?” Furthermore, we investigated the changes in lifestyle patterns during the confinement reflecting neuropsychiatric-like behaviors (sleep patterns, eating, drinking and smoking habits, and medication use). Participants answered a questionnaire aimed at evaluating change in sleep (hours), caloric food, alcohol and tobacco consumption, use of anxiolytics/antidepressants, sleeping pills, and analgesics during the confinement compared with preconfinement (eFigure 1, links.lww.com/WNL/C226). The sleep variables were coded to reflect less or more than 7 hours of sleep before and during the confinement. Then, we classified participants under the categories of “No change,” “Decreased,” or “Increased” sleep hours. We categorized the responses to the questions of the rest of the variables as follows: “Decreased” (I have stopped consuming or I have decreased the consumption), “No change” (I have not changed the consumption), and “Increased” (I have increased the consumption moderately or I have increased the consumption significantly).
APOE Genotyping
The APOE genotype was obtained from the allelic combination of the rs429358 and rs7412 variants. APOE status was determined based on the APOE ε4 allele, and the participants were classified as APOE ε4 carriers or APOE ε4 noncarriers.
Neuroimaging and CSF Biomarker Measurements
MRI Acquisitions and MRI-Based AD Signature
Anatomic 3D T1-weighted fast field echo sequence MRIs were obtained with a 3T scanner (Ingenia CX, Philips, the Netherlands) at the Barcelonaβeta Brain Imaging Center with the following parameters: voxel size = 0.75 mm3 isotropic, field of view = 240 × 240 × 180 mm3, flip angle = 8°, repetition time = 9.9 ms, echo time = 4.6 ms, and inversion time = 900 ms in sagittal acquisition. FreeSurfer version 6.0 was used to determine the cortical thickness of regions vulnerable to AD. The so-called AD signature was calculated as the surface-area weighted average of the individual thickness values of the following regions: entorhinal, inferior temporal, middle temporal, and fusiform cortices in both hemispheres.17,27
PET Imaging Acquisitions and Preprocessing
[18F] Flutemetamol PET scans were acquired in a Siemens Biograph mCT (Munich, Germany) after performing a cranial CT scan for attenuation correction. One hundred eighty-five MBq (range 166.5–203.5 MBq) of [18F] flutemetamol was injected to the participants, and 4 frames of 5 minutes each were acquired following the waiting period of 90 minutes. An OSEM3D algorithm with 8 iterations and 21 subsets was used to reconstruct the images with point spread function and time-of-flight corrections into a 1.02 × 1.02 × 2.03 mm matrix. The acquired images were preprocessed using SPM12. Averaged PET images were coregistered to corresponding MRI scans. Following the segmentation of MRIs, the images were normalized to Montreal Neurological Institute (MNI) space together with the PET images. The standardized uptake value ratio (SUVR) was calculated in MNI space from the standard regions (bilateral frontal and parietotemporal areas) and the whole cerebellum as the reference region. We then transformed the SUVR values to the centiloid (CL) scale. Amyloid positivity was ascertained using CL values.28 We defined the cutoff value for CL with a threshold of 12 to classify the participants as Aβ-negative (<12 CL) or Aβ-positive (≥12 CL).
CSF Measurements
IL-6 was measured with the Roche NeuroToolKit, a panel of automated Elecsys and prototype immunoassays (Roche Diagnostics International Ltd, Rotkreuz, Switzerland) on a cobas e411 or e601 instrument at the Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital, Mölndal, Sweden. Glial fibrillary acidic protein (GFAP) and soluble fragment of triggering receptor expressed on myeloid cells 2 (sTREM2), measured with the Roche NeuroToolKit, and also reflecting neuroinflammatory processes,29 were used to test the specificity of the associations between IL-6 and anxiety-depression. We used the log10‐transformed versions of the IL-6 and GFAP measurements for the analyses as they were not normally distributed.29 All participants were blinded to the results of their CSF and amyloid PET assessments.
Statistical Analysis
The characteristics of the samples were defined with means and SDs or medians and ranges for continuous variables and frequencies and percentages for categorical variables. With descriptive purposes, we investigated the differences by sex, caregiver status, Aβ positivity in preconfinement HADS scores, and stress-related measurements (PSS and BRS) with t tests. We also reported raw HADS change scores by group and explored the differences between these groups in lifestyle changes during the confinement with χ2 analyses.
In our main analyses, we performed 2 sets of multivariable linear regression models with HADS total scores during the confinement as the outcome variable. First, we investigated whether AD-related biomarkers showed cross-sectional associations with HADS total scores in the biomarker sample. To this end, we included Aβ-positivity, CSF IL-6, and cortical thickness in AD signature regions as independent variables and demographics, APOE ε4 status, and caregiver status as covariates in the model. We also investigated the factors associated with HADS total scores in the whole sample considering the demographics, APOE ε4 status, and caregiver status as independent variables. In a second step, we performed the models adjusting by preconfinement anxiety-depression levels in the subgroup of participants with available preconfinement HADS scores (see the Results section). In these models, we also controlled for the interindividual variability in the time lag between preconfinement and confinement HADS assessments.
As sensitivity analysis, we explored whether anxiety or depression (HADS-Anxiety and HADS-Depression as dependent variables) drove the results of the main analyses. In addition, considering the higher prevalence of anxiety and depression in women,11 we performed sex-stratified analyses to investigate whether the tested associations were driven by women.
All analyses were performed using RStudio v1.4.1103-4 and SPSS 27 (IBM, Armonk, NY) statistical software. Statistical significance was considered when the results yielded a 2-tailed p value lower than 0.05.
Data Availability
The data supporting the findings of the current study may be available on a reasonable request from the ALFA study management team.
