Abstract
Purpose
To investigate the prevalence, risk factors of intestinal carbapenem-resistant Enterobacteriaceae (CRE) colonization and bloodstream infection (BSI) caused by CRE in allogeneic hematopoietic stem cell transplantation (allo-HSCT) recipients.
Methods
We analyzed the clinical data of 185 patients with hematological malignancies who underwent allo-HSCT from May 2019 to December 2021. All patients received regular CRE monitoring by rectal swab during allo-HSCT, and some CRE strains were further identified for carbapenemase phenotypes. The rates, distribution and risk factors of CRE colonization, CRE-induced BSI were analyzed.
Results
CRE was detected in 44 of 185 recipients, with colonization rate of 23.8%. A total of 46 strains of CRE were isolated, including 22 Escherichia coli, 17 Klebsiella pneumoniae, three Klebsiella oxytoca, two Enterobacter hormaechei, and two other Enterobacteriaceae. Among the 19 strains identified with carbapenemase phenotypes, eight strains of E. coli produced metal β-lactamase, five K. pneumoniae produced serine carbapenemase, two K. pneumoniae produced metal β-lactamase, two K. oxytoca produced metal β-lactamase, a Citrobacter malonic acid-free produced metal β-lactamase and a Citrobacter freundii produced metal β-lactamase. In 10 patients developed with CRE-related BSI, the types and combined drug sensitivity of strains detected by rectal swab were highly consistent with blood culture. Multivariate analysis revealed that pulmonary infection, perianal infection and carbapenem application in the 3 months pre-transplant were independent risk factors for rectal CRE colonization, while rectal colonization with carbapenem-resistant K. pneumoniae (CR-KP) was an independent risk factor for CRE-induced BSI. The mortality rate within 30 days of CRE-related BSI was 50.0%, and patients receiving multi-drug therapy within 24 hours showed slightly lower mortality than that in the single-drug treatment group.
Conclusion
Allo-HSCT patients with CRE-induced BSI have poor prognosis, and CR-KP rectal colonization is an independent risk factor for CRE-related BSI. Rectal swab screening during allo-HSCT could provide early warning for later CRE-induced BSI.
Keywords: allogeneic hematopoietic stem cell transplantation, carbapenem-resistant enterobacteriaceae, bloodstream infection, intestinal colonization
Introduction
In recent years, due to the progress of HLA matching, GVHD prevention and treatment, and support care, allo-HSCT has significantly improved the prognosis of patients with hematological malignancies.1 However, the outcome of infection during allo-HSCT is still dismal and their treatment represents an unmet clinical need.2 Based on 2018 US data, infection was the third most common cause of death among allo-HSCT recipients who died within 100 days post-transplantation, at 20%, and the second most common cause of death after relapse, after day +100.3 Additionally, the emergence and prevalence of drug-resistant bacteria has become a major challenge in the field of global public health, especially the infection situation caused by carbapenem-resistant enterobacteriales (CRE). Allo-HSCT patients have extremely particular immunological deficiency due to combined utilization of radiotherapy, chemotherapy and immunosuppressants during the process of transplantation, and are high-risk population of CRE infection.4,5 The drug resistance of Enterobacteriaceae to carbapenems is becoming increasingly serious, and the significant impact of CRE on morbidity and mortality in allo-HSCT recipients is now widely appreciated.
Bloodstream infection (BSI) is a fatal complication in the early stage of allo-HSCT, with an incidence of 13.6% to 38.9% and a mortality rate of 26.9–36.5%.6–11 Nevertheless, the mortality rate of BSI induced by CRE was obviously elevated, reaching more than 50%.12 Some studies have indicated that there might exist remarkable correlations between CRE colonization and CRE-induced BSI.13–15 Furthermore, Gorrie et al showed that the intestinal tract is the reservoir of Enterobacteriaceae bacteria, and intestinal colonization of CRE strains is an important risk factor for their migration to other parts of the body and serious secondary infection.16 At present, there are few studies regarding the correlation between CRE intestinal colonization and the later occurrence of CRE-related BSI in allo-HSCT patients.
To explore the early forecasting effect of intestinal CRE screening for the subsequent occurrence of CRE-induced BSI and its guiding value for clinical preemptive anti-infection treatment in allo-HSCT patients, we retrospectively investigate the prevalence and epidemiology of CRE intestinal colonization, the carbapenemase phenotypes, prognostic factors for the acquisition of CRE colonization and the later occurrence of CRE-induced BSI in these certain population.
Materials and Methods
Patients
A total of 185 patients with hematological diseases who received allo-HSCT in the Hematology Department of the First Affiliated Hospital of Zhengzhou University from May 2019 to December 2021 were selected. All patients received periodical rectal swabs to monitor CRE intestinal colonization. Among them, there were 59 cases of acute myeloid leukemia (AML), 63 cases of acute lymphoblastic leukemia (ALL), 30 cases of myelodysplastic syndrome (MDS), 24 cases of severe aplastic anemia (SAA), three cases of paroxysmal nocturnal hemoglobinuria (PNH), three cases of lymphoma, one case of chronic myeloid leukemia (CML) and two cases of mixed acute leukemia (MAL). The study protocol was approved by the Institutional Review Board on Medical Ethics at the First Affiliated Hospital of Zhengzhou University. Informed consent was obtained from all patients to use their personal information for research purposes in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The defined endpoint analysis for the last follow-up was conducted on May 31, 2022.
Intestinal CRE Screening
Each cohort patient was systematically screened for CRE colonization during allo-HSCT. Generally, we implemented continuous weekly rectal screening until hematopoietic reconstruction. Rectal swabs were used to detect intestinal CRE and its drug sensitivity. A total of 185 rectal swabs were collected from non-repetitive patients. After cleaning their hands, the collector wore disposable examination gloves and assisted the patient in the lateral decubitus position to expose the haunch. After wetting with saline, the cotton swab was gently inserted into the patient’s anus for deeper than 5cm, swiveled for three circles, removed and inserted vertically into the sampling tube. Then the collector tightened the caps, cleaned the anus with gauze, and assisted the patient to take a comfortable lying position. Specimens were collected and submitted by specially trained clinical staff to ensure obtaining and transferring qualified samples timely.