Results
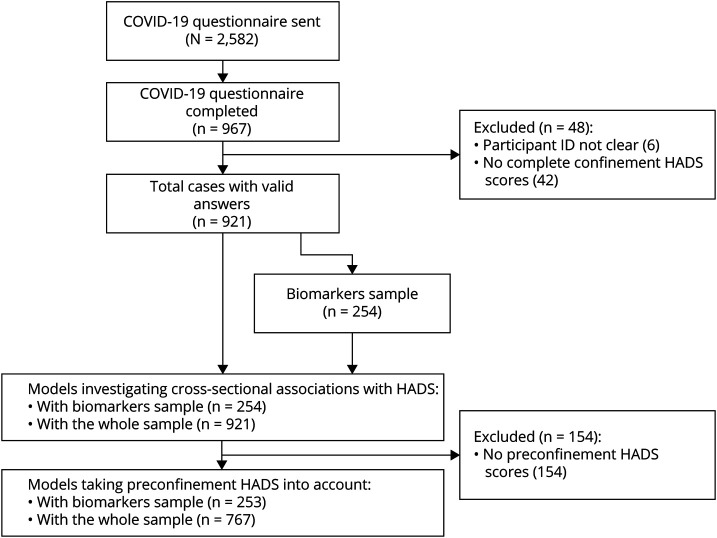
From the ALFA participants who accepted to participate in the study, those with complete HADS evaluation during the confinement were included in the present study (N = 921/2,582, 35.67%). Of these, 254 participants had available AD biomarker data (biomarker sample, Figure 1). The majority of participants were residing in the northeast region of Spain, Catalonia (eFigure 2, links.lww.com/WNL/C226). Participants who accepted to participate in the current study (compared with those who declined [N = 1,661]) had significantly higher years of education (t2580 = 3.87; p < 0.001) and lower preconfinement HADS scores (t1754 = −2.68; p = 0.007).
Figure 1. Flow Diagram Illustrating the Recruitment and Number of Participants Included in Cross-sectional and Longitudinal Analyses.
HADS = Hospital Anxiety and Depression Scale.
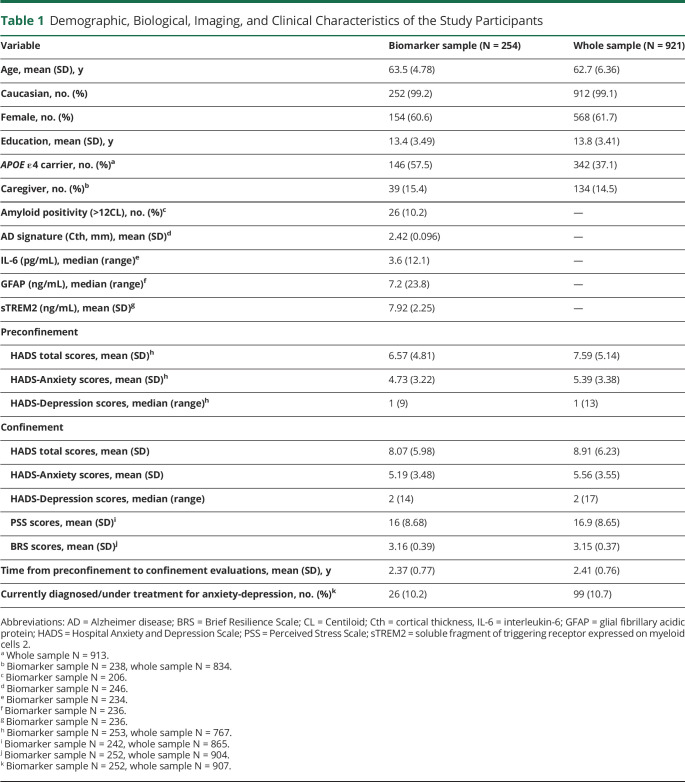
Table 1 shows the demographic, biological, imaging, and clinical data of the biomarker sample and whole sample included in the study. A total of 253 (99.35%) in the biomarker sample and 767 (83.28%) in the whole sample had preconfinement HADS scores. In brief, there were 61.7% of women (N = 568), 14.5% of caregivers (N = 134), 99.1% of White Caucasian (0.9% Latinos), 10.2% (N = 26) of Aβ-positive participants (biomarker sample only), and 10.7% (N = 99) with a self-reported clinical diagnosis of anxiety or depression during the confinement (Table 1).
Table 1.
Demographic, Biological, Imaging, and Clinical Characteristics of the Study Participants
Fifty-one percent of all participants (N = 473) completed the HADS in May, 46.5% (N = 428) in June, 1.4% (N = 13) in July, and 0.8% (N = 7) in August. Thirty-six percent (N = 330) of all participants completed the HADS during the de-escalation phase 0, 13.1% (N = 121) during phase 1, 35.9% (N = 332) during phase 2, 11% (N = 102) during phase 3, and 3.9% (N = 36) during phase 4. The month or phase of the confinement when the HADS was completed did not show any effect on the total anxiety-depression scores (eFigure 3, links.lww.com/WNL/C226).
Participants in the whole sample had significantly higher preconfinement HADS scores (t766 = 40.8; p < 0.001), were younger (t920 = 255.7; p < 0.001), and had higher years of education (t920 = 122.4; p < 0.001) than participants in the biomarker sample. The biomarker sample included a higher number of APOE ε4 carriers (X2 = 32.7; p < 0.001).
Association of Confinement HADS Total Scores With Confinement-Related Variables
In the absence of a control condition, we used proxies of length and intensity of the confinement to assess its association with HADS measurements. Participants who started the confinement at an earlier date or who were confined in smaller-size dwellings did not show higher HADS scores. However, anxiety-depression scores were higher in participants who went outdoors less frequently (F = 21.4, p < 0.001) and in those who did not have any open-air space (e.g., garden, terrace, and balcony) at their dwellings (F = 4.24, p = 0.04).
Preconfinement and Confinement HADS Measurements
In the preconfinement evaluation, the majority of participants scored within the normal ranges of HADS-Anxiety (76.7%) and HADS-Depression (96%).24 During the confinement, 16.6% of the participants showed a significant increase (p < 0.001) in anxious symptomatology (10.8% changed from normal to borderline, 2.5% from borderline to probable, and 3.3% from normal to probable), and 9.9% showed a significant increase (p < 0.001) in depressive symptomatology (6.1% changed from normal to borderline, 0.9% from borderline to probable, and 2.9% from normal to probable). The change in clinical HADS categories from preconfinement to confinement is provided by sex and caregiver status in eTable 1, links.lww.com/WNL/C226.
In the preconfinement evaluations, women had significantly higher total anxiety-depression scores than men (biomarker sample: t245.4 = 3.87; p < 0.001, whole sample: t765 = 4.65; p < 0.001). Caregivers also showed higher HADS scores at the preconfinement as compared to noncaregivers (biomarker sample: t235 = 2.67; p = 0.008, whole sample: t832 = 3.21; p < 0.001). Aβ-positive or Aβ-negative participants did not show any difference in preconfinement HADS total scores (t203 = 1.84; p = 0.067).
Raw mean change in HADS scores (biomarker sample: 1.5, whole sample: 1.32) was higher in women (1.55 ± 6) vs men (0.72 ± 4.6), younger (1.68 ± 5.7) vs older (0.76 ± 5.3) adults, noncaregivers (1.4 ± 5.5) vs caregivers (0.29 ± 5), Aβ-positive (1.81 ± 7.7) vs Aβ-negative (1.6 ± 5.9) participants, APOE ε4 noncarriers (1.35 ± 5.1) vs APOE ε4 carriers (1.04 ± 6.1), participants with lower CSF IL-6 levels (1.85 ± 5.9) vs higher IL-6 levels (1.09 ± 5.6), and those with lower years of education (1.4 ± 5.3) vs higher years of education (1.04 ± 5.6). The mean HADS total, HADS-Anxiety, and HADS-Depression scores by sex, caregiver, and Aβ status during preconfinement and confinement are shown in eFigure 4, links.lww.com/WNL/C226.
Differences in Stress-Related Measurements and Lifestyle Changes by Sex, Caregiver Status, and Amyloid Status
During the confinement, women had higher PSS scores than men (biomarker sample: t240 = 1.97; p = 0.05, whole sample: t863 = 3.47; p < 0.001). The 2 groups did not show any difference in BRS scores (biomarker sample: t250 = −0.58; p = 0.562; whole sample: t858.3 = 0.69; p = 0.493). Compared with noncaregivers, caregivers had higher PSS scores (biomarker sample: t228 = 2.07; p = 0.04, whole sample: t181.5 = 4.56; p < 0.001). Nevertheless, caregivers had higher BRS scores than noncaregivers (whole sample only: t826 = 2.23; p = 0.05). There were no significant differences between Aβ-positive or Aβ-negative participants in PSS (t193 = 1.24; p = 0.216) or BRS (t202 = 1.56; p = 0.12) scores.
Regarding the analyses investigating the changes in lifestyle patterns, we observed sex differences in hours of sleep and food consumption (women>men, X2 = 7.52; p = 0.023; X2 = 37.5; p < 0.001, respectively). In addition, we observed a significant difference between caregivers and noncaregivers in food consumption (caregivers>noncaregivers, X2 = 6.41; p = 0.041). There were no significant differences between Aβ-positive or Aβ-negative participants in any of the investigated lifestyle domain (eTable 2, links.lww.com/WNL/C226).
Factors Associated With Total Anxiety-Depression During the Confinement
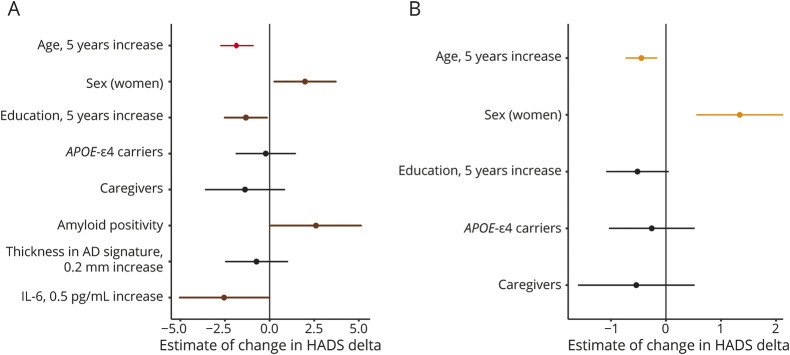
In the biomarker sample, Aβ positivity (B = 3.73; 95% CI 1.1–6.36; p = 0.006), but not thickness in AD signature (B = −5.31; 95% CI −14.5 to 3.87; p = 0.255) or CSF IL-6 (B = −5.13; 95% CI −10.4 to 0.11; p = 0.055), showed a cross-sectional association with greater total anxiety-depression scores independent of age, sex, and years of education. When the model was adjusted by preconfinement HADS scores, higher preconfinement anxiety-depression scores were associated with greater confinement HADS scores and the association between Aβ positivity and HADS total scores remained significant. In addition, lower levels of CSF IL-6 were associated with greater HADS total scores irrespective of the preconfinement anxiety-depression level (Figure 2A and Table 2).
Figure 2. Forest Plots Showing the Multivariable Linear Associations With HADS Total Scores During the Confinement.
The figure shows the estimated amount of change (95% CI) in HADS total scores for a given difference in each factor. (A) Biomarker sample. (B) Whole sample. Both models are adjusted by preconfinement HADS scores and the individual variability between preconfinement and confinement HADS assessments. The colors on the figure represent: black = nonsignificant p value, brown = p < 0.05, orange ≤ 0.01, and red ≤ 0.001. AD = Alzheimer disease; HADS = Hospital Anxiety and Depression Scale; IL-6 = interleukin-6.
Table 2.