Blood Culture Collection
If the patient presented chills or early fever during allo-HSCT (oral temperature measured at a single time ≥38.3℃ or ≥38.0℃ for more than one hour; axillary temperature ≥38℃ or ≥37.7℃ for more than one hour), 10mL peripheral blood was collected according to the standard protocols. Blood was drawn twice consecutively to culture aerobic bacteria and anaerobic bacteria, respectively. CRE-induced BSI was diagnosed with the collection of blood cultures that yielded a CRE strain.
Strain Isolation and Antimicrobial Susceptibility Testing
All CRE strains were further isolated from rectal swabs, and positive rectal swab samples from the same patient were counted only once. VITEK MS IVD 3.0 mass spectrometer, VITEK2 Compact automatic microbial identification and drug sensitivity analysis system were used for bacterial culture, identification and drug sensitivity detection according to the relevant protocols in the National Clinical Laboratory Operation Procedures. The targeted bacteria were Enterobacteriaceae bacteria, including E. coli, K. pneumoniae, Enterobacter cloacae, Enterobacter huesei and so on, and the results were read according to the standards issued by the Committee on Clinical and Laboratory Standardization (CLSI).17 All of them were resistant to at least one carbapenem on the basis of antimicrobial susceptibility testing results determined by the broth microdilution method, with the criteria of MIC of ≥2 mg/L for ertapenem, ≥4 mg/L for imipenem, and ≥4 mg/L for meropenem.
Carbapenemase Laboratory Test
Modified carbapenem inactivation method (mCIM) and modified EDTA-carbapenem inactivation method (eCIM): eCIM is used to distinguish serine carbapenemase from metalase on the premise that mCIM results are positive. A positive eCIM indicates the production of metalase, while a negative eCIM indicates the production of serine carbapenemase (the production of metalase cannot be ruled out, and there is a possibility of the coexistence of serine carbapenemase and metalase).18
Risk Factors for CRE Colonization
Colonization was defined as the existence of at least one positive rectal swab sample for CRE.19 We conducted a case-control study to explore the risk factors and clinical outcomes of patients colonized with CRE. Patients with CRE colonization were included as cases. Patients without CRE colonization were identified as controls. Clinical and epidemiological data, including the demographics, underlying diseases, remission status before allo-HSCT, agranulocytosis duration, ATG usage, C-reactive protein (CRP), procalcitonin (PCT), invasive procedures prior to the isolation of CRE, previous exposures of antibiotic within 3 months, ICU stay and invasive operation history, history of rectal swab screening, history of BSI, diarrhea, oral mucositis, pulmonary disease, perianal infection, and the clinical outcomes, were extracted from the patient’s electronic medical records system and clinical microbiology laboratory database.
Statistical Analysis
Patients with and without colonization with CRE were compared for risk factors. We assessed differences in demographic, clinical, and transplantation parameters using Chi-squared or Fisher’s exact test for categorical variables and Mann–Whitney U-test for continuous variables, depending on data distributions. Univariate models were built to determine the outcomes of interest based on baseline characteristics, including patient age, gender, primary disease, duration of agranulocytosis, carbapenem application in the 3 months prior to transplantation, history of previous rectal swab screening, history of previous BSI, ICU admission, diarrhea, oral mucositis, pulmonary disease and perianal infection. Variables that were deemed significant (p < 0.05) based on the univariate models were then used to build a multivariate (MVA) model. SPSS 22.0 statistical software was used for analysis. All p values are two-sided, and p < 0.05 was considered statistically significant.
Results
Patients’ Characteristics
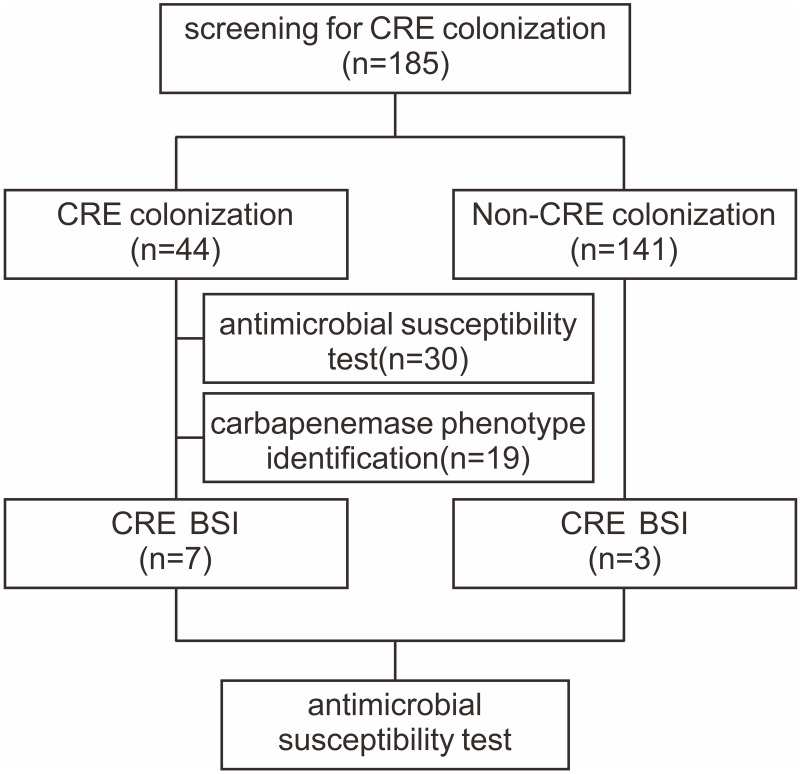
In this study, 185 patients undergoing allo-HSCT were voluntarily screened by rectal swabs for a total of 1009 times. The median age was 30 years (13–57 years). There were 114 male (61.6%) and 71 female (38.4%). Fifty-nine patients received donor-recipient HLA-related matched HSCT, 95 patients underwent HLA-haploidentical HSCT and 31 patients underwent HLA-unrelated HSCT. Among them, 126 cases adopted myeloablative conditioning regimen, 30 utilized intensified conditioning regimen and 29 used reduced conditioning regimen. The median numbers of infused total nucleated cell were 11.57×108/kg (3.95–20.91), and CD34+ cells were 6.76×106/kg (3.66–14.99), respectively. The flow-chart of the current study is depicted in Figure 1. Forty-four patients with positive CRE screening results were included in the CRE group, and 141 patients with negative CRE screening results were included in the non-CRE group. Table 1 shows overall characteristics of patients with or without colonization of CRE. There were no significant differences in terms of age, gender, disease type, duration of agranulocytosis, previous positive history of rectal swab screening, history of previous BSI, ICU admission, invasive procedures, diarrhea, oral mucositis, usage of cephalosporins, fluoroquinolones and penicillins antibiotics between CRE group and non-CRE group.
Figure 1.
The flow-chart of the present study.
Table 1.
Baseline Data of Two Groups of Patients
| Factors | CRE Group (n=44) | Non-CRE Group (n=141) | P-value |
|---|---|---|---|
| Median age, years | 34 (15–57) | 29 (13–57) | 0.074 |
| Gender, n (%) | 0.453 | ||
| Male | 25 (56.8) | 89 (63.1) | |
| Female | 19 (43.2) | 52 (36.9) | |
| Primary Diseases, n (%) | 0.470 | ||
| AML | 14 (31.8) | 45 (31.9) | |
| ALL | 17 (38.6) | 46 (32.6) | |
| MDS | 7 (15.9) | 23 (16.3) | |
| SAA | 4 (9.1) | 20 (14.3) | |
| PNH | 2 (4.6) | 1 (0.7) | |
| Lymphoma | 0 (0) | 3 (2.1) | |
| CML | 0 (0) | 1 (0.7) | |
| MAL | 0 (0) | 2 (1.4) | |
| Duration of agranulocytosis, day (range) | 18 (7–46) | 16 (6–60) | 0.230 |
| Carbapenem application in the 3 months prior to allo-HSCT, n (%) | 0.012 | ||
| Yes | 24 (54.5) | 47 (33.3) | |
| No | 20 (45.5) | 94 (66.7) | |
| History of previous rectal swab screening, n (%) | 0.519 | ||
| Yes | 4 (9.1) | 7 (5.0) | |
| No | 40 (90.9) | 134 (95.0) | |
| History of previous BSI, n (%) | 0.526 | ||
| Yes | 6 (13.6) | 25 (17.7) | |
| No | 38 (86.4) | 116 (82.3) | |
| ICU admission, n (%) | 1.000 | ||
| Yes | 5 (11.4) | 14 (9.9) | |
| No | 39 (88.6) | 127 (90.1) | |
| Invasive procedures, n (%) | 0.829 | ||
| Yes | 9 (20.5) | 31 (22.0) | |
| No | 35 (79.5) | 110 (78.0) | |
| Diarrhea, n (%) | 0.116 | ||
| Yes | 25 (56.8) | 61 (43.3) | |
| No | 19 (43.2) | 80 (56.7) | |
| Oral mucositis, n (%) | 0.859 | ||
| Yes | 36 (81.8) | 117 (83.0) | |
| No | 8 (18.2) | 24 (17.0) | |
| Pulmonary disease, n (%) | 0.031 | ||
| Yes | 36 (81.8) | 91 (64.5) | |
| No | 8 (18.2) | 50 (35.5) | |
| Perianal infections, n (%) | 0.008 | ||
| Yes | 10 (22.7) | 10 (7.1) | |
| No | 34 (77.3) | 131 (92.9) | |
| Polymyxin, n (%) | 0.002 | ||
| Yes | 6 (13.6) | 2 (1.4) | |
| No | 38 (86.4) | 139 (98.6) | |
| Glycopeptides, n (%) | 0.000 | ||
| Yes | 35 (54.5) | 42 (29.8) | |
| No | 9 (45.5) | 99 (70.2) | |
| Tigecycline, n (%) | 0.003 | ||
| Yes | 11 (25.0) | 10 (7.1) | |
| No | 33 (75.0) | 131 (92.9) | |
| Cephalosporins, n (%) | 0.440 | ||
| Yes | 21 (47.7) | 58 (41.1) | |
| No | 23 (52.3) | 83 (58.9) | |
| Fluoroquinolones, n (%) | 0.378 | ||
| Yes | 23 (52.3) | 68 (48.2) | |
| No | 21 (47.7) | 73 (51.8) | |
| Penicillins, n (%) | 0.194 | ||
| Yes | 11 (25.0) | 23 (16.3) | |
| No | 33 (75.0) | 118 (83.7) |
Abbreviations: BSI, bloodstream infection; CRE, carbapenem-resistant enterobacteriaceae; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; MDS, myelodysplastic syndrome; SAA, severe aplastic anemia; PNH, paroxysmal nocturnal hemoglobinuria; CML, chronic myeloid leukemia; MAL, mixed acute leukemia; allo-HSCT, allogeneic hematopoietic stem cell transplantation.
CRE Distribution and Identification of Enzyme Type
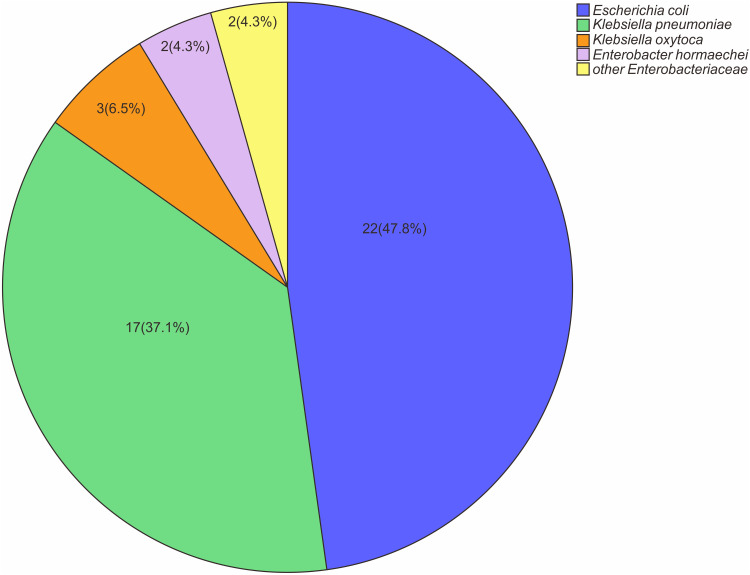
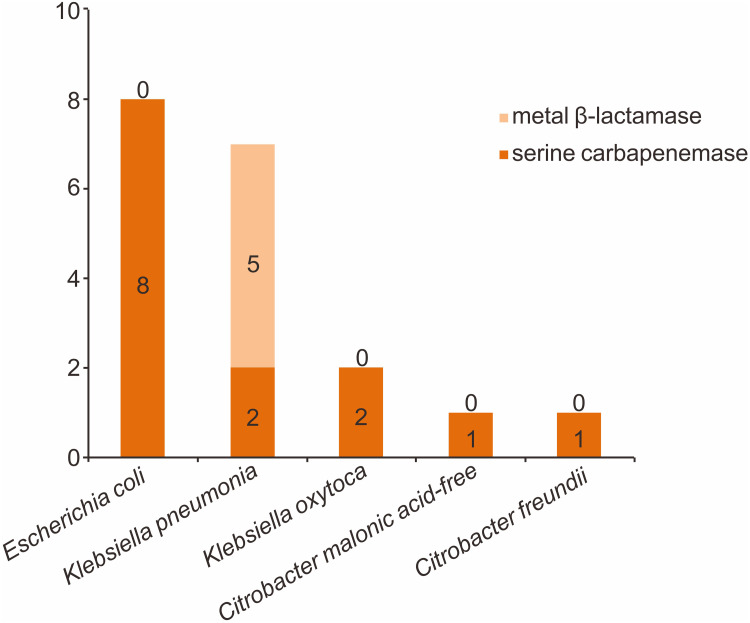