Results From the Analyses of Covariance With HADS Total Scores During the Confinement as the Dependent Variable
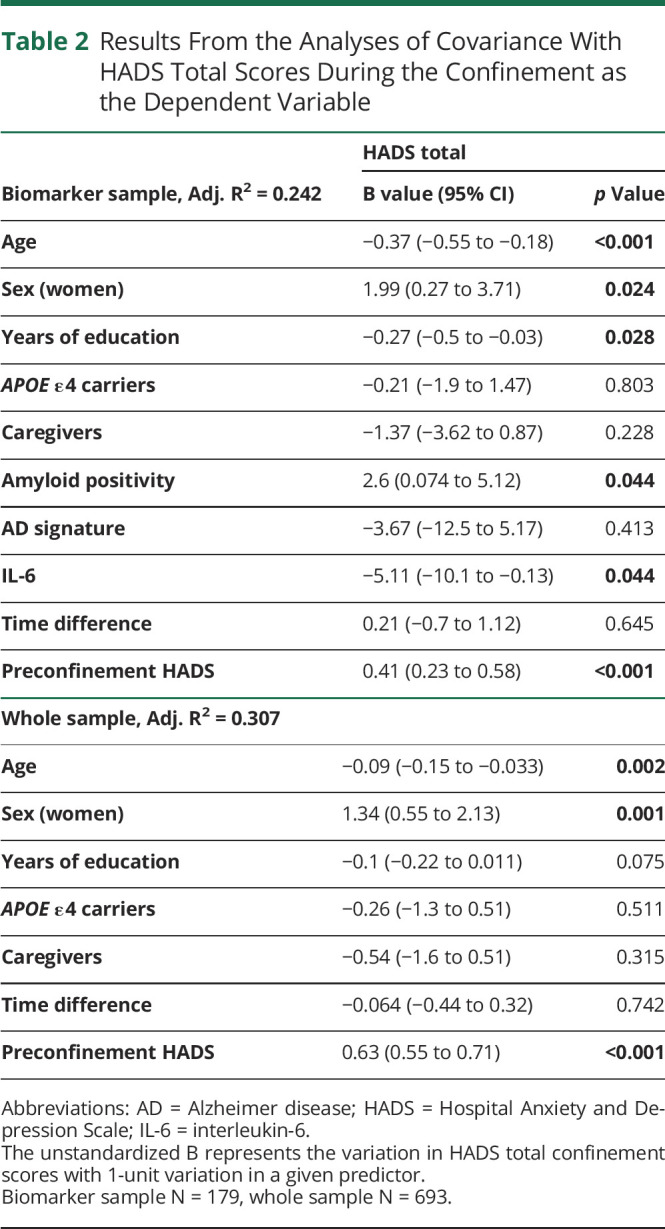
In the whole sample, younger age (B = −0.12; 95% CI −0.18 to −0.052; p < 0.001), being a woman (B = 1.95; 95% CI 1.1–2.79; p ≤ 0.001), lower years of education (B = −0.16; 95% CI −0.28 to −0.042; p = 0.008), and being a caregiver (B = 1.37; 95% CI 0.24–2.5; p = 0.018) showed cross-sectional associations with greater HADS total scores during the confinement. Higher preconfinement HADS scores were associated with greater confinement HADS scores. The associations of age and sex with total anxiety-depression scores were still significant after controlling for the preconfinement HADS scores (Figure 2B and Table 2).
Models with HADS subscales (anxiety and depression) as dependent variables showed similar associations in both samples. Among the AD-related biomarkers, only β-amyloid positivity showed a specific association with greater HADS-Anxiety scores irrespective of the preconfinement anxiety level (eTable 3, links.lww.com/WNL/C226).
Sensitivity Analyses
Amyloid Results
Given the relatively small percentage of Aβ-positive subjects in our cohort, we confirmed the robustness of the results with continuous CL levels that showed an association with HADS total scores irrespective of preconfinement HADS scores (B = 0.055; 95% CI 0.005–0.1; p = 0.031).
Models Excluding the Participants Who Are Currently Diagnosed With or Under the Treatment of Anxiety-Depression
We performed our main analyses without the participants with self-reported clinical diagnosis and/or under the treatment of anxiety-depression. Our main results in the biomarker sample or the whole sample remained significant in these analyses. In addition, IL-6 showed a cross-sectional association with greater HADS total scores during the confinement (B = −5.13; 95% CI −9.98 to −0.27; p = 0.039).
Associations With Neuroinflammation Markers
We investigated whether the observed association between neuroinflammation and HADS total scores was specific to IL-6 as hypothesized. To this end, we performed our main model in the biomarker sample including the sTREM2 and GFAP while taking into account of the preconfinement HADS scores. Results showed that the association between neuroinflammation and anxious-depressive symptoms was restricted to IL-6 because STREM2 and GFAP did not show any association with HADS (sTREM2, B = −0.31, p = 0.13; GFAP, B = 1.63, p = 0.618).
Adjustments by Measurements of Stress
The models considering the preconfinement anxiety-depression levels were adjusted by PSS and BRS scores to evaluate whether stress-related variables had any confounding effect on the reported results. The main results remained significant after this adjustment (eTable 4, links.lww.com/WNL/C226).
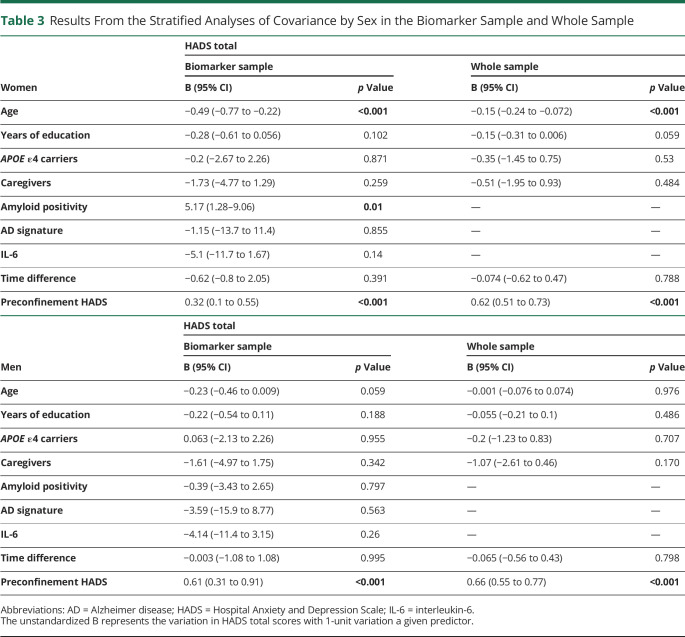
Stratified Analyses by Sex
The results of the sex-stratified analyses adjusted by preconfinement HADS levels are reported in Table 3. These analyses revealed that women are driving the associations reported above (Table 3).
Table 3.