Forty-six CRE strains were isolated from 44 patients with positive rectal swab screening, and the carriage rate was 23.8% (44/185). Twenty-two strains of E. coli were the most common, followed by 17 strains of K. pneumoniae, three strains of K. oxytoca, two strains of E. hormaechei and two strains of other enterobacteriaceae. Specific pathogen distribution is shown in Figure 2. We further carried out combined drug susceptibility test for 30 strains of pathogens. Among carbapenems, the resistance rate of imipenem was higher than that of meropenem (83.3% vs 76.7%). The resistance rate to cephalosporin and enzyme inhibitor compound preparations were all higher. Surprisingly, the resistance rate of cefoperazone sulbactam reached 100%; meanwhile, the resistance rate to tigecycline was lowest, only 6.7%. Furthermore, 19 strains were identified for carbapenemase phenotype. Among them, fourteen isolated strains produced metal β-lactamase (NDM), including eight strains of E. coli, two strains of K. pneumoniae, two strains of K. oxytoca, and other two strains. In addition, five isolated strains produced serine carbapenemase (KPC), all from K. pneumoniae, as shown in Figure 3.
Figure 2.
Microbial characteristics of CRE strains isolated from 44 patients with positive CRE colonization screening.
Figure 3.
Carbapenemase phenotypes of CRE strains isolated from 44 patients with positive CRE colonization screening.
Risk Factors for the Acquisition of CRE Colonization
The risk factors for the acquisition of rectal CRE colonization in allo-HSCT recipients are shown in Table 2. The following factors were significantly associated with CRE colonization in univariate analysis using non-CRE colonization as control group: carbapenem use, pulmonary disease, perianal infection, applications of polymyxin, glycopeptides and tigecycline antibiotics in prior 3 months. Further multivariate analysis demonstrated that pulmonary disease, perianal infection and carbapenem application in prior 3 months were independent risk factors for the acquisition of rectal CRE colonization in allo-HSCT patients.
Table 2.
Multivariate Analysis of Risk Factors Associated with CRE Colonization
| Variable | OR | 95% CI | P-value |
|---|---|---|---|
| Pulmonary disease | 2.587 | 1.050–6.375 | 0.039 |
| Perianal infections | 3.160 | 1.105–9.032 | 0.032 |
| Carbapenems usage | 3.111 | 1.393–6.949 | 0.006 |
| Polymyxin usage | 4.857 | 0.608–38.803 | 0.136 |
| Glycopeptides usage | 1.063 | 0.472–2.393 | 0.883 |
| Tigecycline usage | 1.643 | 0.485–5.571 | 0.425 |
Abbreviations: CRE, carbapenem-resistant enterobacteriaceae; OR, odds ratio; CI, confidence interval.
Risk Factors of BSI Caused by CRE
Throughout the study period, there were 46 patients with BSI during allo-HSCT, and the incidence of BSI was 24.9%. We further divided 46 patients into two groups according to the pathogens isolated from peripheral blood culture. Patients with CRE isolated from peripheral blood culture were included in the CRE-induced BSI group, and patients with non-CRE pathogens isolated from peripheral blood culture were included in the non-CRE-induced BSI group. The general clinical characteristics of the two groups are shown in Table 3. Compared with the non-CRE-induced BSI group, the proportions of carbapenem-resistant K. pneumoniae (CR-KP) rectal colonization, the history of carbapenem application within the previous 3 months, and the history of invasive procedures were significantly higher in the CRE group. Multivariate analysis showed that the rectal colonization of CR-KP (OR, 19.783; 95% CI, 1.984 to 197.301; P = 0.011) was an independent risk factor for BSI induced by CRE (Table 4).
Table 3.
Baseline Data of Patients with CRE BSI and Non-CRE BSI
| Factors | CRE BSI (n=10) | Non-CRE BSI (n=36) | P-value |
|---|---|---|---|
| Median age, years (range) | 40 (16–54) | 32 (14–57) | 0.224 |
| Gender, n (%) | 1.000 | ||
| Male | 7 (70.0) | 24 (66.7) | |
| Female | 3 (30.0) | 12 (33.3) | |
| Primary Diseases, n (%) | 1.000 | ||
| AML | 4 (40.0) | 10 (27.8) | |
| ALL | 1 (10.0) | 9 (25.0) | |
| MDS | 2 (20.0) | 5 (13.8) | |
| AA | 3 (30.0) | 9 (25.0) | |
| PNH | 0 (0) | 1 (2.8) | |
| Lymphoma | 0 (0) | 1 (2.8) | |
| MAL | 0 (0) | 1 (2.8) | |
| Duration of agranulocytosis | 22 (15–46) | 19 (7–60) | 0.377 |
| Type of transplant, n (%) | 1.000 | ||
| Matched related donor | 2 (20.0) | 12 (33.3) | |
| Mismatched related donor | 6 (60.0) | 18 (50.0) | |
| Matched unrelated donor | 2 (20.0) | 5 (13.9) | |
| Mismatched unrelated donor | 0 (0) | 1 (2.8) | |
| Remission status, n (%) | 0.443 | ||
| CR | 5 (50.0) | 25 (69.4) | |
| PR | 5 (50.0) | 11 (30.6) | |
| ATG in regimen, n (%) | 0.777 | ||
| Yes | 7 (70.0) | 29 (80.6) | |
| No | 3 (30.0) | 7 (19.4) | |
| CR-KP rectal colonization, n (%) | 0.000 | ||
| Yes | 6 (60.0) | 2 (5.6) | |
| No | 4 (40.0) | 34 (94.4) | |
| PCT, ng/mL (range) | 0.6 (0.2–22.1) | 0.8 (0.07–70.9) | 0.461 |
| CRP, mg/L (range) | 113.6 (66.4–294.6) | 90.3 (8.1–318.0) | 0.305 |
| Carbapenem application in the 3 months prior to transplantation, n (%) | 0.009 | ||
| Yes | 8 (80.0) | 10 (27.8) | |
| No | 2 (20.0) | 26 (72.2) | |
| History of previous rectal swab screening, n (%) | 0.635 | ||
| Yes | 2 (20.0) | 3 (8.3) | |
| No | 8 (80.0) | 33 (91.7) | |
| History of previous BSI, n (%) | 0.624 | ||
| Yes | 3 (30.0) | 6 (16.7) | |
| No | 7 (70.0) | 30 (83.3) | |
| ICU admission, n (%) | 1.000 | ||
| Yes | 2 (20.0) | 5 (13.9) | |
| No | 8 (80.0) | 31 (86.1) | |
| Invasive procedures, n (%) | 0.009 | ||
| Yes | 5 (50.0) | 3 (8.3) | |
| No | 5 (50.0) | 33 (91.7) | |
| Diarrhea, n (%) | 0.840 | ||
| Yes | 4 (40.0) | 18 (50.0) | |
| No | 6 (60.0) | 18 (50.0) | |
| Oral mucositis, n (%) | 0.929 | ||
| Yes | 8 (80.0) | 26 (72.2) | |
| No | 2 (20.0) | 10 (27.8) | |
| Pulmonary disease, n (%) | 0.243 | ||
| Yes | 10 (100.0) | 28 (77.8) | |
| No | 0 (0.0) | 8 (22.2) | |
| Perianal infections, n (%) | 0.049 | ||
| Yes | 4 (40.0) | 3 (8.3) | |
| No | 6 (60.0) | 33 (91.7) |
Abbreviations: BSI, bloodstream infection; CRE, carbapenem-resistant enterobacteriaceae; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; MDS, myelodysplastic syndrome; SAA, severe aplastic anemia; PNH, paroxysmal nocturnal hemoglobinuria; CML, chronic myeloid leukemia; MAL, mixed acute leukemia; CR, complete remission; PR, partial remission; ATG, antithymocyte globulin; PCT, procalcitonin; CRP, C-reactive protein.
Table 4.
Multivariate Analysis of Risk Factors for CRE BSI
| Risk Factors | OR | 95% CI | P-value |
|---|---|---|---|
| CR-KP rectal colonization | 19.783 | 1.984–197.301 | 0.011 |
| Carbapenem application | 4.147 | 0.524–32.823 | 0.178 |
| Invasive procedures | 7.392 | 0.767–71.265 | 0.084 |
| Perianal infections | 0.210–24.881 | 0.497 |
Abbreviations: CR-KP, carbapenem-resistant K. pneumoniae; OR, odds ratio; CI, confidence interval.
Correlation Analysis Between CRE Colonization and CRE-Induced BSI
Among 185 patients who underwent regular rectal swab screening for CRE, 46 patients developed BSI, with an incidence of 24.9% (46/185), including 45 patients (97.8%) in the granulocytopenia stage. The median time from the day of agranulocytosis to BSI was 7 days (0–24 days). Among these patients who experienced BSI, ten patients were induced with CRE, with an incidence of 5.4% (10/185). And the median time from the day of agranulocytosis to CRE-induced BSI was 5 days (1–17 days). Among the 44 patients with CRE rectal colonization, seven patients experienced subsequent CRE-related BSI, with an incidence of 15.9% (7/44). Among the 141 patients without CRE rectal colonization in the control group, three patients developed subsequent CRE-induced BSI, with an incidence of 2.1% (3/141). The incidence of CRE-related BSI in the rectal colonization group was significantly higher than in those patients without colonization, and the difference was statistically significant (P = 0.001).
Among the 10 patients with CRE-related BSI during allo-HSCT in the experimental group, seven patients were positive for rectal swab screening, and the intestinal colonization rate of CRE was 70.0% (7/10). The results of CRE strains screened were consistent with the results of pathogenic bacteria isolated from peripheral blood culture, and carbapenem-resistant K. pneumoniae accounted for 85.7% (6/7). Carbapenem-resistant E. coli accounted for 14.3% (1/7). Additionally, combining drug susceptibility tests showed that the resistances to imipenem, meropenem, amikacin, tigecycline, colistin, levofloxacin, piperacillin-sulbactam, cefoperazone-sulbactam and cephalosporins were exactly consistent with the results of CRE strains isolated from blood peripheral.
We then compared the efficacy of active empirical antibacterial therapy for patients who experienced CRE-related BSI between CRE colonization and non-CRE colonization groups. For the 10 patients with CRE-related BSI during allo-HSCT, seven cases were from the colonization group, and three were from non-CRE colonization groups, and they were all treated with empirical anti-infective therapy at the time of fever. The specific antimicrobial protocols within 24 hours and associated mortality rates are outlined in Table 5. In the colonization group, the mortality of patients receiving multi-drug combination therapy within 24 hours was lower than that of patients using single drug treatment group; however, the difference was not statistically remarkable.
Table 5.
Specific Antimicrobial Protocols Within 24 Hours and Associated Mortality Rates Between CRE Colonization Group and Control Group
| Antimicrobial Protocol | CRE Group | Non-CRE Group | ||
|---|---|---|---|---|
| Alive | Dead | Alive | Dead | |
| Carbapenem | 0 | 1 | 0 | 1 |
| Carbapenem+tigecycline/polymyxin | 2 | 1 | 1 | 1 |
| Carbapenem+tigecycline+polymyxin | 2 | 1 | 0 | 0 |
Abbreviation: CRE, carbapenem-resistant enterobacteriaceae.
Discussion
To our knowledge, this is the first reported study to assess the prevalence, independent risk factors, and outcomes for the acquisition of CRE colonization, the carbapenemase phenotype, CRE bacteremia in hematological diseases patients receiving allo-HSCT. In the current study, the incidence of positive CRE colonization with rectal swab screening was 23.8% (44/185). Moreover, the incidence of CRE-related BSI in the rectal colonization group was significantly higher than in patients without colonization. These reinforcing evidences indicated that in the high-risk allo-HSCT population where CRE infections are frequent, active surveillance for detecting CRE colonization might be useful in order to administer an effective empirical antimicrobial and prevent transmission to non-colonized patients in the ward.