Results From the Stratified Analyses of Covariance by Sex in the Biomarker Sample and Whole Sample
Adjustments by Changes in Lifestyle Patterns
We observed differences in hours of sleep and/or food consumption by sex and caregiver status during the confinement. Therefore, we adjusted our main analyses by hours of sleep and food consumption to control for a potential confounding effect of these variables on the associations tested. Following these adjustments, the previously observed associations did not change. Changes in sleep hours, however, showed an association with HADS total scores (F = 6.23; p = 0.002).
Discussion
The main results of the present study in CU adults at an increased risk for developing AD were as follows: (1) Aβ positivity and lower CSF IL-6 levels measured 2.4 years before the pandemic were associated with greater anxious-depressive symptomatology during the COVID-19 confinement; (2) the results were mainly seen in women and were independent of demographics, stress-related measurements, and changes in lifestyle patterns during the confinement; and (3) women and caregivers presented higher anxious-depressive symptoms during the confinement.
Our sample consisted of adults with low burden of anxiety-depression before the COVID-19 pandemic. Even so, and in line with previous reports,1 16.6% and 9.9% of the participants showed clinically significant increases in anxious-depressive symptoms, respectively. The change in HADS total scores in the biomarker sample (1.5) is considered a significant difference in clinical settings.30 Furthermore, we did not find any effect of specific confinement phase or month on the anxious-depressive symptoms. However, we observed that participants going outdoors less frequently (once a week or less during the confinement and de-escalation phases) and those spending the confinement in a dwelling without any open-air space showed higher anxiety-depression. Overall, these results support that our sample showed modest but clinically meaningful changes associated with the COVID-19 confinement.
β-amyloid positivity was associated with greater anxious-depressive symptoms during the confinement irrespective of the preconfinement anxiety-depression level. This association was driven by anxiety symptoms. These results are consistent with cross-sectional31,32 and longitudinal33,34 data showing that Aβ burden is associated with neuropsychiatric symptoms in CU adults. The results were independent of self-reported perceived stress and stress resilience. However, these associations might be mediated by the physiologic stress response.35 The dysregulation of the hypothalamic-pituitary-adrenal axis may result in a chronic stress response and in elevated adrenal glucocorticoids. This may increase the Aβ deposition and accumulation of tau, which ultimately can cause damage of brain structure and function.16,36,37 Contrary to our expectations, but in line with this temporality of events, brain integrity in AD-related regions was not associated with anxiety-depression. This may indicate that the increase in neuropsychiatric symptomatology in preclinical AD might precede brain atrophy.38 However, these associations may exist with other brain regions not included in our AD signature such as the insula.39
Previous findings suggest neuroinflammation as a mechanism by which anxiety and depression are linked to AD pathophysiology.16,40 Our results showed that the associations between neuroinflammation and anxiety-depression are specific to IL-6 but not to other neuroinflammation markers such as GFAP and sTREM2. Elevated IL-6 levels have been reported previously in subjects with depression19 and in patients with AD.16,18 Moreover, long-lasting stressful events, such as the pandemic, may induce the expression of IL-6.41 Unexpectedly, however, we observed that participants with lower CSF IL-6 levels showed higher anxiety-depression during the confinement irrespective of their anxiety-depression level at the preconfinement. A possible explanation is that the levels of IL-6 might be lower in preclinical AD as reported in early onset AD,42 and therefore, the associations with anxiety-depression might be different in preclinical AD. Future studies with longitudinal data are required to replicate our results and investigate whether the association of IL-6 with neuropsychiatric symptomatology is different throughout the AD continuum.
Our findings showed that the associations between anxiety-depression and Aβ were driven by women. We also observed greater changes in sleep patterns in women than men, which is a factor associated with increasing amyloid levels.43 Although sleep patterns showed an association with greater anxiety-depression, the association with Aβ remained significant after adjustments by sleep. Women also showed greater changes in eating patterns than men. The results are in line with previous research reporting higher prevalence of neuropsychiatric symptomatology11 and higher cognitive vulnerability to AD-related pathophysiology in women.44 Altogether, these findings point out the necessity to address whether the associations of neuropsychiatric symptomatology with AD pathologies in preclinical AD are driven by women. They may also suggest that sex-specific mechanisms linking anxiety-depression and AD exist. Future studies are required to evaluate the biological and sociocultural factors that may explain differences in pathophysiologic and neuropsychiatric profiles between women and men.
In the whole sample, being a woman and being a caregiver were independently associated with greater anxious-depressive symptoms during the confinement. These results are consistent with previous findings,45-47 showing higher perceived stress, anxiety, and depression during the pandemic in women and caregivers. One explanation for higher anxiety-depression observed in caregivers could be related to taking care of patients with chronic illnesses (e.g., dementia).13 Further studies accounting for the condition of the care recipient and the caregiver burden can elucidate whether confinement had an additive effect on the mental health burden of the caregivers. Furthermore, the observed associations in women, but not in caregivers, were independent of the anxiety-depression levels measured before the pandemic. The pandemic may have exacerbated system-level deficits and disparities that could have increased anxiety-depression in women.46 Regarding the caregivers, they showed higher stress resilience than noncaregivers during the confinement, suggesting that they may have more cognitive resources to cope. This may explain the results that did not indicate an increase in anxious-depressive symptoms in caregivers when their preconfinement anxiety-depression levels were taken into account.48
Finally, we observed associations of younger age and lower education level with greater anxious-depressive symptoms during the confinement. These results are consistent with the literature and could be explained by unique stressors (e.g., job loss or unemployment)49 or false beliefs and insufficient information about the pandemic in younger or lower-educated adults.50
This study is not free of limitations. First, our study focuses on participants at an increased risk for developing AD because the majority of our sample have an FH of sporadic AD. The lack of a control group limits the generalizability of our results to the general population and does not allow disentangling the contribution of the natural history of the disease to the observed increases in anxious-depressive symptoms. In the same vein, whether the observed results are attributable to the effect of the confinement itself or to the general effect of the pandemic remains unclear, as they are temporally overlapping and interrelated events. Nevertheless, individual differences in confinement intensity (going outdoors less than once a week and spending the confinement in a dwelling without an open-air space) showed associations with greater anxiety-depression, which supports our interpretation. Furthermore, the study design does not allow studying the causal effects between β-amyloid and anxiety-depression. Future studies are required to investigate whether anxiety and depression precede amyloid or are a consequence of it. In addition, our approach for the missing data was to exclude the participants with missing data, and this may have led to less power to detect some effects. Finally, the percentage of amyloid positivity was low (10%). However, our results were robust across continuous amyloid measurements.