CRE infections have been emerging rapidly and posing serious challenges to clinical management in the context of allo-HSCT. Thus, timely and efficient diagnosis, strict and evidence-based infection control measures, and prompt and effective therapy are of paramount importance.20–22 Rectal colonization of CRE is a risk factor for bacterial translocation leading to subsequent endogenous CRE infections.23 Therefore, active surveillance in patients who are exposed to CRE-infected or -colonized patients is recommended as part of infection control. The overall rate of CRE colonization in patients with hematologic and oncologic patients may vary between 3% and 29%.24,25 Nevertheless, studies regarding CRE colonization in allo-HSCT patients are rare. In a CRE surveillance study, six of the 46 patients (13%) who received reduced-intensity umbilical blood transplantation were colonized with P aeruginosa. Moreover, the BSI rate has been reported to be higher in colonized patients than in those without colonization (50% vs 7.5%, respectively).26 Another study reported that the rate of P aeruginosa colonization was 7.3% among 794 allo-HSCT patients, but other gram-negative bacteria were not screened for colonization in the study.27 In the current study, the incidence of positive CRE colonization with rectal swab screening was 23.8% (44/185). Our high prevalence might arise from a relatively stronger conditioning regimen, GVHD preventive protocols and a high percentage of carbapenem use in the course of transplantation. For clinical practice and the design of early intervention strategies, assessments of risk factors for CRE are particularly relevant. In studies on CRE colonization, the independent risk factors for colonization were found as antibiotic usage such as carbapenem and fluoroquinolone in the month before colonization.28 One possibility is that inappropriate combined antibiotic usage may disrupt the gastrointestinal microflora and eradicate susceptible competing strains, thus elevating the incidence of CRE colonization. Our findings showed that pulmonary disease, perianal infection and carbapenem application in prior 3 months were independent risk factors for rectal CRE colonization. Further more extensive studies may need to identify risk factors for CRE colonization.
In general, carbapenem resistance for enterobacteriacea arises from two main mechanisms: (i) acquisition of carbapenemase genes that encode for enzymes capable of degrading carbapenems, it most frequently occurs via transfer of plasmids, which usually co-harbor β-lactamases and other resistance determinants, rendering these carbapenemase-producing Enterobacteriaceae (CPE) strains multi-drug resistant (MDR) or extensively drug-resistant (XDR), or (ii) a decrease in the uptake of antibiotics by a qualitative or/and quantitative deficiency of porin expression in association with overexpression of β-lactamases that possess very weak affinity for carbapenems.29 Therefore, rapid detection and characterization of carbapenemase types in colonized CRE strains according to the Ambler classification will lead to improved guidance on the implementation of infection control measures. As these deleterious pathogens may spread throughout health-care facilities, closer attention to infection control measures and stewardship of the carbapenem-containing drugs should be paid in order to control selection of even more detrimental pathogens. In the current study, 19 strains were identified for carbapenemase phenotype. Among them, sixteen isolated strains produced metal β-lactamase, including eight strains of E. coli, two strains of K. pneumoniae, two strains of K. oxytoca, and other two strains. Five isolated strains produced serine carbapenemase (KPC), all from K. pneumoniae. Although the dissemination mechanism is still unclear, our findings highlighted the importance of the identification of enzyme types for the subsequent anti-infective therapy in allo-HSCT recipients.
BSI due to CRE is a fatal complication in patients receiving allo-HSCT and is remarkably associated with dismal prognosis.30 Some studies have reported that an active surveillance strategy reduces BSI frequency during the granulocytopenia period.31,32 Moreover, the European Society of Clinical Microbiology and Infectious Diseases guideline recommends active screening culture to reduce the spread of MDR pathogens.33 Girmenia et al found that 39.2% of patients with CRE colonization subsequently developed CRE-related BSI, which was significantly more frequent than the rate of general hospitalized patients (16.5%).34 In our study, 46 patients developed BSI, with an incidence of 24.9% (46/185), including 45 patients (97.8%) in the granulocytopenia stage. The incidence of CRE-related BSI in the rectal colonization group was significantly higher than in those patients without colonization. Furthermore, compared with the non-CRE-induced BSI group, the proportions of CR-KP rectal colonization, the history of carbapenem application within prior 3 months, and the history of invasive procedures were significantly higher in the CRE group. Multivariate analysis showed that the rectal colonization of CR-KP was an independent risk factor for BSI induced by CRE. Thus, stricter infection control measures such as screening patients for asymptomatic colonization and implementation of contact precautions are urgently needed to limit the CRE dissemination, especially in vulnerable patients who were afflicted by invasive procedures, have CR-KP rectal colonization, and were previously exposed to carbapenem within prior 3 months.
Due to the lack of effective antibiotic treatment, the mortality of CRE-related BSI after allo-HSCT is high, approximately at 51–65%.35–37 An appropriate antimicrobial therapy is crucial for allo-HSCT patients, and it generally depends on the patient’s microflora and the antimicrobial susceptibility pattern of bacteria. Increasing studies have revealed the importance of preemptive intervention in preventing and treating CRE BSI. The empirical treatment of infections might be associated with surveillance and patient’s colonization.38,39 We found that the mortality of patients receiving multi-drug combination therapy within 24 hours in the colonization group was lower than that of patients using single drug treatment group; however, the difference was not statistically remarkable, probably owing to the small sample size. Thus, targeted managements such as multi-drug combination therapy for colonized patients may contribute to reduce the incidence and mortality of BSI induced by CRE, therefore improving the prognosis of these immunocompromised allo-HSCT patients. Other decolonization protocols using oral gentamicin or fecal microbiota transplant still need to be deeply explored for definite effectiveness.
We have to acknowledge several major limitations of this study. First, the design was non-randomized and the sample size was small. We reported observations based on historical data and were thus unable to perform a detailed analysis. Second, rectal swabs screening alone may have underestimated the CRE detection rate in this study. We did not compare the colonized and infected CRE strains using molecular methods because these tests have not been conducted in clinical laboratory of our hospital. Third, the analysis did not include the conditioning regimen, graft-versus-host disease (GVHD), comorbidities, and treatment for BSI. Therefore, it was difficult to perform a detailed survival analysis accurately. A multicenter, prospective study is being carried out in our center to further demonstrate the optimal surveillance opportunity for CRE colonization in allo-HSCT patients.
Conclusion
In summary, screening of patients for CRE colonization may have a remarkable role in preventing the occurrence of BSI due to CRE. This study also demonstrated that surveillance for intestinal colonization caused by CRE in patients receiving allo-HSCT might be useful in guiding to administer appropriate empirical antimicrobial treatment. Strategies that help identify allo-HSCT recipients at risk of CRE and reduce inappropriate empiric antibiotic therapy are paramount to reduce mortality.
Acknowledgments
The present work was supported and funded by the National Natural Science Foundation of China (No. 81900181), Key Scientific Research Project of Henan University (No. 20A320021, 22A320017), Joint Co-construction Project of Henan Medical Technology Research Plan (No. 2018020028) and Key Science and Technology Project of Henan Province (No. SBGJ202103054).
Ethical Statement
This study has been approved by the ethics review committee of the research project of the First Affiliated Hospital of Zhengzhou University, and has obtained relevant certificates.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. This study complies with the Declaration of Helsinki.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Xu LP, Lu PH, Wu DP, et al.; Chinese Blood and Marrow Transplantation Registry Group. Hematopoietic stem cell transplantation activity in China 2019: a report from the Chinese Blood and Marrow Transplantation Registry Group. Bone Marrow Transplant. 2021;56(12):2940–2947. doi: 10.1038/s41409-021-01431-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sahin U, Toprak SK, Atilla PA, Atilla E, Demirer T. An overview of infectious complications after allogeneic hematopoietic stem cell transplantation. J Infect Chemother. 2016;22(8):505–514. doi: 10.1016/j.jiac.2016.05.006 [DOI] [PubMed] [Google Scholar]
- 3.D’Souza A, Fretham C, Lee SJ, et al. Current use of and trends in hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant. 2020;26(8):e177–e182. doi: 10.1016/j.bbmt.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Duin D. Carbapenem-resistant enterobacteriaceae: what we know and what we need to know. Virulence. 2017;8(4):379–382. doi: 10.1080/21505594.2017.1306621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan L, Sun J, Xu X, Huang S. Epidemiology and risk factors of rectal colonization of carbapenemase-producing enterobacteriaceae among high-risk patients from ICU and HSCT wards in a university hospital. Antimicrob Resist Infect Control. 2020;9(1):155. doi: 10.1186/s13756-020-00816-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kikuchi M, Akahoshi Y, Nakano H, et al. Risk factors for pre- and post-engraftment bloodstream infections after allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis. 2015;17(1):56–65. doi: 10.1111/tid.12345 [DOI] [PubMed] [Google Scholar]
- 7.Mikulska M, Raiola AM, Galaverna F, et al. Pre-engraftment bloodstream infections after allogeneic hematopoietic cell transplantation: impact of T cell-replete transplantation from a haploidentical donor. Biol Blood Marrow Transplant. 2018;24(1):109–118. doi: 10.1016/j.bbmt.2017.08.024 [DOI] [PubMed] [Google Scholar]
- 8.Youssef A, Hafez H, Madney Y, et al. Incidence, risk factors, and outcome of blood stream infections during the first 100 days post-pediatric allogeneic and autologous hematopoietic stem cell transplantations. Pediatr Transplant. 2020;24(1):e13610. doi: 10.1111/petr.13610 [DOI] [PubMed] [Google Scholar]
- 9.Cao W, Guan L, Li X, et al. Clinical analysis of bloodstream infections during agranulocytosis after allogeneic hematopoietic stem cell transplantation. Infect Drug Resist. 2021;14:185–192. doi: 10.2147/IDR.S280869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mikulska M, Del Bono V, Bruzzi P, et al. Mortality after bloodstream infections in allogeneic haematopoietic stem cell transplant (HSCT) recipients. Infection. 2012;40(3):271–278. doi: 10.1007/s15010-011-0229-y [DOI] [PubMed] [Google Scholar]
- 11.Stoma I, Karpov I, Milanovich N, Uss A, Iskrov I. Risk factors for mortality in patients with bloodstream infections during the pre-engraftment period after hematopoietic stem cell transplantation. Blood Res. 2016;51(2):102–106. doi: 10.5045/br.2016.51.2.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu Y, Xiao T, Wang Y, et al. Socioeconomic burden of bloodstream infections caused by carbapenem-resistant enterobacteriaceae. Infect Drug Resist. 2021;14:5385–5393. doi: 10.2147/IDR.S341664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao Y, Chen M, Cai M, et al. An analysis of risk factors for carbapenem-resistant enterobacteriaceae infection. J Glob Antimicrob Resist. 2022;30:191–198. doi: 10.1016/j.jgar.2022.04.005 [DOI] [PubMed] [Google Scholar]
- 14.Dickstein Y, Edelman R, Dror T, Hussein K, Bar-Lavie Y, Paul M. Carbapenem-resistant Enterobacteriaceae colonization and infection in critically ill patients: a retrospective matched cohort comparison with non-carriers. J Hosp Infect. 2016;94(1):54–59. doi: 10.1016/j.jhin.2016.05.018 [DOI] [PubMed] [Google Scholar]
- 15.Tamburini FB, Andermann TM, Tkachenko E, Senchyna F, Banaei N, Bhatt AS. Precision identification of diverse bloodstream pathogens in the gut microbiome. Nat Med. 2018;24(12):1809–1814. doi: 10.1038/s41591-018-0202-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorrie CL, Mirceta M, Wick RR, et al. Gastrointestinal carriage is a major reservoir of Klebsiella pneumoniae infection in intensive care patients. Clin Infect Dis. 2017;65(2):208–215. doi: 10.1093/cid/cix270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woods GL, Brown-Elliott BA, Conville PS, et al. Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes. 2nd ed. Wayne (PA): Clinical and Laboratory Standards Institute; 2011. [PubMed] [Google Scholar]
- 18.Lee YT, Huang TW, Liu IF, et al. The prediction values of carbapenemase detection methods and carbapenem susceptibility testing for clinical outcomes of patients with Acinetobacter bacteremia under carbapenem treatment. J Microbiol Immunol Infect. 2022;55(2):257–265. doi: 10.1016/j.jmii.2021.03.013 [DOI] [PubMed] [Google Scholar]