Overall, our findings showed a negative effect of COVID-19 confinement on mental health in people at an increased risk for AD and support the link between neuropsychiatric symptomatology and brain Aβ burden in preclinical AD, notably in women. Future studies are warranted to investigate the consequences of the pandemic and related confinement on mental health and on the clinical prognosis of individuals at the preclinical stage of AD.
Acknowledgment
This publication is part of the Alzheimer's and Families (ALFA) project. The authors would like to express their sincerest gratitude to the ALFA project participants and relatives without whom this research would have not been possible. The authors would also like to thank Dr. Gregory Lecouvey (INSERM, Caen, France), Dr. Fanny Degeilh (LMU, University Hospital Munich, Germany) and Dr. Joe Winer (Stanford University, California, USA) for their advice regarding the evaluations and analyses of mental health and sleep variables. The authors thank Roche Diagnostics International Ltd for providing the kits to measure IL-6 CSF biomarker. ELECSYS, COBAS, and COBAS E are trademarks of Roche. The Roche NeuroToolKit is a panel of exploratory prototype assays designed to robustly evaluate biomarkers associated with key pathologic events characteristic of AD and other neurological disorders, used for research purposes only and not approved for clinical use. Authors also thank GE Healthcare for kindly providing the [18F] flutemetamol doses of ALFA+ study participants. Authors would also like to thank Dr. Prashanthi Vemuri (Mayo Clinic, US) for her scientific input.
Glossary
- AD
Alzheimer disease
- ALFA
ALzheimer and FAmilies
- Aβ
β-amyloid
- BRS
Brief Resilience Scale
- CDR
Clinical Dementia Rating
- CL
Centiloid
- CU
cognitively unimpaired
- FH
family history
- GFAP
glial fibrillary acidic protein
- HADS
Hospital Anxiety and Depression Scale
- IL-6
interleukin-6
- MNI
Montreal Neurological Institute
- PSS
Perceived Stress Scale
- sTREM2
soluble fragment of triggering receptor expressed on myeloid cells 2
- SUVR
standardized uptake value ratio
Appendix. Authors
Appendix 2. Coinvestigators

Footnotes
CME Course: NPub.org/cmelist
COVID-19 Resources: NPub.org/COVID19
Study Funding
This research was supported by Alzheimer's Association research grants (AARG 2019-AARG-644641 and AARG 2019-AARG-644641-RAPID) to E.M. Arenaza-Urquijo. E.M. Arenaza-Urquijo holds a Ramón y Cajal fellowship (RYC2018-026053-I) and a grant of the Ministry of Science and Innovation (PID2019-111514RA-I00). The research leading to these results has received funding from la Caixa Foundation (LCF/PR/GN17/10300004), the Alzheimer's Association, and an international anonymous charity foundation through the TriBEKa Imaging Platform project. Additional support has been received from the Universities and Research Secretariat, Ministry of Business and Knowledge of the Catalan Government under grant 2017-SGR-892.
Disclosure
O. Grau-Rivera receives funding from the Alzheimer's Association Research Fellowship Program (2019-AARF-644568). I. Suridjan is a full-time employee and shareholder of Roche Diagnostics International Ltd. G. Kollmorgen is a full-time employee of Roche Diagnostics GmbH. A. Bayfield is a full-time employee and shareholder of Roche Diagnostics GmbH. H. Zetterberg is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2018-02532), the European Research Council (#681712), Swedish State Support for Clinical Research (#ALFGBG-720931), the Alzheimer Drug Discovery Foundation (ADDF), USA (#201809-2016862), the AD Strategic Fund and the Alzheimer's Association (#ADSF-21-831376-C, #ADSF-21-831381-C, and #ADSF-21-831377-C), the Olav Thon Foundation, the Erling-Persson Family Foundation, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden (#FO2019-0228), the European Union's Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No 860197 (MIRIADE), and the UK Dementia Research Institute at UCL. Dr. Zetterberg has also served at scientific advisory boards and/or as a consultant for Alector, Eisai, Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics, Nervgen, AZTherapies, CogRx, and Red Abbey Labs, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, and Biogen, and is a cofounder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). M. Suárez-Calvet receives funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (Grant agreement No. 948677), from the Instituto de Salud Carlos III (PI19/00155), and from the Spanish Ministry of Science, Innovation and Universities (Juan de la Cierva Programme grant IJC2018-037478-I). Dr. Suárez-Calvet has also served as a consultant and at advisory boards for Roche Diagnostics International Ltd and has given lectures in symposia sponsored by Roche Diagnostics, S.L.U and Roche Farma, S.A. J. Domingo Gispert is supported by the Spanish Ministry of Science and Innovation (RYC-2013-13054) and the Agencia Estatal de Investigación Proyectos de I+D+i Retos Investigación (RTI2018-102261-B-I00). Dr. Domingo Gispert has also received research support from GE Healthcare, Roche Diagnostics, and Hoffmann-La Roche and speaker's fees from Philips and Biogen. The other authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Brooks SK, Webster RK, Smith LE, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet (London, England). 2020;395(10227):912-920. doi: 10.1016/S0140-6736(20)30460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nochaiwong S, Ruengorn C, Thavorn K, et al. Global prevalence of mental health issues among the general population during the coronavirus disease-2019 pandemic: a systematic review and meta-analysis. Sci Rep. 2021;11(1):10173. doi: 10.1038/s41598-021-89700-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gimson A, Schlosser M, Huntley JD, Marchant NL. Support for midlife anxiety diagnosis as an independent risk factor for dementia: a systematic review. BMJ Open. 2018;8(4):e019399. doi: 10.1136/bmjopen-2017-019399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geerlings MI, den Heijer T, Koudstaal PJ, Hofman A, Breteler MMB. History of depression, depressive symptoms, and medial temporal lobe atrophy and the risk of Alzheimer disease. Neurology. 2008;70(15):1258-1264. doi: 10.1212/01.wnl.0000308937.30473.d1. [DOI] [PubMed] [Google Scholar]