- 19.Zuckerman T, Benyamini N, Sprecher H, et al. SCT in patients with carbapenem resistant Klebsiella pneumoniae: a single center experience with oral gentamicin for the eradication of carrier state. Bone Marrow Transplant. 2011;46(9):1226–1230. doi: 10.1038/bmt.2010.279 [DOI] [PubMed] [Google Scholar]
- 20.Zeng Q, Xiang B, Liu Z. Profile and antibiotic pattern of blood stream infections of patients receiving hematopoietic stem cell transplants in Southwest China. Infect Drug Resist. 2022;15:2045–2054. doi: 10.2147/IDR.S358926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Satlin MJ, Walsh TJ. Multidrug-resistant enterobacteriaceae, pseudomonas aeruginosa, and vancomycin-resistant enterococcus: three major threats to hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2017;19(6):e12762. doi: 10.1111/tid.12762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sahitya DSK, Jandiyal A, Jain A, et al. Prevention and management of carbapenem-resistant Enterobacteriaceae in haematopoietic cell transplantation. Ther Adv Infect Dis. 2021;8:20499361211053480. doi: 10.1177/20499361211053480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoma I, Littmann ER, Peled JU, et al. Compositional flux within the intestinal microbiota and risk for bloodstream infection with gram-negative bacteria. Clin Infect Dis. 2021;73(11):e4627–e4635. doi: 10.1093/cid/ciaa068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petrosillo N, Giannella M, Lewis R, Viale P. Treatment of carbapenem-resistant Klebsiella pneumoniae: the state of the art. Expert Rev Anti Infect Ther. 2013;11(2):159–177. doi: 10.1586/eri.12.162 [DOI] [PubMed] [Google Scholar]
- 25.Ben-David D, Maor Y, Keller N, et al. Potential role of active surveillance in the control of a hospital-wide outbreak of carbapenem-resistant Klebsiella pneumoniae infection. Infect Control Hosp Epidemiol. 2010;31(6):620–626. doi: 10.1086/652528 [DOI] [PubMed] [Google Scholar]
- 26.Narimatsu H, Kami M, Miyakoshi S, et al. Value of pretransplant screening for colonization of Pseudomonas aeruginosa in reduced-intensity umbilical cord blood transplantation for adult patients. Ann Hematol. 2007;86(6):449–451. doi: 10.1007/s00277-007-0260-3 [DOI] [PubMed] [Google Scholar]
- 27.Nesher L, Rolston KV, Shah DP, et al. Fecal colonization and infection with Pseudomonas aeruginosa in recipients of allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis. 2015;17(1):33–38. doi: 10.1111/tid.12323 [DOI] [PubMed] [Google Scholar]
- 28.Young BE, Lye DC, Krishnan P, Chan SP, Leo YS. A prospective observational study of the prevalence and risk factors for colonization by antibiotic resistant bacteria in patients at admission to hospital in Singapore. BMC Infect Dis. 2014;14(1):298. doi: 10.1186/1471-2334-14-298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nordmann P, Dortet L, Poirel L. Carbapenem resistance in enterobacteriaceae: here is the storm! Trends Mol Med. 2012;18(5):263–272. doi: 10.1016/j.molmed.2012.03.003 [DOI] [PubMed] [Google Scholar]
- 30.Yang TT, Luo XP, Yang Q, et al. Different screening frequencies of carbapenem-resistant enterobacteriaceae in patients undergoing hematopoietic stem cell transplantation: which one is better? Antimicrob Resist Infect Control. 2020;9(1):49. doi: 10.1186/s13756-020-0706-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang XL, Wu SH, Shi PF, et al. [Active screening of intestinal carbapenem-resistant enterobacteriaceae in high-risk patients admitted to the hematology wards and its effect evaluation]. Zhonghua Xue Ye Xue Za Zhi. 2020;41(11):932–936. Chinese. doi: 10.3760/cma.j.issn.0253-2727.2020.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pang F, Jia XQ, Zhao QG, Zhang Y. Factors associated to prevalence and treatment of carbapenem-resistant Enterobacteriaceae infections: a seven years retrospective study in three tertiary care hospitals. Ann Clin Microbiol Antimicrob. 2018;17(1):13. doi: 10.1186/s12941-018-0267-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bilavsky E, Schwaber MJ, Carmeli Y. How to stem the tide of carbapenemase-producing enterobacteriaceae?: proactive versus reactive strategies. Curr Opin Infect Dis. 2010;23(4):327–331. doi: 10.1097/QCO.0b013e32833b3571 [DOI] [PubMed] [Google Scholar]
- 34.Girmenia C, Rossolini GM, Piciocchi A, et al.; Gruppo Italiano Trapianto Midollo Osseo (GITMO); Gruppo Italiano Trapianto Midollo Osseo GITMO. Infections by carbapenem-resistant Klebsiella pneumoniae in SCT recipients: a nationwide retrospective survey from Italy. Bone Marrow Transplant. 2015;50(2):282–288. doi: 10.1038/bmt.2014.231 [DOI] [PubMed] [Google Scholar]
- 35.Demiraslan H, Cevahir F, Berk E, Metan G, Cetin M, Alp E. Is surveillance for colonization of carbapenem-resistant gram-negative bacteria important in adult bone marrow transplantation units? Am J Infect Control. 2017;45(7):735–739. doi: 10.1016/j.ajic.2017.01.006 [DOI] [PubMed] [Google Scholar]
- 36.Pouch SM, Satlin MJ. Carbapenem-resistant enterobacteriaceae in special populations: solid organ transplant recipients, stem cell transplant recipients, and patients with hematologic malignancies. Virulence. 2017;8(4):391–402. doi: 10.1080/21505594.2016.1213472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Satlin MJ, Cohen N, Ma KC, et al. Bacteremia due to carbapenem-resistant enterobacteriaceae in neutropenic patients with hematologic malignancies. J Infect. 2016;73(4):336–345. doi: 10.1016/j.jinf.2016.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Satlin MJ, Calfee DP, Chen L, et al. Emergence of carbapenem-resistant enterobacteriaceae as causes of bloodstream infections in patients with hematologic malignancies. Leuk Lymphoma. 2013;54(4):799–806. doi: 10.3109/10428194.2012.723210 [DOI] [PubMed] [Google Scholar]
- 39.Liang Q, Chen J, Xu Y, Chen Y, Huang M. Active surveillance of carbapenem-resistant gram-negative bacteria to guide antibiotic therapy: a single-center prospective observational study. Antimicrob Resist Infect Control. 2022;11(1):89. doi: 10.1186/s13756-022-01103-0 [DOI] [PMC free article] [PubMed] [Google Scholar]