- 5.Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 2006;63(5):530-538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green RC, Cupples LA, Kurz A, et al. Depression as a risk factor for Alzheimer disease: the MIRAGE Study. Arch Neurol. 2003;60(5):753-759. doi: 10.1001/archneur.60.5.753. [DOI] [PubMed] [Google Scholar]
- 7.Steenland K, Karnes C, Seals R, Carnevale C, Hermida A, Levey A. Late-life depression as a risk factor for mild cognitive impairment or Alzheimer's disease in 30 US Alzheimer's disease centers. J Alzheimers Dis. 2012;31(2):265-275. doi: 10.3233/JAD-2012-111922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donovan NJ, Amariglio RE, Zoller AS, et al. Subjective cognitive concerns and neuropsychiatric predictors of progression to the early clinical stages of Alzheimer disease. Am J Geriatr Psychiatry. 2014;22(12):1642-1651. doi: 10.1016/j.jagp.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geda YE, Knopman DS, Mrazek DA, et al. Depression, apolipoprotein E genotype, and the incidence of mild cognitive impairment: a prospective cohort study. Arch Neurol. 2006;63(3):435-440. doi: 10.1001/archneur.63.3.435. [DOI] [PubMed] [Google Scholar]
- 10.Mielke MM. Sex and gender differences in Alzheimer's disease dementia. Psychiatr Times. 2018;35(11):14-17. [PMC free article] [PubMed] [Google Scholar]
- 11.2021 Alzheimer's disease facts and figures. Alzheimers Dement. 2021;17(3):327-406. doi: 10.1002/alz.12328 [DOI] [PubMed] [Google Scholar]
- 12.Sallim AB, Sayampanathan AA, Cuttilan A, Ho R. Prevalence of mental health disorders among caregivers of patients with Alzheimer disease. J Am Med Dir Assoc. 2015;16(12):1034-1041. doi: 10.1016/j.jamda.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Li Q, Zhang H, Zhang M, et al. Prevalence and risk factors of anxiety, depression, and sleep problems among caregivers of people living with neurocognitive disorders during the COVID-19 pandemic. Front Psychiatry. 2020;11:590343. doi: 10.3389/fpsyt.2020.590343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lavretsky H, Siddarth P, Kepe V, et al. Depression and anxiety symptoms are associated with cerebral FDDNP-PET binding in middle-aged and older nondemented adults. Am J Geriatr Psychiatry. 2009;17(6):493-502. doi: 10.1097/jgp.0b013e3181953b82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pichet Binette A, Vachon-Presseau É, Morris J, et al. , Dominantly Inherited Alzheimer Network DIAN, PREVENT-AD Research Group. Amyloid and tau pathology associations with personality traits, neuropsychiatric symptoms, and cognitive lifestyle in the preclinical phases of sporadic and autosomal dominant Alzheimer's disease. Biol Psychiatry. 2021;89(8):776-785. doi: 10.1016/j.biopsych.2020.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dafsari FS, Jessen F. Depression—an underrecognized target for prevention of dementia in Alzheimer's disease. Transl Psychiatry. 2020;10(1):160-213. doi: 10.1038/s41398-020-0839-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. doi: 10.1002/alz.12131. Milà-Alomà M, Salvadó G, Gispert JD, et al. Amyloid beta, tau, synaptic, neurodegeneration, and glial biomarkers in the preclinical stage of the Alzheimer's continuum. Alzheimers Dement J Alzheimers Assoc. 2020;16(10):1358-1371. doi:10.1002/alz.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blum-Degen D, Müller T, Kuhn W, Gerlach M, Przuntek H, Riederer P. Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer's and de novo Parkinson's disease patients. Neurosci Lett. 1995;202(1-2):17-20. doi: 10.1016/0304-3940(95)12192-7. [DOI] [PubMed] [Google Scholar]
- 19.Sasayama D, Hattori K, Wakabayashi C, et al. Increased cerebrospinal fluid interleukin-6 levels in patients with schizophrenia and those with major depressive disorder. J Psychiatr Res. 2013;47(3):401-406. doi: 10.1016/j.jpsychires.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Molinuevo JL, Gramunt N, Gispert JD, et al. The ALFA project: a research platform to identify early pathophysiological features of Alzheimer's disease. Alzheimers Dementia (N Y). 2016;2(2):82-92. doi: 10.1016/j.trci.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosconi L, Rinne JO, Tsui WH, et al. Increased fibrillar amyloid-{beta} burden in normal individuals with a family history of late-onset Alzheimer's disease. Proc Natl Acad Sci U S A. 2010;107(13):5949-5954. doi: 10.1073/pnas.0914141107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arenaza-Urquijo EM, Salvadó G, Operto G, et al. , ALFA Study. Association of years to parent's sporadic onset and risk factors with neural integrity and Alzheimer biomarkers. Neurology. 2020;95(15):e2065-e2074. doi: 10.1212/WNL.0000000000010527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spanish Royal Decree 463/2020, of March 14th, 2020, declaring the state of alarm in Spain to manage the health crisis situation caused by COVID-19. Ramón y Cajal Abogados. 2020. Accessed November 11, 2021. ramonycajalabogados.com/es/node/2063.
- 24.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361-370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 25.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385-396. doi: 10.2307/2136404. [DOI] [PubMed] [Google Scholar]
- 26.Smith BW, Dalen J, Wiggins K, Tooley E, Christopher P, Bernard J. The brief resilience scale: assessing the ability to bounce back. Int J Behav Med. 2008;15(3):194-200. doi: 10.1080/10705500802222972. [DOI] [PubMed] [Google Scholar]
- 27.Jack CR, Wiste HJ, Weigand SD, et al. Defining imaging biomarker cut points for brain aging and Alzheimer's disease. Alzheimers Demen J. 2017;13(3):205-216. doi: 10.1016/j.jalz.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salvadó G, Molinuevo JL, Brugulat-Serrat A, et al. , Alzheimer's Disease Neuroimaging Initiative, for the ALFA Study. Centiloid cut-off values for optimal agreement between PET and CSF core AD biomarkers. Alzheimers Res Ther. 2019;11(1):27. doi: 10.1186/s13195-019-0478-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milà-Alomà M, Salvadó G, Gispert JD, et al. , ALFA study. Amyloid beta, tau, synaptic, neurodegeneration, and glial biomarkers in the preclinical stage of the Alzheimer's continuum. Alzheimers Demen J. 2020;16(10):1358-1371. doi: 10.1002/alz.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puhan MA, Frey M, Büchi S, Schünemann HJ. The minimal important difference of the hospital anxiety and depression scale in patients with chronic obstructive pulmonary disease. Health Qual Life Outcomes. 2008;6:46. doi: 10.1186/1477-7525-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krell-Roesch J, Lowe VJ, Neureiter J, et al. Depressive and anxiety symptoms and cortical amyloid deposition among cognitively normal elderly persons: the Mayo Clinic Study of Aging. Int Psychogeriatr. 2018;30(2):245-251. doi: 10.1017/S1041610217002368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yasuno F, Kazui H, Morita N, et al. High amyloid-β deposition related to depressive symptoms in older individuals with normal cognition: a pilot study. Int J Geriatr Psychiatry. 2016;31(8):920-928. doi: 10.1002/gps.4409. [DOI] [PubMed] [Google Scholar]
- 33.Donovan NJ, Locascio JJ, Marshall GA, et al. , Harvard Aging Brain Study. Longitudinal association of amyloid beta and anxious-depressive symptoms in cognitively normal older adults. Am J Psychiatry. 2018;175(6):530-537. doi: 10.1176/appi.ajp.2017.17040442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perin S, Harrington KD, Lim YY, et al. , AIBL Research Group. Amyloid burden and incident depressive symptoms in preclinical Alzheimer's disease. J Affect Disord. 2018;229:269-274. doi: 10.1016/j.jad.2017.12.101. [DOI] [PubMed] [Google Scholar]
- 35.Herman JP, McKlveen JM, Ghosal S, et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. 2016;6(2):603-621. doi: 10.1002/cphy.c150015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dong H, Csernansky JG. Effects of stress and stress hormones on amyloid-beta protein and plaque deposition. J Alzheimers Dis. 2009;18(2):459-469. doi: 10.3233/JAD-2009-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Canet G, Hernandez C, Zussy C, Chevallier N, Desrumaux C, Givalois L. Is AD a stress-related disorder? Focus on the HPA axis and its promising therapeutic targets. Front Aging Neurosci. 2019;11:269. doi: 10.3389/fnagi.2019.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ng KP, Chiew H, Rosa-Neto P, Kandiah N, Ismail Z, Gauthier S. Associations of AT(N) biomarkers with neuropsychiatric symptoms in preclinical Alzheimer's disease and cognitively unimpaired individuals. Transl Neurodegener. 2021;10(1):11. doi: 10.1186/s40035-021-00236-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holt-Gosselin B, Tozzi L, Ramirez CA, Gotlib IH, Williams LM. Coping strategies, neural structure, and depression and anxiety during the COVID-19 pandemic: a longitudinal study in a naturalistic sample spanning clinical diagnoses and subclinical symptoms. Biol Psychiatry Glob Open Sci. 2021;1(4):261-271. doi: 10.1016/j.bpsgos.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hurley LL, Tizabi Y. Neuroinflammation, neurodegeneration and depression. Neurotox Res. 2013;23(2):131-144. doi: 10.1007/s12640-012-9348-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hüll M, Strauss S, Berger M, Volk B, Bauer J. The participation of interleukin-6, a stress-inducible cytokine, in the pathogenesis of Alzheimer's disease. Behav Brain Res. 1996;78(1):37-41. doi: 10.1016/0166-4328(95)00213-8. [DOI] [PubMed] [Google Scholar]
- 42.Yamada K, Kono K, Umegaki H, et al. Decreased interleukin-6 level in the cerebrospinal fluid of patients with Alzheimer-type dementia. Neurosci Lett. 1995;186(2-3):219-221. doi: 10.1016/0304-3940(95)11318-q. [DOI] [PubMed] [Google Scholar]
- 43.Lim MM, Gerstner JR, Holtzman DM. The sleep–wake cycle and Alzheimer's disease: what do we know? Neurodegener Dis Manag. 2014;4(5):351-362. doi: 10.2217/nmt.14.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA. Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch Gen Psychiatry. 2005;62(6):685-691. doi: 10.1001/archpsyc.62.6.685. [DOI] [PubMed] [Google Scholar]
- 45.O'Connor RC, Wetherall K, Cleare S, et al. Mental health and well-being during the COVID-19 pandemic: longitudinal analyses of adults in the UK COVID-19 Mental Health & Wellbeing study. Br J Psychiatry. 2021;218(6):326-333. doi: 10.1192/bjp.2020.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giuntella O, Hyde K, Saccardo S, Sadoff S. Lifestyle and mental health disruptions during COVID-19. Proc Natl Acad Sci U S A. 2021;118(9):e2016632118. doi: 10.1073/pnas.2016632118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bergmann M, Wagner M. The impact of COVID-19 on informal caregiving and care receiving across Europe during the first phase of the pandemic. Front Public Health. 2021;9:673874. doi: 10.3389/fpubh.2021.673874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harmell AL, Chattillion EA, Roepke SK, Mausbach BT. A review of the psychobiology of dementia caregiving: a focus on resilience factors. Curr Psychiatry Rep. 2011;13(3):219-224. doi: 10.1007/s11920-011-0187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giorgi G, Lecca LI, Alessio F, et al. COVID-19-Related mental health effects in the workplace: a narrative review. Int J Environ Res Public Health. 2020;17(21):E7857. doi: 10.3390/ijerph17217857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Melki J, Tamim H, Hadid D, Makki M, El Amine J, Hitti E. Mitigating infodemics: the relationship between news exposure and trust and belief in COVID-19 fake news and social media spreading. PLoS One. 2021;16(6):e0252830. doi: 10.1371/journal.pone.0252830. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of the current study may be available on a reasonable request from the ALFA study management team.